Body temperature in tropical forager-farmers declined as much in the past two decades as among Americans in the past 150 years.
Abstract
Normal human body temperature (BT) has long been considered to be 37.0°C. Yet, BTs have declined over the past two centuries in the United States, coinciding with reductions in infection and increasing life expectancy. The generality of and reasons behind this phenomenon have not yet been well studied. Here, we show that Bolivian forager-farmers (n = 17,958 observations of 5481 adults age 15+ years) inhabiting a pathogen-rich environment exhibited higher BT when first examined in the early 21st century (~37.0°C). BT subsequently declined by ~0.05°C/year over 16 years of socioeconomic and epidemiological change to ~36.5°C by 2018. As predicted, infections and other lifestyle factors explain variation in BT, but these factors do not account for the temporal declines. Changes in physical activity, body composition, antibiotic usage, and thermal environment are potential causes of the temporal decline.
INTRODUCTION
Recent analysis of three U.S. cohorts spanning almost two centuries revealed a 0.03°C decline per decade in normal body temperature (BT) of adults, leading the authors to infer that “humans in high-income countries currently have a mean body temperature 1.6% lower [36.4°C] than in the pre-industrial era” (1). This effect was robust to potential confounders such as demographics, ambient temperature, time of day, and comorbidities. One hypothesis is that historical reductions in infectious disease lowered both systemic inflammation and energetic costs of immune responses during the 19th and 20th centuries, resulting in lower BT (1). Yet, it is not known whether BT <37.0°C is normal outside of high-income countries, nor have the contributions of infection and immune activation to temporal change in BT been extensively tested. BT is an easy-to-measure vital sign of human health, and thus, understanding factors affecting BT variation is of global public health interest.
Here, we test this hypothesis in a population-representative cohort of Tsimane Amerindians of the Bolivian Amazon using 16 years of mixed longitudinal data. Tsimane live a subsistence lifestyle without access to running water or sanitation and experience high exposure to diverse pathogens, indicated by elevated immune activation biomarkers throughout life [e.g., leukocytes, erythrocyte sedimentation rate (ESR), C-reactive protein, interleukin-6, and immunoglobulin E (2–4)]. Infections, particularly respiratory infections, are responsible for roughly half of all Tsimane deaths, and life expectancy at birth was in the low 40s until the late 20th century, before increasing by over a decade by the turn of the century (5). Tsimane also exhibit endemic polyparasitism, including various helminths (6), and helminthic infection is associated with higher resting metabolism in this population (7). These conditions lead us to predict higher normal BT among Tsimane than residents of high-income countries. We also predict higher BT among infected than noninfected Tsimane. At the same time, the rapid pace of improvements in health and lifestyle over recent decades may be lowering BT, as reported in the United States (1). Health conditions have been changing throughout rural Bolivia, including the Tsimane territory, over the past two decades with improved public health infrastructure and access to markets. Government-sponsored vaccination campaigns and improved access to social security income and medical services (e.g., hospitals and pharmacies) are among some of the other changes accompanying epidemiological transition over the 16-year study period. The Tsimane setting thus presents a rare opportunity to test whether a rural tropical population shows higher BT than commonly reported in high-income countries, whether BT has declined over time, potentially due to improved conditions, and if so, whether BT decline over time is associated with a lower infectious burden.
RESULTS
The Tsimane Health and Life History Project (THLHP) has been studying Tsimane health and lifestyle since 2002 with continuous biomedical surveillance (8). BT is measured as a routine part of clinical exams performed by project physicians in Tsimane villages roughly annually or every other year (n = 17,958 exams for 5481 individuals in 110 villages over 16 years; tables S1 to S3, and fig. S1). Following (1), our analysis focused on changes in “habitual” BT by excluding cases with extreme measurements (<35° or >39°C). During the earliest study period from 2002 to 2006, unadjusted mean BTs for adult women and men were 37.02°C [95% confidence interval (CI): 36.98 to 37.06] and 36.94°C (95% CI: 36.90 to 36.97), respectively (Fig. 1). By the latest study period from 2012 to 2018, BT dropped 0.45°C for women and 0.49°C for men (tables S2 and S4). To assess temporal changes, we model BT as a function of measurement date using multilevel models adjusting for age, sex, height, and weight and including random intercepts for individual, community, and attending physician (see Materials and Methods). The rate of BT decline in the baseline model is −0.55°C per decade (P < 0.001), with men showing a slightly steeper rate of decline than women (−0.60°C per decade, Pdate × sex < 0.01) (Table 1: model 1).
Fig. 1. BT of adults (>15 years) by age, sex, and time period.
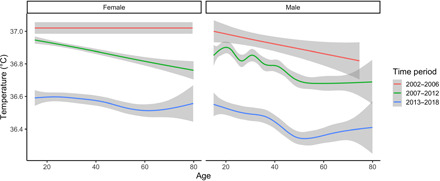
Shown are unadjusted values smoothed with generalized additive models using a cubic spline. Time periods are divided as October 2002–June 2006 (n = 1269 observations, oral temperature), July 2006–July 2012 (n = 10,496, tympanic), and August 2013–December 2018 (n = 6193, tympanic).
Table 1. Mixed-effects models of BT.
All models include random intercepts for individual ID, community ID, and physician ID.
| Dependent variable: BT | |||
| Model 1 | Model 2 | Model 3 | |
| Predictors | Base | Base + weather | Base + weather + diseases |
| Height (cm) | −0.001 | −0.002 | −0.001 |
| (−0.003, 0.0004) | (−0.003, 0.0001) | (−0.003, 0.0001) | |
| Weight (kg) | 0.001 | 0.001* | 0.001** |
| (−0.0001, 0.002) | (0.0003, 0.002) | (0.0005, 0.002) | |
| Age (years) | −0.003*** | −0.003*** | −0.003*** |
| (−0.004, −0.003) | (−0.004, −0.002) | (−0.003, −0.002) | |
| Sex (reference = female) | 0.171* | 0.139 | 0.142 |
| (0.003, 0.339) | (−0.025, 0.303) | (−0.022, 0.305) | |
| Male: age | −0.001 | −0.001 | −0.001 |
| (−0.002, 0.0002) | (−0.002, 0.0002) | (−0.001, 0.0002) | |
| Date (years) | −0.055*** | −0.054*** | −0.054*** |
| (−0.061, −0.049) | (−0.060, −0.049) | (−0.060, −0.049) | |
| Male: date | −0.005** | −0.005* | −0.005* |
| (−0.010, −0.001) | (−0.009, −0.001) | (−0.009, −0.001) | |
| Method (reference = “ear”) | −0.050* | −0.040* | −0.046* |
| (−0.090, −0.010) | (−0.080, −0.001) | (−0.085, −0.007) | |
| Season (reference = “dry”) | 0.041*** | 0.040*** | |
| (0.024, 0.058) | (0.023, 0.057) | ||
| Average ambient temperature | 0.029*** | 0.029*** | |
| (°C) | (0.027, 0.031) | (0.027, 0.031) | |
| Infectious/parasitic diseases | −0.017 | ||
| (Yes/no) | (−0.034, 0.001) | ||
| Blood diseases | −0.003 | ||
| (Yes/no) | (−0.031, 0.025) | ||
| Nervous system/sensory | −0.016 | ||
| (Yes/no) | (−0.035, 0.003) | ||
| Respiratory system | 0.075*** | ||
| (Yes/no) | (0.058, 0.092) | ||
| Digestive system | −0.007 | ||
| (Yes/no) | (−0.021, 0.008) | ||
| Genitourinary system | 0.025** | ||
| (Yes/no) | (0.008, 0.043) | ||
| Skin/subcutaneous | 0.038* | ||
| (Yes/no) | (0.001, 0.075) | ||
| Musculoskeletal system | −0.012 | ||
| (Yes/no) | (−0.026, 0.003) | ||
| Constant | 39.412*** | 38.574*** | 38.533*** |
| (39.069, 39.754) | (38.235, 38.913) | (38.192, 38.874) | |
| Observations | 16,830 | 16,830 | 16,830 |
| R2 (marginal/conditional) | 0.13/0.41 | 0.16/0.45 | 0.17/0.45 |
| Log likelihood | −9891.67 | −9444.39 | −9421.74 |
| Akaike information criterion | 19,809.35 | 18,918.77 | 18,889.48 |
*P < 0.05.
**P < 0.01.
***P < 0.001.
From this baseline model, we considered whether temporal changes in local ambient temperature and seasonality can explain Tsimane BT reductions (fig. S2). Wet season months (November–April) and higher ambient daily temperature are both associated with higher BT but do not affect the magnitude of temporal BT declines (Table 1: model 2). We next considered the role of clinical diagnoses [International Classification of Diseases, 10th revision (ICD-10) classifications] assigned by Bolivian physicians working on the THLHP. Respiratory and genitourinary infections—which represent two of the most common categories of infection among Tsimane—are associated with BT elevations of 0.075°C (95% CI: 0.058 to 0.092; P < 0.001) and 0.025°C (95% CI: 0.008 to 0.043; P < 0.01), respectively, but the rate of BT decline remains unaltered in models adjusting for these and six other diagnosis categories (−0.54°C per decade, P < 0.001; Table 1: model 3; Fig. 2).
Fig. 2. Predicted BT by study date.
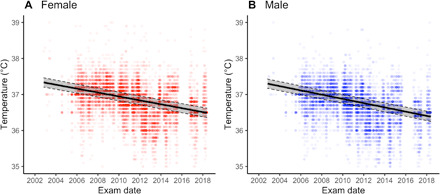
Shown for women (A) and men (B). Shaded region outlined by dashed line denotes 95% CI. Based on model 3 from Table 1. All covariates are set to population mean.
The magnitude of BT decline over time was robust to other model specifications (table S5), including nonlinearity in the pattern of BT decline, considering finer-grained seasonal effects (e.g., by month instead of wet versus dry season), and restricting the sample to adults age 40+ years (table S5). For a subset of individuals with available data on immune activation and anemic status (n = 3189 individuals, 6719 observations), inclusion of ESR (a general indicator of inflammation), hemoglobin (Hb) (indicator of anemia), and leukocyte count (indicator of immune activation) accounts for additional variation in BT but does not affect the estimated rate of BT decline (table S5: model 6). Using standard cutoffs for elevated ESR, anemia, or leukocytosis does not affect results (table S6). Further restricting analysis to the 7% of adults who were diagnosed by THLHP physicians as “healthy” (i.e., were not assigned any ICD-10 diagnoses at the time of BT reading, n = 1056 individuals; n = 1188 observations) still results in a strong temporal BT decline (−0.45°C per decade, P < 0.0001) (table S5: model 5).
DISCUSSION
Tsimane BT hovered around 37.0°C in the early 2000s before declining to about 36.5°C in roughly two decades of rapid socioeconomic change. While German physician Carl Wunderlich popularized 37.0°C as normal core BT for healthy adults in 1867, he considered the range to vary from 36.2° to 37.5°C. Normal variation in tympanic temperature has been cited as 35.4° to 37.8°C (9) and 35.76° to 37.52°C (10). Many have reported normothermic temperatures <37.0°C in studies throughout the 20th century (9, 11). This pattern may be especially common in tropical populations that tend to have depressed resting metabolic rates (RMRs) (12). Protsiv et al. (1) showed a sustained decline of roughly −0.03°C per decade from the mid–19th century to the present in Americans, followed by a recent flattening or even increasing trend in BT (fig. S3C). Here, we show relatively high BT before 2006, followed by a decline of ~0.5°C per decade in the Bolivian Amazon, much steeper than that observed in the United States (fig. S3A).
BT reflects a combination of passive heat loss to the environment and physiological processes such as cellular metabolism, circadian rhythms, immune function, and disease state. The time period covered in our study encompasses many changes that could affect these processes, resulting in either an alteration in thermoregulatory set points or shifts in the common physiological state experienced by Tsimane over time. Just as U.S. life expectancy improved substantially over the period of observed BT decline (from ~39 to 76 years from 1860 to 2000), Tsimane life expectancy also improved, especially among adults, between early-mid and the late 20th century (from ~43 to 54 years), although it still lags far behind the life expectancy of high-income countries (5, 13). Modest reductions in normal BT have been linked to lower mortality both prospectively (14) and in experimental models (15). The present findings thus suggest that rapid declines in BT may reflect improved health, survivorship, and living conditions affecting thermoregulation among rural indigenous Bolivians over the past two decades.
As reported in (1), infection and inflammation are associated with elevated BT, and elevated core BT helps facilitate generation and differentiation of CD8+ cytotoxic T cells (16). According to model 3 (Table 1), having a diagnosed respiratory infection, a +10 mm/hour elevation in ESR, and a +5000 cells/liter elevation in leukocyte count combined are associated with 0.14°C higher BT (table S5). Over the past two decades, Tsimane have had greater access to medical treatment, including vaccines, aspirin, ibuprofen, and other nonsteroidal anti-inflammatory drugs. Greater access to treatment is consistent with lower systemic inflammation and immune activation over time, as evidenced by significantly decreased ESR (a general biomarker of inflammation) (Fig. 3A and table S7) and resting metabolism over the study period (Fig. 3C). Although respiratory illnesses have declined over the 16-year study period, other infections and health problems are more variable and remain fairly prevalent (Fig. 3, G and H, and table S3). We speculate that duration and impact of illness on physiology may now be lower than in the past, partly due to greater access to medical treatment and improved condition. The elevation in BT from having a greater number of diagnoses across ICD-10 categories is indeed lower in later study years (βdate × # conditions = −0.006, P < 0.0001; table S9). On the basis of this model, being diagnosed with two morbid conditions in 2002 is associated with 0.19°C higher BT than being diagnosed with the same conditions in 2018. A smaller effect in the same direction holds when focusing only on respiratory illness, the clinical category with the largest effect in Table 1 (0.06°C higher in 2002 than in 2018).
Fig. 3. Secular trends in biomarkers and disease ecology for Tsimane adults (>15 years).
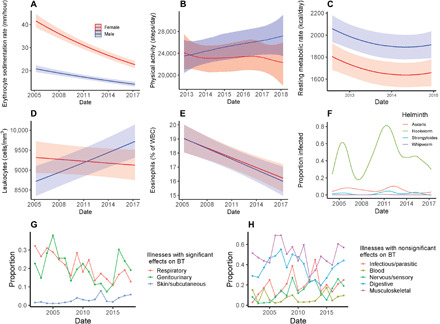
Blue and red represent males and females, respectively, in (A) to (E). (A, D, and E) Predicted means (±95% CI) by sex across the study period from models of ESR (log-transformed), leukocyte count (WBC) (log-transformed), and eosinophils (% of WBC). (B) Physical activity, as average steps per day, from triaxial accelerometry (see Materials and Methods). (C) is RMR (kcal/day) based on indirect calorimetry (using Cosmed Fitmate MED). (F) Modeled prevalence of four helminth species, based on direct microscopy from fecal smears. (G and H) Per-year means of age-standardized prevalence by illness categories based on clinical ICD-10 diagnoses, separated by whether or not the category was a significant predictor of BT. All models adjust for age, sex, and season (shown “wet”) and include study community and individual ID random effects. Models in (A), (D), and (E) also adjust for age2, sex*age, and sex*age2. Model in (B) is a Bayesian generalized additive multilevel model using a thin-plate smoothing spline for date interacted with sex, adjusting for sex, age, age2, sex*age, season, and a community random effect. Model in (C) also adjusts for weight, height, ambient temperature, time of day of measurement, and time since last meal. Models in (F) are Bayesian generalized additive multilevel models fit separately by helminth species using a thin-plate smoothing spline for date, adjusting for age, season, and community and individual ID random effects. Model results for (A), (C), (D), and (E) are shown in table S7, and model results for (B) and (F) are shown in table S8.
Despite the possibility that changing infection rates and reduced morbidity can explain some of the temporal decline in BT, several lines of evidence suggest that these changes alone are insufficient. First, explicit consideration of medical diagnoses and immune biomarkers in our models helps explain overall variation in BT but does not diminish the effects of sustained temporal declines in Tsimane BTs. Second, Tsimane today show BTs as low as those reported in high-income countries with low infectious burden, despite the fact that Tsimane still experience a comparatively high prevalence of illnesses (Fig. 3, F to H). Third, previous large-scale studies have failed to identify links between inflammation and BT; for example, a study of >240,000 observations on 35,488 U.K. adults found no clear relationship between BT and the inflammatory biomarker C-reactive protein (14).
These observations suggest that factors other than inflammation and immune activation are likely to influence BT. The greater application of antibiotics, especially for villages near town with greater access to medical care, may also reduce the diversity of microbial populations living within individuals, including those in the gut. Given that microbial thermogenesis contributes to measurable differences in core BT in other organisms (17), the increased application of antibiotics may be lowering BT via both direct reductions of active infections and indirect reductions of heat produced by gut microbes (18, 19).
Changes in helminthic infection rates may also moderate the secular decline in BT. Helminths have anti-inflammatory and immune regulatory effects (20, 21), which could lower core BT. On the other hand, helminth infection has been shown to increase resting metabolism (7), which might otherwise raise core BT. The effects of helminths on human BT have rarely been explored [but see (22)]. While helminth infections are common and endemic among Tsimane (6), rates of infection, especially of hookworm, are variable over time (Fig. 3F). Helminth infection in our sample was not associated with BT (β = 0.003; 95% CI: −0.03 to 0.03), nor did its addition to model 3 of Table 1 affect temporal BT decline. However, it is possible that the intensity of worm burden is lower now than in the past because of deworming campaigns and greater use of footwear; this is consistent with eosinophils comprising a smaller proportion of leukocytes over time (Fig. 3E).
Protsiv et al. (1) suggest that improved access over time to heating and cooling technology may help explain the U.S. temporal decline in BT. Controlled ambient temperature from heating and air conditioning increases time spent in the thermoneutral zone, whereby lower energy expenditure (a proxy for BT) is required to support thermoregulation. Tsimane houses often lack walls, and air conditioning and indoor heating have never existed in Tsimane villages, most of which still lack electricity; hence, Tsimane are less buffered against fluctuations in ambient temperature. The difference in minimum and maximum daily temperatures declined by about −1.66°C (P < 0.0001; adjusting for a month) from 2002 to 2018 (fig. S2), consistent with a narrower range in ambient temperature fluctuation over time. However, when added to model 3 of Table 1, higher daily ambient temperature range was associated with lower BT (β = −0.0027, P < 0.05), and adding ambient temperature range did not alter the magnitude of the temporal BT decline. We speculate that improved access to warm clothing and blankets and more insulated housing over the past ~15 years may have reduced energy metabolism allocated to thermoregulation during the colder months. Consistent with this notion, we find that lower daily minimum ambient temperature is associated with higher BT in later study years (βmin ambient temp × year = 0.0009, P < 0.0001; interaction term added to model 3 of Table 1), although including this interaction term does not reduce the magnitude of the temporal BT decline.
Our models adjusted for village of residence as a random effect, because villages differ in their proximity to health services, available infrastructure (e.g., clean water from wells), and other amenities. However, some of this variation is captured by distance to the market town of San Borja. Residents of more remote villages had 0.11° to 0.13°C higher BTs than residents located near town in early study years, but by 2014, there were no BT differences between near and distant villages (fig. S3). If BT is a valid aggregate proxy for general health, then this could suggest that over the past two decades, health conditions and possibly other amenities that we did not account for in our models have improved more in remote areas.
In addition to changing epidemiological conditions over time, other aspects of socioeconomic change are likely to be involved in temporal BT decline. Changes in BT over time may reflect increasing body mass index (BMI) or body fat percentage. The relationship between body size or composition and BT is complex: Although some studies report an inverse association between obesity (BMI > 30) and BT [e.g., (23, 24)], other studies do not (25–27). Nonetheless, it has been suggested that lower core BT in obese subjects may reflect increased metabolic efficiency (28), and thus, obesity may be associated with core BT via the energetic cost of thermogenesis. Such an effect could be important for the Tsimane, who have experienced rapid, recent changes in body mass and composition (29). Our models indicate that height and weight are largely unassociated with BT (Table 1), and alternative model specifications show that BMI and body fat percentage have minimal effect on the temporal trends observed despite evincing significant positive and negative relationships with BT, respectively (table S10). Although our findings do not link body size and composition to temporal trends in BT, the effects of body mass and composition deserve consideration in the clinical interpretation of BT.
Another possibility is that changes in physical activity over time have driven temporal reductions in BT. Habitual physical activity is associated with increased basal metabolic rate (30), which, in turn, can increase core BT (28). Moderate or habitual levels of physical activity raise metabolic rate over prolonged periods of time (31), and BTs correlate positively with objectively measured physical activity (32, 33). Greater physical activity in an experimental mouse model led to elevated core BT not only during active periods but also during periods of inactivity (34). Reduced physical activity due to lifestyle changes may be particularly relevant for explaining the substantial reductions in BT between the National Health and Nutrition Examination Survey (NHANES) and Strategies to Reduce Injuries and Develop Confidence in Elders (STRIDE) cohorts (1975–2017) reported in (1). Among Americans, work- and transportation-related physical activity has declined, and inactivity has increased over this period (35, 36). For Tsimane, we hypothesized that a gradual transition away from a subsistence economy due to increasing market access, particularly in villages near towns, could be responsible for recent decreases in physical activity, but we found little evidence to suggest that physical activity was decreasing in Tsimane, at least since 2013 (Fig. 3B). The role of habitual physical activity on core BT remains an interesting candidate for further study.
A final possibility is changes in sleep duration over time. Reduced sleep duration can disrupt the circadian rhythm of thermoregulation, appetite, metabolism, and core BT. However, sleep behavior is unlikely to explain temporal changes in BT among Tsimane or Americans. We have data on sleep behavior only for a restricted period, and although electricity now exists in a few villages (37), the overall magnitude of temporal declines in sleep duration due to environmental or lifestyle changes is limited. Similarly, despite claims of secular declines in sleep duration in the United States due to changes in technology and lifestyle, reviews and meta-analyses of both self-reports and actigraphy studies show little evidence of sustained decline over the past 50+ years (38). Furthermore, modest decreases in sleep duration, even if they were to exist, likely have minimal impact on BT. A recent experimental study showed that a large (50%) reduction in sleep duration (7 to 3.5 hours) over three nights led to a mean reduction of core BT of only 0.07°C (39).
Our study has several limitations. First, we did not use the same thermometer over the entire study period, although our results are conservative and robust to the type of thermometer used (Table 1 and tables S5, S6, and S9 to S11). Second, our sample size is smaller during the earliest study years. Third, we did not account for pregnancy or lactation, although these also did not vary over the study period. Fourth, we did not account for hydration status of participants. Tsimane show nontrivial prevalence of hypohydration (40), which may lead to increased core BT, especially during physical activity (41). Hydration status would only affect the temporal trend of lower BT if hypohydration became less common over the study period. However, analysis of urine-specific gravity shows no consistent pattern of hypohydration between 2006 and 2014 (fig. S6). Last, we did not record time of day during BT assessments, and diurnal core BT can vary by up to 1°C within individuals over the course of a day (10). Clinical exams were performed consistently between 7:00 a.m. and 5:00 p.m. over the entire study period, without any biased timing over the study period, and so, not adjusting for time of day is unlikely to directionally bias results. Greater attention to intraindividual changes in BT beyond time of day could further illuminate the effects we describe and help reduce confusion over clinical applications of BT for medical diagnostics (42). Despite the limitations, it is worth noting that the amount of variation explained in our main model [Table 1, model 3: marginal R2 = 0.17 (for fixed effects only) and conditional R2 = 0.45 (includes both fixed and random effects) (43)] exceeds the <10% explained in similar work in the United States and the United Kingdom (1, 14).
In conclusion, our findings support prior observations of declining BT in high-income countries coinciding with changing epidemiological and socioeconomic conditions and with normal BTs currently below the long-cited 37.0°C guideline. Tsimane BT was closer to 37.0°C during an early period of higher mortality and then dropped steeply in under two decades on a trajectory similar to that observed in the United States over two centuries. Our findings demonstrate both a wide variation of BT among adults and a broad suite of variables that influence BT in a pathogen-rich environmental context. While we were able to address several potential explanations of the secular trend, further study into the causes and implications of BT change over time remains an open avenue for human biology. The broad physiological changes that accompany lower core BT are evident beyond the context of urbanization and industrialization and include the rural tropics during a period of socioeconomic and epidemiological transition.
MATERIALS AND METHODS
Study population
The Tsimane are a natural fertility population of forager-horticulturalists in the Beni Department of lowland Bolivia (population, ~16,000). They inhabit 110+ villages ranging in size from 40 to 550 inhabitants. Their diet has remained largely traditional, with more than 90% of calories coming from horticulture (plantains, manioc, and rice), fishing, hunting, and gathering fruits (29, 44). More recently, increasing foods and additives from markets have entered the Tsimane diet, especially in communities closer to roads and towns (29). No villages have running water, and most villages lack electricity, plumbing, and public sanitation. Latrines and wells were constructed or repaired in a growing number of villages over the past decade, and limited electricity now exists in a handful of communities near town (45).
Tsimane Health and Life History Project
Since 2002, a mobile team of trained Bolivian physicians, laboratory technicians, and anthropologists has visited 18 to 90 villages per wave (each wave is 1 to 2 years) and conducted systematic clinical evaluations with no exclusion criteria. Certain clinical evaluation protocols were limited to adults aged 40+ years given the project’s focus on aging (8). As part of the evaluation, the team collected data for each participant on medical history, current symptoms and diagnoses (ICD-10), anthropometry, hematology, and health biomarkers from fecal and urine analysis.
BT data (n = 17,958 observations) exist for 5481 Tsimane age 15+ years measured from 2002 to 2018, covering birth years 1914–2003 (fig. S1). An oral mercury thermometer was used from the beginning of the study until mid-2006, while tympanic Braun digital thermometers (Thermoscan 5) were used thereafter. As in Protsiv et al. (1), we eliminated extreme temperature values [i.e., <35°C (n = 41) and >39°C (n = 16)]. The final dataset including complete data contains 16,830 observations on 5370 individuals (tables S1 and S2).
The use of an upper threshold of 39°C to eliminate cases of acute fever is arbitrary and greater than some standard clinical cutoffs (i.e., the American College of Critical Medicine and Infectious Disease Society classify fever as BT ≥38.3°C). To investigate the effect of different upper thresholds, we reran our analyses and that of Protsiv et al. (1), removing any data ≥38°C (tables S11 and S12). This change did not affect the results of either study.
Other THLHP data reveal changes in environment and behavior over the two-decade study period (Fig. 3). Anthropometric indices include weight (kg) and body fat (%) as measured by bioelectric impedance using a Tanita scale (BF series) and BMI (kg/m2). Height was measured using a portable Seca 213 stadiometer.
In-field blood analysis of fasting venous samples provided estimates of ESR (Westergren method) and leukocyte [white blood cell (WBC)] count. WBC count is a biomarker of immune activation and possibly infection, while ESR is a nonspecific indicator of inflammation (46). Leukocytosis was defined as WBC > 10.0 × 109 cells/liter, and elevated ESR was defined as ESR > 20 mm/hour (ages 15 to 44) or >30 mm/hour (ages 45+) (see table S6). Hb and WBC count were measured with the QBC Autoread Plus Dry Hematology System (Drucker Diagnostics, State College, PA).
Physical activity was measured as daily step count based on ~3-day samples using a waist-worn triaxial ActiGraph GT3X accelerometer (47). Accelerometers were initialized using a participant’s ID, weight, age, sex, and a 30-Hz resampling rate using ActiGraph’s ActiLife software. Participants were instructed as to the proper positioning of the accelerometers on the right hip and asked to only remove the accelerometers before submersion in water (e.g., bathing in the river) and to reapply them directly after leaving the water. Raw data were downloaded and converted to 1-min epochs using ActiLife version 6.13.4. We used the “low-frequency” extension filter from ActiLife to convert raw accelerations to steps. Non-wear periods, considered any period of 60-min or longer with accelerometry counts at zero, were excluded. Only days with a minimum of 10 hours of detected wear time were included for analysis. Daily step counts were calculated as the average steps per minute over the valid wear time multiplied by 60 × 24 to get the average daily step count.
RMR was assessed with a Cosmed Fitmate MED indirect calorimeter (7). RMR is modeled as a quadratic function of date using linear regression, adjusting for sex, age, weight, height, and ambient temperature. Some deviations from standard protocol were required given remote field conditions, thus requiring additional model adjustments, including time of day and time since last meal [see (7) for details].
Data analysis
We performed a series of multilevel regression models: Model 1 includes height, weight, age, sex, date, and age*sex and age*date interactions, as well as a control for the type of thermometer used. Model 2 adds to model 1: season (wet = November to April versus dry) and average daily ambient temperature, downloaded from meteostat.net for the nearest weather station in San Borja, Beni, Bolivia (fig. S2). Model 3 adds to model 2 eight clinical categories based on THLHP physician diagnoses using International Classification of Diseases (ICD-10) codes assigned to Clinical Classifications Software (CCS) categories (overall prevalence in descending order: musculoskeletal and connective tissue: 46.9%; digestive system: 36.8%; infectious/parasites: 19.7%; respiratory system: 19.3%; genitourinary system: 17.5%; nervous system and sensory: 14.4%; blood and blood-forming organs: 5.8%; skin: 3.1%). All models include individual ID, community ID, and physician ID as random intercept effects. Our modeling approach thus permits comparison of how climate and individual condition contribute to temporal differences in BT. The order in which different thermometers were used during the study period (oral mercury thermometer followed by tympanic digital) is conservative against any methodology-based expectation of declining BT over time as tympanic temperatures are sometimes reported to trend slightly (0.3° to 0.6°C) higher than oral temperature. However, a comparison of the methods in a rural tropical region found no significant differences between methods (48). Precision on the mercury and ear thermometers is approximately ±0.2°C. Nevertheless, we included a variable in models for type of instrument used.
Additional models explore other indicators of infection or compromised health (table S5), changes in these indicators over time (table S7), and the effects of body composition (table S10). The sample sizes for these models vary based on missing data due to selective sampling by the THLHP (8). In general, our sampling scheme here focused on all adults age 40+ years and a stratified random sample of those age 15 to 39 years. Indicators include ESR, leukocyte count (WBC), and Hb. Elevated ESR indicates nonspecific, systemic inflammation, whereas elevated WBC suggests immune activation. Low Hb is an indicator of anemia status, which acts as an additional proxy of nutritional status and condition. For a smaller subset (n = 5821 observations on 2221 individuals), microscopy of eggs or larvae from fecal smears on wet mounts was conducted to identify the presence or absence of four intestinal helminth species: hookworm (Necator americanus), large roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura), and threadworm (Strongyloides stercoralis) [see (6) for methodological details]. Probability of species-specific infections was estimated using Bayesian generalized additive multilevel models with a binomial error distribution for the presence of each helminth species (coded as a binary variable) and a thin-plate smoothing spline for time, adjusting for age, season, and random effects for community ID and individual ID (using Stan via the brms package in R).
Ethics statement
All methods and procedures were approved and conducted in accordance with guidelines set by the Institutional Review Boards of University of California, Santa Barbara (#12–496) and University of New Mexico (#07-157 and #15-133). Informed consent was given by the Tsimane Government (Gran Consejo Tsimane), village leaders, and all study participants directly.
Supplementary Material
Acknowledgments
We thank Tsimane for their hospitality and collaboration and THLHP personnel for their herculean effort and dedication. Funding: The THLHP has been funded by the NIH/National Institute on Aging (NIA) (R01AG024119, R56AG024119, and RF1AG054442) and the NSF (BCS0136274, BCS0422690, and RAPID BCS1440212). J.S. acknowledges the Institute for Advanced Study in Toulouse (IAST) funding from the French National Research Agency (ANR) under grant ANR-17-EURE-0010 (Investissements d’Avenir program). Author contributions: M.G. conceived the study. M.G. and T.S.K. wrote the paper. T.S.K. and M.G. analyzed the data. M.G., H.K., J.S., and B.T. direct THLHP and manage data collection. E.C.L., D.E.R., R.Q.G., I.M.S., and J.C.A. collected data. D.C., E.S., S.A., P.L.H., A.V.J., and T.S.K. helped organize datasets. All authors revised the paper and approved the final draft. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials, and/or available for download at https://osf.io/3q4nw/. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/44/eabc6599/DC1
REFERENCES AND NOTES
- 1.Protsiv M., Ley C., Lankester J., Hastie T., Parsonnet J., Decreasing human body temperature in the United States since the Industrial Revolution. eLife 9, e49555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurven M., Kaplan H., Winking J., Finch C., Crimmins E. M., Aging and inflammation in two epidemiological worlds. J. Gerontol. A Biol. Sci. Med. Sci. 63, 196–199 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell A. D., Trumble B. C., Maldonado Suarez I., Stieglitz J., Beheim B. A., Snodgrass J. J., Kaplan H., Gurven M., Immune function in Amazonian horticulturalists. Ann. Hum. Biol. 43, 382–396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasunilashorn S., Finch C. E., Crimmins E. M., Vikman S. A., Stieglitz J., Gurven M., Kaplan H., Allayee H., Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious load. Biodemography Soc. Biol. 57, 33–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurven M., Kaplan H., Supa A. Z., Mortality experience of Tsimane Amerindians of Bolivia: Regional variation and temporal trends. Am. J. Hum. Biol. 19, 376–398 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Blackwell A. D., Martin M., Kaplan H., Gurven M., Antagonism between two intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proc. R. Soc. B Biol. Sci. 280, 20131671 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurven M. D., Trumble B. C., Stieglitz J., Yetish G., Cummings D., Blackwell A. D., Beheim B. A., Kaplan H. S., Pontzer H., High resting metabolic rate among Amazonian forager-horticulturalists experiencing high pathogen burden. Am. J. Phys. Anthropol. 161, 414–425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurven M., Stieglitz J., Trumble B., Blackwell A. D., Beheim B., Davis H., Hooper P., Kaplan H., The Tsimane Health and Life History Project: Integrating anthropology and biomedicine. Evol. Anthropol. 26, 54–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sund-Levander M., Forsberg C., Wahren L. K., Normal oral, rectal, tympanic and axillary body temperature in adult men and women: A systematic literature review. Scand. J. Caring Sci. 16, 122–128 (2002). [DOI] [PubMed] [Google Scholar]
- 10.I. I. Geneva, B. Cuzzo, T. Fazili, W. Javaid, in Open Forum Infectious Diseases (Oxford Univ. Press, 2019), vol. 6, pp. ofz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackowiak P. A., Wasserman S. S., Levine M. M., A critical appraisal of 98.6°F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA 268, 1578–1580 (1992). [PubMed] [Google Scholar]
- 12.Henry C. J., Rees D. G., New predictive equations for the estimation of basal metabolic rate in tropical peoples. Eur. J. Clin. Nutr. 45, 177–185 (1991). [PubMed] [Google Scholar]
- 13.H. Kaplan, P. L. Hooper, J. Stieglitz, M. Gurven, The causal relationship between fertility and infant mortality: Prospective analyses of a population in transition, in Population in the Human Sciences: Concepts, Models, Evidence (Oxford University Press, 2015), p. 361. [Google Scholar]
- 14.Obermeyer Z., Samra J. K., Mullainathan S., Individual differences in normal body temperature: Longitudinal big data analysis of patient records. BMJ 359, j5468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti B., Sanchez-Alavez M., Winsky-Sommerer R., Morale M. C., Lucero J., Brownell S., Fabre V., Huitron-Resendiz S., Henriksen S., Zorrilla E. P., de Lecea L., Bartfai T., Transgenic mice with a reduced core body temperature have an increased life span. Science 314, 825–828 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Mace T. A., Zhong L., Kilpatrick C., Zynda E., Lee C.-T., Capitano M., Minderman H., Repasky E. A., Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J. Leukoc. Biol. 90, 951–962 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg E., Zilber-Rosenberg I., Do microbiotas warm their hosts? Gut Microbes 7, 283–285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller A., Mitchell D., Oral antibiotics reduce body temperature of healthy rabbits in a thermoneutral environment. J. Basic Clin. Physiol. Pharmacol. 10, 1–13 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Bowers S. J., Thompson R. S., Mika A., Greenwood B. N., Fleshner M., 150. Disruption of the gut microbiome with oral antibiotic reduces core body temperature and disrupts diurnal rhythms of locomotor activity, but not sleep, in rats. Brain Behav. Immun. 40, e43 (2014). [Google Scholar]
- 20.Rook G. A. W., Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology 126, 3–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guigas B., Molofsky A. B., A worm of one’s own: How helminths modulate host adipose tissue function and metabolism. Trends Parasitol. 31, 435–441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nacher M., Singhasivanon P., Traore B., Dejvorakul S., Phumratanaprapin W., Looareesuwan S., Gay F., Short report: Hookworm infection is associated with decreased body temperature during mild Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 65, 136–137 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Rising R., Keys A., Ravussin E., Bogardus C., Concomitant interindividual variation in body temperature and metabolic rate. Am. J. Physiol. Endocrinol. Metab. 263, E730–E734 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Kim H., Richardson C., Roberts J., Gren L., Lyon J. L., Cold hands, warm heart. The Lancet 351, 1492 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Rising R., Fontvieille A. M., Larson D. E., Spraul M., Bogardus C., Ravussin E., Racial difference in body core temperature between Pima Indian and Caucasian men. Int. J. Obes. Relat. Metab. Disord. 19, 1–5 (1995). [PubMed] [Google Scholar]
- 26.Eriksson H., Svärdsudd K., Larsson B., Welin L., Ohlson L. O., Wilhelmsen L., Body temperature in general population samples: The study of men born in 1913 and 1923. Acta Med. Scand. 217, 347–352 (1985). [PubMed] [Google Scholar]
- 27.Heikens M. J., Gorbach A. M., Eden H. S., Savastano D. M., Chen K. Y., Skarulis M. C., Yanovski J. A., Core body temperature in obesity. Am. J. Clin. Nutr. 93, 963–967 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landsberg L., Young J. B., Leonard W. R., Linsenmeier R. A., Turek F. W., Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Trans. Am. Clin. Climatol. Assoc. 120, 287–295 (2009). [PMC free article] [PubMed] [Google Scholar]
- 29.Kraft T. S., Stieglitz J., Trumble B. C., Martin M., Kaplan H., Gurven M., Nutrition transition in 2 lowland Bolivian subsistence populations. Am. J. Clin. Nutr. 108, 1183–1195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjödin A. M., Forslund A. H., Westerterp K. R., Andersson A. B., Forslund J. M., Hambraeus L. M., The influence of physical activity on BMR. Med. Sci. Sports Exerc. 28, 85–91 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Mischler I., Vermorel M., Montaurier C., Mounier R., Pialoux V., Péquignot J.-M., Cottet-Emard J.-M., Coudert J., Fellmann N., Prolonged daytime exercise repeated over 4 days increases sleeping heart rate and metabolic rate. Can. J. Appl. Physiol. 28, 616–629 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Aoyagi Y., Park S., Cho S., Shephard R. J., Objectively measured habitual physical activity and sleep-related phenomena in 1645 people aged 1–91 years: The Nakanojo Community Study. Prev. Med. Rep. 11, 180–186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Someren E. J. W., Raymann R. J. E. M., Scherder E. J. A., Daanen H. A. M., Swaab D. F., Circadian and age-related modulation of thermoreception and temperature regulation: Mechanisms and functional implications. Ageing Res. Rev. 1, 721–778 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Weinert D., Waterhouse J., The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol. Behav. 90, 246–256 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Church T. S., Thomas D. M., Tudor-Locke C., Katzmarzyk P. T., Earnest C. P., Rodarte R. Q., Martin C. K., Blair S. N., Bouchard C., Trends over 5 decades in US occupation-related physical activity and their associations with obesity. PLOS ONE 6, e19657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brownson R. C., Boehmer T. K., Luke D. A., Declining rates of physical activity in the United States: What are the contributors? Annu. Rev. Public Health 26, 421–443 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Yetish G., Kaplan H., Gurven M., Wood B., Pontzer H., Manger P. R., Wilson C., McGregor R., Siegel J. M., Natural sleep and its seasonal variations in three pre-industrial societies. Curr. Biol. 25, 2862–2868 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youngstedt S. D., Goff E. E., Reynolds A. M., Kripke D. F., Irwin M. R., Bootzin R. R., Khan N., Jean-Louis G., Has adult sleep duration declined over the last 50+ years? Sleep Med. Rev. 28, 69–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibi M., Kubota C., Mizuno T., Aritake S., Mitsui Y., Katashima M., Uchida S., Effect of shortened sleep on energy expenditure, core body temperature, and appetite: A human randomised crossover trial. Sci. Rep. 7, 39640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bethancourt H. J., Swanson Z. S., Nzunza R., Huanca T., Conde E., Kenney W. L., Young S. L., Ndiema E., Braun D., Pontzer H., Rosinger A. Y., Hydration in relation to water insecurity, heat index, and lactation status in two small-scale populations in hot-humid and hot-arid environments. Am. J. Hum. Biol., e23447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buono M. J., Wall A. J., Effect of hypohydration on core temperature during exercise in temperate and hot environments. Pflugers Arch. 440, 476–480 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Sund-Levander M., Grodzinsky E., Time for a change to assess and evaluate body temperature in clinical practice. Int. J. Nurs. Pract. 15, 241–249 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa S., Johnson P. C. D., Schielzeth H., The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin M. A., Lassek W. D., Gaulin S. J., Evans R. W., Woo J. G., Geraghty S. R., Davidson B. S., Morrow A. L., Kaplan H. S., Gurven M. D., Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: Controlled comparisons with a US sample. Matern. Child Nutr. 8, 404–418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinkel K. A., Costa M. E., Kraft T. S., Stieglitz J., Cummings D. K., Gurven M., Kaplan H., Trumble B. C., Relationship of sanitation, water boiling, and mosquito nets to health biomarkers in a rural subsistence population. Am. J. Hum. Biol. 32, e23356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brigden M. L., Clinical utility of the erythrocyte sedimentation rate. Am. Fam. Physician 60, 1443–1450 (1999). [PubMed] [Google Scholar]
- 47.Gurven M., Jaeggi A. V., Kaplan H., Cummings D., Physical activity and modernization among Bolivian Amerindians. PLOS ONE 8, e55679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chue A. L., Moore R. L., Cavey A., Ashley E. A., Stepniewska K., Nosten F., McGready R., Comparability of tympanic and oral mercury thermometers at high ambient temperatures. BMC. Res. Notes 5, 356 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/44/eabc6599/DC1


