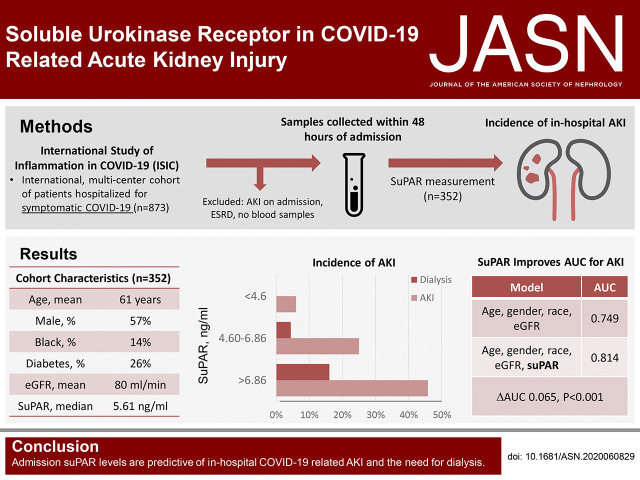
Significance Statement
Nearly half of hospitalized patients with coronavirus disease 2019 (COVID-19) develop AKI, with 20% requiring dialysis. High levels of soluble urokinase plasminogen activator receptor (suPAR)—an immune mediator of kidney injury—predispose patients to AKI in various clinical scenarios, including critical illness. High suPAR levels modulate mitochondrial respiration and induce the generation of reactive oxygen species in proximal tubular cells, sensitizing them to additional insults. In a multicenter observational study, the authors measured plasma suPAR in 352 patients with COVID-19 within 48 hours of their hospitalization. They found suPAR levels to be strongly associated with incident AKI, independent of such clinical characteristics as kidney function and inflammatory biomarkers, and predictive of the need for dialysis. SuPAR may be a key component of the pathophysiology of AKI in COVID-19.
Keywords: acute renal failure, AKI, urokinase, SuPAR, SARS-CoV-2, coronavirus, dialysis, renal replacement therapy, CRP, COVID-19
Visual Abstract
Abstract
Background
AKI commonly occurs in patients with coronavirus disease 2019 (COVID-19). Its pathogenesis is poorly understood. The urokinase receptor system is a key regulator of the intersection between inflammation, immunity, and coagulation, and soluble urokinase plasminogen activator receptor (suPAR) has been identified as an immunologic risk factor for AKI. Whether suPAR is associated with COVID-19–related AKI is unknown.
Methods
In a multinational observational study of adult patients hospitalized for COVID-19, we measured suPAR levels in plasma samples from 352 adult patients that had been collected within 48 hours of admission. We examined the association between suPAR levels and incident in-hospital AKI.
Results
Of the 352 patients (57.4% were male, 13.9% were black, and mean age was 61 years), 91 (25.9%) developed AKI during their hospitalization, of whom 25 (27.4%) required dialysis. The median suPAR level was 5.61 ng/ml. AKI incidence rose with increasing suPAR tertiles, from a 6.0% incidence in patients with suPAR <4.60 ng/ml (first tertile) to a 45.8% incidence of AKI in patients with suPAR levels >6.86 ng/ml (third tertile). None of the patients with suPAR <4.60 ng/ml required dialysis during their hospitalization. In multivariable analysis, the highest suPAR tertile was associated with a 9.15-fold increase in the odds of AKI (95% confidence interval [95% CI], 3.64 to 22.93) and a 22.86-fold increase in the odds of requiring dialysis (95% CI, 2.77 to 188.75). The association was independent of inflammatory markers and persisted across subgroups.
Conclusions
Admission suPAR levels in patients hospitalized for COVID-19 are predictive of in-hospital AKI and the need for dialysis. SuPAR may be a key component of the pathophysiology of AKI in COVID-19.
Over 7.5 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with >425,000 deaths as of June of 2020 attributed to coronavirus disease 2019 (COVID-19).1 Although initially thought of as a mainly respiratory disease, close to 50% of patients hospitalized with COVID-19 develop AKI.2–7 The burden of AKI in COVID-19 has placed significant strain on resources for RRT.8 In an observational study of 3235 hospitalized patients with COVID-19 across the Mount Sinai Health System in New York, AKI occurred in 1406 patients (46%), of whom 280 (20%) required dialysis.7 Two-thirds of AKI cases were moderate to severe.7 The majority of patients had evidence of proteinuria.4,5 Other large studies from New York and China have reported similar findings.2–6 Most importantly, AKI is strongly associated with poor survival in COVID-19.4,6 Although attention has been directed to evidence of infection of renal tubular epithelium and podocytes by the SARS-CoV-2 virus,9,10 the pathophysiology of AKI in COVID-19 is likely multifactorial and includes aggravation of prior risk factors, acute inflammation, cardiorenal syndrome, hemodynamic instability, and hypovolemia.11,12 The incidence of AKI in COVID-19 is similar to that reported in other pandemics, notably H1N1 influenza, suggesting that the systemic inflammatory response may be the main contributor to AKI in the setting of viral-related critical illness.13,14 There are no therapies or strategies to reduce the incidence of AKI in this patient population.15–17
We have identified soluble urokinase plasminogen activator receptor (suPAR) as an immune mediator of kidney injury.18–28 The urokinase receptor system is a key regulator of the intersection between inflammation, immunity, and coagulation.29 suPAR is produced by cleavage of membrane-bound uPAR in response to inflammatory stimuli such as viruses, and cardiovascular risk factors such as smoking and diabetes mellitus.30–32 High suPAR levels predispose patients to AKI in various clinical scenarios including critical illness, likely by modulating mitochondrial respiration and inducing reactive oxygen species generation in proximal tubular cells, sensitizing them to additional damage.20,33,34 Most importantly, adverse effects of suPAR on the kidneys are abrogated by the use of an anti-suPAR mAb in experimental models, suggesting that suPAR is a promising therapeutic target to mitigate AKI.19–21,35 suPAR levels are dramatically elevated in COVID-19 and may be a key mediator of COVID-19–related AKI.36 We hypothesize that blood suPAR levels in patients hospitalized for COVID-19 are predictive of incident AKI. To test our hypothesis, we investigated the association between admission suPAR levels and incident AKI in patients hospitalized for COVID-19 enrolled in the International Study of Inflammation in COVID-19 (ISIC).
Methods
The International Study of Inflammation in COVID-19
The ISIC is an ongoing multicenter observational study whose primary purpose is to characterize levels of various biomarkers of inflammation and their association with in-hospital outcomes of patients with COVID-19. Participating centers include: the University of Michigan in Ann Arbor, MI; Copenhagen University Hospital in Hvidovre, Denmark; Attikon University Hospital in Athens, Greece; the University of Thessaly, Greece; the University Hospital of Dusseldorf, Germany; and Charité University Medicine Berlin, Germany (Supplemental Material). Inclusion criteria were: (1) adult (≥18 years old) patients hospitalized primarily for COVID-19; (2) a confirmed SARS-CoV-2 infection diagnosed through RT-PCR test of nasopharyngeal or oropharyngeal samples; and (3) at least one blood sample collected during the hospitalization and stored for biomarker testing. Patients with confirmed SARS-CoV-2 infection who were not primarily admitted for COVID-19 were excluded. Institutional review board approval and consent procedures were obtained separately at each site according to local institutional policies.
The data collected were granular and extensive, including details at presentation, past medical history, home medications, inpatient medical therapy, hospitalization course, and outcomes. Clinical outcomes included several in-hospital events: respiratory failure, AKI, myocardial injury, thromboembolism, and coagulopathy, all accompanied by laboratory testing. We collected all biomarker data available including levels of C-reactive protein (CRP), D-dimer, ferritin, and lactate dehydrogenase (LDH). The data were extracted through manual chart review by at least two reviewers per site and entered in an online data repository managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Michigan.37,38 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.
Study Design and Definitions
For the purpose of this substudy of ISIC, we only included patients who had a blood sample collected within 48 hours of admission. Patients with a history of ESKD on dialysis, and those with AKI on admission (by comparing admission serum creatinine with measures within 1 year of admission), were excluded (Supplemental Figure 1). Overall, 352 participants of ISIC met inclusion criteria. The primary outcome was incident AKI during the hospitalization, defined on the basis of the Kidney Disease Improving Global Outcomes (KDIGO) criteria39: AKI stage 1 was defined as a 1.5–1.9-fold increase in serum creatinine relative to the value on admission or a ≥0.3 mg/dl absolute increase in creatinine. Stage 2 AKI was defined as a 2.0–2.9-fold increase in serum creatinine compared with admission levels. Lastly, stage 3 AKI was defined as a threefold increase in serum creatinine compared with admission, a rise in creatinine to ≥4 mg/dl, or initiation of RRT.
Assessment of Kidney Function
Serum creatinine measurements on admission and all subsequent values during the index hospitalization were obtained from electronic medical records. The serum creatinine level at the time of suPAR measurement was used as the baseline value for all analyses. suPAR was measured on the day of admission in 327 (92.9%), and between 24 and 48 hours of admission in 25 (7.1%) patients. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.40
Measurement of suPAR
suPAR was measured in plasma at participating centers using a commercially available ELISA (suPARnostic assay by ViroGates, Birkerød, Denmark) by experienced technicians blinded to the clinical data. The suPARnostic assay uses a double mAb sandwich assay to human suPAR. Kits have a lower detection limit of 100 pg/ml and intra- and interassay variation of 2.75% and 9.17%, respectively, as determined by the assay manufacturer.
Statistical Analyses
We first report cohort characteristics stratified by AKI and suPAR tertiles. Continuous variables are presented as means (±SD) or as medians (25th to 75th interquartile range [IQR]) for normally and non-normally distributed data, respectively. Categoric variables are presented as proportions (%). To compare patients with and without AKI, and across AKI stages or suPAR tertiles, we used ANOVA or Kruskal–Wallis for continuous variables, and chi-squared tests for categoric variables. We used Spearman-rank to assess correlations between suPAR and inflammatory biomarkers (CRP, D-dimer, ferritin, and LDH), and linear regression after log-transformation of the aforementioned markers (base 2, interpreted as per 100% increase) to identify those that were independently associated with suPAR.
To identify determinants of in-hospital AKI, we used logistic regression with AKI as the binary dependent variable in models that incorporated age, sex, race, country of enrollment (United States versus Europe), body mass index (BMI), diabetes mellitus, hypertension, coronary artery disease, congestive heart failure, oxygen saturation on admission, and eGFR derived from serum creatinine level obtained from the sample in which suPAR was measured. Each biomarker was evaluated in a separate model that included the aforementioned clinical characteristics in addition to suPAR.
We explored the association between suPAR and incident AKI using two approaches: binary logistic regression with in-hospital AKI as the dependent variable reporting odds ratio (OR), and modeling time to AKI using Cox regression hazards modeling reporting hazard ratio. Model 1 was unadjusted, including suPAR as tertiles with the first tertile being the reference. Model 2 was adjusted for age, sex, race, diabetes mellitus, hypertension, and eGFR to adjust for risk factors. Model 3 incorporated the aforementioned variables including oxygen saturation and CRP levels to adjust for disease severity.
For visualization, we plotted the daily percentage change in creatinine from admission stratified by suPAR tertiles using locally weighed smoothing. To assess suPAR’s effect on risk discrimination, we plotted receiver operating characteristic curves and computed the area under the curve (AUC) for model 0 incorporating age, sex, race, and admission eGFR, and for model 1 which includes variables from model 0 in addition to suPAR. We compared the AUCs for both models using DeLong’s test.41
Lastly, we investigated the possibility of effect modification attributed to differences in baseline characteristics. We tested for interaction and computed ORs for the association between suPAR levels and AKI in relevant subgroups. Two-tailed P values ≤0.05 were considered statistically significant. Analyses were performed using SPSS 24 (IBM, NY) and R (R Core Team, 2014).42
Results
Cohort Characteristics
Among 873 participants of ISIC, 352 met inclusion criteria for our study (Supplemental Figure 1). One hundred and 48 patients (42.0%) were admitted at the University of Michigan, and 204 (57.9%) at European centers (Supplemental Tables 1 and 2). Overall, the cohort had a mean age of 61 (range, 19–95) and consisted of 57.4% male and 13.9% Black patients (Table 1). The mean admission eGFR was 80 ml/min per 1.73 m2, and 25.6% of patients had an eGFR<60 ml/min per 1.73 m2. A total of 91 (25.9%) patients developed AKI during the hospitalization, among whom 43 (47.3%) had at least moderate AKI (stage 2 or 3 KDIGO), and 25 (27.5%) required RRT. The mean admission serum creatinine in patients with AKI was 1.3 mg/dl (SD 0.9), whereas the mean peak creatinine was 2.8 mg/dl (SD 2.0). All patients requiring dialysis were in the intensive care unit. The median number of days on RRT was 5 (range, 1–12). Biomarkers of inflammation including CRP, ferritin, D-dimer, and LDH were significantly higher in patients with AKI compared with those without AKI (Table 1). In multivariable analysis, male sex and eGFR were independently associated with AKI. Younger age, lower eGFR, and lower oxygen saturation were associated with incident RRT. Among the biomarkers, only suPAR and CRP were independently associated with AKI (Table 2).
Table 1.
Clinical characteristics and inflammatory markers stratified by AKI
| Characteristic | Cohort (n=352) | No AKI (n=261) | AKI (n=91) | P Valuea |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age in yr, mean (SD) | 61 (16) | 60 (17) | 65 (15) | 0.011 |
| Male, n (%) | 202 (57.4) | 138 (52.9) | 64 (70.3) | 0.004 |
| Black, n (%) | 49 (13.9) | 27 (10.3) | 22 (24.2) | 0.001 |
| Enrolled in the United States, n (%) | 148 (42.0) | 95 (36.4) | 53 (58.2) | <0.001 |
| BMI kg/m2, mean (SD) | 30 (7) | 30 (7) | 32 (7) | 0.03 |
| Diabetes mellitus, n (%) | 91 (25.9) | 54 (20.7) | 37 (40.7) | <0.001 |
| Hypertension, n (%) | 169 (48.0) | 110 (42.1) | 59 (64.8) | <0.001 |
| Coronary artery disease, n (%) | 34 (9.7) | 22 (8.4) | 12 (13.2) | 0.19 |
| Congestive heart failure, n (%) | 29 (8.2) | 14 (5.4) | 15 (16.5) | 0.001 |
| Admission eGFR ml/min per 1.73 m2, mean (SD)b | 80 (27) | 85 (25) | 67 (29) | <0.001 |
| Admission eGFR<60 ml/min per 1.73 m2, n (%)b | 90 (25.6) | 51 (19.5) | 39 (42.9) | <0.001 |
| Oxygen saturation on admission, % | 94 (6) | 95 (5) | 92 (7) | <0.001 |
| Required mechanical ventilation, n (%) | 77 (21.9) | 22 (8.4) | 55 (60.4) | <0.001 |
| Required dialysis, n (%) | 25 (7.1) | 0 (0.0) | 25 (27.5) | <0.001 |
| Biomarkersc | ||||
| suPAR ng/ml, median (IQR) | 5.61 (4.00–7.88) | 5.05 (3.51–6.70) | 7.38 (5.50–10.38) | <0.001 |
| CRP mg/dl, median (IQR) | 5.70 (2.58–12.50) | 4.90 (2.00–9.80) | 14.25 (7.80–22.80) | <0.001 |
| Ferritin ng/ml, median (IQR) | 495 (234–1160) | 465 (205–998) | 595 (299–1350) | 0.01 |
| D-dimer mg/L, median (IQR) | 0.72 (0.44–1.71) | 0.63 (0.40–1.23) | 1.10 (0.56–2.17) | 0.005 |
| LDH IU/L, median (IQR) | 322 (232–472) | 295 (220–407) | 427.00 (313–592) | <0.001 |
P value for the comparison between groups with and without AKI.
Calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
CRP levels were available in 301 participants, ferritin in 207 participants, D-dimer in 204 participants, and LDH in 256 participants.
Table 2.
Determinants of incident AKI and need for dialysis in hospitalized patients with COVID-19
| Characteristic | AKI (n=91) | RRT (n=25) | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Model 0: clinical characteristics | ||||
| Age, per 10 yr | 0.91 (0.71 to 1.15) | 0.42 | 0.56 (0.37 to 0.86) | 0.007 |
| Male | 3.00 (1.65 to 5.46) | <0.001 | 1.77 (0.65 to 4.82) | 0.27 |
| Black | 1.99 (0.88 to 4.46) | 0.10 | 3.77 (0.58 to 24.43) | 0.16 |
| Institution (United States versus Europe) | 0.57 (0.30 to 1.08) | 0.08 | 14.49 (2.40 to 87.58) | 0.004 |
| BMI, per 5 kg/m2 | 1.03 (0.83 to 1.27) | 0.80 | 0.91 (0.61 to 1.36) | 0.66 |
| Diabetes mellitus | 1.46 (0.78 to 2.72) | 0.24 | 2.30 (0.80 to 6.58) | 0.12 |
| Hypertension | 1.13 (0.59 to 2.14) | 0.72 | 1.53 (0.48 to 4.87) | 0.47 |
| Coronary artery disease | 0.74 (0.29 to 1.92) | 0.54 | 0.61 (0.11 to 3.47) | 0.57 |
| Congestive heart failure | 1.35 (0.51 to 3.58) | 0.55 | 0.76 (0.13 to 4.58) | 0.76 |
| eGFR, per 5 ml/min per 1.73 m2 | 0.87 (0.81 to 0.92) | <0.001 | 0.81 (0.72 to 0.91) | <0.001 |
| Oxygen saturation, per 1% | 0.96 (0.91 to 1.00) | 0.06 | 0.91 (0.86 to 0.97) | 0.002 |
| Models 1–5: biomarkersa | ||||
| suPAR, per 100% increaseb | 2.47 (1.60 to 3.79) | <0.001 | 4.01 (2.01 to 7.99) | <0.001 |
| CRP, per 100% increase | 1.37 (1.09 to 1.72) | 0.04 | 1.11 (0.81 to 1.53) | 0.53 |
| Ferritin, per 100% increase | 1.02 (0.81 to 1.27) | 0.89 | 1.23 (0.78 to 1.94) | 0.37 |
| D-dimer, per 100% increase | 1.09 (0.87 to 1.36) | 0.46 | 1.01 (0.68 to 1.50) | 0.97 |
| LDH, per 100% increase | 1.17 (0.69 to 2.00) | 0.56 | 2.06 (0.89 to 4.77) | 0.07 |
Each biomarker was evaluated in a separate model that included the listed clinical characteristics in addition to suPAR.
Serum creatinine–derived eGFR measured at the time of suPAR sample collection was used in Model 1 assessing the association between suPAR and AKI. eGFR was measured at the time of suPAR collection. CRP levels were available in 301 participants, ferritin in 207 participants, D-dimer in 204 participants, and LDH in 256 participants.
suPAR Level and Its Determinants
The median suPAR level in the entire cohort was 5.61 ng/ml (IQR, 4.00–7.88), close to threefold higher than that of healthy individuals (2.1 ng/ml).43 Patients in the highest suPAR tertile were more likely to be men, to be Black, and to have higher BMI, diabetes mellitus, hypertension, congestive heart failure, and lower eGFR on admission (Supplemental Table 3). suPAR levels correlated positively with CRP, D-dimer, ferritin, and LDH, independently of clinical characteristics (Supplemental Tables 4 and 5). Only congestive heart failure and eGFR were independently associated with suPAR levels (Supplemental Table 5).
suPAR and In-Hospital AKI in Patients with COVID-19
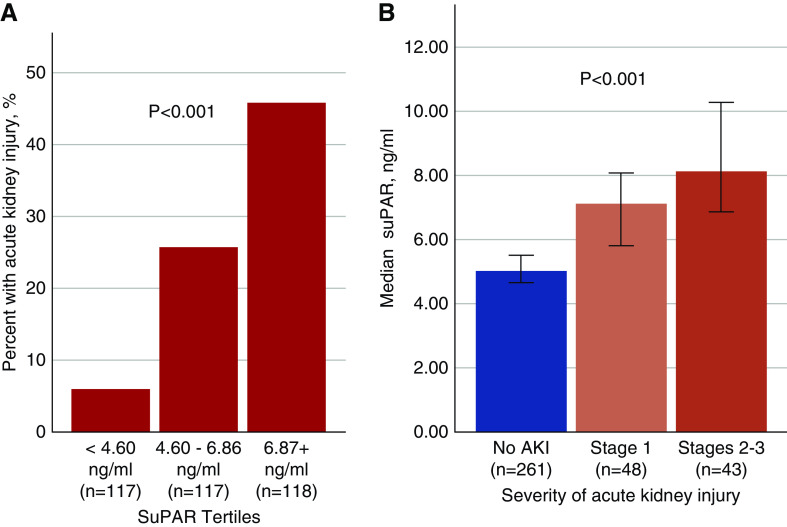
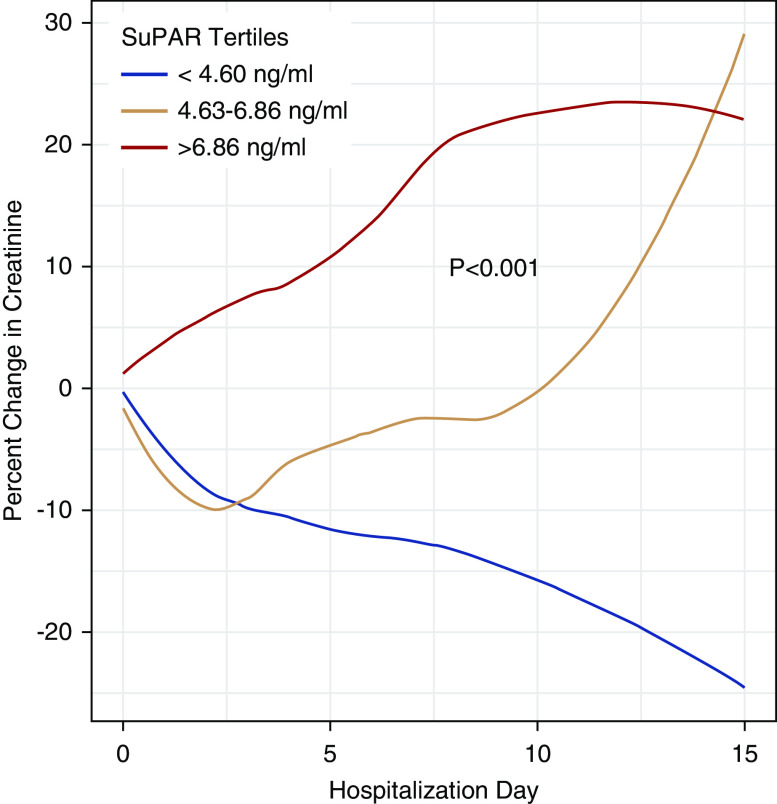
Patients who developed AKI during their hospitalization had a median suPAR level 61.6% higher than that of patients who did not develop AKI (Table 1). We found a step-wise rise in the incidence of AKI with increasing suPAR tertiles, from a 6% incidence in patients with suPAR<4.60 ng/ml (first tertile), to a 45.8% incidence of AKI in patients with suPAR levels >6.86 ng/ml (Figure 1A). Severity of in-hospital AKI correlated with admission suPAR levels: patients with KDIGO stage 2–3 AKI had significantly higher suPAR levels compared with those with mild AKI (Figure 1B). Serum creatinine levels steadily increased in patients with suPAR levels in the second and third tertiles, whereas they declined in patients in the first tertile (Figure 2). None of the patients with suPAR<4.60 ng/ml (first tertile) needed dialysis. Among those who required dialysis (n=25), we found a strong correlation between suPAR levels and the duration of RRT (r=0.74, P<0.001). The median duration of RRT in the third suPAR tertile (>6.86 ng/ml) was 6 days (IQR 3–8), compared with 3 days (IQR 1–3) in the second suPAR tertile (4.60–6.86 ng/ml), P=0.03.
Figure 1.
AKI stratified by suPAR levels. Bar graphs showing (A) the percentage of patients with incident AKI stratified by suPAR tertiles, and (B) the median suPAR levels across AKI stages. Note: stages 2–3 AKI here exclude patients on dialysis, which are shown in a separate bar graph. P value is derived from the chi-squared test in (A) and Kruskal–Wallis test in (B).
Figure 2.
Percent change in creatinine throughout the hospitalization stratified by suPAR tertiles. We plotted the daily percentage creatinine change relative to admission creatinine stratified by suPAR tertiles using locally weighted smoothing. P value derived from linear mixed model examining the interaction between the creatinine slope and suPAR tertiles. Patients in second and third tertiles had steadily increasing creatinine levels compared with patients in the first suPAR tertile.
Multivariable and Sensitivity Analyses
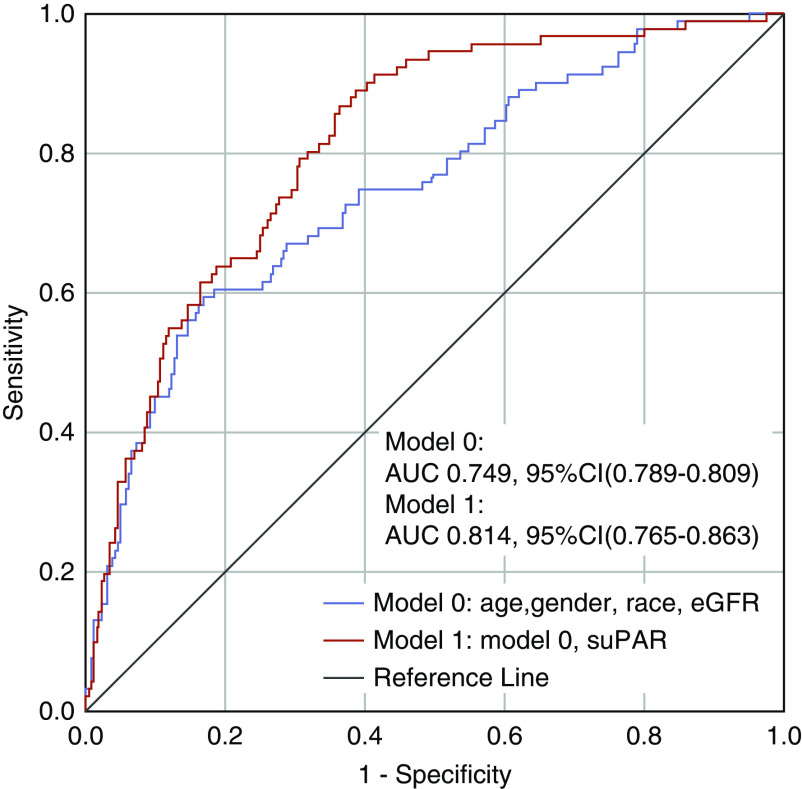
In unadjusted analysis, patients in the second and third suPAR tertiles had a 5.42-fold (95% confidence interval [95% CI], 2.27 to 12.93) and a 13.26-fold (95% CI, 5.69 to 30.88) increase in the odds of developing AKI, respectively, compared with the first tertile (Figure 3A). The highest suPAR tertile remained strongly associated with incident AKI (aOR, 9.15; 95% CI, 3.64 to 22.93) after adjusting for age, sex, race, and eGFR. Although the second tertile of suPAR was not associated with incident RRT (OR, 5.18; 95% CI, 0.60 to 45.0), patients in the third suPAR tertile had a 22.26-fold increase in the odds of requiring RRT (95% CI, 2.93 to 169.29). The association between suPAR and RRT was not attenuated by adjusting for age, male sex, race, and eGFR (aOR, 22.86; 95% CI, 2.77 to 188.75). When examined as a continuous variable, a doubling of suPAR was associated with a 3.18-fold increase in the odds of developing AKI (95% CI, 2.18 to 4.63), and 3.28-fold odds of requiring RRT (95% CI, 1.93 to 5.57). The association was mildly attenuated after adjusting for clinical characteristics including eGFR (Model 2) and CRP (Model 3). Findings were consistent in a time-to-event analysis (Figure 3B). None of the other inflammatory biomarkers were associated with incident AKI (Table 2). The AUC for suPAR in predicting AKI was 0.741 (95% CI, 0.684 to 0.798), whereas the AUC for admission eGFR was 0.675 (95% CI, 0.609 to 0.740) (Supplemental Figure 2). Addition of suPAR to a model including age, sex, race, and admission eGFR significantly improved the AUC for predicting AKI (ΔAUC 0.065, P<0.001; Figure 4).
Figure 3.
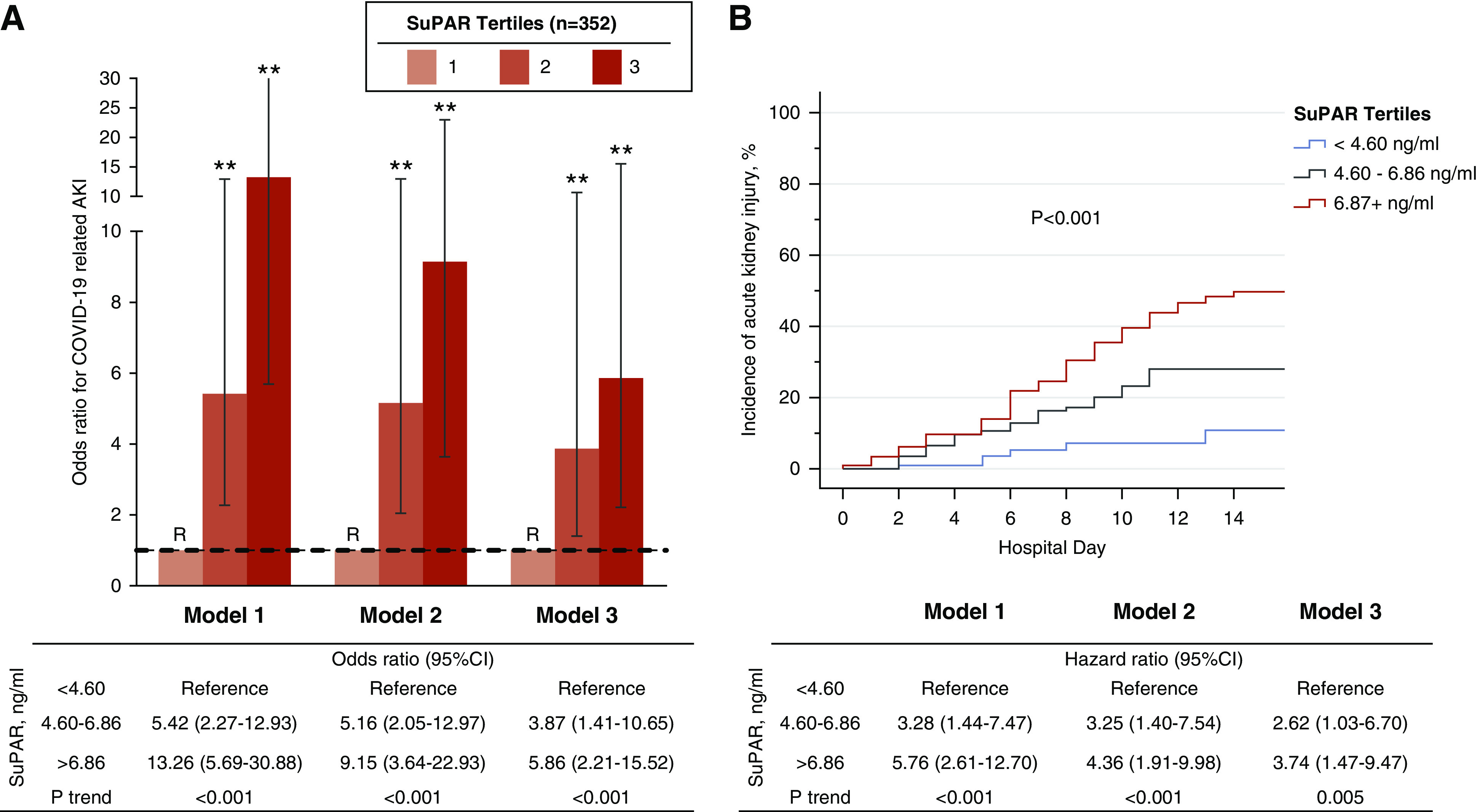
SuPAR and odds of COVID-19–related AKI. (A) Bar graph depicting ORs and 95% CIs for AKI according to admission suPAR tertiles. (B) Kaplan–Meier curves showing cumulative incidence of AKI stratified by suPAR tertiles with log-rank P value. Cox regression modeling hazard ratios are reported in the accompanying table. Model 1 was unadjusted. Model 2 was adjusted for age, sex, race, diabetes mellitus, hypertension, and eGFR at the time of sample collection for suPAR. Model 3 incorporated the aforementioned variables including oxygen saturation and CRP levels. Tertile 1 was the reference (R) group in all models. **P<0.001. Data for model 3 were available in 301 participants.
Figure 4.
Addition of suPAR improves the AUC for predicting AKI. Receiver operating characteristic curve for predicting AKI. Model 0 includes age, sex, race, and creatinine-derived eGFR at the time of suPAR measurement. Model 1 includes all aforementioned characteristics in addition to suPAR as a continuous variable.
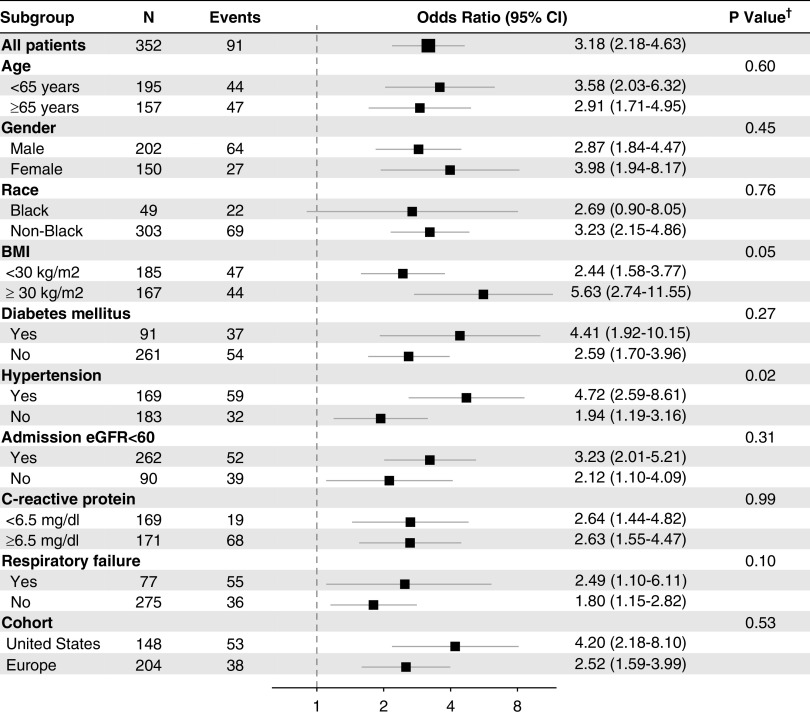
In sensitivity analyses, the association between suPAR and AKI did not differ according to day of sample collection (P=0.71), and persisted across relevant subgroups (Figure 5), but was notably stronger in obese patients (BMI>30 kg/m2, interaction P value=0.05) and nonhypertensive patients (interaction P value=0.02). Exclusion of patients in whom suPAR levels were measured 24–48 hours after admission yielded similar findings (Supplemental Table 6).
Figure 5.
SuPAR and AKI across subgroups. Forest plot depicting the risk of AKI on the basis of suPAR levels as a continuous variable according to subgroups. suPAR levels were log-transformed (base 2) given their skewed distribution. †P value for the interaction between subgroups.
Discussion
A characterizing feature of severe COVID-19 is the induction of the innate immune response and massive cytokine release, including suPAR.36 We report for the first time the association between suPAR and AKI in a multinational cohort of patients hospitalized for COVID-19. We found admission suPAR levels in these patients to be a strong predictor of in-hospital AKI. Patients in the lowest suPAR tertile (<4.60 ng/ml) had a low incidence of AKI (6%), with none requiring dialysis, whereas patients in the third tertile (>6.86 ng/ml) had an incidence of AKI of 45.8% with 16.1% requiring dialysis. The association was independent of eGFR, disease severity, or other biomarkers of inflammation and persisted across subgroups. In light of recent studies showing a role for suPAR as an immune-mediator of kidney injury, our findings highlight suPAR as a potential contributor to the large burden of AKI in COVID-19.
suPAR is the circulating form of uPAR, a glycosyl-phosphatidylinositol-anchored (GPI) three-domain (DI, DII, and DIII) receptor protein encoded by the PLAUR gene, and expressed on a variety of cells, including immune cells, endothelial cells, and podocytes.19,44,45 The specific physiologic role of suPAR is unclear. Its levels in circulation reflect the aggregate activity of the uPAR system with respect to innate immune activity, proteolysis, and extracellular matrix remodeling.44,46,47 Although expressed at low levels in normal conditions, uPAR is induced during leukocyte activation and differentiation in response to environmental stimuli such as smoking and certain RNA viruses.48–50 Several proinflammatory conditions with high suPAR levels have a high incidence of kidney disease, including cardiovascular risk factors, autoimmune diseases, lung disease, atherosclerosis, cancer, heart failure, and critical illness.51–57 High levels of suPAR in circulation are strongly predictive of progressive kidney dysfunction, with prolonged exposure directly affecting the kidneys via pathologic activation of αvβ3 integrin expressed on podocytes, which induces small GTPase signaling (Rac-1) and podocyte foot process effacement leading to proteinuria and eventually chronic kidney dysfunction.18,19,21,28 Transgenic mice over-expressing suPAR develop proteinuric kidney disease and more-severe AKI after contrast injection.20,21,28 We found suPAR levels in COVID-19 to be almost threefold higher than in healthy persons (2.1 ng/ml),43 and as high as in critically ill patients who subsequently develop AKI.25
The pathophysiology of AKI in COVID-19 is unclear.58 Autopsy reports in patients with COVID-19 have revealed significant proximal tubular injury, and glomerular thrombi with lymphocyte and macrophage infiltration.9 Although a hypercoagulable state and microangiopathy are thought to be common mechanisms for both respiratory failure and AKI, D-dimer levels were not independently associated with AKI in our cohort. Direct infection of the kidney has also been shown, but its contribution to injury is unclear.9,58,59 Nevertheless, AKI in the setting of sepsis is complex and multifactorial, involving both intrinsic (risk factors and comorbidities) and extrinsic processes (viral infection, hypovolemia, nephrotoxic drugs), with the immune system and acute inflammation having a central role and suPAR being one potential mediator.11,60
suPAR may be implicated in the pathogenesis of COVID-19–related AKI through multiple mechanisms. It has been previously shown to facilitate viral entry into kidney cells through αvβ3 integrin in hemorrhagic fever secondary to hantavirus, leading to disruption in cell permeability and AKI.61–64 Whether SARS-CoV-2 infects kidney cells more readily in the presence of high suPAR needs to be explored. suPAR was initially thought to be involved in FSGS.18,19 Interestingly, histopathologic findings of FSGS and severe proteinuria have been reported in Black patients with COVID-19 and specifically those with high-risk homozygous APOL1 variants.10,65,66 We have previously shown that high suPAR levels and high-risk homozygous APOL1 variants are synergistic in activating αvβ3 integrins and in their association with the progression of kidney disease in two cohorts of Black patients.23 The same mechanism may be at play in patients with severe COVID-19. suPAR also affects proximal tubular cells, altering mitochondrial respiration and inducing oxidative stress.20 Other reports have suggested that high suPAR promotes fibrosis of the tubules in an integrin-dependent manner.19,21,67,68 These deleterious changes to podocytes and tubular cells have been abrogated by the use of anti-uPAR monoclonal antibodies in experimental models.18–20,69 Ultimately, only an in-human intervention assessing the effect of lowering suPAR on the incidence of AKI can provide definitive conclusion on its role as an immune-mediator of kidney injury.
The study has several strengths. It is a multinational study that relied on granular collection of clinical, laboratory, and outcome data throughout the COVID-19 hospitalization. Patients with a positive COVID-19 test who were not primarily admitted for COVID-19 were excluded from this cohort, allowing us to derive conclusions specifically related to severe COVID-19. We acknowledge a few limitations to the study. Patients from European centers were not consecutively enrolled, lending a risk of selection bias. However, the range of suPAR levels in this cohort was wide, thus limiting the effect of a selection bias on the validity of the association between suPAR and AKI. The association between suPAR and AKI was also similar when examining the European and the University of Michigan cohorts separately. Although COVID-19 is associated with a high incidence of proteinuria, we could not assess urine protein systematically assessed in ISIC.
In conclusion, suPAR levels were independently associated with incident AKI in patients hospitalized for COVID-19. In light of recent experimental data suggesting that suPAR is an immune-mediator of kidney injury, targeting suPAR should be considered as a strategy to mitigate AKI in COVID-19 and other patient populations at high risk of AKI.
Disclosures
O. Anderson and J. Eugen-Olsen are named inventors on patents related to suPAR. J. Eugen-Olsen is a cofounder, shareholder, and chief scientific officer of ViroGates. J. Eugen-Olsen has a patent suPAR as a marker of low-grade inflammation issued to ViroGates. J. Reiser is cofounder of Trisaq, a biotechnology company developing drugs targeting suPAR. S. Hayek and J. Reiser are members of the scientific advisory board of Trisaq. J. Reiser has a patent US20110212083—Role of soluble uPAR in the Pathogenesis of Proteinuric Kidney Disease with royalties paid to Trisaq, a patent US9867923—Reducing Soluble urokinase Receptor in the Circulation with royalties paid to Miltenyi, a patent JP2016530510—Non-Glycosylated suPAR Biomarkers and Uses thereof with royalties paid to Trisaq, a patent US20160296592—Methods/Compositions for the Treatment of Proteinuric Diseases with royalties paid to Trisaq, a patent US9144594—Dynamin Mediated Diseases with royalties paid to Trisaq, and a patent US8809386—Dynamin Ring Stabilizers with royalties paid to Trisaq. All remaining authors have nothing to disclose.
Funding
S. Hayek is funded by National Heart, Lung, and Blood Institute grant 1R01HL153384-01 and the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor (U-M G024231) award. E.J. Giamarellos-Bourboulis is supported by the Hellenic Institute for the Study of Sepsis. F. Tacke is supported through intramural funds from Charité Universitaetsmedizin Berlin and the Berlin Institute of Health.
Supplementary Material
Acknowledgments
The authors acknowledge the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication. The authors are grateful to the services of the Microbiome Core supported by grant U2CDK110768, especially Chris Blair, the Michigan Clinical Research Unit including Wrenn Woodard and Dexter Hobdy, and the University of Michigan Medical School Central Biorepository for providing biospecimen storage, management, and distribution services in support of the research reported in the publication.
Dr. Salim S. Hayek is supported by the Michigan Center for Contextual Factors in Alzheimer’s Disease (P30-AG059300), the Michigan Institute for Clinical & Health Research (UL1-TR002240), the Mi-Kickstart Early-Stage Funding Award (CASE-244578), and personal fees from Trisaq, outside the submitted work. Dr. Rodica Pop-Busui reports grants from AstraZeneca, personal fees from Bayer, personal fees from Novo Nordisk, and personal fees from Boehringer Ingelheim, outside the submitted work. Dr. Subramaniam Pennathur is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK081943), outside the submitted work. Dr. Frank Tacke reports grants from Allergan, BMS, Inventiva, Gilead, and Galapagos, and personal fees from Allergan, BMS, Inventiva, Gilead, Pfizer, Abbott, Alnylam, and Novartis, outside the submitted work. Dr. Evangelos J. Giamarellos-Bourboulis reports grants from Abbot CH, grants and personal fees from InflaRx GmbH, grants from Axis Shield, grants and personal fees from XBiotech InC, personal fees from Angelini Italy, grants and personal fees from BioMerieux, grants from Horizon 2020 ImmunoSep, grants from Marie-Curie ITN gran European Sepsis Academy, and grants from Novartis, outside the submitted work. Dr. Jesper Eugen-Olsen reports personal fees from ViroGates, outside the submitted work. Dr. Jochen Reiser reports personal fees from Biomarin, grants from National Institutes of Health, grants from Nephcure, other from TRISAQ, grants from Thermo BCT, personal fees from Astellas, personal fees from Massachusetts General Hospital, personal fees from Genentech, personal fees from Up to Date, personal fees from Merck, personal fees from Incepetionsci, and personal fees from GLG, outside the submitted work.
Dr. Salim S. Hayek designed the study. Dr. Tariq U. Azam, Dr. Husam R. Shadid, Dr. Pennelope Blakely, Dr. Patrick O’Hayer, Dr. Hanna Berlin, Dr. Michael Pan, Dr. Izzet Altintas, Dr. Jens Tingleff, Dr. Maria-Evangelia Adami, Dr. Nicky Solomonidi, Dr. Maria Tsilika, Dr. Pinkus Tober-Lau, Dr. Eleni Arnaoutoglou, Dr. Athanasios Chalkias, and Dr. Sven H. Loosen collected the data and performed quality control. Dr. Salim S. Hayek and Dr. Lili Zhao analyzed the data. Dr. Salim S. Hayek wrote the first draft of the manuscript. Dr. Tariq U. Azam, Dr. Husam R. Shadid, Dr. Pennelope Blakely, Dr. Patrick O’Hayer, Dr. Salim S. Hayek, Dr. Subramaniam Pennathur, Dr. Rodica Pop-Busui, Ove Andersen, Dr. Frank Tacke, Dr. Athanasios Chalkias, Dr. Sven H. Loosen, Dr. Evangelos J. Giamarellos-Bourboulis, Dr. Jesper Eugen-Olsen, and Dr. Jochen Reiser provided expert interpretation of the findings, and provided critical revisions to the manuscript. All coauthors had full access to the data and take responsibility for the integrity and accuracy of the data analysis. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Salim S. Hayek, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Elizabeth Anderson, Danny Perry, Abbas Bitar, Rayan Kaakati, Lili Zhao, Peiyao Zhao, Jesper Eugen-Olsen, Izzet Altintas, Jens Tingleff, Marius Stauning, Morten Baltzer Houlind, Mette B. Lindstrøm, Ove Andersen, Hejdi Gamst-Jensen, Line Jee Hartmann Rasmussen, Christian Rasmussen, Jan O. Nehlin, Thomas Kallemose, Imran Parvaiz, Evangelos J. Giamarellos-Bourboulis, Maria-Evangelia Adami, Nicky Solomonidi, Maria Tsilika, Maria Saridaki, Vasileios Lekakis, Sven Loosen, Tom Luedde, Verena Keitel, Athanasios Chalkias, Eleni Arnaoutoglou, Ioannis Pantazopoulos, Eleni Laou, Konstantina Kolonia, Anargyros Skoulakis, Frank Tacke, Pinkus Tober-Lau, Raphael Mohr, Florian Kurth, Leif Erik Sander, and Christoph Jochum
DATA SHARING STATEMENT
Data from ISIC can be made available upon request through a collaborative process. Please contact penegonz@med.umich.edu for additional information.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060829/-/DCSupplemental.
Supplemental Figure 1. Patient selection flowchart.
Supplemental Figure 2. ROC curves for suPAR and admission eGFR.
Supplemental Table 1. Participating centers and number of patients included in the substudy.
Supplemental Table 2. Clinical characteristics and inflammatory biomarkers stratified by continent of enrollment.
Supplemental Table 3. Clinical characteristics and inflammatory biomarkers stratified by suPAR tertiles.
Supplemental Table 4. Correlation between suPAR and inflammatory biomarkers.
Supplemental Table 5. Determinants of suPAR levels.
Supplemental Table 6. Determinants of incident acute kidney injury and need for dialysis in hospitalized patients with COVID-19 after excluding 25 patients with suPAR levels measured 24–48 hours after admission.
References
- 1.Dong E, Du H, Gardner L: An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 20: 533–534, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Northwell Covid-19 Research Consortium; Northwell Nephrology Covid-19 Research Consortium : Acute kidney injury in patients hospitalized with covid-19. Kidney Int 98: 209–218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Northwell Covid-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 323: 2052–2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. : Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. : Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 395: 1763–1770, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Baweja M, et al. : Acute kidney injury in hospitalized patients with COVID-19. medRxiv, 2020 [Google Scholar]
- 8.Chua HR, MacLaren G, Choong LH, Chionh CY, Khoo BZE, Yeo SC, et al. : Ensuring sustainability of continuous kidney replacement therapy in the face of extraordinary demand: Lessons from the COVID-19 pandemic. Am J Kidney Dis 76: 392–400, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. : Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA: Collapsing glomerulopathy in a patient with Coronavirus Disease 2019 (COVID-19). Kidney Int Rep 5: 935–939, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco C, Bellomo R, Kellum JA: Acute kidney injury. Lancet 394: 1949–1964, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Ronco C, Reis T, Husain-Syed F: Management of acute kidney injury in patients with COVID-19. Lancet Respir Med 8: 738–742, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdulkader RC, Ho YL, de Sousa Santos S, Caires R, Arantes MF, Andrade L: Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin J Am Soc Nephrol 5: 1916–1921, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettilä V, Webb SA, Bailey M, Howe B, Seppelt IM, Bellomo R: Acute kidney injury in patients with influenza A (H1N1) 2009. Intensive Care Med 37: 763–767, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Solomon R, Werner C, Mann D, D’Elia J, Silva P: Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med 331: 1416–1420, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin SS, et al. PRESERVE Trial Group : Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 378: 603–614, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, James MT: Acute kidney injury. Ann Intern Med 167: ITC66-ITC80, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. : Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. : Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Hayek SS, Leaf DE, Samman Tahhan A, Raad M, Sharma S, Waikar SS, et al. : Soluble urokinase receptor and acute kidney injury. N Engl J Med 382: 416–426, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, et al. : Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med 23: 100–106, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayek SS, Ko YA, Awad M, Ahmed H, Gray B, Hosny KM, et al. : Cardiovascular disease biomarkers and suPAR in predicting decline in renal function: A prospective cohort study. Kidney Int Rep 2: 425–432, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, et al. : A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 23: 945–953, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayek SS, Landsittel DP, Wei C, Zeier M, Yu ASL, Torres VE, et al. : Soluble urokinase plasminogen activator receptor and decline in kidney function in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 30: 1305–1313, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al. : Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo S, Coresh J, Tin A, Rebholz CM, Chen TK, Hayek SS, et al. : Soluble urokinase-type plasminogen activator receptor in black Americans with CKD. Clin J Am Soc Nephrol 13: 1013–1021, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefer F, Trachtman H, Wühl E, Kirchner M, Hayek SS, Anarat A, et al. ESCAPE Trial Consortium and the 4C Study Group : Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr 171: e172914, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei C, Li J, Adair BD, Zhu K, Cai J, Merchant M, et al. : uPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J Clin Invest 129: 1946–1959, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Rosso M, Margheri F, Serratì S, Chillà A, Laurenzana A, Fibbi G: The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Curr Pharm Des 17: 1924–1943, 2011. [DOI] [PubMed] [Google Scholar]
- 30.De Witte H, Sweep F, Brunner N, Heuvel J, Beex L, Grebenschikov N, et al. : Complexes between urokinase-type plasminogen activator and its receptor in blood as determined by enzyme-linked immunosorbent assay. Int J Cancer 77: 236–242, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Sier CF, Sidenius N, Mariani A, Aletti G, Agape V, Ferrari A, et al. : Presence of urokinase-type plasminogen activator receptor in urine of cancer patients and its possible clinical relevance. Lab Invest 79: 717–722, 1999. [PubMed] [Google Scholar]
- 32.Gustafsson A, Ajeti V, Ljunggren L: Detection of suPAR in the saliva of healthy young adults: Comparison with plasma levels. Biomark Insights 6: 119–125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iversen E, Houlind MB, Eugen-Olsen J: Soluble urokinase receptor and acute kidney injury. N Engl J Med 382: 2166–2167, 2020. [DOI] [PubMed] [Google Scholar]
- 34.Mossanen JC, Pracht J, Jansen TU, Buendgens L, Stoppe C, Goetzenich A, et al. : Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci 18: 1662, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dal Monte M, Cammalleri M, Pecci V, Carmosino M, Procino G, Pini A, et al. : Inhibiting the urokinase-type plasminogen activator receptor system recovers STZ-induced diabetic nephropathy. J Cell Mol Med 23: 1034–1049, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ: Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care 24: 187, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap Consortium : The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95: 103208, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120: c179–c184, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988. [PubMed] [Google Scholar]
- 42.R Core Team : R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. 2014. Available at: http://www.R-project.org/. Accessed June 1, 2020
- 43.Chew-Harris J, Appleby S, Richards AM, Troughton RW, Pemberton CJ: Analytical, biochemical and clearance considerations of soluble urokinase plasminogen activator receptor (suPAR) in healthy individuals. Clin Biochem 69: 36–44, 2019. [DOI] [PubMed] [Google Scholar]
- 44.Thunø M, Macho B, Eugen-Olsen J: suPAR: The molecular crystal ball. Dis Markers 27: 157–172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, et al. : Structure of human urokinase plasminogen activator in complex with its receptor. Science 311: 656–659, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Isselbacher EM: Thoracic and abdominal aortic aneurysms. Circulation 111: 816–828, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Smith HW, Marshall CJ: Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11: 23–36, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Solberg H, Ploug M, Høyer-Hansen G, Nielsen BS, Lund LR: The murine receptor for urokinase-type plasminogen activator is primarily expressed in tissues actively undergoing remodeling. J Histochem Cytochem 49: 237–246, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Plesner T, Ralfkiaer E, Wittrup M, Johnsen H, Pyke C, Pedersen TL, et al. : Expression of the receptor for urokinase-type plasminogen activator in normal and neoplastic blood cells and hematopoietic tissue. Am J Clin Pathol 102: 835–841, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Nykjaer A, Møller B, Todd RF 3rd, Christensen T, Andreasen PA, Gliemann J, et al. : Urokinase receptor. An activation antigen in human T lymphocytes. J Immunol 152: 505–516, 1994. [PubMed] [Google Scholar]
- 51.Gussen H, Hohlstein P, Bartneck M, Warzecha KT, Buendgens L, Luedde T, et al. : Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care 7: 26, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diederichsen MZ, Diederichsen SZ, Mickley H, Steffensen FH, Lambrechtsen J, Sand NPR, et al. : Prognostic value of suPAR and hs-CRP on cardiovascular disease. Atherosclerosis 271: 245–251, 2018. [DOI] [PubMed] [Google Scholar]
- 53.Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL: suPAR: A new biomarker for cardiovascular disease? Can J Cardiol 31: 1293–1302, 2015. [DOI] [PubMed] [Google Scholar]
- 54.Lyngbæk S, Marott JL, Sehestedt T, Hansen TW, Olsen MH, Andersen O, et al. : Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol 167: 2904–2911, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, et al. : Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events [published correction appears in J Am Heart Assoc 4: e000563, 2015 10.1161/JAHA.114.000563]. J Am Heart Assoc 3: e001118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersen O, Eugen-Olsen J, Kofoed K, Iversen J, Haugaard SB: Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol 80: 209–216, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Langkilde A, Petersen J, Klausen HH, Henriksen JH, Eugen-Olsen J, Andersen O: Inflammation in HIV-infected patients: Impact of HIV, lifestyle, body composition, and demography - a cross sectional cohort study. PLoS One 7: e51698, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Covid-19 and ACE2 in cardiovascular, Lung, and kidney working Group : Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 31: 1380–1383, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. : Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, et al. Acute Dialysis Quality Initiative Consensus XIII Work Group : Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J Am Soc Nephrol 27: 371–379, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER: beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci U S A 95: 7074–7079, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mustonen J, Outinen T, Laine O, Pörsti I, Vaheri A, Mäkelä S: Kidney disease in Puumala hantavirus infection. Infect Dis (Lond) 49: 321–332, 2017. [DOI] [PubMed] [Google Scholar]
- 63.Mittler E, Dieterle ME, Kleinfelter LM, Slough MM, Chandran K, Jangra RK: Hantavirus entry: Perspectives and recent advances. Adv Virus Res 104: 185–224, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hägele S, Müller A, Nusshag C, Reiser J, Zeier M, Krautkrämer E: Motility of human renal cells is disturbed by infection with pathogenic hantaviruses. BMC Infect Dis 18: 645, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al. : Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 98: 228–231, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, et al. : COVID-19-Related glomerulopathy: A report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med 2: 488–492, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han R, Hu S, Qin W, Shi J, Hou Q, Wang X, et al. : C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis [published correction appears in JCI Insight 4: e130986, 2019 10.1172/jci.insight.130986]. JCI Insight 4: e122912, 2019 [Google Scholar]
- 68.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, et al. PodoNet and FSGS CT Study Consortia : Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 23: 2051–2059, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincenti F, Rashmi P, Da Silva AA, Shoji J, Craik C, Sarwal M: Neutralization of upar with an anti-upar antibody ameliorates recurrent FSGS sera induced podocyte injury. Nephrol Dial Transpl 35[Suppl 3]: gfaa140, 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.