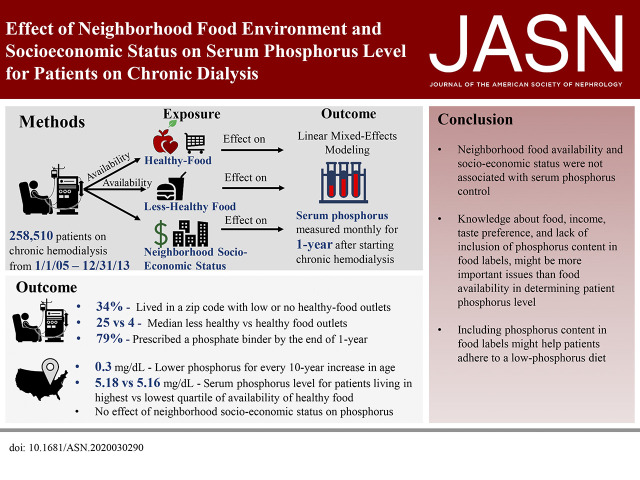
Significance Statement
Persistently elevated blood phosphorus levels, which are common among patients with ESKD who undergo dialysis, are associated with a heightened risk of death. In this study, the authors used data from a national dialysis provider to investigate whether higher availability of healthier, low-phosphorus food choices, such as fruits and vegetables, in a patient’s neighborhood is associated with better phosphorus levels. However, they found no meaningful association between better availability of healthy food in the patients’ residential neighborhoods or in neighborhoods around the dialysis center and better phosphorus control. These findings suggest that rather than neighborhood availability of healthy food, health literacy, individual patient food preferences, and challenges in interpreting food labels might be the main determinants of diet-related phosphorus levels.
Keywords: phosphate binders, dialysis, outcomes, hyperphosphatemia, nutrition
Visual Abstract
Abstract
Background
Elevated blood phosphorus levels are common and associated with a greater risk of death for patients receiving chronic dialysis. Phosphorus-rich foods are prevalent in the American diet, and low-phosphorus foods, including fruits and vegetables, are often less available in areas with more poverty. The relative contributions of neighborhood food availability and socioeconomic status to phosphorus control in patients receiving dialysis are unknown.
Methods
Using longitudinal data from a national dialysis provider, we constructed hierarchical, linear mixed-effects models to evaluate the relationships between neighborhood food environment or socioeconomic status and serum phosphorus level among patients receiving incident dialysis.
Results
Our cohort included 258,510 patients receiving chronic hemodialysis in 2005–2013. Median age at dialysis initiation was 64 years, 45% were female, 32% were Black, and 15% were Hispanic. Within their residential zip code, patients had a median of 25 “less-healthy” food outlets (interquartile range, 11–40) available to them compared with a median of four “healthy” food outlets (interquartile range, 2–6). Living in a neighborhood with better availability of healthy food was not associated with a lower phosphorus level. Neighborhood income also was not associated with differences in phosphorus. Patient age, race, cause of ESKD, and mean monthly dialysis duration were most closely associated with phosphorus level.
Conclusions
Neither neighborhood availability of healthy food options nor neighborhood income was associated with phosphorus levels in patients receiving chronic dialysis. Modifying factors, such as nutrition literacy, individual-level financial resources, and adherence to diet restrictions and medications, may be more powerful contributors than food environment to elevated phosphorus.
Elevated serum phosphorus levels can increase the risk of cardiovascular disease and mortality, yet the Dialysis Outcomes and Practice Patterns Study found that only 44% of patients receiving chronic dialysis achieved optimal phosphorus control.1 An important contributor to elevated phosphorus levels is its ubiquitous presence in food, either occurring naturally or used as a preservative.2,3 Because conventional (three times a week) hemodialysis removes only a modest quantity of phosphorus from the body, 4–6 physicians prescribe oral phosphorus binders that must be consumed with meals and reduce intestinal absorption of phosphorus. Unfortunately, these medications can cause gastrointestinal distress, impose a serious pill burden, are inconvenient for patients, and add to health care costs.7,8 Despite routine prescribing of binders and nutritional counseling, phosphorus levels are persistently elevated in thousands of patients receiving dialysis in the United States.
The intestinal absorption of phosphorus varies depending on the source of food: organic or inorganic, presence of phosphorus additives, and the presence of phytates, which can reduce the absorption of phosphorus.3 The Western diet is typically low in soluble fiber and phytates, with the major source of phytates being whole grains in breads and ready-to-eat cereals, and it also tends to be high in readily absorbable phosphorus included as additives in processed foods to enhance flavor and improve shelf life.9 Over 70% of phosphorus from processed foods, red meat, dairy, and sodas is absorbed.3,10 In contrast, because of the presence of phytates, only 30%–40% of phosphorus present in grains, legumes, fruits, and vegetables is absorbed.11
Although food labels are intended to provide the consumer with easily understood and relevant information about nutritional content, the US Food and Drug Administration does not require food manufacturers to display the phosphorus content.12,13 When the phosphorus content of food is listed on labels, the label can be challenging to interpret,14 or the phosphorus content may be inaccurately stated.15–18 For example, a survey of food labels of bestselling, branded grocery products in Northeast Ohio showed that phosphorus additives were extremely common in prepared frozen foods, packaged meat, and bread and baked goods.2 The same study also found that phosphorus additive–free meals were often more expensive than meals with additives.2 Additionally, many patients on chronic dialysis have low health and nutrition literacy and limited income, creating further barriers to selecting meals low in phosphorus.19–22
In summary, foods rich in phosphorus are prevalent in the American diet. 23,24 Geographic areas with more poverty often have less availability of low-phosphorus alternatives, including fruits and vegetables.25–28 Because of the phosphorus-rich content of many types of food, we hypothesized that restrictions on food availability might affect phosphorus control for patients on dialysis, acknowledging that dietary choices are complex and depend on multiple factors, including gustatory preferences, sociocultural norms, and comorbidities such as diabetes, income, and nutrition literacy. Using data from a national dialysis provider linked to US Census data on food outlets and income, we characterized the neighborhood environment for patients on dialysis. We quantified the effect of neighborhood food access and socioeconomic status (SES) on longitudinal serum phosphorus concentrations.
Methods
We used data from a national dialysis provider to assemble a cohort of adult patients (aged ≥18 years) receiving chronic hemodialysis therapy between January 1, 2005, and December 31, 2013. We included all patients on incident chronic dialysis who received conventional, in-center hemodialysis. We excluded patients who received home hemodialysis, peritoneal dialysis, and nocturnal dialysis. We also excluded patients who were on dialysis for <90 days. Data collected during the initial 90 days after starting dialysis (the baseline exposure period) were used to determine the baseline characteristics for each patient.
Outcome
Our primary outcome was serum phosphorus concentration. All patients had the opportunity of 1 year of follow-up after starting dialysis. The initial date for outcome measurement was the first day after the baseline exposure period (day 91). Phosphorus was measured serially during usual dialysis care and every patient in our dataset could contribute up to nine phosphorus values (one for each month). Patients were censored from analyses if they changed providers, discontinued dialysis, or died before the end of 1 year after starting dialysis. It is routine clinical practice among dialysis centers to measure monthly serum electrolyte levels, including phosphorus.29 In instances where the dataset contained more than one phosphorus level measured in a month, we selected the first measurement.
Exposures
Our primary exposure was the individual patient’s neighborhood food environment, on the basis of the patient’s home zip code. In instances where a patient did not have a zip code listed in the database at the time of dialysis initiation, we used the first available zip code for the patient.
To ascertain the food environment, we first identified food outlets within each zip code by using data from the US Census.30 We defined food outlets as either healthy or less-healthy, using the criteria defined by the Centers for Disease Control and Prevention Modified Retail Food Environment Index (further described in Supplemental Appendix 1).31 Finally, we divided zip codes into quartiles on the basis of food availability (low availability, medium-low availability, medium-high availability, and high availability of healthy food).
Our secondary exposure was SES, as measured by the Agency for Healthcare Related Quality (AHRQ) SES index.32 The AHRQ SES Index reflects the following domains: occupation (percentage unemployed), income, wealth, education, and housing (Supplemental Appendix 2). We then divided patients’ residential zip codes into four quartiles on the basis of the SES index that was determined at the zip code level.
Covariates
Covariates included patient demographic and clinical characteristics (age at dialysis initiation, sex, race, history of diabetes, and cause of ESKD). Because our dataset had detailed information about all dialysis treatments, we extracted information on the following time-updated variables: mean time patients spent on dialysis each month (measured in minutes), phosphorus binder use, and the number of dialysis treatments missed each month.
Statistical Analyses
We used descriptive statistics to compare baseline characteristics for patients according to their neighborhood availability of healthy and less-healthy food (low availability, medium-low availability, medium-high availability, and high availability).
Neighborhood Food Environment and Serum Phosphorus Level
Because of the longitudinal nature of our dataset, we fit hierarchical, linear mixed-effects models to account for time-updated variables and clustering of patients (within dialysis units and zip codes). For the primary analysis, we included variables that we identified a priori as clinically important for serum phosphorus control (see Covariates, above). We used the Akaike information and Bayesian information criteria to guide model selection.
Neighborhood SES and Serum Phosphorus Level
Similar to the prior model, to assess the relationship between neighborhood SES and the outcome of serum phosphorus level, we fit a hierarchical, linear mixed-effects model. First, we analyzed the effect of neighborhood SES alone on serum phosphorus level. We then included neighborhood food availability in the model, and, finally, we included an interaction term to evaluate the relationship between availability of food and SES regarding serum phosphorus levels.
Secondary Analyses
To examine the effect of food environment surrounding a dialysis unit (versus the patient residential neighborhood) on phosphorus control, we characterized the food environment around each dialysis unit. Again, applying criteria defined by the Centers for Disease Control and Prevention Modified Retail Food Environment Index, we first located dialysis units, and then constructed geographic boundaries using drivable distance around each dialysis unit. We geocoded the location of every dialysis unit in our dataset using R package “ggmap,” and then, using Network Analyst extension in ArcGIS Desktop 10.6.1 (Environmental Systems Research Institute, Redlands, CA), we identified 0.6-mile (1-km) driving distances around each dialysis unit. We then identified the number of food outlets (healthy and less healthy) located within the geographical boundary surrounding every dialysis unit in our dataset. Finally, we divided availability of healthy food outlets around dialysis units into quartiles (low availability, medium-low availability, medium-high availability, and high availability).
All analyses were completed using STATA version 15.1 (StataCorp, College Station, TX), R version 3.5.3 (R Core Team, Vienna, Austria), and ArcGIS version 10.6.1.
Results
Neighborhood Environment of the Patient’s Residential Zip Code
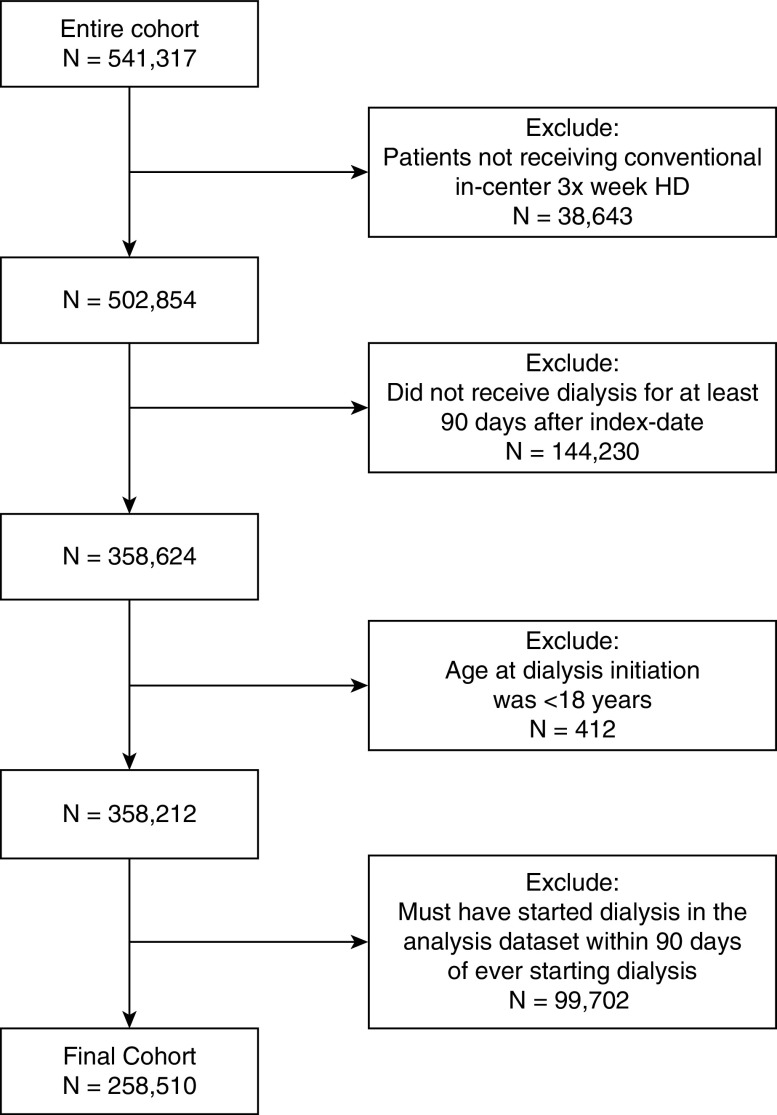
Our cohort consisted of 258,510 patients on chronic, in-center hemodialysis (Figure 1). The mean number of phosphorus values for each patient was 7.7 (SD 2.3). The median age of the cohort was 64 years (interquartile range [IQR], 53–74 years), 45% were female, 32% were Black, and 15% were Hispanic (Table 1). Diabetes was the most common cause of ESKD (48%), followed by hypertension (30%) and GN (10%). Of the entire cohort, 14% lived in a rural zip code.
Figure 1.
Flowchart for cohort generation. HD, hemodialysis.
Table 1.
Demographic characteristics of patients by availability of healthy food outlets
| Characteristic | Healthy Food Outlet Availability, Quartilea | ||||
|---|---|---|---|---|---|
| Lowest | Medium-Low | Medium-High | Highest | P Value | |
| Median number of healthy food outlets (IQR) | 1 (0–2) | 3 (3–4) | 5 (5–6) | 9 (7–11) | |
| No. of patients | 87,622 | 64,850 | 46,005 | 59,594 | |
| Age, yr, median (IQR) | 64 (53–74) | 64 (53–74) | 64 (53–74) | 63 (52–74) | <0.001 |
| Female | 38,773 (44.3%) | 29,323 (45.2%) | 20,449 (44.5%) | 26,512 (44.5%) | 0.002 |
| Black race | 27,379 (31.2%) | 22,779 (35.1%) | 14,720 (32.0%) | 16,898 (28.4%) | |
| Cause of ESKD | |||||
| GN | 8708 (10.3%) | 6060 (9.6%) | 4314 (9.7%) | 5316 (9.2%) | <0.001 |
| Diabetes mellitus | 40,536 (47.8%) | 29,663 (47.1%) | 20,969 (47.0%) | 28,350 (48.9%) | |
| Hypertension | 24,711 (29.1%) | 19,437 (30.8%) | 13,851 (31.0%) | 17,680 (30.5%) | |
| PKD/CAKUT | 1750 (2.1%) | 1271 (2.0%) | 889 (2.0%) | 1028 (1.8%) | |
| Other | 9095 (10.7%) | 6576 (10.4%) | 4598 (10.3%) | 5572 (9.6%) | |
| Income quartile | |||||
| Lowest | 32,064 (36.6%) | 22,547 (34.8%) | 13,899 (30.2%) | 19,164 (32.2%) | <0.001 |
| Medium-low | 19,961 (22.8%) | 15,418 (23.8%) | 11,370 (24.7%) | 14,684 (24.6%) | |
| Medium-high | 21,706 (24.8%) | 16,258 (25.1%) | 13,059 (28.4%) | 17,033 (28.6%) | |
| Highest | 13,891 (15.9%) | 10,627 (16.4%) | 7677 (16.7%) | 8713 (14.6%) | |
| SES category | |||||
| Lowest | 28,126 (33.6%) | 19,802 (30.7%) | 12,417 (27.1%) | 22,888 (38.4%) | <0.001 |
| Medium-low | 17,925 (21.4%) | 13,086 (20.3%) | 9492 (20.7%) | 12,266 (20.6%) | |
| Medium-high | 16,702 (19.9%) | 13,195 (20.5%) | 9710 (21.2%) | 9752 (16.4%) | |
| Highest | 20,973 (25.0%) | 18,416 (28.6%) | 14,175 (31.0%) | 14,688 (24.6%) | |
| Geographic region | |||||
| Urban | 68,613 (78.4%) | 55,263 (85.2%) | 41,000 (89.1%) | 56,485 (94.8%) | <0.001 |
| Rural | 18,931 (21.6%) | 9586 (14.8%) | 4990 (10.9%) | 3103 (5.2%) | |
IQR, interquartile range; PKD, polycystic kidney disease; CAKUT, congenital anomalies of the kidney and urinary tract.
Quartiles were divided on healthy food availability among all zip codes in the United States.
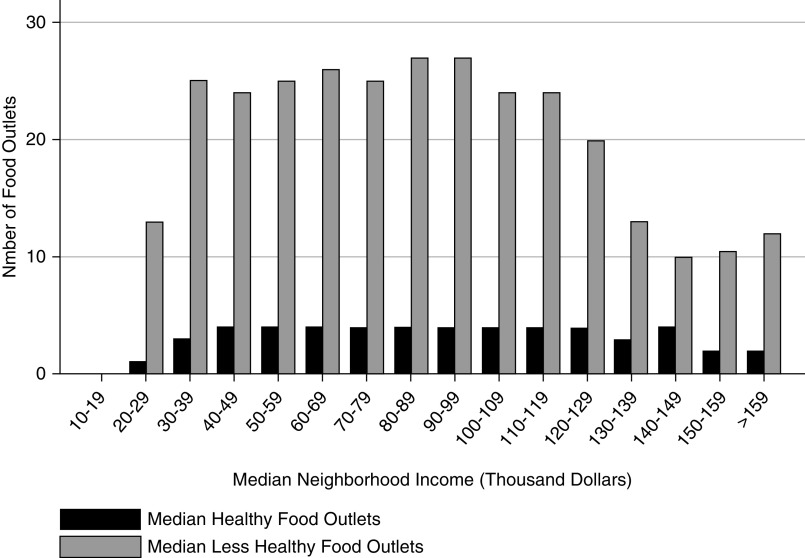
Availability of healthy food outlets varied widely (Table 1). Zip codes in the lowest quartile of availability of healthy food had a median of one healthy food outlet (IQR, 0–2), whereas zip codes in the highest quartile had a median of nine healthy food outlets (IQR, 7–11). A total of 9% of the cohort lived in a zip code without a single healthy food outlet, and 31% lived in a zip code with at least six healthy food outlets (29% in an urban zip code and 1.9% in a rural zip code). Regardless of neighborhood income, there were fewer healthy food outlets compared with less-healthy food outlets (Figure 2). Overall, there were a median of 25 less-healthy food outlets (IQR, 11–40) compared with a median of four healthy food outlets (IQR, 2–6) per neighborhood. We also found that the median number of less-healthy food outlets declined in neighborhoods with incomes above $130,000 (12 versus 25 less-healthy food outlets in neighborhoods with incomes ≤$130,000).
Figure 2.
Most patients live in neighborhoods with healthy food outlets, but less-healthy food outlets are more common than healthy food outlets in neighborhood categories defined by median income.
Neighborhood Environment of the Dialysis Unit
Our cohort consisted of 2914 dialysis units located over a wide geographic area (Supplemental Figure 1). In the immediate proximity of a dialysis unit (1 km around a dialysis unit), there was a median of one healthy and one less-healthy food outlet (Supplemental Table 1). Surprisingly, we also found only weak correlation between neighborhood SES of the dialysis unit zip code and the residential zip code of the patient (Supplemental Table 2). For instance, 42% of patients who lived in a zip code classified as the lowest quartile of SES went to a dialysis unit in a zip code classified as having a higher quartile of SES.
Effect of Healthy Food Outlet Availability and SES on Phosphorus Control
The mean phosphorus value was 5.2 mg/dl (SD 1.47). Nearly 34% of patients had a phosphorus value >5.5 mg/dl in the first month of the observation period, and over 16% of patients had a phosphorus value >6.5 mg/dl during the same time period. The proportion of patients with a phosphorus value greater than either 5.5 mg/dl or 6.5 mg/dl did not vary across the observation period (Supplemental Figure 2).
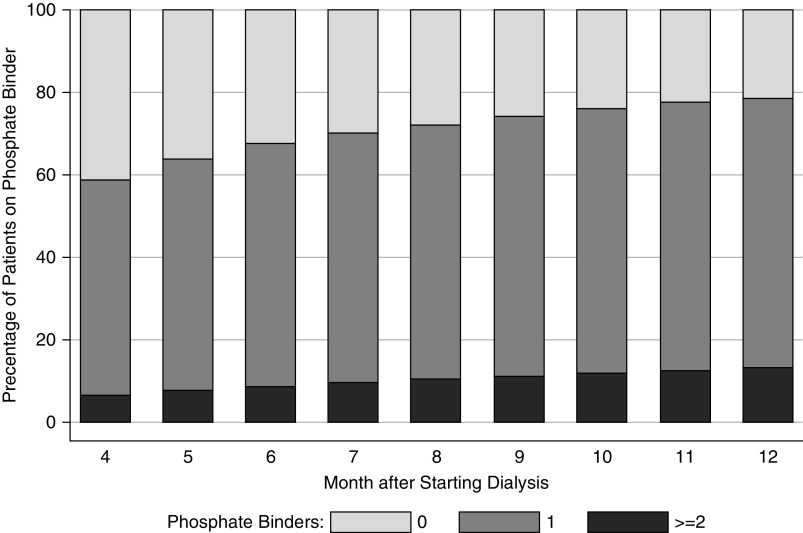
The proportion of patients with an elevated phosphorus (phosphorus >5.5 mg/dl) at any time during the observation period did not vary between quartiles of access to healthy food or SES derived from patient residence. A mixed-effects, linear regression model did not show a statistically significant association between access to food and change in phosphorus control over time, even after accounting for prescriptions for phosphorus binders for patients residing in either urban or rural zip codes (Supplemental Table 3 and 4, Table 2). Similarly, we did not find a statistically significant association between neighborhood SES and change in phosphorus over time (Supplemental Table 5, Table 3). Notably, phosphorus binder use increased from 59% to 79% over the course of the study period (Figure 3).
Table 2.
Mixed-effects linear model demonstrating the relationship between neighborhood availability of healthy food and serum phosphorus levels
| Variablea | Base Model | Base Model+Month | Base Model+Month+AHRQ SES Index | Base Model+Month+AHRQ SES Index+Demographics | Base Model+Month+AHRQ SES Index+Demographics+Time-updated variables |
|---|---|---|---|---|---|
| Healthy Food Availability | |||||
| Lowest quartile | Reference | Reference | Reference | Reference | Reference |
| Medium-low quartile | 0.0035 | 0.0037 | 0.0044 | 0.0034 | 0.0038 |
| SEM | 0.0078 | 0.0078 | 0.0079 | 0.0077 | 0.0077 |
| P value | 0.66 | 0.64 | 0.58 | 0.66 | 0.62 |
| Medium-high quartile | 0.0121 | 0.0124 | 0.0108 | 0.0045 | 0.0040 |
| SEM | 0.0079 | 0.0079 | 0.0081 | 0.0078 | 0.0078 |
| P value | 0.13 | 0.12 | 0.19 | 0.57 | 0.61 |
| Highest quartile | 0.0288 | 0.0292 | 0.0223 | 0.0028 | 0.003 |
| SEM | 0.0081 | 0.0081 | 0.0083 | 0.008 | 0.008 |
| P value | <0.001 | <0.001 | 0.007 | 0.73 | 0.71 |
The base model only includes access to healthy food outlets. The month is the month during which the laboratory value was measured in exposure period after starting dialysis. Demographics were age, sex, race, and cause of ESKD. Time-updated variables were time spent on dialysis, treatments missed per month, and phosphorus binder use.
Analyses limited to urban zip codes.
Table 3.
Mixed-effects linear model demonstrating the relationship between neighborhood SES and serum phosphorus levels
| Variablea | Base Model | Base Model+Month | Base Model+Month+Demographics | Base Model+Month+Demographics+Time-updated variables |
|---|---|---|---|---|
| Neighborhood SES Index | ||||
| Lowest quartile | Reference | Reference | Reference | Reference |
| Medium-low quartile | 0.0022 | 0.002 | 0.0104 | 0.0102 |
| SEM | 0.0081 | 0.0081 | 0.0078 | 0.0078 |
| P value | 0.78 | 0.81 | 0.19 | 0.19 |
| Medium-high quartile | −0.0327 | −0.0332 | −0.0009 | −0.0015 |
| SEM | 0.0082 | 0.0082 | 0.0081 | 0.0081 |
| P value | <0.001 | <0.001 | 0.91 | 0.86 |
| Highest quartile | −0.0635 | −0.0646 | 0.017 | 0.015 |
| SEM | 0.0077 | 0.0078 | 0.0077 | 0.0077 |
| P value | <0.001 | <0.001 | 0.03 | 0.05 |
The base model only includes neighborhood AHRQ SES Index. The month is the month during which the laboratory value was measured in the exposure period after starting dialysis. Demographics were age, sex, race, and cause of ESKD. Time-updated variables were time spent on dialysis, treatments missed per month, and phosphorus binder use.
Analyses limited to urban zip codes.
Figure 3.
Increasing proportion of patients are on a phosphorus binder over time, after starting dialysis.
Instead, patient age and race were the strongest determinants of serum phosphorus level. We found that older patients had a lower serum phosphorus than younger patients (serum phosphorus was 0.3 mg/dl lower for every 10-year increase in age; P<0.01). We also found that patients who self-identified as Black or Hispanic had a slightly lower phosphorus level (compared with White patients, the serum phosphorus of Black patients was lower by 0.2 mg/dl and the serum phosphorus of Hispanic patients was lower by 0.1 mg/dl; both P<0.01).33
Secondary analyses examining the association between access to healthy and less-healthy food within 0.6 miles (1 km) of a dialysis unit again did not show a relationship of access to food outlets with serum phosphorus level (Supplemental Tables 6 and 7).
Discussion
Elevated serum phosphorus is a pervasive problem for patients receiving chronic dialysis and has a negative association with survival. Management of hyperphosphatemia involves substantial dietary restrictions and significant pill burden. Because diet is a major contributor to phosphorus levels, we evaluated the relationship between access to healthy food, socioeconomic environment, and phosphorus control among patients on chronic dialysis. Although most patients in our study lived in neighborhoods with low access to healthy food, we did not find a clinically meaningful relationship between neighborhood food environment and hyperphosphatemia.
Our finding of a lack of association between neighborhood SES and healthy food options and serum phosphorus levels should be considered in the context of prior high-quality analyses by Gutiérrez et al.34 Using data from the National Health and Nutrition Examination Survey, the Gutiérrez group demonstrated that individual income level predicts phosphorus control for patients with CKD who are not yet on dialysis. There are several explanations for this observed difference. First, because of the nature of our dataset, our study evaluated SES at the neighborhood level, whereas the study by Gutiérrez et al. was at the individual level. Ascertainment of SES at the individual level may better predict individual-level outcomes, such as phosphorus. Second, patients on dialysis have frequent laboratory monitoring. Dialysis providers (and the Centers for Medicare and Medicaid Services) monitor serum phosphorus levels as a marker of quality of care, which may lead to providers intensifying treatments when serum phosphorus levels are high.29 Another possibility is that cultural and personal food preferences, as well as actual food cost, and not food availability, may drive selection of a high-phosphorus diet. This hypothesis is supported by a recent analysis in which patients with CKD enrolled in the Chronic Renal Insufficiency Cohort study, who had better access to grocery stores, did not have a healthier diet score.35 Specifically, a lack of knowledge about which foods to choose, and inability to make further restrictions on an already challenging set of dietary recommendations because of diabetes, heart disease, or hyperkalemia, may inhibit the ability of patients to control phosphorus even when healthy food is locally available.2 Finally, the US Food and Drug Administration does not require manufactures to report the phosphorus content on food labels, when even items such as unprocessed meats have phosphorus added to prolong their shelf life. The lack of phosphorus content in food labels, prevalence of phosphorus-containing additives, and health literacy all might be contributing to difficulty controlling serum phosphorus levels.
Even if patients have access to healthy food, individual food preference can vary significantly and affect overall food choice. Qualitative work by our group and others found that many patients on dialysis have difficulty identifying foods high in phosphorus.2,22 In a small pilot trial, our group found that financial incentives and additional nutritional counseling led to only a modest improvement in phosphorus control.22 It is possible that patients with high access to healthy food and personalized counseling may still make dietary choices that cause high-phosphorus intake. Furthermore, healthy food options may still lead to consumption of meals that are high in phosphorus.27 Sharon Moe and others have proposed that the renal community needs to reimagine kidney nutrition counseling, such that advice should involve asking patients to avoid canned, boxed, or prepackaged foods, instead of providing patients with handouts about phosphorus. This advice alone might help in reducing serum phosphorus levels.36
Our study has several limitations. Although we had access to detailed, longitudinal laboratory values and prescribed medications, we did not have exact dosing information, nor could we capture medication adherence in our dataset. Another limitation is that our analysis relied on North American Industry Classification System codes to specify if a food outlet was healthy or not, which has the potential to misclassify food outlets and might not be updated regularly.37,38 A third limitation of our approach is that we assumed that the neighborhood environment immediately surrounding patients’ homes would be similar to the aggregate food environment within their zip code. We acknowledge that zip codes can vary in size, and that a smaller geographic boundary (US Census block or tract) may have better accuracy at predicting an individual’s SES; however, we did not have access to individual patient addresses.39–41 Similarly, because of the nature of our dataset, we did not have access to high-granularity data on individual-level SES indicators (e.g., household income, education level), which might be a stronger predictor of health-related outcomes, but is also complex to measure. Finally, the AHRQ SES Index was initially described using US Census data from 2000, before the study period; however, we compared the AHRQ SES Index to updated US Census data from 2007 to 2011, and we found excellent concordance in predicting neighborhood socioeconomic index (Supplemental Figure 3).
Despite the fact that 30% of dialysis centers and 33.7% of patients receiving dialysis in our study were located within geographic regions with either no access or low access to healthy food, neighborhood food environment and socioeconomic environment did not affect serum phosphorus control. These results suggest that the main determinants of phosphorus control may instead be food choice, access to effective educational tools, and medication adherence. Understanding a patient’s income, food availability near where they live, and their personal preferences can help develop personalized dietary plans to reduce dietary phosphorus intake. Future remedies to improve phosphorus control may require addressing these issues as well as the pervasive problem of inadequate labeling.
Disclosures
P. Reese is an associate editor for the American Journal of Kidney Disease. All remaining authors have nothing to disclose.
Funding
V. Potluri’s work was supported by an American Society of Nephrology Ben J. Lipps grant, and a National Kidney Foundation satellite dialysis clinical investigator grant. J. Cohen’s work was supported by National Institutes of Health grant K23-HL133843. P. Reese’s work was supported by National Institute of Allergy and Infectious Diseases grant K24AI146137, and he consulted for Collaborative Healthcare Research & Data Analytics (COHRDATA) on epidemiology medications to improve laboratory parameters, including potassium among patients on dialysis, during the conduct of the study.
Supplementary Material
Acknowledgments
Dr. Peter P. Reese received investigator-initiated grants from CVS Caremark and Merck to support research on medication adherence (focus: Statins), grants from Investigator-initiated grants from Merck and Massachusetts General (with AbbVie) to support research into transplantation using organs from donors with hepatitis C virus, all outside the submitted work.
Dr. Vishnu S. Potluri, Dr. Deirdre Sawinski, and Dr. Peter P. Reese designed the study. Dr. Vishnu S. Potluri, Vicky Tam, Dr. Justine Shults, Dr. Deirdre Sawinski, Dr. Jordana B. Cohen, and Dr. Peter P. Reese were involved in analyzing the data. Dr. Vishnu S. Potluri and Dr. Peter P. Reese drafted and revised the manuscript. All authors were involved in the interpretation of the results and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020030290/-/DCSupplemental.
Supplemental Appendix 1. Methodology for estimating the number of healthy and less-healthy food outlets within patient residential zip code and dialysis unit neighborhood.
Supplemental Appendix 2. Components of the AHRQ SES Index.
Supplemental Figure 1. Geographic distribution of dialysis units included in the final cohort.
Supplemental Figure 2. The proportion of patients with serum phosphorus >5.5 mg/dl or >6.5 mg/dl is greater among patients who are younger.
Supplemental Figure 3. Correlation between AHRQ SES Index from US Census data from 2000 and SES Index calculated using US Census data from 2007 to 2011.
Supplemental Table 1. Availability of healthy and less-healthy food outlets in the neighborhood of the dialysis unit.
Supplemental Table 2. Correlation between zip code SES for dialysis unit and patient home location.
Supplemental Table 3. Individual components of the mixed-effects linear model demonstrating the relationship between availability of healthy food outlets and serum phosphorus levels.
Supplemental Table 4. Individual components of the mixed-effects linear model examining the relationship between neighborhood SES index and serum phosphorus levels.
Supplemental Table 5. Mixed-effects linear model examining the relationship between availability of healthy food outlets within 0.6 miles (1 km) of a dialysis unit on serum phosphorus level.
Supplemental Table 6. Mixed effects linear model examining the relationship between availability of less-healthy food within 0.6 miles (1 km) of a dialysis unit on serum phosphorus level.
References
- 1.Port FK, Pisoni RL, Bommer J, Locatelli F, Jadoul M, Eknoyan G, et al. : Improving outcomes for dialysis patients in the international Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 1: 246–255, 2006. [DOI] [PubMed] [Google Scholar]
- 2.León JB, Sullivan CM, Sehgal AR: The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr 23: 265–270.e2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, et al. : Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 519–530, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Daugirdas JT: Removal of phosphorus by hemodialysis. Semin Dial 28: 620–623, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Gutzwiller JP, Schneditz D, Huber AR, Schindler C, Gutzwiller F, Zehnder CE: Estimating phosphate removal in haemodialysis: An additional tool to quantify dialysis dose. Nephrol Dial Transplant 17: 1037–1044, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Kerr PG, Lo A, Chin M, Atkins RC: Dialyzer performance in the clinic: Comparison of six low-flux membranes. Artif Organs 23: 817–821, 1999. [DOI] [PubMed] [Google Scholar]
- 7.St Peter WL, Wazny LD, Weinhandl ED: Phosphate-binder use in US dialysis patients: Prevalence, costs, evidence, and policies. Am J Kidney Dis 71: 246–253, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Pannu N, Manns B: Oral phosphate binders in patients with kidney failure. N Engl J Med 362: 1312–1324, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Schlemmer U, Frølich W, Prieto RM, Grases F: Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res 53[Suppl 2]: S330–S375, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD: Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol 5: 683–692, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cupisti A, Kovesdy CP, D’Alessandro C, Kalantar-Zadeh K: Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients 10: 261, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borgi L: Inclusion of phosphorus in the nutrition facts label. Clin J Am Soc Nephrol 14: 139–140, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo MS, Sherman RA, Uribarri J: Dietary phosphate and the forgotten kidney patient: A critical need for FDA regulatory action. Am J Kidney Dis 73: 542–551, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan C, Sayre SS, Leon JB, Machekano R, Love TE, Porter D, et al. : Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 301: 629–635, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Sherman RA, Mehta O: Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin J Am Soc Nephrol 4: 1370–1373, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winger RJ, Uribarri J, Lloyd L: Phosphorus-containing food additives: An insidious danger for people with chronic kidney disease. Trends Food Sci Technol 24: 92–102, 2012 [Google Scholar]
- 17.Calvo MS, Moshfegh AJ, Tucker KL: Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv Nutr 5: 104–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan CM, Leon JB, Sehgal AR: Phosphorus-containing food additives and the accuracy of nutrient databases: Implications for renal patients. J Ren Nutr 17: 350–354, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor DM, Fraser SDS, Bradley JA, Bradley C, Draper H, Metcalfe W, et al. ; ATTOM Investigators : A systematic review of the prevalence and associations of limited health literacy in CKD. Clin J Am Soc Nephrol 12: 1070–1084, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollock JB, Jaffery JB: Knowledge of phosphorus compared with other nutrients in maintenance dialysis patients. J Ren Nutr 17: 323–328, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cupisti A, Ferretti V, D’Alessandro C, Petrone I, Di Giorgio A, Meola M, et al. : Nutritional knowledge in hemodialysis patients and nurses: Focus on phosphorus. J Ren Nutr 22: 541–546, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Reese PP, Mgbako O, Mussell A, Potluri V, Yekta Z, Levsky S, et al. : A pilot randomized trial of financial incentives or coaching to lower serum phosphorus in dialysis patients. J Ren Nutr 25: 510–517, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA: Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: Evidence from a nationally representative cross-sectional study. BMJ Open 8: e020574, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldridge AS, Huffman MD, Taylor F, Xavier D, Bright B, Van Horn LV, et al. : The healthfulness of the US packaged food and beverage supply: A cross-sectional study. Nutrients 11: 1704, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez JJ, Isakova T, Anderson CAM, Boulware LE, Wolf M, Scialla JJ: Food access, chronic kidney disease, and hypertension in the U.S. Am J Prev Med 49: 912–920, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolak M, Bradley M, Block DR, Pool L, Garg G, Toman CK, et al. : Urban foodscape trends: Disparities in healthy food access in Chicago, 2007-2014. Health Place 52: 231–239, 2018. [DOI] [PubMed] [Google Scholar]
- 27.Glanz K, Sallis JF, Saelens BE, Frank LD: Nutrition Environment Measures Survey in Stores (NEMS-S): Development and evaluation. Am J Prev Med 32: 282–289, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Powell LM, Slater S, Mirtcheva D, Bao Y, Chaloupka FJ: Food store availability and neighborhood characteristics in the United States. Prev Med 44: 189–195, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services (CMS) : End Stage Renal Disease (ESRD) quality measure development and maintenance mineral and bone disorder clinical technical expert panel summary report. CMS ed., 2013. Available at: https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mineralandbonedisordertepsummary-report.pdf. Accessed November 25, 2019
- 30.US Census Bureau : County Business Patterns Datasets, 2013. Available at: https://www.census.gov/programs-surveys/cbp/data/datasets.html. Accessed November 25, 2019
- 31.Centers for Disease Control and Prevention (CDC) : Census Tract Level State Maps of the Modified Retail Food Environment Index (mRFEI), 2011. Available at: https://www.cdc.gov/obesity/downloads/census-tract-level-state-maps-mrfei_TAG508.pdf. Accessed November 28, 2016
- 32.Bonito AJ, Bann C, Eicheldinger C, Carpenter L: Creation of New Race-Ethnicity Codes and Socioeconomic Status (SES) Indicators for Medicare Beneficiaries, Rockville, MD, Agency for Healthcare Research and Quality, 2008 [Google Scholar]
- 33.Moshfegh A, Kovalchik A, Clemens J; Food Surveys Research Group : Phosphorus Intake of Americans: What We Eat in America, NHANES 2011-2012, 2016. Available at: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/15_Phosphorus_intake_1112.pdf. Accessed November 26, 2019 [Google Scholar]
- 34.Gutiérrez OM, Isakova T, Enfield G, Wolf M: Impact of poverty on serum phosphate concentrations in the third national health and nutrition examination survey. J Ren Nutr 21: 140–148, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madrigal JM, Cedillo-Couvert E, Ricardo AC, Appel LJ, Anderson CAM, Deo R, et al. ; CRIC Study Investigators : Neighborhood food outlet access and dietary intake among adults with chronic kidney disease: Results from the chronic renal insufficiency cohort study. J Acad Nutr Diet 120: 1151–1162.e3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe S: Phosphate: Time for a Fresh Look at Dietary Control? 2011. Available at: https://www.kidneynews.org/kidney-news/special-sections/stones-and-bones/phosphate-time-for-fresh-look-dietary-control. Accessed May 20, 2020
- 37.Caspi CE, Sorensen G, Subramanian SV, Kawachi I: The local food environment and diet: A systematic review. Health Place 18: 1172–1187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong MS, Peyton JM, Shields TM, Curriero FC, Gudzune KA: Comparing the accuracy of food outlet datasets in an urban environment. Geospat Health 12: 546, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R: Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US). J Epidemiol Community Health 57: 186–199, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD; Multiple Risk Factor Intervention Trial (MRFIT) Research Group : ZIP-code-based versus tract-based income measures as long-term risk-adjusted mortality predictors. Am J Epidemiol 164: 586–590, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Krieger N, Chen JT, Waterman PD, Soobader M-J, Subramanian SV, Carson R: Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter?: The public health disparities geocoding project. Am J Epidemiol 156: 471–482, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.