Significance Statement
Predicting outcomes on the basis of renal histology after a radical nephrectomy has been limited to studies with small sample sizes, short follow-ups, and only a descriptive characterization of histology. In this study of 936 patients followed up for a median of 6.4 years after establishing a new baseline kidney function following radical nephrectomy, the authors used morphometric methods to quantitatively characterize microstructural features on large renal wedge sections. Findings of larger nephrons and more extensive glomerulosclerosis and interstitial fibrosis predicted progressive CKD; interstitial fibrosis also predicted mortality from causes other than cancer. These findings provide insight into the important microstructural features of “generic” CKD in patients without a specific kidney disease and support the use of quantitative methods to evaluate renal histology after a radical nephrectomy to determine patients’ long-term risks.
Keywords: Nephrectomy, kidney biopsy, interstitial fibrosis, glomerulosclerosis, nephron, progression of chronic renal failure, glomerulus, mortality risk
Abstract
Background
Nephron hypertrophy and nephrosclerosis may be important determinants of CKD and mortality. However, studies of outcomes associated with these microstructural features have been limited to small tissue specimens from patients selected for either good kidney health or known kidney disease.
Methods
To determine whether microstructural features are predictive of progressive CKD and mortality outcomes, we studied patients who underwent a radical nephrectomy for a tumor. Large wedge sections of renal parenchyma distal to the tumor were stained and scanned into high-resolution images; we annotated the cortex and all glomeruli to calculate glomerular volume, cortex volume per glomerulus, and percentage of globally sclerotic glomeruli. Morphometric measurements also included percentages of artery luminal stenosis and interstitial fibrosis/tubular atrophy (IF/TA) of the cortex. At follow-up visits every 6–12 months, we determined which patients experienced progressive CKD (defined as dialysis, kidney transplantation, or a 40% decline from postnephrectomy eGFR). Cox models for these outcomes were adjusted for age, sex, body mass index, hypertension, diabetes, smoking, eGFR, and proteinuria.
Results
Among 936 patients (mean age, 64 years; postnephrectomy baseline eGFR, 48 ml/min per 1.73 m2), 117 progressive CKD events, 183 noncancer deaths, and 116 cancer deaths occurred during a median follow-up of 6.4 years. Larger glomerular volume, larger cortex per glomerulus, and higher percentage of globally sclerotic glomeruli or IF/TA predicted progressive CKD. Higher percentage IF/TA also predicted noncancer mortality. Microstructural features did not predict cancer mortality or recurrence.
Conclusions
After a radical nephrectomy, larger nephrons and nephrosclerosis predicted progressive CKD, and IF/TA predicted noncancer mortality. Morphometric analysis of renal parenchyma can predict noncancer clinical events in patients long after their radical nephrectomy.
Most knowledge on how kidney structural pathology predicts adverse outcomes is on the basis of highly select groups of patients who have an overt nephropathy (declining GFR, substantial proteinuria, or active urine sediment) that justifies the risk of obtaining a kidney biopsy. Less is known about the relationship between kidney structural pathology and outcomes in the general population. Larger nephrons and nephrosclerosis (also described as “chronic changes”) are modestly predictive of intermediate outcomes (hypertension, proteinuria, and GFR decline) in living kidney donors.1 However, living kidney donors are selected on health such that they have a normal GFR, normal urine protein, and few, if any, risk factors for CKD. Further, the study of structural-outcome associations in these nephrology populations has relied on small needle core tissue biopsies with limited precision for structural pathology.
Patients who undergo a radical nephrectomy for a tumor (usually renal cancer) are a unique population for the study of kidney structure-outcome associations. Although these patients often have significant comorbidities, they are a population that is selected neither for the absence of nephropathy (such as living kidney donors) nor for the presence of nephropathy (such as patients biopsied by a nephrologist). Further, large wedge sections of kidney parenchyma distal to the tumor allow for accurate and precise characterization of structural pathology. Prior kidney structure-outcome associations in patients undergoing nephrectomy have used small samples (49–222 patients) with short follow-up and had limited and only descriptive characterization of the structural pathology.2–7 Adjustment for baseline kidney function has been lacking or inadequate. Many of these studies also included patients who had a partial nephrectomy. However, the small rim of nontumor kidney parenchyma surrounding the tumor in partial nephrectomy tissue samples is often distorted by the tumor itself.
The College of American Pathologists recommends evaluation of the nontumor parenchyma after nephrectomy.8 Although pathologists will detail any specific glomerular or interstitial disease processes they detect on histology, measures of nephron size, such as glomerular volume, and accurate measures of nephrosclerosis (e.g., interstitial fibrosis/tubular atrophy [IF/TA]) are usually not reported. Notably, larger glomeruli and nephrosclerosis are frequently present in the parenchyma of patients with tumors.3,7,9 However, the clinical and/or pathophysiologic importance of these features for predicting the risk of progressive CKD or mortality is unclear. Specifically, it is uncertain whether microstructural features predict these outcomes independent of readily available kidney function and CKD risk factor assessments. Thus, we performed a large study to predict outcomes from morphometric measures among patients who underwent a radical nephrectomy for tumor. Our objective was to determine whether nephron size and nephrosclerosis were predictive of progressive CKD, noncancer mortality, cancer mortality, or cancer recurrence.
Methods
Study Population
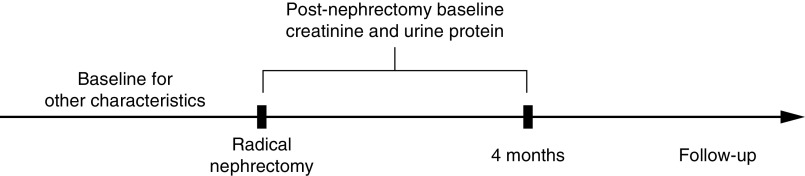
The study sample was an expansion of a previously described cohort of patients who are part of the Mayo Clinic Nephrectomy Registry.10,11 This cohort has undergone a radical nephrectomy for a renal tumor at Mayo Clinic Minnesota from 2000 to 2015. We only studied patients who underwent a complete unilateral nephrectomy for a renal tumor (usually renal cancer) with no metastatic lesions or positive lymph nodes at the time of surgery. Medical comorbidities did not necessarily exclude the patient from undergoing radical nephrectomy unless surgical risk outweighed the benefits; thus, this older cohort was enriched with CKD risk factors. The goal was to study long-term changes in kidney function predicted by kidney structural findings rather than early changes in kidney function directly due to the nephrectomy. Thus, baseline kidney function (serum creatinine and proteinuria) was defined using postnephrectomy levels obtained during the first 4 months after the nephrectomy (Figure 1). The standard follow-up visit with laboratory testing was at 3 months after surgery, and this provided time for postsurgical recovery. Patients without any serum creatinine testing between the nephrectomy and 4 months after nephrectomy were excluded. Other baseline characteristics were defined at or just prior to the nephrectomy. Patients with cancer recurrence, death, or kidney failure during the first 4 months after the nephrectomy were excluded, and follow-up outcomes were assessed after the time point of 4 months postnephrectomy. Patients with a diffuse specific kidney disease on histology (other than mild-moderate diabetic nephropathy), severe and diffuse tubulointerstitial inflammation, severe ischemia with IF/TA (>90%), a large focal scar, or end stage kidney histology (thinned and nearly completely scarred cortex) were also excluded. Segmental sclerosis was rare and minimal in this cohort.11 The study was approved by the Mayo Clinic Institutional Review Board.
Figure 1.
Patients were followed from the time of their nephrectomy. Baseline for serum creatinine and urine protein was defined during the first 4 months postnephrectomy in order to study the nonsurgical changes in kidney function during follow-up. Thus, follow-up for outcomes started after 4 months postnephrectomy. Baseline for other characteristics was defined at or just prior to the nephrectomy.
Kidney Function and Risk Factors
The medical records of patients with renal tumor were reviewed to obtain baseline age, sex, body mass index (BMI), serum creatinine (corrected to standardized values if assayed prestandardization), 24-hour urine protein, hypertension (as documented in medical record), diabetes mellitus (as documented in medical record), cigarette smoking, and renal cancer type/stage. The eGFR was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.12 If multiple serum creatinine levels were available, the one closest to but before 4 months after the nephrectomy was used for baseline. Postnephrectomy baseline eGFR levels were a mean 3.5 ml/min per 1.73 m2 higher during the first month postnephrectomy compared with months 2–4. However, findings were reported without correction for this 3.5-ml/min per 1.73 m2 difference as findings were not substantively different with correction (not shown). The 24-hour urine protein excretion was estimated from a spot urine protein-osmolality ratio.13 Among patients who had both prenephrectomy (up to 1 year before) and postnephrectomy (up to 4 months after) urine protein testing, the median levels were not meaningfully different (n=559; 135 mg/24 h [prenephrectomy] versus 123 mg/24 h [postnephrectomy]; P=0.18, Wilcoxon signed-rank test). Thus, prenephrectomy urine protein levels were used for the postnephrectomy baseline if a postnephrectomy level was not obtained during the first 4 months. The renal tumor volume was calculated using the ellipsoid formula from the three orthogonal linear dimensions of the tumor (a, b, c) on the preoperative computed tomography or magnetic resonance imaging scan [volume =(1/6)π×a×b×c].14
Kidney Microstructure
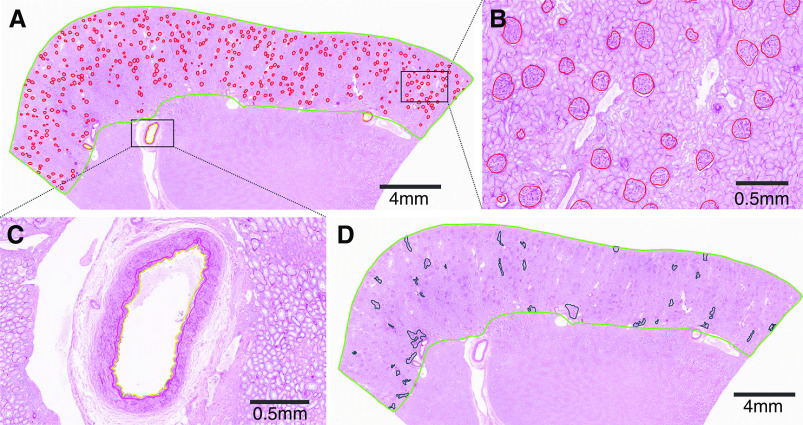
The stored formalin-fixed whole-kidney specimens were retrieved to obtain a large wedge section of parenchyma that was distant from the tumor-involved tissue, which was then embedded in paraffin. A 3-µm-thick section was cut from the paraffin-embedded tissue block, stained with periodic acid–Schiff, and scanned into high-resolution digital images (Aperio XT system scanner; Leica Microsystems, Inc., Buffalo Grove, IL; http:/www.aperio.com). Biopsy images were morphometrically analyzed by investigators masked to age and other clinical characteristics to minimize bias. The scanned digital images were magnified with Image Scope software (version 12.2.2.5015 Aperio) onto a large touch-screen tablet. The entire cortex was outlined. At high magnification, every glomerular cross-sectional profile was traced (Figure 2, A and B) to calculate the mean nonsclerotic glomerular tuft volume and the volume of cortex per (nonsclerotic) glomerulus (Supplemental Material).15,16 This cortex per glomerulus is intended to be a measure of the average size of normal nephrons, but IF/TA contributes to part of the volume of the cortex. Thus, we also calculated nonfibrotic cortex per glomerulus by using only the area of cortex that did not contain IF/TA (Supplemental Material). The mean cross-sectional tubular area for 1 mm2 of cortex was obtained in the middle of the wedge section, approximately equidistant between the capsule and corticomedullary junction using the same approach our group previously applied to kidney donor biopsies (Supplemental Figure 1, Supplemental Material).17 The number of globally sclerotic glomeruli (GSG) divided by the number of all glomeruli (sclerosed or nonsclerosed) was used to calculate percentage GSG.10,18 Arteriosclerosis was assessed by the average percentage luminal stenosis (from intimal thickening) using the three most orthogonal small- to medium-sized arteries on the wedge section.11 The percentage luminal stenosis was determined from the area of intima divided by the area of intima and lumen (Figure 2C). IF/TA was assessed for the entire cortex. To determine percentage IF/TA, the total areas of all traced regions containing IF/TA were divided by the total area of cortex, as previously described (Figure 2D).11
Figure 2.
Morphometry was performed on wedge sections. (A) An example of a periodic acid–Schiff-stained wedge section, with cortex outline in green trace. (B) Nonsclerotic glomeruli (red trace) and GSG were traced manually. (C) An example of an artery with labeled media to intima boundary (red trace) and intima to lumen (yellow trace) to quantify percentage luminal stenosis. (D) Percentage interstitial fibrosis (black trace) was assessed in the whole cortex.
Outcomes
As part of the postsurgical care to monitor for cancer recurrence and CKD, patients had a postoperative follow-up visit every 3–6 months during the first year and 6–12 months thereafter. The last follow-up visit allowed in this study was April 6, 2019. These visits included a prescheduled serum creatinine level. Patients who did not return were contacted by phone to determine vital status, and if alive, they were surveyed or had outside medical records reviewed for recent serum creatinine levels, dialysis, and kidney transplantation. Progressive CKD was defined by a 40% or more decline in eGFR, dialysis or kidney transplantation, or development of an eGFR below 10 ml/min per 1.73 m2. If the patient died, the death certificate was obtained to determine the cause of death. If the death certificate could not be obtained, cause of death was verified with the patient’s local physician or family. Cause of death was divided into cancer mortality and noncancer mortality.
Statistical Analyses
The risks of progressive CKD, noncancer mortality, cancer mortality, and cancer recurrence were assessed using separate Cox proportional hazards models. Competing risk models were not used because the goal was to understand biologic determinants of each outcome rather than to determine true incidence rates. Thus, Cox models for progressive CKD were censored at last eGFR or death; Cox models for noncancer mortality and for cancer morality were censored at last follow-up or death. The risk of each of these outcomes was assessed for each doubling of the microstructural features for nephron size (glomerular volume, cortex per glomerulus, and tubular area) and for nephrosclerosis (percentage IF/TA, percentage GSG, and percentage luminal stenosis). This was done by using the log2 transform of the microstructure features. Models were unadjusted; adjusted for age, sex, BMI, hypertension, diabetes, and smoking at baseline; further adjusted for postnephrectomy baseline eGFR; and further adjusted for postnephrectomy baseline proteinuria. The C statistics for models were computed and then corrected for overfitting with tenfold crossvalidation. Models on the basis of clinical predictors alone, structural predictors alone, and clinical and structural predictors were compared using the Wald test or likelihood ratio test, as appropriate. Spline plots were generated to assess whether the hazard ratios had a nonlinear association with the outcomes. A sensitivity analysis assessed the risk of progressive CKD with censoring at cancer recurrence. Another analysis assessed the risk of progressive CKD with structural predictors in obese (BMI>30 kg/m2) versus nonobese patients. We also assessed whether the tumor size and stage of renal cancer affected the risk of progressive CKD or noncancer mortality.
Results
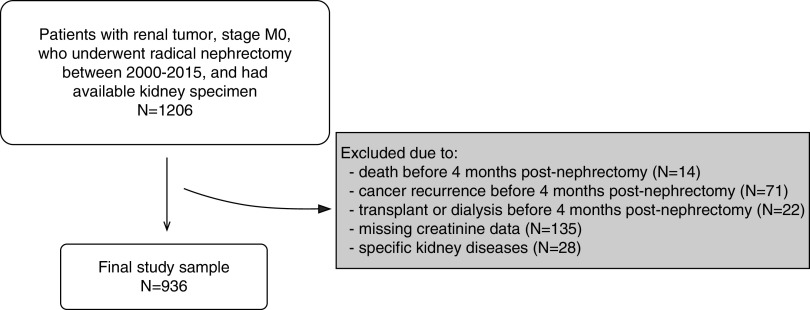
We identified 1206 patients at Mayo Clinic who had a radical nephrectomy from 2000 to 2015. There were 107 patients excluded due to death, cancer recurrence, dialysis, or kidney transplantation within 4 months of surgery. Another 135 patients were excluded due to missing serum creatinine. Twenty-eight patients were excluded for specific kidney diseases observed on histology (Supplemental Table 1). After exclusions, there were 936 patients in the final sample to study progressive CKD and mortality outcomes (Figure 3). Patients with findings on biopsy consistent with hypertensive nephrosclerosis or diabetic nephropathy were not excluded. Clinical characteristics, tumor findings, biopsy findings, and outcomes of the studied cohort are summarized in Table 1.
Figure 3.
Selection of study sample. Patients had to survive cancer-free and without kidney failure for the first 4 months after the nephrectomy. M0, no metastasis.
Table 1.
Cohort characteristics of 936 patients with renal tumor
| Cohort Characteristic | Value |
|---|---|
| Baseline clinical characteristics | |
| Age, yr | 63.7 (12.0) |
| Men, % | 593 (63.4%) |
| White | 861 (92.0%) |
| BMI, kg/m2 | 30.7 (6.7) |
| Hypertension, % | 614 (65.6%) |
| Diabetes mellitus, % | 119 (12.7%) |
| Current smoker, % | 127 (13.6%) |
| Baseline tumor burden | |
| Tumor stages | |
| Benign tumor or tumor could not be staged | 69 (7.4%) |
| 1A | 109 (11.6%) |
| 1B | 220 (23.5%) |
| 2A | 113 (12.1%) |
| 2B | 64 (6.8%) |
| 3A | 286 (30.6%) |
| 3B | 55 (5.9%) |
| 3C | 12 (1.3%) |
| 4 | 8 (0.9%) |
| Tumor volume, cm3 | 97 [40–226] |
| Kidney function | |
| Prenephrectomy creatinine, mg/dl | 1.1 (0.3) |
| Prenephrectomy eGFR, ml/min per 1.73 m2 | 71.9 (18.3) |
| Postnephrectomy baseline 24-h urine protein, mga | 124 [72–272] |
| Postnephrectomy baseline creatinine, mg/dl | 1.5 (0.4) |
| Postnephrectomy baseline eGFR, ml/min per 1.73 m2 | 48.0 (13.2) |
| Baseline kidney microstructural features | |
| Biopsy cortex area, mm2 | 141.2 (57.2) |
| No. of sampled glomeruli | 349 (161) |
| NSG volume, mm3 | 0.0028 (0.0011) |
| Cortex volume per glomerulus, mm3 | 0.076 (0.035) |
| Tubular cross-sectional area, μm2 | 5989 (1478) |
| IF/TA, % | 4.2 (8.2) |
| GSG, % | 9.1 (9.3) |
| Artery luminal stenosis, % | 55.5 (15.1) |
| Outcomes during follow-up | |
| Progressive CKD | 117 (12.5%) |
| Noncancer death, % | 183 (19.6%) |
| Cancer death, % | 116 (12.4%) |
| Cancer recurrence, % | 236 (25.2%) |
Data are presented as mean (SD), median [25%–75%], or n (%). NSG, nonsclerotic glomeruli.
Proteinuria was only available on 816 patients.
The median time to progressive CKD or last available eGFR was 5.3 years. The median number of follow-up serum creatinine levels was 14 (interquartile range, 9–24) per patient. Progressive CKD occurred in 117 patients during follow-up (36 required dialysis or received a kidney transplant). Progressive CKD was predicted by microstructural features of larger nephron size and more severe nephrosclerosis in unadjusted analysis (Table 2). The risk of progressive CKD with larger glomerular volume, larger cortex per glomerulus, larger nonfibrotic cortex per glomerulus, higher percentage IF/TA, and higher percentage GSG persisted even after adjusting for age, sex, CKD risk factors, eGFR, and proteinuria. Repeating the analysis with censoring at cancer recurrence modestly strengthened the prediction of progressive CKD by glomerular volume, cortex per glomerulus, percentage IF/TA, and percentage GSG (Supplemental Table 2). These associations were similar in patients with BMI<30 kg/m2 compared with those with BMI≥30 kg/m2 (Supplemental Table 3). Figure 4 shows the unadjusted higher risk of progressive CKD with larger glomerular volume, larger cortex per glomerulus, higher percentage IF/TA, and higher percentage GSG. Spline plots (not shown) revealed these associations to be linear across the observed range of these microstructural predictors (log scaled). Of the clinical predictors, only older age, higher BMI, and higher proteinuria were independently predictive of progressive CKD; tumor stage and tumor volume were not predictive of progressive CKD (Supplemental Table 4). A model predicting progressive CKD on the basis of microstructural predictors alone showed similar predictive discrimination to a model on the basis of clinical predictors alone (C statistic: 0.695 versus 0.687; Wald test P=0.37). However, a model on the basis of microstructural and clinical predictors outperformed a model on the basis of clinical predictors alone (C statistic: 0.724 versus 0.687; likelihood ratio test P=0.001).
Table 2.
Structural predictors of CKD progression from 4 months after a radical nephrectomy
| Predictor (per Doubling) | Unadjusted HR (95% CI) | P Value | Adjusted for Age, Sex, BMI, HTN, DM, and Smoking HR (95% CI) | P Value | Further Adjusted for eGFR HR (95% CI) | P Value | Further Adjusted for eGFR and Proteinuriaa HR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Nephron size | ||||||||
| Nonsclerotic glomerular volume | 2.41 (1.69 to 3.42) | <0.001 | 2.49 (1.68 to 3.69) | <0.001 | 2.40 (1.62 to 3.55) | <0.001 | 2.25 (1.45 to 3.49) | <0.001 |
| Cortex per glomerulus | 2.58 (1.90 to 3.50) | <0.001 | 2.37 (1.69 to 3.31) | <0.001 | 2.25 (1.60 to 3.15) | <0.001 | 2.07 (1.40 to 3.04) | <0.001 |
| Nonfibrotic cortex per glomerulus | 2.33 (1.69 to 3.20) | <0.001 | 2.11 (1.50 to 2.96) | <0.001 | 2.01 (1.43 to 2.82) | <0.001 | 1.84 (1.25 to 2.69) | <0.001 |
| Tubular cross-sectional area | 1.78 (1.05 to 3.02) | 0.03 | 1.64 (0.94 to 2.83) | 0.08 | 1.57 (0.91 to 2.73) | 0.11 | 1.48 (0.81 to 2.71) | 0.20 |
| Nephrosclerosis, % | ||||||||
| IF/TA | 1.28 (1.18 to 1.40) | <0.001 | 1.21 (1.09 to 1.33) | <0.001 | 1.19 (1.08 to 1.32) | <0.001 | 1.17 (1.05 to 1.31) | 0.006 |
| GSG | 1.54 (1.33 to 1.77) | <0.001 | 1.39 (1.17 to 1.65) | <0.001 | 1.34 (1.12 to 1.60) | 0.001 | 1.32 (1.08 to 1.62) | 0.006 |
| Artery luminal stenosis | 2.03 (1.29 to 3.20) | 0.002 | 1.57 (0.98 to 2.51) | 0.06 | 1.51 (0.95 to 2.42) | 0.08 | 1.26 (0.76 to 2.10) | 0.37 |
CKD progression was defined as eGFR reduction by ≥40%, need for dialysis, or kidney transplantation. Sample size was 936 with 117 events. HR, hazard ratio; 95% CI, 95% confidence interval; HTN, hypertension; DM, diabetes mellitus.
Proteinuria subgroup analysis had 816 patients with 95 events.
Figure 4.
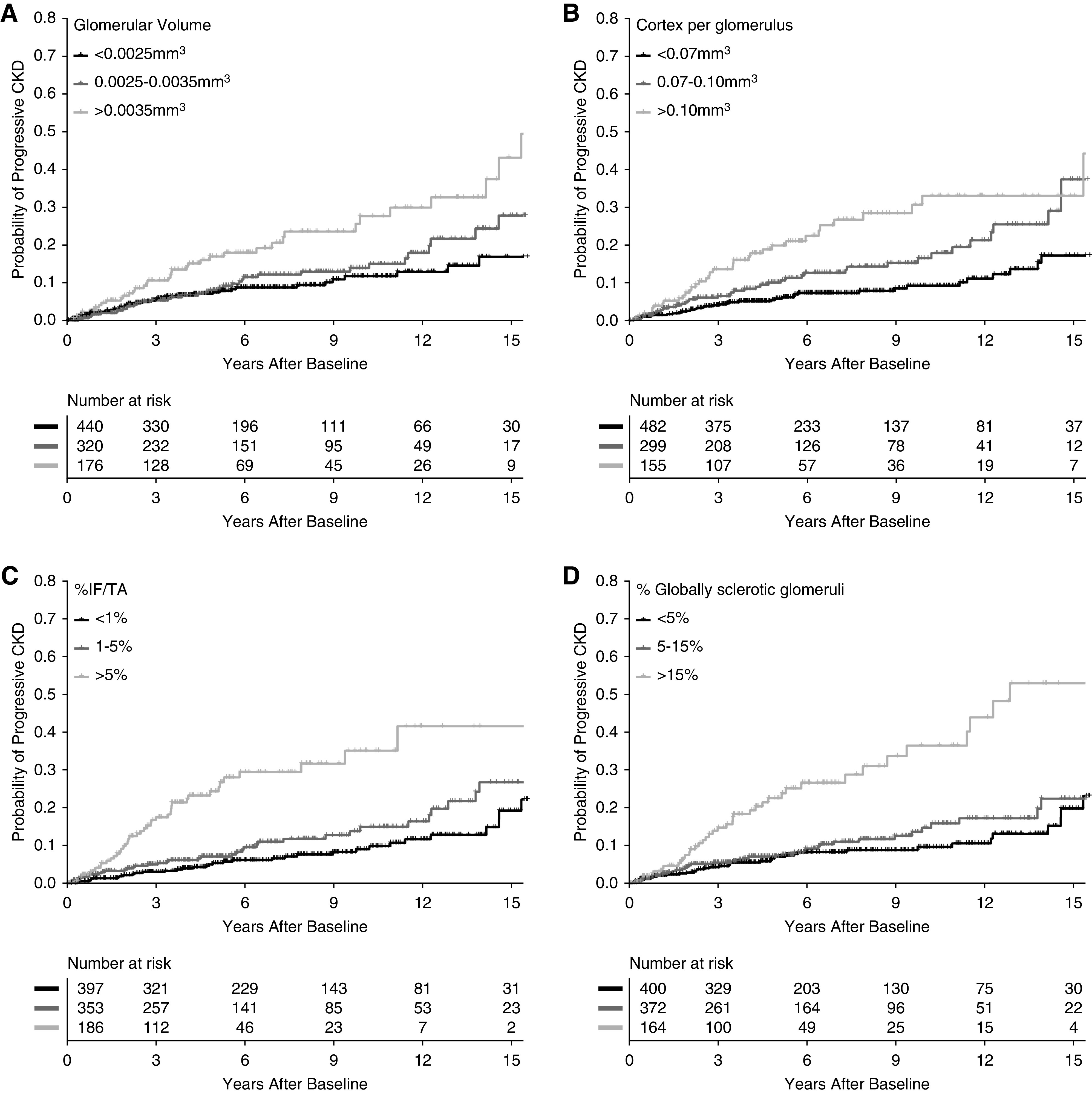
Risk of progressive CKD with microstructural predictors. Cumulative incidence of progressive CKD increased with (A) larger glomerular volume, (B) larger cortex per glomerulus, (C) higher percentage IF/TA, and (D) higher percentage GSG.
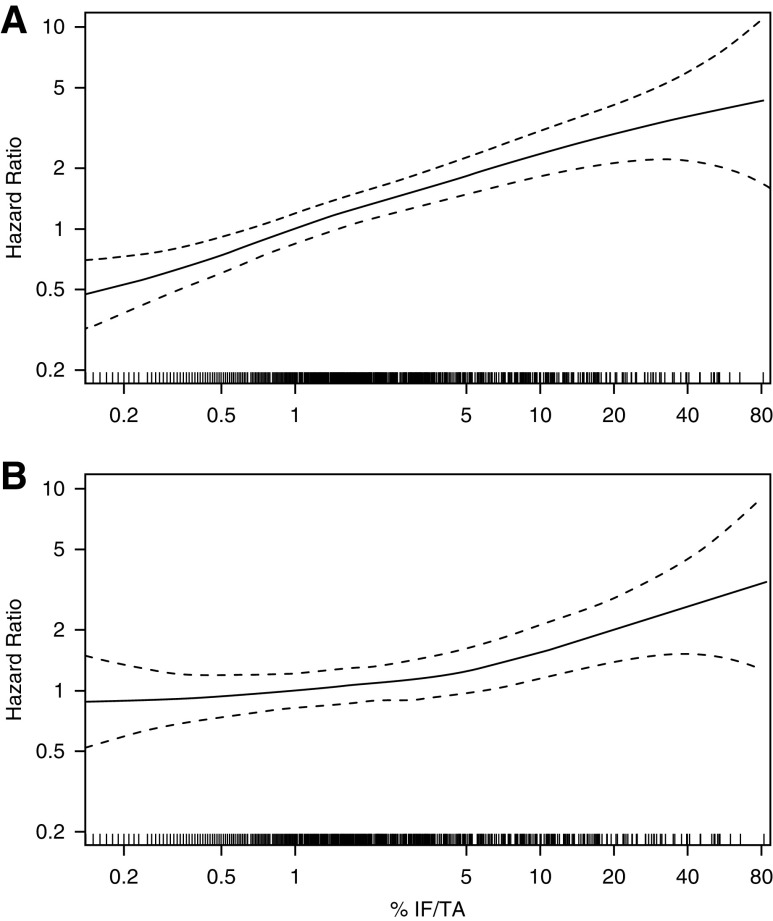
The median time to death or last follow-up was 6.4 years. There were 299 deaths; 183 were due to noncancer mortality, and 116 were due to cancer mortality. Noncancer mortality was predicted by larger cortex per glomerulus and higher levels of nephrosclerosis in unadjusted analysis (Table 3). The risk of noncancer mortality with percentage IF/TA persisted even after adjusting for age, sex, CKD risk factors, eGFR, and proteinuria. The unadjusted risk of noncancer mortality progressively increased with higher levels of percentage IF/TA (log scaled) in a linear relationship (Figure 5). However, the risk of noncancer mortality in the fully adjusted model was nonlinear; the risk only began to increase after percentage IF/TA exceeded about 5%. Of the clinical characteristics, only older age, men, higher BMI, and higher proteinuria were independently predictive of noncancer mortality (Supplemental Table 5). A model predicting noncancer mortality on the basis of microstructural predictors alone showed weaker predictive discrimination than a model on the basis of clinical predictors alone (C statistic: 0.701 versus 0.779; Wald test P<0.001). Further, a model on the basis of microstructural and clinical predictors was not clearly more predictive than a model on the basis of clinical predictors alone (C statistic: 0.792 versus 0.779; likelihood ratio test P=0.06). Cancer mortality was not predicted by any of the microstructural findings in unadjusted or adjusted models (Supplemental Table 6). Cancer recurrence was predicted by higher percentage IF/TA in unadjusted analysis but not after full adjustment for clinical characteristics (Supplemental Table 7).
Table 3.
Structural predictors of noncancer mortality from 4 months after a radical nephrectomy
| Predictor (per Doubling) | Unadjusted HR (95% CI) | P Value | Adjusted for Age, Sex, BMI, HTN, DM, and Smoking HR (95% CI) | P Value | Further Adjusted for eGFR HR (95% CI) | P Value | Further Adjusted for eGFR and Proteinuriaa HR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|---|
| Nephron size | ||||||||
| Nonsclerotic glomerular volume | 1.27 (0.96 to 1.69) | 0.10 | 1.38 (1.01 to 1.87) | 0.04 | 1.37 (1.01 to 1.85) | 0.05 | 1.32 (0.95 to 1.84) | 0.10 |
| Cortex per glomerulus | 1.58 (1.23 to 2.04) | <0.001 | 1.35 (1.03 to 1.76) | 0.03 | 1.33 (1.02 to 1.74) | 0.04 | 1.23 (0.92 to 1.65) | 0.15 |
| Nonfibrotic cortex per glomerulus | 1.32 (1.02 to 1.71) | 0.04 | 1.16 (0.90 to 1.51) | 0.25 | 1.15 (0.88 to 1.49) | 0.31 | 1.07 (0.81 to 1.41) | 0.64 |
| Tubular cross-sectional area | 1.44 (0.95 to 2.18) | 0.09 | 1.35 (0.88 to 2.08) | 0.17 | 1.34 (0.87 to 2.05) | 0.19 | 1.25 (0.80 to 1.96) | 0.32 |
| Nephrosclerosis, % | ||||||||
| IF/TA | 1.28 (1.20 to 1.37) | <0.001 | 1.14 (1.05 to 1.24) | 0.001 | 1.14 (1.05 to 1.23) | 0.002 | 1.13 (1.04 to 1.23) | 0.005 |
| GSG | 1.51 (1.35 to 1.69) | <0.001 | 1.10 (0.96 to 1.27) | 0.17 | 1.09 (0.94 to 1.26) | 0.26 | 1.11 (0.94 to 1.30) | 0.24 |
| Artery luminal stenosis | 2.44 (1.66 to 3.59) | <0.001 | 1.32 (0.89 to 1.95) | 0.16 | 1.31 (0.89 to 1.94) | 0.17 | 1.27 (0.83 to 1.95) | 0.27 |
Sample size was 936 with 183 events. HR, hazard ratio; 95% CI, 95% confidence interval; HTN, hypertension; DM, diabetes mellitus.
Proteinuria subgroup analysis had 816 patients with 161 events.
Figure 5.
More severe percentage fibrosis is a predictor of noncancer mortality. (A) In unadjusted analysis, cumulative incidence of noncancer mortality is worse with increasing percentage fibrosis. (B) After adjusting for all clinical characteristics, fibrosis >5% associated with increased risk of noncancer mortality. Curves represent hazard ratios relative to 1% fibrosis, and dashed curves represent 95% confidence intervals.
Discussion
In this study, we identified several microstructural features of the nontumor kidney parenchyma that were predictive of progressive CKD and noncancer mortality. By assessing risks at least 4 months after the nephrectomy, these analyses determined the long-term effect of microstructural features on kidney health and morality. These structure-outcome associations were independent of clinical characteristics and routine laboratory tests obtained at the time of a radical nephrectomy, including kidney function tests. Several measures of larger nephron size and nephrosclerosis were predictive of progressive CKD, whereas only IF/TA was predictive of noncancer mortality. The lack of microstructural features associating with cancer mortality or cancer recurrence served as a “negative control” for these data. Indeed, censoring at cancer recurrence only increased the strength of each microstructural association with progressive CKD. These data identify important microstructural predictors of progressive CKD and noncancer mortality in a population that is neither healthy nor has a disease that would typically warrant a kidney biopsy.
Hypertrophy of glomeruli and tubules occurs early in many kidney diseases, including diabetic nephropathy.19,20 This glomerular hypertrophy may lead to proteinuria via a disorganized glomerular structure that loses efficiency in preventing the leakage of protein.21 The risk of progressive CKD with larger nephron size remained even after adjustment for proteinuria. Reduction in the number of functioning nephrons from the radical nephrectomy itself will further glomerular enlargement and glomerular hypertension in the remaining nephrons.22,23 As a consequence of glomerulomegaly, podocyte dysfunction and detachment can lead to collapse of the glomerular tuft with ensuing global glomerulosclerosis.21 Larger cortex per (nonsclerosed) glomerulus also predicted progressive CKD. Cortex per glomerulus is a measure of nephron size in healthy populations16 but may less accurately reflect nephron size in less healthy populations. Specifically, cortex per glomerulus may be inflated from IF/TA replacing normal cortex. To address this, we performed analysis using nonfibrotic cortex per glomerulus (to more accurately reflect the size of healthy-appearing nephrons), but this predicted progressive CKD to a similar extent. This suggests that any bias caused by IF/TA with the use of cortex per glomerulus to estimate nephron size is minimal.
Nephrosclerosis also predicted progressive CKD. Specifically, percentage IF/TA and percentage GSG were predictive of progressive CKD even after adjustment for eGFR and proteinuria and for risk factors including diabetes and hypertension. Because percentage artery luminal stenosis did not predict progressive CKD, ischemic injury from arteriosclerosis is unlikely to be the primary pathway by which glomerulosclerosis and interstitial fibrosis predict progressive CKD in this population. The underlying cause of CKD in many patients is unknown or is simply attributed to hypertension.24 Because persons with diffuse specific kidney diseases on histology were excluded, this nephrosclerosis may reflect a type of “generic” CKD, possibly from past AKI events. However, histologic assessment was limited to a periodic acid–Schiff staining alone, and further studies are needed to determine the etiologies of nephrosclerosis.
The percentage IF/TA was predictive of noncancer mortality independent of clinical characteristics, including eGFR and proteinuria. Interestingly, percentage IF/TA was continuously associated with noncancer mortality in unadjusted analysis, but after adjusting for kidney function and CKD risk factors, the noncancer mortality risk with percentage IF/TA seems to have a threshold effect. Specifically, the risk of noncancer mortality was only increased for levels of percentage IF/TA above about 5%. This could imply that the mortality risk associated with mild percentage IF/TA is already detected with routine kidney function and CKD risk factor assessment. However, the mortality risk from moderate to severe percentage IF/TA requires a kidney biopsy to detect. Because larger nephron size and higher percentage GSG were not also predictive of noncancer mortality, the noncancer mortality predicted by percentage IF/TA may not be due to a renal-limited disease. Rather, interstitial fibrosis in the kidney may be reflective of systemic “end organ damage,” affecting multiple tissues beyond the kidney. Fibrosis is well recognized as the final pathology for many disease processes that can affect multiple organs.25
Prior work that has evaluated measures of nephron size and nephrosclerosis as predictors of progressive CKD has largely been done in living kidney donors. To some extent, these studies in healthy populations mirror those in this current population enriched with comorbidities. Larger glomeruli and percentage IF/TA abnormal for age predict an eGFR<60 ml/min per 1.73 m2, and larger cortex per glomerulus predicts higher albuminuria early after kidney donation.1 Another study looked at long-term outcomes of 310 donors and found that glomerulosclerosis and increased interstitial fibrosis predict a more pronounced eGFR decline after donation.26 Recipients of kidneys from living donors who have higher percentage IF/TA and larger cross-sectional tubular area are at a higher risk for graft failure.27 Studies in patients with specific kidney diseases have also found measures of nephrosclerosis and nephron size to be predictive of progressive CKD.28–31 This study demonstrates that in a population that does not have a specific kidney disease that would typically warrant a kidney biopsy (other than diabetic nephropathy and hypertensive nephrosclerosis), underlying larger nephron size and nephrosclerosis are predictors of progressive CKD.
These findings have specific relevance to the medical care of patients who undergo a radical nephrectomy for a renal tumor and can be compared to prior kidney structure-outcome studies in this patient group. A study of 73 patients followed for a mean of 1 year did not find glomerulosclerosis, arteriosclerosis, or interstitial fibrosis to predict eGFR decline.6 Conversely, a study of 156 patients followed a mean of 4 years found that arteriosclerosis, glomerulosclerosis, and interstitial fibrosis predicted a rise in serum creatinine.3 Another study in 49 patients found that for each 10% increase of glomerulosclerosis, there was a 9% decrease in eGFR at 6 months postoperative follow-up.2 A study in 222 patients followed for a mean of 4 years found glomerulosclerosis, arteriosclerosis, and interstitial fibrosis in adjacent non-neoplastic kidney tissue to predict eGFR decline.5 Overall, this study expands on this prior literature with a larger sample size, longer follow-up, less distorted histology by excluding partial nephrectomy specimens, focus on the nonsurgical decline in eGFR after nephrectomy, accurate and quantitative morphologic measures of nephrosclerosis, assessment of nephron size as a predictor, and more thorough adjustment for CKD risk factors and kidney function (including proteinuria).
These findings may have implications for the management of patients who have a renal tumor. First, histologic assessment of the nontumor parenchyma is recommended by the guidelines from the International Collaboration on Cancer Reporting.8 Indeed, 28 of 1206 (2.3%) had histologic evidence of severe and diffuse patterns of specific kidney diseases. Even when a specific kidney disease is not present, assessment of nephron size and nephrosclerosis provides prognostic information for CKD progression beyond CKD risk factors, eGFR, and proteinuria. First, patients at higher risk for progressive CKD on the basis of microstructural features may warrant closer monitoring to treat CKD risk factors and monitor for CKD progression. Second, these findings provide further support for preferential use of partial rather than radical nephrectomies when possible.32,33 Even if a patient has relatively normal kidney function, these data suggest there could still be subclinical nephrosclerosis and nephron enlargement with an increased risk for progressive CKD such that nephron-sparing surgery would be preferred. Third, identifying larger nephrons may guide the targeted use of antihypertensive agents or other medications to reduce glomerular hypertension and hypertrophy. Currently, nephron size is only assessed with the subjective descriptor of glomerulomegaly by the pathologist. More refined assessment of nephron size using artificial intelligence–based image analysis to measure the size of glomeruli and tubules may be needed to make the morphometric evaluation of nephrectomy specimens practical.34
Our study has several limitations. Although efforts were made to confirm the cause of the death, the cause of death was sometimes obtained from the death certificate and may not always be accurate. The study population was predominantly white, thus precluding meaningful analyses to assess for any race differences. Proteinuria was estimated from urine protein-osmolality ratios, and a more accurate assessment of glomerular proteinuria with 24-hour urine albumin was not available. However, urine protein-osmolality ratio is still a more accurate assessment of proteinuria than the more common dipstick urine protein that is confounded by urine concentration. We used Weibel and Gomez15 stereology models to calculate mean glomerular volume and cortex per glomerulus. The model assumes that glomeruli are spheres and that there is 10% variation in the distributions of glomerular volume within a biopsy section. There is a significant degree of tissue shrinkage due to formalin fixation and paraffin embedding.35 However, this occurs in all wedge sections, and thus, was unlikely to cause a differential bias. Microstructural measurements were only available on a single large wedge biopsy section, and serial sections were not available to identify atubular glomeruli.36
However, our study also has several notable strengths. We studied patients who underwent radical nephrectomy with a larger sample size and longer follow-up than prior studies in this patient group. Moreover, rather than using only descriptive visual assessment or reliance on subjective scoring system by a pathologist, we used a precise morphometric approach in quantifying all microstructural measurements on wedge sections.
In summary, this study demonstrated that larger nephrons and nephrosclerosis predicted the long-term risk of CKD progression after a radical nephrectomy. Only interstitial fibrosis predicted the risk of noncancer mortality. There is an opportunity to morphometrically assess the nontumor histology of radical nephrectomy specimens in order to identify prognostic information that may be of particular importance to patients who were surgically cured of their cancer and for whom prevention of kidney failure is now the primary concern.
Disclosures
All authors have nothing to disclose.
Funding
This study was supported with funding from National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK090358. A. Denic was supported by the Robert W. Fulk Career Development Award Fund in Nephrology Research. W. Kremers reports grants from the National Institutes of Health, during the conduct of the study. L. Lerman reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases, during the conduct of the study. A. Rule reports grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, during the conduct of the study.
Supplementary Material
Acknowledgments
We thank Miloš Denić for assistance with computer algorithms for processing of biopsy annotations data.
W. Kremers reports grants from AstraZeneca, Biogen, and Roche, outside the submitted work. L. Lerman reports personal fees from AstraZeneca, grants from Novo Nordisk, and personal fees and nonfinancial support from Weijian Technologies, outside the submitted work. J. Lieske reports grants and other from Alnylam and Dicerna; grants from Allena, OxThera, Retrophin, and Siemens; and other from Novobiome, Orfan, and Synlogic, outside the submitted work.
A. Denic, H. Elsherbiny, and A. Rule designed the study; A. Denic, B. Leibovich, L. Ricaurte Archila, and R. Thompson collected or provided the data; A. Denic, W. Kremers, A. Mullan, and A. Rule analyzed the data; A. Denic, H. Elsherbiny, and A. Rule drafted the manuscript; and all authors contributed to revisions and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040449/-/DCSupplemental.
Supplemental Figure 1. An example of mean tubular area measurement.
Supplemental Material. Methods.
Supplemental Table 1. Specific kidney diseases excluded.
Supplemental Table 2. Structural predictors of CKD progression censoring for cancer recurrence.
Supplemental Table 3. Structural predictors of CKD progression by BMI<30 or ≥30 kg/m2.
Supplemental Table 4. Multivariable models for predicting CKD progression.
Supplemental Table 5. Multivariable models for predicting noncancer mortality.
Supplemental Table 6. Structural predictors of cancer mortality.
Supplemental Table 7. Structural predictors of cancer recurrence.
References
- 1.Issa N, Vaughan LE, Denic A, Kremers WK, Chakkera HA, Park WD, et al. : Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant 19: 1989–1998, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautam G, Lifshitz D, Shikanov S, Moore JM, Eggener SE, Shalhav AL, et al. : Histopathological predictors of renal function decrease after laparoscopic radical nephrectomy. J Urol 184: 1872–1876, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Salvatore SP, Cha EK, Rosoff JS, Seshan SV: Nonneoplastic renal cortical scarring at tumor nephrectomy predicts decline in kidney function. Arch Pathol Lab Med 137: 531–540, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK: Kidney pathological changes in metabolic syndrome: A cross-sectional study. Am J Kidney Dis 53: 751–759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandina R, Moreira Leite KR, Gregório EP, Fernandes KBP, Srougi M: Histologic abnormalities in non-neoplastic renal parenchyma and the risk of chronic kidney disease following radical nephrectomy. Urology 100: 158–162, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Birendra R, John NT, Duhli N, Devasia A, Kekre N, Manojkumar R: Histopathological analysis of the non-tumour parenchyma following radical nephrectomy: Can it predict renal functional outcome? Int Braz J Urol 43: 655–660, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bijol V, Mendez GP, Hurwitz S, Rennke HG, Nosé V: Evaluation of the nonneoplastic pathology in tumor nephrectomy specimens: Predicting the risk of progressive renal failure. Am J Surg Pathol 30: 575–584, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Delahunt B, Srigley JR, Judge M, Amin M, Billis A, Camparo P, et al. : Dataset for the reporting of renal biopsy for tumour: Recommendations from the International Collaboration on Cancer Reporting (ICCR). J Clin Pathol 72: 573–578, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Henriksen KJ, Meehan SM, Chang A: Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: A review of 246 cases. Am J Surg Pathol 31: 1703–1708, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Denic A, Mathew J, Nagineni VV, Thompson RH, Leibovich BC, Lerman LO, et al. : Clinical and pathology findings associate consistently with larger glomerular volume [published correction appears in J Am Soc Nephrol 29: 2445, 2018 10.1681/ASN.2018060653]. J Am Soc Nephrol 29: 1960–1969, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denic A, Ricaurte L, Lopez CL, Narasimhan R, Lerman LO, Lieske JC, et al. : Glomerular volume and glomerulosclerosis at different depths within the human kidney. J Am Soc Nephrol 30: 1471–1480, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA: Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson DM, Anderson RL: Protein-osmolality ratio for the quantitative assessment of proteinuria from a random urinalysis sample. Am J Clin Pathol 100: 419–424, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Choi SM, Choi DK, Kim TH, Jeong BC, Seo SI, Jeon SS, et al. : A comparison of radiologic tumor volume and pathologic tumor volume in renal cell carcinoma (RCC). PLoS One 10: e0122019, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weibel ER, Gomez DM: A principle for counting tissue structures on random sections. J Appl Physiol 17: 343–348, 1962. [DOI] [PubMed] [Google Scholar]
- 16.Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, et al. : Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis 68: 58–67, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M, et al. : Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 9: 1892–1902, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, et al. : The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28: 313–320, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hostetter TH: Hypertrophy and hyperfunction of the diabetic kidney. J Clin Invest 107: 161–162, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallon V, Komers R: Pathophysiology of the diabetic kidney. Compr Physiol 1: 1175–1232, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, et al. : Glomerular aging and focal global glomerulosclerosis: A podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogo AB: Glomerular hypertension, abnormal glomerular growth, and progression of renal diseases. Kidney Int Suppl 75: S15–S21, 2000. [PubMed] [Google Scholar]
- 23.Schnaper HW: Remnant nephron physiology and the progression of chronic kidney disease. Pediatr Nephrol 29: 193–202, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ku E, Lee BJ, Wei J, Weir MR: Hypertension in CKD: Core curriculum 2019. Am J Kidney Dis 74: 120–131, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR: Fibrosis: Ultimate and proximate causes. J Clin Invest 124: 4673–4677, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahmy LM, Massie AB, Muzaale AD, Bagnasco SM, Orandi BJ, Alejo JL, et al. : Long-term renal function in living kidney donors who had histological abnormalities at donation. Transplantation 100: 1294–1298, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Issa N, Lopez CL, Denic A, Taler SJ, Larson JJ, Kremers WK, et al. : Kidney structural features from living donors predict graft failure in the recipient. J Am Soc Nephrol 31: 415–423, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M, et al. : Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int 93: 1175–1182, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuboi N, Kawamura T, Koike K, Okonogi H, Hirano K, Hamaguchi A, et al. : Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol 5: 39–44, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto H, Kawamura T, Okonogi H, Tsuboi N, Miyazaki Y, Yokoo T: The role of a low glomerular density and being overweight in the etiology of proteinuria in CKD patients without known glomerular diseases. Clin Exp Nephrol 18: 911–917, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haruhara K, Tsuboi N, Sasaki T, Amano H, Tanaka M, Koike K, et al. : Volume ratio of glomerular tufts to bowman capsules and renal outcomes in nephrosclerosis. Am J Hypertens 32: 45–53, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Lau WL, Rhee CM, Harley K, Kovesdy CP, Sim JJ, et al. : Risk of chronic kidney disease after cancer nephrectomy. Nat Rev Nephrol 10: 135–145, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Kim SP, Thompson RH, Boorjian SA, Weight CJ, Han LC, Murad MH, et al. : Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol 188: 51–57, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Hermsen M, de Bel T, den Boer M, Steenbergen EJ, Kers J, Florquin S, et al. : Deep learning-based histopathologic assessment of kidney tissue. J Am Soc Nephrol 30: 1968–1979, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulladosa X, Moreso F, Narváez JA, Grinyó JM, Serón D: Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol 14: 2662–2668, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Marcussen N: Atubular glomeruli in renal artery stenosis. Lab Invest 65: 558–565, 1991. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.