Abstract
Background
The ideal approach for a total hip arthroplasty (THA) would be kind to soft tissues, have the lowest complication rates and be easily reproducible. Although there have been several attempts to find the best approach for THA in the last decade, a definitive answer has not been found. We performed a prospective study to compare the direct anterior and posterior approaches for THA in terms of hospital length of stay, functional outcome, pain, implant position, complications and surgical time.
Methods
A prospective, randomized, multicentre clinical study was conducted between February 2011 and July 2013, with an average follow-up of 55 months. Patients undergoing the direct anterior or posterior approach for THA were enrolled. Hospital length of stay, surgical time and complications were documented. The Harris Hip Score and visual analogue scale were used to monitor functional outcome and pain until 5 years postoperatively. Radiologic analysis was used to assess implant position.
Results
Fifty-five patients (28 undergoing the direct anterior approach, 27 undergoing the posterior approach) were enrolled in this study. Length of stay, functional outcome, pain, implant position and complications were similar for the 2 approaches. There was a trend toward a better functional outcome for patients who underwent the direct anterior approach in the first 3 months postoperatively, with a peak at 4 weeks (Harris Hip Score 76.7 v. 68.7; p = 0.08). Average surgical time for the direct anterior approach was significantly longer (69.9 v. 45.7 min; p = 0.002).
Conclusion
The direct anterior approach for THA appears to be a safe and effective option. However, there is no significant difference in hospital length of stay or postoperative recovery between the 2 approaches.
Clinical trial registration
Abstract
Contexte
L’approche idéale pour l’arthroplastie totale de la hanche (ATH) serait douce pour les tissus mous, aurait le taux de complications le plus bas et serait facilement reproductible. Dans les 10 dernières années, on a tenté à de nombreuses reprises de déterminer quelle est la meilleure approche, sans obtenir de réponse concluante. Nous avons mené une étude prospective visant à comparer la durée du séjour à l’hôpital, les résultats fonctionnels, la douleur, la position de l’implant, les complications et le temps de chirurgie associés aux approches antérieure directe et postérieure pour l’ATH.
Méthodes
Un essai clinique randomisé prospectif multicentrique a été mené auprès de patients ayant subi une ATH par voie antérieure directe ou postérieure entre février 2011 et juillet 2013; le suivi moyen était de 55 mois. La durée du séjour à l’hôpital, le temps de chirurgie et les complications ont été notés. Le score de Harris pour la hanche et l’échelle analogique visuelle ont servi au suivi des résultats fonctionnels et de la douleur dans les 5 ans suivant l’opération. Des clichés radiologiques ont été analysés pour évaluer la position de l’implant.
Résultats
Au total, 55 patients ont été recrutés (28 ayant subi une ATH par voie antérieure directe, et 27, une ATH par voie postérieure). La durée du séjour, les résultats fonctionnels, la douleur, la position de l’implant et les complications étaient sensiblement les mêmes, quelle que soit l’approche utilisée. Dans les 3 premiers mois suivant l’opération, les patients ayant subi une ATH par voie antérieure directe avaient tendance à présenter de meilleurs résultats fonctionnels que les autres, en particulier à la quatrième semaine postopératoire (score de Harris pour la hanche : 76,7 c. 68,7; p = 0,08). Le temps de chirurgie moyen pour l’approche antérieure directe était significativement plus long (69,9 c. 45,7 min; p = 0,002).
Conclusion
La voie antérieure directe semble être une approche efficace et sûre. Aucune différence significative n’a toutefois été observée entre les 2 approches quant à la durée du séjour à l’hôpital ou au rétablissement postopératoire.
Enregistrement de l’essai
The best approach for a total hip arthroplasty (THA) is still a matter of debate. The ideal approach would be kind to soft tissues, have the lowest complication rates and be easily reproducible. Although there have been several attempts to resolve this issue in the last decade, controversy remains.
There are 3 classic approaches for THA, each with disadvantages: the direct anterior approach, the posterior approach and the lateral approach. The main drawback of the posterior approach (PA), the most commonly used approach in the United States and probably worldwide,1,2 is the disinsertion of the external rotators. Posterior dislocation is a potential complication of this approach, although recent studies have showed no increased risk when adequate capsulorrhaphy or enhanced posterior soft tissue repair was performed.3–6 Relying on the Hueter interval, the direct anterior approach (DAA) has a steep learning curve7–9 but is by its very nature truly intermuscular and internervous.10 Two studies have found less iatrogenic muscular damage, as seen on magnetic resonance imaging, with the DAA than with the lateral or posterior approaches.11,12
Consequently, proponents of the DAA infer that the associated postoperative recuperation will be easier and faster and could lead to a better functional outcome. Although recent literature has showed promising short-term results in some respects,7,13–16 other studies have failed to demonstrate long-term advantages13,14,17 or any advantage9,18–21 of the DAA over other surgical approaches to the hip. Furthermore, reported complication rates associated with the DAA have been relatively high in some series.21,22 Therefore, we performed a prospective study to compare the DAA and the PA in terms of hospital stay as the primary outcome and functional recovery, pain, implant position, complications and surgical time as secondary outcomes.
Methods
Patients
This was a multicentre, prospective, randomized clinical trial that enrolled patients who underwent total hip arthroplasties between February 2011 and July 2013. The trial was registered retrospectively with ClinicalTrials.gov (NCT-03673514). Institutional review board approval was granted for this study by the Comité d’éthique de la recherche of the CIUSSS NIM Hôpital du Sacré-Coeur de Montréal (CER 2010-415). After providing written informed consent to participate in this study, patients meeting the inclusion criteria underwent surgical treatment using the DAA or PA, according to a randomization process. This process used random blocks of 2 and 4, ensuring that group allocation was equal throughout the recruitment period. Group allocation was made immediately before surgery by an independent research coworker using sequentially numbered, sealed envelopes containing the designated surgical approach.
Inclusion criteria were as follows: primary total hip replacement because of osteoarthrosis or osteonecrosis, and patients older than 50 years. Patients were excluded if they suffered from inflammatory arthritis, had previously undergone any ipsilateral hip surgery, had a proximal femoral deformity, had a body mass index over 40, had an active infection, had severe contralateral hip disease (Tönnis grade 3 or any dysplasia), had neuromuscular pathology or required structural bone grafts. Patients were enrolled by the 2 surgeons involved in the study (H.G, B.B.).
Clinical and radiologic assessment
Clinical follow-up was scheduled at 2 weeks, 4 weeks, 3 months, 6 months, 1 year, 2 years and 5 years postoperatively. The Harris Hip Score (HHS) and visual analogue scale (VAS) were used to monitor pain and functional outcome at these visits. Data were collected by an independent research assistant at the outpatient clinic of both surgeons.
Radiologic analysis assessed implant position, limb lengthening, and potential implant-related complications. Radiography was performed at subsequent follow-up visits and consisted of an anteroposterior (AP) pelvic radiograph and a lateral projection of the hip.23 Two independent observers (K.M., P.D.), not involved with patients or surgeries, reviewed all postoperative radiographs independently. Cup version was measured with the Lewinnek method. Radiographic analysis of radiolucency and sclerotic changes around the implants was performed using the DeLee zones for the acetabulum and the Gruen zones for the femoral stem. An orthopedic templating software (Orthoview) was used for surgical planning. Preoperatively, an AP scaled radiograph of the pelvis was obtained on which the desired limb length and offset were planned, according to the contralateral side. During surgery, abductor tension (shuck test and hip pistoning) and the kick test were performed by the surgeon to confirm the accuracy of preoperative planning and hip stability.
Surgical procedure
Two fellowship-trained surgeons in 2 separate hospitals performed all procedures. Before the initiation of this study, both surgeons had each performed more than 100 cases with each approach.
Patients underwent either the PA or DAA. The PA to the hip, which is considered to be a minimally invasive approach because of the smaller operative scar (under 10 cm), has been described by many authors24–27 and could potentially yield better results.28 The modified Hueter approach, based on the Smith-Peterson approach, was performed for the direct anterior minimally invasive surgery. 29,30 A traction table was used for DAA as the surgeons were trained to use this method. No intraoperative fluoroscopy was used for implant confirmation.
The 2 groups of patients received the same implants (Quadra-H stem and Versacup hip system, Medacta), with metal on polyethylene bearing. All implants were non-cemented. Surgical approaches were compared in terms of surgical time (skin to skin), length of hospital stay and complications.
Study blinding
Because of the nature of the intervention, it was impossible for the investigator and the patients to be blinded. However, the decision to discharge patients was made by physiotherapists who were blinded to treatment group assignment, and they made the decision on the basis of objective criteria. These criteria were as follows: (a) the patient had to be able to autonomously transfer from bed/chair to the upright position, (b) the patient had to be able to walk with a walking aid, (c) the patient had to be able to climb stairs in a safe way and (d) the patient’s pain had to be controlled by painkillers. No restrictions were recommended for either group. The same rehabilitation and pain protocols were used for the 2 groups. In addition, statistical analyses were performed by an independent consultant who remained blinded to treatment group assignment.
Statistical analysis
The sample size was determined by power analysis (statistical power of 0.80, α of 0.05) with a large effect size (0.80) and an allocation ratio of 1 using G*Power,31 yielding 26 patients per group for a total of 52 patients. Primary and secondary outcome continuous variables were analyzed using the Student t test. The Mann–Whitney U test was used when data were not equally distributed. Nominal variables were analyzed using χ2 tests. All p values were 2-tailed, and the significance level was set at 0.05. All analyses were performed using SPSS version 25.0 software.
Results
Fifty-five total hip athroplasties were performed on 50 patients between February 2011 and July 2013. Demographic data did not differ in terms of age, American Society of Anesthesiologists (ASA) class and body mass index. Only sex distribution differed significantly between the 2 groups (Table 1). One patient in the PA group was lost to follow-up after 6 months; 4 patients (2 in the PA group, 2 in the DAA group) were lost to follow-up after 1 year. All 50 other patients completed the 5-year follow-up. In our cohort of 55 patients, the percentage of hip dysplasia was similar in the 2 groups (7.1% in the DAA group v. 7.4% in the PA group). Only dysplasias of Hartofilakidis type A were present; there were no low or high hip dislocations.
Table 1.
Characteristics of patients who underwent the direct anterior or posterior approach for total hip arthroplast
| Characteristic | Approach | p value | |
|---|---|---|---|
| PA n = 27 |
DAA n = 28 |
||
| Age, yr, mean ± SD | 68.9 ± 8.8 | 70.4 ± 9.1 | 0.52 |
| ASA class, mean ± SD | 2.0 ± 0.8 | 1.8 ± 0.7 | 0.38 |
| Body mass index, mean ± SD | 26.5 ± 4.3 | 27.6 ± 4.4 | 0.36 |
| Sex, no. | 0.042 | ||
| Men | 18 | 11 | |
| Women | 9 | 17 | |
ASA = American Society of Anesthesiologists; DAA = direct anterior approach; PA = posterior approach; SD = standard deviation.
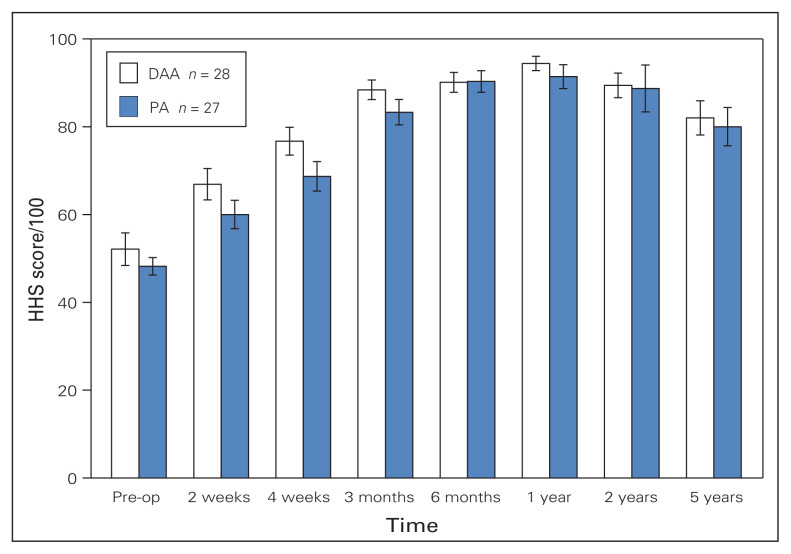
The mean length of hospital stay was similar in the 2 groups: 3.5 days (range 2–10 d) for PA and 3.8 days (range 2–7 d) for DAA (Table 2). Functional preoperative HHS scores were similar for the 2 groups and HHS scores indicated a trend toward faster functional recovery with the DAA until 4 weeks postoperatively (Mann–Whitney U test, 76.7 v. 68.7; p = 0.08). However, no statistical difference was found in terms of overall functional recovery, with similar VAS and HHS scores at each visit for both groups. Figure 1 shows the HHS scores over time. None of the scores for any of the subcategories of the HHS were significantly different at any postoperative time (Table 2).
Table 2.
Length of stay, surgical time, and pain and functional outcomes in patients who underwent the direct anterior or posterior approach for total hip arthroplasty
| Variable | Approach | p value | |
|---|---|---|---|
| PA n = 27 |
DAA n = 28 |
||
| Length of stay, d, mean ± SD | 3.5 ± 2.2 | 3.8 ± 1.8 | 0.53 |
| Surgical time, min, mean ± SD | 45.7 ± 17.9 | 59.9 ± 12.7 | 0.002 |
| VAS score, mean ± SD | |||
| Preoperative | 6.9 ± 2.1 | 5.0 ± 2.4 | 0.029 |
| Postoperative | |||
| 2 wk | 2.1 ± 2.0 | 2.0 ± 2.0 | 0.79 |
| 4 wk | 1.6 ± 1.9 | 1.4 ± 2.0 | 0.63 |
| 3 mo | 1.1 ± 1.9 | 1.0 ± 1.7 | 0.66 |
| 6 mo | 0.4 ± 1.0 | 0.4 ± 0.8 | 0.61 |
| 1 yr | 0.6 ± 1.2 | 0.3 ± 0.5 | 0.38 |
| 2 yr | 1.0 ± 1.9 | 0.5 ± 0.8 | 1.00 |
| HHS score, mean ± SD | |||
| Preoperative | 48.2 ± 10.1 | 52.1 ± 19.7 | 0.66 |
| Postoperative | |||
| 2 wk | 60.0 ± 15.1 | 66.9 ± 17.1 | 0.12 |
| 4 wk | 68.7 ± 16.8 | 76.7 ± 16.4 | 0.08 |
| 3 mo | 83.3 ± 15.1 | 88.4 ± 11.8 | 0.18 |
| 6 mo | 90.3 ± 12.3 | 90.1 ± 11.3 | 1.00 |
| 1 yr | 91.4 ± 13.0 | 94.4 ± 8.0 | 0.72 |
| 2 yr | 88.7 ± 20.0 | 89.4 ± 11.9 | 0.58 |
| 5 yr | 80.0 ± 20.4 | 82.0 ± 19.8 | 0.72 |
DAA = direct anterior approach; HHS = Hip Harris Score; PA = posterior approach; VAS = visual analogue scale; SD = standard deviation.
Fig. 1.
Comparison of Harris Hip Score (HHS) values preoperatively and at different time points postoperatively for patients who underwent the direct anterior approach (DAA) and posterior approach (PA) for total hip arthroplasty. The maximum score possible is 100. Preop = preoperative.
Preoperative VAS pain scores were significantly different between the groups (Mann–Whitney U test, p = 0.029), but postoperative scores were similar at each visit for the 2 groups (Table 2).
Radiologic analysis confirmed that the implants were well positioned in terms of version (Student t test, DAA group 26.9°, range 11° to 45°; PA group 21.9°, range 4° to 41°; p = 0.103) and abduction (Student t test, DAA group 43.3°, range 20° to 56°; PA group 39.8°, range 24° to 49°; p = 0.064), with both surgical approaches (Table 3). The data suggest more anteversion and abduction with the DAA, but none of these values were significant. Cup abduction was statistically different between the 2 surgeons, with a mean difference of 4.8° (Student t test, p = 0.013). With regard to the mean planned leg length discrepancy, the reconstructed hip was 2.2 mm (range −14 to 9 mm) shorter than the ideal planned length for DAA, and a 1.0 mm (range −9 to 10 mm) leg lengthening was reported, compared with the ideal planned length, for PA (Student t test, p = 0.061) (Table 3). Acetabular and femoral implants showed no migration or progressive radiolucency at the latest follow-up.
Table 3.
Radiologic assessment of postoperative pelvic radiographs for acetabular implants and preoperative versus planned leg length for patients who underwent the posterior or direct anterior approach for total hip arthroplasty
| Variable | Approach | p value | |
|---|---|---|---|
| PA n = 27 |
DAA n = 28 |
||
| Implant | |||
| Cup anteversion, degrees, mean ± SD | 21.9 ± 13.4 | 26.9 ± 8.6 | 0.10 |
| Cup abduction, degrees, mean ± SD | 39.8 ± 5.4 | 43.3 ± 8.4 | 0.06 |
| Preoperative LLI, mm, mean ± SD | −2.4 ± 4.3 | −2.2 ± 4.8 | 0.87 |
| Planned leg length discrepancy, mm, mean ± SD | 1.0 ± 5.6 | −2.2 ± 6.8 | 0.06 |
DAA = direct anterior approach; LLI = lower leg inequality; PA = posterior approach, SD = standard deviation.
Few complications were documented for the 2 groups. Two intraoperative periprosthetic fractures occurred in the PA group and were found to be stable (not requiring fixation) according to surgeon assessment: 1 occurred on the acetabular side and the other on the femoral side. These 2 patients were advised to refrain from weight-bearing activities for a period of 6 weeks; both patients had good outcomes and did not require revision surgery. One patient in the DAA group experienced an early prosthetic joint infection with Enterococcus faecalis and was successfully treated with débridement, lavage and intravenous antibiotics.
Surgical time was significantly longer in the DAA group (59.9 min, range 24 to 85 min) than in the PA group (45.7 min; range 26 to 85 min), for a mean difference of 14 minutes (Mann–Whitney U test, p = 0.002). Surgical duration was significantly different between the 2 surgeons (Student t test, p < 0.001); however, each surgeon was significantly faster using the PA (Table 2). The distribution of patients who underwent the DAA and PA was similar for the 2 surgeons.
Discussion
The DAA approach has been widely promoted in recent years as the only truly minimally invasive hip approach, using the intermuscular and internervous interval. The major benefit of the DAA is thought to be a shorter length of stay, accelerated rehabilitation and less postoperative pain. To our knowledge only a few randomized controlled trials have compared clinical outcomes between the DAA and PA,13,32–34 and there is a need for larger multicentre, prospective, randomized controlled trials, as these are sparse.35 Higgins and colleagues concluded, following a meta-analysis, that current evidence comparing outcomes for the DAA versus the PA failed to demonstrate clear superiority of either approach.36 Our findings suggest that the choice of surgical approach should be based on patient characteristics and surgeon experience and preference. As patient satisfaction and clinical follow-up were similar with the 2 approaches and surgical duration was shorter with the PA, both surgeons favour the PA.
Length of stay was similar for the 2 approaches in our study (mean 3.5 d for PA and 3.8 d for DAA). The length of stay in our study may appear to be overly long, but it could have been because of the exacting criteria for hospital discharge, which were applied for both approaches. There was a recent decrease in length of stay for our THA patients resulting from better patient education, which also increased the number of ambulatory THA.
The literature suggests that functional recovery is accelerated in patients who undergo the DAA. However, most of these studies have been retrospective and have had small sample sizes. Barrett and colleagues assessed this issue in a randomized controlled trial comparing 43 patients who underwent the DAA with 44 patients who underwent the PA and showed that DAA patients performed better in the immediate postoperative period.13 More DAA patients were climbing stairs normally and walking freely at 6 weeks. There were no significant differences at later time points. Studies by Reininga and colleagues and Ward and colleagues found improved gait after surgery for both approaches without any significant difference between the 2.32,33 In the present study, functional outcome seemed to improve in the early postoperative period (first 3 months), with an HHS score that almost reached statistical significance at 1-month follow-up. However, this was not borne out in the long-term follow-up. Miller and colleagues reviewed 13 prospective studies comparing DAA and PA in the first 90 days postoperatively and found better hip function, less pain and lower narcotic consumption in the DAA group.16
Findings similar to those of our study have recently been published.32–34 A prospective clinical trial of 54 patients, randomly assigned to undergo the DAA or PA, showed a faster voluntary cessation of use of all walking aids in the DAA group (22 v. 28 d). At 3 weeks, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function scores and SF-12 mental scores favoured the PA group. There were no differences in HHS, SF-12 or WOMAC scores at long-term follow-up.32 Another study comparing the DAA and mini-posterior approaches found no difference in return to activities of daily living, length of stay, complication rate and use of pain medication. However, the DAA group had a higher HHS score at 8 weeks, but these patients also had a higher VAS score for pain in the hospital, and fewer patients returning to work.33 In 2017, Cheng and colleagues also reported a randomized trial involving 72 patients who underwent either the DAA or the PA approach for primary hip arthroplasty.37 They found no difference between the groups with regard to total scores for primary outcomes with the WOMAC, EuroQol and 10 m walk test. Functional outcomes may be improved in the early postoperative period when the DAA is performed; however, this advantage seems to be short lived with negligible long-term benefits. Furthermore, postoperative pain was comparable in both groups at each recorded time.
Implant positioning showed that cup anteversion seemed to be greater with the DAA, but this difference was not significant. Conversely, Barrett and colleagues found significantly more inclination and less anteversion with the DAA, suggesting that it is probably a surgeon-dependent factor.13 Surgeons using an anterior approach will usually avoid too much anteversion to prevent anterior dislocation. All cups were placed within the Lewinnek safe zone with no outliers. There was no statistically significant difference in planned leg lengthening between the cohorts.
Previous reports have demonstrated increased risks of postoperative complications and/or poor outcomes during the surgeon’s learning curve period when adopting the DAA.21,35 Others have indicated a theoretical advantage for the DAA in terms of dislocation risk over the PA. In the present study, a low complication rate was seen with both approaches with no dislocations. Only 1 patient was reoperated for infection and then for a periprosthetic fracture of the femur after sustaining a fall.
Surgical time was significantly longer with the DAA than with the PA. Two meta-analyses concluded that the mean operative time was significantly longer with the DAA and many also reported a steep learning curve.35–38 The only study that found a significantly longer surgical time with the PA39 compared 1 surgeon who performed the DAA surgeon with 2 surgeons who performed the PA. Therefore, these results could be attributed to the surgeon.
The strengths of our study include the random assignment of patients, with few lost to follow-up, and a complete clinical and radiologic follow-up at different periods for a mean of 55 months. Patients were evaluated for discharge by a physiotherapist who was blinded to study group allocation, according to established criteria, unlike a previous publication by Barrett and colleagues.13
Limitations
Limitations of the study include the relatively small number of patients. However, most studies analyzing prospective outcomes following orthopedic surgeries have had a similar sample size. Patients were not equally distributed in the 2 groups in terms of sex, and preoperative VAS score was higher in the PA group, which could have affected the results.
Conclusion
This study could not demonstrate significant advantages for the PA or the DAA in the early nor in the late post-operative recovery. Length of hospital stay was similar in the 2 groups, with a low complication rate for both approaches. Surgical time was greater in the DAA group.
Acknowledgements
The authors thank Julie Fournier for data collection, Ian Massé for statistical analysis and Kathleen Beaumont for manuscript review and preparation.
Footnotes
Competing interests: G. Laflamme and S. Leduc have received consulting fees from Stryker, outside the submitted work. B. Benoit has received consulting fees from Medacta, Stryker and Bioventus, outside the submitted work. The Hôpital Sacré-Coeur de Montréal has received funding for research and educational purposes from Arthrex, ConMed, Depuy, Linvatec, Medacta, Smith & Nephew, Stryker, Synthes, Tornier, Wright and Zimmer Biomet. No other competing interests were declared.
Funding: K. Moerenhout was supported by a grant for an arthroplasty fellowship awarded by the Édouard Samson fund from Hôpital Sacré-Coeur de Montréal; the Fonds de bourses Swiss Orthopaedics, Switzerland; and the Fonds de Perfectionnement du CHUV (Centre hospitalier universitaire Vaudois), Switzerland. There were no sources of outside funding for this study.
Data sharing: The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributors: K. Moerenhout designed the study. P. Derome, S. Leduc, H. Gaspard and B. Benoit acquired the data, which K. Moerenhout, P. Derome, Y. Laflamme analyzed. K. Moerenhout, P. Derome, Y. Laflamme wrote the manuscript, which Y. Laflamme, S. Leduc, H. Gaspard and B. Benoit critically revised. All authors gave final approval of the article to be published.
References
- 1.Waddell J, Johnson K, Hein W, et al. Orthopaedic practice in total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY) Am J Orthop. 2010;39(Suppl):5–13. [PubMed] [Google Scholar]
- 2.Chechik O, Khashan M, Lador R, et al. Surgical approach and prosthesis fixation in hip arthroplasty worldwide. Arch Orthop Trauma Surg. 2013;133:1595–600. doi: 10.1007/s00402-013-1828-0. [DOI] [PubMed] [Google Scholar]
- 3.Kwon MS, Kuskowski M, Mulhall KJ, et al. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34–8. doi: 10.1097/01.blo.0000218746.84494.df. [DOI] [PubMed] [Google Scholar]
- 4.Kim YS, Kwon SY, Sun DH, et al. Modified posterior approach to total hip arthroplasty to enhance joint stability. Clin Orthop Relat Res. 2008;466:294–9. doi: 10.1007/s11999-007-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iorio R, Specht LM, Healy WL, et al. The effect of EPSTR and minimal incision surgery on dislocation after THA. Clin Orthop Relat Res. 2006;447:39–42. doi: 10.1097/01.blo.0000218750.14989.ef. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Li L, Narava M, et al. Is soft tissue repair a right choice to avoid early dislocation after THA in posterior approach? BMC Surg. 2017;17:60. doi: 10.1186/s12893-017-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zawadsky MW, Paulus M, Murray P, et al. Early outcome comparison between the direct anterior approach and the mini-incision posterior approach for primary total hip arthroplasty: 150 consecutive cases. J Arthroplasty. 2014;29:1256–60. doi: 10.1016/j.arth.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics. 2008;31(Suppl 2) [PubMed] [Google Scholar]
- 9.Rathod PA, Bhalla S, Deshmukh AJ, et al. Does fluoroscopy with anterior hip arthroplasty decrease acetabular cup variability compared with a nonguided posterior approach? Clin Orthop Relat Res. 2014;472:1877–85. doi: 10.1007/s11999-014-3512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judet J, Judet H. Anterior approach in total hip arthroplasty. Presse Med. 1985;14:1031–3. [PubMed] [Google Scholar]
- 11.Bremer AK, Kalberer F, Pfirrmann CW, et al. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93:886–9. doi: 10.1302/0301-620X.93B7.25058. [DOI] [PubMed] [Google Scholar]
- 12.Agten CA, Sutter R, Dora C, et al. MR imaging of soft tissue alterations after total hip arthroplasty: comparison of classic surgical approaches. Eur Radiol. 2017;27:1312–21. doi: 10.1007/s00330-016-4455-7. [DOI] [PubMed] [Google Scholar]
- 13.Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs postero-lateral approach for total hip. J Arthroplasty. 2013;28:1634–8. doi: 10.1016/j.arth.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Nakata K, Nishikawa M, Yamamoto K, et al. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24:698–704. doi: 10.1016/j.arth.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Schweppe ML, Seyler TM, Plate JF, et al. Does surgical approach in total hip arthroplasty affect rehabilitation, discharge disposition, and readmission rate? Surg Technol Int. 2013;23:219–27. [PubMed] [Google Scholar]
- 16.Miller L, Gondusky J, Bhattacharyya S, et al. Does surgical approach affect outcomes in total hip arthroplasty through 90 days of follow-up? A systematic review with meta-analysis. J Arthroplasty. 2018;33:1296–302. doi: 10.1016/j.arth.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Graves SC, Dropkin BM, Keeney BJ, et al. Does surgical approach affect patient-reported function after primary THA? Clin Orthop Relat Res. 2016;474:971–81. doi: 10.1007/s11999-015-4639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugano N, Takao M, Sakai T, et al. Comparison of mini-incision total hip arthroplasty through an anterior approach and a posterior approach using navigation. Orthop Clin North Am. 2009;40:365–70. doi: 10.1016/j.ocl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Martin CT, Pugely AJ, Gao Y, et al. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28:849–54. doi: 10.1016/j.arth.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez JA, Deshmukh AJ, Rathod PA, et al. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res. 2014;472:455–63. doi: 10.1007/s11999-013-3231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spaans AJ, van den Hout JA, Bolder SB. High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop. 2012;83:342–6. doi: 10.3109/17453674.2012.711701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wayne N, Stoewe R. Primary total hip arthroplasty: a comparison of the lateral Hardinge approach to an anterior mini-invasive approach. Orthop Rev (Pavia) 2009;1:e27. doi: 10.4081/or.2009.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston RC, Fitzgerald RH, Jr, Harris WH, et al. Clinical and radiographic evaluation of total hip replacement. A standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72:161–8. [PubMed] [Google Scholar]
- 24.Gibson A. Posterior exposure of the hip joint. J Bone Joint Surg Br. 1950;32-B:183–6. doi: 10.1302/0301-620X.32B2.183. [DOI] [PubMed] [Google Scholar]
- 25.Marcy GH, Fletcher RS. Modification of the posterolateral approach to the hip for insertion of femoral-head prosthesis. J Bone Joint Surg Am. 1954;36-A:142–3. [PubMed] [Google Scholar]
- 26.Moore AT. Metal hip joint; a new self-locking vitallium prosthesis. South Med J. 1952;45:1015–9. doi: 10.1097/00007611-195211000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Moore AT. The self-locking metal hip prosthesis. J Bone Joint Surg Am. 1957;39-A:811–27. [PubMed] [Google Scholar]
- 28.Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision posterior approach to total hip arthroplasty. J Arthroplasty. 2014;29:1970–82. doi: 10.1016/j.arth.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Petersen M. Approach to and exposure of the hip joint for mold arthroplasty. J Bone Joint Surg Am. 1949;31-A:40–6. [PubMed] [Google Scholar]
- 30.Hueter C. Grundriss der Chirurgie. Leipzig: FCW Vogel; 1883. [Google Scholar]
- 31.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 32.Reininga IH, Stevens M, Wagenmakers R, et al. Comparison of gait in patients following a computer-navigated minimally invasive anterior approach and a conventional posterolateral approach for total hip arthroplasty: a randomized controlled trial. J Orthop Res. 2013;31:288–94. doi: 10.1002/jor.22210. [DOI] [PubMed] [Google Scholar]
- 33.Ward SR, Jones RE, Long WT, et al. Functional recovery of muscles after minimally invasive total hip arthroplasty. Instr Course Lect. 2008;57:249–54. [PubMed] [Google Scholar]
- 34.Taunton MJ, Mason J, Odum S, et al. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty. 2014;29:169–72. doi: 10.1016/j.arth.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Poehling-Monaghan KL, Kamath AF, Taunton MJ, et al. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473:623–31. doi: 10.1007/s11999-014-3827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins BT, Barlow D, Heagerty N, et al. Anterior vs. posterior approach for total hip arthroplasty, a systematic review and meta-analysis. J Arthroplasty. 2015;30:419–34. doi: 10.1016/j.arth.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Cheng TE, Wallis JA, Taylor NF, et al. A prospective randomized clinical trial in total hip arthroplasty-comparing early results between the direct anterior approach and the posterior approach. J Arthroplasty. 2017;32:883–90. doi: 10.1016/j.arth.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 38.Meermans G, Konan S, Das R, et al. The direct anterior approach in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2017;99-B:732–40. doi: 10.1302/0301-620X.99B6.38053. [DOI] [PubMed] [Google Scholar]
- 39.Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93:1392–8. doi: 10.2106/JBJS.J.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]