Abstract
Background:
Reduced fetal growth increases the risk for adverse health outcomes. Growing evidence suggests that metal exposures contribute to reduced fetal growth, but little is known about the effects of complex metal mixtures.
Objectives:
We investigated the impact of a complex mixture of metals on birth weight for gestational age (BW for GA) in the Maternal and Developmental Risks from Environmental and Social Stressors study, a predominately lower-income Hispanic pregnancy cohort in Los Angeles, California.
Methods:
Cadmium (Cd), cobalt (Co), mercury (Hg), nickel (Ni), molybdenum (Mo), lead (Pb), antimony (Sb), tin (Sn), and thallium (Tl) were measured by inductively coupled plasma mass spectrometry (ICP-MS) in maternal urine samples collected in early pregnancy (median GA: 13.1 wk). Speciated urinary arsenic (As) ( As) was measured by high-performance liquid chromatography coupled to ICP-MS. Primary analyses focused on a mixture of seven metals that have previously been associated individually with fetal growth (i.e., As, Cd, Co, Hg, Ni, Pb, Tl) (). In exploratory analyses, we additionally examined three metals that have been less studied in relation to fetal growth (i.e., Mo, Sb, Sn). Covariate-adjusted Bayesian kernel machine regression was used to investigate metal mixture associations with BW for GA -scores.
Results:
In primary analyses, Hg and Ni ranked highest as predictors of BW for GA. An inverse linear association was estimated for Hg, whereas a positive association was estimated for Ni at low-to-moderate concentrations. A potential interaction between Hg and Ni was also identified. In our exploratory analysis, Sb ranked highest as a predictor of BW for GA, followed by Hg and Ni.
Conclusions:
Our findings suggest that in this understudied population, Hg may reduce fetal growth, whereas Ni may promote fetal growth. We also identified Sb as a potential metal of concern for this population, which merits additional investigation. https://doi.org/10.1289/EHP7201
Introduction
Birth weight (BW) is an indicator of cumulative fetal growth that has been closely related to health risk later in life (Barker and Thornburg 2013). Rates of low BW have been increasing in the United States (Martin et al. 2018; Womack et al. 2018). This may have important consequences for public health given that low BW is an established risk factor for a diverse number of adverse health outcomes, including morbidity and mortality in infancy (McCormick 1985), cognitive deficits (Oudgenoeg-Paz et al. 2017), and cardiovascular disease and metabolic syndrome in adulthood (Visentin et al. 2014). Fetal growth is impacted by a variety of maternal and environmental factors, such as maternal age, diet, and in utero tobacco smoke exposure (Nardozza et al. 2017), and a growing body of evidence indicates that prenatal exposure to toxic metals and metalloids (hereafter referred to collectively as metals), including arsenic (As), cadmium (Cd), mercury (Hg), lead (Pb), and thallium (Tl), also contributes to reduced fetal growth (Ballester et al. 2018; Hoffman 2000; Khoshhali et al. 2020; Kim et al. 2017; Kippler et al. 2010, 2012; Mikelson et al. 2019; Milton et al. 2017; Rabito et al. 2014; Ramón et al. 2009a; Rodosthenous et al. 2017; Thomas et al. 2015; Vejrup et al. 2014; Vigeh et al. 2018; Xia et al. 2016). In contrast, essential trace elements are important for normal fetal growth and development. For example, cobalt (Co) is a component of vitamin B12 that has been associated with higher BW (Mikelson et al. 2019). In addition, some metals, such as nickel (Ni) exhibit both toxic and nutritional properties (Nielsen 2012), and both adverse and protective effects have been reported in relation to fetal growth (Cabrera-Rodríguez et al. 2018; Deyssenroth et al. 2018; Jalali and Koski 2018; Pedersen et al. 2016; Sun et al. 2018; Vaktskjold et al. 2007).
Although humans are typically exposed to a mixture of toxic and essential elements, most studies have evaluated metals individually in relation to fetal growth. Yet, in combination, certain metals may exert toxic effects even at relatively low levels of exposure owing to synergistic effects (Claus Henn et al. 2014), whereas other metals may act antagonistically by inhibiting each other’s absorption or tissue uptake (Davidson et al. 2015). Although several recent studies have begun examining simple mixtures (e.g., three elements) in relation to fetal growth (Cabrera-Rodríguez et al. 2018; Cassidy-Bushrow et al. 2019; Signes-Pastor et al. 2019), few studies have considered complex mixtures (Deyssenroth et al. 2018; Govarts et al. 2016), which are more representative of human exposures. This has been particularly understudied in minority populations in the United States. The objective of the present study was, therefore, to investigate the impact of early pregnancy exposure to a complex mixture of metals on fetal growth in a cohort of predominately lower-income Hispanic mother–newborn pairs in urban Los Angeles.
Methods
Study Population
The Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) study is an ongoing, prospective pregnancy cohort, which began in November 2015 and has been described previously (Bastain et al. 2019). Briefly, participants are recruited from four prenatal care providers in Los Angeles, California, including two community health clinics, one county hospital prenatal clinic, and one private obstetrics and gynecology practice. A small number of participants are also recruited through self-referral from community meetings and local advertisements. Most of the participating clinics serve predominately lower-income Hispanic populations. Women are eligible to participate in the MADRES study if their pregnancy is at of gestation at the time of recruitment, they are , and they can speak either English or Spanish fluently. Exclusion criteria included the following: a) human immunodeficiency virus–positive status; b) having a physical, mental, or cognitive disability that would prevent participation in the study or the ability to provide informed consent; c) current incarceration; and d) multiple gestation. Informed consent was obtained from each participant at study entry, and the study was approved by the University of Southern California’s Institutional Review Board.
Given evidence that fetal growth trajectories are largely determined by conditions in early pregnancy (Bloomfield et al. 2006) and evidence that early pregnancy may be a particularly sensitive window for metals exposures (Cheng et al. 2017; Rabito et al. 2014; Vigeh et al. 2018), the present study focused on participants who enrolled before 20 wk of gestation and provided a urine sample at their first study visit. We restricted our analyses to 262 participants who a) enrolled prior to urine metals analysis (fall 2019); b) had not withdrawn from the study; and c) had complete covariate information. Overall, this subset of 262 participants was similar to all MADRES study participants who enrolled prior to fall 2019 at of gestation although these participants were slightly older and there was a larger percentage of foreign-born Hispanic and a smaller percentage of non-Hispanic black participants (Table S1).
Urine Collection
Spot urine samples were collected by participants during their first study visit [median gestational age (GA): 13.1 wk] in a sterile specimen container. Samples were transported on ice to the laboratory within 1 h. One and one-half–milliliter aliquots were then stored at in sterile cryovials (VWR).
Urine Metals
Urine metals analysis was performed by NSF International in collaboration with the Children’s Health Exposure Analysis Resource (CHEAR). Metals were measured using inductively coupled plasma mass spectrometry (ICP-MS) based on the Centers for Disease Control and Prevention method 3018.3, with modifications for the expanded metals panel and the Thermo Scientific iCAP™ RQ instrument (serial number RQ0029). The elements measured for this panel include the following: antimony (Sb), As, barium, beryllium, Cd, cesium, Co, copper, chromium, Hg, manganese, molybdenum (Mo), Ni, Pb, platinum, tin (Sn), Tl, tungsten, uranium, vanadium, and zinc. All quality control samples, blanks, and urine samples were diluted 10-fold in a diluent consisting of 2% nitric acid () solution containing the internal standards and gold. Standards were prepared in 1% trace metal–grade and diluted 10-fold with a diluent consisting of 2% solution containing the internal standards in order to minimize any matrix effect. The rinse solution for the instrument was 1% trace metal–grade . The samples were analyzed in two analysis modes: a) standard (default) for the majority of metals, and b) kinetic energy discrimination for vanadium, chromium, As, Mo, and Cd. Percentages of coefficient of variation were between 0.8% and 7.0% for all elements. Five field blanks were sent out for testing, and 96% of measures were below the limits of detection (LODs).
For our primary analysis, we restricted to metals a) that were above the LOD for of participants and b) for which urine is considered an accepted matrix for assessing exposure (ATSDR 1992; Chou et al. 2007; Faroon et al. 2004, 2012; Fay and Ingerman 2005; Risher and DeWoskin 1999). Our primary analysis was further restricted to metals for which there was evidence that they may impact fetal growth (Cabrera-Rodríguez et al. 2018; Deyssenroth et al. 2018; Hoffman 2000; Jalali and Koski 2018; Khoshhali et al. 2020; Kim et al. 2017; Mikelson et al. 2019; Milton et al. 2017; Pedersen et al. 2016; Rabito et al. 2014; Rodosthenous et al. 2017; Sun et al. 2018; Vaktskjold et al. 2007; Vejrup et al. 2014; Vigeh et al. 2018). Six metals (As, Cd, Co, Hg, Ni, Tl) met all three criteria. We also included Pb in our primary analysis given its established toxicity, even though blood is the preferred matrix for exposure assessment (Sommar et al. 2014). In exploratory analyses, we included three additional metals that met the first two criteria: Mo, Sb, and Sn but that have been less studied in relation to fetal growth. Importantly, the As measure from the CHEAR metals panel is total As, which is composed of many different As species and metabolites, including arsenobetaine (AsB), which is a nontoxic form of As derived from fish and seafood (Tseng 2009). Therefore, we replaced the total As measure from this panel with total speciated urinary As data, as described in more detail in the next section. The number (percentage) of samples below the LOD for the elements retained from the metals panel were as follows: Cd: 85 (32.4%), Co: 3 (1.1%), Hg: 3 (1.1%), Mo: 2 (0.8%), Ni: 33 (12.6%), Pb: 46 (17.6%), Sb: 88 (33.6%), Sn: 36 (13.7%), and Tl: 65 (24.8%). Values below the LOD were replaced with the LOD divided by the square root of 2 given that this method has been recommended for measures that are not highly skewed (geometric standard deviations ) (Hornung and Reed 1990).
Speciated Urinary As
Speciated urinary As was measured by the Arizona Laboratory for Emerging Contaminants (Michel‐Ramirez et al. 2020), using methods previously described by the Centers for Disease Control and Prevention (Branch and Jones 2004). Briefly, arsenite (), arsenate (), monomethyl arsenic (MMA), dimethyl arsenic (DMA), and AsB were measured by high-performance liquid chromatography, using the Hamilton PRP-X100 column, coupled to ICP-MS. Working calibration standards were prepared daily for each As species at concentrations ranging from 0.2 to . For quality control, a mid-range calibration check sample was prepared using mixed species standard. For each batch of 30 samples, at least 3 samples were spiked with a low-to-mid–range standard to monitor As recovery for each species. Evaluation of recovery was calculated by comparing the sum of the individual species to the reported total As measure. Measures were considered acceptable if they were within of the total As concentration. LODs across four analytical runs ranged from 0.011 to for , 0.020 to for , 0.020 to for MMA, and 0.014 to for DMA. Values below the LOD were set to the LOD divided by the square root of 2. Total speciated urinary arsenic (i.e., excluding AsB) was calculated by summing the inorganic As metabolites (), MMA, and DMA. This variable was used as the primary As measure for all statistical analyses.
Specific Gravity
Urine specific gravity (SG) was measured by a refractometer (Itago), and urinary metal concentrations were adjusted for SG to account for urine dilution using the following formula: , where , , and (Boeniger et al. 1993).
BW for GA -Scores
To evaluate fetal growth, we calculated sex-specific BW for GA z-scores using a representative U.S. reference (Aris et al. 2019). This reference was selected because it uses obstetric estimates of GA at birth and was updated recently, reflecting current trends in obesity and gestational diabetes, which can impact fetal growth (Aris et al. 2019). BW measures were abstracted from medical records. If this information was missing (), BW values were filled in using proxy-reported information obtained from the mothers. Best estimates of GA at birth were ascertained using a hierarchy of methods. A first-trimester ( GA) ultrasound measurement of crown–rump length was considered the most preferred and was used if available (). If unavailable, a second-trimester ( GA) ultrasound measurement of fetal biparietal diameter was used (). If measures from an early ultrasound were unavailable, GA at birth was determined based on the physician’s best clinical estimate, abstracted from the medical records (). If none of these measures were available, GA at birth was estimated using self-reported last menstrual period dating ().
Covariates
Questionnaires were administered in either English or Spanish, depending on the participant’s preferred language. Maternal self-reported prepregnancy weight, race, ethnicity, and birth country were determined from a questionnaire that was administered during the first study visit. In this questionnaire, participants were also asked if they resided with a smoker during their pregnancy. In questionnaires administered during their first, second, and third trimesters, participants were additionally asked if they had ever smoked during the pregnancy. Maternal standing height was measured twice by stadiometer (Perspectives Enterprises; Model PE-AIM-101). Maternal prepregnancy body mass index (BMI) was calculated using the self-reported prepregnancy weight and measured height values (in kilograms per meter squared). Each participant’s age was determined using the date that she consented and her birth date. A combined variable indicating race by ethnicity and birth place was created based on the participant’s self-reported race (white, Asian, black or African American, Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, more than one race), ethnicity (Hispanic vs. non-Hispanic), and birth country (United States vs. other). This variable was collapsed into five categories: non-Hispanic white, non-Hispanic black, non-Hispanic other, Hispanic born in the United States, and Hispanic born outside the United States. A combined variable was also created for any smoke exposure during the pregnancy that was based on any self-reported maternal smoking during the pregnancy or the participant sharing a residence with a smoker during the pregnancy. Information on newborn sex was abstracted from medical records. If this information was missing from the maternal medical records, it was filled in using reports from a questionnaire administered to the mothers 7–14 d after birth. Maternal hemoglobin and hematocrit measures were also abstracted from medical records for the pregnancy period. Using GA-specific cutoffs for these measures (ACOG 2008), participants were classified as ever vs. never being anemic during the pregnancy.
Statistical Analyses
Statistical analyses were conducted in R (version 3.6.2; R Development Core Team). A priori, we hypothesized that toxic (e.g., As, Cd, Hg, Pb, Tl) and essential (e.g., Co) elements would act in opposing directions and further hypothesized that associations between essential/nutritional elements (e.g., Co, Ni) and BW for GA would be nonlinear. Therefore, we used Bayesian kernel machine regression (BKMR) (Bobb et al. 2015) as our primary mixture modeling approach because it is a flexible method that does not constrain associations to a single direction, accommodates nonlinear associations between exposures and outcomes, and evaluates all possible synergistic and antagonistic relationships between mixture components without specifying these a priori. Using the bkmr R package (Bobb et al. 2018), we chose the variable selection option and ran 200,000 Markov chain Monte Carlo (MCMC) iterations using the default priors. The first half of iterations was used as burn-in. To reduce potential autocorrelation, we thinned the chains, selecting every 25th iteration. Model convergence was inspected using trace plots.
For the primary analysis, we used a hypothesis-driven approach and collectively evaluated the seven selected urinary elements (As, Cd, Co, Hg, Ni, Pb, Tl) in relation to BW for GA z-scores, using the following model: , where function represents the exposure–response function using the Gaussian kernel machine representation, the coefficient represents effect estimates for the Cth covariate for the ith individual, and represents the model residuals. Metals were generally right-skewed and were, therefore, to reduce the influence of extreme values. In an exploratory analysis, we ran a similar BKMR model that included three additional metals that have been less studied in relation to fetal growth: Mo, Sb, and Sn. Three participants had unusually low urinary Mo concentrations () and were, therefore, excluded from the exploratory analysis. We also identified two extreme high outliers for urinary Sb () (Rosner 1983), who were also excluded. Hypothesized confounders and precision variables were identified using a directed acyclic graph (Figure S1) (Shrier and Platt 2008). Final models were adjusted for recruitment site, maternal age, prepregnancy BMI, race by ethnicity and birth place, any maternal anemia in pregnancy, any smoke exposure (maternal or other) in pregnancy, and urinary AsB [an objective biomarker of fish and seafood consumption (Navas-Acien et al. 2011)]. Because urinary AsB was right-skewed, it was to reduce the influence of extreme values. Given that the BW for GA z-scores were generated separately for male and female infants, infant sex was not included as a covariate in the model. We also examined whether results were sensitive to additional adjustment for the GA at urine collection. All metals and continuous covariates were centered and scaled. Metals that ranked highly based on their BKMR posterior inclusion probabilities (PIPs) were further investigated using generalized additive models (GAMs), using the mgcv R package (version 1.8-31; R Development Core Team) (Wood 2015). We also used GAMs to determine whether associations from the primary model were robust after a) excluding extreme metal outliers [identified by Rosner’s test (Rosner 1983)] and b) adjusting for SG as a covariate as an alternative approach to account for urine dilution.
Although BKMR can identify potential interactions between pairs of mixture components, it does not generate PIPs for these interactions. Therefore, we also investigated possible synergistic and antagonistic relationships between the seven elements by applying a new method (Antonelli et al. 2020) that uses Bayesian semiparametric regression and sparsity-inducing priors to generate PIPs for interactions in addition to main effects. To conduct this analysis, we used the NLinteraction R package, specifying 200,000 MCMC iterations (half of which were removed for burn-in) and using the default options (Antonelli et al. 2020). For this method, the exposure–response relationships are modeled using natural cubic splines; 2 degrees of freedom were selected for these splines based on the Watanabe-Akaike information criterion (Antonelli et al. 2020).
Given that both BKMR and NLinteraction are sensitive to the choice of model priors, we conducted sensitivity analyses for each approach. For BKMR, we compared results after varying the parameter , which controls the smoothness of the exposure–outcome relationships. We investigated both a lower () and higher () degree of smoothness. For NLinteraction, we examined the impact of varying the threshold parameter , which influences the likelihood of an exposure being included in the function, from the default of 0.10 to a less conservative value of 0.25. We also evaluated the NLinteraction results after manually changing the parameter, which controls the variance for the slab component of the prior, instead of estimating this parameter using the default empirical Bayes approach (Antonelli et al. 2020); we compared both a small (0.005) and larger (1) value for .
Results
Participant Characteristics
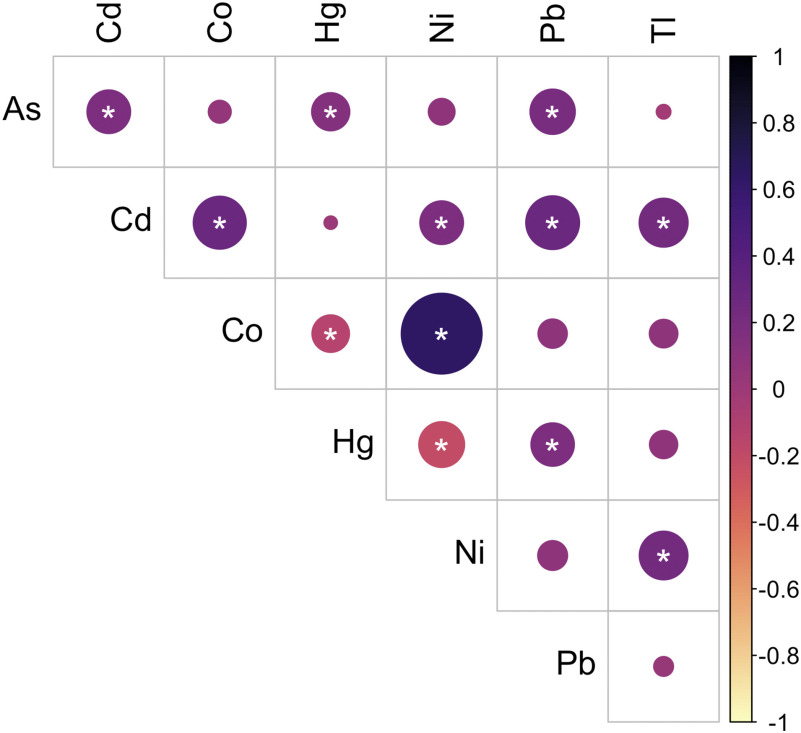
Characteristics of participants in the present study are shown side by side with all MADRES study participants who enrolled prior to fall 2019 at of gestation and the subset of participants who were excluded from this study (Table S1). Participants in the present study were between 18 and 45 years of age at study enrollment. The median (range) prepregnancy BMI was . The majority of participants were Hispanic (44.3% Hispanic and born outside the United States, 34.4% Hispanic and born in the United States). Approximately 33% of the participants experienced anemia during their pregnancy, and 8.0% reported either smoking during their pregnancy or residing with a smoker. Urinary AsB concentrations ranged from to with a median of . Urinary metal concentrations are shown in Table 1, and Pearson correlations between metals are shown in Figure 1. The majority of metals were positively correlated with each other, and the strongest positive correlation was between Co and Ni (, ). However, Co and Hg were significantly inversely correlated with each other (, ), as were Ni and Hg (, ).
Table 1.
Specific gravity-adjusted urinary metal concentrationsa ().
| Metals | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|
| Metals included in primary analysis | |||||
| As () | 1.09 | 4.50 | 5.81 | 8.09 | 325.97 |
| Cd () | 0.14 | 0.26 | 1.07 | ||
| Co () | 0.39 | 0.59 | 0.98 | 7.51 | |
| Hg () | 0.62 | 1.02 | 2.12 | 16.10 | |
| Ni () | 1.94 | 2.88 | 4.38 | 34.91 | |
| Pb () | 0.50 | 1.59 | 3.69 | 37.89 | |
| Tl () | 0.03 | 0.07 | 0.14 | 0.59 | |
| Metals additionally evaluated in exploratory analysisb | |||||
| Mo () | 14.9 | 42.9 | 56.8 | 80.7 | 359.3 |
| Sb () | 0.08 | 0.12 | 0.67 | ||
| Sn () | 0.27 | 0.49 | 0.97 | 26.03 | |
Note: As, arsenic; Cd, cadmium; Co, cobalt; Hg, mercury; LOD, limit of detection; Mo, molybdenum; Ni, nickel; Pb, lead; Sb, antimony; Sn, tin; Tl, thallium.
Values below the LOD were imputed to the LOD divided by the square root of 2 for all statistical analyses.
.
Figure 1.
Pearson correlations between urinary metals (). Stronger correlations are indicated by darker shades and larger circles. *, . See Table S7 for corresponding numeric data. Note: As, arsenic; Cd, cadmium; Co, cobalt; Hg, mercury; Ni, nickel; Pb, lead; Tl, thallium.
Primary Analysis
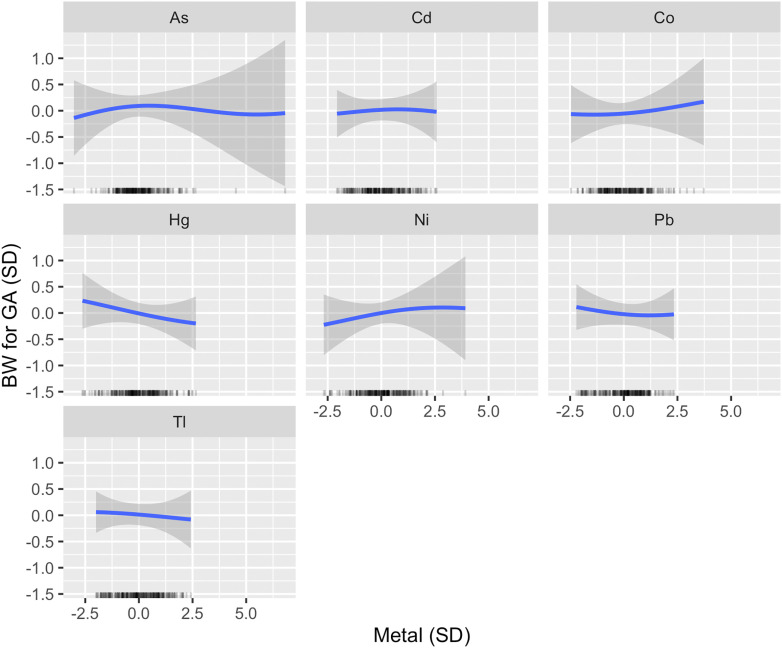
Using BKMR, Hg was estimated to have the highest PIP followed by Ni (Table 2). When setting all other metals to their median, a linear inverse association was estimated between urinary Hg and BW for GA (Figure 2), such that an increase from the 25th to 75th percentile was associated with a [95% credible interval (CI): , 0.09] standard deviation (SD) difference in BW for GA. Varying Ni from its 25th to 75th percentile was associated with a 0.10 (95% CI: , 0.35) SD difference in BW for GA (Figure 2). Visually, the Ni association with BW for GA appeared potentially nonlinear, with a positive association estimated at low-to-moderate concentrations only. However, the CIs were very wide at high levels of Ni, likely due to the small number of participants represented. For Hg and Ni, the estimates obtained after varying the smoothing parameter were very similar to those obtained using the default specifications (Figure S2). Although less pronounced, BKMR also identified inverse associations between both Pb and Tl with BW for GA, and a weak positive association for Co (Figure 2). The association between Cd and BW for GA appeared null, and a very weak inverted U-shaped relationship was estimated between As and BW for GA (Figure 2). Estimates for all metals obtained from the primary model were similar to those obtained by a model that additionally adjusted for the GA at urine collection (Figure S3).
Table 2.
Individual metal posterior inclusion probabilities.
| Metal | Primary analysis | Exploratory analysis |
|---|---|---|
| PIP | PIP | |
| As | 0.30 | 0.39 |
| Cd | 0.28 | 0.35 |
| Co | 0.31 | 0.38 |
| Hg | 0.40a | 0.46a |
| Ni | 0.35a | 0.46a |
| Pb | 0.29 | 0.41 |
| Tl | 0.29 | 0.38 |
| Mo | — | 0.41 |
| Sb | — | 0.58a |
| Sn | — | 0.45 |
Note: PIPs from a Bayesian kernel machine regression model, which was adjusted for maternal age, prepregnancy BMI, recruitment site, race by ethnicity and birth place, any maternal anemia in pregnancy, tobacco smoke exposure in pregnancy, and urinary arsenobetaine. Metals and urinary arsenobetaine were . Metals and all continuous covariates were also centered and scaled. —, not applicable; As, arsenic; BMI, body mass index; Cd, cadmium; Co, cobalt; Hg, mercury; Mo, molybdenum; Ni, nickel; Pb, lead; PIP, posterior inclusion probability; Sb, antimony; Sn, tin; Tl, thallium.
The highest-ranking elements for each model.
Figure 2.
Univariate exposure–response functions for primary analysis (). Associations between each metal and BW for GA z-score (with corresponding 95% credible intervals) are shown setting all other metals to their median, adjusting for maternal age, prepregnancy BMI, recruitment site, race by ethnicity and birth place, any maternal anemia in pregnancy, tobacco smoke exposure in pregnancy, and urinary arsenobetaine (AsB). A rug plot showing the distribution of the specified metal is shown along the x-axis of each panel. Metals and urinary AsB were . Metals and all continuous covariates were also centered and scaled. Note: As, arsenic; BMI, body mass index; BW, birth weight; Cd, cadmium; Co, cobalt; GA, gestational age; Hg, mercury; Ni, nickel; Pb, lead; Tl, thallium.
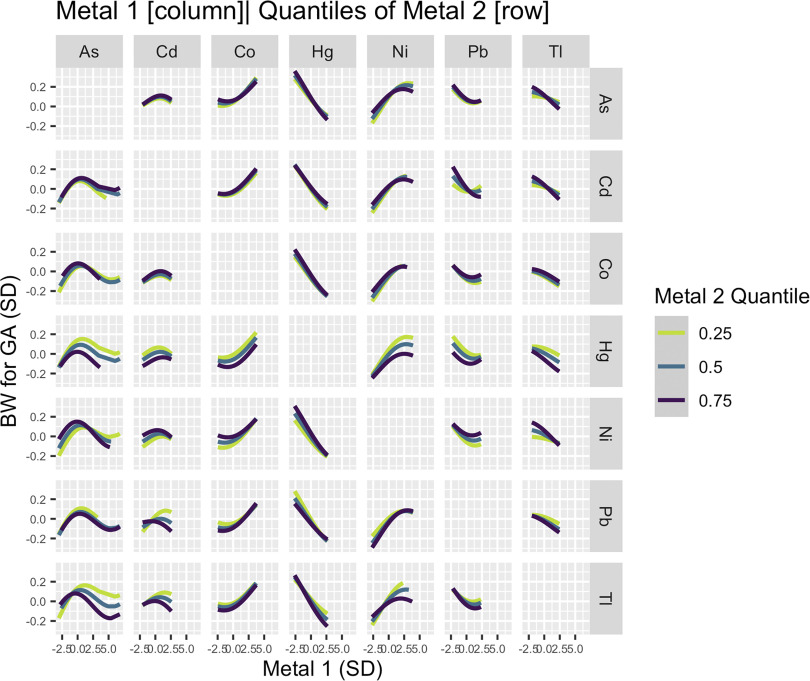
Several potential interactions between pairs of metals were identified visually using BKMR (Figure 3). The positive association between Ni and BW for GA appeared to be attenuated at higher levels of Hg and Tl. In contrast, the inverse association between Hg and BW for GA appeared stronger at higher levels of Ni. In addition, the inverted U-shaped relationship between As and BW for GA varied by levels of Hg and Tl. For As concentrations below the inflection point, the positive association between As and BW for GA appeared stronger at lower quantiles of Hg and Tl. For As concentrations above the inflection point, the inverse association between As and BW for GA was stronger at higher quantiles of Hg and Tl. We also estimated a positive association for Cd at lower levels of Pb and Tl, a more pronounced inverse association for Pb and BW for GA at higher levels of Cd or lower levels of Hg or Ni and a stronger inverse association for Tl at higher levels of all metals except Co (Figure 3).
Figure 3.
Bivariate exposure–response functions for primary analysis (). Associations between each element (columns) and BW for GA z-score, setting a second element (rows) to its 25th, 50th, and 75th percentile and all other elements to their median, adjusting for maternal age, prepregnancy BMI, recruitment site, race by ethnicity and birth place, any maternal anemia in pregnancy, tobacco smoke exposure in pregnancy, and urinary arsenobetaine (AsB). Metals and urinary AsB were . Metals and all continuous covariates were also centered and scaled. Note: As, arsenic; BMI, body mass index; BW, birth weight; Cd, cadmium; Co, cobalt; GA, gestational age; Hg, mercury; Ni, nickel; Pb, lead; Tl, thallium.
Using the novel NLinteraction method (Antonelli et al. 2020), we additionally estimated PIPs for pairwise interactions between element pairs. The interaction between Hg and Ni ranked highest of all possible metal pairs (Table S2). However, the PIPs for all pairwise interactions were very small. Compared with BKMR, the individual metal PIPs were also small when using the NLinteraction method, although the metal rankings were consistent across methods (Table S3). The individual metal PIPs and pairwise interaction PIPs were larger after increasing the NLinteraction threshold parameter from the default value of 0.10 to a less conservative value of 0.25 (Tables S3 and S4). However, metal rankings were similar, with Hg and Ni ranking highest both for their main effects and pairwise interaction. The individual metal PIPs and pairwise interaction PIPs also increased after setting to a very small value (0.005), instead of estimating this parameter using the default empirical Bayes approach (Table S3 and S5), but the metal rankings remained similar. In contrast, all PIPs approached 0 when manually setting this parameter to a higher value () (Tables S3 and S6).
Given that a) the PIPs estimated for Hg and Ni ranked highest both when using BKMR and NLinteraction and b) the PIP for the interaction between Hg and Ni ranked highest of all possible pairwise interactions when using NLinteraction, we used GAMs to further investigate their associations with BW for GA. Similar to BKMR, GAMs estimated an inverse linear association between urinary Hg and BW for GA (approximate ) (Figure S4). They also estimated a positive association between urinary Ni and BW for GA at low-to-moderate concentrations (approximate ), with a wide degree of uncertainty at the high end of exposure owing to few participants falling in this range (Figure S5). Similar to the BKMR results, the inverse association between Hg and BW for GA appeared stronger among individuals with higher levels of Ni (Figure S4), and the positive association for Ni and BW for GA appeared to be attenuated in a dose-dependent manner with increasing levels of Hg (Figure S5) (approximate for tensor product smooth term for Hg and Ni ). On the log-scale, no extreme values were identified for urinary Hg using Rosner’s test. However, one extreme high value was identified for urinary Ni (). Results were similar after excluding this extreme outlier (Figures S4 and S5) and also after adjusting for SG as a covariate to account for urine dilution instead of applying an SG correction directly to the metal concentrations (Figures S6 and S7). However, in both sets of sensitivity analyses, the Ni–fetal growth relationship appeared linear across the full range of urinary Ni concentrations (Figures S5 and S7).
Exploratory Analysis
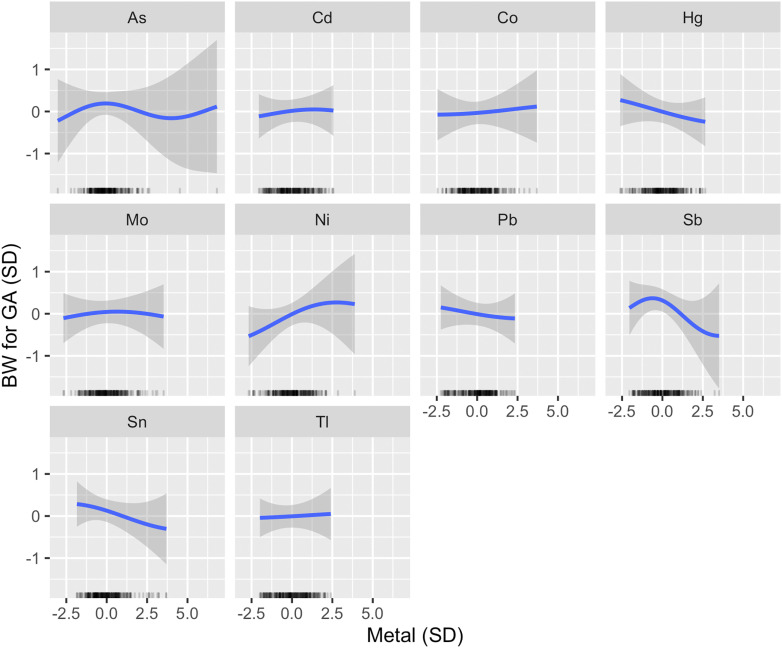
In an exploratory analysis that examined a larger mixture of metals, Sb ranked highest as a predictor of BW for GA, followed by Hg and Ni (Table 2). An inverse association was estimated between Sb and BW for GA at moderate-to-high concentrations (Figure 4). Results for Hg and Ni were consistent with the primary model (Figure 4). Although Sn did not rank as highly as these metals, an inverse and linear association was estimated with BW for GA (Figure 4). A very weak inverted U-shape association was identified for Mo and BW for GA (Figure 4).
Figure 4.
Univariate exposure–response functions for exploratory analysis (). Associations between each metal and BW for GA z-score (with corresponding 95% credible intervals) are shown setting all other metals to their median, adjusting for maternal age, prepregnancy BMI, recruitment site, race by ethnicity and birth place, any maternal anemia in pregnancy, tobacco smoke exposure in pregnancy, and urinary arsenobetaine (AsB). A rug plot showing the distribution of the specified metal is shown along the x-axis of each panel. Metals and urinary AsB were . Metals and all continuous covariates were also centered and scaled. Note: As, arsenic; BMI, body mass index; BW, birth weight; Cd, cadmium; Co, cobalt; GA, gestational age; Hg, mercury; Mo, molybdenum; Ni, nickel; Pb, lead; Sb, antimony; Sn, tin; Tl, thallium.
Discussion
Growing evidence suggests that toxic metal exposures adversely affect fetal growth (Ballester et al. 2018; Cabrera-Rodríguez et al. 2018; Deyssenroth et al. 2018; Hoffman 2000; Jalali and Koski 2018; Khoshhali et al. 2020; Kim et al. 2017; Kippler et al. 2010; Mikelson et al. 2019; Milton et al. 2017; Nielsen 2012; Pedersen et al. 2016; Rabito et al. 2014; Ramón et al. 2009a; Rodosthenous et al. 2017; Sun et al. 2018; Thomas et al. 2015; Vaktskjold et al. 2007; Vejrup et al. 2014; Vigeh et al. 2018), but the majority of studies have focused on individual metals. Because metals may behave differently in the presence of metal co-exposures owing to synergistic or antagonistic relationships (Claus Henn et al. 2014; Davidson et al. 2015), studies have begun investigating metal mixture impacts on fetal growth (Cabrera-Rodríguez et al. 2018; Cassidy-Bushrow et al. 2019; Deyssenroth et al. 2018; Govarts et al. 2016; Signes-Pastor et al. 2019). Using a novel mixture modeling method that can account for possible nonlinear relationships and interactions between mixture components (Bobb et al. 2015), we examined the impact of a complex mixture of seven elements on BW for GA. In our primary analysis, we focused on seven elements, selected based on prior evidence that they may influence fetal growth, and identified Hg and Ni as the highest-ranking predictors of BW for GA. Setting other metals to their median, an inverse linear association was estimated for Hg and BW for GA and a positive association was estimated for Ni at low-to-moderate concentrations. In an exploratory analysis that evaluated a larger panel of metals, Sb ranked highest as a predictor of BW for GA; an inverse linear association was estimated.
Our findings for Hg are consistent with several previous studies that also reported inverse associations between Hg exposure and fetal growth (Ballester et al. 2018; Kim et al. 2017; Ou et al. 2015; Ramón et al. 2009a; Thomas et al. 2015; Vigeh et al. 2018). Although findings from previous studies on Ni and fetal growth have been mixed (Cabrera-Rodríguez et al. 2018; Deyssenroth et al. 2018; Jalali and Koski 2018; Sun et al. 2018; Vaktskjold et al. 2007), several studies have similarly reported that Ni may promote fetal growth, exhibited by greater increases in fetal head circumference (Jalali and Koski 2018) or a reduced odds of being small for GA (Deyssenroth et al. 2018; Vaktskjold et al. 2007). Ni is not an essential element, but it does have nutritional properties that may promote fetal growth (Nielsen 2012). For example, studies in both animal models (Stangl et al. 2000) and humans (Katko et al. 2008) have demonstrated that Ni supplementation can reverse vitamin B12 deficiency and hyperhomocysteinemia, which are risk factors for low BW and fetal growth restriction (Hogeveen et al. 2012; McGee et al. 2018). Although the magnitudes of the associations for Hg and Ni were small, they were similar to effect estimates previously reported for other metals and BW for GA (Claus Henn et al. 2016; Rodosthenous et al. 2017).
Using BKMR, we also identified a potential interaction between Hg and Ni, and we similarly identified a suggestive interaction () between this pair of metals when using GAMs. Both sets of results suggest that the positive association between Ni and BW for GA estimated at low-to-moderate concentrations may be attenuated by higher levels of Hg. It also suggests that the inverse association between Hg and BW for GA may be stronger at higher levels of Ni. The latter finding could be due to the apparent attenuation of the positive Ni–fetal growth association at high levels of Ni. However, there were very few participants with Ni concentrations at the high end of exposure, and the shape of the Ni–fetal growth relationship was sensitive to an extreme outlier for Ni and the method used to account for urine dilution. Future studies that span a wider range of urinary Ni levels are therefore needed to better understand the shape of this relationship, particularly at high concentrations. Importantly, one limitation of BKMR is that it does not quantify the importance of potential interactions. Therefore, we also applied a novel mixture modeling approach (NLinteraction) that can generate PIPs for interactions between mixture components (Antonelli et al. 2020). Using this method, we found the interaction between Hg and Ni to rank highest of all possible metal pairs. However, the PIP was extremely small. Thus, we cannot rule out the possibility that this may have been a chance finding.
Although the magnitudes were small, the directions of associations for Co, Pb, and Tl with fetal growth were consistent with previous studies (Govarts et al. 2016; Hu et al. 2015; Mikelson et al. 2019; Rabito et al. 2014; Rodosthenous et al. 2017; Xia et al. 2016). The weak inverse association estimated for Pb may be explained in part by the use of urine as a matrix because blood concentrations are more sensitive to inter-individual differences in Pb exposure (Sommar et al. 2014). Given that urine is an accepted matrix for both Co and Tl (ATSDR 1992; Faroon et al. 2004), the weak associations estimated for these metals may be due to the particular levels represented in the MADRES study. For example, maternal urinary Tl levels were much lower [geometric mean (GM): ] in the MADRES study compared with a cohort of mother–newborn pairs in China (GM: ) that identified an association between Tl and risk of low BW (Xia et al. 2016).
Although numerous studies have estimated inverse associations between Cd exposure and fetal growth (Khoshhali et al. 2020), the overall association between urinary Cd and BW for GA appeared null in the MADRES study. This could be due to the low urinary Cd concentrations in this population or to potential confounding from an unmeasured dietary factor given that diet is the main source of Cd exposure among nonsmokers (Faroon et al. 2012). For example, vegetable intake has been associated with both Cd exposure and increases in fetal growth (Faroon et al. 2012; Ramón et al. 2009b) and could induce a spurious positive association between these two variables. This is especially plausible for MADRES study participants because the prevalence of maternal smoking during pregnancy is very low in this population (2.7%), consistent with the overall prevalence among Hispanic women in the United States (1.8%) (Drake et al. 2018), and maternal environmental tobacco smoke exposure during pregnancy is also lower among Hispanic women (Hoshiko et al. 2019). Diet is, therefore, likely the main source of Cd exposure for this population.
Similar to the findings for Cd, the overall association between urinary As and BW for GA also appeared null. This finding differs from those of most previous studies, which have estimated inverse associations between As exposure and fetal growth, even at low levels of exposure (Milton et al. 2017). In fact, in a previous study that compared multiple biomarkers of As exposure (Howe et al. 2020), we estimated an inverse association between maternal hair As and BW in the MADRES study but did not observe a similar trend for urinary As (Howe et al. 2020). This discrepancy may be due to the different arsenicals present in hair vs. urine. Although hair is thought to primarily reflect inorganic As, urine represents a complex mixture of arsenicals, with dimethyl arsenicals predominating (National Research Council 2013). These dimethyl arsenicals can reflect metabolized inorganic As, but they may also reflect As from dietary sources, such as metabolized arsenosugars and arsenolipids, which are derived from fish and seafood and thought to be nontoxic (Navas-Acien et al. 2011). Although models were adjusted for urinary AsB (Navas-Acien et al. 2011), a biomarker of fish and seafood consumption, we cannot rule out the possibility of residual confounding.
Although most of the metals evaluated in this study were lower in MADRES study participants or were similar to concentrations reported for pregnant women in the National Health and Nutrition Examination Survey (NHANES) or other pregnancy cohorts, we found urinary Hg and Pb concentrations to be higher on average (Ashrap et al. 2020; Lewis et al. 2018; Watson et al. 2020). Fish/seafood is the main source of methylHg for most populations, and this form of Hg can be demethylated in the intestine and excreted into urine (Li et al. 2019). However, the majority of MADRES study participants reported rarely or never consuming fish/seafood (Farzan et al. 2020). Furthermore, the correlation between AsB, a biomarker of fish/seafood consumption (Navas-Acien et al. 2011), and urinary Hg was very weak (, ), which suggests that other sources of Hg exposure may be important. Another potential dietary source of Hg exposure could be rice (Davis et al. 2014) given that the majority of MADRES study participants reported consuming rice frequently (Farzan et al. 2020), whereas nondietary sources may include dental amalgams or the use of skin whitening/lightening creams (Copan et al. 2015; Peregrino et al. 2011). Although Pb exposure has declined in many parts of the world because of the removal of Pb additives from gasoline, exposure from industrial sources, such as Pb smelters, is a growing concern in urban areas, including Los Angeles (Johnston and Hricko 2017). Pb-based paint, which is still prevalent in older homes in the United States (Kennedy et al. 2016), and certain imported food and spices, such as chili powder (Handley et al. 2017; Hore et al. 2019), may also contribute to exposure.
In an exploratory analysis, we investigated a larger panel of metals, including Sb. Associations for Hg and Ni remained robust after accounting for these additional metals. However, Sb ranked highest as a predictor of BW for GA; an inverse association was estimated between Sb and BW for GA at moderate-to-high concentrations. Although few studies have investigated the impacts of Sb on fetal growth, we are aware of one previous study that similarly identified an association between cord blood Sb concentrations and risk for low BW (Cabrera-Rodríguez et al. 2018). Importantly, the urinary Sb concentrations in the MADRES study were comparable to levels reported for pregnant women in the NHANES (Watson et al. 2020). This suggests that Sb may adversely impact fetal growth even at relatively low levels of exposure, which merits additional investigation.
The present study had many strengths, including the prospective design, the measurement of a multimetals panel to evaluate complex metal mixture exposures in early pregnancy, focusing on an understudied population at higher risk for multipollutant burdens (Cushing et al. 2015; Shim et al. 2017), the use of a mixture modeling approach that simultaneously accounts for nonlinear relationships and synergistic and antagonistic relationships (Bobb et al. 2015), and the application of a novel method that formally investigates interactions between mixture components (Antonelli et al. 2020). However, our study also had several important limitations. Most notably, we were underpowered to evaluate possible differences by fetal sex, which have been reported for certain metals (Cassidy-Bushrow et al. 2019; Govarts et al. 2016; Kippler et al. 2012; Milton et al. 2017; Signes-Pastor et al. 2019; Sun et al. 2018), and we measured urinary Pb concentrations to profile Pb exposure. Although urinary Pb does capture inter-individual differences in exposure, it is less sensitive than blood Pb (Sommar et al. 2014), which may have biased results toward the null. An additional limitation of our study was the use of a single spot urine sample to assess metals exposure, which may reflect only recent exposure for some metals (Wang et al. 2016). The use of a single urine measurement also precluded our ability to compare metal exposures across different windows in pregnancy. However, previous studies that measured metals at multiple time points in pregnancy have identified the early prenatal period as a particularly sensitive window (Cheng et al. 2017; Rabito et al. 2014; Vigeh et al. 2018). Another important consideration is that some metals are mobilized from bone or are metabolized more efficiently as the pregnancy progresses (Gardner et al. 2011; Gulson et al. 1997). However, our results were robust after adjusting for the GA at urine collection. Finally, we cannot rule out the possibility of unmeasured or residual confounding, particularly from diet, as detailed dietary information was not obtained for MADRES study participants in early pregnancy.
Given that reduced fetal growth has been associated with a broad range of health consequences later in life (Barker and Thornburg 2013), identifying modifiable factors that impact fetal growth is critical. Of the seven elements evaluated in our primary analysis, urinary Hg was identified as the element of greatest concern because of its inverse association with fetal growth and possible antagonistic relationship with Ni. In exploratory analyses, Sb was found to be an even stronger predictor of reduced fetal growth. Identifying the major sources of Hg and Sb in this population is, therefore, essential. Although the downstream health consequences of our findings are currently unknown, the MADRES cohort was designed to follow children through the first 5 y of life. The impacts of prenatal exposure to these metals on early life growth, adiposity, and other outcomes can, therefore, be directly examined in future studies.
Supplementary Material
Acknowledgments
C.G.H. conceptualized the study, acquired funding support for the study, was involved in data acquisition, conducted the statistical analyses, and wrote the first draft of the manuscript. B.C.H. supervised the statistical analyses and provided feedback on the manuscript. S.P.E. supervised the statistical analyses and provided feedback on the manuscript. S.F.F. acquired funding support for the study, was involved in data acquisition, and provided feedback on the statistical analyses and manuscript. B.H.G. provided feedback on the manuscript. T.A.C., T.L.H., D.F., and M.J.R. were involved in data acquisition and provided feedback on the manuscript. J.D.M. was involved in data acquisition and provided feedback on the statistical analyses and manuscript. T.M.B. acquired funding support for the study and provided feedback on the statistical analyses and manuscript. C.V.B. acquired funding support for the study, conceived of the original study hypothesis, supervised the statistical analyses, and provided feedback on the manuscript. L.A., D.L., A.Q., and S.T. facilitated participant recruitment and provided feedback on the manuscript.
We thank the MADRES study participants, the study staff, and our community clinic partners for their many contributions to this work. We also thank the Arizona Laboratory for Emerging Contaminants at the University of Arizona, Tucson, Arizona, which performed the urinary metals analyses, and M. Bixby (Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai) from the CHEAR Data Center.
This work was supported by National Institutes of Health grants K99 ES030400 (C.G.H.), P50 ES026086 (C.V.B.), 4UH3OD023287-03 (C.V.B.), P30ES007048, and U.S. Environmental Protection Agency grant 83615801-0 (C.V.B.). B.C.H. and S.F.F. are supported by Pathway to Independence Awards from the National Institute of Environmental Health Sciences (NIEHS; R00 ES022986 and R00 ES024144). S.F.F. is also supported by a University of Southern California Provost’s Fellowship. This work was additionally supported by grants U2CES026555 and U2CES026553 from the NIEHS as part of the Children’s Health Exposure Analysis Resource (CHEAR). A portion of the data used in this study was generated by the CHEAR Program with grant support from the NIEHS and are publicly available (https://www.doi.org/10.36043/1945_177, https://www.doi.org/10.36043/1945_159). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of these funding organizations.
References
- ACOG (American College of Obstetrician and Gynecologists). 2008. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol 112(1):201–207, PMID: 18591330, 10.1097/AOG.0b013e3181809c0d. [DOI] [PubMed] [Google Scholar]
- Antonelli J, Mazumdar M, Bellinger D, Christiani D, Wright R, Coull B. 2020. Estimating the health effects of environmental mixtures using Bayesian semiparametric regression and sparsity inducing priors. Ann Appl Stat 14(1):257–275, 10.1214/19-AOAS1307. [DOI] [Google Scholar]
- Aris IM, Kleinman KP, Belfort MB, Kaimal A, Oken E. 2019. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics 144(1):e20190076, PMID: 31201230, 10.1542/peds.2019-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, et al. 2020. Predictors of urinary and blood metal(loid) concentrations among pregnant women in northern Puerto Rico. Environ Res 183:109178, PMID: 32007748, 10.1016/j.envres.2020.109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 1992. Toxicological Profile for Thallium. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=309&tid=49 [accessed 19 October 2020]. [PubMed]
- Ballester F, Iñiguez C, Murcia M, Guxens M, Basterretxea M, Rebagliato M, et al. 2018. Prenatal exposure to mercury and longitudinally assessed fetal growth: relation and effect modifiers. Environ Res 160:97–106, PMID: 28968527, 10.1016/j.envres.2017.09.018. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Thornburg KL. 2013. The obstetric origins of health for a lifetime. Clin Obstet Gynecol 56(3):511–519, PMID: 23787713, 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- Bastain TM, Chavez T, Habre R, Girguis MS, Grubbs B, Toledo-Corral C, et al. 2019. Study design, protocol and profile of the Maternal and Developmental Risks from Environmental and Social Stressors (MADRES) pregnancy cohort: a prospective cohort study in predominantly low-income Hispanic women in urban Los Angeles. BMC Pregnancy Childbirth 19(1):189, PMID: 31146718, 10.1186/s12884-019-2330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Harding JE. 2006. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed 91(4):F299–F304, PMID: 16790736, 10.1136/adc.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):10, PMID: 30126431, 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: 25532525, 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54(10):615–627, PMID: 8237794, 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Branch N, Jones RL. 2004. Laboratory Procedure Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/UAS_UASS_I_MET.pdf [access 31 August 2020].
- Cabrera-Rodríguez R, Luzardo OP, González-Antuña A, Boada LD, Almeida-González M, Camacho M, et al. 2018. Occurrence of 44 elements in human cord blood and their association with growth indicators in newborns. Environ Int 116:43–51, PMID: 29649776, 10.1016/j.envint.2018.03.048. [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Wu KHH, Sitarik AR, Park SK, Bielak LF, Austin C, et al. 2019. In utero metal exposures measured in deciduous teeth and birth outcomes in a racially-diverse urban cohort. Environ Res 171:444–451, PMID: 30735952, 10.1016/j.envres.2019.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang B, Zheng T, Hu J, Zhou A, Bassig BA, et al. 2017. Critical windows of prenatal exposure to cadmium and size at birth. Int J Environ Res Public Health 14(1):58, PMID: 28075368, 10.3390/ijerph14010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S, Harper C, Ingerman L, Llados F, Colman J, Chappell L, et al. 2007. Toxicological Profile for Arsenic. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- Claus Henn B, Coull BA, Wright RO. 2014. Chemical mixtures and children’s health. Curr Opin Pediatr 26(2):223–229, PMID: 24535499, 10.1097/MOP.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Hopkins MR, Jim R, Amarasiriwardena C, Christiani DC, et al. 2016. Prenatal arsenic exposure and birth outcomes among a population residing near a mining-related Superfund site. Environ Health Perspect 124(8):1308–1315, PMID: 26859631, 10.1289/ehp.1510070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copan L, Fowles J, Barreau T, McGee N. 2015. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel). Int J Environ Res Public Health 12(9):10943–10954, PMID: 26364641, 10.3390/ijerph120910943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing L, Faust J, August LM, Cendak R, Wieland W, Alexeeff G. 2015. Racial/ethnic disparities in cumulative environmental health impacts in California: evidence from a statewide environmental justice screening tool (CalEnviroScreen 1.1). Am J Public Health 105(11):2341–2348, PMID: 26378826, 10.2105/AJPH.2015.302643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T, Ke Q, Costa M. 2015. Selected molecular mechanisms of metal toxicity and carcinogenicity. In: Handbook on the Toxicology of Metals, vol. 1 4th ed Nordberg G, Fowler BA, Nordberg M, eds. London, UK: Elsevier, 173–196. [Google Scholar]
- Davis MA, Gilbert-Diamond D, Karagas MR, Li Z, Moore JH, Williams SM, et al. 2014. A dietary-wide association study (DWAS) of environmental metal exposure in US children and adults. PLoS One 9(9):e104768, PMID: 25198543, 10.1371/journal.pone.0104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyssenroth MA, Gennings C, Liu SH, Peng S, Hao K, Lambertini L, et al. 2018. Intrauterine multi-metal exposure is associated with reduced fetal growth through modulation of the placental gene network. Environ Int 120:373–381, PMID: 30125854, 10.1016/j.envint.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake P, Driscoll AK, Mathews T. 2018. Cigarette Smoking during Pregnancy: United States, 2016. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- Faroon O, Abadin H, Keith S, Osier M, Chappell LL, Diamond G, et al. 2004. Toxicological Profile for Cobalt. Atlanta, GA: U.S. Department of Health and Human Services, Public Service, Agency for Toxic Substances and Disease Registry. [Google Scholar]
- Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. 2012. Toxicological Profile for Cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Service, Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- Farzan SF, Howe CG, Chavez TA, Hodes TL, Johnston JE, Habre R, et al. 2020. Demographic predictors of urinary arsenic in a low-income predominantly Hispanic pregnancy cohort in Los Angeles. J Expo Sci Environ Epidemiol, PMID: 32719440, 10.1038/s41370-020-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay M, Ingerman L. 2005. Toxicological Profile for Nickel. Atlanta, GA: U.S. Department of Health and Human Services, Public Service, Agency for Toxic Substances and Disease Registry. [Google Scholar]
- Gardner RM, Nermell B, Kippler M, Grandér M, Li L, Ekström E-C, et al. 2011. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod Toxicol 31(2):210–218, PMID: 21078382, 10.1016/j.reprotox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, et al. 2016. Combined effects of prenatal exposures to environmental chemicals on birth weight. Int J Environ Res Public Health 13(5):495, PMID: 27187434, 10.3390/ijerph13050495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulson BL, Jameson CW, Mahaffey KR, Mizon KJ, Korsch MJ, Vimpani G. 1997. Pregnancy increases mobilization of lead from maternal skeleton. J Lab Clin Med 130(1):51–62, PMID: 9242366, 10.1016/S0022-2143(97)90058-5. [DOI] [PubMed] [Google Scholar]
- Handley MA, Nelson K, Sanford E, Clarity C, Emmons-Bell S, Gorukanti A, et al. 2017. Examining lead exposures in California through state-issued health alerts for food contamination and an exposure-based candy testing program. Environ Health Perspect 125(10):104503, PMID: 29084633, 10.1289/EHP2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RS. 2000. Thallium poisoning during pregnancy: a case report and comprehensive literature review. J Toxicol Clin Toxicol 38(7):767–775, PMID: 11192464, 10.1081/CLT-100102390. [DOI] [PubMed] [Google Scholar]
- Hogeveen M, Blom HJ, den Heijer M. 2012. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr 95(1):130–136, PMID: 22170376, 10.3945/ajcn.111.016212. [DOI] [PubMed] [Google Scholar]
- Hore P, Alex-Oni K, Sedlar S, Nagin D. 2019. A spoonful of lead: a 10-year look at spices as a potential source of lead exposure. J Public Health Manag Pract 25(suppl 1):S63–S70, PMID: 30507772, 10.1097/PHH.0000000000000876. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Hoshiko S, Pearl M, Yang J, Aldous KM, Roeseler A, Dominguez ME, et al. 2019. Differences in prenatal tobacco exposure patterns among 13 race/ethnic groups in California. Int J Environ Res Public Health 16(3):458, PMID: 30764487, 10.3390/ijerph16030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CG, Farzan SF, Garcia E, Jursa T, Iyer R, Berhane K, et al. 2020. Arsenic and birth outcomes in a predominately lower income Hispanic pregnancy cohort in Los Angeles. Environ Res 184:109294, PMID: 32145549, 10.1016/j.envres.2020.109294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zheng T, Cheng Y, Holford T, Lin S, Leaderer B, et al. 2015. Distributions of heavy metals in maternal and cord blood and the association with infant birth weight in China. J Reprod Med 60(1–2):21–29, PMID: 25745747. [PMC free article] [PubMed] [Google Scholar]
- Jalali LM, Koski KG. 2018. Amniotic fluid minerals, trace elements, and prenatal supplement use in humans emerge as determinants of fetal growth. J Trace Elem Med Biol 50:139–145, PMID: 30262271, 10.1016/j.jtemb.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Johnston JE, Hricko A. 2017. Industrial lead poisoning in Los Angeles: anatomy of a public health failure. Environ Justice 10(5):162–167, PMID: 30687453, 10.1089/env.2017.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katko M, Kiss I, Karpati I, Kadar A, Matyus J, Csongradi E, et al. 2008. Relationship between serum nickel and homocysteine concentration in hemodialysis patients. Biol Trace Elem Res 124(3):195–205, PMID: 18465090, 10.1007/s12011-008-8139-2. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Lordo R, Sucosky MS, Boehm R, Brown MJ. 2016. Evaluating the effectiveness of state specific lead-based paint hazard risk reduction laws in preventing recurring incidences of lead poisoning in children. Int J Hyg Environ Health 219(1):110–117, PMID: 26472219, 10.1016/j.ijheh.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshhali M, Rafiei N, Farajzadegan Z, Shoshtari-Yeganeh B, Kelishadi R. 2020. Maternal exposure to cadmium and fetal growth: a systematic review and meta-analysis. Biol Trace Elem Res 195(1):9–19, PMID: 31401745, 10.1007/s12011-019-01819-y. [DOI] [PubMed] [Google Scholar]
- Kim BM, Chen MH, Chen PC, Park H, Ha M, Kim Y, et al. 2017. Path analysis of prenatal mercury levels and birth weights in Korean and Taiwanese birth cohorts. Sci Total Environ 605–606:1003–1010, PMID: 28693105, 10.1016/j.scitotenv.2017.06.151. [DOI] [PubMed] [Google Scholar]
- Kippler M, Hoque AMW, Raqib R, Ohrvik H, Ekström EC, Vahter M. 2010. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett 192(2):162–168, PMID: 19854248, 10.1016/j.toxlet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Kippler M, Wagatsuma Y, Rahman A, Nermell B, Persson LÅ, Raqib R, et al. 2012. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: a longitudinal study in rural Bangladesh. Reprod Toxicol 34(4):504–511, PMID: 22985739, 10.1016/j.reprotox.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Basu N, Gauthier AM, Cantoral A, Mercado-García A, et al. 2018. Urinary metal concentrations among mothers and children in a Mexico City birth cohort study. Int J Hyg Environ Health 221(4):609–615, PMID: 29703512, 10.1016/j.ijheh.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin X, Zhao J, Cui L, Wang L, Gao Y, et al. 2019. Intestinal methylation and demethylation of mercury. Bull Environ Contam Toxicol 102(5):597–604, PMID: 30515547, 10.1007/s00128-018-2512-4. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. 2018. Births: final data for 2017. Natl Vital Stat Rep 67(8):1–50, PMID: 30707672. [PubMed] [Google Scholar]
- McCormick MC. 1985. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 312(2):82–90, PMID: 3880598, 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- McGee M, Bainbridge S, Fontaine-Bisson B. 2018. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr Rev 76(6):469–478, PMID: 29529267, 10.1093/nutrit/nuy006. [DOI] [PubMed] [Google Scholar]
- Michel‐Ramirez G, Recio‐Vega R, Lantz RC, Gandolfi AJ, Olivas‐Calderon E, Chau BT, et al. 2020. Assessment of YAP gene polymorphisms and arsenic interaction in Mexican women with breast cancer. J Appl Toxicol 40(3):342–351, PMID: 31631368, 10.1002/jat.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikelson CK, Troisi J, LaLonde A, Symes SJK, Thurston SW, DiRe LM, et al. 2019. Placental concentrations of essential, toxic, and understudied metals and relationships with birth outcomes in Chattanooga, TN. Environ Res 168:118–129, PMID: 30296639, 10.1016/j.envres.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AH, Hussain S, Akter S, Rahman M, Mouly TA, Mitchell K. 2017. A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. Int J Environ Res Public Health 14(6):556, PMID: 28545256, 10.3390/ijerph14060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardozza LMM, Caetano ACR, Zamarian ACP, Mazzola JB, Silva CP, Marçal VMG, et al. 2017. Fetal growth restriction: current knowledge. Arch Gynecol Obstet 295(5):1061–1077, PMID: 28285426, 10.1007/s00404-017-4341-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. 2013. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic. Washington, DC: National Academies Press. [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. 2011. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res 111(1):110–118, PMID: 21093857, 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen FH. 2012. Manganese, molybdenum, boron, chromium, and other trace elements. In: Present Knowledge in Nutrition. 10th ed Erdman JW. Jr, Macdonald IA, Zeisel SH, eds. Hoboken, NJ: John Wiley & Sons, 586–607. [Google Scholar]
- Ou L, Chen C, Chen L, Wang H, Yang T, Xie H, et al. 2015. Low-level prenatal mercury exposure in north China: an exploratory study of anthropometric effects. Environ Sci Technol 49(11):6899–6908, PMID: 25936461, 10.1021/es5055868. [DOI] [PubMed] [Google Scholar]
- Oudgenoeg-Paz O, Mulder H, Jongmans MJ, van der Ham IJM, Van der Stigchel S. 2017. The link between motor and cognitive development in children born preterm and/or with low birth weight: a review of current evidence. Neurosci Biobehav Rev 80:382–393, PMID: 28642071, 10.1016/j.neubiorev.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Gehring U, Beelen R, Wang M, Giorgis-Allemand L, Andersen AMN, et al. 2016. Elemental constituents of particulate matter and newborn’s size in eight European cohorts. Environ Health Perspect 124(1):141–150, PMID: 26046983, 10.1289/ehp.1409546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peregrino CP, Moreno MV, Miranda SV, Rubio AD, Leal LO. 2011. Mercury levels in locally manufactured Mexican skin-lightening creams. Int J Environ Res Public Health 8(6):2516–2523, PMID: 21776243, 10.3390/ijerph8062516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabito FA, Kocak M, Werthmann DW, Tylavsky FA, Palmer CD, Parsons PJ. 2014. Changes in low levels of lead over the course of pregnancy and the association with birth outcomes. Reprod Toxicol 50:138–144, PMID: 25461912, 10.1016/j.reprotox.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Ramón R, Ballester F, Aguinagalde X, Amurrio A, Vioque J, Lacasaña M, et al. 2009a. Fish consumption during pregnancy, prenatal mercury exposure, and anthropometric measures at birth in a prospective mother-infant cohort study in Spain. Am J Clin Nutr 90(4):1047–1055, PMID: 19710189, 10.3945/ajcn.2009.27944. [DOI] [PubMed] [Google Scholar]
- Ramón R, Ballester F, Iñiguez C, Rebagliato M, Murcia M, Esplugues A, et al. 2009b. Vegetable but not fruit intake during pregnancy is associated with newborn anthropometric measures. J Nutr 139(3):561–567, PMID: 19158218, 10.3945/jn.108.095596. [DOI] [PubMed] [Google Scholar]
- Risher J, DeWoskin R. 1999. Toxicological Profile for Mercury. Atlanta, GA: U.S. Department of Health and Human Services, Public Service, Agency for Toxic Substances and Disease Registry. [Google Scholar]
- Rodosthenous RS, Burris HH, Svensson K, Amarasiriwardena CJ, Cantoral A, Schnaas L, et al. 2017. Prenatal lead exposure and fetal growth: smaller infants have heightened susceptibility. Environ Int 99:228–233, PMID: 27923585, 10.1016/j.envint.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B. 1983. Percentage points for a generalized ESD many-outlier procedure. Technometrics 25(2):165–172, 10.1080/00401706.1983.10487848. [DOI] [Google Scholar]
- Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. 2017. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: the United States NHANES, 2007–2012. J Toxicol Environ Health A 80(9):502–512, PMID: 28703686, 10.1080/15287394.2017.1330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier I, Platt RW. 2008. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 8:70, PMID: 18973665, 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Doherty BT, Romano ME, Gleason KM, Gui J, Baker E, et al. 2019. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environ Epidemiol 3(5):e068, PMID: 31844832, 10.1097/EE9.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommar JN, Hedmer M, Lundh T, Nilsson L, Skerfving S, Bergdahl IA. 2014. Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J Expo Sci Environ Epidemiol 24(1):51–57, PMID: 23443239, 10.1038/jes.2013.4. [DOI] [PubMed] [Google Scholar]
- Stangl GI, Roth-Maier DA, Kirchgessner M. 2000. Vitamin B-12 deficiency and hyperhomocysteinemia are partly ameliorated by cobalt and nickel supplementation in pigs. J Nutr 130(12):3038–3044, PMID: 11110865, 10.1093/jn/130.12.3038. [DOI] [PubMed] [Google Scholar]
- Sun X, Jiang Y, Xia W, Jin S, Liu W, Lin X, et al. 2018. Association between prenatal nickel exposure and preterm low birth weight: possible effect of selenium. Environ Sci Pollut Res Int 25(26):25888–25895, PMID: 29961220, 10.1007/s11356-018-2622-x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W. 2015. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: the MIREC study. Environ Res 140:430–439, PMID: 25967284, 10.1016/j.envres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Tseng CH. 2009. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol 235(3):338–350, PMID: 19168087, 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Vaktskjold A, Talykova LV, Chashchin VP, Odland JO, Nieboer E. 2007. Small-for-gestational-age newborns of female refinery workers exposed to nickel. Int J Occup Med Environ Health 20(4):327–338, PMID: 18165195, 10.2478/v10001-007-0034-0. [DOI] [PubMed] [Google Scholar]
- Vejrup K, Brantsæter AL, Knutsen HK, Magnus P, Alexander J, Kvalem HE, et al. 2014. Prenatal mercury exposure and infant birth weight in the Norwegian Mother and Child Cohort Study. Public Health Nutr 17(9):2071–2080, PMID: 24103413, 10.1017/S1368980013002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeh M, Nishioka E, Ohtani K, Omori Y, Matsukawa T, Koda S, et al. 2018. Prenatal mercury exposure and birth weight. Reprod Toxicol 76:78–83, PMID: 29360564, 10.1016/j.reprotox.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E, Cosmi E. 2014. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 237(2):391–399, PMID: 25463063, 10.1016/j.atherosclerosis.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Wang Y-X, Feng W, Zeng Q, Sun Y, Wang P, You L, et al. 2016. Variability of metal levels in spot, first morning, and 24-hour urine samples over a 3-month period in healthy adult Chinese men. Environ Health Perspect 124(4):468–476, PMID: 26372665, 10.1289/ehp.1409551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CV, Lewin M, Ragin-Wilson A, Jones R, Jarrett JM, Wallon K, et al. 2020. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999–2016. Environ Res 183:109208, PMID: 32058143, 10.1016/j.envres.2020.109208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack LS, Rossen LM, Martin JA. 2018. Singleton low birthweight rates, by race and Hispanic origin: United States, 2006–2016. NCHS Data Brief 306:1–8, PMID: 29616897. [PubMed] [Google Scholar]
- Wood S. 2015. mgcv: mixed GAM computation vehicle with automatic smoothness estimation. R package version 1:29. https://cran.r-project.org/web/packages/mgcv/index.html [accessed 20 October 2020].
- Xia W, Du X, Zheng T, Zhang B, Li Y, Bassig BA, et al. 2016. A case–control study of prenatal thallium exposure and low birth weight in China. Environ Health Perspect 124(1):164–169, PMID: 26009470, 10.1289/ehp.1409202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.