Abstract
Objective
Risk factors for in-hospital mortality in confirmed COVID-19 patients have been summarized in numerous meta-analyses, but it is still unclear whether they vary according to the age, sex and health conditions of the studied populations. This study explored these variables as potential mortality predictors.
Methods
A systematic review was conducted by searching the MEDLINE, Scopus, and Web of Science databases of studies available through July 27, 2020. The pooled risk was estimated with the odds ratio (p-OR) or effect size (p-ES) obtained through random-effects meta-analyses. Subgroup analyses and meta-regression were applied to explore differences by age, sex and health conditions. The MOOSE guidelines were strictly followed.
Results
The meta-analysis included 60 studies, with a total of 51,225 patients (12,458 [24.3%] deaths) from hospitals in 13 countries. A higher in-hospital mortality risk was found for dyspnoea (p-OR = 2.5), smoking (p-OR = 1.6) and several comorbidities (p-OR range: 1.8 to 4.7) and laboratory parameters (p-ES range: 0.3 to -2.6). Age was the main source of heterogeneity, followed by sex and health condition. The following predictors were more markedly associated with mortality in studies with patients with a mean age ≤60 years: dyspnoea (p-OR = 4.3), smoking (p-OR = 2.8), kidney disease (p-OR = 3.8), hypertension (p-OR = 3.7), malignancy (p-OR = 3.7), diabetes (p-OR = 3.2), pulmonary disease (p-OR = 3.1), decreased platelet count (p-ES = -1.7), decreased haemoglobin concentration (p-ES = -0.6), increased creatinine (p-ES = 2.4), increased interleukin-6 (p-ES = 2.4) and increased cardiac troponin I (p-ES = 0.7). On the other hand, in addition to comorbidities, the most important mortality predictors in studies with older patients were albumin (p-ES = -3.1), total bilirubin (p-ES = 0.7), AST (p-ES = 1.8), ALT (p-ES = 0.4), urea nitrogen (p-ES), C-reactive protein (p-ES = 2.7), LDH (p-ES = 2.4) and ferritin (p-ES = 1.7). Obesity was associated with increased mortality only in studies with fewer chronic or critical patients (p-OR = 1.8).
Conclusion
The prognostic effect of clinical conditions on COVID-19 mortality vary substantially according to the mean age of patients.
PROSPERO registration number
CRD42020176595.
Introduction
The rapid global spread of COVID-19 beginning in December 2019 [1] and the countless associated health, social and economic impacts have resulted in an exponential increase in scientific publications from independent groups and global coalitions [2]. In addition to aspects related to the spread of the disease, the profile and symptoms of patients [3, 4] and the drugs being developed to prevent and treat it [5], much interest has been devoted to potential predictors of hospital mortality from COVID-19. The identification of clinical aspects observed at hospital admission among patients with a worse prognosis has been the focus of many meta-analyses. Although the accumulated evidence consistently indicates that worse prognosis is related to old age [6], the male sex [7] and the presence of comorbidities [8–10], it is still unclear whether other potential predictors of mortality, such as smoking, clinical symptoms or laboratory parameters, vary between populations with different sociodemographic and epidemiological profiles.
To the best of our knowledge, no meta-analysis specifically designed to assess the role of age, sex and health condition of the studied populations with a comprehensive list of potential clinical predictors of mortality in hospitalized patients with confirmed COVID-19 has been reported. Thus far, Zhang et al. [11] observed that the association between hypertension and mortality was stronger in studies with patients who are less than 50 years old (OR = 6.4) than in those 50 years old and older (OR = 2.6), although the authors included only six studies in their meta-analysis. In another study, Pranata et al. [12] used meta-regression methods and found an association between hypertension and increased composite worse outcome, including mortality, which is influenced by sex (more strongly when the population is less than 55% male patients), but not age. While the call for epidemiological data to be presented by age and sex groups has not been met [13], alternative proposals are requiered to provide a deeper understanding of how mortality risk factors vary according to the sociodemographic and epidemiological profiles of patients.
Additionally, with the extraordinary speed and quantity of publications on COVID-19 mortality predictors that have emerged in recent months, some meta-analyses have included studies with overlapping data from patients from the same hospitals [10, 14, 15] and preprint studies [8, 16, 17]. Although such methodological aspects are partially justified limitations considering the urgency for evidence, it is important to make efforts to overcome these limitations to yield results with greater precision and reliability.
Therefore, this systematic review and meta-analysis adds to the available evidence by synthesizing the results of primary studies and providing pooled effect size estimators for a comprehensive set of potential predictors of in-hospital mortality in patients with confirmed COVID-19. Concretely, we estimated the potential risk associated with symptoms and pre-existing chronic conditions and comorbidities reported on admission, and with specific laboratory parameters such as routine blood tests, coagulation, liver and kidney function and inflammatory factors. Subgroup analyses and meta-regression explored whether these factors vary according to age, sex and baseline health conditions of the studied populations.
Methods
This systematic review with meta-analyses is reported according to the MOOSE guidelines [18] followed the recommendations of the Cochrane Collaboration Handbook [19] and was registered in PROSPERO (registration number: CRD42020176595). No ethics approval was required given the nature of the study.
Search strategy and study selection
We systematically searched the MEDLINE (via PubMed), Scopus and Web of Science databases from December 2019 to July 27, 2020. The search was specifically focused on peer-reviewed studies that analysed demographic characteristics, clinical status, pre-existing comorbidities, and laboratory parameters on hospital admission as potential risk factors for higher mortality in subjects with confirmed COVID-19. The full search strategy is presented in the (S1 Appendix). The literature search was complemented by reviewing the citations of the articles considered eligible for the systematic review. No language restriction was applied.
The criteria for the inclusion of studies were as follows: (i) participants—100 and more patients with confirmed COVID-19; (ii) design—observational studies (prospective or retrospective) with primary individual data for each mortality outcome group, i.e., nonsurvivors and survivors; (iii) exposure variables—sociodemographic characteristics, clinical symptoms and signs, pre-existing comorbidities and the laboratory parameters routine blood tests, coagulation indicators, liver and kidney function and inflammatory factors; and (iv) outcome—all patients followed up to definitive hospital discharge or COVID-19 mortality.
The criteria for the exclusion of studies were as follows: (i) noneligible publication types, such as case series, family case studies or editorials and letters to the editor; (ii) studies with no deaths registered; and (iii) studies including only patients with specific diseases (cancer, organ transplantation, etc.) or from specific occupations or conditions (health professionals, prisoners, etc.). The literature search and the selection of the studies were independently conducted by two reviewers (AM and IC-R), and disagreements were solved by consensus or through the involvement of a third researcher (CA-B).
Data extraction and risk of bias assessment
The following data were extracted from the original reports: (1) date of publication; (2) clinical setting; (3) timing of subjects’ admission to the hospital; (4) sample characteristics (sample size, percentage of nonsurvivors, percentage of patients followed-up until discharge or death, percentage of men, mean or median age).
Data extraction and quality assessment were independently performed by two reviewers (AM and CA-B), and inconsistencies were solved by consensus or by involving a third researcher (IC-R). When there were different units of measurement for the same parameter, we converted them to enable the meta-analyses.
The Quality In Prognosis Studies (QUIPS) [20] was used to assess the risk of bias and quality of the included studies, as presented in the (S2 Table).
Statistical analyses and data synthesis
The odds ratio (OR) and its respective 95% confidence interval (CI) were calculated for nonsurvivors and survivors among exposed and nonexposed individuals for each potential predictor. Regarding the laboratory indicators, we calculated the following for each parameter: i) the pooled mean estimate for nonsurvivors and survivors, ii) the pooled mean difference (MD) between nonsurvivors and survivors, and iii) the effect size (ES) statistic, using Cohen’s d index [21]. These mean values and mean differences were calculated and presented in the unit of measurement most frequently adopted for each laboratory parameter. Values in different units of measure were converted to the reference unit when necessary.
The DerSimonian and Laird method [22] was used to compute the pooled mean estimates, MD, ES and the OR and its respective 95% CI. Meta-analyses with random-effects models were performed for each potential predictor. The heterogeneity of the results across studies was assessed using the I2 statistic [23]. We selected a random effect model because moderate or high heterogeneity (I2 >50%) was observed in 38 of 41 predictors under analysis. Because some studies conducted in China obtained data from the same hospital and time interval, we only considered the study with later end date when analysing each predictor to avoid overrepresentation of data from the same patients [24]. We provided a list of those studies and the order of preference considered for analysis in the (S2 Appendix).
Subgroups meta-analyses were applied when the percentage of total variation across studies due to heterogeneity was moderate or high (I2 >50%) [23]. The pooled OR (p-OR) and ES (p-ES) of each potential predictor was recalculated in subgroups of studies defined according the proportion of patients who were elderly, men and in poor health condition. For this purpose, we considered two groups of predominant age (older adults: the mean age of patients was >60 years; younger adults: ≤60 years) [6], two groups of predominant sex (≥60% of male patients; <60%) and two groups of predominant health condition (poor: all patients were in critical or severe condition or the most prevalent chronic condition was >50%; regular: studies that did not fit the poor criteria). The subgroup classification of the included studies and the corresponding values considered are available in the (S1 Table).
For the purpose of meta-regression, the continuous mean age (years), as well as the proportion of male sex and of the highest prevalence of chronic condition or critical status were considered as continuous adjustment variables. Thus, three models adjusted the pooled-OR for age, sex and health condition for each predictor and the percentage of residual variation due to heterogeneity was generated [25].
Sensitivity analyses were conducted to assess the robustness of the summary estimates and to determine whether any particular study accounted for a large proportion of the heterogeneity. Additionally, post-hoc sensitivity analyses were performed by removing studies with a moderate risk of bias in more than one domain of the QUIPS tool [20].
Finally, publication bias was evaluated by visual inspection of funnel plots and by using the method proposed by Egger, considering a p-value of <0.10 to be statistically significant [26].
Statistical analyses were performed using the commands metan and metareg of STATA SE software, version 15 (StataCorp, College Station, TX, USA). All analyses were performed by two reviewers (AM and IC-R).
Results
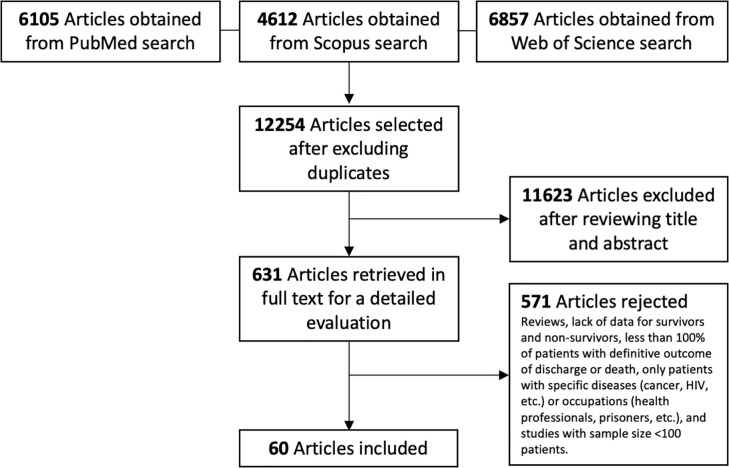
We identified 60 studies [27–86] that met the inclusion criteria (Fig 1). The studies were conducted in hospitals from 13 countries. The timing of hospital admission of subjects ranged from December 24, 2019 and May 17, 2020. The mean age of the populations included ranged between 40 and 73 years, with sample sizes ranging from 100 to 7,371 participants. In total, 51,225 patients were included, 12,458 of whom (24.3%) were nonsurvivors (Table 1).
Fig 1. Flow diagram of the literature search and studies selection.
Table 1. Characteristics of the included studies.
| Authors | Country | Patient’s admission | Patients, n | Deaths, n (%) | Male sex, % | Agea (years) | |
|---|---|---|---|---|---|---|---|
| From | To | ||||||
| Aloisio E et al. [27] | Italy | Feb 21, 2020 | Mar 31, 2020 | 427 | 89 (20.8) | 68.60 | 61.3 ± 6.6 |
| Amit M et al. [28] | Israel | Mar 5, 2020 | Apr 27, 2020 | 156 | 87 (55.8) | 69.00 | 71.5 ± 6.3 |
| Asghar MS et al. [29] | Pakistan | Mar, 2020 | Apr, 2020 | 100 | 22 (22.0) | 69.00 | 52.6 ± 15.7 |
| Baqui P et al. [30] | Brazil (Central-South) | Feb 27, 2020 | May 4, 2020 | 6021 | 2457 (40.8) | 58.00 | 58.2 ± 17.8 |
| Brazil (North) | Feb 27, 2020 | May 4, 2020 | 1350 | 871 (64.5) | 58.90 | 58.8 ± 19.4 | |
| Bonetti G et al. [31] | Italy | Mar 1, 2020 | Mar 30, 2020 | 144 | 70 (48.6) | 66.70 | 69.1 ± 8.9 |
| Borghesi A et al. [32] | Italy | Mar 4, 2020 | Mar 24, 2020 | 302 | 65 (21.5) | 64.20 | 67.0 ± 5.8 |
| Borobia AM et al. [33] | Spain | Feb 25, 2020 | Apr 19, 2020 | 2226 | 460 (20.7) | 48.20 | 61.5 ± 9.2 |
| Brill SE et al. [34] | UK | Mar 10, 2020 | Apr 8, 2020 | 410 | 173 (42.2) | 59.8 | 69.9 ± 7.0 |
| Cao J et al. [35] | China | Jan 3, 2020 | Feb 1, 2020 | 102 | 17 (16.7) | 52.00 | 53.0 ± 8.7 |
| Carter B et al. [36] | UK/Italy | Feb 27, 2020 | Apr 28, 2020 | 1564 | 425 (27.2) | 57.70 | 73.0 ± 6.3 |
| Chen F et al. [37] | China | Jan 1, 2020 | Feb 15, 2020 | 660 | 82 (12.4) | 44.70 | 53.0 ± 9.8 |
| Chen R et al. [38] | China | NA | Mar 22, 2020 | 548 | 103 (18.8) | 57.10 | 56.0 ± 14.5 |
| Chen T et al. [39] | China | Jan 13, 2020 | Feb 12, 2020 | 274 | 113 (41.2) | 62.40 | 59.5 ± 7.5 |
| Cheng A et al. [40] | China | Feb 8, 2020 | Mar 11, 2020 | 305 | 85 (27.9) | 60.00 | 63.3 ± 5.5 |
| Ciceri F et al. [41] | Italy | Feb 25, 2020 | Mar 24, 2020 | 386 | 95 (24.6) | 71.80 | 65.7 ± 7.4 |
| Deng Y et al. [42] | China | Jan 1, 2020 | Feb 21, 2020 | 225 | 109 (48.4) | 55.10 | 55.1 ± 14.1 |
| Du RH et al. [43] | China | Dec 25, 2019 | Feb 7, 2020 | 179 | 21 (11.7) | 54.20 | 57.6 ± 13.7 |
| Gao S et al. [44] | China | Jan 23, 2020 | Feb 29, 2020 | 210 | 35 (16.7) | 48.00 | 71.5 ± 2.9 |
| Garcia PDW et al. [45] | Switzerland, Spain, Italy, France, Germany | NA | Apr 22, 2020 | 639 | 97 (15.2) | 75.10 | 62.5 ± 5.2 |
| Gavin W et al. [46] | USA | Mar 1, 2020 | Mar 31, 2020 | 140 | 18 (12.9) | 51.40 | 60.0 ± 6.9 |
| Gayam V et al. [47] | USA | Mar 1, 2020 | Apr 9, 2020 | 408 | 132 (32.4) | 56.60 | 66.5 ± 5.8 |
| Harmouch F et al. [48] | USA | Mar 1, 2020 | Apr 15, 2020 | 560 | 81 (14.5) | 57.10 | 63.5 ± 21.2 |
| Hu H et al. [49] | China | Feb 7, 2020 | Mar 7, 2020 | 105 | 19 (18.1) | 50.90 | 60.8 ± 16.3 |
| Huang J et al. [50] | China | Jan 25, 2020 | Mar 24, 2020 | 299 | 16 (5.4) | 53.50 | 53.4 ± 16.7 |
| Hwang JM et al. [51] | South Korea | Feb 1, 2020 | Mar 25, 2020 | 103 | 26 (25.2) | 50.00 | 67.6 ± 15.3 |
| Khalil K et al. [52] | UK | Mar 7, 2020 | 7 Apr, 2020 | 220 | 58 (26.4) | 59.10 | 66.9 ± 17.0 |
| Klang E et al. [53] | USA (≤50y) | Mar 1, 2020 | May 17, 2020 | 572 | 60 (10.5) | 69.40 | 40.7 ± 3.9 |
| USA (>50y) | Mar 1, 2020 | May 17, 2020 | 2834 | 1076 (37.9) | 55.20 | 71.1 ± 6.1 | |
| Krishnan S et al. [54] | USA | Mar 10, 2020 | Apr 15, 2020 | 152 | 92 (60.5) | 62.50 | 66.0 ± 13.0 |
| Laguna-Goya R et al. [55] | Spain | Mar 10, 2020 | Apr 12, 2020 | 501 | 36 (7.2) | 63.30 | 52.0 ± 4.6 |
| Li Q et al. [56] | China | Jan 20, 2020 | Apr 4, 2020 | 1449 | 122 (8.4) | 51.00 | 55.5 ± 6.9 |
| Lieberman-Cribbin W et al. [57] | USA | Feb 29, 2020 | Apr 24, 2020 | 6245 | 1128 (18.1) | NA | NA |
| Liu Q et al. [58] | China | Feb 1, 2020 | Mar 13, 2020 | 336 | 34 (10.1) | 50.30 | 62.5 ± 5.2 |
| Long H et al. [59] | China | Jan 18, 2020 | Mar 5, 2020 | 115 | 23 (20.0) | 57.40 | 63.6 ± 13.9 |
| Luo M et al. [60] | China | Jan 9, 2020 | Mar 31, 2020 | 1018 | 201 (19.7) | 51.20 | 60.0 ± 5.8 |
| Luo X et al. [61] | China | Jan 30, 2020 | Feb 20, 2020 | 289 | 84 (29.1) | 50.30 | 55.8 ± 8.4 |
| Luo Y et al. (a) [62] | China | Feb, 2020 | Apr, 2020 | 739 | 51 (6.9) | 49.40 | 60.1 ± 15.2 |
| Luo Y et al. (b) [63] | China | Feb, 2020 | Apr, 2020 | 1115 | 129 (11.6) | 50.50 | 59.9 ± 15.3 |
| Masetti C et al. [64] | Italy | Feb 28, 2020 | Apr 10, 2020 | 229 | 33 (14.4) | 64.60 | 60.7 ± 14.2 |
| Mikami T et al. [65] | USA | Mar 12, 2020 | Apr 17, 2020 | 2820 | 806 (28.6) | 54.50 | 65.5 ± 9.1 |
| Okoh AK et al. [66] | USA | Mar 10, 2020 | Apr 10, 2020 | 251 | 97 (38.6) | 51.00 | 61.8 ± 7.2 |
| Pan F et al. [67] | China | Jan 27, 2020 | Mar 19, 2020 | 124 | 89 (71.8) | 68.50 | 66.9 ± 5.5 |
| Rastad H et al. [68] | Iran | Feb 20, 2020 | Mar 25, 2020 | 2957 | 301 (10.2) | 53.70 | 54.8 ± 16.9 |
| Richardson S et al. [69] | USA (18-65y) | Mar 1, 2020 | Apr 4, 2020 | 1175 | 87 (7.4) | 62.3 | 63.3 ± 6.6 |
| USA (>65y) | Mar 1, 2020 | Apr 4, 2020 | 1425 | 466 (32.7) | 57.1 | ||
| Rivera-Izquierdo M et al. [70] | Spain | Mar 16, 2020 | Apr 10, 2020 | 238 | 61 (25.6) | 55.00 | 64.7 ± 15.4 |
| Ruan Q et al. [71] | China | Dec 31, 2019 | Jan 31, 2020 | 150 | 68 (45.3) | 68.00 | 56.8 ± 15.0 |
| Salacup G et al. [72] | USA | Mar 1, 2020 | Apr 24, 2020 | 242 | 52 (21.5) | 51.00 | 66.5 ± 5.2 |
| Shah P et al. [73] | USA | Mar 2, 2020 | May 6, 2020 | 522 | 92 (17.6) | 41.80 | 62.0 ± 6.3 |
| Shang Y et al. [74] | China | Jan 1, 2020 | Mar 27, 2020 | 113 | 49 (43.4) | 64.60 | 65.6 ± 4.8 |
| Shi S et al. [75] | China | Jan 1, 2020 | Feb 23, 2020 | 671 | 62 (9.2) | 48.00 | 62.0 ± 6.3 |
| Soares RCM et al. [76] | Brazil | Feb 29, 2020 | Jun 11, 2020 | 1152 | 456 (39.6) | 57.10 | NA |
| Sun H et al. [77] | China | Jan 29, 2020 | Mar 5, 2020 | 244 | 121 (49.6) | 54.50 | 69.7 ± 3.7 |
| Wang K et al. [78] | China | Jan 7, 2020 | Feb 11, 2020 | 340 | 33 (9.7) | 48.20 | 48.3 ± 15.4 |
| Xu B et al. [79] | China | Dec 26, 2019 | Mar 1, 2020 | 145 | 28 (19.3) | 52.40 | 60.9 ± 6.5 |
| Yan X et al. [80] | China | Jan 11, 2020 | Mar 13, 2020 | 1004 | 40 (4.0) | 49.10 | 61.3 ± 6.0 |
| Yang Q et al. [81] | China | Jan 1, 2020 | Feb 29, 2020 | 226 | 50 (22.1) | 50.00 | 53.9 ± 17.1 |
| Yang X et al. [82] | China | Dec, 2019 | Feb 25, 2020 | 1476 | 238 (16.1) | 52.60 | 57.6 ± 6.8 |
| Ye W et al. [83] | China | Jan 1, 2020 | Mar 16, 2020 | 349 | 52 (14.9) | 49.60 | 53.5 ± 13.8 |
| Yu C et al. [84] | China | Jan 14, 2020 | Feb 28, 2020 | 1464 | 212 (14.5) | 50.30 | 62.5 ± 5.8 |
| Zhang JJ et al. [85] | China | Dec 29, 2019 | Feb 16, 2020 | 289 | 49 (17.0) | 53.30 | 56.0 ± 19.1 |
| Zhou F et al. [86] | China | Dec 29, 2019 | Jan 31, 2020 | 191 | 54 (28.3) | 62.00 | 56.3 ± 6.1 |
NA: not available.
a When the mean or median were available for nonsurvivors and survivors, the weighted mean age for the total sample was calculated. When only the median and interquartile range for the total sample were available, the mean age was calculated according to the method proposed by Hozo SP, Djulbegovic B and Hozo I (BMC Med Res Methodol. 2005, 5:13).
Subjects with dyspnoea (p-OR = 2.5) and current smokers (p-OR = 1.6) were more likely to die than those without these conditions. All comorbidities were significantly associated with nonsurvival (p-ORs from 1.8 to 4.7). Headache (p-OR = 0.5), diarrhoea (p-OR = 0.6), vomiting (p-OR = 0.6), cough (p-OR = 0.7) and fever (p-OR = 0.8) were associated with a significantly lower risk of death. Moderate or high heterogeneity was detected in the analysis of almost all clinical symptoms and comorbidities (Table 2). All forest plots are available in the (S3 Appendix).
Table 2. Meta-analyses of sociodemographic and clinical potential predictors of in-hospital mortality.
| Potential predictor | Studies, n | Total patients, n | Non-survivors, n (% exposed) | Survivors, n (% exposed) | Pooled odds ratio (95% CI) | p-value | I2 |
|---|---|---|---|---|---|---|---|
| Symptoms and signs (yes/no) | |||||||
| Dyspnoea | 10 | 7577 | 1545 (63.5) | 6032 (40.4) | 2.54 (1.84, 3.50)* | <0.001 | 80.8 |
| Fatigue | 9 | 5884 | 954 (35.1) | 4930 (22.1) | 1.30 (0.59, 2.87) | 0.52 | 94.4 |
| Fever | 17 | 10665 | 2069 (63.3) | 8596 (63.5) | 0.78 (0.64, 0.95)* | 0.013 | 53.2 |
| Myalgia | 9 | 6608 | 1114 (19.2) | 5494 (22.3) | 0.77 (0.36, 1.64) | 0.50 | 92.8 |
| Cough | 15 | 11911 | 2121 (62.6) | 9790 (67.3) | 0.74 (0.61, 0.91)* | 0.003 | 63.7 |
| Vomiting | 6 | 5361 | 879 (7.7) | 4482 (10.3) | 0.60 (0.40, 0.89)* | 0.011 | 32.0 |
| Diarrhoea | 12 | 9491 | 1846 (10.0) | 7645 (15.8) | 0.61 (0.45, 0.82)* | 0.001 | 54.3 |
| Headache | 9 | 7384 | 1417 (8.6) | 5967 (14.2) | 0.52 (0.30, 0.90)* | 0.020 | 74.9 |
| Chronic condition (yes/no) | |||||||
| Obesity | 17 | 20289 | 6885 (14.2) | 13404 (17.1) | 1.09 (0.84, 1.41) | 0.53 | 82.9 |
| Smoking habit | 23 | 18844 | 4436 (19.3) | 14408 (16.2) | 1.55 (1.24, 1.96)* | <0.001 | 73.1 |
| Unspecified comorbidity | 13 | 7644 | 1412 (77.7) | 6232 (43.6) | 4.70 (3.19, 6.91)* | <0.001 | 77.3 |
| Stroke | 5 | 1310 | 228 (14.9) | 1082 (4.3) | 4.15 (1.80, 9.59)* | 0.001 | 45.5 |
| Kidney disease | 30 | 25413 | 7746 (14.8) | 17667 (6.7) | 3.20 (2.52, 4.06)* | <0.001 | 75.8 |
| Cardiovascular disease | 32 | 27052 | 7642 (30.8) | 19410 (14.0) | 2.98 (2.51, 3.53)* | <0.001 | 75.9 |
| Hypertension | 37 | 21388 | 4963 (61.4) | 16425 (39.8) | 2.61 (2.19, 3.17)* | <0.001 | 79.0 |
| Malignancy | 22 | 12687 | 2635 (15.8) | 10052 (6.9) | 2.36 (1.77, 3.15)* | <0.001 | 54.3 |
| Diabetes | 38 | 25498 | 5673 (33.8) | 19825 (20.4) | 2.12 (1.79, 2.52)* | <0.001 | 77.9 |
| Pulmonary disease | 31 | 24748 | 7848 (13.3) | 16900 (7.5) | 2.00 (1.39, 2.88)* | <0.001 | 88.0 |
| Liver disease | 13 | 13617 | 4432 (2.5) | 9185 (2.7) | 1.80 (1.35, 2.39)* | <0.001 | 6.2 |
* p-value <0.05.
Both survivors and nonsurvivors COVID-19 patients presented abnormal values for haemoglobin, D-dimer, albumin and all inflammatory factors. On the other hand, lymphocytes and neutrophil count, urea nitrogen and aspartate transaminase (AST) were out of the reference range only in nonsurvivors. Normal values were observed for both groups for leucocyte and platelet count, prothrombin time, activated partial thromboplastin time (APTT), total bilirubin, alanine transaminase (ALT) and creatinine (Table 3). The pooled mean showed worse values for all laboratory parameters in nonsurvivors, except for APTT, for which no significant difference was found between survival groups. Finally, the highest pooled effect sizes were observed for albumin (p-ES = -2.6), C-reactive protein (CRP) (p-ES = 2.5), urea nitrogen (p-ES = 2.4), interleukin-6 (p-ES = 2.3), neutrophil count (p-ES = 2.2), lactate dehydrogenase (LDH) (p-ES = 2.2), and lymphocyte count (p-ES = -2.0). The heterogeneity between studies for all laboratory parameters was high (I2 >90.0%) (Table 3).
Table 3. Meta-analyses of body mass index and laboratorial potential predictors of in-hospital mortality.
| Potential predictor | Reference range | Studies, n | Non-survivors | Survivors | Effect estimates | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | Pooled Mean | Patients, n | Pooled Mean (95% CI) | Pooled Mean difference (95%CI) | Effect size (95% CI) | p-value | I2 | |||
| (95% C I) | ||||||||||
| BMI (kg/m2) | 18.5–24.9 | 6 | 1237 | 27.54 | 2887 | 27.88 | -0.13 | -0.01 | 0.925 | 89.1 |
| (26.91, 28.18) | (26.73, 29.03) | (-0.70, 0.44) | (-0.29, 0.27) | |||||||
| Routine blood tests | ||||||||||
| Lymphocyte count(x109/L) | 1.0–4.0 | 26 | 3121 | 0.69 | 12876 | 1.06 | -0.36 | -1.97 | <0.001 | 99.3 |
| (0.57, 0.82) | (0.91, 1.21) | (-0.52, -0.20) | (-2.55, -1.38)* | |||||||
| Leucocyte count (x109/L) | 3.6–11.0 | 26 | 2515 | 8.56 | 10959 | 5.92 | 2.75 | 1.79 | <0.001 | 98.6 |
| (8.09, 9.03) | (5.06, 6.23) | (2.02, 3.49) | (1.32, 2.25)* | |||||||
| Neutrophil count (x109/L) | 1.8–6.3 | 19 | 2195 | 7.18 | 9313 | 4.53 | 2.69 | 2.20 | <0.001 | 99.0 |
| (6.77, 7.59) | (3.75, 5.30) | (1.89, 3.48) | (1.57, 2.83)* | |||||||
| Platelet count (x109/L) | 140.0–400.0 | 18 | 1927 | 174.81 | 6917 | 212.04 | -36.06 | -1.37 | <0.001 | 97.7 |
| (162.65, 186.96) | (200.81, 223.28) | (-49.34, -22.77) | (-1.78, -0.95)* | |||||||
| Haemoglobin (g/L) | Men 140–170 | 16 | 1096 | 127.56 | 8798 | 130.84 | -3.47 | -0.41 | 0.018 | 97.2 |
| Women 120–150 | (125.83, 129.28) | (127.91, 133.76) | (-5.84, -1.09) | (-0.75, -0.07)* | ||||||
| Coagulation | ||||||||||
| D-dimer (mg/L) | 0–0.5 | 27 | 2460 | 4.17 | 8804 | 0.91 | 3.22 | 1.76 | <0.001 | 97.6 |
| (3.65, 4.70) | (0.73, 1.09) | (2.84, 3.61) | (1.39, 2.13)* | |||||||
| Prothrombin time (s) | 10.0–14.0 | 11 | 626 | 12.66 | 2345 | 11.67 | 0.93 | 1.35 | <0.001 | 97.2 |
| (7.92, 17.40) | (7.54, 15.80) | (0.45, 1.40) | (0.73, 1.97)* | |||||||
| APTT (s) | 30–40 | 10 | 671 | 34.17 | 2020 | 33.50 | 0.79 | 0.28 | 0.112 | 91.9 |
| (31.38, 36.97) | (30.58, 36.42) | (-0.13, 1.71) | (-0.07, 0.63) | |||||||
| Liver function/aggression | ||||||||||
| Albumin (g/L) | 3.5–5.5 | 17 | 1145 | 29.50 | 7222 | 34.20 | -4.70 | -2.63 | <0.001 | 99.0 |
| (26.24, 32.76) | (31.15, 37.26) | (-6.14, -3.26) | (-3.45, -1.80)* | |||||||
| Total bilirubin (mmol/L) | 3.0–22.0 | 10 | 618 | 10.72 | 1908 | 8.97 | 1.86 | 0.79 | <0.001 | 94.5 |
| (6.29, 15.15) | (6.64, 11.29) | (0.93, 2.78) | (0.34, 1.24)* | |||||||
| AST (U/L) | 14.0–36.0 | 21 | 2098 | 49.53 | 9518 | 34.43 | 17.41 | 1.61 | <0.001 | 97.5 |
| (45.36, 53.69) | (31.50, 37.36) | (13.99, 20.83) | (1.22, 2.00)* | |||||||
| ALT (U/L) | 9.0–52.0 | 25 | 2373 | 32.45 | 10542 | 30.16 | 2.18 | 0.44 | <0.001 | 96.3 |
| (30.18, 34.72) | (27.97, 32.34) | (0.09, 4.28) | (0.39, 0.49)* | |||||||
| Kidney function | ||||||||||
| Urea nitrogen (mmol/L) | 2.5–6.1 | 14 | 798 | 12.06 | 3737 | 5.80 | 6.64 | 2.43 | <0.001 | 96.7 |
| (10.33, 13.79) | (5.31, 6.29) | (5.19, 8.09) | (1.90, 2.97)* | |||||||
| Creatinine (μmmol/L) | 46.0–92.0 | 21 | 1442 | 90.09 | 8173 | 68.33 | 21.72 | 1.28 | <0.001 | 96.8 |
| (66.44, 113.74) | (48.74, 87.92) | (16.72, 26.71) | (0.97, 1.59)* | |||||||
| Inflammatory factors | ||||||||||
| CRP (mg/L) | 0.5–10.0 | 30 | 3322 | 133.18 | 13338 | 64.39 | 68.31 | 2.49 | <0.001 | 98.9 |
| (112.77, 153.59) | (54.96, 73.82) | (53.11, 83.50) | (2.01, 2.96)* | |||||||
| Interleukin-6 (pg/mL) | 0–7 | 12 | 1734 | 73.87 | 5735 | 29.88 | 43.64 | 2.31 | <0.001 | 99.1 |
| (57.99, 89.76) | (25.08, 34.68) | (30.92, 56.35) | (1.47, 3.15)* | |||||||
| LDH (U/L) | 140.0–280.0 | 19 | 2230 | 506.89 | 9667 | 328.02 | 180.26 | 2.21 | <0.001 | 98.5 |
| (336.59, 677.18) | (216.07, 439.97) | (131.02, 229.51) | (1.70, 2.72)* | |||||||
| Procalcitonin (ng/mL) | 0–0.15 | 18 | 2045 | 0.63 | 6367 | 0.16 | 0.52 | 1.58 | <0.001 | 97.9 |
| (0.52, 0.74) | (0.13, 0.19) | (0.42, 0.62) | (1.14, 2.02)* | |||||||
| Ferritin (ng/L) | Men 24–336 | 13 | 1663 | 1396.19 | 4404 | 797.98 | 603.94 | 1.56 | <0.001 | 98.2 |
| Women 11–307 | (1229.84, 1562.55) | (685.52, 910.44) | (383.27, 824.60) | (1.00, 2.12)* | ||||||
| Cardiac troponin-I (ng/mL) | 0–0.04 | 15 | 1161 | 0.05 | 3852 | 0.07 | 0.02 | 0.91 | 0.022 | 98.9 |
| (0.05, 0.06) | (0.04, 0.10) | (0.02, 0.02) | (0.13, 1.70)* | |||||||
| ESR (mm/h) | 0–15 | 6 | 358 | 57.15 | 1369 | 48.12 | 9.57 | 0.34 | <0.001 | 91.5 |
| (32.28, 82.02) | (35.05, 61.19) | (1.75, 17.39) | (0.22, 0.47)* | |||||||
* p-value <0.05.
ALT: alanine transaminase; APTT: activated partial thromboplastin time, AST: aspartate transaminase; BMI: body mass index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate, LDH: lactate dehydrogenase.
Subgroup analysis are presented in Table 4. The following predictors were associated with worse prognosis in all age, sex and health status subgroups: dyspnoea, unspecified comorbidity, cardiovascular disease, hypertension, diabetes, kidney disease, pulmonary disease and cancer, lymphocytes, leukocytes, neutrophils and platelets count, D-dimer, prothrombin time, AST, urea nitrogen, creatinine, C-reactive protein, Interleukine-6, LDH and procalcitonin. The clinical symptoms fatigue, fever and myalgia, and the laboratory indicators APTT and erythrocyte sedimentation rate (ESR) were not associated with mortality in any of the subgroups. Obesity was only associated with increased mortality in studies with fewer chronic or critical patients (p-OR = 1.8). When body mass index (BMI) was examined as a continuous variable, risk of death decreased as BMI increased (p-ES = -0.2) in studies with a mean age of the patients of >60 years (the corresponding p-ES was not calculated for younger patients as only one study was available in this group) (Table 4).
Table 4. Subgroup meta-analysesa of sociodemographic and clinical potential predictors of in-hospital mortality according to the predominant age, sex and health condition of the patients.
| Potential predictor | Predominant age | Predominant sex | Predominant health conditionb | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | >60 years | n | ≤60 years | n | ≥60% Men | n | <60% Men | n | Poor | n | Regular | |||||||
| Symptoms and signs | ||||||||||||||||||
| Dyspnoea | 4 | 1.59 | 0.0 | 5 | 4.29 | 72.8 | 0 | - | 10 | 2.54 | 80.8 | 2 | 1.72 | 0.0 | 8 | 2.90 | 80.9 | |
| (1.34, 1.89)* | (2.51, 7.34)* | (1.84, 3.50)* | (1.41, 2.10)* | (1.94, 4.35)* | ||||||||||||||
| Fatigue | 6 | 1.42 | 93.9 | 3 | 1.08 | 90.5 | 3 | 1.48 | 0.0 | 7 | 1.25 | 96.5 | 5 | 1.03 | 38.0 | 4 | 1.75 | 97.5 |
| (0.57, 3.53) | (0.30, 3.87) | (0.96, 2.29) | (0.47, 3.29) | (0.71, 1.49) | (0.33, 9.12) | |||||||||||||
| Fever | 9 | 0.76 | 42.1 | 7 | 0.90 | 31.5 | 4 | 0.64 | 0.0 | 14 | 0.80 | 63.0 | 8 | 0.70 | 56.9 | 9 | 0.82 | 54.4 |
| (0.57, 1.00) | (0.69, 1.19) | (0.39, 1.03) | (0.64, 1.00) | (0.49, 1.00) | (0.63, 1.07) | |||||||||||||
| Myalgia | 5 | 0.50 | 95.8 | 4 | 1.09 | 25.7 | 1 | - | - | 8 | 0.87 | 93.5 | 4 | 0.49 | 96.9 | 5 | 0.98 | 41.8 |
| (0.13, 1.87) | (0.75, 1.60) | (0.40, 1.87) | (0.10, 2.44) | (0.65, 1.47) | ||||||||||||||
| Cough | 7 | 0.80 | 59.8 | 7 | 0.77 | 68.4 | 1 | - | - | 14 | 0.74 | 65.9 | 4 | 0.85 | 61.4 | 11 | 0.72 | 76.1 |
| (0.59, 1.10) | (0.55, 1.07) | (0.60, 0.91)* | (0.52, 1.41) | (0.57, 0.91)* | ||||||||||||||
| Headache | 4 | 0.26 | 34.1 | 4 | 0.99 | 57.8 | 0 | - | - | 9 | 0.52 | 74.9 | 2 | 0.19 | 0.0 | 7 | 0.73 | 58.8 |
| (0.14, 0.49)* | (0.42, 2.37) | (0.30, 0.90)* | (0.13, 0.28)* | (0.41, 1.33) | ||||||||||||||
| Chronic condition | ||||||||||||||||||
| Obesity | 11 | 0.90 | 79.7 | 5 | 1.62 | 78.5 | 6 | 1.59 | 91.0 | 11 | 1.03 | 73.7 | 10 | 0.77 | 78.9 | 7 | 1.76 | 79.3 |
| (0.67, 1.20) | (0.92, 2.83) | (0.60, 4.25) | (0.82, 1.29) | (0.56, 1.06) | (1.19, 2.61)* | |||||||||||||
| BMI | 5 | -0.23 | 52.6 | 1 | - | - | 2 | -0.05 | 0.0 | 4 | 0.05 | 92.6 | 3 | -0.08 | 0.0 | 3 | 0.15 | 94.9 |
| (-0.36, -0.10)* | (-0.24, 0.14) | (-0.36, 0.46) | (-0.24, 0.08) | (-0.39, 0.69) | ||||||||||||||
| Smoking | 13 | 1.21 | 30.0 | 9 | 2.78 | 76.3 | 5 | 2.62 | 89.7 | 18 | 1.37 | 50.2 | 12 | 1.14 | 24.4 | 11 | 2.34 | 80.9 |
| (1.04, 1.41)* | (1.43, 5.39)* | (0.81, 8.47) | (1.14, 1.64)* | (0.95, 1.37) | (1.52, 3.59)* | |||||||||||||
| Unspecified comorbidity | 8 | 5.08 | 80.6 | 5 | 3.90 | 58.8 | 7 | 3.38 | 45.9 | 7 | 5.98 | 87.9 | 9 | 5.04 | 77.7 | 4 | 3.61 | 52.6 |
| (2.83, 9.13)* | (2.47, 6.17)* | (2.32, 4.91)* | (3.17, 11.30)* | (3.06, 8.30)* | (2.11, 6.17)* | |||||||||||||
| Kidney disease | 21 | 3.06 | 76.0 | 8 | 3.80 | 67.2 | 7 | 3.17 | 43.7 | 23 | 3.21 | 79.8 | 18 | 3.07 | 77.1 | 12 | 3.39 | 75.5 |
| (2.30, 4.08)* | (2.33, 6.19)* | (1.76, 5.71)* | (2.46, 4.18)* | (2.18, 4.32)* | (2.34, 4.92)* | |||||||||||||
| Cardiovascular disease | 21 | 3.02 | 78.6 | 10 | 3.02 | 69.7 | 9 | 2.45 | 38.6 | 23 | 3.20 | 80.9 | 16 | 2.90 | 83.0 | 16 | 2.89 | 62.1 |
| (2.34, 3.91)* | (2.27, 4.03)* | (1.79, 3.37)* | (2.61, 3.91)* | (2.12, 3.97)* | (2.37, 3.52)* | |||||||||||||
| Hypertension | 27 | 2.31 | 78.2 | 10 | 3.73 | 68.1 | 12 | 2.51 | 38.2 | 25 | 2.65 | 84.2 | 23 | 2.46 | 81.6 | 14 | 2.85 | 74.8 |
| (1.91, 2.80)* | (2.60, 5.35)* | (2.00, 3.16)* | (2.11, 3.32)* | (1.93, 3.14)* | (1.93, 3.14)* | |||||||||||||
| Malignancy | 15 | 2.10 | 55.2 | 7 | 3.69 | 21.1 | 7 | 2.41 | 0.0 | 15 | 2.47 | 65.4 | 14 | 2.09 | 58.1 | 8 | 3.27 | 16.5 |
| (1.56, 2.83)* | (1.94, 7.00)* | (1.75, 3.33)* | (1.64, 3.72)* | (1.52, 2.88)* | (1.93, 5.53)* | |||||||||||||
| Diabetes | 26 | 1.85 | 72.5 | 11 | 3.17 | 81.9 | 10 | 1.85 | 0.0 | 28 | 2.21 | 83.1 | 23 | 1.96 | 75.1 | 15 | 2.44 | 82.2 |
| (1.55, 2.20)* | (1.95, 5.15)* | (1.49, 2.28)* | (1.80, 2.73)* | (1.58, 2.43)* | (1.79, 3.33)* | |||||||||||||
| Pulmonary disease | 20 | 1.83 | 41.8 | 9 | 3.10 | 58.8 | 10 | 1.88 | 0.0 | 20 | 2.17 | 64.9 | 15 | 1.94 | 42.7 | 16 | 2.11 | 93.1 |
| (1.44, 2.32)* | (1.95, 4.93)* | (1.42, 2.50)* | (1.66, 2.84)* | (1.45, 2.59)* | (1.15, 3.88)* | |||||||||||||
| Routine blood tests | ||||||||||||||||||
| Lymphocyte count | 16 | -2.00 | 98.7 | 11 | -1.86 | 99.5 | 9 | -1.63 | 96.1 | 18 | -2.13 | 99.5 | 12 | -1.96 | 98.8 | 15 | -1.97 | 99.5 |
| (-2.53, -1.46)* | (-3.13, -0.59)* | (-2.15, -1.12)* | (-2.95, -1.31)* | (-2.83, -1.10)* | (-2.77, -1.16)* | |||||||||||||
| Leucocyte count | 11 | 1.57 | 98.6 | 15 | 1.94 | 98.7 | 18 | 2.11 | 94.2 | 9 | 1.19 | 98.8 | 14 | 1.74 | 97.6 | 12 | 1.84 | 99.0 |
| (0.79, 2.36)* | (1.32, 2.57)* | (1.51, 2.70)* | (0.72, 1.65)* | (1.16, 2.32)* | (1.11, 2.57)* | |||||||||||||
| Neutrophil count | 12 | 2.08 | 98.9 | 8 | 2.37 | 99.0 | 5 | 1.88 | 96.3 | 15 | 2.30 | 99.2 | 9 | 2.09 | 97.4 | 11 | 2.29 | 99.3 |
| (1.37, 2.80)* | (1.12, 3.62)* | (1.10, 2.66)* | (1.52, 3.09)* | (1.38, 2.79)* | (1.35, 3.22)* | |||||||||||||
| Platelet count | 11 | -1.16 | 96.4 | 7 | -1.69 | 98.1 | 8 | -0.78 | 88.5 | 10 | -1.84 | 98.6 | 10 | -1.11 | 96.5 | 8 | -1.68 | 98.5 |
| (-1.56, -0.75)* | (-2.58, -0.80)* | (-1.13, -0.43)* | (-2.48, -1.19)* | (-1.66, -0.57)* | (-2.39, -0.97)* | |||||||||||||
| Haemoglobin | 10 | -0.29 | 94.3 | 6 | -0.60 | 98.0 | 6 | -0.44 | 96.2 | 10 | -0.39 | 97.8 | 9 | -0.30 | 95.9 | 7 | -0.55 | 97.3 |
| (-0.82, 0.24) | (-1.02, -0.19)* | (-1.14, 0.27) | (-0.80, 0.03) | (-0.79, 0.19) | (-0.98, -0.12)* | |||||||||||||
| Coagulation | ||||||||||||||||||
| D-dimer | 18 | 1.78 | 97.8 | 10 | 1.72 | 96.8 | 10 | 1.52 | 91.4 | 18 | 1.90 | 98.2 | 14 | 2.04 | 96.0 | 14 | 1.48 | 98.4 |
| (1.32, 2.24)* | (1.09, 2.35)* | (1.15, 1.89)* | (1.38, 2.42)* | (1.57, 2.51)* | (0.90, 2.06)* | |||||||||||||
| Prothrombin time | 8 | 1.37 | 97.4 | 3 | 1.30 | 97.5 | 4 | 1.09 | 97.7 | 7 | 1.50 | 97.0 | 7 | 1.30 | 97.7 | 4 | 1.44 | 96.4 |
| (0.56, 2.18)* | (0.18, 2.43)* | (0.02, 2.16)* | (0.70, 2.29) * | (0.40, 2.19)* | (0.55, 2.33)* | |||||||||||||
| APTT | 5 | 0.44 | 95.7 | 5 | 0.11 | 69.5 | 3 | 0.22 | 95.4 | 7 | 0.30 | 91.1 | 5 | 0.44 | 95.7 | 5 | 0.11 | 69.5 |
| (-0.31, 1.18) | (-0.13, 0.35) | (-0.66, 1.11) | (-0.09, 0.70) | (-0.31, 1.18) | (-0.13, 0.35) | |||||||||||||
| Liver function/aggression | ||||||||||||||||||
| Albumin | 11 | -3.05 | 98.9 | 6 | -1.85 | 99.2 | 7 | -2.16 | 98.1 | 10 | -2.96 | 99.1 | 8 | -2.73 | 98.8 | 9 | -2.53 | 99.0 |
| (-4.11, -1.99)* | (-3.40, -0.30)* | (-3.14, -1.18)* | (-4.12, 1.79) | (-3.93, -1.53)* | (-3.67, -1.40)* | |||||||||||||
| Total bilirubin | 6 | 0.66 | 80.8 | 4 | 0.98 | 6 | 0.85 | 96.2 | 4 | 0.69 | 88.4 | 5 | 0.59 | 79.4 | 5 | 0.98 | 97.0 | |
| (0.35, 0.97)* | (-0.18, 2.14) | (0.14, 1.57)* | (0.20, 1.18)* | (0.26, 0.93)* | (0.11, 1.84)* | |||||||||||||
| AST | 14 | 1.79 | 97.8 | 7 | 1.25 | 97.2 | 9 | 0.96 | 92.1 | 12 | 2.09 | 97.1 | 11 | 1.64 | 97.7 | 10 | 1.59 | 96.4 |
| (1.28, 2.29)* | (0.50, 2.00)* | (0.55, 1.38)* | (1.65, 2.53)* | (0.99, 2.30)* | (1.15, 2.02)* | |||||||||||||
| ALT | 15 | 0.42 | 93.1 | 10 | -0.01 | 97.9 | 10 | -0.03 | 97.8 | 15 | 0.45 | 12 | 0.40 | 92.3 | 13 | 0.12 | 97.6 | |
| (0.16, 0.68)* | (-0.66, 0.65) | (-0.73, 0.67) | (0.22, 0.67)* | (0.07, 0.73)* | (-0.30, 0.54) | |||||||||||||
| Kidney function | ||||||||||||||||||
| Urea nitrogen | 9 | 2.66 | 97.4 | 5 | 2.03 | 94.8 | 5 | 2.31 | 93.5 | 9 | 2.51 | 97.6 | 7 | 2.63 | 97.8 | 7 | 2.25 | 94.7 |
| (1.92, 3.40)* | (1.25, 2.82)* | (1.62, 3.00)* | (1.73, 3.28)* | (1.71, 3.54)* | (1.63, 2.88)* | |||||||||||||
| Creatinine | 14 | 1.30 | 94.4 | 8 | 2.42 | 98.1 | 8 | 1.21 | 93.3 | 14 | 1.86 | 97.5 | 12 | 1.76 | 97.3 | 10 | 1.46 | 94.8 |
| (0.95, 1.66)* | (1.56, 3.29)* | (0.75, 1.66)* | (1.34, 2.39)* | (1.19, 2.34)* | (1.02, 1.89)* | |||||||||||||
| Inflammatory factors | ||||||||||||||||||
| CRP | 20 | 2.69 | 99.0 | 11 | 2.11 | 98.6 | 11 | 2.25 | 98.9 | 19 | 2.67 | 98.9 | 16 | 2.26 | 98.8 | 15 | 2.73 | 98.7 |
| (2.07, 3.32)* | (1.30, 2.93)* | (1.20, 3.29)* | (2.10, 3.24)* | (1.56, 2.96)* | (2.11, 3.35)* | |||||||||||||
| Interleukin-6 | 6 | 2.23 | 99.4 | 6 | 2.39 | 98.8 | 5 | 2.10 | 92.2 | 7 | 2.45 | 99.4 | 5 | 1.35 | 96.1 | 7 | 3.01 | 98.9 |
| (0.92, 3.54)* | (1.19, 3.59)* | (1.59, 2.61)* | (1.23, 3.67)* | (0.69, 2.00)* | (2.06, 3.97)* | |||||||||||||
| LDH | 13 | 2.43 | 98.7 | 7 | 1.81 | 98.1 | 9 | 2.47 | 98.3 | 11 | 2.00 | 98.3 | 9 | 2.29 | 97.4 | 11 | 2.15 | 98.9 |
| (1.74, 3.12)* | (0.93, 2.69)* | (1.54, 3.40)* | (1.34, 2.66)* | (1.51, 3.06)* | (1.45, 2.85)* | |||||||||||||
| Procalcitonin | 11 | 1.64 | 97.0 | 7 | 1.50 | 98.2 | 6 | 1.63 | 95.8 | 12 | 1.55 | 98.4 | 10 | 2.22 | 96.8 | 8 | 0.78 | 98.7 |
| (1.20, 2.07)* | (0.53, 2.46)* | (0.95, 2.31)* | (0.98, 2.13)* | (1.66, 2.79)* | (0.04, 1.52)* | |||||||||||||
| Ferritin | 9 | 1.74 | 98.0 | 4 | 1.11 | 98.8 | 7 | 1.48 | 97.0 | 6 | 1.66 | 98.9 | 6 | 0.44 | 96.7 | 7 | 2.54 | 98.3 |
| (1.16, 2.32)* | (-0.80, 3.02) | (0.75, 2.21)* | (0.69, 2.62)* | (-0.23, 1.11) | (1.69, 3.40)* | |||||||||||||
| Cardiac troponin I | 10 | 1.01 | 99.2 | 5 | 0.72 | 93.7 | 7 | 1.71 | 97.6 | 8 | 0.22 | 99.1 | 6 | 1.52 | 98.9 | 9 | 0.51 | 99.0 |
| (-0.11, 2.13) | (0.08, 1.36)* | (0.82, 2.59)* | (-0.92, 1.37) | (0.32, 2.73)* | (-0.61, 1.62) | |||||||||||||
| ESR | 3 | 0.14 | 90.6 | 3 | 0.80 | 94.1 | 1 | - | - | 5 | 0.17 | 82.6 | 2 | 0.12 | 95.0 | 4 | 0.62 | 92.3 |
| (-0.36, 0.65) | (-0.32, 1.91) | (-0.17, 0.51) | (-0.80, 1.05) | (-0.09, 1.33) | ||||||||||||||
ALT: alanine transaminase; APTT: activated partial thromboplastin time, AST: aspartate transaminase; BMI: body mass index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate, LDH: lactate dehydrogenase.
* p-value <0.05, n: number of studies.
a Values indicate the pooled risk estimators for each predictor (odds ratio for categorical and effect size for BMI and biochemical variables).
b The “poor” group comprises studies in which all patients were critical or severe or the prevalence of any of the comorbidities was >50%.
The following predictors were more markedly associated with worse prognosis in studies with younger patients (mean age ≤60 years), a lower proportion of men (<60%) and better health (<50% with chronic/critical/severe condition): smoking, dyspnoea, hypertension, malignancy, diabetes, kidney and pulmonary disease, increased neutrophil and decreased platelet count, and increased interleukin-6. Decreased haemoglobin only predicted higher mortality in younger patients (p-ES = -0.6) and in those with better health (p-ES = -0.6). Increased creatinine levels showed worse prognosis value in younger patients (p-ES = 2.4) than in older patients (p-ES = 1.3), while the effect size for abnormal nitrogen urea was moderately higher in older patients (p-ES = 2.7) than in younger patients (p-ES = 2.0) (Table 4).
All indicators of liver function or aggression were more strongly associated with mortality in studies with older patients, as albumin (p-ES = -3.1 for older patients and -1.9 for younger patients) and aspartate transaminase (p-ES = 1.8 for older patients and 1.3 for younger patients). In the case of total bilirubin and alanine transaminase, small-sized effects were found only in studies with older patients (p-ES = 0.7 and 0.4, respectively) (Table 4).
Abnormal values of C-reactive protein, LDH, procalcitonin and ferritin were the inflammatory indicators with greater effect size in studies with older patients (p-ES = 2.7, 2.4, 1.6 and 1.7, respectively) than in those with younger patients (p-ES = 2.1, 1.8, 1.5 and 1.1, respectively). On the other hand, abnormal values of cardiac troponin I predicted worse prognosis only in studies with younger patients (p-ES = 0.7), a higher proportion of men (p-ES = 1.7) and worse health conditions (p-ES = 1.5) (Table 4).
Meta-regression showed that the mean age of patients is an important source of heterogeneity when examining the potential predictive effect of dyspnoea, BMI, smoking, hypertension, malignancy and pulmonary disease (Table 5).
Table 5. Meta-regression models adjusted for characteristics of the included studies according to the age, sex and health condition of the patients.
| Potential predictor | Original I2 | Age-adjusted modela | Sex-adjusted modelb | Health-adjusted modelc |
|---|---|---|---|---|
| I2 residual | I2 residual | I2 residual | ||
| Dyspnoea | 80.8 | 68.0* | 83.0 | 78.4 |
| Fatigue | 94.4 | 94.8 | 94.4 | 77.7 |
| Fever | 53.2 | 33.5 | 41.7 | 55.9 |
| Myalgia | 92.8 | 93.6 | 91.4 | 93.3 |
| Cough | 63.7 | 64.9 | 65.7 | 50.4 |
| Headache | 74.9 | 73.3 | 73.9 | 0.0** |
| Obesity | 82.9 | 79.9 | 84.0 | 80.3 |
| BMI | 89.1 | 75.0** | 90.8 | 89.5 |
| Smoking habit | 73.1 | 62.0* | 74.9 | 72.7 |
| Unspecified comorbidity | 77.3 | 76.9 | 74.6 | 67.8 |
| Kidney disease | 75.8 | 69.6 | 76.5 | 76.5 |
| Cardiovascular disease | 75.9 | 74.6 | 72.8 | 76.5 |
| Hypertension | 79.0 | 69.0** | 77.9 | 79.2 |
| Malignancy | 54.3 | 8.7* | 56.2 | 52.7 |
| Diabetes | 77.9 | 71.8 | 76.6 | 78.4 |
| Pulmonary disease | 88.0 | 40.9* | 54.7 | 86.3 |
| Lymphocyte count | 99.3 | 99.2 | 99.3 | 99.4 |
| Leucocyte count | 98.6 | 98.6 | 98.4 | 98.6 |
| Neutrophil count | 99.0 | 98.9 | 98.9 | 98.9 |
| Platelet count | 97.7 | 96.9 | 96.3 | 98.2 |
| Haemoglobin | 97.2 | 97.4 | 97.4 | 96.2 |
| D-dimer | 97.6 | 97.5 | 97.6 | 97.8 |
| Prothrombin time | 97.2 | 97.4 | 96.9 | 97.7 |
| APTT | 91.9 | 91.5 | 92.8 | 94.6 |
| Albumin | 99.0 | 98.9 | 98.7 | 98.9 |
| Total bilirubin | 94.5 | 95.1 | 95.1 | 85.2 |
| AST | 97.5 | 97.6 | 95.9 | 97.6 |
| ALT | 96.3 | 96.4 | 95.8 | 96.4 |
| Urea nitrogen | 96.7 | 96.9 | 96.8 | 97.2 |
| Creatinine | 96.8 | 96.9 | 96.8 | 96.6 |
| CRP | 98.9 | 98.9 | 98.8 | 98.9 |
| Interleukin-6 | 99.1 | 99.2 | 98.9 | 98.6 |
| LDH | 98.5 | 98.6 | 98.4 | 98.4 |
| Procalcitonin | 97.9 | 97.6 | 98.0 | 97.9 |
| Ferritin | 98.2 | 98.3 | 98.3 | 96.9 |
| Cardiac troponin-I | 98.9 | 98.9 | 98.7 | 98.4 |
| ESR | 91.5 | 93.2 | 54.6** | 90.6 |
* p-value <0.05
** p-value <0.01.
ALT: alanine transaminase; APTT: activated partial thromboplastin time, AST: aspartate transaminase; BMI: body mass index; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate, LDH: lactate dehydrogenase.
a Meta-regression model adjusted for the estimated mean age (y) of each study. When the mean or median were available for nonsurvivors and survivors, the weighted mean age for the total sample was calculated. When only the median and interquartile range for the total sample were available, the mean age was calculated according to the method proposed by Hozo SP, Djulbegovic B and Hozo I (BMC Med Res Methodol. 2005, 5:13).
b Meta-regression model adjusted for the proportion of male sex in each study.
c Meta-regression model adjusted for the highest prevalence of chronic conditions or critical/severe status of patients in each study.
The pooled-ES estimates were not substantially modified when the data of individual studies were removed from the analysis one at a time. No relevant differences were observed in sensitivity analyses when studies with moderate risk of bias in more than one domain of the QUIPS tool were excluded, as presented in S2 and S3 Tables.
There was evidence of publication bias in both funnel plot asymmetry and Egger’s test for cough, unspecified comorbidity, cardiovascular disease, hypertension, malignancy, diabetes, haemoglobin, prothrombin time, urea nitrogen and C-reactive protein. All funnel plots and Egger’s test p-values are available in the (S3 Appendix).
Discussion
This meta-analysis included 60 studies with primary data from more than 50,000 patients with confirmed COVID-19, of whom a quarter died. Higher in-hospital mortality risk was found for patients with dyspnoea; a smoking habit; pulmonary, cardiovascular, cerebrovascular, kidney and liver diseases; hypertension; diabetes; and malignancy. Among laboratory parameters, the highest mortality risk was observed for routine blood tests (lymphocyte, leucocyte, neutrophil and platelet counts and haemoglobin), coagulation indicators (D-dimer and prothrombin time), liver function and aggression markers (albumin, total bilirubin, aspartate and alanine transaminase), kidney function markers (urea nitrogen and creatinine), and inflammatory factors (CRP, IL-6, LDH, procalcitonin, ferritin and cardiac troponin). In subgroup analyses and meta-regression, age was the main source of heterogeneity, followed by sex and health condition.
Age played an important role in subgroup analyses. For instance, the increased mortality risks were higher in studies with younger patients than those in studies with older adult patients regarding dyspnoea (p-OR = 4.3 vs. 1.6) and smoking (p-OR = 2.8 vs. 1.2). Additionally, the risks associated with comorbidities were also higher among younger populations, in agreement with the findings of a previous meta-analysis on hypertension [11]. Sex differences may be considered, since smoking increased the risk of death only in studies with lower proportion of male patients. It has been suggested that this could be due to the higher prevalence of chronic conditions and smoking and poorer immunological status in men [87]. Moreover, smoking is more frequent in men than in women, and smokers usually have chronic respiratory conditions, and dyspnoea is a frequent symptom [88]. Although this cluster effect may explain part of the increased risk in smokers, it has been suggested that nicotine can directly affect the putative virus receptor (ECA2) and lead to harmful signalling in the lung epithelial cells, which may contribute to worse prognosis of respiratory viral infections [89]. The interpretation of these findings may indicate that dyspnoea and smoking are more markedly associated with worse prognosis in populations with less exposure to the main risk factors for mortality in COVID-19, that is, populations with advanced age, a high proportion of men and a high prevalence of comorbidities.
Among the symptoms and clinical signs studied, only dyspnoea was associated with higher mortality risk in this meta-analysis. Surprisingly, this risk was reduced in the presence of fever, cough, vomiting, diarrhoea and headache, which are the most prevalent symptoms in COVID-19 [3, 4, 90]. Other meta-analyses found only borderline [8] or no significant association between these symptoms and mortality [10], although all included less studies than the present meta-analysis. We cannot explain these unexpected relationships, although some of them have been reported elsewhere [91]. However, it could be hypothesized that the reporting of these symptoms is affected by information bias and may include single variable symptoms that are mild and occasional (characteristic of mild cases of the disease) or strong and persistent (justifying a worse prognosis given the advanced stage of the disease). Additionally, these associations were not consistent in the subgroup analyses, reinforcing that these symptoms, unlike dyspnoea, may not differentiate patients with worse prognosis. Pulmonary impairment results in low ventilation and oxygenation, which is expressed clinically by dyspnoea [92]. Although dyspnoea is frequent in COVID-19 patients [93–95], its detection on hospital admission highlights the need for specific attention compared with patients without this respiratory symptom, particularly if they are younger adults.
In the present study, a higher mortality risk was observed for all studied comorbidities. The overall risk estimators we found are similar to those of other meta-analyses for smoking [96, 97]; dyspnoea [8]; pulmonary [10, 96, 98], cardiovascular [8, 10, 98, 99], cerebrovascular [10, 99], kidney [10] and liver diseases [17]; hypertension [10, 98]; diabetes [10, 98, 100]; and malignancy [101]. In addition to confirming that chronic conditions lead to worse prognosis, the present study suggests that the association with worse prognosis is of greater magnitude for patients with lower mean age, lower percentage of men and lower prevalence of chronic or critical health condition, although they are also important predictors of mortality in the other subgroups. Similarly, obesity was associated with increased mortality only in studies with fewer chronic or critical patients. Two previous meta-analyses found significant associations between obesity and increased risk of adverse or severe outcomes, although mortality was not specifically addressed [102, 103]. Thus far, current evidence suggests that excess weight and obesity are relevant to adverse COVID-19 outcomes [104–107]. However, our results suggest that BMI is probably a more prominent prognostic factor in patients with fewer comorbidities.
Abnormal levels of laboratory parameters common in infectious conditions were especially associated with mortality when combining the results of the studies included in this review. Haemoglobin, D-dimer, albumin and all inflammatory factors were impaired in all patients, while lymphocyte and neutrophil counts, AST and urea nitrogen were impaired only for non-survivors. The highest risk was observed for the inflammatory factors, albumin and kidney function indicators, although significant effect sizes were observed for all parameters included in this meta-analysis, except for APTT, results consistent with previous meta-analyses [108–110]. The fact that APTT is not a good marker of COVID-related poor coagulation suggests that D-dimer and prothrombin time may be more sensitive indicators for regulating the anticoagulant doses in these patients [111].
In subgroup analyses, the most important mortality predictors in studies with older patients were liver function-related indicators (albumin, total bilirubin, AST and ALT), urea nitrogen and inflammatory factors including C-reactive protein, LDH and ferritin. Likewise, other authors observed that, compared to younger patients, older COVID-19 patients had higher levels of AST, ALT, C-reactive protein and LDH [90], which illustrates the potential for more serious organ damage caused by SARS-CoV-2 infection in the elderly. According to our results, high values in these specific biochemical markers could be used for early identification of elderly patients with worse prognosis. On the other hand, factors noted in younger patients included decreased platelet count, decreased haemoglobin, increased creatinine, increased interleukin-6 and increased cardiac troponin I. Although reduced haemoglobin was related to worse prognosis in the main analysis, confirming previous results [110], this association was detected only in low-risk populations in the subgroup analysis (younger and healthier patients). The significant effect (p-ES = -0.6) found for these associations indicates that even small reductions in haemoglobin may identify those with the lowest chance of survival among these patients. Future studies are needed to examine whether differences in the magnitude of effect sizes point to age-specific indicators for worse prognosis of SARS-CoV-2 infection and whether these variations are due to other variables not available in the included studies to explain the high and persistent heterogeneity between them, such as social and cultural factors related to gender rather than biological sex factors [13].
Some methodological aspects must be considered when interpreting the results presented. First, all included studies had a retrospective design, which is related to some concerns regarding the risk of bias in the selection of participants and in the identification of the outcome [112]. In addition, they were based on medical records fulfilled in a period during which the hospitals were likely to be overwhelmed due to the increased demand caused by SARS-CoV-2 infections, which could result in some underreporting or even inaccuracy of important data [113, 114]. Second, studies included in this review included only in-hospital patients, among whom the intrinsic mortality risk is higher. Third, moderate and high heterogeneity was observed in the meta-analysis of almost all risk factors; however, we could not explore most of the results in subgroup analyses, as there were limited available data in the included studies. Finally, publication bias was observed in one of four of the studied predictors. This is a limitation shared with previous meta-analyses [10, 115] and it indicates that some specific results should be confirmed when more studies are available.
In addition to the clinical implications resulting from the precise identification of predictors of COVID-19-related mortality with suggestions for possible specificities according to the profile of the patients, this systematic review also has implications for future studies. For example, as the evidence indicates that COVID-19 is potentially more dangerous for elderly populations and men, we emphasize that the following studies should present primary data separated according to age and sex groups, allowing the assessment of specific risks according to these characteristics [13]. Last, other potential risk factors not included in this review may be relevant for patients with different demographic and epidemiological profiles. For example, some emerging evidence indicates that the prevalence of rigors, wheeze and hyposmia [3, 116] is high in COVID-19 patients.
In conclusion, this study provides robust evidence on the relative importance of age, sex and health conditions of the patients to the prognostic value of clinical and biochemical parameters observed in COVID-19 patients around the world. Therefore, epidemiological data stratified by age, sex and baseline chronic conditions are required to allow for more accurate decisions considering predictors of COVID-mortality.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by FEDER funds.
References
- 1.World Health Organization W. Coronavirus disease 2019 (COVID-19). Situation Report– 72. 2020. [Google Scholar]
- 2.Coalition C-CR. Global coalition to accelerate COVID-19 clinical research in resource-limited settings. The Lancet. 2020. Epub April 2, 2020 10.1016/S0140-6736(20)30798-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765 Epub 2020/06/24. 10.1371/journal.pone.0234765 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeley P, Buchanan D, Carolan C, Pivodic L, Tavabie S, Noble S. Symptom burden and clinical profile of COVID-19 deaths: a rapid systematic review and evidence summary. BMJ Support Palliat Care. 2020. Epub 2020/05/30. 10.1136/bmjspcare-2020-002368 . [DOI] [PubMed] [Google Scholar]
- 5.Karlsen APH, Wiberg S, Laigaard J, Pedersen C, Rokamp KZ, Mathiesen O. A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment. PLoS One. 2020;15(8):e0237903 Epub 2020/08/21. 10.1371/journal.pone.0237903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonanad C, García-Blas S, Tarazona-Santabalbina F, Sanchis J, Bertomeu-González V, Fácila L, et al. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J Am Med Dir Assoc. 2020;21:915–8. Epub 2020/07/18. 10.1016/j.jamda.2020.05.045 Epub 2020 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J, et al. Systematic Review and Meta-Analysis of Sex-Specific COVID-19 Clinical Outcomes. Front Med (Lausanne). 2020;7:348–. Epub 2020/07/17. 10.3389/fmed.2020.00348 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: A Systematic Review and Meta-analysis of Clinical Characteristics, Risk factors and Outcomes. J Med Virol. 2020. Epub 2020/08/14. 10.1002/jmv.26424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020. Epub 2020/06/24. 10.1111/dom.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020. Epub 2020/05/23. 10.1002/jmv.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Wu J, Sun X, Xue H, Shao J, Cai W, et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: A meta-analysis. Epidemiol Infect. 2020;148:e106 Epub 2020/05/29. 10.1017/S095026882000117X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320926899 Epub 2020/05/16. 10.1177/1470320320926899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396(10250):532–3. Epub 2020/08/18. 10.1016/S0140-6736(20)31748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid Chronic Diseases are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:668–78. Epub 2020/06/04. 10.14336/AD.2020.0502 eCollection 2020 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, et al. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467–74. Epub 2020/05/20. 10.1016/j.ijid.2020.05.055 Epub 2020 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdi A, Jalilian M, Ahmadi Sarbarzeh P, Vlaisavljevic Z. Diabetes and COVID-19: A systematic review on the current evidences. Diabetes Res Clin Pract. 2020:108347–. Epub 2020/07/28. 10.1016/j.diabres.2020.108347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020:1–9. Epub 2020/07/30. 10.1007/s12072-020-10078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–12. Epub 2000/05/02. 10.1001/jama.283.15.2008 . [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S. Chapter 7: selecting studies and collecting data. In: Collaboration C, editor. Cochrane Handbook of Systematic Reviews of Interventions 2011. [Google Scholar]
- 20.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. Epub 2013/02/20. 10.7326/0003-4819-158-4-201302190-00009 . [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, MI, USA: Lawrence Earlbaum Associates; 1988. p. 20–6. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. Epub 1986/09/01. 10.1016/0197-2456(86)90046-2 . [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauchner H, Golub RM, Zylke J. Editorial Concern-Possible Reporting of the Same Patients With COVID-19 in Different Reports. Jama. 2020. Epub 2020/03/17. 10.1001/jama.2020.3980 . [DOI] [PubMed] [Google Scholar]
- 25.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 2002;21:1559–73. 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. Epub 1997/10/06. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloisio E, Chibireva M, Serafini L, Pasqualetti S, Falvella FS, Dolci A, et al. A comprehensive appraisal of laboratory biochemistry tests as major predictors of COVID-19 severity. Arch Pathol Lab Med. 2020. Epub 2020/07/11. 10.5858/arpa.2020-0389-SA [DOI] [PubMed] [Google Scholar]
- 28.Amit M, Sorkin A, Chen J, Cohen B, Karol D, Tsur AM, et al. Clinical Course and Outcomes of Severe Covid-19: A National Scale Study. J Clin Med. 2020;9 Epub 2020/07/28. 10.3390/jcm9072282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asghar MS, Haider Kazmi SJ, Ahmed Khan N, Akram M, Ahmed Khan S, Rasheed U, et al. Clinical Profiles, Characteristics, and Outcomes of the First 100 Admitted COVID-19 Patients in Pakistan: A Single-Center Retrospective Study in a Tertiary Care Hospital of Karachi. Cureus. 2020;12:e8712–e. Epub 2020/07/24. 10.7759/cureus.8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M, Baqui Bica IMVEAvdSMP. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8:e1018–e26. Epub 2020/07/06. 10.1016/S2214-109X(20)30285-0 Epub 2020 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonetti G, Manelli F, Patroni A, Bettinardi A, Borrelli G, Fiordalisi G, et al. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica, Italy. Clinical Chemistry and Laboratory Medicine. 2020;58:1100–5. Epub 2020/06/24. 10.1515/cclm-2020-0459 [DOI] [PubMed] [Google Scholar]
- 32.Zigliani Borghesi, Borghesi A, Zigliani A, Golemi S, Carapella N, Maculotti P, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–3. Epub 2020/05/22. 10.1016/j.ijid.2020.05.021 Epub 2020 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borobia AM, Carcas AJ, Arnalich F, Álvarez-Sala R, Monserrat-Villatoro J, Quintana M, et al. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J Clin Med. 2020;9 Epub 2020/06/10. 10.3390/jcm9061733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brill SE, Jarvis HC, Ozcan E, Burns TLP, Warraich RA, Amani LJ, et al. COVID-19: a retrospective cohort study with focus on the over-80s and hospital-onset disease. BMC medicine. 2020;18:194–. Epub 2020/06/27. 10.1186/s12916-020-01665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/04/03. 10.1093/cid/ciaa243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter B, Collins JT, Barlow-Pay F, Rickard F, Bruce E, Verduri A, et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial study (COVID in Older PEople). J Hosp Infect. 2020. Epub 2020/07/24. 10.1016/j.jhin.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Sun W, Sun S, Li Z, Wang Z, Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China. Clin Transl Med. 2020. Epub 2020/06/09. 10.1002/ctm2.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146(1):89–100. Epub 2020/05/15. 10.1016/j.jaci.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091–m. Epub 2020/03/29. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng A, Hu L, Wang Y, Huang L, Zhao L, Zhang C, et al. Diagnostic performance of initial blood urea nitrogen combined with D-Dimer levels for predicting in-hospital mortality in COVID-19 patients. Int J Antimicrob Agents. 2020:106110–. Epub 2020/07/28. 10.1016/j.ijantimicag.2020.106110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castagna Ciceri, Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509–. Epub 2020/06/15. 10.1016/j.clim.2020.108509 Epub 2020 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chinese medical journal. 2020. Epub 2020/03/27. 10.1097/CM9.0000000000000824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Du, Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. European Respiratory Journal. 2020;55 Epub 2020/04/10. Print 2020 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao S, Jiang F, Jin W, Shi Y, Yang L, Xia Y, et al. Risk factors influencing the prognosis of elderly patients infected with COVID-19: a clinical retrospective study in Wuhan, China. Aging (Albany NY). 2020;12:12504–16. Epub 2020/07/12. 10.18632/aging.103631 Epub 2020 Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendel Garcia Fumeaux TGPHDMMJR-CFSRAHMPPD. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020. 10.1016/j.eclinm.2020.100449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavin W, Campbell E, Zaidi A, Gavin N, Dbeibo L, Beeler C, et al. "Clinical Characteristics, Outcomes and Prognosticators in Adult Patients Hospitalized with COVID-19". Am J Infect Control. 2020. Epub 2020/07/12. 10.1016/j.ajic.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gayam V, Chobufo MD, Merghani MA, Lamichanne S, Garlapati PR, Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2020. Epub 2020/07/17. 10.1002/jmv.26306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harmouch F, Shah K, Hippen J, Kumar A, Goel H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J Med Virol. 2020. Epub 2020/07/28. 10.1002/jmv.26347 [DOI] [PubMed] [Google Scholar]
- 49.Hu Yao NQYH, Hu H, Yao N, Qiu Y. Comparing Rapid Scoring Systems in Mortality Prediction of Critically Ill Patients With Novel Coronavirus Disease. Acad Emerg Med. 2020;27:461–8. Epub 2020/04/21. 10.1111/acem.13992 Epub 2020 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng Huang, Huang J, Cheng A, Kumar R, Fang Y, Chen G, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020. Epub 2020/05/15. 10.1002/jmv.26003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim Hwang, Hwang JM Kim JH, Park JS Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurological Sciences. 2020:1–8. Epub 2020/07/10. 10.1007/s10072-020-04541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khalil K, Agbontaen K, McNally D, Love A, Mandalia S, Banya W, et al. Clinical characteristics and 28-day mortality of medical patients admitted with COVID-19 to a central London teaching hospital. J Infect. 2020. Epub 2020/06/21. 10.1016/j.jinf.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than 50. Obesity (Silver Spring). 2020. Epub 2020/05/24. 10.1002/oby.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan S, Patel K, Desai R, Sule A, Paik P, Miller A, et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020;67:110005–. Epub 2020/07/25. 10.1016/j.jclinane.2020.110005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagunas-Rangel FA. Neutrophil-to-Lymphocyte ratio and Lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J Med Virol. 2020. Epub 2020/04/04. 10.1002/jmv.25819 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Li, Li Q, Cao Y, Chen L, Wu D, Yu J, et al. Hematological features of persons with COVID-19. Leukemia. 2020:1–10. Epub 2020/06/13. 10.1038/s41375-019-0660-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lieberman-Cribbin W, Rapp J, Alpert N, Tuminello S, Taioli E. The Impact of Asthma on Mortality in Patients With COVID-19. Chest. 2020. Epub 2020/06/12. 10.1016/j.chest.2020.05.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Q, Song NC, Zheng ZK, Li JS, Li SK, Liu Song. Laboratory findings and a combined multifactorial approach to predict death in critically ill patients with COVID-19: a retrospective study. Epidemiology and Infection. 2020;148:e129–e. Epub 2020/07/01. 10.1017/S0950268820001442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long H, Nie L, Xiang X, Li H, Zhang X, Fu X, et al. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. Biomed Res Int. 2020;2020:6159720–. Epub 2020/07/01. 10.1155/2020/6159720 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo M, Liu J, Jiang W, Yue S, Liu H, Wei S, et al. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI insight. 2020;5 Epub 2020/06/17. 10.1172/jci.insight.139024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, et al. Prognostic value of C-reactive protein in patients with COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/05/24. 10.1093/cid/ciaa641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo Y, Mao L, Yuan X, Xue Y, Lin Q, Tang G, et al. Prediction Model Based on the Combination of Cytokines and Lymphocyte Subsets for Prognosis of SARS-CoV-2 Infection. Journal of Clinical Immunology. 2020:1–10. Epub 2020/07/15. 10.1007/s10875-020-00821-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo Y, Xue Y, Mao L, Yuan X, Lin Q, Tang G, et al. Prealbumin as a Predictor of Prognosis in Patients With Coronavirus Disease 2019. Front Med (Lausanne). 2020;7:374–. Epub 2020/07/17. 10.3389/fmed.2020.00374 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masetti C, Generali E, Colapietro F, Voza A, Cecconi M, Messina A, et al. High mortality in COVID-19 patients with mild respiratory disease. European Journal of Clinical Investigation. 2020:e13314–e. Epub 2020/06/15. 10.1111/eci.13314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, et al. Risk Factors for Mortality in Patients with COVID-19 in New York City. Journal of General Internal Medicine. 2020:1–10. Epub 2020/07/02. 10.1007/s11606-020-05983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sossou Okoh, Okoh AK, Sossou C, Dangayach NS, Meledathu S, Phillips O, et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. International Journal for Equity in Health. 2020;19:93–. Epub 2020/06/12. 10.1186/s12939-020-01208-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17:1281–. Epub 2020/06/18. 10.7150/ijms.46614 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karim Rastad, Rastad H, Karim H, Ejtahed HS, Tajbakhsh R, Noorisepehr M, et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr. 2020;12:57–. Epub 2020/07/10. 10.1186/s13098-020-00565-9 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirsch Richardson, Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA—Journal of the American Medical Association. 2020;323:2052–9. Epub 2020/04/23. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rivera-Izquierdo del Carmen Valero-Ubierna MRDJLF-GMÁM-DST-MAR-CMG-MA, Rivera-Izquierdo M, Del Carmen Valero-Ubierna M, Jl Rd, Fernández-García MÁ, Martínez-Diz S, et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLoS One. 2020;15:e0235107–e. Epub 2020/06/26. 10.1371/journal.pone.0235107 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive care medicine. 2020. Epub 2020/03/04. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lo Salacup, Salacup G, Lo KB, Gul F, Peterson E, De Joy R, et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: A single tertiary center cohort. J Med Virol. 2020. Epub 2020/07/04. 10.1002/jmv.26252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Owens Shah, Shah P, Owens J, Franklin J, Mehta A, Heymann W, et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Annals of Medicine. 2020:1–7. Epub 2020/07/06. 10.1080/07853890.2020.1791356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Shang. Scoring systems for predicting mortality for severe patients with COVID-19. EClinicalMedicine. 2020. 10.1016/j.eclinm.2020.100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi Shi, Shi S, Qin M, Cai Y, Liu T, Shen B, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. European Heart Journal. 2020;41:2070–9. Epub 2020/05/12. 10.1093/eurheartj/ehaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soares RCM, Mattos LR, Raposo LM. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020. Epub 2020/07/20. 10.4269/ajtmh.20-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ning Sun, Sun H, Ning R, Tao Y, Yu C, Deng X, et al. Risk Factors for Mortality in 244 Older Adults With COVID-19 in Wuhan, China: A Retrospective Study. J Am Geriatr Soc. 2020;68:E19–e23. Epub 2020/05/10. 10.1111/jgs.16533 Epub 2020 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang K, Zuo P, Liu Y, Zhang M, Zhao X, Xie S, et al. Clinical and laboratory predictors of in-hospital mortality in patients with COVID-19: a cohort study in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. Epub 2020/05/04. 10.1093/cid/ciaa538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect. 2020;81:e51–e60. Epub 2020/04/22. 10.1016/j.jinf.2020.04.012 Epub 2020 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Yan, Yan X, Li F, Wang X, Yan J, Zhu F, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross-sectional study. J Med Virol. 2020. Epub 2020/05/28. 10.1002/jmv.26061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Q, Zhou Y, Wang X, Gao S, Xiao Y, Zhang W, et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: a propensity score-matching analysis. Respir Res. 2020;21(1):172 Epub 2020/07/08. 10.1186/s12931-020-01435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469-. Epub 2020/04/18. 10.1111/jth.14848 Epub 2020 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Ye, Ye W, Chen G, Li X, Lan X, Ji C, et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21:169–. Epub 2020/07/06. 10.1186/s12931-020-01428-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu C, Lei Q, Li W, Wang X, Liu W, Fan X, et al. Clinical Characteristics, Associated Factors, and Predicting COVID-19 Mortality Risk: A Retrospective Study in Wuhan, China. American Journal of Preventive Medicine. 2020;59:168–75. Epub 2020/06/23. 10.1016/j.amepre.2020.05.002 Epub 2020 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang JJ, Cao YY, Tan G, Dong X, Wang BC, Lin J, et al. Clinical, radiological and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2020. Epub 2020/07/15. 10.1111/all.14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. Epub 2020/03/15. 10.1016/S0140-6736(20)30566-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Austad SN, Fischer KE. Sex Differences in Lifespan. Cell metabolism. 2016;23(6):1022–33. Epub 2016/06/16. 10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386(10002):1447–56. Epub 2015/10/16. 10.1016/S0140-6736(15)00340-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olds JL, Kabbani N. Is nicotine exposure linked to cardiopulmonary vulnerability to COVID-19 in the general population? The FEBS journal. 2020. Epub 2020/03/20. 10.1111/febs.15303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neumann-Podczaska A, Al-Saad SR, Karbowski LM, Chojnicki M, Tobis S, Wieczorowska-Tobis K. COVID 19—Clinical Picture in the Elderly Population: A Qualitative Systematic Review. Aging Dis. 2020;11:988–1008. Epub 2020/08/09. 10.14336/AD.2020.0620 eCollection 2020 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gil-Rodrigo A, Miró Ò, Piñera P, Burillo-Putze G, Jiménez S, Martín A, et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias. 2020;32(4):233–41. Epub 2020/07/22. . [PubMed] [Google Scholar]
- 92.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls. Treasure Island (FL)2020. [PubMed] [Google Scholar]
- 93.Cao Y, Liu X, Xiong L, Cai K. Imaging and Clinical Features of Patients With 2019 Novel Coronavirus SARS-CoV-2: A systematic review and meta-analysis. J Med Virol. 2020. Epub 2020/04/04. 10.1002/jmv.25822 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, Villamizar-Pena R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel medicine and infectious disease. 2020:101623 Epub 2020/03/18. 10.1016/j.tmaid.2020.101623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol. 2020. Epub 2020/02/29. 10.1002/jmv.25735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS One. 2020;15:e0233147–e. Epub 2020/05/12. 10.1371/journal.pone.0233147 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J Med Virol. 2020. Epub 2020/08/05. 10.1002/jmv.26389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nandy K, Salunke A, Pathak SK, Pandey A, Doctor C, Puj K, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr. 2020;14:1017–25. Epub 2020/07/08. 10.1016/j.dsx.2020.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949 Epub 2020/05/16. 10.1016/j.jstrokecerebrovasdis.2020.104949 Epub 2020 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. Epub 2020/04/26. 10.1016/j.dsx.2020.04.018 Epub 2020 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian Y, Qiu X, Wang C, Zhao J, Jiang X, Niu W, et al. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int J Cancer. 2020. Epub 2020/07/20. 10.1002/ijc.33213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Földi M, Farkas N, Kiss S, Zádori N, Váncsa S, Szakó L, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes Rev. 2020. Epub 2020/07/21. 10.1111/obr.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. Epub 2020/07/30. 10.1016/j.ijid.2020.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes Metab Syndr. 2020;14:655–9. Epub 2020/05/22. 10.1016/j.dsx.2020.05.020 Epub 2020 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020;28(7):1195–9. Epub 2020/04/10. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262–. Epub 2020/05/19. 10.1016/j.metabol.2020.154262 Epub 2020 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of 10.1002/oby.22859 Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity (Silver Spring). 2020;28(7):1200–4. Epub 2020/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qiu P, Zhou Y, Wang F, Wang H, Zhang M, Pan X, et al. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res. 2020:1–10. Epub 2020/08/01. 10.1007/s40520-019-01392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med. 2020;76:97–9. Epub 2020/04/30. 10.1016/j.ejim.2020.04.043 Epub 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa-Díaz ZM, et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020:1–11. Epub 2020/08/21. 10.1007/s10654-020-00616-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol. 2020;76:122–4. Epub 2020/05/11. 10.1016/j.jacc.2020.05.001 Epub 2020 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szklo M, Javier Nieto F. Epidemiology: beyond the basics. 2nd ed ed London: Jones and Bartlett Publishers International; 2007. [Google Scholar]
- 113.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. Bmj. 2020:m1198 10.1136/bmj.m1198 [DOI] [PubMed] [Google Scholar]
- 114.Mahase E. Covid-19: death rate is 0.66% and increases with age, study estimates. Bmj. 2020;369:m1327 Epub 2020/04/03. 10.1136/bmj.m1327 . [DOI] [PubMed] [Google Scholar]
- 115.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36–. Epub 2020/06/03. 10.1186/s40560-020-00453-4 eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. The Laryngoscope. 2020. Epub 2020/04/03. 10.1002/lary.28692 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.