Abstract
Plant species of the Poaceae family are not only used as fodder and forage but also contribute substantially to the treatment of various health disorders, particularly in livestock. Consequently, the present study was aimed to document the therapeutic uses of Poaceae practiced by the inhabitants of the Punjab Province for the treatment of various veterinary health disorders. Semi structured interviews, group discussion and field walks were conducted to collect the data. Quantitative indices including cultural significance index (CSI), relative frequency of citations (RFC), fidelity level (FL), relative popularity level (RPL), and Jaccard Index (JI) were used for the data analysis. Traditional uses of 149 species belonging to 60 genera and 16 tribes of 5 sub families of Poaceae were recorded. Whole plants and leaves were the most consistently used parts with 40.94 and 29.53%. The plants were mainly given orally as fodder (59 reports) without processing followed by decoction (35 reports). Most of the species were employed to treat infectious diseases (25.93%), and digestive disorders (14.10%). Triticum aestivum had the highest CSI, RFC and RPL levels at 8.00, 0.96, 1.00, respectively, followed by Oryza sativa and Poa annua. Likewise, T. aestivum and Saccharum spontaneum had 100% FL and ROP. Jaccard index ranged from 12.25 to 0.37. Twelve plant species namely Chrysopogon zizanioides (anti-inflammatory), Pennisetum lanatum (improve bull fertility), Cymbopogon citratus (glandular secretion), Sorghum saccharatum and Themeda triandra (malaria), Aristida funiculate (anticancer), Koeleria argentia (skin allergies), Tetrapogon villosus (antibacterial), Cynodon radiatus (eyes infection), Sporobolus nervosa (Jaundice), Enneapogon persicus (antifungal), and Panicum repens (dysfunctional cattle organs) were reported for the first time, with novel ethnoveterinary uses. The inhabitants of the study area had a strong association with their surrounding plant diversity and possessed significant knowledge on therapeutic uses of Poaceae to treat various health disorders in animals. Plant species with maximum cultural and medicinal values could be a potential source of novel drugs to cure health disorders in animals and human as well.
Introduction
Botanical taxa belonging to the family Poaceae are the most substantial component of agricultural crops and livestock feed as well as the main sources of economy and revenue for many people of the rural areas around the globe [1]. The majority of livestock depends on forage grasses and natural pastures. Native plant species of Poaceae are a cost-effective source of nutrients for livestock and contribute significantly to conserve the soil integrity, water supply and air quality [2]. The major constraints for improved productivity of livestock is the low quality of forage available during the dry season, that cannot meet the nutrient requirements of grazing ruminants [3]. Rural populations worldwide use grasses as a source of feed for domestic animals and as medicines to treat health disorders in animals and humans [4].
Many scientific studies have recorded ethnoveterinary data on medicinal plants in different part of the world like, Kenya [5], Italy [6], Canada [7], South Africa [8], Pakistan [9], Brazil [10], Argentina [11], India [12], Nigeria [13], Spain [14] and Uganda [15], nevertheless Poaceae remained among the least explored plant family. In the past, grasses rich in nutrition were preferred over those that have therapeutically important compounds [4]. Grasses are of particular importance in traditional health care system due to the presence of biologically active compound like alkaloids, flavonoids and saponins [16]. The presence of alkaloids makes them highly resistant against foreign microbes while flavonoids have been shown to have anti-inflammatory, anticancer, and antiviral activity, and also help animals to repair oxidative cell damage [17]. Cynodon dactylon, Sacchrum spontaneum, and Imperata cylindrica have been found to be effective in inflammation and fungal diseases in animals [18]. Moreover, Chloris barbata is used as disinfectant [19], while Heteropogon contortus has anti-microbial, anti-cancer, anti-inflammatory properties, and it also increases milk production in livestock [20].
The local inhabitants of rural areas of Punjab widely use grasses for ethnoveterinary purposes. For example, Cynodon dactylon, Eleusine indica, Bromus japonicus, Phragmites australis, Eragrostis minor and Desmostachya bipinnata are reported to treat various stomach problems whereas Sorghum bicolor, Brachiaria ramosa, Arundo donax, Chrysopogon zizanioides, and Panicum antidotale are used to treat microbial infections in cattle [4]. Indigenous people with long history of livestock rearing may have developed precious information about the potential forage resources [21]. They prefer to use grasses as fodder because these are highly palatable than other form of fodders [22]. Their palatability also depends on animal choice and may also be linked with their seasonal availability, morphological and chemical nature of plant [23]. Animals generally prefer fresh foliage over dried because leaves of grasses are rich source of protein and cellulose, and have low lignin than forbs and shrubs [24]. Grasses beside the nutritional and healthcare services also reduce the grazing pressure on other palatable species and improve the productivity in livestock [25].
Nevertheless, different workers have been reported the traditional uses of plant species from different areas of Punjab [26–34], but a little is known about the therapeutic potential of grasses for the treatment of various diseases in livestock [4]. Consequently, the present study was conducted with the aim to document the ethnomedicinal uses of plant species of Poaceae family from Punjab province of Pakistan, traditionally used for the treatment of various health disorders in livestock. Another objective was to explore the cultural significance of species and their popularity among different tribes on the basis of their usage in animal healthcare.
Materials and methods
Ethics statement
The study was authorized by the Institutional Human and Animal Ethics Committee through No. HPR/HAEC/254/2019. Verbal informed consent was obtained from each informant before conducting the interview process.
Study area
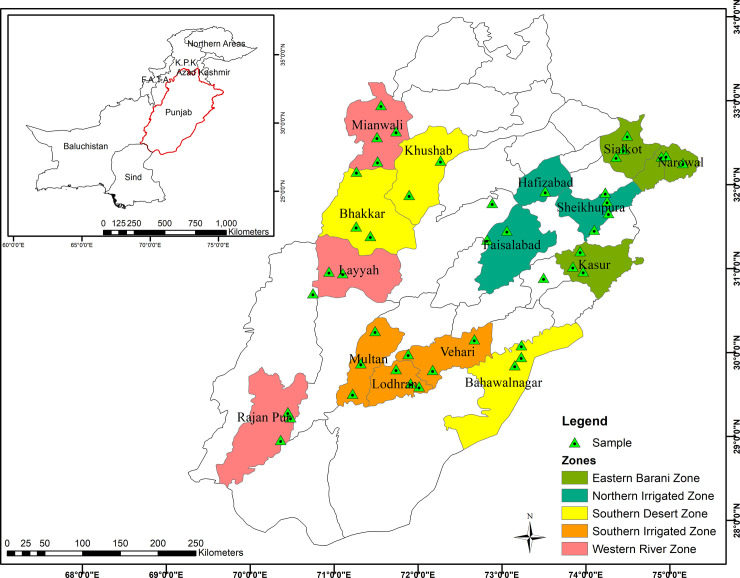
Punjab is the second largest province of Pakistan after Baluchistan. It encompasses of 205,344 km2 and is located between latitudes 27.42° and 34.02° N and longitudes 69.81° and 75.23° E at the northwestern edge of the geological Indian plate in South Asia [35]. Punjab is comprised of 36 districts and 5 agro-ecological zones [36] i.e. eastern barani zone, northern irrigated zone, southern desert zone, southern irrigated zone, and western river zone (Fig 1). Of these, northern irrigated zone and south desert zone are the two largest zones with large difference in cultural groups and ethnobotanical practices [4]. Most of the areas in Punjab consist of fertile alluvial plains heavily irrigated by 5 rivers namely Jehlum, Ravi, Chenab and Sutlej. Small deserts areas can be found in southern Punjab and Sulaiman Range. The variation in temperature and rainfall occurs all over the year. Soil of the study area is generally sandy, clay, and loamy [37]. Most of the areas experience foggy weather during winter and hot weather in summer while the average annual temperature ranges from -2°C to 45°C. June is the hottest and January is the coldest month of the year. Average annual rainfall of last five year is about 479.8 mm. The northern parts of the province receive a reasonable amount of rainfall through the year as compared to the drier southern part. Almost half of the rainfall occur during the month of July and August averaging about 255 mm. Punjab is home to over half of the total population of Pakistan. The ethnic composition of the area is quite diverse comprising of different tribes and communities. Rana, Gujjar, Butt, Rayain are the major ethnic groups. Most of the people speak Urdu and Punjabi languages followed by Saraiki. English language is used in government offices. Compared to the other provinces, Punjab has highest literacy rate. Moreover, Punjab contributes major share in the economy of Pakistan in terms of GDP. The economy of the people in the province is based on agriculture, and wheat is the widely cultivated crop with significant production of rice, cotton, corn, sugarcane, pulses and jute. The major occupation of the rural communities is farming, and they depend on agricultural means and livestock management to support livelihood. The inhabitants of the Punjab province have diverse traditional knowledge and practices because of linguistic and cultural variations. In agricultural lands like Punjab, grasses are preferred over other medicinal herbs and shrubs [4] because they are common, highly palatable and easy to process in order to cure livestock ailments [38].
Fig 1. Map of study area showing different ecological zones and sampling sites of Punjab, Pakistan.
Data collection
Data on ethnomedicinal uses of grasses were collected through group discussions, semi-structured interviews and open and closed ended questionnaires during field visits in 2016–17, following the methods reported previously [39–41]. Prior to collect information, permission was obtained from the head of local government and prior informed consent from all local informants. In total, 271 participants including both men and women, traditional practitioners, village leaders, shepherds, cattle holders who worked in local farms and some senior household animal owners were interviewed. Demographic information (Table 1) about the participants was gathered by adopting a method of [42] and analysed in Microsoft Excel 365. Questionnaires were first developed in English, later translated in local languages, i.e. Punjabi, Saraiki and Urdu. Before conducting interviews, prior informed consent was also obtained from the participants after briefing the research objectives. No further ethical approval was required as there is lacking explicit rules or regulations pertaining to the practices of ethnomedicinal uses of plants or animals in Pakistan. However, participants were allowed to discontinue the interviews at any time.
Table 1. Demographic data about informants of the study area.
| Variable | Demographic categories | Numbers | Percentage |
|---|---|---|---|
| Gender | Females | 167 | 61.62 |
| Males | 104 | 38.37 | |
| Age | Upto 20 years | 31 | 11.43 |
| 21–40 years | 73 | 26.93 | |
| 41–60 years | 119 | 43.91 | |
| 61–80 years | 48 | 17.71 | |
| Occupation | Domestic cattle holders | 121 | 44.64 |
| Nomads | 80 | 29.52 | |
| Farm cattle holders | 70 | 25.83 | |
| Education levels | Illiterate | 49 | 18.08 |
| Primary | 77 | 28.41 | |
| Middle | 53 | 19.56 | |
| Intermediate | 37 | 13.65 | |
| Graduate | 28 | 10.33 | |
| Master | 16 | 5.90 | |
| M.Phil. | 9 | 3.32 | |
| Ph.D. | 2 | 0.74 | |
| Traditioinal practitioners | |||
| ≤ 3 | 34 | 12.55 | |
| 3–5 years | 66 | 24.35 | |
| 5–10 years | 61 | 22.51 | |
| 10–15 years | 55 | 20.30 | |
| ≥ 15 years | 55 | 20.30 |
Collected plant specimens were identified with the help of the Flora of Pakistan, and names were verified through literature [43], www.efloras.org/index.aspx and Kew grass data base (https://www.kew.org/data/grasses-db/index.htm). For voucher specimen, standard herbarium techniques [44, 45] were strictly followed. All plants were labelled and deposited in the herbarium at the Department of Botany, University of Gujarat, Punjab, Pakistan and their voucher specimens were preserved for the furture record.
Cultural significance index (CSI)
The relationship between use reports of a given species and agreement among the informant knowledge was attributed through cultural significance index (CSI). It was calculated following a method of [46] using formula:
Where i is the management of species having considerable impact on community (a species cultivated, managed or operated by any mean is awarded score of 2 and the value 1 is awarded if species is yet free from any kind of manipulation), e is the use preference of the informant for one plant species over another species for a specific purpose (value 2 is for preferred species and value 1 is for non-preferred species), c is use frequency of a plant species (value 2 is attributed to a high potential plant species being considerably used by informants and value 1 is awarded to a rarely cited species), correction factor (CF) is level of the informant consensus which comes from species citation divided by the number of citations of the most mentioned species.
Relative frequency of citation (RFC)
RFC is to set up the priority order among the listed species and its value is depended on the numbers of participants who have mentioned a particular species as a medicinal plant or good fodder indicating its significance. The RFC was estimated with the help of the following equation following [47].
Where, FC is the number of participants who stated a particular plant species as an excellent medicinal plant and N is the total number of participants included in the study.
Use value (UV)
Use value (UV) was calculated by applying a standard procedure reported previously [48, 49].
Where U is the total number of use reports mentioned by informants for a given plant and n is the total number of informants interviewed for a given plant species. UV close to 1 indicates many use reports for a given plant and its importance among informants.
Correlation study
Correlation between CSI, RFC and UVs was tested using Pearson’s correlation in SPSS 16.0 (SPSS for Windows, Chicago, IL, USA).
Fidelity level (FL)
FL comes from the percentage of informant knowledge who report the uses of a plant species for an ailment and was determined using formula as previously reported by [48, 49].
Where, Np is the number of participants who reported the use of a grass for a specific purpose and N is the sum of participants who claimed the use of a grass for any purpose. High level of FL reflects the high use of plant species in specific disease in the study area.
Relative popularity level (RPL)
Plant healing potential cannot be differentiated when species show same fidelity level. In order to differentiate the healing potential of species with same FL values, the relative popularity level is calculated, which is the ratio between ailments cured by a specific plant and total number of informants who reporting that disease. Base of RPL, plant species are divided into popular and non-popular groups. Popular species are those reported by more than half number of informants or above and rest of the species are declared as non-popular. For popular plant species RPL was arbitrarily selected equal to 1 that represents the complete popularity of a species for the cure of ailments and 0 value represents that no ailment was treated by this species [36].
Rank order priority (ROP)
Plant species having different FL and RPL values were attributed with the correction factor (ROP) to rank properly the reported species. The ROP was calculated by multiplying FL and RPL values as elucidated earlier [47].
Jaccard index (JI)
The data presented in our study was compared with already published data in the adjacent areas of Himalayan territory using Jaccard index by appraising percentage of reported species and their medicinal uses [5].
Where, a represent the number of plants in an area A, b is the number of plants in area B and c is the number of plants common to area A and B.
Results and discussion
Demographic features
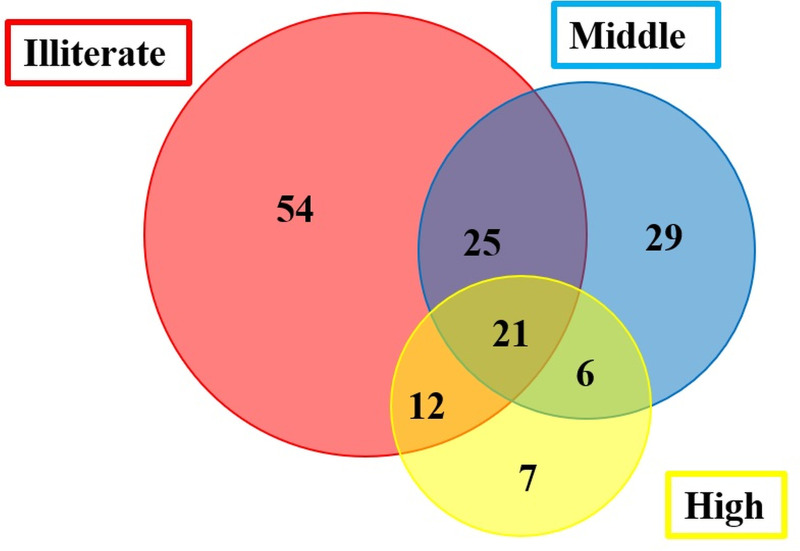
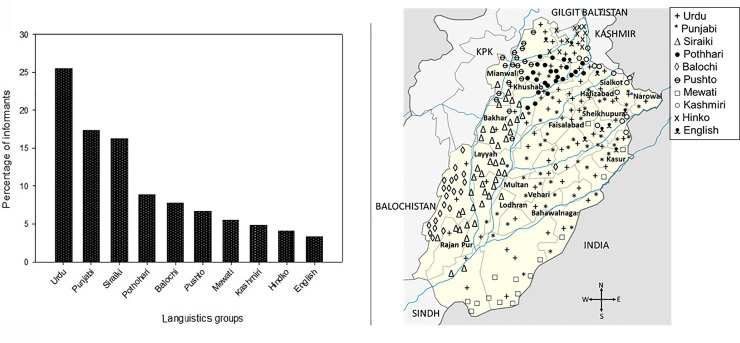
Data were collected from 271 informants (167 females and 104 males) of ages between 20 to 80 years (Table 1), including domestic cattle holders (44.64%), nomads (29.52%), and farm cattle holders (25.83%), and Traditional practitioners (TP) were classified into five groups based on their experience, such TP with less than 3 years of experience (34), 3–5 years (66). 5–10 years (61), 10–15 years (55), and more than 15 years (55). About, 18.08% informants were illiterate while others were educated up to master and PhD level. The plant citations were grouped in three education groups such as Illiterate, middle (primary to secondary), and high (secondary to PhD) and are represented through Venn diagram (Fig 2). The total plant species cited by each group were roughly equal to 112, 76 and 46 for the illiterate, middle and high education groups, respectively. About 54 species were only cited by illiterate people, 29 by middle, and 7 by highly educated people. Furthermore, there were common citations between different groups: 25 between illiterate and middle, 12 between illiterate and high, and 6 between middle and high. A total of 21 species were used by all three groups. From this analysis, it is easy to perceive that the level of indigenous knowledge on medicinal uses of the plant species of family Poaceae was more prevalent in illiterate participants and less educated people, while the more educated informants were less conversant on the ethnomedicinal uses of plant species, chiefly of Poaceae taxa. This difference in ethnomedicinal knowledge could be linked to the fact that educated people have highlevel of exposure to modernization and their dependence on allopathic medicines which have already been reported in literature [50, 51]. Most of the previous studies were focused on a single community with one ethic group and same culture [52], but in the last few decades, ethnobiologists have shown more interest in cross cultural variation of traditional knowledge of different communities and ethnic groups [53]. Because of this, we also collected data on ethno-veterinary uses of Poaceae from different ethnic groups i.e. Punjabi, Gujjar, Butt, Khawaja, Arayeen, and Rana. These groups have diverse culture and languages such as Urdu, Punjabi, Saraiki, Pothare, Balochi, Pashto, Mewatti, Kashmiri, Hindku, and English (25.46, 17.34, 16.24, 8.86, 7.75, 6.64, 5.54, 4.80, 4.06 and 3.32%, respectively (Fig 3).
Fig 2. Venn diagram representing the overlap of taxa cited by different education groups.
a) Illiterate, b) Middle education (primary to secondary), and c) Higher education (secondary to PhD).
Fig 3. Linguistic wise classification and geographic distribution of the informants.
Taxonomic description of Poaceae species
In total, 149 species of Poaceae, belonging to 18 tribes were documented, which were used to treat various animal health disorders, classified into 12 major disease categories. Of these, 56% were of perennial nature and rest were annual herbs (S1 Table).
Plant part (s) used
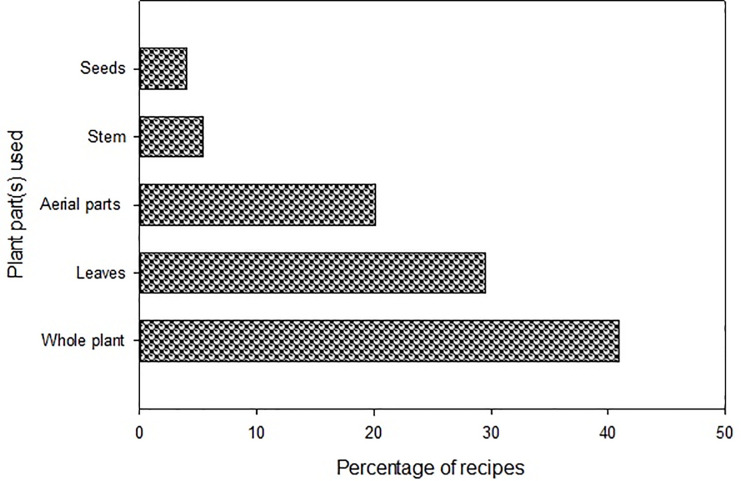
As depicted in Fig 4 and S1 Table, about 41% recipes were based on whole plants while leaves, aerial parts (without roots), stem, and seeds contributed to the remaing 59% of recipes. As the majority of the Poaceae taxa are small annual herbs with shallow roots, therefore, they are easy to pull out as a whole plant and utilized to treat various diseases [4]. Likewise, leaves are easy to collect and are rich in health beneficial secondary metabolites which contribute significantly to the treatment and prevention of health disorders [50, 54, 55]. Leaves have also been reported previously as one of the most consistently used plant part for grazing and medicinal purposes [26, 36, 56].
Fig 4. Proportion of plant part (s) used in different recipes for the disease management.
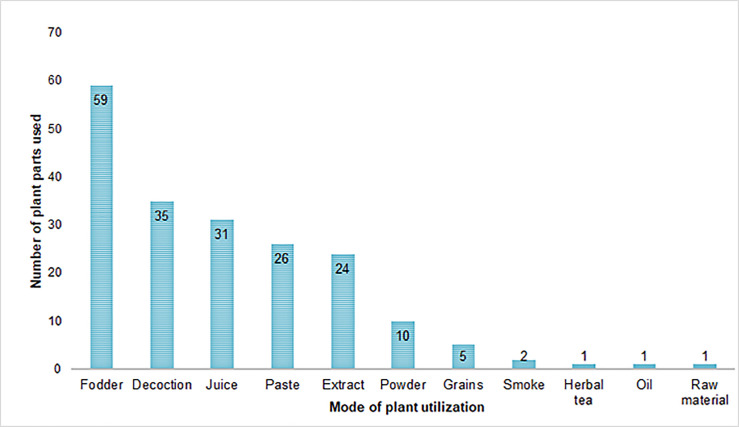
Method of preparation and administration
As shown in Fig 5 and S1 Table, most of the plants were given orally as fodder (59 reports) without processing them in crude preparation followed by decoction (35 reports), juice (31 reports), paste (26 reports), extract (24 reports), powder (10 reports), grains (5 reports), smoke (2 reports) and herbal tea, oil, and processed raw material (1 report each). Offering plants as fodder to animals is the best way to treat a specific disease without having side effects. Crude preparation as decoction by boiling the plant parts in water for the treatment of various ailments is the most common practice among the ethnic communities in Punjab. Powder is prepared by grinding the dried plant parts and paste is made from crushing the fresh or dried plant parts with water or oil [34]. Futhermore, herbal preparations used to treat internal diseases i.e. gastrointestinal disorders, fever, and pain etc. were usually administrated orally, while for joint pain, skin infections topical method was used. Interestingly, there is a significant trend of multi-plant formulation devised by semiprofessional herbalists and traditional practitioners. In that case, powder of more than one plant/plant part is orally administered with water known as “Phakki”. There are certain cases with such recipes where overdose and malpractice resulted in adverse drug reactions.
Fig 5. Commonly used methods in the preparation of herbal recipies.
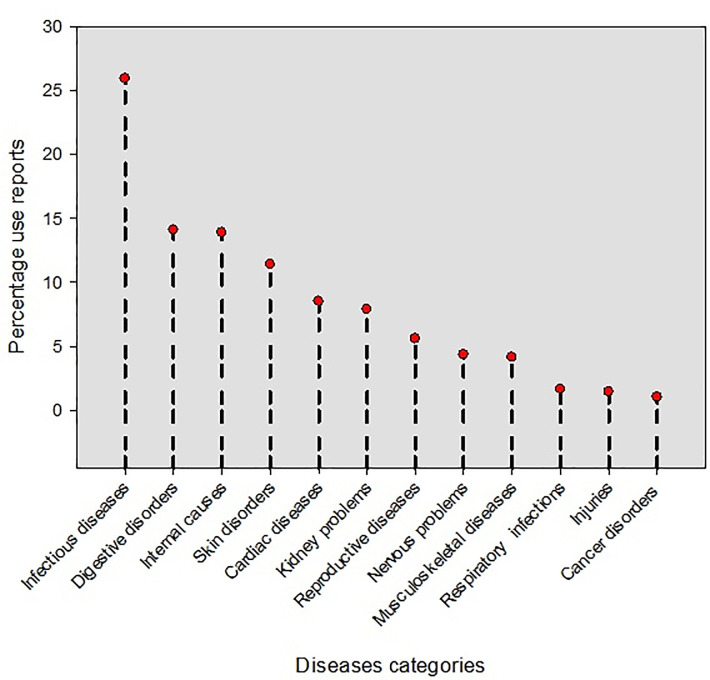
Major disease categories and use reportes
Inhabitants of the study area used 149 plant species to treat various health disorders in cattle, which were grouped into 12 major disease categories (S1 Table, Fig 6). The maximum number of use reports of plant species was reported for infectious diseases (25.93%), followed by digestive disorders (14.10%), internal causes (13.90%), skin diseases (11.41%), cardiac disorders (8.50%), kidney problems (7.88%), reproductive diseases (5.60%), nervous problems (4.35%), musculoskeletal diseases (4.14%), respiratory infections (1.65%), injuries (1.45%), and cancer (1.03%). Grasses mainly, Eulaliopsis binata, Saccharum bengalense, Sorghum halepense, Phragmites australis, Paspalidium punctatum, Pennisetum divisum, P. lanatum Paspalum dilatatum, Setaria intermedia, and Brachiaria ramosa were used to cure infectious diseases in livestock. Likewise, most of the skin disorders were treated with Aristida adscensionis, Koeleria argentia, Cynodon dactylon, Acrachne racemosa, Panicum turgidum, Setaria glauca and Hordeum vulgare.
Fig 6. Major disease categories with use reports (%) in the study area.
[Infectious diseases: cholera, tick infestation, typhoid fever, pneumonia, foot and mouth diseases, malaria, hemorrhagic fever, toothache, viral, fungal and bacterial infection, oral infections. Digestive disorders: stomachache, indigestion, gastro enteritis, abdominal pain, appetite, constipation, dysentery, diarrhea, dyspepsia. Internal causes: jaundice, toxification, piles, and general weakness. Skin disorders: allergies, herps, measles, soreness, shingles, itching. Cardiac diseases: blood pressure, heart palpitations, inflammation, anemia, diabetic. Kidney problems: urinary problems, urinary tract infections. Reproductive diseases: sexual disorder, premature ejaculation, menstrual discharge, dysfunctional organs, leucorrhoea, infertility. Nervous problems: hysteria, nervous exhaustion, headaches. Musculoskeletal diseases: bone fractures. Respiratory infections: bronchitis, cough. Injuries: skins injuries or cutting, wound healings. Cancer disorders: tumors.].
In previous reports, B. ramosa and S. bicolor have been used in microbial infections [57], and S. halepense is primarily used for infectious diseases in livestock [58]. Futhermore, D. bipinnata, E. indica and E. minor are used to treat digestive disorders [4]. Likewise, A. donax, C. dactylon and D. annulatum are effective in gastrointestinal problems in animals [59–61]. Additionally, E. indica is use to relieve abdominal pain [62] and C. citratus is used as anti-bacterial, anti-fungal and anti-inflammatory agent [63]. We found D. aegyptium as detoxifier and antiallergic plant (S1 Table), but according to Kumar et al. [64], this plant has anti-inflammatory, anti-cancer, anti-microbial properties. Stem of H. vulgare is ground and mixed with water in order to increase the weight of cattle in Hawassa Zuria District, Sidama zone, Southern Ethiopia [65]. In Salt Range of Pakistan, flour made from Hordeum vulgare is used to cure jaundice [66, 67], D. bipinnata is diuretic and anti-amenorrhea [68], and root powder used to cure rheumatism while root infusion is used in urinary infections [69]. The grass V. zizanoidesis is antiseptic, demulcent and anti-inflammatory [70]. We reported that paste made from the leaves of C. dactylon is used to treat skin injuries in animals and juice is given for healing bone fractures. Additionally, root decoction is given to cattle having respiratory problem [67]. A decoction made from S. bicolor is given in in case of typhoid [71] whereas Themeda anathera is refrigerant and blood purifier [72]. The grass D. bipinnata is anti-amenorrhea and diuretic [73], while decoction made from the whole plant of C. jwarancusa is given in abdominal pain, tumor and unconsciousness [74]. We reported that E. indica is used in abdominal pain, which is in line with the study of Lans et al. [7]. Paste made from A. mutica is used to cure fungal diseases in livestock [75]. Zareen et al. [38] described that besides improving digestion, B. bladhii is also used as stored food and fodder for both livestock and wild ruminants. We found that extract made from aerial parts of Z. mays is used as detoxifier, nerve tonic and antiseptic while Zia-ud-Din et al. [57] reported that seeds of the same plant are mixed with oil to manage tick infection in livestock. We documented that the paste made from leaves of I. cylindrica is given to cattle in order to control microbial infections while Sushama and Nishteswar [76] reported that this plant is neuroprotective and possess anticancer properties. Juice extracted from leaves and roots of S. spontaneum improves appetite and gives relieve in inflammation, urinary problems, and abdominal pain [61]. Dicanthium annulatum is used in indigestion and menorrhagia [77].
During a detailed survey of literature review on ethnoveterinary practices, we have observed that ethno-veterinary literature on Poaceae used is fragmentary in Pakistan. To date, only few reports on ethno-veterinary investigations are available from different parts of Pakistan [9, 56, 61, 78–100]. On the other hand, rich data on ethnoveterinary practices is available from other parts of the world, for example, Africa, Orma land- Kenya [101], Nigeria [102], Zimbabwe [103], India [104], China [105], Netherlands [106], America [107], Canada [7] and Brazil [108]. Keep in view the current scenario, the current study is a continuance of earlier explorations for the improvement of records on the ethno-veterinary medication in Pakistan.
Cultural significance index (CSI)
The cultural significance index is used to compute the importance of individual plant used by indigenous people. In CSI, the recognition or reputation of species is linked to its functions to the people and are considered auxiliary element in the cultural recognition of a plant [109, 110]. We observed that cultural importance of each species fluctuates between local communities. For instance, CSI values varied from 0.13 to 8.00, markedly affected by the preference, management and frequency of use by the local inhabitants (Table 2). This difference was influenced by the level of knowledge, the cultural settings, and the local conditions.
Table 2. Tribe wise distribution of plants of Poaceae with frequency of citations (FC), relative frequency of citations (RFC), use value (UV) and cultural significance index (CSI) values.
| Tribe | S. # | Botanical name | FC (n) | RFC | UV | CSI |
|---|---|---|---|---|---|---|
| Andropogoneae | 1. | Apluda mutica L. | 47 | 0.17 | 0.56 | 0.18 |
| 2. | Bothriochloa bladhii (Retz.) S.T. Blake | 220 | 0.81 | 0.21 | 1.68 | |
| 3. | Chrysopogon aucheri (Boiss.) Stapf | 70 | 0.26 | 0.47 | 0.27 | |
| 4. | Chrysopogon serrulatus Trin. | 86 | 0.32 | 0.31 | 0.33 | |
| 5. | Chrysopogon zizanioides (L.) Roberty | 111 | 0.41 | 0.12 | 0.84 | |
| 6. | Cymbopogon citratus (DC) Stapf | 175 | 0.65 | 0.89 | 2.68 | |
| 7. | Cymbopogon commutatus (steud) Stapf. | 88 | 0.32 | 0.74 | 0.34 | |
| 8. | Cymbopogon jwarancusa (Jones.) Schult | 168 | 0.62 | 0.66 | 2.57 | |
| 9. | Cymbopogon martini (Roxb.) W. Watson | 53 | 0.20 | 0.81 | 0.81 | |
| 10. | Dichanthium annulatum (Forssk.) Stapf | 251 | 0.93 | 0.17 | 1.92 | |
| 11. | Dichanthium foveolatum (Delile) Roberty | 98 | 0.36 | 0.19 | 1.5 | |
| 12. | Eulaliopsis binata (Retz) C.E.Hubb. | 96 | 0.35 | 0.09 | 0.03 | |
| 13. | Heteropogon contortus (L.) P Beauv. ex. Roem & Schult | 172 | 0.63 | 0.10 | 2.62 | |
| 14. | Imperata cylindrica (L.) Raeuschel | 183 | 0.68 | 0.23 | 2.80 | |
| 15. | Saccharum arundinaceum Retz. | 202 | 0.75 | 0.46 | 1.55 | |
| 16. | Saccharum bengalense Retz. | 233 | 0.86 | 0.75 | 3.56 | |
| 17. | Saccharum spontaneum L | 116 | 0.43 | 0.80 | 1.77 | |
| 18. | Saccharum ravennae L. | 145 | 0.54 | 0.55 | 1.11 | |
| 19. | Saccharum officinarum L. | 233 | 0.86 | 0.91 | 7.14 | |
| 20. | Sorghum bicolor (L.) Moench | 223 | 0.82 | 0.91 | 3.56 | |
| 21. | Sorghum saccharatum (L.) Moench | 178 | 0.66 | 0.64 | 0.68 | |
| 22. | Sorghum halepense (L.) Pers. | 219 | 0.81 | 0.60 | 3.35 | |
| 23. | Themeda anathera (Nees) Hack | 89 | 0.33 | 0.25 | 0.34 | |
| 24. | Themeda triandra Forsk. | 134 | 0.49 | 0.15 | 0.51 | |
| 25. | Vetiveria zizanioides (L.) Nash | 144 | 0.53 | 0.72 | 2.12 | |
| 26. | Zea mays L. | 221 | 0.82 | 0.74 | 6.77 | |
| Aristideae | 27. | Aristida adscensionis L. | 143 | 0.53 | 0.34 | 1.15 |
| 28. | Aristida cyanantha Nees ex Steud. | 99 | 0.37 | 0.36 | 0.37 | |
| 29. | Aristida funiculata Trin. & Rupr. | 55 | 0.20 | 0.29 | 0.21 | |
| 30. | Aristida hystricula Edgew, | 156 | 0.58 | 0.20 | 0.59 | |
| 31. | Aristida mutabilis Trin. & Rupr. | 51 | 0.19 | 0.14 | 0.25 | |
| 32. | Stipagrostis plumosa (L.) Munro ex T.Anderson | 161 | 0.59 | 0.10 | 1.23 | |
| Arundineae | 33. | Arundo donax L. | 225 | 0.83 | 0.48 | 3.44 |
| 34. | Phragmites australis (Cav.) Trin. ex Steud. | 48 | 0.18 | 0.39 | 0.36 | |
| 35. | Phragmites karka (Retz.) Trin. ex Steud. | 159 | 0.59 | 0.35 | 1.22 | |
| Aveneae | 36. | Avena fatua L. | 149 | 0.55 | 0.54 | 1.14 |
| 37. | Agrostis gigantea Roth. | 87 | 0.32 | 0.27 | 0.33 | |
| 38. | Agrostis viridis Gouan | 107 | 0.39 | 0.71 | 0.41 | |
| 39. | Avena sativa L. | 239 | 0.92 | 0.86 | 7.33 | |
| 40. | Avena sterilis (Dur.) Gill & Magne | 39 | 0.14 | 0.28 | 0.15 | |
| 41. | Koeleria argentina Griseb. | 79 | 0.29 | 0.63 | 0.30 | |
| 42. | Phalaris minor Retz. | 176 | 0.65 | 0.22 | 1.34 | |
| 43. | Polypogon monspeliensis (L.) Desf. | 97 | 0.36 | 0.49 | 0.37 | |
| 44. | Trisetum clarkei (Hook. f.) R. R. Stewart | 55 | 0.20 | 0.33 | 0.21 | |
| 45. | Polypogon fugax Nees ex Steud | 45 | 0.17 | 0.37 | 0.17 | |
| Bromeae | 46. | Bromus catharticus Vahl | 70 | 0.26 | 0.18 | 0.26 |
| Bambuseae | 47. | Bambusa glaucescens (Willd.) Merr. | 183 | 0.68 | 0.56 | 0.70 |
| Bromeae | 48. | Bromus japonicus Thunb. | 82 | 0.30 | 0.37 | 0.31 |
| 49. | Bromus pectinatus Thunb. | 67 | 0.25 | 0.17 | 0.25 | |
| 50. | Bromus sericeus Drobov | 113 | 0.42 | 0.24 | 0.43 | |
| Chlorideae | 51. | Tetrapogon cenchriformis (A. Rich.) Clayton | 77 | 0.28 | 0.19 | 0.29 |
| 52. | Tetrapogon tenellus (Roxb.) Chiov. | 144 | 0.53 | 0.15 | 0.55 | |
| 53. | Tetrapogon villosus Desf.. | 43 | 0.16 | 0.21 | 0.16 | |
| Cynodonteae | 54. | Chloris gayana Kunth | 129 | 0.48 | 0.10 | 0.49 |
| 55. | Chloris barbata Sw. | 228 | 0.84 | 0.16 | 0.87 | |
| 56. | Chloris dolichostachya Lag. | 137 | 0.51 | 0.02 | 0.52 | |
| 57. | Chloris virgata Sw. | 211 | 0.78 | 0.26 | 1.62 | |
| 58. | Cynodon dactylon (L.) Pers. | 249 | 0.92 | 0.60 | 7.34 | |
| 59. | Cynodon radiaus Roth. | 105 | 0.39 | 0.38 | 0.40 | |
| Danthonieae | 60. | Schismus arabicus Nees | 216 | 0.80 | 0.24 | 0.83 |
| Eragrostideae | 61. | Acrachne racemosa (Heyne ex Roth) Ohwi | 132 | 0.41 | 0.31 | 1.02 |
| 62. | Aeluropus lagopoides (L.) Thwaites | 71 | 0.26 | 0.33 | 0.27 | |
| 63. | Dactyloctenium aristatum Link. | 46 | 0.17 | 0.30 | 0.17 | |
| 64. | Dactyloctenium aegyptium (L.) Wild. | 178 | 0.66 | 0.30 | 2.73 | |
| 65. | Dactyloctenium scindicum Boiss. | 98 | 0.36 | 0.25 | 0.75 | |
| 66. | Desmostachya bipinnata L. Stapf | 196 | 0.72 | 0.41 | 1.50 | |
| 67. | Eragrostis amabilis (L.) Wight & Arn. | 205 | 0.76 | 0.46 | 0.78 | |
| 68. | Eragrostis atrovirens (Desf.) Trin. ex. Steud. | 81 | 0.30 | 0.34 | 0.31 | |
| 69. | Eragrostis barrelieri Dav. | 127 | 0.47 | 0.24 | 0.48 | |
| 70. | Eragrostis ciliaris (L.) R. Br | 224 | 0.86 | 0.15 | 0.85 | |
| 71. | Eragrostis cilianensis (All.) Janch. | 148 | 0.55 | 0.14 | 0.56 | |
| 72. | Eragrostis japonica (Thunb.) Trin. | 48 | 0.18 | 0.12 | 0.72 | |
| 73. | Eragrostis pectinacea. (Michx.) Nees | 139 | 0.51 | 0.11 | 0.53 | |
| 74. | Eragrostis minor Host. | 219 | 0.81 | 0.13 | 1.68 | |
| 75. | Eragrostis pilosa (L.) P. Beauve | 52 | 0.19 | 0.22 | 0.21 | |
| 76. | Eragrostis papposa (Roem. & Schult.) Steud. | 147 | 0.57 | 0.19 | 0.56 | |
| 77. | Leptochloa panicea (Retz.) Ohwi | 39 | 0.14 | 0.21 | 0.31 | |
| 78. | Leptochloa chinensis (L.) Nees | 67 | 0.25 | 0.32 | 0.25 | |
| 79. | Eleusine indica (L.) Gaertn | 143 | 0.53 | 0.34 | 2.3 | |
| 80. | Sporobolus virginicus (L.) Kunth | 46 | 0.17 | 0.35 | 0.17 | |
| Paniceae | 81. | Sporobolus nervosa (Hocshsst.) | 145 | 0.54 | 0.28 | 0.55 |
| Eragrostideae | 82. | Dactyloctenium aristatum Link | 231 | 0.85 | 0.29 | 0.88 |
| 83. | Dactyloctenium scindicum Boiss. | 189 | 0.70 | 0.19 | 0.72 | |
| Hainardeae | 84. | Parapholis strigosa (Dum.) C. E. Hubbard | 43 | 0.16 | 0.13 | 0.16 |
| Oryzeae | 85. | Oryza sativa L. | 255 | 0.94 | 0.76 | 7.81 |
| Pappophoreae | 86. | Enneapogon shimpranus (Hochst. ex A. Rich) Renvoize | 69 | 0.25 | 0.36 | 0.264 |
| 87. | Enneapogon persicus Boiss. | 46 | 0.17 | 0.04 | 0.17 | |
| 88. | Enneapogon desvauxii P. Beauv. | 56 | 0.21 | 0.37 | 0.21 | |
| Paniceae | 89. | Digitaria nodosa Parl. | 201 | 0.74 | 0.52 | 0.77 |
| 90. | Brachiaria distachya (L.) Stapf. | 151 | 0.56 | 0.19 | 1.14 | |
| 91. | Brachiaria deflexa (Schumach.) C.E.Hubb. ex Robyns | 169 | 0.62 | 0.27 | 0.65 | |
| 92. | Brachiaria mutica (Forssk.) Stapf. | 142 | 0.52 | 0.33 | 2.26 | |
| 93. | Brachiaria eruciformis (sm) Griseb. | 159 | 0.59 | 0.26 | 0.61 | |
| 94. | Brachiaria adspersa (Trin.) Parodi | 184 | 0.68 | 0.11 | 0.70 | |
| 95. | Brachiaria ovalis Stapf | 148 | 0.55 | 0.09 | 0.56 | |
| 96. | Cenchrus biflorus Roxb. | 163 | 0.60 | 0.16 | 1.25 | |
| 97. | Cenchrus ciliaris L. | 238 | 0.69 | 0.46 | 1.82 | |
| 98. | Cenchrus prieurii (Kunth.) Marie | 173 | 0.64 | 0.27 | 0.66 | |
| 99. | Cenchrus pennisetiformis Steud. | 186 | 0.69 | 0.24 | 1.42 | |
| 100. | Cenchrus setiger Vahl. | 135 | 0.50 | 0.08 | 0.52 | |
| 101. | Digitaria pennata (Hochst.) T.Cooke | 52 | 0.19 | 0.07 | 0.21 | |
| 102. | Digitaria ciliaris (Retz.) Koeler | 147 | 0.54 | 0.42 | 0.56 | |
| 103. | Digitaria longiflora (Retz.) Pers. | 88 | 0.32 | 0.16 | 0.68 | |
| 104. | Digitaria stricta Rotch | 221 | 0.82 | 0.25 | 0.85 | |
| 105. | Digitaria radicosa (Presl) Miq. | 67 | 0.25 | 0.10 | 0.25 | |
| 106. | Digitaria sanguinalis (L.) scop. | 173 | 0.64 | 0.58 | 0.66 | |
| 107. | Digitaria setigera Roth. | 232 | 0.86 | 0.32 | 0.88 | |
| 108. | Digitaria violascens Link. | 51 | 0.19 | 0.12 | 0.21 | |
| 109. | Echinochloa colona (L.) Link | 182 | 0.67 | 0.21 | 1.38 | |
| 110. | Echinochloa crus-galli (L.) P. Beauv. | 98 | 0.36 | 0.28 | 0.74 | |
| 111. | Ochthochloa compressa (Forssk.) Hilu. | 152 | 0.56 | 0.33 | 0.58 | |
| 112. | Panicum antidotale Retz. | 191 | 0.70 | 0.73 | 0.73 | |
| 113. | Panicum atrosanguineum Hochst. Ex A. Rich | 43 | 0.26 | 0.57 | 0.16 | |
| 114. | Panicum maximum Jacq. | 139 | 0.51 | 0.61 | 2.13 | |
| 115. | Panicum turgidum Forssk | 81 | 0.30 | 0.68 | 0.31 | |
| 116. | Panicum sumatrense Roth. | 104 | 0.38 | 0.62 | 0.39 | |
| 117. | Paspalidium geminatum (Forssk.) Stapf | 93 | 0.34 | 0.44 | 0.35 | |
| 118. | Paspalidium flavidum (Retz.) A. Camus | 86 | 0.32 | 0.47 | 0.33 | |
| 119. | Paspalidium punctatum (Burm.) A. Camus | 41 | 0.15 | 0.24 | 0.15 | |
| 120. | Paspalum paspaloides (Michx.) Scribner. | 84 | 0.31 | 0.08 | 0.32 | |
| 121. | Panicum repens L. | 213 | 0.39 | 0.15 | 1.64 | |
| 122. | Pennisetum divisum (Forssk. ex J.F.Gmel.) Henrard | 94 | 0.35 | 0.22 | 0.72 | |
| 123. | Pennisetum glaucum (L.) R.Br. | 100 | 0.37 | 0.18 | 0.76 | |
| 124. | Pennisetum americanum (L.) Leeke | 38 | 0.14 | 0.09 | 0.15 | |
| 125. | Pennisetum orientale Rich | 211 | 0.78 | 0.55 | 0.81 | |
| 126. | Sporobolus ioclados (Nees. ex. Trin.) Nees. | 90 | 0.33 | 0.75 | 0.34 | |
| 127. | Paspalum dilatatum Poir. | 36 | 0.13 | 0.33 | 0.14 | |
| 128. | Setaria glauca (L.) P. Beauv | 228 | 0.84 | 0.48 | 0.87 | |
| 129. | Setaria intermedia Roem. & Schult. | 220 | 0.81 | 0.36 | 0.84 | |
| 130. | Setaria italica (L.) P.Beauv. | 97 | 0.36 | 0.24 | 0.74 | |
| 131. | Setaria pumila (Poir) Roem. & Schult. | 189 | 0.70 | 0.20 | 1.44 | |
| 132. | Setaria verticillata (L.) P. Beauv. | 167 | 0.62 | 0.23 | 1.28 | |
| 133. | Setaria viridis (L.) P. Beauv. | 241 | 0.89 | 0.16 | 0.92 | |
| 134. | Brachiaria prostrata (Lam) Griseb | 231 | 0.85 | 0.33 | 0.88 | |
| 135. | Brachiaria ramosa (L.) Stapf | 201 | 0.74 | 0.07 | 0.77 | |
| 136. | Pennisetum lansatum Klotzsch. | 160 | 0.59 | 0.06 | 0.61 | |
| 137. | Urochloa panicoides P. Beauv. | 143 | 0.53 | 0.11 | 0.55 | |
| 138. | Urochloa setigera (Retz.) Stapf. | 233 | 0.86 | 0.09 | 0.89 | |
| Poeae | 139. | Dactylis glomerata L. | 101 | 0.37 | 0.09 | 0.38 |
| 140. | Lolium temulentum L. | 35 | 0.13 | 0.68 | 0.13 | |
| 141. | Lolium persicum Boiss. & Hohen. | 119 | 0.44 | 0.39 | 0.45 | |
| 142. | Poa annua L. | 251 | 0.93 | 0.86 | 7.69 | |
| 143. | Poa infirma Kunth | 249 | 0.92 | 0.72 | 0.95 | |
| Triticeae | 144. | Hordeum vulgare L. | 107 | 0.39 | 0.83 | 0.82 |
| 145. | Triticum aestivum L. | 261 | 0.96 | 0.93 | 8.00 | |
| Zoysieae | 146. | Leptothrium senegalense (Kunth) Clayton | 154 | 0.57 | 0.25 | 0.59 |
| 147. | Tragus berteronianus Schult. | 65 | 0.24 | 0.07 | 0.25 | |
| 148. | Tragus racemosus (L.) All. | 54 | 0.20 | 0.06 | 0.21 | |
| 149. | Tragus roxburghii Panigrahi | 83 | 0.31 | 0.04 | 0.32 |
The highest CSI value was found for T. aestivum (8.00), which is extensively used in anti-cancer and gastrointestinal disorders in livestock. The other 6 species with CSI were O. sativa (0.81), P. annua (7.69), C. dactylon (7.34), A.sativa (7.33), S. officinarum (7.14) and Z. mays (6.77). These species were highly cited and preferentially used by the informants to be used in therapeutic and other purposes but also cultivated and used different kind of management tools. The preference and frequency of use and quality of medicinal use are the factors that determine the cultural importance of plants. The high CSI values of plant species also reflect the fact that more a species is available to the users of a community for medicinal purpose, it becomes more important. For instance, T. aestivum is well known herb which is mentioned in Ayurveda herbal system. It has been used in many dietary supplements and is extremely valuable for treating various ailments such as kidney malfunctioning, immune modulator, joint swelling and bacterial infection [111]. Furthermore, species reported with high cultural significance are not only important for ethnic groups of Punjab but also for the other ethnic group of the world. Therefore, these species have accumulated a lot of traditional knowledge that has been transmitted through direct experience over the time in next generations. Grasses represented with lowest CSI values were, L. temulentum (0.13), P. dilatatum (0.14), P. typhoidum (0.15), P. punctatum (0.15), A. sterilis (0.15), P. atrosanguineum (0.16), P. strigosa (0.16), T. villosus (0.16), E. persicus (0.17), S. arabicus (0.17), D. aristatum (0.17), P. fugax (0.17), and A. mutica (0.18). The results obtained in our study are in agreement with the study of Wong [112], who observed that plants which are not easily available are less popular in the community, hence have lower CSI value. Nevertheless, Albuquerque et al. [113] proposed a weak correlation between use and availability of plants and further suggested that species with greater cultural significance tend to become vulnerable or rare locally.
Relative frequency of citations (RFC) and use value (UV)
Relative frequency of citations reveals the importance of each grass among indigenous communities of Punjab, ethno-veterinary medicines and primary health care of animals to make them healthy and productive. It is calculated from the citation frequency of informants claiming the use of a plant species divided by the total number of informants who participated in the survey to share their indigenous knowledge [5]. In our work, RFC ranges 0.96 to 0.14 (Table 2).
The maximum RFC value was obtained for T. aestivum (0.96). The other high citation species and their respective values were O. sativa (0.94), P. annua (0.92), D. annulatum (0.92), C. dactylon (0.91), P. infirma (0.91) and A. sativa (0.91), S. viridis (0.88), S. officinarum (0.85), S. bengalense (0.85), U. setigera (0.85), D. setigera (0.85) and B. prostrata (0.85), C. barbata (0.84), S. glauca (0.84), S. bicolor (0.82), Z. mays (0.81), S. intermedia (0.81), S. halepense (0.80), and E. minor (0.80). These resutls highlight the fact that grasses are known to local culture for a long period of time and majority of the people in different regions of the Punjab were not fully aware of the medicinal potential of Poaceae due to thier poor educational background.
Relative frequency of citation highlights the importance of individual species among local communities based on the number of uses [114, 115]. It has been suggested that plants with high RFC values should be involved in biological, phytochemical and pharmacological studies for further investigation of drug development [56]. Furthermore, such plants must be conserved on priority basis due to the threat of over exploitation and extensive use by the community [19]. Values of RFC are very dynamic as it changes with area to area and depends on the folk knowledge of the native people. It is well known that species with low RFC values are not necessarily less important [116]. Their low values may represent the low knowledge of the local people especially the younger ones, who are not aware of the uses of these species.
We observed that plants with high RFC values depict their dominancy in the study area and indigenous people are more familiar with them. They prefer these plants over others because of their availability and positive role in traditional health system. These results are in line with the appearance hypothesis which explains that local people have greater knowledge of ethnomedicinal use on plants which are more common in an area [58]. Additionally, common plants would allow local people to gain more experience of their properties and consequently would have a greater probability of being introduced into the local culture [56].
Use value is an important index to identify the plant species which are extensively used among indigenous communities [117]. In our case, use values ranged from 0.02 to 0.93 (Table 2) and species with high UV were, T. aestivum (0.93), S. bicolor (0.91), C. citratus (0.89), A. sativa (0.86) P. annua (0.86), H. vulgare (0.83), and C. martini (0.81), and S. spontaneum (0.80). The high use values of species show their importance in the traditional medicine system [118], which can be attributed with the fact that they are the first choice of the traditional healers for the treatment of ailments and local inhabitants are familiar with these plants [58, 119]. Species with low use values were, C. dolicostachya (0.02), E. persicus (0.04), P. repens (0.05), P. lanatum (0.06) and T. racemosus (0.06). The low use value of plants may relect less distribution of species in the area and low ethnomedicinal knowledge of informants [58, 120]. On the other hand, high use citations of T. aestivum could be due to its prevelance, high nutritional values, and cheap source of forage with the additional benefit of potential medicinal properties.
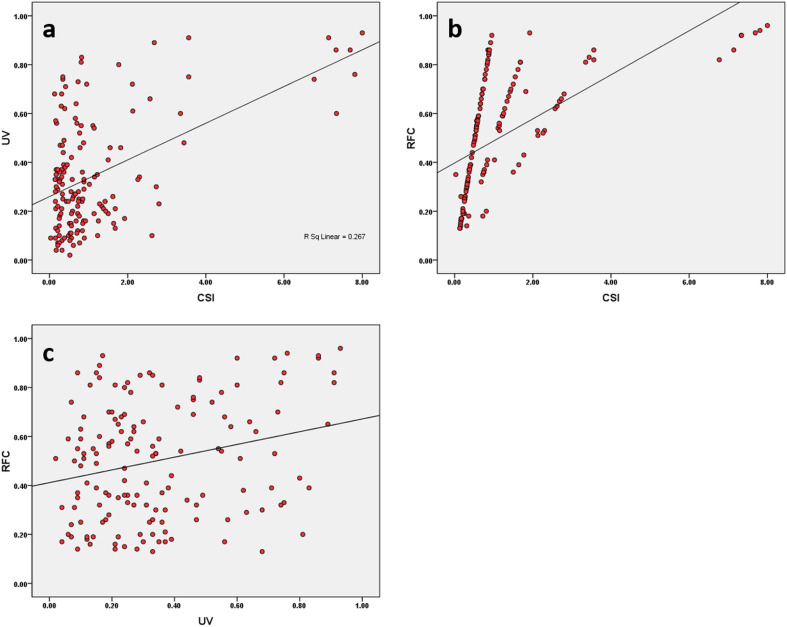
Correlation among ethnobotanical indices
The relationship among ethnobotanical indices i.e. CSI, RFC and UV were found significant at the 0.01 level (Fig 7, S2 Table). A positive correlation was present between CSI and RFC (r = 0.59**), followed by CSI and UV (r = 0.51**) and RFC and UV (r = 0.25**). The positive correlation provides evidence of cultural importance of each species and relative importance of the use of plants. The fact that these indices are strongly correlated means that their patterns across species match.
Fig 7. The scatter plot representing correlation.
a) UV vs CSI, b) RFC vs SCI, and c) UV vs RFC.
Fidelity level (FL)
The fidelity level of 24 most important species ranged from 14.3 to 100% (Table 3). Triticum aestivum and Saccharum spontaneum shows 100% fidelity level for tumor and urinary problems. Other species with high FL values were: C. jwarancusa (typhoid fever), V. zizanioides (stomach pain), S. officinarum (digestive disorders), T. tenellus (antimicrobial), D. bipinnata (digestive problems), S. bengalense (oral infections), E. minor (anti-inflammatory), O. sativa (wound healing), D. aegyptium (jaundice), P. minor (cough) and C. virgata (bone fracture) with FL 98, 94, 84, 80, 78, 76, 75, 74, 71, 71 and 70%, respectively. The high fidelity score of a species highlights the existence of a disease in the study area and utilization of plant species by the local people in order to cure it [94, 95]. Plants are important and cheap source of medicine to treat multiple health issues in animals [121]. The traditional ethno-veterinary system has played a significant role in animal production especially in the rural areas where livestock diseases are locally treated [122, 123].
Table 3. Highly utilized plant species with FL, RPL and ROP.
| Species name | Iu | NA | Major ailments | Ip | FL | RPL | ROP |
|---|---|---|---|---|---|---|---|
| Triticum aestivum | 84 | 3 | Anticancer | 84 | 100 | 1 | 100 |
| Saccharum officinarum | 90 | 3 | Digestive disorders | 76 | 84 | 1 | 84 |
| Sorghum halepense | 80 | 4 | Infectious diseases | 55 | 69 | 1 | 69 |
| Cymbopogon jwarancusa | 84 | 4 | Typhoid fever | 82 | 98 | 1 | 98 |
| Saccharum bengalense | 71 | 7 | Oral disorders | 54 | 76 | 1 | 76 |
| Saccharum spontaneum | 71 | 4 | Urinary pain | 71 | 100 | 1 | 100 |
| Oryza sativa | 68 | 3 | Diarrhea | 50 | 74 | 1 | 74 |
| Vetiveria zizanioides | 65 | 3 | Stomach pain | 61 | 94 | 1 | 94 |
| Arundo donax | 62 | 5 | Blood pressure | 37 | 60 | 0.98 | 58 |
| Bambusa glaucescens | 57 | 4 | Allergies | 30 | 53 | 0.92 | 48 |
| Phragmites karka | 53 | 2 | Nervous problems | 25 | 47 | 0.86 | 41 |
| Imperata cylindrica | 51 | 6 | Piles | 25 | 49 | 0.86 | 42 |
| Cynodon dactylon | 42 | 8 | Anemia | 24 | 57 | 0.79 | 45 |
| Setaria pumila | 34 | 2 | Oral infection | 21 | 62 | 0.74 | 46 |
| Bromus japonicus | 29 | 2 | Constipation | 17 | 59 | 0.74 | 43 |
| Sporobolus ioclados | 29 | 5 | Liver disorder | 14 | 48 | 0.66 | 32 |
| Octhochloa compressa | 21 | 4 | Kidney pain | 14 | 67 | 0.58 | 39 |
| Panicum antidotale | 19 | 2 | Antibacterial | 10 | 53 | 0.53 | 28 |
| Phalaris minor | 14 | 1 | Cough | 10 | 71 | 0.5 | 36 |
| Dactyloctenium aegyptium | 14 | 4 | Jaundice | 10 | 71 | 0.45 | 32 |
| Tetrapogon tenellus | 10 | 4 | Antimicrobial | 8 | 80 | 0.45 | 36 |
| Chloris virgata | 10 | 2 | Bon fracture | 7 | 70 | 0.41 | 29 |
| Desmostachya bipinnata | 9 | 4 | Digestive problems | 7 | 78 | 0.36 | 28 |
| Eragrostis minor | 8 | 2 | Anti-inflammatory | 6 | 75 | 0.29 | 22 |
Iu: Sum of participants who claimed the use of a grass for any purpose NA: Number of ailments treated Ip: Number of participants who reported the use of a grass for specific purpose FL: Fidelity level RPL: Relative popularity level ROP: Rank order priority.
Relative popularity of species
One hundred and forty-nine species were mentioned for different kind of diseases by 271 informants, interviewed during this study. Of these, 125 species were reported by fewer than 8 informants and therefore were excluded for further discussion. The rest of the 24 species were reported by more than 7 informants (Table 3). For species cited by 8 to 62 informants, the number of uses per species increased progressively (S1 Fig) with increasing number of informants interviewed showing positive correlation (r, 0.149). Conversely, species mentioned by more than 65 informants, the average number of uses per species did not increase with increasing number of informants. About sixteen plant species mentioned by 62 informants were grouped as unpopular whereas, eight species reported by 65 or more informants were classified as popular.
Species with high popularity level (1.0 RPL) were: T. aestivum, S. officinarum, S. halepense; C. jwarancusa, S. bengalense, S. spontaneum, O. sativa and V. zizanioides. The healing potential of each species may vary and is expressed by its FL value [36]. Rank order priority index can be used as correction factor to rank plants properly with different fidelity level [48, 49]. Out of 24 species only 9 attained 50% or above ROP values. This can be attributed to the decreasing popularity of herbal medicines in the study area. T. aestivum and S. spontaneum were reported with highest ROP value (100%), trailed by C. jwarancusa (98%), V. zizanioides (94%), S. officinarum (84%), S. bengalense (76%), O.sativa (74%), S. halepense (69%) and A. donax (58%). The rest of the species presented less than 50% of ROP values. The high popularity of these species can be attributed to their high nutritional values [4] and may be linked to the fact that local farmers are aware of these species and they frequently used them for the treatment of various ailments in the livestock. This is an agreement with similar findings of previously reported studies conducted in the same province, Punjab [4, 36]. Our study is also consistent with the findings of on the status of healing potential of medicinal plants in Palestinian area [48, 49] and medicinal plants among Bedouins communities in Negev desert [46].
Jaccard index (JI)
The Jaccard Index was performed in order to develop a relationship between this study and previously reported studies, one from other regions of Pakistan (within Pakistan) and other from outside the Pakistan. This helped in finding a novelty in uses because ethnobotanical information may vary with respect to cultural differences and ethnic origin [53, 55]. Therefore, a comprehensive research with high understanding of ethnobotanical folk knowledge is mandatory for better judgement [124], and exploration of traditional knowledge in order to find novelty in work and possible drug discovery [99, 125].
Relationship with studies from Pakistan
In comparison of our study with other studies form Pakistan, the JI ranged from 12.25 to 0.37 (Table 4). The highest JI (12.25) was found with previous report from Central Punjab [4], followed by the study conducted in Layyah, Punjab, Pakistan [125] with 5.61 JI, Swat, KPK, Pakistan [58] with 3.70 JI, Gujrat, Pakistan [126] with 3.63 JI and Hafizabad Punjab, Pakistan [36] with 3.63 JI. The lowest JI such as 0.37, 0.46, and 0.47 was recorded from Abbatobad, KPK, Pakistan [21], Hungu, Pakistan [73], and Mohmand Agency, FATA, Pakistan [51], respectively. Our study shares the greatest number of common species (53) with the study from Central Punjab, Pakistan [4] with 71.7% similar uses and 28.3% dissimilar uses (Table 4). Similarly, 11 species are common between our study and a study form Layyah, Punjab, Pakistan that reported a total of 78 species [125] with 7 species having similar uses and 5 species having dissimilar uses. 8 species, in our study, were found common to the studies reported from Gujrat, Punjab, Pakistan [126], Hafizabad, Punjab, Pakistan [36], and Swat, KPK, Pakistan [58] where a total of 88, 85 and 83 species, respectively, were recorded. Other studies from Pakistan only share 1 to 4 species common to our study.
Table 4. Comparison between this study and other studies from Pakistan.
Evidences based comp.
| Study area | Journal name | Ref. | TRS | CPBA | PPAA | PPSA | PSU | PDU | % SU | % DU | JI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| District Attock, Punjab, Paksitan | Pak. J. Bot. | [27] | 43 | 1 | 42 | 148 | 0 | 1 | 0.00 | 2.33 | 0.52 |
| Talagang, Punjab, Pakistan | Braz. J. Pharmacol. | [127] | 101 | 3 | 98 | 146 | 1 | 2 | 0.99 | 1.98 | 1.24 |
| Gujrat, Punjab, Pakistan | Ethnobotanical Leaflets | [126] | 88 | 8 | 80 | 141 | 7 | 1 | 7.95 | 1.14 | 3.63 |
| Hafizabad, Punjab, Pakistan | Plosone | [36] | 85 | 8 | 87 | 141 | 6 | 2 | 7.06 | 2.35 | 3.63 |
| Lakki Marwat KPK, Pakistan | J. Ethnopharmacol. | [118] | 62 | 3 | 59 | 146 | 1 | 2 | 1.61 | 3.23 | 1.48 |
| Central Punjab, Pakistan | J. Ethnobiol Ethnomed | [4] | 53 | 53 | 0 | 96 | 38 | 15 | 71.70 | 28.30 | 12.25 |
| Layyah, Punjab, Pakisstan | Ind. Res. J. Pharm. Sci. | [125] | 78 | 11 | 69 | 138 | 7 | 4 | 8.97 | 5.13 | 5.61 |
| Toba Tek Singh, Punjab, Pakistan | Ind. Res. J. Pharm. Sci. | [119] | 17 | 4 | 13 | 145 | 0 | 4 | 0.00 | 23.53 | 2.59 |
| Abbottabad, KPK, Pakistan | J. Ethnopharmacol. | [73] | 120 | 1 | 119 | 148 | 0 | 1 | 0.00 | 0.83 | 0.37 |
| Sawat, KPK, Pakistan | Pak. J. Bot. | [58] | 83 | 8 | 75 | 141 | 5 | 3 | 6.02 | 3.61 | 3.70 |
| Karak, KPK, Pakistan | J. Ethnopharmacol. | [128] | 46 | 3 | 43 | 146 | 3 | 0 | 6.52 | 0.00 | 1.61 |
| Hangu, KPK, Pakistan | Evid Based Comp. Alt. Med. | [129] | 67 | 1 | 66 | 148 | 0 | 1 | 0.00 | 1.49 | 0.46 |
| Tehsil Kabal, KPK, Pakistan | J. Bot | [130] | 138 | 3 | 135 | 146 | 1 | 2 | 0.72 | 1.45 | 1.07 |
| Bajaur Agency, FATA, Pakistan | J. Ethnobiol Ethnomed. | [51] | 64 | 1 | 63 | 148 | 1 | 0 | 1.56 | 0.00 | 0.47 |
Ref. References, TRS: Total reported species, CPBA: Common plants of both areas, PPAA: Plants only present in the aligned area. PPSA: Plants only present in the study area, PSU: Plants with similar uses, PDU: Plants with different uses
Relationship with studies from outside the Pakistan
From outside Pakistan, the JI ranged from 0.49 to 1.99 (Table 5). The higher JI such as 1.99, 1.97 and 1.73 was found with the studies conducted in Ogun State, Nigeria [71], Terai Forest, Western Nepal [131], and tropical regions of Nigeria [132], respectively. The lower JI such as 0.49, 0.50, 0.50 and 0.60 was found with the studies reported from Eastern Amazon, Brazil [133], Tamil Nadu India [134], Baitadi & Darchula, Nepal [135], and Bandarban, Bangladesh [55], respectively. In contrast to Pakistan, our study shares only few common species with the studies from outside the Pakistan (Table 5). [71] reported 63 species from Ogun State, Nigeria and only 4 species are common to our study with 1.95% similar uses. Similarly, 4 species are found common to the studies conducted in Tropical regions of Nigeria by [132] and Guimaras Island, Philippines by [13] who have reported 93 and 142 species with 3.23 and 0.70% similar uses, respectively. overall, the percentage similar uses ranged from 0 to 4.55% and percentage dissimilar uses ranged from 0 to 4.76%.
Table 5. Comparison between this study and other studies from outside the Pakistan.
Evidences based comp.
| Study area | Journal name | Ref. | TRS | CPBA | PPAA | PPSA | PSU | PDU | % SU | % DU | JI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tamil Nadu, India | Braz. J. Pharmacol. | [135] | 54 | 1 | 53 | 148 | 1 | 0 | 1.85 | 0.00 | 0.50 |
| Western Himalaya, India | J. Ethnobiol Ethnomed. | [136] | 78 | 2 | 76 | 147 | 1 | 1 | 1.28 | 1.28 | 0.90 |
| Bandarban, Bangladesh | Front. Pharmacol. | [55] | 159 | 1 | 9 | 147 | 0 | 1 | 0.00 | 0.63 | 0.64 |
| Baitadi & Darchula, Nepal | J. Ethnobiol Ethnomed. | [134] | 53 | 1 | 52 | 148 | 0 | 1 | 0.00 | 1.89 | 0.50 |
| Terai Forest, Western Nepal | J. Ethnobiol. Ethnomed. | [131] | 66 | 2 | 62 | 145 | 2 | 0 | 3.03 | 0.00 | 1.97 |
| Ogun State, Nigeria | Amer. J. Plant Sci. | [71] | 63 | 4 | 59 | 145 | 1 | 3 | 1.59 | 4.76 | 1.99 |
| Tropical regions of Nigeria | J. Ethnopharmacol. | [132] | 93 | 4 | 89 | 145 | 3 | 1 | 3.23 | 1.08 | 1.73 |
| Guimaras Island, Philippines | J. Ethnopharmacol. | [137] | 142 | 4 | 138 | 145 | 1 | 3 | 0.70 | 2.11 | 1.45 |
| Switzerland | J. Ethnobiol Ethnomed. | [138] | 22 | 1 | 21 | 148 | 1 | 0 | 4.55 | 0.00 | 0.60 |
| Uige, Northern Angola | J. Ethnobiol Ethnomed. | [139] | 122 | 3 | 118 | 146 | 0 | 3 | 0.00 | 2.46 | 1.14 |
| Eastern Amazon, Brazil | J. Ethnopharmacol. | [131] | 56 | 1 | 55 | 148 | 0 | 1 | 0.00 | 1.79 | 0.49 |
Ref. References, TRS: Total reported species, CPBA: Common plants of both areas, PPAA: Plants only present in the aligned area. PPSA: Plants only present in the study area, PSU: Plants with similar uses, PDU: Plants with different uses
In general, the high degree of similar type of plant uses may indicate same cultural practices among communities, and similar type of vegetation may reflect same type of floral diversity and climate in those areas [41]. It has been reported that neighboring indigenous communities share more common traditional practice of plants as medicines in order to cure various ailments and this is because of more social trade and sharing of ethnomedicinal knowledge among native groups [140, 141]. In contrast, a low similarity index indicates less sharing of medicinal knowledge and low social interaction that could have been happened in the past bringing more difference in ethnobotanical practices [51]. Geological isolation of ethnic groups and plants resulted a significant change in vegetation structure and therapeutic uses of indigenous plants and this may be a reason for loss of ethnobotanical information [44]. A low degree of similarity index of our study with other studies conducted in same province or in other parts of Pakistan indicated that either a little attention has been paid towards grasses in these studies and zero (0) percent similarity index show that grasses were totally ignored. Therefore, this study represents the first comprehensive report on ethno-veterinary knowledge of indigenous grasses of the whole province of Punjab.
Novelty in ethno-veterinary uses
Therapeutic uses of plant species of family Poaceae were compared with previous reports from different parts of the Pakistan and with other studies conducted in the neighboring areas including India, Bangladesh and Nepal to find out the novelty index. The data shows significant differences in the usage of plant species and their parts used to treat health disorders in animals. Out of 149 species, ethnomedicinal uses of 12 plant species such as C. zizanioides (anti-inflammatory), P. lanatum (improve bull fertility), C. citratus (glandular secretion), S. saccharatum and T. triandra (malaria), A. funiculate (anticancer), K. argentia (skin allergies), T. villosus (antibacterial), C. radiates (eyes infection), S. nervosa (Jaundice), E. persicus (antifungal), and P. repens (dysfunctional cattle organs) have rarely been reported so far from this region and rest of the world. It is noteworthy that most of these plants have not been explored pharmacologically. Therefore, they could be used for in-depth phytochemical screening and bioactivity assays in order to validate their traditional uses. Moreover, less familiar P. annua, D. annulatum, P. infirma, S. viridis, S. bengalense, U. setigera, D. setigera, B. prostrata, C. barbata, S. glauca, S. intermedia and E. minor with high citations should also be investigated in detail.
Conclusion
The present study is the first comprehensive report with emphasis on the use of Poaceae in traditional ethno-veterinary medicine. A total of 149 species with therapeutic uses were reported, which represents new bioresources for pharmacological studies and drug discovery. Our study revealed that ethnobotanical knowledge of Poaceae is less prevailing in the rural areas of Punjab compared to other medicinal plants. Therefore, ethnomedicinal applications of the members of this family should be reported from other parts of the country, which would be helpful in conserving precious medicinal plant knowledge and its application in novel drug discovery.
Supporting information
(DOCX)
Correlation analysis between a) UV vs CSI, b) RFC vs SCI, and c) UV vs RFC.
(DOC)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Geng Y, Hu G, Ranjitkar S, Wang Y, Bu D, Pei S, et al. Prioritizing fodder species based on traditional knowledge: a case study of mithun (Bos frontalis) in Dulongjiang area, Yunnan Province, Southwest China. Journal of Ethnobiology and Ethnomedicine. 2017; 13: 24–38. 10.1186/s13002-017-0153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunes AT, Lucena RF, dos Santos MV, Albuquerque UP. Local knowledge about fodder plants in the semi-arid region of Northeastern Brazil. Journal of Ethnobiology and Ethnomedicine. 2015; 11: 12–23. 10.1186/1746-4269-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matlebyane MM. Relationship between chemical composition, in vitro digestibility and locally based feeding value rankings and medicinal use of some common forages for ruminant livestock in three area of the Capricon of limpopo Province, South Africa (Doctoral Dissertation, MSc Dissertation). School of Agricultural and Environmental Sciences, Faculty of Sciences, Health and Agriculture. University of Limpopo, South Africa: p.108. [Google Scholar]
- 4.Harun N, Chaudhry AS, Shaheen S, Ullah K, Khan F. Ethnobotanical studies of fodder grass resources for ruminant animals, based on the traditional knowledge of indigenous communities in Central Punjab Pakistan. Journal of Ethnobiology and Ethnomedicine. 2017; 13–56. 10.1186/s13002-017-0138-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Njoroge GN, Bussmann RW. Herbal usage and informant consensus in ethnoveterinary management of cattle diseases among the Kikuyus (Central Kenya). Journal of ethnopharmacology. Journal of Ethnopharmacology. 2006; 108: 332–339. 10.1016/j.jep.2006.05.031 [DOI] [PubMed] [Google Scholar]
- 6.Pieroni A, Giusti ME, De Pasquale C, Lenzarini C, Censorii E, Gonzáles-Tejero MR, et al. Circum-Mediterranean cultural heritage and medicinal plant uses in traditional animal healthcare: a field survey in eight selected areas within the RUBIA project. Journal of Ethnobiology and Ethnomedicine. 2006; 2–16. 10.1186/1746-4269-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lans C, Turner N, Khan T, Brauer G, Boepple W. Ethnoveterinary medicines used for ruminants in British Columbia, Canada. Journal of Ethnobiology and Ethnomedicine. 2007; 3–11. 10.1186/1746-4269-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGaw LJ, Eloff JN. Ethnoveterinary use of southern African plants and scientific evaluation of their medicinal properties. Journal of Ethnopharmacology. 2008; 119: 559–574. 10.1016/j.jep.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 9.Farooq Z, Iqbal Z, Mushtaq S, Muhammad G, Iqbal MZ, Arshad M. Ethnoveterinary practices for the treatment of parasitic diseases in livestock in Cholistan desert (Pakistan). Journal of Ethnopharmacology. 2008; 118: 213–219. 10.1016/j.jep.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 10.Monteiro MV, Bevilaqua CM, Palha MD, Braga RR, Schwanke K, Rodrigues ST, et al. Ethnoveterinary knowledge of the inhabitants of Marajó Island, Eastern Amazonia, Brazil. Acta Amazon. 2011; 41: 233–242. [Google Scholar]
- 11.Martínez GJ, Luján MC. Medicinal plants used for traditional veterinary in the Sierras de Córdoba (Argentina): an ethnobotanical comparison with human medicinal uses. Journal of Ethnobiology and Ethnomedicine. 2011; 7–23. 10.1186/1746-4269-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma R, Manhas RK, Magotra R. Ethnoveterinary remedies of diseases among milk yielding animals in Kathua, Jammu and Kashmir, India. Journal of Ethnopharmacology. 2012; 141: 265–272. 10.1016/j.jep.2012.02.027 [DOI] [PubMed] [Google Scholar]
- 13.Offiah NV, Makama S, Elisha IL, Makoshi MS, Gotep JG, Dawurung CJ, et al. Ethnobotanical survey of medicinal plants used in the treatment of animal diarrhoea in Plateau State, Nigeria. BMC Veterinary Research. 2011; 7–36. 10.1186/1746-6148-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benítez G, González-Tejero MR, Molero-Mesa J. Knowledge of ethnoveterinary medicine in the Province of Granada, Andalusia, Spain. Journal of Ethnopharmacology. 2012; 139: 429–439. 10.1016/j.jep.2011.11.029 [DOI] [PubMed] [Google Scholar]
- 15.Winter K, McClatchey W. Quantifying evolution of cultural interactions with plants: implications for managing diversity for resilience in social-ecological systems. Functional Ecosystem and Communities. 2008; 2(1): 1–10. [Google Scholar]
- 16.Babu RH, Savithramma N. Phytochemical screening of underutilized species of Poaceae. An International Journal. 2013; 1(10): 947–951. [Google Scholar]
- 17.Anyasor GN, Ogunwenmo O, Oyelana OA, Akpofunure BE. Phytochemical constituents and antioxidant activities of aqueous and methanol stem extracts of Costus afer Ker Gawl.(Costaceae). African Journal of Biotechnology. 2010; 9(31): 4880–4884. [Google Scholar]
- 18.Nunes AT, Lucena RF, dos Santos MV, Albuquerque UP. Local knowledge about fodder plants in the semi-arid region of Northeastern Brazil. Journal of Ethnobiology and Ethnomedicine. 2015; 11(1): 12–23. 10.1186/1746-4269-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Church DC. The Ruminant animals. In: Digestive physiology and nutrition. Prospect Heights, IL: Waveland Press; 1993; 33. [Google Scholar]
- 20.Provenza FD, Villalba JJ, Dziba LE, Atwood SB, Banner RE. Linking herbivore experience, varied diets, and plant biochemical diversity. Small Ruminant Research. 2003; 49(3): 257–74. [Google Scholar]
- 21.Kayani SA, Masood AY, Achakzai AK, Anbreen S. Distribution of secondary metabolites in plants of Quetta-Balochistan. Pakistan Journal of Botany. 2007; 39: 1179–1179. [Google Scholar]
- 22.Okoli IC, Ebere CS, Uchegbu MC, Udah CA, Ibeawuchi II. A survey of the diversity of plants utilized for small ruminant feeding in south-eastern Nigeria. Agriculture, Ecogystems and Environment. 2003; 96(1): 147–154. [Google Scholar]
- 23.Nahed J, Villafuerte L, Grande D, Pérez-Gil F, Alemán T, Carmona J. Fodder shrub and tree species in the Highlands of southern Mexico. Animal Feed Science and Technology. 1997; 68(3): 213–223. [Google Scholar]
- 24.Stur WW, Ibrahim T, Tuhulele M, Binh LH, Gabunada F, Nakamanee IG, et al. Adaptation of forages to climate, soils and use in smallholder farming systems in Southeast Asia. InACIAR PROCEEDINGS 2000. (pp. 112–119). ACIAR; 1998. [Google Scholar]
- 25.Ahmad F, Hameed M, Ashraf M, Ahmad M, Khan A, Nawaz T, et al. Role of leaf epidermis in identification and differentiation of grasses in tribe Chlorideae (Poaceae) from Pakistan. Journal of Medicinal Plant Research. 2012; 6(10): 1955–1960. [Google Scholar]
- 26.Arshad M, Nisar MF, Majeed A, Ismail S, Ahmad M. Ethnomedicinal flora in district Sialkot, Punjab, Pakistan. Middle East Journal of Scientific Research. 2011; 9(2): 209–214. [Google Scholar]
- 27.Noor MJ, Kalsoom U. Ethnobotanical studies of selected plant species of Ratwal village, district Attock, Pakistan. Pakistan Journal of Botany. 2011; 43(2): 781–786. [Google Scholar]
- 28.Ikram S, Bhatti KH, Parvaiz M. Ethnobotanical studies of aquatic plants of district Sialkot, Punjab (Pakistan). Journal of Medicinal Plant Research. 2014; 2:58–63. [Google Scholar]
- 29.Mahmood A, Mahmood A, Malik RN, Shinwari ZK. Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. Journal of Ethnopharmacology. 2013; 148(2): 714–723. 10.1016/j.jep.2013.05.035 [DOI] [PubMed] [Google Scholar]
- 30.Parvaiz M. Ethnobotanical studies on plant resources of mangowal, district Gujrat, Punjab, Pakistan. Avicenna Journal of Phytomedicine. 2014; 4(5):364–370. [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi R, Maqsood MU, Arshad MU, Chaudhry AK. Ethnomedicinal uses of plants by the people of Kadhi areas of Khushab, Punjab, Pakistan. Pak J Bot. 2011; 1; 43(1):121–133. [Google Scholar]
- 32.Qureshi R, Waheed A, Arshad MU, Umbreen T. Medico-ethnobotanical inventory of tehsil Chakwal, Pakistan. Pakistan Journal of Botany. 2009; 41(2): 529–538. [Google Scholar]
- 33.Sardar AA, Khan Z, Perveen A, Zereen A. Appraisal of ethnobotanical uses of the wetland plants of Punjab, Pakistan. African Journal of Traditional, complementary and Alternative Medicines. 2015; 12: 9–13. [Google Scholar]
- 34.Choudhury PR, Choudhury MD, Ningthoujam SS, Das D, Nath D, Talukdar AD. Ethnomedicinal plants used by traditional healers of North Tripura district, Tripura, North East India. Journal of Ethnopharmacology. 2015; 66: 135–148. [DOI] [PubMed] [Google Scholar]
- 35.Rehman F, Sajjad A, Mengal MA, Taj MK, Mengal MA, Mengal MH, et al. Antimicrobial activity of selected indigenous medicinal herbs against human pathogenic bacteria. Pure Applied Biology. 2017; 6(2): 740–747. [Google Scholar]
- 36.Umair M, Altaf M, Abbasi AM. An ethnobotanical survey of indigenous medicinal plants in Hafizabad district, Punjab-Pakistan. PLOS One. 2017; 1–22. 10.1371/journal.pone.0177912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zereen A, Khan Z. A survey of ethnobotanically important trees of Central Punjab, Pakistan. Biologia (Pakistan). Biologia (Pakistan). 2012; 58; (1 & 2):21–30. [Google Scholar]
- 38.Zareen A, Khan Z, Ajaib M. Ethnobotanical evaluation of the shrubs of Central Punjab, Pakistan. Biologia (Pakistan). 2013; 59(1): 139–147. [Google Scholar]
- 39.Heinrich M, Edwards S, Moerman DE, Leonti M. Ethnopharmacological field studies: a critical assessment of their conceptual basis and methods. Journal of Ethnopharmacology. 2009; 124: 1–17. 10.1016/j.jep.2009.03.043 [DOI] [PubMed] [Google Scholar]
- 40.Martin GJ. Ethnobotany-A People and Plants conservation manual. 1995; pp. 268. [Google Scholar]
- 41.Ahmad R, Ahmad N, Naqvi AA, Shehzad A, Al-Ghamdi MS. Role of traditional Islamic and Arabic plants in cancer therapy. Journal of Traditional and Complementary Medicine. 2017; 7(2): 195–204. 10.1016/j.jtcme.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards SV, Kingan SB, Calkins JD, Balakrishnan CN, Jennings WB, Swanson WJ, et al. Speciation in birds: genes, geography, and sexual selection. Proc. National Academy of Science USA. 2005; 102: 6550–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali SI, Qaiser M. (Eds.). Flora of Pakistan Karachi. 1993–2015. Nos. 194–220.
- 44.Jain SK, Rao RR. A Handbook of Field and Herbarium Methods. Today and Tomorrow Printers and Publishers, New Delhi: 1977. [Google Scholar]
- 45.Alexiades M. (ed). Selected Guidelines for Ethnobotanical Research: A Field Manual. Advances in Economic Botany, vol.10 The New York Botanical Garden, Bronx: 1996. [Google Scholar]
- 46.da Silva M, Moraes AM, Nishikawa MM, Gatti MJ, de Alencar MV, Brandão LE, et al. Inactivation of fungi from deteriorated paper materials by radiation. International Biodeterioration & Biodegradation. 2006; 57(3): 163–167. [Google Scholar]
- 47.Friedman J, Yaniv Z, Dafni A, Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. Journal of Ethnopharmacology. 1986; 16: 275–287. 10.1016/0378-8741(86)90094-2 [DOI] [PubMed] [Google Scholar]
- 48.Ali-Shtayeh MS, Yaniv Z, Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. Journal of Ethnopharmacology. 2000; 73: 221–232. 10.1016/s0378-8741(00)00316-0 [DOI] [PubMed] [Google Scholar]
- 49.Giday M, Asfaw Z, Woldu Z, Teklehaymanot T. Medicinal plant knowledge of the Bench ethnic group of Ethiopia: an ethnobotanical investigation. Journal of Ethnobiology and Ethnomedicine. 2009; 5: 34 10.1186/1746-4269-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aziz MA, Khan AH, Adnan M, Ullah H. Traditional uses of medicinal plants used by Indigenous communities for veterinary practices at Bajaur Agency, Pakistan. Journal of Ethnobiology and Ethnomedicine. 2018; 14(1): 11 10.1186/s13002-018-0212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: Healers' consensus and cultural importance. Social Science and Medicine. 1998; 47(11): 1859–1871. 10.1016/s0277-9536(98)00181-6 [DOI] [PubMed] [Google Scholar]
- 52.Leonti M, Cabras S, Weckerle CS, Solinas MN, Casu L. The causal dependence of present plant knowledge on herbals—contemporary medicinal plant use in Campania (Italy) compared to Matthioli (1568). Journal of Ethnopharmacology. 2010; 130(2): 379–391. 10.1016/j.jep.2010.05.021 [DOI] [PubMed] [Google Scholar]
- 53.Bano A, Ahmad M, Hadda TB, Saboor A, Sultana S, Zafar M, et al. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. Journal of Ethnobiology and Ethnomedicine. 2014; 10: 43–71. 10.1186/1746-4269-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faruque MO, Uddin SB, Barlow JW, Hu S, Dong S, Cai Q, et al. Quantitative ethnobotany of medicinal plants used by indigenous communities in the Bandarban District of Bangladesh. Frontier in Pharmacology. 2018; 6(9): 40 10.3389/fphar.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah A, Rahim S. Ethnomedicinal uses of plants for the treatment of malaria in Soon Valley, Khushab, Pakistan. Journal of Ethnopharmacology. 2017; 200: 84–106. 10.1016/j.jep.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 56.Zia-ud-Din S, Zafar I, Khan MN, Jonsson NN, Muhammad S. Documentation of ethnoveterinary practices used for treatment of different ailments in a selected hilly area of Pakistan. International Journal of Agriculture and Biology. 2010; 12: 353–358. [Google Scholar]
- 57.Khan MA, Khan MA, Hussain M, Ghulam GM. An ethnobotanical inventory of himalayan region Poonch Valley Azad Kashmir (Pakistan). Ethnobotany Research and Application. 2010; 8: 107–123. [Google Scholar]
- 58.Tariq A, Mussarat S, Adnan M, AbdElsalam NM, Ullah R, Khan AL. Ethnoveterinary study of medicinal plants in a tribal society of Sulaiman range. Science World Journal. 2014; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abbasi AM, Khan MA, Khan N, Shah MH. Ethnobotanical survey of medicinally important wild edible fruits species used by tribal communities of Lesser Himalayas-Pakistan. Journal of Ethnopharmacology. 2013; 148(2): 528–536. [DOI] [PubMed] [Google Scholar]
- 60.Nisar MF, Jaleel F, Haider SM, Toor Y, Ismail S, Arfan M, et al. Exploration of ethno-medicinal plants and their ritual uses in Bahawalnagar, Pakistan. Middle East Journal of Scientific Research. 2014; 21(9): 1466–1471. [Google Scholar]
- 61.Ettebong EO, Nwafor PA, Okokon JE. In vivo antiplasmodial activities of ethanolic extract and fractions of Eleusine indica. Asian Pacific Journal of Tropical Medicine. 2012; 5:673–676. 10.1016/S1995-7645(12)60105-9 [DOI] [PubMed] [Google Scholar]
- 62.Shah GM, Ahmad M, Arshad M, Khan MA, Zafar M, Sultana S. Ethno-phyto-veterinary medicines in northern Pakistan. Journal of Animal and Plant Sciences. 2012; 22(3): 791–797. [Google Scholar]
- 63.Kumar V, Rauf FB, Sulthana B, Satish MK, Mangilal T. Evaluation of antimicrobial activity of ethanolic extract of Dactyloctenium aegyptium. International Journal of Pharmaceutical Research. 2015; 5(12): 338–343 [Google Scholar]
- 64.Tefera BN, Kim YD. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2019; 15–25. 10.1186/s13002-019-0292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mishra N, Panda T, Singh RR. Phototherapeutic Veterinary Practice in Kendrapara, District, Odisha, India. International Journal of Science and Nature. 2015; 6(2): 228–237. [Google Scholar]
- 66.Ahmad F, Khan MA, Ahmad M, Zafar M, Mahmood T, Jabeen A, et al. Ethnomedicinal uses of grasses in salt range region of northern Pakistan. Journal of Medicinal Plant Research. 2010; 4(5): 362–369. [Google Scholar]
- 67.Ahmad F, Khan MA, Ahmad M, Zafar M, Nazir A, Marwat SK. Taxonomic studies of grasses and their indigenous uses in the salt range area of Pakistan. African Journal of Biotechnology. 2009; 8(2): 231–249. [Google Scholar]
- 68.Tomar A. Folk medicinal uses of plant roots from Meerut district, Uttar Pradesh. Indian Journal of Traditional Knowledge. 2009; 8(2): 298–301. [Google Scholar]
- 69.Khan ZR, Chiliswa P, Ampong-Nyarko K, Smart LE, Polaszek A, Wandera J, et al. Utilisation of wild gramineous plants for management of cereal stemborers in Africa. Insect Science and Its Application. 1997. 17 (1): 143–150 [Google Scholar]
- 70.Shosan LO, Fawibe OO, Ajiboye AA, Abeegunrin TA, Agboola DA. Ethnobotanical survey of medicinal plants used in curing some diseases in infants in Abeokuta South Local Government Area of Ogun State, Nigeria. American Journal of Plant Science. 2014; 5: 3258–3268. [Google Scholar]
- 71.Amjad MS, faisal Qaeem M, Ahmad I, Khan SU, Chaudhari SK, Malik NZ, et al. Descriptive study of plant resources in the context of the ethnomedicinal relevance of indigenous flora: A case study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLOS one. 2017; 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kayani S, Ahmad M, Zafar M, Sultana S, Khan MP, Ashraf MA, et al. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies-Abbottabad, Northern Pakistan. Journal of Ethnopharmacology. 2014; 156, 47–60. 10.1016/j.jep.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 73.Prasad SK, Jain D, Kumar M, Hemalatha S. Antioxidant and antibacterial potential of different fractions from roots of Eriosema chinense Vogel. Journal of Pharmaceutical Research. 2013; 3: 135–146. [Google Scholar]
- 74.Saha S, Anisuzzman M, Islam MK, Mondal H, Talukder C. Antibacterial and cytotoxic potential of Dalbergia spinosa Roxb. leaves. International Journal of Pharmaceutical Science and Research. 2013. 4(1): 512–515. [Google Scholar]
- 75.Sushama B, Nishteswar K. Review of anti-oxidant herbal drugs WSR to madhuraskandha (charakasamhita). International Journal of Ayurvedic Herbal Medicine. 2014; 4:1480–1493. [Google Scholar]
- 76.Lapuz AM, Arabiran RD, Sembrano TM, Albaniel JR, Paet JC, Maini HA. Preformulation and Evaluation of Antibacterial and Anti-Inflammatory Activities of Saccharum spontaneum Linne Root Extract Cream. International Journal of Chemical Engineering and Applications. 2016; 7 (3): 204–208. [Google Scholar]
- 77.Sher H, Hussain F, Mulk S, Ibrar M. Ethnoveternary plants of Shawar Valley, District Swat, Pakistan. Pakistan Journal of Plant Science. 2004; 10(1): 35–40. [Google Scholar]
- 78.Iqbal Z, Jabbar A, Akhtar MS, Muhammad G, Lateef M. Possible role of ethnoveterinary medicine in poverty reduction in Pakistan: use of botanical anthelmintics as an example. Journal of Agriculture and Social Sciences. Journal of Agriculture and Social Science. 2005. 1 (2): 187–195. [Google Scholar]
- 79.Muhammad F, Monteiro-Riviere NA, Riviere JE. Comparative in vivo toxicity of topical JP-8 jet fuel and its individual hydrocarbon components: identification of tridecane and tetradecane as key constituents responsible for dermal irritation. Toxicological Pathology. 2005. 33(2): 258–66. 10.1080/01926230590908222 [DOI] [PubMed] [Google Scholar]
- 80.Jabbar A, Raza MA, Iqbal Z, Khan MN. An inventory of the ethnobotanicals used as anthelmintics in the southern Punjab (Pakistan). Journal of Ethnopharmacology. 2006. 108(1): 152–154. [DOI] [PubMed] [Google Scholar]
- 81.Tipu MA, Akhtar MS, Anjum MI, Raja ML. New dimension of medicinal plants as animal feed. Pakistan Veterinary Journal. 2006. 26 (3): 144–148. [Google Scholar]
- 82.Dilshad SM, Iqbal Z, Muhammad G, Iqbal A, Ahmed N. An inventory of the ethnoveterinary practices for reproductive disorders in cattle and buffaloes, Sargodha district of Pakistan. Journal of Ethnopharmacology. 2008; 117(3): 393–402. [DOI] [PubMed] [Google Scholar]
- 83.Deeba F. 2009. a. Documentation of Ethno-veterinary Practices in Urban and Periurban Areas of Faisalabad (Pakistan) Doctoral dissertation. University of Agriculture, Faisalabad. [Google Scholar]
- 84.Deeba F, Muhammad G, Iqbal Z, Hussain, I. 2009. b. Survey of ethno-veterinary practices used for different ailments in dairy animals in peri-urban areas of Faisalabad (Pakistan). International Journal of Agriculture and Biology. 11 (5): 535–541. [Google Scholar]
- 85.Khan FM. Ethno-veterinary medicinal usage of flora of Greater Cholistan desert (Pakistan). Pakistan Veterinary Journal. 2009; 29(2): 75–80. [Google Scholar]
- 86.Dilshad SR, Rehman NU, Ahmad N, Iqbal A. Documentation of ethnoveterinary practices for mastitis in dairy animals in Pakistan. Pakistan Veterinary Journal. 2010; 30(3): 167–171. [Google Scholar]
- 87.Raziq A, de Verdier K, Younas M. Ethnoveterinary treatments by dromedary camel herders in the Suleiman Mountainous Region in Pakistan: an observation and questionnaire study. Journal of Ethnobiology and Ethnomedicine. 2010; 6(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.ul Islam M, Anwar Z, Tabassum S, Khan SA, Zeb AC, Abrar M, et al. Plants of Ethno-Veterinary Uses of Tunglai Mountain Baffa Mansehra, Pakistan. International Journal of Veterinary and Animal Advances. 2012; 4(3): 221–224. [Google Scholar]
- 89.Sindhu ZU, Ullah S, Rao ZA, Iqbal Z, Hameed M. Inventory of ethno-veterinary practices used for the control of parasitic infections in district Jhang, Pakistan. International Journal of Agriculture and Biology. 2012; 1(6): 922–928. [Google Scholar]
- 90.Khan MA, Khan MA, Hussain M. Ethno veterinary medicinal uses of plants of Poonch Valley Azad Kashmir. Pakistan Journal of Weed Science Research. 2012; 18(4), 495–507. [Google Scholar]
- 91.Akhtar N, Rashid A, Murad W, Bergmeier E. Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. Journal of Ethnobiology and Ethnomedicine. 2013; 9(1): 25 10.1186/1746-4269-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed MJ, Murtaza G. A study of medicinal plants used as ethnoveterinary: harnessing potential phytotherapy in Bheri, district Muzaffarabad (Pakistan). Journal of Ethnopharmacology. 2015; 15: 209–214. [DOI] [PubMed] [Google Scholar]
- 93.Hassan H, Murad W, Tariq A, Ahmad A. Ethnoveterinary study of medicinal plants in Malakand Valley, District Dir (Lower), Khyber Pakhtunkhwa, Pakistan. Irish Veterinary Research. 2014; 67(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mussarat S, Amber R, Tariq A, Adnan M, AbdElsalam NM, Ullah R, et al. Ethnopharmacological assessment of medicinal plants used against livestock infections by the people living around Indus river. BioMed Research International. 2014; 1–14. 10.1155/2014/616858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akhtar MS, Iqbal Z, Khan MN, Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Ruminant Research. 2000; 38(2): 99–107. [Google Scholar]
- 96.Iqbal Z, Khan MN, Qudoos A. Parasitic research on domesticated animals of Pakistan. University Grants Commission; 2002. [Google Scholar]
- 97.Lateef M, Iqbal Z, Khan MN, Akhtar MS, Jabbar A. Anthelmintic activity of Adhatoda vesica roots. International Journal of Agriculture and Biology. 2003; 5(1): 86–90. [Google Scholar]
- 98.Iqbal Z, Lateef M, Ashraf M, Jabbar A. Anthelmintic activity of Artemisia brevifolia in sheep. Journal of Ethnopharmacology. 2004; 1: 265–268. [DOI] [PubMed] [Google Scholar]
- 99.Bachaya HA, Iqbal Z, Khan MN, Jabbar A. Anthelmintic activity of Ziziphus nummularia (bark) and Acacia nilotica (fruit) against Trichostrongylid nematodes of sheep. Journal of Ethnopharmacology. 2009; 22(2): 325–329. [DOI] [PubMed] [Google Scholar]
- 100.Abubakar S. Ethno veterinary medicine in Orma land-Kenya. Center for Tropical Veterinary Medicine. UK (MSc Dissertation): University of Edinburgh;1999. [Google Scholar]
- 101.Alawa JP, Jokthan GE, Akut K. Ethnoveterinary medical practice for ruminants in the subhumid zone of northern Nigeria. Preventive Veterinary Medicine. 2002; 54(1): 79–90. 10.1016/s0167-5877(01)00273-2 [DOI] [PubMed] [Google Scholar]
- 102.Matekaire T, Bwakura TM. Ethnoveterinary medicine: a potential alternative to orthodox animal health delivery in Zimbabwe. International Journal of Applied Research in Veterinary. 2004; 4: 269–273. [Google Scholar]
- 103.Somvanshi R. Veterinary medicine and animal keeping in ancient India. Asian Agriculture History. 2006; 2: 133–146. [Google Scholar]
- 104.Shen S, Qian J, Ren J. Ethnoveterinary plant remedies used by Nu people in NW Yunnan of China. Journal of Ethnobiology and Ethnomedicine. 2010; 6(1): 24 10.1186/1746-4269-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lans C, Brown G. Observations on ethnoveterinary medicines in Trinidad and Tobago. International Journal of Applied Research in Veterinary Medicine. 1998; 2:125–142. 10.1016/s0167-5877(97)00055-x [DOI] [PubMed] [Google Scholar]
- 106.Lans C, Turner N. Organic parasite control for poultry and rabbits in British Columbia, Canada. Journal of Ethnobiology and Ethnomedicine. 2011; 7(1): 21 10.1186/1746-4269-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Souto WM, Mourao JS, Barboza RR, Mendonca LE, Lucena RF, Confessor MV, et al. Medicinal animals used in ethnoveterinary practices of the'Cariri Paraibano', NE Brazil. Journal of Ethnobiology and Ethnomedicine. 2011; 7(1):30 10.1186/1746-4269-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turner NJ. “The importance of a rose”: evaluating the cultural significance of plants in Thompson and Lillooet Interior Salish. American Anthropologist. 1988; 90(2): 272–290. [Google Scholar]
- 109.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009; 16(3): 259–266. 10.1016/j.ccr.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panwar P, Du X, Sharma V, Lamour G, Castro M, Li H, et al. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. Journal of Biological Chemistry. 2013; 8: 5940–5950. 10.1074/jbc.M112.419689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wong JL. The biometrics of non-timber forest product resource assessment: a review of current methodology. Departmetnt for International Development (DFID) 2000. [Google Scholar]
- 112.Albuquerque UP, Andrade LD, Silva AC. Use of plant resources in a seasonal dry forest (Northeastern Brazil). Acta Botânica Brasílica. 2005; 19(1): 27–38. [Google Scholar]
- 113.Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds, MSJ. Ethnobotanical study of some Ghanaian anti-malarial plants. Journal of Ethnopharmacology. 2005; 99: 273–279. 10.1016/j.jep.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 114.Vitalini S, Iriti M, Puricelli C, Ciuchi D, Segale A, Fico G. Traditional knowledge on medicinal and food plants used in ValSan Giacomo (Sondrio, Italy) an alpine ethnobotanical study. Journal of Ethnopharmacology. 2013; 145:517–529. 10.1016/j.jep.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 115.Camou-Guerrero A, Reyes-Garcí V, Martí nez-Ramos M, Casas A. Knowledge and use value of plant species in a Rará muri community: a gender perspective for conservation. Human Ecology. 2008; 36: 259–272. [Google Scholar]
- 116.Vendruscolo G, Mentz A. Ethnobotanical survey of the medicinal plants used by the community of Ponta Grossa neighborhood, Porto Alegre, Rio Grande doSul, Brazil. Iheringia Serie Botanica. 2006, 61: 83–103. [Google Scholar]
- 117.Ullah S, Khan MR, Shah NA, Shah SA, Majid M, Farooq MA. Ethnomedicinal plant use value in the Lakki Marwat District of Pakistan. Journal of Ethnopharmacology. 2014; 158: 412–422. 10.1016/j.jep.2014.09.048 [DOI] [PubMed] [Google Scholar]
- 118.Choudhary BA, Aslam MS, Ijaz AS, Uzair M, Awan AJ, Khan TR. Survey of Ethno-Medicinal Weeds of District Toba Tek Singh, Punjab, Pakistan. Indian Research Journal of Pharmacy and Science. 2014; 2: 124–132. [Google Scholar]
- 119.Macuja JCO, Ruedas L.N, Espa˜na R.C. Utilization of cellulose from Luffacylindrica fiber as binder in acetaminophen tablets. Advance Environmantal Chemistry. 2015; 1–8. [Google Scholar]
- 120.Moreki JC. Documentation of ethnoveterinary practices used in family poultry in Botswana. Veterinary World. 2013; 1: 18–21. [Google Scholar]
- 121.Pieroni A, Rexhepi B, Nedelcheva A, Hajdari A, Mustafa B, Kolosova V, et al. One century later: the folk botanical knowledge of the last remaining Albanians of the upper Reka Valley, Mount Korab Western Macedonia. Journal of Ethnopharmacology. 2013; 9(1): 1–22. 10.1186/1746-4269-9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Sanzo P, Laura D, M, Emilia M, Vincenzo DF. Medicinal and useful plants in the tradition of Rotonda, Pollino National Park, Southern Italy. Journal of Ethnobiology and Ethnomedicine. 2013; 9: 1–19. 10.1186/1746-4269-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rahman A, Margaret L, Sarawat R, Nauruj J, Hashima E, Nasreen. Community perceptions of behaviour change communication interventions of the maternal neonatal and child health programme in rural Bangladesh: an exploratory study. BMC Health Services Research. 2016; 16: 389 10.1186/s12913-016-1632-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ladio AH, Lozada M, Weigandt M. Comparison of traditional wild plant knowledge between aboriginal communities inhabiting arid and forest environments in Patagonia, Argentina. Journa of Arid Environment. 2007; 69: 695–715. [Google Scholar]
- 125.Hussain M, Muhammad W, Muhammad SA, Muhammad SA, Awang SM. Ethnomedicinal survey of plants of Thal Desert Choubara District Layyah, Punjab, Pakistan. Indian Research Journal of Pharmacy and Science. 2015; 6: 235–250. [Google Scholar]
- 126.Hussain K, Nisar MF, Majeed A, Nawaz K, Bhatti KH. Ethnomedicinal survey for important plants of Jalalpur Jattan, district Gujrat, Punjab. Pakistan. Ethnobotanical Leaflets. 2010; 14(7): 807–825. [Google Scholar]
- 127.126. Rehman MN, Ahmad M, Sultana S, Zafar M, Edwards S. Relative popularity level of medicinal plants in Talagang, Punjab Province, Pakistan. Revista Brasileira de Farmacognosia. 2017; 6:751–75. [Google Scholar]
- 128.Khattak NS, Nouroz F, Ur Rahman I, Noreen S. Ethnoveterinary uses of medicinal plants of district Karak, Pakistan. Journal of Ethnopharmacology. 2015; 171: 273–279. 10.1016/j.jep.2015.05.048 [DOI] [PubMed] [Google Scholar]
- 129.Khan I, AbdElsalam NM, Fouad H, Tariq A, Ullah R, Adnan M. Application of ethnobotanical indices on the use of traditional medicines against common diseases. Evidence-based Complementary and Alternative Medicine. 2014; 21: 10.1155/2014/635371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahmad I, Ibrar M, Ali N. Ethnobotanical study of Tehsil Kabal, Swat District, KPK, Pakistan. Journal of Botany. 2011; 2011: 10.1155/2011/368572 [DOI] [Google Scholar]
- 131.Singh AG, Kumar A, Tewari DD. An ethnobotanical survey of medicinal plants used in Terai forest of western Nepal. Journal of Ethnobiology and Ethnomedicine. 2012; 8: 1–19. 10.1186/1746-4269-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adebayo JO, Krettli AU. Potentials of anti-malarias from Nigerian plants: A review. Journal of Ethnopharmacology. 2011; 13: 289–302. [DOI] [PubMed] [Google Scholar]
- 133.Ritter R.A. Maria VBM, Frederico OBM, Silvane TR, Marina LS, Jean CRS, Maria DCP, et al. Ethnoveterinary knowledge and practices at Colares island, Pará state, eastern Amazon, Brazil. Journal of Ethnopharmacology. 2012; 2: 346–352. 10.1016/j.jep.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 134.Kunwar RM, Maria F, Mary C, Rainer WB, Khum B, Thapa- M, et al. Cross-cultural comparison of plant use knowledge in Baitadi and Darchula districts, Nepal Himalaya. Journal of Ethnobiology and Ethnomedicine. 2018; 14–40. 10.1186/s13002-018-0215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parthiban R, Subramaniyan V, Srinivasan P, Jobu GEMY. Quantitative traditional knowledge of medicinal plants used to treat livestock diseases from Kudavasal taluk of Thiruvarur district, Tamil Nadu, India. Revista Brasileira de Farmacognocia. 2016; 26: 109–121. [Google Scholar]
- 136.Singh A, Nautiyal MC, Kunwar RM, Bussmann RW. Ethnomedicinal plants used by local inhabitants of Jakholi block, Rudraprayag district, western Himalaya, India. Journal of Ethnobiology and Ethnomedicine. 2017; 1: 49–77. 10.1186/s13002-017-0178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ong HG, Kim YD. Quantitative ethnobotanical study of the medicinal plants used by the Ati Negrito indigenous group in Guimaras Island, Philippines. Journal of Ethnopharmacology. 2014; 157: 228–242. 10.1016/j.jep.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 138.Mayer M, Zbinden M, Vogl CR, Ivemeyer S, Meier B, Amorena M, et al. Swiss ethnoveterinary knowledge on medicinal plants–a within-country comparison of Italian speaking regions with north-western German speaking regions. Journal of Ethnobiology and Ethnomedicine. 2017; 13: 10.1186/s13002-016-0106-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Göhre A, Toto-Nienguesse AB, Futuro M, Neinhuis C, Lautenschläger T. Plants from disturbed savannah vegetation and their usage by Bakongo tribes in Uíge, Northern Angola. Journal of Ethnobiology and Ethnomedicine. 2016; 12: 42 10.1186/s13002-016-0116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Esakkimuthu S, Sylvester SD, Mutheeswaran S, Gabriel MP, Pandikumar P, Ignacimuthu S, et al. A study on food-medicine continuum among the non-institutionally trained siddha practitioners of Tiruvallur district, Tamil Nadu, India. Journal of Ethnobiology and Ethnomedicine. 2018; 14–45. 10.1186/s13002-018-0215-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khan MP, Ahmad M, Zafar M, Sultana S, Ali MI, Sun H. Ethnomedicinal uses of Edible Wild Fruits (EWFs) in Swat Valley, Northern Pakistan. Journal of Ethnopharmacology. 2015; 173: 191–203. 10.1016/j.jep.2015.07.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Correlation analysis between a) UV vs CSI, b) RFC vs SCI, and c) UV vs RFC.
(DOC)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.