Abstract
The Sentinel System is a national electronic postmarketing resource established by the US Food and Drug Administration to support assessment of the safety and effectiveness of marketed medical products. It has built a large, multi-institutional, distributed data network that contains comprehensive electronic health data, covering about 700 million person-years of longitudinal observation time nationwide. With its sophisticated infrastructure and a large selection of flexible analytic tools, the Sentinel System permits rapid and secure analyses, while preserving patient privacy and health-system autonomy. The Sentinel System also offers enhanced capabilities, including accessing full-text medical records, supporting randomized clinical trials embedded in healthcare delivery systems, and facilitating effective collection of patient-reported data using mobile devices, among many other research programs. The nephrology research community can use the infrastructure, tools, and data that this national resource offers for evidence generation. This review summarizes the Sentinel System and its ability to rapidly generate high-quality, real-world evidence; discusses the program’s experience in, and potential for, addressing gaps in kidney care; and outlines avenues for conducting research, leveraging this national resource in collaboration with Sentinel investigators.
Keywords: U.S. Food and Drug Administration, Sentinel Initiative, epidemiology, outcomes research, real-world evidence, electronic health data
The US Food and Drug Administration’s (FDA’s) Sentinel Initiative, launched in 2008, is a national postmarketing safety surveillance system for regulated medical products.1 The Sentinel System (Sentinel) is routinely used by the FDA to study utilization and performance of marketed medical products at a population level. Evidence generated by Sentinel informs regulatory actions, such as safety communications and label revisions.2 Although Sentinel’s mandate is to support the FDA’s public health mission, the FDA encourages broader use of the system’s infrastructure and capabilities by non-FDA parties, with the goal of the system becoming a national resource for evidence generation. Researchers in nephrology may, therefore, use Sentinel’s capabilities to generate real-world evidence and improve kidney care. In this review, we describe Sentinel’s capabilities; discuss its experience in, and potential for, addressing gaps in kidney care; and outline avenues for research in collaboration with Sentinel investigators.
FDA’s Sentinel and Its Capabilities
Sentinel was developed by the FDA in partnership with numerous health plans and scientific collaborators. Over time, Sentinel has created a large, national, multi-institutional, distributed data network of electronic health information and developed a sophisticated infrastructure that permits rapid and secure analyses, while preserving patient privacy.3–5 The partnership between the Sentinel Operations Center and the large network of data and academic partners enables access to rich electronic healthcare data and scientific and technical expertise. Sentinel and its capabilities enable rapid generation of high-quality, real-world evidence (Figure 1).
Figure 1.
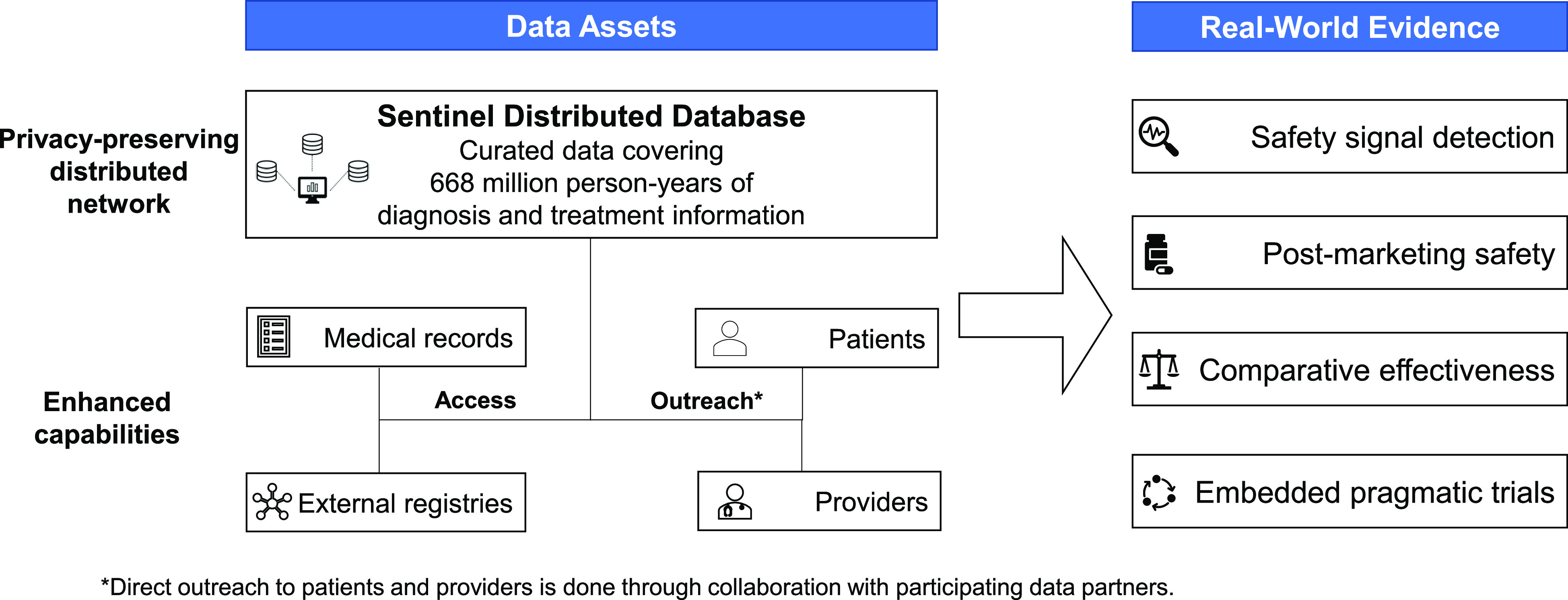
An illustration of Sentinel's data assets and enhanced capabilities to generate real-world evidence using its privacy-preserving distributed network and infrastructure.
Sentinel’s national data network, the Sentinel Distributed Database, includes routinely collected electronic health data from 17 data partners6,7 across the United States, totaling about 700 million person-years of observation. These data include administrative claims from several large national insurers and integrated delivery networks, components of electronic health records (EHRs) from a selection of integrated delivery networks, and inpatient EHRs from a network of 178 US hospitals. The Sentinel Distributed Database is a distributed network in that each data partner retains its data behind institutional firewalls and maintains both physical and operational control over the use of data—there is no central repository of data.8,9 Each data partner regularly transforms their data into the Sentinel Common Data Model format, meaning that, despite the varied sources, the data are organized the same way for Sentinel analyses. Robust, routine processes are in place to ensure the data pass quality checks and are curated as expected.
The population in the Sentinel Distributed Database includes individuals covered by commercial plans including Medicare Advantage, beneficiaries of Medicare Fee-for-Service, and some Medicaid recipients. Longitudinal information on reimbursed medical and outpatient pharmacy services is captured for the covered population.10 Data partners refresh their datasets quarterly or annually, usually with an intentional 6- to 9-month lag to ensure completeness. Accelerated access to data in near real-time is possible when there are specific needs. Nearreal-time data have lags of as little as days for EHRs, and 1–2 months for claims data. Of note, accelerated access to data in near real-time typically requires trade-offs between completeness and timeliness, and incurs additional costs. Additionally, Sentinel routinely works with detailed inpatient data, including laboratory results from the nation’s largest hospital network that covers approximately 5% of inpatient care in the country.11 The large size of the Sentinel Distributed Database7 and its inclusion of diverse populations enable evaluation of rare events12 and generation of evidence, often with great precision. In addition, several data partners contribute laboratory result data that are typically unavailable in administrative claims databases, including results for tests like hemoglobin A1C, creatine kinase, and creatinine.13 Notably, approximately 47 million individuals in the Sentinel Distributed Database have at least one creatinine measurement. Although potential selection biases and other caveats apply to the use of this information, these measurements provide a foundation for assessment of renal function. Sentinel has also linked records for nearly 5 million mother-baby pairs, allowing routine evaluation of outcomes in neonates and infants after antenatal exposure to medical products.
To conduct analyses, scientists at the Sentinel Operations Center first work with scientific staff from the FDA to design studies and then create analytic computer programs, which the Operations Center distributes to individual data partners. Data partners opt in to run these programs on their data and return summary-level information to the Sentinel Operations Center, where it is aggregated across sites for reporting to the FDA and, ultimately, the public. In comparative analyses, Sentinel uses propensity score–based methods combined with risk set–based, data-sharing approaches to aggregate the summary-level data provided by the data partners to estimate overall effect estimates.14,15 These analytic and data-sharing approaches produce results identical to those obtained from pooled patient-level data analysis. Patient-level analytic datasets can also be created and returned to the Sentinel Operations Center, when necessary. Shared patient-level datasets are either fully deidentified—for instance, through date shifting or grouping of ages and geographic locations—or they contain the minimum necessary information required to address the question at hand. These exchanges are facilitated by a software platform (PopMedNet) that ensures secure transmission of information in compliance with operational and governance processes of all participating organizations.16
Sentinel has developed a library of flexible “tools” (analytic computer programs, typically in the SAS programming language) to answer frequent questions from the FDA in a rapid manner, such as utilization patterns of medical products among specific cohorts of patients, occurrence of health outcomes of interest, and the evaluation of medication safety. Efforts are underway to determine the potential role of Sentinel in effectiveness research.17 These tools can be used to perform new-user cohort studies with propensity-score or multiple-factor matching, and self-controlled risk-interval analyses.18 Studies have shown that these tools are capable of reproducing well-established product-outcome associations, and, in general, produce findings comparable to those derived using de novo programming.19,20 The library of tools also includes signal identification programs21 that use both cohort and self-controlled designs and perform analysis with tree-based scan statistics,22,23 information component temporal pattern discovery,24 or sequence symmetry analysis.25
Enhanced Capabilities of Sentinel
In addition to routine querying using administrative data and the analytic tools described above, Sentinel data partners can obtain full-text medical records when necessary. When a chart review is required, direct identifiers in the charts are redacted and relevant information is abstracted before being used for expert adjudication. When requested, 70%–90% of records are obtainable for review. Reviews of full-text records have provided essential information for validating algorithms to identify important diseases12 or to confirm case status for specific investigations.26 When necessary, data partners can also link to external data sources, including birth or vaccine registries.27–29
Sentinel can also support the assessment and optimization of care delivery by facilitating the conduct of embedded randomized trials. The FDA created the FDA-Catalyst program to enable direct contact with patients and providers in Sentinel through collaboration with participating data partners. This program enhances the capabilities of Sentinel to support new research initiatives, moving beyond observational assessments of safety and effectiveness to interventional studies embedded in real-world delivery systems. The IMplementation of a randomized controlled trial to imProve treatment with oral AntiCoagulanTs in patients with Atrial Fibrillation (IMPACT-AFib) study is the first randomized, pragmatic, clinical trial through the FDA-Catalyst program. This large trial aims to evaluate the effect of patient- and provider-level education on the initiation of oral anticoagulants among patients with atrial fibrillation who may benefit from their use.30,31 In this trial, the Sentinel infrastructure is used to identify eligible patients and providers and to conduct the analysis, assessing initiation of therapy and stroke and bleeding outcomes.
Sentinel can also facilitate effective collection of information directly from patients that is not routinely collected in administrative claims or EHRs. Augmenting patient-reported information with existing health data can bring the patient perspective into real-world evidence and expand the capacity of comparative effectiveness and safety studies. Sentinel has developed FDA MyStudies—a patient-facing, mobile-device application available in the public domain—to serve this purpose.32–34 In compliance with federal laws (Federal Information Security Management Act) and the Code of Federal Regulations (Title 21 CFR Part 11), the application facilitates the direct input of data by patients (e.g., outcomes, over-the-counter medication use, and other patient-experience data) and secure linkage of this self-reported data35 to their information in the Sentinel Distributed Database, supporting prospective studies, registries, and randomized trials.
Limitations of Sentinel
Although Sentinel and its capabilities enable rapid generation of high-quality, real-world evidence, there are some limitations. The majority of the data in the Sentinel Distributed Database are administrative claims, which usually lack information on potentially important confounding factors such as body mass index, smoking, alcohol consumption, or other lifestyle-related factors. The Sentinel Distributed Database also does not have information on genetic markers of disease severity, and has varying access of laboratory test results and race information across data partners. Linking detailed inpatient hospital data, including exposure to specific medications, with claims data remains challenging. Individuals covered by commercial health plans also tend to have a shorter follow-up time than those covered by Medicare or Medicaid, making long-term treatment evaluations difficult. Moreover, Sentinel analyses have unknown generalizability to populations without health insurance. Lastly, Sentinel has limited information on patients with ESKD, for whom information on dialysis timing, modality, and outcomes may be incomplete. Important variability exists in missing laboratory results for serum creatinine across data partners.36,37
How the FDA Has Used Sentinel
The FDA routinely uses Sentinel’s >10 billion pharmacy-dispensing and medical encounters to evaluate the utilization, safety, and effectiveness of marketed medical products. Evidence generated by Sentinel has informed revision of product labels or issuance of drug-safety communications. For example, during a safety assessment prompted by international evidence that suggested a higher risk of birth defects associated with etanercept use, the FDA used Sentinel to study the utilization of TNF-α inhibitors in pregnant women in the United States.38 In 19,681 pregnancies where the expectant mother had at least one chronic inflammatory condition between 2004 and 2015, etanercept was found to be the most frequently used agent. This finding was one source of evidence considered by the FDA for the Pregnancy and Lactation Labeling Rule Conversion Safety Labeling Change for etanercept.39
Findings generated from Sentinel analyses are also routinely discussed in advisory committee meetings at the FDA to inform regulatory decision making. In preparation for an advisory committee meeting focused on the development of opioid-sparing and opioid-replacement drugs for acute pain in November 2018, the FDA conducted an analysis within Sentinel to evaluate the duration of opioid analgesic use after surgeries.2,40 The analysis included 820,767 patients initiating opioids immediately after one of 11 common surgical procedures. These data, along with other non-Sentinel studies, indicated that for certain indications, such as less-invasive procedures, opioid exposure could be reduced. The analysis also indicated variation in opioid analgesic needs by patient and procedure. These findings were discussed in the advisory committee meeting to inform guidance.2,41–43
The FDA is required to consider whether using the FDA Adverse Event Reporting System and Sentinel are sufficient to answer the question of interest before requiring manufacturers to conduct postmarketing studies that are often resource intensive.44 Sentinel’s Active Risk Identification and Analysis (ARIA) System can efficiently conduct many safety analyses. From 2016 through 2019, the FDA conducted 394 analyses in ARIA.45 As an example, Sentinel’s ARIA system was recently deemed sufficient to study the risk of renal cell carcinoma following treatment with canagliflozin.46
Experience with Sentinel in Nephrology
Sentinel has been used to conduct several analyses of direct relevance to nephrology, assessing patient populations (e.g., acute or CKD), outcomes (e.g., kidney stones, rhabdomyolysis, and acute kidney failure), or exposures (e.g., nephrotoxic medications). Table 1 highlights select examples. Sentinel investigators developed algorithms to identify health outcomes, such as AKI47 and rhabdomyolysis,48 using administrative data. The system has also been used to perform many studies of conditions closely related to kidney disease, including hypertension, diabetes, or autoimmune diseases.2
Table 1.
A selection of nephrology-related assessments completed in Sentinel
| Scope | Analysis Type | Nephrology-Related Assessments |
|---|---|---|
| Descriptive | Summary information: Characterizes the size of patient groups on the basis of drug products, diagnosis codes, and procedure codes | Number of patients with acute kidney failure82 |
| Number of patients on dialysis83 | ||
| Number of patients using the following medications that are either renally eliminated or nephrotoxic: analgesics, anti-infectives, antiseizure agents, cardiovascular agents, bisphosphonates, quinine, allopurinol, tenofovir, tolvaptan, ticlopidine84–96 | ||
| Background rates: Measures the rate of an event (exposure, outcome, or condition) | Rate of CKD52 | |
| Rate of kidney stones in patients with prior exposure to antidiabetic agents97 | ||
| Rate of oral anticoagulant use before diagnosis of cutaneous small vessel vasculitis and AKI98 | ||
| Rate of oral anticoagulant use in patients with atrial fibrillation and preexisting CKD99 | ||
| Incidence or incidence rates: Measures the incidence or incidence rate of a health outcome of interest during exposed time | Rates of celiac disease after treatment with angiotensin receptor blockers100 | |
| Rates of kidney stones after treatment with antiepileptic medications101 | ||
| Incidence of treatment with urate-lowering therapies after diagnosis of gout disease102 | ||
| Rates of rhabdomyolysis and/or creatine kinase laboratory result abnormalities after new use of statins or angiotensin-converting enzyme inhibitors103 | ||
| Rates of kidney failure after administration of intravenous Ig104 | ||
| Medical product utilization: Characterizes duration of treatment | Treatment episode characteristics for patients newly initiating the following medications that are either renally eliminated or nephrotoxic: antiepileptic medications, cardiovascular medications, quinine, antidepressants, antibiotics, antiarrhythmics105–107 | |
| Treatment episode characteristics for patients who are pregnant and newly initiating the following medications that are either nephrotoxic or affect the renal excretory function: statins, angiotensin-converting enzyme inhibitors, anticoagulants, antidepressants, gadolinium-based contrast agents108,109 | ||
| Comparative | Propensity score analysis: Estimates treatment effects in the propensity score–matched cohorts) | Risk of angioedema after treatment with drugs targeting the RAAS51 |
| Risk of nonmelanoma skin cancer after treatment with hydrochlorothiazide110 |
These studies have informed a variety of research and regulatory decisions at the FDA. An example is a study in patients with gout. The Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial reported increased cardiovascular mortality with febuxostat, a urate-lowering medication.49 Prompted by these findings, the FDA used Sentinel to determine the generalizability of the CARES findings by evaluating real-world use of febuxostat. Compared with patients in the CARES trial, those initiating febuxostat in routine care were older, more likely to be female, with a lower burden of preexisting cardiovascular disease or CKD, and less severe gout.50 This assessment contributed to a determination that there is a patient population with gout in which the risk-benefit profile of febuxostat is likely to be favorable for the treatment of hyperuricemia.2
Sentinel has also conducted several comparative analyses of nonkidney safety events following treatment with medications that interface with the kidney. In one study, Sentinel assessed the cardiovascular safety of drugs targeting the renin-angiotensin-aldosterone system (RAAS).51 Using an active-comparator, new-user study design and propensity score stratification to adjust for confounding, the study evaluated the risk of angioedema after initiation of treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, the direct renin inhibitor aliskiren, or β-blockers. The study found a three-fold increased risk of angioedema following treatment with angiotensin-converting enzyme inhibitors or aliskiren relative to β-blockers, but not with angiotensin receptor blockers. Differential excess risks found in this study contributed to the understanding of comparative safety of these drugs, and demonstrated that not all drugs targeting RAAS have similar adverse event profiles.
Potential Applications of Sentinel’s Infrastructure and Capabilities in Nephrology
There are many opportunities to use Sentinel’s capabilities to address nephrology-related topics other than those directed by the FDA (Table 2). In this section, we discuss a selection of research themes that can be potentially addressed using its infrastructure. The Sentinel Distributed Database includes over a million patients with CKD.52 Existing analytic tools in Sentinel can be used to examine the longitudinal healthcare experience of patients with CKD and evaluate the safety of medical products used in this population. Furthermore, Sentinel can assess the burden of comorbidities and polypharmacy in the CKD population.
Table 2.
Possible use cases for leveraging Sentinel infrastructure in nephrology research
| Type of Assessment | Example Drugs or Drug Classes |
|---|---|
| Safety | |
| Sentinel could be used to facilitate the identification of rare or difficult-to-detect ADRs (both kidney and nonkidney related) through postmarketing surveillance | Nonkidney ADRs |
| ESA-associated cardiovascular outcomes | |
| SGLT2 inhibitors and risk for necrotizing skin and soft tissue infections or amputation | |
| DOAC-related hemorrhage in patients with kidney disease or dialysis dependence | |
| HIF inhibitors and risk for cancer when used for anemia in patients with CKD | |
| Denosumab-associated severe hypocalcemia in advanced-stage CKD | |
| Kidney ADRs | |
| Immune checkpoint inhibitor–related acute and chronic nephrotoxicity | |
| PPI-associated acute interstitial nephritis and CKD | |
| Synergistic risk of toxicity when multiple potentially nephrotoxic agents are used in combination (e.g., the “triple whammy” of NSAIDs, RAAS blockade, and diuretics) | |
| Effectiveness | |
| Sentinel could be used to assess congruence with clinical trial findings using real-world data. This may apply to new therapeutics designed to prevent or slow the progression of kidney outcomes, or other comorbidities in patients with kidney disease | Kidney-related effectiveness outcomes |
| SGLT2 inhibitors for kidney protection in patients with cardiovascular disease, diabetes, or CKD | |
| HIF inhibitors for anemia management in patients with CKD | |
| Nonkidney-related effectiveness outcomes in the subpopulation with kidney disease | |
| DOAC effectiveness for stroke prevention in patients with advanced stage CKD and atrial fibrillation | |
| Optimization of care delivery | |
| FDA-Catalyst can facilitate direct outreach with healthcare providers and patients to facilitate implementation of evidence-based practices in patients with identified care gaps | Use of RAAS blockade for hypertension or proteinuria in patients with CKD, with or without diabetes |
| Introduction of statin therapy in patients with CKD to improve cardiovascular outcomes and delay progression | |
| Survey providers regarding the viability of non-NSAID alternatives for pain management in patients with CKD | |
| Pneumococcal vaccination in adults with CKD aged 18–64 yr old | |
ADR, adverse drug reaction; ESA, erythropoietin-stimulating agent; DOAC, direct oral anticoagulant; HIF, hypoxia-inducible factor; PPI, proton pump inhibitor; NSAID, nonsteroidal anti-inflammatory drug.
Sentinel’s safety signal identification and evaluation capabilities can be used to monitor kidney- or nonkidney-related adverse events of newly approved medications at a population level, including drug-associated nephrotoxicity manifesting as AKI. Although drug-associated nephrotoxicity is common, affecting 14%–26% patients worldwide,53 it is often not well established at the time of market entry. Although many medications have a type-A nephrotoxicity pattern (dose related) and an acute onset (<7 days), medications with a type-B pattern (idiosyncratic) or a subacute or chronic onset may be difficult to detect in preapproval clinical trials.54 Therefore, it is necessary to monitor and quantify risks postmarketing and identify conditions or characteristics that exacerbate these risks. As a recent example, immune checkpoint inhibitor therapy has been approved for a variety of malignant conditions including melanoma, renal cell carcinoma, non–small cell lung cancer, and urothelial carcinoma. As of 2018, about 44% of patients with cancer were eligible for treatment with one of these therapies.55 Evidence from observational studies indicated an association between immune checkpoint inhibitors and drug-associated nephrotoxicity, in particular acute tubulointerstitial nephritis.56,57 Yet, the size of these observational studies remains small compared with the scope of their use, which challenges the ability to draw conclusions about true incidence and risk factors for nephrotoxicity. Because drug-associated nephrotoxicity can be studied in claims data using validated algorithms,58 Sentinel’s capabilities can be readily leveraged to evaluate acute and chronic nephrotoxicity associated with immune checkpoint inhibitor therapy in a more diverse, generalizable population.
Sentinel can also be used to study real-world effectiveness of newly approved kidney protective medications. For example, whereas clinical trials of sodium-glucose cotransporter-2 (SGLT2) inhibitors demonstrated a reduction in risk for incident or worsening nephropathy in patients with diabetes,59,60 few observational studies had yet evaluated their effectiveness in real-world settings until recently.61 Sentinel could be used to address unanswered questions, including the role for SGLT2 inhibition without RAAS blockade and the safety of SGLT2 inhibition in the presence of diuretics.62 Approximately 475,000 patients in Sentinel initiated treatment with canagliflozin, dapagliflozin, or empagliflozin between March 2013 and June 2018.63 With this large sample size and the overall capabilities of the system, including the ability to extract detailed clinical information from full-text medical records, robust evidence on real-world safety and, potentially, effectiveness can be generated.
Sentinel can also be leveraged to evaluate interventions targeting optimization of delivery of care in patients with CKD by conducting embedded, pragmatic trials. Data suggest that suboptimal delivery of care leads to elevated risk for cardiovascular morbidity and mortality for patients with CKD.64 As an example, an embedded, pragmatic trial—analogous to the IMPACT-AFib study described above—might be used to assess various approaches to introduce statin therapy in patients with CKD in a rapid and resource-efficient manner. Such interventions could delay progression of kidney disease and improve cardiovascular outcomes in these patients.65
Opportunities for Researchers to Leverage Sentinel’s Capabilities
Use of Sentinel and its enhanced capabilities by non-FDA investigators requires the oversight of an institutional review board and depends on voluntary participation by the Sentinel data partners. Opportunities for researchers, delivery systems, and others interested in leveraging the capabilities of Sentinel are described below.
Researchers in academia and industry may use Sentinel for multisite observational or interventional studies through collaborations with partner organizations, working directly with individual Sentinel data partners, or engaging with the Sentinel Operations Center. Investigations can be facilitated via several entities, including the Reagan-Udall Foundation’s Innovation in Medical Evidence Development and Surveillance (IMEDS) program,66,67 the National Institutes of Health (NIH) Collaboratory Distributed Research Network,68–70 and the Biologics and Biosimilars Collective Intelligence Consortium (BBCIC).71 Recent IMEDS studies have focused on the incidence of venous thromboembolism among patients receiving routine clinical care for rheumatoid arthritis,72 the safety of oral contraceptives, and the effect of a drug class label change on medication use and outcomes.73 Investigators funded by the NIH or other not-for-profit sponsors can initiate or participate in research via the NIH Collaboratory Distributed Research Network. Collaboratory Distributed Research Network topics relevant to nephrology have included drug-induced toxicity,74 medication use in the elderly,75 and reducing polypharmacy in at-risk populations.76 Investigations that focus on biologics or biosimilars can leverage the BBCIC. Similar consortiums can also be created to focus on organ system–related topics, like kidney disease. Additionally, external investigators may work directly with Sentinel data partners and/or the Operations Center; several studies have already taken place through these collaborations.77,78 Costs of conducting a research project with Sentinel may vary with the complexity of the effort, number of data partners, timeliness of data access, need to obtain original medical records, and other requirements. Researchers interested in initiating any of the aforementioned collaborations may first contact the Sentinel Operations Center using https://www.sentinelinitiative.org/contact-us.
Researchers may also directly benefit from Sentinel by using its publicly available resources available at www.sentinelinitiative.org. The Sentinel Common Data Model,13 analytic tools,18,79 and data source translation code80,81 are publicly available for researchers to transform their own data and run queries. Study protocols, specifications, and analytic packages used for regulatory analyses are also publicly available on Sentinel’s website to facilitate adaptation by the broader research community in other environments.
Conclusion
Rigorously designed and appropriately analyzed observational studies can help generate evidence with greater external validity. With a large distributed data network, flexible tools, and a sophisticated infrastructure, Sentinel is a national resource for evidence generation. The unique advantages of Sentinel are (1) its size—with 700 million person-years of observation, the Sentinel Distributed Database is larger than other claims data sources; (2) its ability to obtain full-text records; (3) its ability to link to external sources such as registries; and (4) the ability to facilitate direct outreach to patients and providers included in the Sentinel Distributed Database. In addition to these data assets, Sentinel includes a thriving network of scientific partners from across the country that have expertise about their own delivery systems that allows informed interpretation of these data for the conduct of rigorous research. Many opportunities exist for the nephrology research community to directly collaborate with Sentinel or use its publicly available resources to generate evidence and improve kidney care for the millions of Americans at risk for, or living with, kidney diseases.
Disclosures
R. Ball reports he is an author on US Patent 9,075,796, “Text mining for large medical text datasets and corresponding medical text classification using informative feature selection.” E. Barreto provides consultation to FAST BioMedical for work unrelated to this manuscript. All remaining authors have nothing to disclose.
Funding
This project was supported by US FDA under master agreement number HHSF223201400030I. E. Barreto (principal investigator) is supported by a National Institute of Allergy and Infectious Diseases grant K23AI143882.
Acknowledgments
We thank Ms. Madison Cebular for her technical assistance with the reference management software.
Many thanks are due to the Sentinel Investigator team at the following data partner institutions: Aetna, a CVS Health company (Blue Bell, PA); Blue Cross Blue Shield of Massachusetts (Boston, MA); Department of Population Health Sciences, Duke University School of Medicine (Durham, NC), through the Centers for Medicare and Medicaid Services which provided data; Harvard Pilgrim Health Care Institute (Boston, MA); HealthCore, Inc., Translational Research for Affordability and Quality (Alexandria, VA); HealthPartners Institute (Minneapolis, MN); Humana, Inc., Healthcare Research (Miramar, FL); Kaiser Permanente Colorado Institute for Health Research (Aurora, CO); Kaiser Permanente Center for Integrated Health Care Research Hawai’i (Honolulu, HI); Kaiser Permanente Mid-Atlantic States, Mid-Atlantic Permanente Research Institute (Rockville, MD); Kaiser Permanente Northern California, Division of Research (Oakland, CA); Kaiser Permanente Northwest Center for Health Research (Portland, OR); Kaiser Permanente Washington Health Research Institute (Seattle, WA); Marshfield Clinic Research Institute (Marshfield, WI); Meyers Primary Care Institute (Worcester, MA); OptumInsight Life Sciences Inc. (Boston, MA); and Vanderbilt University Medical Center, Department of Health Policy (Nashville, TN), through the TennCare Division of the Tennessee Department of Finance and Administration.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Robb MA, Racoosin JA, Sherman RE, Gross TP, Ball R, Reichman ME, et al.: The US Food and Drug Administration’s Sentinel Initiative: Expanding the horizons of medical product safety. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 9–11, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Sentinel Initiative: Completed ARIA assessments & impact, 2017. Available at: https://www.sentinelinitiative.org/assessments/aria-overview/completed-aria-assessments-impact. Accessed August 28, 2020
- 3.Platt R, Brown JS, Robb M, McClellan M, Ball R, Nguyen MD, et al.: The FDA sentinel initiative - an evolving national resource. N Engl J Med 379: 2091–2093, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R: Developing the Sentinel System--a national resource for evidence development. N Engl J Med 364: 498–499, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Sentinel Initiative: Sentinel Initiative. Available at: https://www.sentinelinitiative.org/. Accessed December 10, 2019
- 6.Sentinel Initiative: Sentinel data partners. Available at: https://www.sentinelinitiative.org/sentinel/data/data-partners. Accessed May 29, 2020
- 7.Sentinel Initiative: Key database statistics, 2017. Available at: https://www.sentinelinitiative.org/about/key-database-statistics. Accessed August 31, 2020
- 8.Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R: Distributed health data networks: A practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care 48[Suppl]: S45–S51, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Maro JC, Platt R, Holmes JH, Strom BL, Hennessy S, Lazarus R, et al.: Design of a national distributed health data network. Ann Intern Med 151: 341–344, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Sentinel Initiative: How sentinel gets its data, 2019. Available at: https://www.sentinelinitiative.org/about/how-sentinel-gets-its-data. Accessed August 31, 2020
- 11.HCA Healthcare Inc: HCA healthcare fact sheet. Available at: https://hcahealthcare.com/util/forms/press-kit/HCA-presskit-fact-sheet-a.pdf. Accessed March 3, 2020
- 12.Baker MA, Baer B, Kulldorff M, Zichittella L, Reindel R, DeLuccia S, et al.: Kawasaki disease and 13-valent pneumococcal conjugate vaccination among young children: A self-controlled risk interval and cohort study with null results. PLoS Med 16: e1002844, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sentinel Initiative: Sentinel common data model, 2016. Available at: https://www.sentinelinitiative.org/about/sentinel-common-data-model/sentinel-common-data-model. Accessed August 31, 2020
- 14.Li X, Fireman BH, Curtis JR, Arterburn DE, Fisher DP, Moyneur É, et al.: Validity of privacy-protecting analytical methods that use only aggregate-level information to conduct multivariable-adjusted analysis in distributed data networks. Am J Epidemiol 188: 709–723, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida K, Gruber S, Fireman BH, Toh S: Comparison of privacy-protecting analytic and data-sharing methods: A simulation study. Pharmacoepidemiol Drug Saf 27: 1034–1041, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies M, Erickson K, Wyner Z, Malenfant J, Rosen R, Brown J: Software-enabled distributed network governance: The PopMedNet experience. EGEMS (Wash DC) 4: 1213, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sentinel Initiative: COPD, Asthma, and Respiratory Disease Effectiveness (CARE) for 21st century cures: Feasibility assessments for comparative effectiveness studies. Available at: https://www.sentinelinitiative.org/drugs/assessments/copd-asthma-and-respiratory-disease-effectiveness-care-21st-century-cures. Accessed April 11, 2020
- 18.Sentinel Initiative: Routine querying tools, 2015. Available at: https://www.sentinelinitiative.org/methods-surveillance-tools/routine-querying-tools. Accessed August 31, 2020
- 19.Gagne JJ, Han X, Hennessy S, Leonard CE, Chrischilles EA, Carnahan RM, et al.: Successful comparison of US Food and Drug Administration sentinel analysis tools to traditional approaches in quantifying a known drug-adverse event association. Clin Pharmacol Ther 100: 558–564, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang T-Y, Welch EC, Shinde MU, Platt RW, Filion KB, Azoulay L, et al.: Reproducing protocol-based studies using parameterizable tools-comparison of analytic approaches used by two medical product surveillance networks. Clin Pharmacol Ther 107: 966–977, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Sentinel Initiative: Signal identification in the sentinel system, 2019. Available at: https://www.sentinelinitiative.org/methods-surveillance-tools/signal-identification-sentinel-system. Accessed August 31, 2020
- 22.Kulldorff M, Dashevsky I, Avery TR, Chan AK, Davis RL, Graham D, et al.: Drug safety data mining with a tree-based scan statistic. Pharmacoepidemiol Drug Saf 22: 517–523, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Wang SV, Maro JC, Baro E, Izem R, Dashevsky I, Rogers JR, et al.: Data mining for adverse drug events with a propensity score-matched tree-based scan statistic. Epidemiology 29: 895–903, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norén GN, Hopstadius J, Bate A, Star K, Edwards IR: Temporal pattern discovery in longitudinal electronic patient records. Data Min Knowl Discov 20: 361–387, 2010 [Google Scholar]
- 25.Lai EC-C, Pratt N, Hsieh C-Y, Lin S-J, Pottegård A, Roughead EE, et al.: Sequence symmetry analysis in pharmacovigilance and pharmacoepidemiologic studies. Eur J Epidemiol 32: 567–582, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al.: Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med 370: 503–512, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Yih WK, Lee GM, Lieu TA, Ball R, Kulldorff M, Rett M, et al.: Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) System, 2009-2010. Am J Epidemiol 175: 1120–1128, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen M, Ball R, Midthun K, Lieu TA: The Food and Drug Administration’s post-licensure rapid immunization safety monitoring program: Strengthening the federal vaccine safety enterprise. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 291–297, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Kawai AT, Andrade SE, Zichittella L, Rosofsky R, Carroll KN, Longley E, et al. : CBER Sentinel assessment: Developing the infrastructure to conduct surveillance of birth outcomes following maternal vaccination: A project using influenza vaccines and birth outcomes as a use case, 2019. Available at: https://www.sentinelinitiative.org/sites/default/files/vaccines-blood-biologics/assessments/Developing_Infrastructure_to_Conduct_Surveillance_Birth_Outcomes_Following_Maternal_Vaccination_Final_Report_v2.pdf. Accessed April 9, 2020
- 30.Cocoros NM, Pokorney SD, Haynes K, Garcia C, Al-Khalidi HR, Al-Khatib SM, et al.: FDA-Catalyst—Using FDA’s Sentinel Initiative for large-scale pragmatic randomized trials: Approach and lessons learned during the planning phase of the first trial. Clin Trials 16: 90–97, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Pokorney SD, Cocoros NM, Al-Khalidi H, Haynes K, Al-Khatib S, Garcia C, et al. : FDA-Catalyst protocol: Implementation of a randomized controlled trial to improve treatment with oral anticoagulants in patients with atrial fibrillation (IMPACT-AFib). Available at: https://www.sentinelinitiative.org/sites/default/files/IMPACT-AFib_Protocol_v5.pdf. Accessed March 9, 2020 [DOI] [PMC free article] [PubMed]
- 32.Sentinel Initiative: FDA-catalyst MyStudies app alignment with pragmatic trials and/or registries, 2018. Available at: https://www.sentinelinitiative.org/methods-surveillance-tools/fda-catalyst-projects/fda-catalyst-mystudies-app-alignment-pragmatic. Accessed August 31, 2020
- 33.US Food and Drug Administration: COVID MyStudies application (App), 2019. Available at: http://www.fda.gov/drugs/science-and-research-drugs/fdas-mystudies-application-app. Accessed March 13, 2020
- 34.Rogers J: FDA MyStudies comes to google cloud. Google Cloud Blog, 2020. Available at: https://cloud.google.com/blog/topics/healthcare-life-sciences/fda-mystudies-comes-to-google-cloud/. Accessed March 13, 2020 [Google Scholar]
- 35.Dublin S, Wartko P, Mangione-Smith R: Studying medication safety in pregnancy: A call for new approaches, resources, and collaborations. Pediatrics 146: e20201540, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Raebel MA, Shetterly S, Paolino R, Lu CY, Gagne JJ, Haynes K, et al. : Mini-Sentinel methods: Analytic methods for using laboratory test results in active database surveillance: Final report, 2016. Available at: https://www.sentinelinitiative.org/sites/default/files/Methods/Analytic_Methods_for_Using_Laboratory_Test_Results_In_Active_Database_Surveillance_Final_Report.pdf. Accessed April 9, 2020
- 37.Flory JH, Roy J, Gagne JJ, Haynes K, Herrinton L, Lu C, et al.: Missing laboratory results data in electronic health databases: Implications for monitoring diabetes risk. J Comp Eff Res 6: 25–32, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Eworuke E, Panucci G, Goulding M, Neuner R, Toh S: Use of tumor necrosis factor-alpha inhibitors during pregnancy among women who delivered live born infants. Pharmacoepidemiol Drug Saf 28: 296–304, 2019. [DOI] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration: Drug safety-related labeling changes (SrLC). Available at: https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=540. Accessed March 3, 2020
- 40.Sentinel Initiative: Optimal initial length of opioid prescription, 2019. Available at: https://www.sentinelinitiative.org/assessments/drugs/optimal-initial-length-opioid-prescription. Accessed August 31, 2020
- 41.Sentinel Initiative: Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee: Development of opioid-sparing and opioid-replacement drugs, 2019. Available at: https://www.sentinelinitiative.org/news-events/fda-advisory-committee-meetings/meeting-anesthetic-and-analgesic-drug-products-advisory. Accessed August 31, 2020
- 42.MUS Food and Drug Administration: Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee: Background and rationale for the development of opioid-sparing and opioid-replacement drugs, 2018. Available at: https://www.fda.gov/media/121207/download. Accessed August 31, 2020
- 43.US Food and Drug Administration : FDA briefing document: Anesthetic and Analgesic Drug Products Advisory Committee meeting, 2008. Available at: https://www.fda.gov/media/121203/download. Accessed March 9, 2020
- 44.US Food and Drug Administration: Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee : FDA briefing document 2018. Available at: https://www.fda.gov/media/121203/download. Accessed March 9, 2020
- 45.Sentinel Initiative: Active Risk Identification and Analysis (ARIA) overview, 2016. Available at: https://www.sentinelinitiative.org/assessments/active-risk-identification-and-analysis-aria-overview. Accessed August 31, 2020
- 46.US Food and Drug Administration: Supplement approval; fulfillment of postmarketing requirement. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/204042Orig1s027ltr.pdf. Accessed March 9, 2020
- 47.Sentinel Initiative:Validation of acute kidney injury cases, 2013. Available at: https://www.sentinelinitiative.org/methods-surveillance-tools/health-outcome-interest/validation-acute-kidney-injury-cases. Accessed August 31, 2020
- 48.Freeman C, Leonard C, Archdeacon P, Carnahan R, Chrischilles E, Hennessy S: 16 health outcomes of interest for surveillance preparedness, 2014. Available at: https://www.sentinelinitiative.org/sites/default/files/surveillance-tools/validations-literature/Mini-Sentinel_16_HOIs_Surveillance-Preparedness.pdf. Accessed April 9, 2020
- 49.White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. ; CARES Investigators : Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 378: 1200–1210, 2018. [DOI] [PubMed] [Google Scholar]
- 50.Sentinel Initiative: 2019 ICPE presentation: Characteristics of febuxostat and allopurinol initiators and utilization patterns in real-world settings, 2019. Available at: https://www.sentinelinitiative.org/news-events/publications-presentations/2019-icpe-presentation-characteristics-febuxostat-and. Accessed September 2, 2020
- 51.Toh S, Reichman ME, Houstoun M, Ross Southworth M, Ding X, Hernandez AF, et al.: Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 172: 1582–1589, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Sentinel Initiative: Chronic kidney disease diagnoses, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/chronic-kidney-disease-diagnoses. Accessed September 2, 2020
- 53.Perazella MA: Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol 13: 1897–1908, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, et al.: Phenotype standardization for drug-induced kidney disease. Kidney Int 88: 226–234, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haslam A, Prasad V: Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2: e192535, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al.: Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J Am Soc Nephrol 31: 435–446, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, et al.: The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol 14: 1692–1700, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dormuth CR, Filion KB, Paterson JM, James MT, Teare GF, Raymond CB, et al. ; Canadian Network for Observational Drug Effect Studies (CNODES) Investigators : Higher potency statins and the risk of new diabetes: Multicentre, observational study of administrative databases. BMJ 348: g3244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. ; EMPA-REG OUTCOME Investigators : Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016. [DOI] [PubMed] [Google Scholar]
- 60.Seidu S, Kunutsor SK, Cos X, Gillani S, Khunti K; for and on behalf of Primary Care Diabetes Europe : SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: A systematic review and meta-analysis. Prim Care Diabetes 12: 265–283, 2018. [DOI] [PubMed] [Google Scholar]
- 61.Heerspink HJL, Karasik A, Thuresson M, Melzer-Cohen C, Chodick G, Khunti K, et al.: Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): A multinational observational cohort study. Lancet Diabetes Endocrinol 8: 27–35, 2020. [DOI] [PubMed] [Google Scholar]
- 62.Neuen BL, Jardine MJ, Perkovic V: Sodium-glucose cotransporter 2 inhibition: Which patient with chronic kidney disease should be treated in the future? Nephrol Dial Transplant 35[Suppl 1]: i48–i55, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hampp C, Swain RS, Horgan C, Dee E, Qiang Y, Dutcher SK, et al.: Use of sodium-glucose cotransporter 2 inhibitors in patients with type 1 diabetes and rates of diabetic ketoacidosis. Diabetes Care 43: 90–97, 2020. [DOI] [PubMed] [Google Scholar]
- 64.Abdel-Kader K, Greer RC, Boulware LE, Unruh ML: Primary care physicians’ familiarity, beliefs, and perceived barriers to practice guidelines in non-diabetic CKD: A survey study. BMC Nephrol 15: 64, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL: Effects of statins on renal outcome in chronic kidney disease patients: A systematic review and meta-analysis. PLoS One 10: e0132970, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wasser JS; DIA: Global Forum Driving Insights to Action: Innovation in Medical Evidence Development and Surveillance (IMEDS) & the evolution of postmarket safety studies. Available at: https://reaganudall.org/sites/default/files/sites/default/files/IMEDS%20DIAglobal%20Article%208-2017.pdf. Accessed September 29, 2020
- 67.Reagan Udall Foundation : Innovation in medical evidence development and surveillance. Available at: https://reaganudall.org/programs/innovation-medical-evidence-development-and-surveillance-imeds. Accessed September 29, 2020
- 68.Haynes K, Selvam N, Cziraky MJ: Bidirectional data collaborations in distributed research. EGEMS (Wash DC) 4: 1205, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curtis LH, Brown J, Platt R: Four health data networks illustrate the potential for a shared national multipurpose big-data network. Health Aff (Millwood) 33: 1178–1186, 2014. [DOI] [PubMed] [Google Scholar]
- 70.NIH Collaboratory Living Textbook of Pragmatic Clinical Trials: NIH Collaboratory Distributed Research Network (DRN) - rethinking clinical trials. Available at: https://rethinkingclinicaltrials.org/nih-collaboratory-drn/. Accessed February 11, 2020
- 71.McMahill-Walraven CN, Kent DJ, Panozzo CA, Pawloski PA, Haynes K, Marshall J, et al.: Harnessing the biologics and biosimilars collective intelligence consortium to evaluate patterns of care. J Manag Care Spec Pharm 25: 1156–1161, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maro JC, Menzin T, Hornbuckle K, Giles JT, Kavanaugh A, Dörner T, et al. : SAT0140 Risk of venous thromboembolism in rheumatoid arthritis patients treated with biologic and non-biologic dmards. Ann Rheum Dis 77: 932, 2018 [Google Scholar]
- 73.Sobel RE, Bate A, Marshall J, Haynes K, Selvam N, Nair V, et al.: Do FDA label changes work? Assessment of the 2010 class label change for proton pump inhibitors using the Sentinel System’s analytic tools. Pharmacoepidemiol Drug Saf 27: 332–339, 2018. [DOI] [PubMed] [Google Scholar]
- 74.Gewandter JS, Kleckner AS, Marshall JH, Brown JS, Curtis LH, Bautista J, et al.: Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: An NIH Collaboratory study of claims data. Support Care Cancer 28: 2553–2562, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panozzo CA, Curtis LH, Marshall J, Fine L, Wells BL, Brown JS, et al.: Incidence of statin use in older adults with and without cardiovascular disease and diabetes mellitus, January 2008- March 2018. PLoS One 14: e0223515, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.NIH collaboratory living textbook of pragmatic clinical trials: December 6, 2019: Millions more people, stronger collaborations: The new and improved NIH collaboratory distributed research network. Available at: https://dcricollab.dcri.duke.edu/sites/NIHKR/KR/GR-Slides-12-06-19.pdf. Accessed February 11, 2020
- 77.Wu A, McMahon P, Mendelsohn A, Welch E, Gokhale M, McMahill-Walraven C, et al. : Use of mepolizumab among individuals with asthma in the US. Presented at the American Thoracic Society 2020 Annual Meeting, New York, NY, 2020 [Google Scholar]
- 78.Panozzo CA, Purcell G, Andrade S, Griffin M, Haynes K, Lin N, et al. : Safety of trumenba vaccine among pregnant women in the United States: Planning and design of a large-scale multi-site observational study. Pharmacoepidemiol Drug Saf 26: 3–636, 2017. 28547787 [Google Scholar]
- 79.Sentinel Initiative: Public repositories. Available at: https://dev.sentinelsystem.org/repos?visibility=public. Accessed July 14, 2020
- 80.Sentinel Initiative: SAS code for transforming the IBM MarketScan® research Databases (MarketScan) into the sentinel common data model. 2019. Available at: https://www.sentinelinitiative.org/about/sentinel-common-data-model/sas-code-transforming-ibm-marketscan%C2%AE-research-databases-marketscan. Accessed September 2, 2020
- 81.Sentinel Initiative: The Centers for Medicare and Medicaid Services (CMS): Medicare Fee-For-Service (FFS) claims in sentinel common data model format, 2019. Available at: https://www.sentinelinitiative.org/methods-surveillance-tools/software-packages-toolkits/centers-medicare-and-medicaid-services-cms. Accessed September 2, 2020
- 82.Sentinel Initiative: Acute kidney failure diagnoses, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/acute-kidney-failure-diagnoses. Accessed September 2, 2020
- 83.Sentinel Initiative: Dialysis HCPCS procedure codes, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/dialysis-hcpcs-procedure-codes. Accessed September 2, 2020
- 84.Sentinel Initiative: Analgesic use 2, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/analgesic-use-2. Accessed September 2, 2020
- 85.Sentinel Initiative: Anti-infective agents use, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/anti-infective-agents-use. Accessed September 2, 2020
- 86.Sentinel Initiative: Anti-seizure medication use, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/anti-seizure-medication-use. Accessed September 2, 2020
- 87.Sentinel Initiative: Counts and prevalence of alendronate, risedronate, ibandronate, and zolendronate, 2018. Available at: https://www.sentinelinitiative.org/assessments/drugs/counts-and-prevalence-alendronate-risedronate-ibandronate-and-zolendronate. Accessed September 2, 2020
- 88.Sentinel Initiative: Occurrence of selected generic drugs 1, 2012. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-selected-generic-drugs-1. Accessed September 2, 2020
- 89.Sentinel Initiative: Occurrence of selected generic drugs 10, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-selected-generic-drugs-10. Accessed September 2, 2020
- 90.Sentinel Initiative: Occurrence of selected generic drugs 13, 2018. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-selected-generic-drugs-13. Accessed September 2, 2020
- 91.Sentinel Initiative: Occurrence of selected generic drugs 7, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-selected-generic-drugs-7. Accessed September 2, 2020
- 92.Sentinel Initiative: Occurrence of selected generic drugs 9, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-selected-generic-drugs-9. Accessed September 2, 2020
- 93.Sentinel Initiative: Occurrence of selected pediatric drugs 1, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-selected-pediatric-drugs-1. Accessed September 2, 2020
- 94.Sentinel Initiative: Prevalent and incident use of acetazolamide, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/prevalent-and-incident-use-acetazolamide. Accessed September 2, 2020
- 95.Sentinel Initiative: New molecular entities, 2017. Available at: https://www.sentinelinitiative.org/assessments/drugs/new-molecular-entities. Accessed September 2, 2020
- 96.Sentinel Initiative: Counts of commonly used angiostensin-converting enzyme (ACE) inhibitors and beta blockers, 2018. Available at: https://www.sentinelinitiative.org/assessments/drugs/counts-commonly-used-angiostensin-converting-enzyme-ace-inhibitors-and-beta. Accessed September 2, 2020
- 97.Sentinel Initiative: Occurrence of kidney stones, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/occurrence-kidney-stones. Accessed September 2, 2020
- 98.Sentinel Initiative: Cutaneous small vessel vasculitis and acute kidney injury following direct-acting oral anticoagulant use, 2019. Available at: https://www.sentinelinitiative.org/assessments/drugs/cutaneous-small-vessel-vasculitis-and-acute-kidney-injury-following-direct-acting. Accessed September 2, 2020
- 99.Sentinel Initiative: Dabigatran and warfarin use among members with atrial fibrillation and other pre-existing conditions, 2016. Available at: https://www.sentinelinitiative.org/assessments/drugs/dabigatran-and-warfarin-use-among-members-atrial-fibrillation-and-other-pre. Accessed September 2, 2020
- 100.Sentinel Initiative: Angiotension-Receptor Blockers (ARBs), hydrochlorothiazide, atenolol, amlodipine use and celiac disease, 2013. Available at: https://www.sentinelinitiative.org/assessments/drugs/angiotensin-receptor-blockers-arbs-hydrochlorothiazide-atenolol-amlodipine-use-and. Accessed September 2, 2020
- 101.Sentinel Initiative: Antiepileptic Drugs (AEDs) and kidney stones, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/antiepileptic-drugs-aeds-and-kidney-stones. Accessed September 2, 2020
- 102.Sentinel Initiative: Characteristics of gout patients and use of urate-lowering therapies, 2019. Available at: https://www.sentinelinitiative.org/assessments/drugs/characteristics-gout-patients-and-use-urate-lowering-therapies. Accessed September 2, 2020
- 103.Sentinel Initiative: Rhabdomyolysis and/or creatine kinase laboratory results following new use of statins or angiotensin-converting enzyme inhibitors, 2018. Available at: https://www.sentinelinitiative.org/assessments/drugs/rhabdomyolysis-andor-creatine-kinase-laboratory-results-following-new-use-statins. Accessed September 2, 2020
- 104.Sentinel Initiative: IVIg exposure and renal failure, 2015. Available at: https://www.sentinelinitiative.org/assessments/vaccines-blood-biologics/ivig-exposure-and-renal-failure. Accessed September 2, 2020
- 105.Sentinel Initiative: Use of valsartan, angiotensin II receptor blockers (ARB), and angiotensin-converting enzyme (ACE) inhibitors, 2019. Available at: https://www.sentinelinitiative.org/assessments/drugs/use-valsartan-angiotensin-ii-receptor-blockers-arb-and-angiotensin-converting. Accessed September 2, 2020
- 106.Sentinel Initiative: Prevalent and incident use of quinine sulfate, 2014. Available at: https://www.sentinelinitiative.org/assessments/drugs/prevalent-and-incident-use-quinine-sulfate. Accessed September 2, 2020
- 107.Sentinel Initiative: Selected medications and death, with linkage of mini-sentinel distributed database with NDI+, 2018. Available at: https://www.sentinelinitiative.org/assessments/drugs/selected-medications-and-death-linkage-mini-sentinel-distributed-database-ndi. Accessed September 2, 2020
- 108.Sentinel Initiative: Gadolinium-based contrast agents (GBCAs) use in pregnancy, 2017. Available at: https://www.sentinelinitiative.org/assessments/drugs/gadolinium-based-contrast-agents-gbcas-use-pregnancy. Accessed September 2, 2020
- 109.Sentinel Initiative: Drug use among pregnant women with live births, 2016. Available at: https://www.sentinelinitiative.org/assessments/drugs/drug-use-among-pregnant-women-live-births. Accessed September 2, 2020
- 110.Sentinel Initiative: Non-melanoma skin cancer following hydrochlorothiazide use: A propensity score matched analysis, 2019. Available at: https://www.sentinelinitiative.org/assessments/drugs/non-melanoma-skin-cancer-following-hydrochlorothiazide-use-propensity-score. Accessed September 2, 2020


