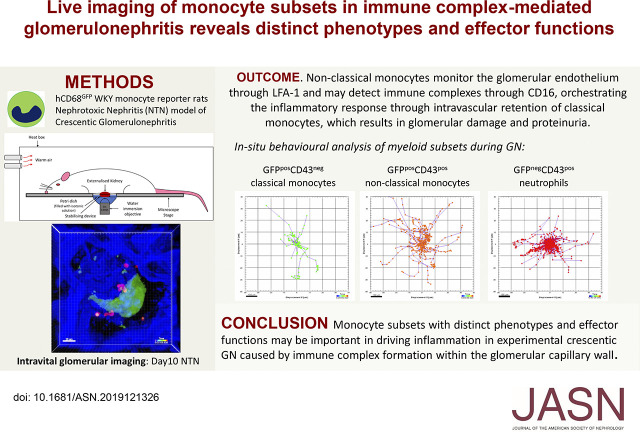
Significance Statement
Monocytes and macrophages are important in mediating crescentic GN (CrGN), but little work has been done to phenotype the subpopulations involved and determine their respective contributions to glomerular inflammation. Using nephrotoxic nephritis in the rat as a clinically relevant experimental model of CrGN, we show that this is a predominantly intravascular disease and that glomerular inflammation and damage is driven by dynamic interactions between intravascular blood monocytes and the endothelium. Monocyte subsets had distinct phenotypes and effector functions: non-classical monocytes were recruited to the glomerulus first, and may orchestrate the inflammatory response. Later recruitment of classical monocytes was associated with glomerular damage and proteinuria. Targeting specific monocyte subpopulations may generate less toxic and more effective therapies for patients with GN.
Keywords: glomerulonephritis, immune complexes, macrophages, immunology
Visual Abstract
Abstract
Background
Immune complexes within glomerular capillary walls cause crescentic GN (CrGN). Monocytes and macrophages are important in mediating CrGN, but little work has been done to phenotype the subpopulations involved and determine their respective contributions to glomerular inflammation.
Methods
Live glomerular imaging using confocal microscopy monitored intravascular monocyte subset behavior during nephrotoxic nephritis (NTN) in a novel WKY-hCD68-GFP monocyte/macrophage reporter rat strain. Flow cytometry and qPCR further analyzed ex vivo the glomerular leukocyte infiltrate during NTN.
Results
Non-classical monocytes surveyed the glomerular endothelium via lymphocyte function-associated antigen 1 (LFA-1) in the steady state. During NTN, non-classical monocytes were recruited first, but subsequent recruitment and retention of classical monocytes was associated with glomerular damage. Monocytes recruited to the glomerular vasculature did not undergo transendothelial migration. This finding suggests that inflammation in immune complex-mediated CrGN is predominantly intravascular, driven by dynamic interactions between intravascular blood monocytes and the endothelium. Glomerular endothelium and non-classical monocytes overexpressed a distinct chemokine axis, which may orchestrate inflammatory myeloid cell recruitment and expression of damage mediators. Reduced classical monocyte recruitment in Lewis rats during NTN confirmed a role for CD16 in mediating glomerular damage.
Conclusions
Monocyte subsets with distinct phenotypes and effector functions may be important in driving inflammation in experimental CrGN resulting from immune complexes formed within the glomerular capillary wall. LFA-1–dependent endothelial surveillance by non-classical monocytes may detect immune complexes through CD16, orchestrating the inflammatory response through intravascular retention of classical monocytes, which results in glomerular damage and proteinuria.
GN is commonly caused by deposition of immune complexes within glomerular capillary walls and is an important cause of ESKD worldwide. Monocytes and their tissue descendants, macrophages, are important in mediating glomerular inflammation caused by immune complexes. Infiltration of the glomerulus with CD68pos mononuclear cells in both human disease and experimental models correlates with disease severity and clinical outcome,1 and inhibiting macrophage recruitment or activation in experimental models ameliorates disease.2–8 However, it is not clear whether infiltrating CD68pos cells are macrophages or monocytes. Moreover, heterogenous populations of these cells exist and relatively little work has been done to phenotype the subpopulations involved and determine their respective contributions to glomerular inflammation. Understanding which leukocyte subpopulations are important in mediating the inflammatory response to immune complex formation within glomerular capillary walls, and the mechanisms by which they cause glomerular injury, may lead to the development of more targeted therapies for GN with reduced toxicity.
Monocytes comprise subsets that have distinct trafficking pathways and effector functions in vivo.9–11 Homologous subsets of classical and non-classical monocytes have been described in humans and mice with conserved differential expression of chemokine receptors and adhesion molecules, suggesting they have important biologic functions in vivo.12 In humans, they are defined by their level of CD14 and CD16 expression and, in mice, by Ly6C expression. Classical monocytes are CD14hiCD16negCX3CR1lo and Ly6ChiCX3CR1lo and non-classical monocytes are CD14loCD16posCX3CR1hi and Ly6CloCX3CR1hi in humans and mice, respectively.10,11 Intermediate monocytes have also been identified, which are lower in abundance, display intermediate Ly6C expression in mice, and are characterized as CD14hiCD16pos in humans. All monocyte subsets express CD68, as do some of their tissue monocyte-derived macrophage descendants.13
Non-classical monocytes continuously patrol the endothelium of blood vessels in the steady state, traveling considerable distances independently of blood flow, suggesting they may perform immune surveillance of the endothelium.9,10 This behavior has been shown in multiple vascular beds, including the capillaries of the kidney cortex in mice,14 and is dependent on firm adhesion to the endothelium, mediated by the β2 integrin CD11a/CD18 (LFA-1) and fractalkine receptor (CX3CR1).9,14 Human CD16pos non-classical monocytes produce proinflammatory cytokines in response to immune complex detection.10 The intravascular location of non-classical monocytes, their unique role in endothelial immune surveillance, and their high level of FcγRIII (CD16) expression would make them ideally situated to detect immune complexes deposited within glomerular capillary walls and trigger subsequent inflammation.10
Indeed, recent elegant work has shown that non-classical monocytes patrol the endothelium of glomerular capillaries in mice, even in the absence of inflammation.15,16 During nephrotoxic nephritis (NTN) caused by anti-GBM antibody deposition, non-classical monocytes increased their duration of retention and promoted neutrophil retention and reactive oxygen species (ROS) production through direct cellular interactions and TNF production.16 This important work has advanced our understanding of myeloid cell behavior within the glomerulus during very early immune complex-mediated GN (the authors examined leukocyte responses exclusively within 5 hours of administration of anti-GBM antibody). However, the behavioral phenotypes and effector functions of myeloid cell subpopulations using live glomerular imaging at later disease time points have not yet been explored.
NTN in the Wistar–Kyoto (WKY) rat strain (caused by anti-GBM antibody deposition) is widely used as a model of immune complex-mediated glomerular inflammation that closely resembles severe crescentic GN in humans.17 It has a well characterized and reproducible time course of glomerular leukocyte infiltration and crescent formation, allowing identification of appropriate time points for investigation. Therefore, we used rat NTN as an experimental model of immune complex-mediated crescentic GN and performed live glomerular imaging using intravital confocal microscopy in a novel transgenic WKY-hCD68-GFP monocyte/macrophage reporter. This permitted real-time in vivo visualization of monocyte subset recruitment and intravascular behavior during both early and established glomerular inflammation. We explored the hypothesis that non-classical monocytes survey the glomerular endothelium, are the first responders to immune complexes formed within the glomerular capillary wall, and orchestrate the subsequent inflammatory response.
Methods
Development of a Novel WKY-hCD68-GFP Transgenic Reporter Rat Strain
A novel transgenic monocyte/macrophage reporter rat strain was developed on a WKY genetic background using the Sleeping Beauty transposon system. This method has been previously described by our group for the generation of other transgenic rat strains.18 To create this novel transgenic strain, the CAGGS promoter in pSB IR-DR(L)-CAGGS-eGFR-pA-IR-DR(R) plasmid was replaced with the human CD68 promoter (hCD68) in order to drive green fluorescent protein (GFP) expression in blood monocytes and tissue macrophages, analogous to the mouse model previously published.13
Animal Husbandry
WKY-hCD68-GFP rats were developed and bred in-house at our facility at Imperial College London. Lewis rats were obtained from Charles River Laboratories. Male rats were used for all experiments. Rats were maintained on a standard diet. All procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act.
Induction of Experimental Crescentic GN
Experimental crescentic GN was induced in male rats using the NTN model, which models in situ immune complex formation and induces severe crescentic GN with associated proteinuria and impairment of kidney function. NTN was induced by intravenous injection of 0.1 ml of nephrotoxic serum, produced in rabbits as described previously.19 Control rats were injected with normal rabbit serum. Rats were euthanized (by exsanguination under isoflurane anesthesia) or imaged (under terminal anesthesia) on days 2, 6, 8, and 10 after induction of NTN. Serum samples were collected during exsanguination for measurement of serum creatinine. Urine was collected by placing animals in metabolic cages for 24 hours with free access to food and water. Proteinuria was determined by the sulfosalicylic acid method.20 Samples of kidney were fixed in 10% formalin for 24 hours and then processed and embedded in paraffin wax. Kidney tissue for frozen sections was fixed in 2% paraformaldehyde for 2 hours, transferred into 13% glucose overnight and then snap frozen in isopentane. For electron microscopy, approximately 1 mm3 sections of kidney cortex were fixed in cacodylate buffered 3% glutaraldehyde. Fixed tissue was then processed using a standard protocol that included postfixation in osmium tetroxide, block staining in uranyl acetate, dehydration through graded alcohols to propylene oxide, and finally, infiltrating and embedding in Spurr resin.
Preparation of the Kidney for Intravital Glomerular Imaging in WKY-hCD68-GFP Rats
Male WKY-hCD68-GFP rats 8–9 weeks of age were given unilateral hydronephrosis by ligating the left ureter under short, recoverable anesthesia, causing progressive atrophy of the kidney parenchyma over 9 weeks, rendering the kidney more translucent and superficializing the glomeruli within the kidney cortex. This permits intravital imaging of the glomerulus in situ by direct transillumination of the kidney using confocal fluorescence microscopy, a protocol which has been validated in mice.15,16,21–23 Imaging was performed under terminal anesthesia, 9 weeks after ureteric ligation, when the rats were 18–19 weeks of age. Inhalational anesthesia with isoflurane was used for induction and maintenance and the rats were maintained at a constant temperature using a heat pad at 37°C. The internal jugular vein was cannulated to enable administration of antibodies (for in vivo cell labeling and blocking studies) and other reagents during imaging. The hydronephrotic left kidney was externalized using a small flank incision, which was then sutured to stabilize the kidney and prevent retraction back into the abdominal cavity. The rats were then transferred onto the stage of an inverted Leica TCS Sp5 Confocal Microscope (Leica Microsystems). A customized stage insert was used to immobilize the kidney in a petri dish containing a sterile, physiologic crystalloid solution (Plasma-Lyte 148, pH 7.4). Intravital glomerular imaging was performed in situ by direct transillumination of the kidney using an immersion system (see Intravital Confocal Microscopy). The microscope was fitted with a heat box maintained at 37°C to enable stable and prolonged anesthesia to be administered.
To visualize the glomerular vasculature, 5 mg of TRITC-conjugated 150 kDa dextran (10 mg/ml) was injected intravenously at the start of imaging. This high-molecular-weight dextran is too large to be filtered by the glomerulus and remains in the vasculature for prolonged periods of time. In vivo antibody labeling of leukocyte subpopulations was performed by intravenous administration of an Alexa Fluor 647–labeled anti-CD43 antibody (W3/13, 25 μg; Biolegend) and/or an Alexa Fluor 555–labeled anti-rat granulocyte antibody (HIS48, 50 μg; Invitrogen) immediately before imaging. In separate experiments, CellRox Deep Red Reagent (40 μl of 2.5 mM solution; Invitrogen) was injected intravenously 20 minutes before imaging to enable in vivo detection of ROS production.
Intravital Confocal Microscopy
Intravital glomerular imaging of the hydronephrotic kidney in WKY-hCD68-GFP transgenic reporter rats was performed using an inverted Leica TCS SP5 confocal microscope (Leica Microsystems) fitted with a ×25 immersion objective (Leica HC Fluotar L 25×/0.95 W). Glomeruli were identified and selected at random for intravital time-lapse imaging. For each glomerulus, Z-stack images (40–50 μm depth), with a 1.5 μm step size, were captured every 30 seconds at a resolution of 512×512 pixels for a total duration of 45 minutes. Approximately two to four glomeruli were imaged per rat. Light was generated from the 488 nm, 561 nm, and 633 nm laser lines. Acquisition settings were optimized and then standardized for all experiments in order to ensure consistency in laser power and detector gain used between rats.
Image Analysis
Maximum intensity projections of time-lapse imaging were generated and analyzed using the Bitplane Imaris software package (version 8.0). Only intravascular leukocytes adherent to the glomerular endothelium were analyzed. Cells were defined as adherent if they remain attached to the glomerular endothelium for two or more consecutive frames. Leukocyte subpopulations were identified and tracked over time using the “surfaces” function within Imaris and the autoregressive motion tracking algorithm. Manual tracking was used when necessary to adjust automatic tracking.
Parameters, including track number, duration, and net displacement length, were calculated using Imaris and used to phenotype changes in the behavior of distinct leukocyte subpopulations during glomerular inflammation. Track number represents cell recruitment and was standardized between experiments as the number of adherent leukocytes per glomerulus per hour. Track duration (dwell time) represents the length of time for which tracked leukocytes remain adherent to the glomerular endothelium before they detach and return to the circulation, i.e., the duration of their retention within the glomerulus. Net displacement length refers to the straight line distance between the start and end of a tracked cell path along the glomerular endothelium and represents the behavioral phenotype of adherent leukocytes, i.e., whether they are migratory or undergo more stationary retention on the endothelium.
CD11a (LFA-1) Inhibition
To investigate the role of the β2 integrin LFA-1 in mediating non-classical monocyte patrolling and the distinct responses of monocyte subsets and neutrophils to immune complex formation within the glomerular capillary wall, in vivo CD11a (LFA-1) inhibition was performed during intravital glomerular imaging of the hydronephrotic kidney, 2 days after induction of NTN. Mouse anti-rat CD11a IgG (WT.1) (2 mg/kg) or an isotype control IgG was injected intravenously at the start of imaging and glomeruli imaged for 2–3 hours after injection.
Glomerular Sieving
In order to analyze and sort leukocyte subpopulations from the glomeruli of rats with NTN using FACS, glomeruli were isolated from kidney tissue and digested to obtain single-cell suspensions using a method adapted from Cook et al.24 These experiments were conducted in rats without unilateral hydronephrosis. Briefly, kidneys were removed from WKY-hCD68-GFP and Lewis rats after exsanguination under terminal isoflurane anesthesia (to remove as much contaminating blood from the glomerular microvasculature as possible). Kidneys were not perfused to avoid disruption of leukocyte-endothelial interactions that otherwise might alter data on the retention of leukocyte subpopulations within the glomerular vasculature during NTN. Cortical strips of kidney were placed in sterile HBSS containing calcium and magnesium. The two kidneys from each rat were pooled and the glomeruli isolated by sieving, as described previously.25 Purified glomeruli were then digested with 1 mg/ml type 4-S collagenase (Sigma), 0.5 mg/ml trypsin (Sigma), and 0.1 mg/ml type 1 DNase (Roche) for 20 minutes at 37°C. Digested glomeruli were then passed once through a 23G needle to obtain single-cell suspensions. Cells were then washed and resuspended in PBS containing 0.5% BSA, ready for staining and FACS.
Preparation of Whole Blood for Analysis of Leukocyte Subpopulations
Blood leukocyte subpopulations in WKY-hCD68-GFP rats (without unilateral hydronephrosis) were analyzed using flow cytometry on whole blood after performing red blood cell lysis. Red blood cell lysis was performed by incubating whole blood in buffer containing ammonium chloride (pH 7.6) for 6 minutes on ice. Remaining leukocytes were then washed and resuspended in sterile PBS containing 0.5% BSA to obtain single-cell suspensions for staining and analysis using flow cytometry.
Flow Cytometry and Cell Sorting
Single-cell suspensions in sterile PBS containing 0.5% BSA (obtained from whole blood or sieved, digested glomeruli) were incubated with the following anti-rat antibodies for 10 minutes at 4°C in polypropylene, round bottom, 96-well plates (Corning): panel 1 (for WKY-hCD68-GFP transgenic reporter rats; all antibodies used at 1:40 dilution) used anti-CD45 (OX-1, V450), anti-CD3 (eBioG4.18, PE), anti-B220 (HIS24, PE), anti-CD161a (3.2.3, PE), granulocyte marker antibody (HIS48, biotin), and anti-CD43 (W3/13, Alexa Fluor 647); panel 2 (for Lewis rats) was the same panel as above, but with addition of anti-rat CD172a (OX-41, FITC) used at a 1:8 dilution. The biotinylated HIS48 antibody was subsequently revealed by incubation with streptavidin-PECy7 (1:300 dilution).
For analysis using flow cytometry, cells were washed and resuspended in sterile PBS containing 0.5% BSA and 1% paraformaldehyde and analyzed on a BD LSRFortessa flow cytometer equipped with violet, blue, yellow-green, and red lasers, using DIVA. Precision Count Beads (Biolegend) were used for quantification of leukocyte subsets.
For FACS, cells were resuspended in sterile PBS containing 0.5% BSA and sorted using a BD FACSAria II flow cytometer. Cells were sorted into sterile PBS containing 0.5% BSA and then centrifuged immediately at 480×g for 5 minutes at 4°C and resuspended in TRI reagent (Sigma) for cell lysis and subsequent RNA extraction.
Cytospin preparations were made from sorted blood leukocyte subpopulations using a standard protocol. Cells were spun onto poly-l-lysine coated slides (Leica Microsystems) at 300 rpm for 3 minutes and allowed to air dry before staining using the Shandon Kwik-Diff Stain (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Quantitative PCR
Total RNA was extracted from sorted glomerular leukocyte subpopulations lysed in TRI reagent (Sigma) using the Direct-zol RNA MicroPrep Kit (Zymoresearch) according to the manufacturer’s protocol. The protocol included a DNA digestion step with DNase I to reduce contaminating genomic DNA. RNA quantity and quality was assessed using OD readings at 260 nm and 280 nm.
RNA was converted to complementary DNA using SuperScript IV Reverse transcription (Invitrogen) with Random Hexamers (Invitrogen) according to the manufacturer’s protocol. Target and housekeeping genes were identified using the Ensembl database and the National Center for Biotechnology Information (NCBI) Reference Sequence number used to design quantitative PCR (qPCR) primers using NCBI-Primer/BLAST (Supplemental Table 1).
qPCR was performed using 100 ng complementary DNA per reaction and the SensiMix SYBR Lo-Rox kit (Bioline). Samples were run in triplicate using a ViiA 7 Real-Time PCR System (Applied Biosystems). Gene expression was calculated using the fold change method (2-ΔΔCT).
Adoptive Transfer
Whole blood was taken from control donor WKY-hCD68-GFP rats via cardiac puncture and exsanguination. Red blood cell lysis was performed as detailed above, cells washed and resuspended in sterile PBS containing 0.5% BSA to obtain single-cell suspension, and GFPpos blood monocytes sorted using a BD FACSAria II flow cytometer. Cells were sorted directly into sterile PBS containing 0.5% BSA maintained at 4°C and then centrifuged immediately at 480×g for 5 minutes at 4°C. The pellet was then resuspended in sterile 0.9% saline for injection and injected into wild-type recipient WKY rats (without unilateral hydronephrosis) with day 8 NTN (when crescents are actively being formed). Recipient rats were euthanized 24 hours later and frozen tissue sections examined using confocal microscopy.
Immunohistochemistry
Infiltrating glomerular monocytes/macrophages were identified in formalin-fixed, paraffin-embedded tissue sections by immunoperoxidase staining using monoclonal mouse anti-rat CD68 antibody (ED-1, dilution 1:500; Bio-Rad). Sections were incubated with primary antibody for 1 hour at room temperature. After washing, sections were incubated with a secondary polymer horseradish peroxidase system for mouse primary antibodies (EnVision Systems; Dako) and developed in 3,3-diaminobenzidine, according to the manufacturer’s instructions. Sections were then rinsed counterstained with filtered Harris hematoxylin (CellPath, Powys, UK), dehydrated, and mounted with DPX mounting medium (Thermo Fisher Scientific, Leicester, UK).
Statistical Analyses
Data are presented as mean±SEM. Statistical differences between groups were analyzed using t test or ANOVA (with Dunnett multiple comparisons test) for parametric data, and Mann–Whitney or Kruskal–Wallis (with Dunn multiple comparisons test) for nonparametric data. Data were tested for normality of distribution using the Shapiro–Wilk normality test. All data were analyzed using the GraphPad Prism 7.0 software package. P values of <0.05 were considered significant.
Study Approval
All procedures were carried out according to the institutional guidelines and the use of experimental animals was performed following the Animal Research: Reporting of In Vivo Experiments guidelines. Animal studies were approved by the UK Home Office.
Results
Transgenic WKY-hCD68-GFP Monocyte/Macrophage Reporter Rat Strain
A novel transgenic rat strain was developed using Sleeping Beauty transposon germline transgenesis, expressing GFP under the hCD68 promoter on a WKY genetic background (Supplemental Figure 1A).
GFP expression in WKY-hCD68-GFP rats occurred exclusively within tissue macrophages and blood monocytes (Supplemental Figure 1, B–D). Blood T and B lymphocytes, NK cells, and neutrophils were GFPneg (Supplemental Figure 1, C and D). GFPpos monocytes expressed high levels of CD68 and CD172a, common monocyte markers (Supplemental Figure 1E). Blood counts in these rats showed typical distribution of monocytes, neutrophils, and lineage-positive cells (Supplemental Figure 1F).
Effect of Transgene Insertion and Unilateral Hydronephrosis on NTN Phenotype
NTN was induced in wild-type WKY and transgenic WKY-hCD68-GFP rats and the phenotype was compared (Supplemental Figure 2). WKY-hCD68-GFP rats developed characteristic histopathologic features of NTN at the expected time points, i.e., marked endocapillary hypercellularity and infiltration of the glomerulus with ED-1pos leukocytes at day 6, followed by crescent formation at day 8. Representative glomerular histology is illustrated in Supplemental Figure 2A. There was no effect of transgene insertion on NTN phenotype, as demonstrated in Supplemental Figure 2, B–E.
Induction of unilateral hydronephrosis did not induce glomerular inflammation at baseline (Supplemental Figure 3, A and B), and did not cause significant proteinuria or a rise in serum creatinine compared with WKY-hCD68-GFP rats without hydronephrosis (Supplemental Figure 3A). The phenotype of NTN in the hydronephrotic kidneys of WKY-hCD68-GFP rats, 9 weeks after ureteric ligation, was examined. There was progressive infiltration of the glomerulus with ED-1pos leukocytes from day 6 NTN, associated with crescent formation and the onset of significant proteinuria (Supplemental Figure 3C). This confirms successful induction of crescentic GN in the hydronephrotic kidney, despite any underlying vasoconstriction and interstitial inflammation generated by ureteric obstruction. Representative histology of NTN in the hydronephrotic kidney is illustrated in Supplemental Figure 3D, confirming typical features of endocapillary hypercellularity, crescent formation, and extensive infiltration of the glomerulus with ED-1pos leukocytes.
Glomerular CD68pos Leukocyte Infiltration during NTN Is the Result of Increased Monocyte Recruitment from the Blood
To identify behavior of intraglomerular monocytes during NTN, we performed time-lapse intravital glomerular imaging in the hydronephrotic kidney of WKY-hCD68-GFP rats, using a similar protocol to that described and validated in mice.15,16,21–23
Live imaging was performed in WKY-hCD68-GFP transgenic rats with NTN and compared with controls. GFPpos blood monocytes within glomerular capillaries were tracked and the dynamics of their recruitment and retention analyzed. GFPpos blood monocytes were observed within glomerular capillaries during the steady state and during NTN (Figure 1A, Supplemental Video 1). Some had prolonged interactions with the endothelium (mean±SEM 3.0±0.4 minutes in controls), and a migratory phenotype typical of “patrolling” monocytes.9,10,14
Figure 1.
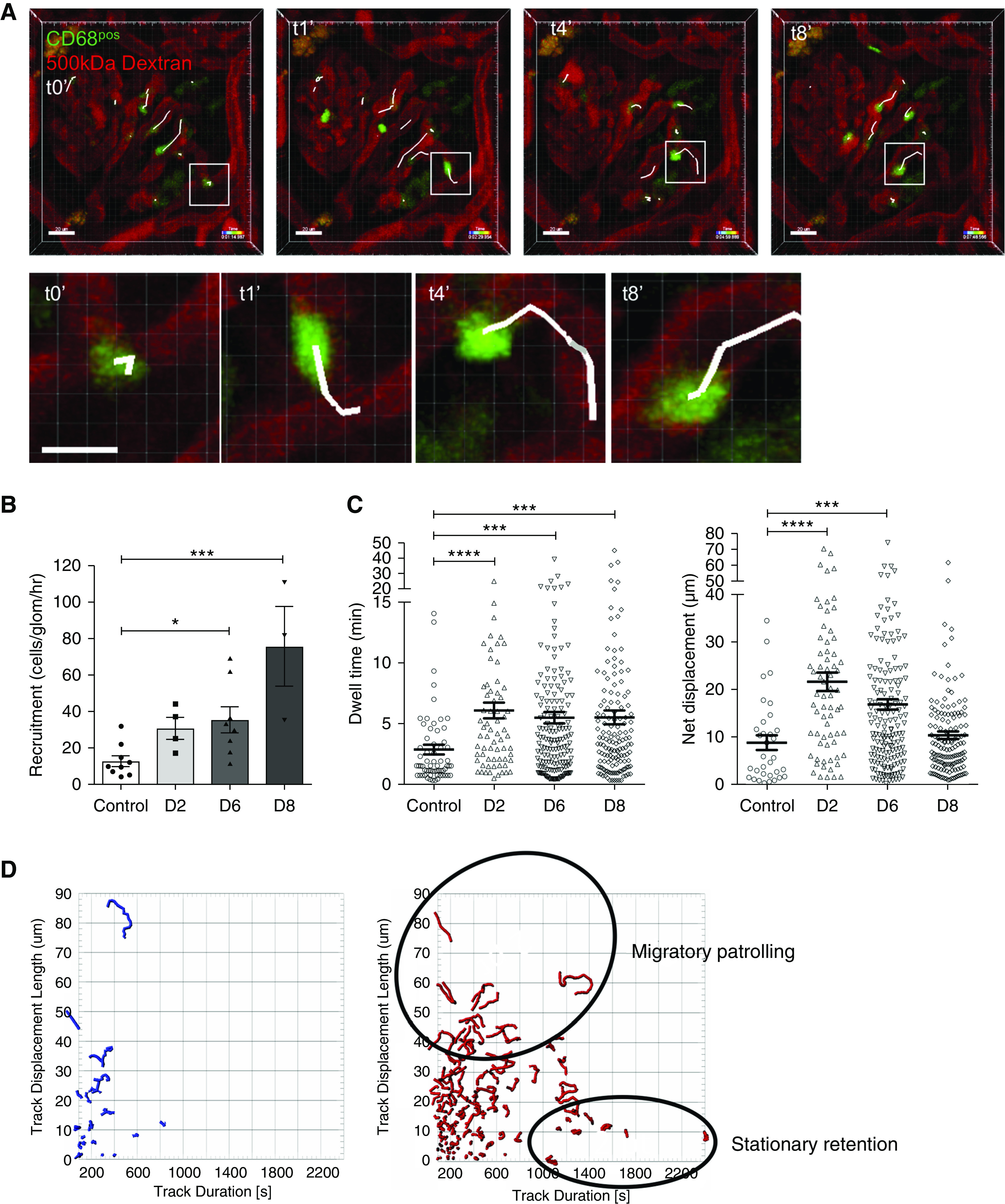
Glomerular CD68 positive leukocyte infiltration during NTN is the result of increased blood monocyte recruitment and retention within glomerular capillaries. (A) Sequence of still frames from live time-lapse glomerular imaging performed in a WKY-hCD68-GFP rat with day 6 NTN to illustrate migrating GFPpos monocytes within the glomerular capillary lumens. WKY-hCD68-GFP rats administered terminal anesthesia and left hydronephrotic kidney externalized through a small flank incision. Glomeruli imaged by direct transillumination of kidney. GFPpos blood monocytes adherent to the glomerular endothelium for at least 2 consecutive time frames were tracked and identified by white tails. Example of tracked GFPpos monocyte migrating along luminal aspect of endothelium of glomerular capillary loop is indicated by white boxes (scale bars, 20 μm). Higher magnification views of tracked GFPpos monocyte shown in lower row of images (scale bar, 10 μm). None of these adherent GFPpos monocytes were seen to undergo transendothelial migration. GFP is shown in green, 500 kDa Dextran-TRITC is shown in red. See also Supplemental Video 1. (B) Total number of adherent GFPpos monocytes per glomerulus per hour (recruitment). Adherent cells defined as those that remained in contact with the glomerular endothelium for at least two consecutive time frames during intravital time-lapse imaging. (C) Duration of retention (dwell time) and movement behavior (net displacement length) of adherent GFPpos monocytes in control animals versus days 2, 6, and 8 NTN. All data presented as mean±SEM. A mean of six glomeruli from at least two separate experiments were analyzed for each time point. Each glomerulus was imaged for 30–45 minutes. *P<0.05; ***P<0.001; ****P<0.001 versus control rats, by ANOVA with Holm–Sidak multiple comparisons test (B), and Kruskal–Wallis with Dunn multiple comparisons test (C). (D) Tracked GFPpos cells plotted in two dimensions according to their net displacement (y axis) and dwell time (x axis). Shapes of tracked cell paths also illustrated. Left plot in blue represents GFPpos cells from control WKY-hCD68-GFP rats. Right plot in red represents GFPpos cells from WKY-hCD68-GFP rats with day 6 NTN (n=2 rats per time point). GFPpos monocytes with distinct movement behaviors identified i.e., migratory patrolling versus stationary retention. D2, day 2 NTN; D6, day 6 NTN; D8, day 8 NTN.
During NTN, the number of GFPpos blood monocytes retained within glomerular capillaries, i.e., their recruitment, increased significantly from mean±SEM 12.8±3.0 cells/glomerulus per hour in controls, to 30.9±6.0, 35.6±7.2, and 75.6±21.9 cells/glomerulus per hours on days 2, 6, and 8 NTN, respectively (Figure 1B). The duration of retention (dwell time) of GFPpos blood monocytes within glomerular capillaries also increased significantly from mean±SEM 3.0±0.4 minutes in controls, to 6.1±0.6, 5.5±0.5, and 5.6±0.6 minutes on days 2, 6, and 8 NTN, respectively (Figure 1C). Moreover, there was preferential recruitment of GFPpos monocytes with a migratory behavioral phenotype during early NTN, i.e., their net displacement lengths increased from mean±SEM 9.0±1.5 μm in controls, to 21.8±2.0 μm on day 2 NTN (Figure 1C).
These data suggest that glomerular infiltration with ED-1pos (CD68pos) leukocytes seen on histology during NTN (Supplemental Figure 2A) is the result of increased monocyte recruitment and retention from the blood. Of note, no transendothelial migration of monocytes into the mesangium was seen at any of these time points during NTN.
Finally, GFPpos monocytes with distinct movement behaviors could be identified when analyzing their net displacement versus dwell time: those that were migratory (with relatively longer displacement lengths) and those that were more stationary (Figure 1D). Moreover, these distinct subsets had different responses to glomerular inflammation during NTN: stationary GFPpos monocytes were retained on the glomerular endothelium for longer and migratory GFPpos monocytes underwent an increase in cell number (Figure 1D, red tracks during NTN versus blue tracks in controls). Together these data suggest monocyte subset–specific responses.
Two Waves of Monocyte Subset Recruitment during NTN
We performed detailed phenotyping of monocytes in WKY-hCD68-GFP rats using flow cytometry to identify two main subpopulations on the basis of CD43 and HIS48 expression26,27 (GFPposCD43hiHIS48int non-classical and GFPposCD43loHIS48hi classical monocytes), and qPCR to confirm that their differential gene expression of phenotypic markers was homologous to the subpopulations identified in mice and humans11,12,28,29 (Supplemental Figure 4).
We examined blood counts of leukocyte subpopulations during NTN and noted a significant monocytosis on days 6 and 8, when glomerular monocyte infiltration peaks (Figure 2A). There was no increase in blood lymphocytes and only a small increase in neutrophils (Supplemental Figure 5A).
Figure 2.
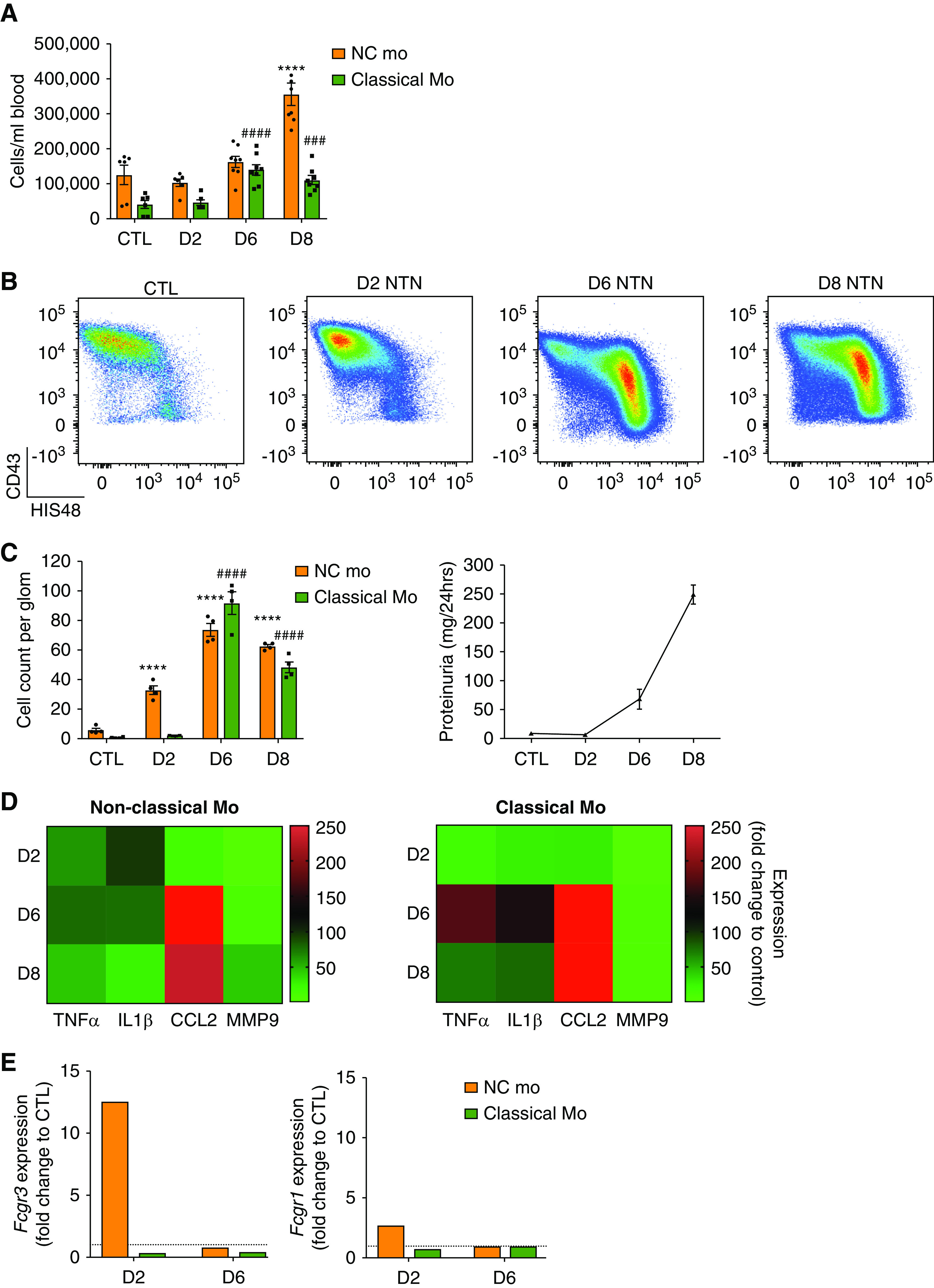
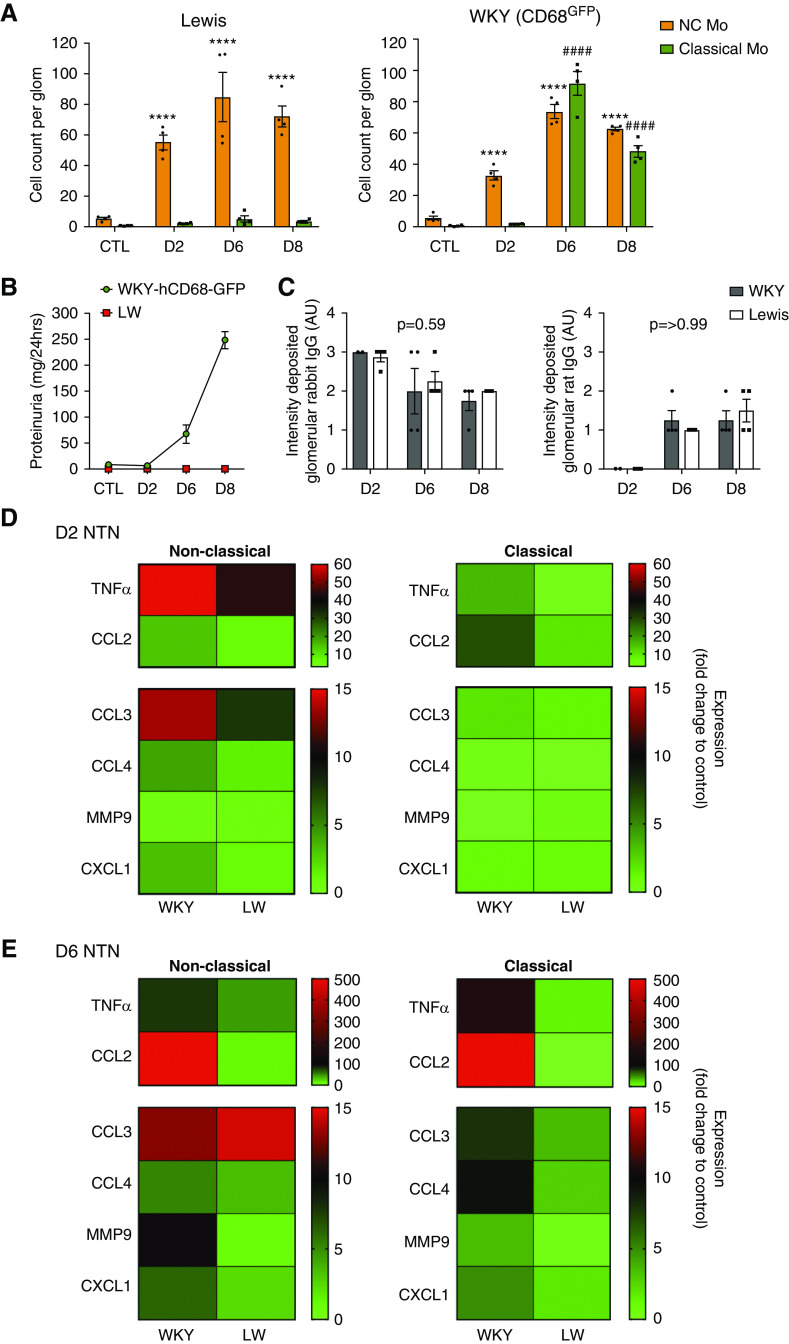
Two waves of monocyte subset recruitment during NTN with distinct effector functions (A) Blood cell counts of monocyte subsets from WKY-hCD68-GFP rats over time with NTN, compared with control (mean±SEM, n=6). ####P<0.001; ###/***P<0.001 relative to respective control population, by ANOVA with Sidak multiple comparisons test. n=6–8 rats per group from two separate experiments. See also Supplemental Figure 4A. (B and C) Ex vivo analysis of glomerular monocyte recruitment during NTN. Glomerular isolates sieved from whole kidneys taken from WKY-hCD68-GFP rats were digested to obtain single-cell suspensions, which were analyzed by flow cytometry. (B) Representative dot plots of monocyte subpopulations in control rats and at each NTN time point are shown and (C) counting beads were used to obtain cell counts per glomerulus at different time points during NTN, compared with control rats. (C) also illustrates proteinuria during NTN, highlighting that onset of proteinuria on day 6 NTN correlates with significant increase in infiltration of glomerulus with classical monocytes. Graphs show mean±SEM. ****/####P<0.001 relative to respective control population, by two-way ANOVA with Dunnett multiple comparisons test. n=4 rats per group from two separate experiments. (D) Heat map summarizing gene expression proinflammatory mediators, analyzed by qPCR, in non-classical and classical monocytes sorted from glomeruli of WKY-hCD68-GFP rats with NTN using FACS. Fold change expression relative to PBMCs from control WKY-hCD68-GFP rats illustrated. Complementary DNA for each time point pooled from n=4 rats; samples were run in triplicate. (E) Gene expression, analyzed by qPCR, of low-affinity FcγRIII (CD16) and high-affinity FcγRI (CD64) in non-classical and classical monocytes sorted from glomeruli of WKY-hCD68-GFP rats with NTN using FACS. Fold change expression relative to PBMCs from control WKY-hCD68-GFP rats illustrated. Complementary DNA from for each time point pooled from n=2 rats; samples were run in triplicate. See also Supplemental Figure 5. Classical Mo, classical monocytes; CTL, control (no NTN); D2, day 2 NTN; D6, day 6 NTN; D8, day 8 NTN; NC Mo, non-classical monocytes.
Infiltration of monocyte subpopulations into the glomerulus during NTN was examined ex vivo using flow cytometry on isolated glomeruli (Figure 2, B and C, Supplemental Figure 5B). In control WKY-hCD68-GFP rats there was a notable population of non-classical monocytes, mean±SEM 5.7±1.2 cells/glomerulus (Figure 2C), supporting the hypothesis that specific retention of non-classical monocytes within glomerular capillaries in the steady state may play a role in endothelial surveillance. There was a marked infiltrate of non-classical monocytes on day 2 NTN (mean±SEM 32.7±2.9 cells/glomerulus versus 5.7±1.2 in controls; P<0.001), suggesting non-classical monocytes are the first responders to immune complex formation within glomerular capillary walls. This was followed by an infiltrate of classical monocytes on day 6 NTN (mean±SEM 91.6±7.5 cells/glomerulus versus 0.6±0.2 cells/glomerulus in controls; P<0.001), which coincided with the onset of proteinuria (Figure 2C). A modest but significant increase in glomerular neutrophils and Linpos cells was seen over the disease course, although this was over ten-fold lower than that of monocytes (Supplemental Figure 5C).
We analyzed the effector functions of infiltrating monocyte subpopulations in WKY-hCD68-GFP rats with NTN ex vivo, examining gene expression of proinflammatory cytokines, chemokines, and damage-mediating agents known to be important in mediating glomerular inflammation in experimental models8,30–37 (Figure 2D). There was early upregulation in gene expression of TNFα and IL-1β by non-classical monocytes (but not by classical monocytes) on day 2 NTN, suggesting that non-classical monocytes may orchestrate the inflammatory response to immune complexes formed within the glomerular capillary wall. Conversely, on day 6 NTN, there was relatively more upregulation in gene expression of TNFα and IL1β by classical monocytes (Figure 2D), suggesting these cells may be important in sustaining the inflammatory response that ultimately results in glomerular damage and proteinuria. CCL2 gene expression was particularly strongly upregulated on day 6 NTN, which corresponds to the time point when glomerular monocyte infiltration is maximal and highlights its importance as a monocyte chemoattractant in NTN.
Next, we showed a marked increase in gene expression of the low-affinity FcγRIII CD16 (Fcgr3) specifically by non-classical monocytes at day 2 NTN (Figure 2E). This suggests non-classical monocytes may detect deposited immune complexes via CD16, a well known mechanism of immune complex receptor–mediated activation.38
Finally, there was increased gene expression of Mertk, Arg1, and Cd14 within both non-classical and classical monocytes infiltrating the glomerulus during NTN, suggesting potential differentiation into monocyte-derived macrophages.39 (Supplemental Figure 5D).
Taken together, these data suggest there are two sequential waves of monocyte subset recruitment during NTN. Non-classical monocytes are the first responders to glomerular immune complex formation and adopt a proinflammatory phenotype that may trigger the subsequent inflammatory response. Later recruitment of classical monocytes coincides with the onset of proteinuria, suggesting this subset may be important in generating a sustained inflammatory response that leads to glomerular damage.
Live Kidney Imaging Reveals Distinct Response of Leukocyte Subpopulations to Immune Complex Detection within the Glomerulus
We used intravital confocal microscopy to examine myeloid cell behavior within the glomerulus in WKY-hCD68-GFP rats with NTN (Figure 3A, Supplemental Videos 2, A–D and 3). In control rats, GFPposCD43pos non-classical monocytes were retained within glomerular capillaries for prolonged periods of time (mean±SEM dwell time 5.8±1.6 minutes, Figure 3B). By comparison, GFPposCD43neg classical monocytes and GFPnegCD43pos neutrophils demonstrated only transient “touch and go” interactions with the glomerular endothelium (mean±SEM dwell time 2.0±0.5 minutes; P<0.001, and 2.5±0.3 minutes; P<0.001, respectively, versus non-classical monocytes). Non-classical monocytes displayed the typical migratory behavior previously described in vascular beds of other species10,14,40 (Supplemental Video 2A). They crawled along the glomerular endothelium, independently of the direction of blood flow, over considerable distances (mean±SEM net displacement length 19.5±3.5 μm). By comparison, classical monocytes and neutrophils underwent stationary retention on the endothelium (net displacement length mean±SEM 8.3±2.0 μm and 7.8±0.5 μm, respectively, versus non-classical monocytes; P<0.001; Figure 3B). These subset-specific differences in migratory patterns within the glomerular capillaries are clearly demonstrated by track plots in which non-classical monocytes have wider, more migratory paths (Figure 3, B–E).
Figure 3.
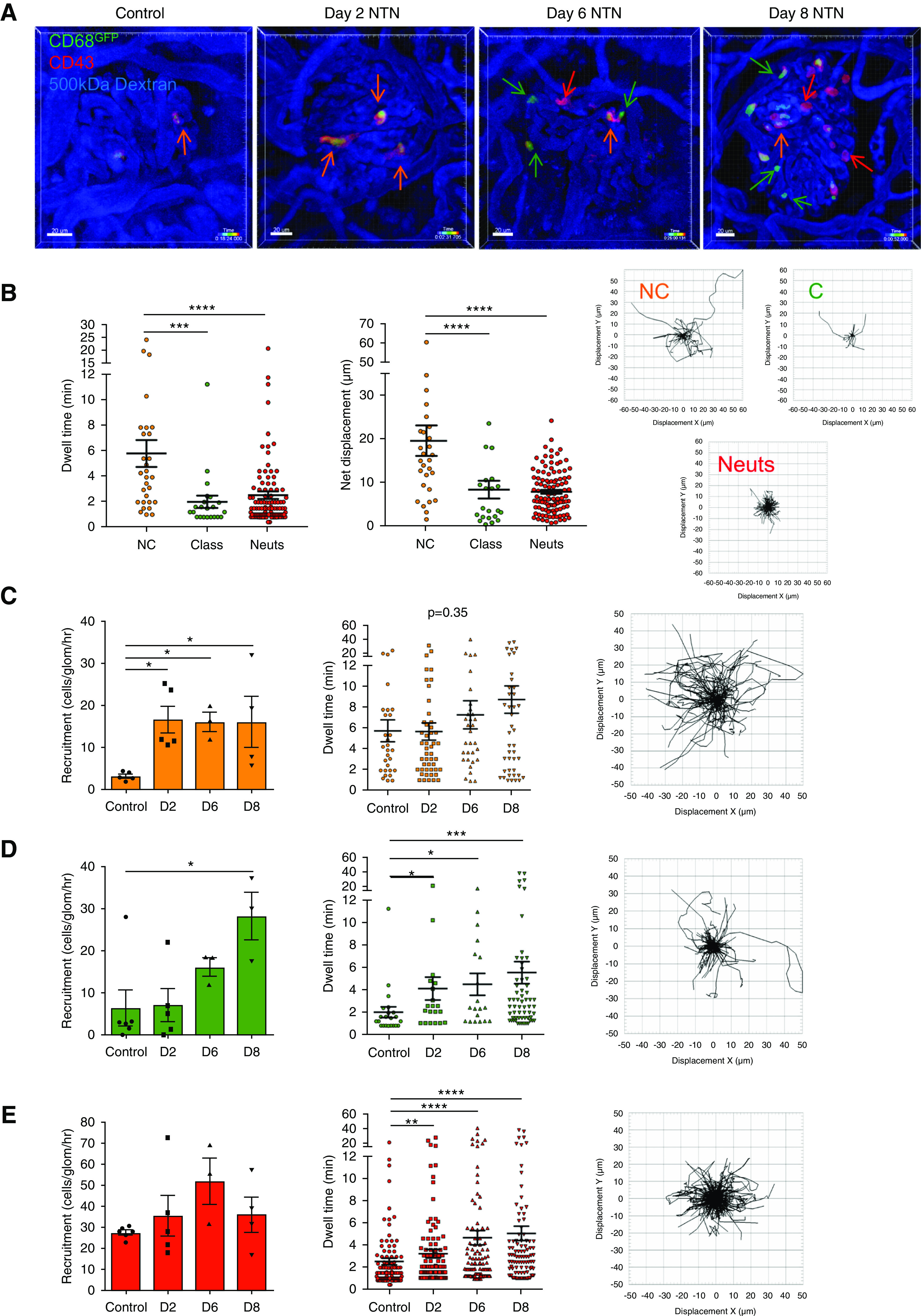
Live glomerular imaging reveals distinct responses of leukocyte subpopulations to immune complex formation within glomerular capillary walls. (A) In vivo antibody labeling with intravenous injection of anti-rat CD43–Alexa Fluor 647 during live glomerular imaging in WKY-hCD68-GFP transgenic rats enables three distinct leukocyte subpopulations to be labeled and tracked in real-time in vivo. Representative still frames from time-lapse imaging shown (scale bars, 20 μm). Orange arrows identify GFPposCD43pos non-classical monocytes, green arrows identify GFPposCD43neg classical monocytes, and red arrows identify GFPnegCD43pos neutrophils. See Supplemental Video 2, A–D. In a separate experiment anti-rat granulocyte antibody HIS48 was injected intravenously to visualize GFPnegHIS48pos neutrophils. This confirmed that the majority of GFPnegCD43pos cells retained within glomerular capillaries during NTN colabel with HIS48, and hence most likely represent neutrophils (see Supplemental Video 3). (B) GFPposCD43pos non-classical monocytes, GFPposCD43neg classical monocytes (Class), and GFPnegCD43pos neutrophils (Neuts) were identified and tracked in postacquisition imaging analysis to determine the duration of their retention within glomerular capillaries (dwell time) and their net displacement in control WKY-hCD68-GFP rats. Displacement track plots of glomerular myeloid populations in the steady state also illustrated (displacement X, μm versus displacement Y, μm plotted, starting position of all tracks aligned at center). Graphs show mean±SEM. ****P<0.001; ***P<0.001, by Kruskal–Wallis with Dunn multiple comparisons test. (C–E) Recruitment, duration of retention (dwell time), and displacement track plots for (C) GFPposCD43pos non-classical monocytes, (D) GFPposCD43neg classical monocytes, and (E) GFPnegCD43pos neutrophils on days 2, 6, and 8 NTN versus controls. All graphs show mean±SEM. *P<0.05; **P<0.01; ***P<0.001; ****P<0.001 versus control, by ANOVA with Holm–Sidak multiple comparisons test for recruitment, and Kruskal–Wallis with Dunn multiple comparisons test for dwell time. A mean of five glomeruli from at least two separate experiments were analyzed for each time point. Each glomerulus imaged for 30–45 minutes. Track plots illustrate leukocyte tracks generated from all time points after aligning their starting positions at the center of the plot (displacement X, μm versus displacement Y, μm plotted). D2, day 2 NTN; D6, day 6 NTN; D8, day 8 NTN.
Retained glomerular myeloid subpopulations displayed distinct responses during glomerular inflammation (Figure 3, C–E). Recruitment of non-classical monocytes increased significantly from mean±SEM 3.2±0.5 to 16.6±3.2, 16.1±2.3, and 16.2±6.0 cells/glomerulus per hour on days 2, 6, and 8 NTN, respectively (P<0.05 versus control) (Figure 3C). By comparison, it was the duration of retention of classical monocytes and neutrophils, rather than their number, which increased: classical monocytes from mean±SEM 2.0±0.5 minutes in controls to 4.1±1.0 (P<0.05), 4.5±1.0 (P<0.05), and 5.5±1.0 minutes (P<0.001) on days 2, 6, and 8 NTN, and neutrophils from mean±SEM 2.5±0.3 minutes to 3.2±0.4 (P<0.01), 4.6±0.6 (P<0.001), and 5.0±0.7 minutes (P<0.001) (Figure 3, D and E). The duration of retention of non-classical monocytes did not change significantly during NTN, but continued to be prolonged e.g., range in mean 5.7–8.7 minutes (Figure 3C).
We did not see any notable transendothelial migration of these subpopulations during NTN, suggesting that myeloid inflammatory responses at these time points are mediated at the luminal side of the endothelial interface, rather than within the mesangium.
CD11a (LFA-1) Is Critical for Non-Classical Monocyte Patrolling and Glomerular Recruitment during NTN
Non-classical monocyte patrolling behavior in mice is dependent on the β2 integrin LFA-1 (CD11a/CD18).9 However, the role of LFA-1 in mediating non-classical monocyte patrolling in the rat has not yet been explored. Therefore, we investigated the effect of in vivo LFA-1 blockade on monocyte subsets and neutrophils during NTN using intravital glomerular imaging.
In vivo LFA-1 inhibition resulted in a highly significant reduction in the recruitment of non-classical monocytes during NTN (Figure 4A), which decreased from mean±SEM 29.9±8.0 to 3.6±1.3 cells/glomerulus per hour in WKY-hCD68-GFP rats treated with anti-CD11a IgG versus isotype control IgG (P<0.01). Non-classical monocytes that remained adherent to the glomerular endothelium in anti-CD11a IgG-treated rats lost their migratory phenotype (net displacement length decreased from mean±SEM 22.2±1.9 μm in control rats to 10.0±1.6 μm; P<0.01; Figure 4B), as illustrated in their track plots in Figure 4C.
Figure 4.
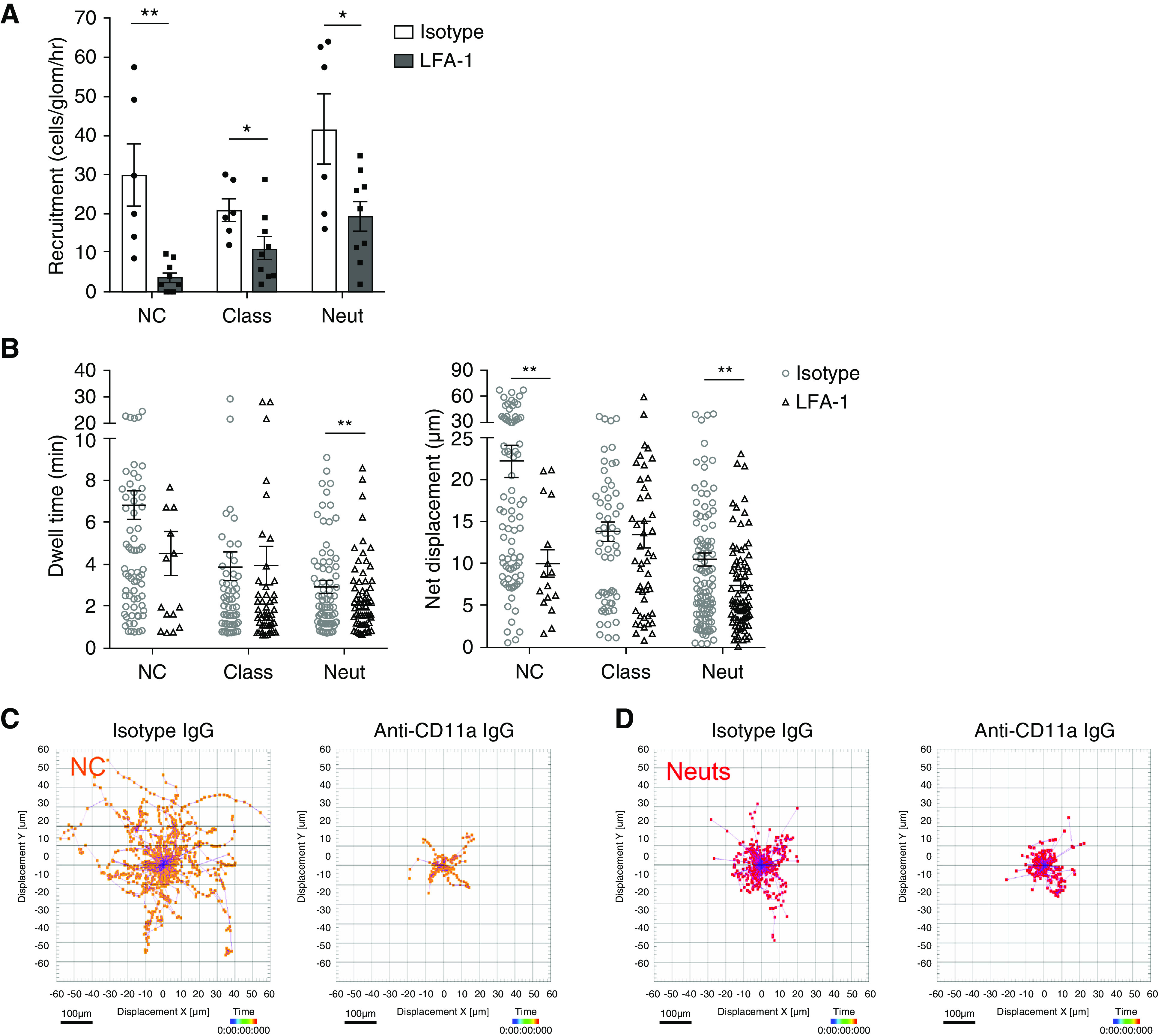
Non-classical monocyte patrolling within glomerular capillaries, and increased recruitment during NTN, is dependent on LFA-1. (A–C) Intravenous injection of 2 mg/kg of mouse anti-rat CD11a IgG was administered to WKY-hCD68-GFP rats in vivo during intravital glomerular imaging on day 2 NTN. Control WKY-hCD68-GFP rats were injected with 2 mg/kg of isotype control IgG. Leukocyte subpopulations were identified by in vivo antibody labeling with anti-rat CD43–Alexa Fluor 647 and cell behavior analyzed, as described in Figure 3. (A and B) Effect of in vivo LFA-1 inhibition on (A) glomerular recruitment and (B) dwell time and net displacement of myeloid cell populations in WKY-hCD68-GFP rats with NTN (day 2). All graphs show mean±SEM. *P<0.05; **P<0.01 versus isotype IgG, by unpaired t test. A mean of eight glomeruli from three separate experiments were analyzed for each group. Each glomerulus was imaged for 30 minutes. (C and D) Track plots for (C) GFPposCD43pos non-classical monocytes and (D) GFPnegCD43pos neutrophils in WKY-hCD68-GFP rats treated with 2 mg/kg of mouse anti-rat CD11a IgG versus isotype control IgG on day 2 NTN. All leukocyte tracks illustrated, after aligning their starting positions at the center of the plots. Displacement X (μm) versus displacement Y (μm) plotted. Scale bar, 100 µm. NC, non-classical monocytes; Class, classical monocytes; Neut, neutrophils.
There were smaller reductions in the recruitment of classical monocytes (from mean±SEM 20.9±2.9 to 11.2±2.9 cells/glomerulus per hour; P<0.05) and neutrophils (from mean±SEM 41.6±9.1 to 19.3±3.8 cells/glomerulus per hour; P<0.05) in anti-CD11a IgG versus isotype IgG-treated rats (Figure 4A).
Retained glomerular neutrophils underwent stationary retention on the endothelium, which was not affected by LFA-1 blockade (Figure 4D). However, their duration of retention was significantly reduced from mean±SEM 2.9±0.3 to 2.0±0.2 minutes (P<0.01) in anti-CD11a versus isotype IgG-treated rats (Figure 4B).
These data suggest that LFA-1 is critical in mediating non-classical monocyte patrolling and glomerular recruitment during NTN.
Imaging Effector Functions of Glomerular Myeloid Cells and Their Accumulation during Crescent Formation
We analyzed ROS production in real-time in vivo in WKY-hCD68-GFP rats using CellRox Deep Red (Figure 5A). Patrolling GFPpos monocytes were an important source of ROS during day 2 NTN (Supplemental Video 4A). By comparison, by day 8 NTN, multiple cell populations were seen to produce ROS, including GFPpos monocytes and large aggregates of GFPneg leukocytes that most likely represent neutrophils (Supplemental Video 4B).
Figure 5.
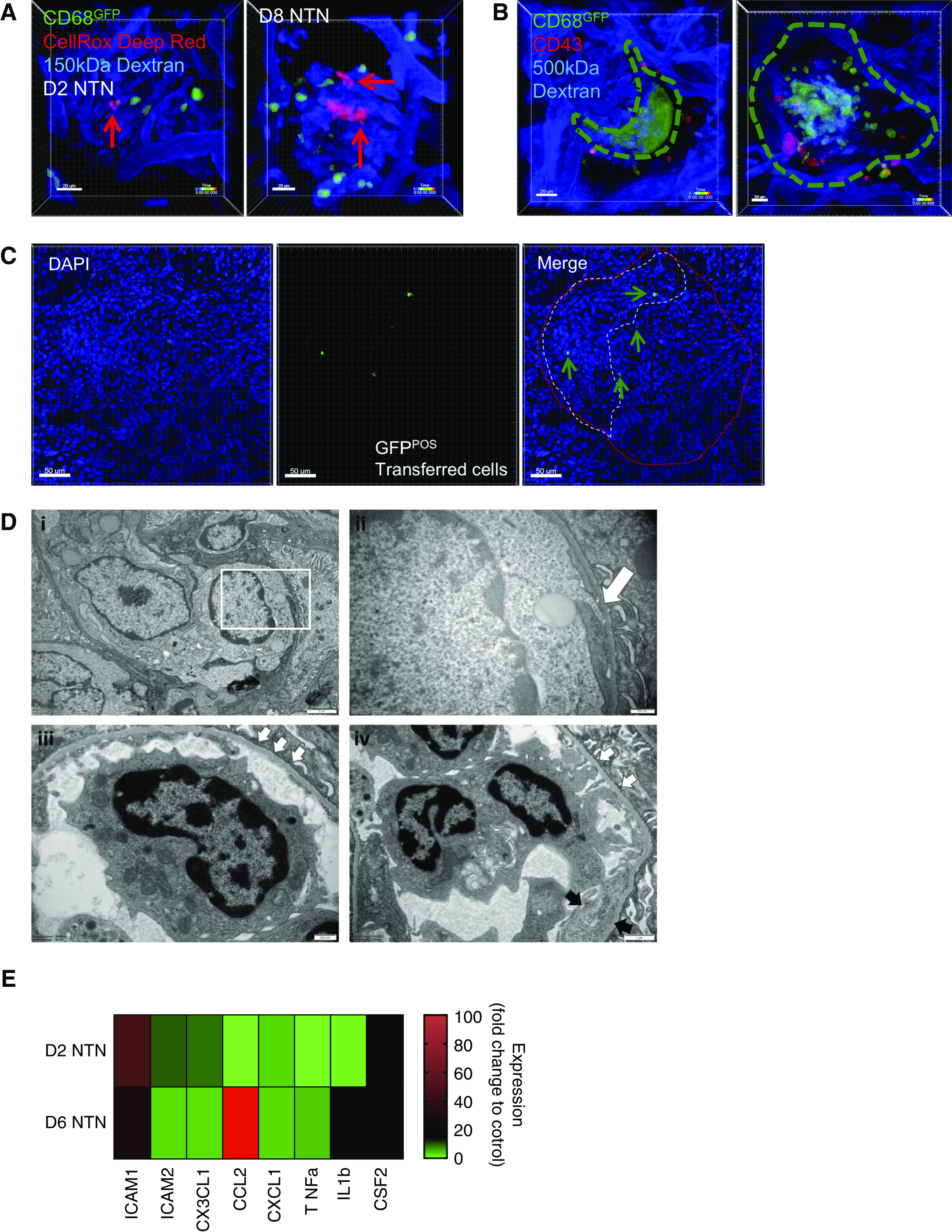
Retained glomerular leukocytes produce ROS during NTN, which may contribute to endothelial inflammation/damage and crescent formation via subendothelial pseudopodia projections. (A) Snapshots of videos (see Supplemental Video 4, A and B) from WKY-hCD68-GFP rats with day 2 (left) and day 8 (right) NTN in which CellRox Deep Red was injected intravenously during intravital glomerular imaging to detect ROS production (red signal) in real time. Red arrows identify ROS positive cells. Scale bars, 20 μm. (B) Live imaging of glomerular crescents performed in WKY-hCD68-GFP rats with day 10 NTN. In vivo antibody labeling with anti-CD43–Alexa Fluor 647 antibody used to label myeloid subpopulations, as in Figure 3. Two snapshots of videos illustrated (see Supplemental Video 5, A and B). Dotted line indicates outline of crescent. Two representative examples of crescents shown: large dynamic GFP aggregate forming typical crescent shape around glomerular tuft (left, Supplemental Video 5A) and more cellular structure with GFPpos monocytes seen within Bowman’s space (right, Supplemental Video 5B). Scale bars, 20 μm. (C) Representative adoptive transfer experiments. A pool of GFPpos monocytes were sorted from donor WKY-hCD68-GFP rats and injected intravenously into wild-type WKY rats on day 8 NTN. Wild-type WKY recipient rats were euthanized 24 hours later and frozen tissue sections were examined using confocal microscopy. GFPpos cells near to and within crescent areas were clearly identified (arrows). Representative glomeruli from two experiments. Scale bars, 50 μm. (D) Ultrastructural examination illustrating the contribution of endothelial cells to glomerular inflammation during NTN: kidney tissue sections obtained at day 6 NTN. (i) Capillary lumina are filled with leukocytes and there is endothelial cell swelling (magnification ×8000). (ii) Intravascular monocytes extend pseudopodia (white arrow) beneath the endothelial cells and come into direct contact with the glomerular basement membrane and hence, presumably, immune complexes [magnified image of the white box in (i), final magnification ×25,000]. (iii) An area of basement membrane denudation is demonstrated with three small white arrows (magnification ×16,500). (iv) An intraluminal monocyte establishes contacts with the basement membrane (white arrows) in areas of glomerular basement membrane denudation. Adjacent endothelium shows increased cytoplasm and loss of fenestrations (black arrows, endothelial cell enlargement) (magnification ×11,500). (E) Glomerular endothelial cells were sorted from glomerular digests of WKY rats with days 2 and 6 NTN using FACS, and gene expression analyzed using qPCR. Multiple gene targets represented. Fold change expression relative to control kidney endothelium from WKY rats. cDNA for each time point pooled from n=2 rats, samples run in triplicate. See also Supplemental Figure 5E for endothelial cell gating strategy.
To identify the contribution of myeloid cells to crescent formation, we performed live glomerular imaging 10 days after induction of NTN, when crescent formation is maximal.17 There was massive accumulation of GFP signal within crescent-like structures and the pattern of the GFP signal within these crescents varied (Figure 5B, Supplemental Video 5, A and B). Interestingly, only GFPposCD43neg classical monocytes were seen within crescents. No non-classical monocytes or neutrophils could be observed.
To understand the origin of CD68pos cells within crescents, which may derive from blood monocytes that transmigrate across glomerular capillary walls to enter Bowman’s space, or from macrophages within the kidney tubulointerstitium that enter Bowman’s space through breaches in Bowman’s capsule,41,42 we performed adoptive transfer experiments. GFPpos monocytes were sorted from donor WKY-hCD68-GFP rats and injected intravenously into wild-type WKY rats with day 8 NTN. Within 24 hours, GFPpos cells were clearly seen near to and within crescents on frozen tissue sections (Figure 5C). Together, these data suggest that blood-derived classical monocytes contribute to crescent formation during NTN.
Contribution of Endothelial Cells to Glomerular Inflammation during NTN
Glomerular endothelial cells were imaged in ultrastructural detail using electron microscopy of kidney tissue sections obtained at day 6 NTN, when there is extensive infiltration of classical monocytes into the glomerular vasculature, corresponding to the onset of proteinuria. At this time point there was evidence of endothelial injury with cell swelling and detachment from the glomerular basement membrane (GBM), allowing intravascular monocytes to extend pseudopodia beneath them and come into direct contact with the GBM and hence, presumably, immune complexes. In some capillary loops significant endothelial cell loss was observed, with areas of denuded GBM (Figure 5D, i–iv).
We also examined gene expression within glomerular endothelial cells obtained from WKY rats with NTN (see Supplemental Figure 5E for gating strategy), demonstrating early upregulation in gene expression of adhesion molecules ICAM-1 and ICAM-2, and the membrane-bound chemokine CX3CL1 on day 2 NTN, followed by strong upregulation of the monocyte chemoattractant CCL2 and IL1β on day 6 NTN (Figure 5E).
Taken together, these data suggest that glomerular endothelial cells themselves actively participate in monocyte recruitment and activation during NTN through upregulation of adhesion molecules, chemokines, and proinflammatory mediators. Additionally, significant endothelial damage occurs during established glomerular inflammation and this may contribute to proteinuria and crescent formation.
NTN in CrGN-Resistant Lewis Rats Suggests a Role for FcγRIII (CD16) in Immune Complex Detection and Suggests Glomerular Damage Is Mediated by Classical Monocytes
As non-classical monocytes are the “first responders” to glomerular immune complex formation during NTN and strongly upregulate gene expression of Fcgr3 (CD16) during early disease (Figure 2E), we hypothesized that this activating FcγRIII may be an important mechanism by which non-classical monocytes detect deposited immune complexes. As there are no anti-rat CD16 blocking antibodies or knockout rat strains available, we compared WKY-hCD68-GFP with Lewis rats with respect to glomerular recruitment of monocyte subsets, and their inflammatory phenotype, during NTN. These two strains have an identical MHC haplotype but differ in their susceptibility to NTN, which is largely because of a polymorphism at Fcgr3.43 The Lewis rat is inherently resistant to NTN and does not develop significant proteinuria or crescent formation.44,45
There was no significant difference in baseline blood counts of leukocyte subpopulations between WKY-hCD68-GFP and Lewis rats (Supplemental Figure 6A). Glomerular monocyte subpopulations in Lewis rats with NTN were identified ex vivo using flow cytometry (Supplemental Figure 6B) and quantified (Figure 6A). As in WKY-hCD68-GFP rats, there was a significant infiltrate of CD43hiHIS48int non-classical monocytes during NTN. However, in contrast to WKY-hCD68-GFP rats, there was no significant increase in the number of CD43loHIS48hi classical monocytes (Figure 6A), and no corresponding proteinuria (Figure 6B). Additionally, there were no significant changes in glomerular neutrophils and Linpos cells in Lewis rats (Supplemental Figure 6C). Glomerular capillary wall deposition of heterologous rabbit IgG and autologous rat IgG did not differ between the two strains (Figure 6C, Supplemental Figure 7).
Figure 6.
Immune complex detection by non-classical monocytes may occur through activating FcγRIII (CD16). (A) Ex vivo analysis of glomerular monocyte recruitment during NTN in Lewis rats, compared with WKY-CD68-GFP rats previously shown in Figure 2C. Glomerular isolates were sieved from whole kidneys taken from Lewis rats, digested to obtain single-cell suspensions, and analyzed by flow cytometry (see also Supplemental Figure 6B). Counting beads were used to obtain cell counts per glomerulus at different time points during NTN in Lewis rat strain and compared with WKY-hCD68-GFP rat strain. Graphs show mean±SEM. ****/####P<0.001, relative to respective control population, by two-way ANOVA with Dunnett multiple comparisons test. n=4 rats per group. (B) Proteinuria in Lewis and WKY rats during NTN compared with control. (C) Intensity of glomerular capillary wall deposition of rabbit (left graph) and rat (right graph) IgG analyzed by direct immunofluorescence on frozen kidney sections. Lewis and WKY rat strains compared by two-way ANOVA with Sidak multiple comparisons test. See also Supplemental Figure 7. (D and E) Heat map summarizing gene expression, analyzed by qPCR, in CD43hiHIS48int non-classical and CD43loHIS48hi classical monocytes sorted from glomeruli of WKY-hCD68-GFP and Lewis rats on (D) day 2 NTN and (E) day 6 NTN, using FACS. Multiple gene targets represented. For all targets except CXCL1, fold change expression relative to PBMCs from control WKY-hCD68-GFP rats is illustrated. For CXCL1, fold change expression relative to Lewis non-classical monocytes on day 2 NTN is illustrated (target not expressed in control PBMCs). Complementary DNA for each time point pooled from n=4 rats; samples were run in triplicate. See also Supplemental Figures 6 and 7. CTL, control; D2, day 2 NTN; D6, day 6 NTN; D8, day 8 NTN; LW, Lewis rats.
Non-classical monocytes in Lewis rats showed less upregulation in expression of all genes analyzed, compared with WKY-hCD68-GFP rats (Figure 6, D and E). Classical monocytes in WKY-hCD68-GFP rats with day 6 NTN demonstrated a highly proinflammatory phenotype (Figure 6E), which was completely absent in Lewis rats (Figure 6, D and E).
Overall, these data suggest non-classical monocytes are recruited to the glomerulus in response to in situ immune complex formation in Lewis rats, but display a markedly attenuated proinflammatory phenotype compared with WKY-hCD68-GFP rats. This may be responsible for the absence of a classical monocyte infiltrate into the glomerulus on day 6 NTN, supporting a role for non-classical monocytes in the orchestration of glomerular inflammation, but classical monocytes in the initiation of glomerular damage and proteinuria.
Discussion
We have developed a novel WKY-hCD68-GFP monocyte/macrophage reporter rat in which GFP expression occurs exclusively within monocytes and tissue macrophages and this complements another recently published CSF1r-mApple transgenic reporter rat.27 In this model GFP expression was seen in neutrophils and B cells, as well as monocyte–macrophage lineage cells.27 We performed detailed phenotyping of monocyte subpopulations in WKY-hCD68-GFP rats, confirming for the first time that rat non-classical and classical monocytes are homologous to those identified in mice and humans,10,11 on the basis of gene expression of key phenotypic markers. Moreover, WKY-hCD68-GFP rats had susceptibility to experimental GN comparable to wild-type WKY rats (Supplemental Figure 2). Hence, this reporter rat strain will be very useful for future research examining the role of monocyte subsets in clinically relevant rat models of GN, and other diseases.
Using NTN as a clinically relevant experimental model of crescentic GN, we have shown that there are two sequential waves of monocyte subset recruitment to the glomerulus with distinct phenotypes and effector functions. In response to immune complex formation within glomerular capillary walls, there is an early influx of non-classical monocytes with a proinflammatory phenotype that may orchestrate the subsequent inflammatory response. This is followed by an infiltrate of classical monocytes on day 6 NTN, which may be important in generating a sustained inflammatory response, resulting in glomerular damage that leads to proteinuria and disease progression. Significant endothelial damage at day 6 NTN was demonstrated in ultrastructural detail using electron microscopy (Figure 5D), which may contribute to proteinuria and crescent formation. Detailed quantification of leukocyte populations within the glomerulus during NTN demonstrated that the number of monocytes per glomerulus was over ten times higher than that of other infiltrating leukocytes, i.e., neutrophils, T and B lymphocytes, and NK cells (Figure 2A, Supplemental Figure 5C).
Using live glomerular imaging in WKY-hCD68-GFP rats, we demonstrated that interactions between recruited glomerular monocytes and the glomerular endothelium are highly dynamic, and that monocytes undergo continuous turnover. Individual cells interact with the endothelium for a period of time before detaching and returning to the circulation to be replaced by new cells recruited from the blood. These adherent monocytes remained in the intravascular compartment and were not seen to extravasate into the mesangium. Moreover, we were able to label these intravascular monocyte subsets in real-time in vivo, showing that they displayed distinct behavioral responses to immune complexes within glomerular capillary walls, i.e., the number of non-classical monocytes retained within the glomerulus increased, whereas it was the duration of retention of classical monocytes (rather than their number) that increased. This has important therapeutic implications as it may be possible to develop more effective therapies for patients with GN by targeting specific monocyte subpopulations and their associated effector functions.
Taken together, our data suggest that the CD68pos leukocyte infiltrate seen histopathologically in NTN (often described as a macrophage infiltrate) represents intravascular accumulation of monocytes and suggests that the inflammatory response to glomerular immune complex formation may be driven by intravascular monocytes, rather than tissue macrophages. More work is now needed to determine whether monocytes infiltrating the glomerulus during NTN differentiate into monocyte-derived macrophages, or whether they retain their monocyte phenotype. Interestingly, recent work in a mouse model of SLE suggests that intravascular patrolling monocytes, rather than macrophages, promote glomerular inflammation and injury.46
We have clearly shown, using live glomerular imaging, that non-classical monocytes constitutively patrol the endothelium of uninflamed glomerular capillaries in rats, as has been shown in mice,16 and that the β2 integrin LFA-1 is critical for non-classical monocyte patrolling and glomerular recruitment during NTN. Existing work has already demonstrated that in vivo inhibition of LFA-1 significantly reduces proteinuria and crescent formation during NTN in the WKY rat.5 We propose that LFA-1 mediated patrolling by non-classical monocytes permits immune surveillance of the glomerular endothelium and is required to promote detection of immune complexes deposited within glomerular capillary walls, and initiate the subsequent inflammatory response.
FcγRIII (CD16) may be an important mechanism by which patrolling non-classical monocytes detect deposited immune complexes during glomerular endothelial surveillance. There is evidence from human GN and experimental models that Fcγ receptors on circulating leukocytes play in important role in the pathogenesis of immune complex-mediated GN.47–51 More recently, in vitro work has shown that interactions between CD16 on CD16hi human monocytes and immune complexes recruit CD16hi monocytes from the circulation to the vascular interface of glomerular capillaries and induce the subsequent inflammatory response.52 In rats, there are no CD16 knockout strains or blocking antibodies available. However, a polymorphism in the Fcgr3 genetic locus is responsible for a large part of the enhanced susceptibility of WKY rats to NTN, compared with NTN-resistant Lewis rats, which have an identical MHC haplotype but lack this polymorphism.43–45 Bone marrow–derived macrophages from WKY rats have enhanced Fc receptor–mediated functions compared with those from Lewis rats.44 We showed that, although equivalent numbers of non-classical monocytes infiltrate into the glomerulus in Lewis compared with WKY rats during early NTN (day 2), they have an attenuated inflammatory phenotype (Figure 6D), which may reflect relative underactivity in downstream FcγRIII-mediated signaling. As Lewis rats do not develop any significant glomerular infiltrate of classical monocytes, or any proteinuria, the production of proinflammatory cytokines and chemokines by non-classical monocytes at day 2 NTN may be necessary to orchestrate this “second wave” of classical monocyte infiltration into the glomerulus, which, in turn, may play a central role in mediating glomerular damage and crescent formation. Because the deposition of heterologous rabbit IgG and autologous rat IgG within the glomerular capillary wall is identical in WKY and Lewis rats with NTN, it is unlikely that differential interactions of non-classical and classical monocytes with deposited rabbit versus rat IgG during NTN drives these two sequential waves of intravascular monocyte subset recruitment. However, whether deposition of autologous rat anti-rabbit IgG contributes, at least in part, to the influx of classical monocytes into the glomerulus on day 6 NTN in WKY rats remains uncertain and will require further study.
Classical monocytes, although seen in abundance during live glomerular imaging in the steady state (Figure 3), have only transient “touch and go” interactions with the glomerular endothelium. Infiltration of the glomerulus with classical monocytes during NTN appears to occur through prolonged interactions with the glomerular endothelium, and may result in glomerular injury through the production of ROS (Figure 5A) and the proinflammatory cytokines TNFα and IL1-β, which are strongly upregulated in classical monocytes sorted from the glomeruli of WKY rats with day 6 NTN (Figure 2D). Interestingly, during intravital glomerular imaging, only CD43negCD68pos classical monocytes, rather than CD43posCD68pos non-classical monocytes, could be seen within crescents, suggesting that classical monocytes may also play an important role in crescent formation.
A number of limitations of our experimental model should be addressed. First, intravital glomerular imaging was performed in the hydronephrotic kidney, as this was necessary to superficialize the glomeruli sufficiently to enable direct transillumination and live imaging of the glomerular vasculature in situ. We have provided control data to confirm that hydronephrosis does not induce glomerular inflammation at baseline, and that NTN within the hydronephrotic kidney had typical histopathologic features, including extensive infiltration of the glomerulus with ED-1pos leukocytes, endocapillary hypercellularity, and crescent formation. This is supported by previous work in mice in which hydronephrosis did not alter the ability of the glomerular vasculature to respond to anti-GBM antibody,23 and in which there was no difference in neutrophil and monocyte behavior, analyzed using intravital microscopy, between hydronephrotic and unobstructed kidneys.15 Hence, it is unlikely that any vasoconstriction or interstitial inflammation generated by the underlying hydronephrosis had a significant effect on the phenotype and effector functions of intravascular glomerular leukocytes, or their interactions with the endothelium in our model.
Second, NTN is a model in which glomerular inflammation is driven both by deposition of heterologous rabbit IgG and by the subsequent adaptive immune response to this planted foreign antigen. This may limit the generalizability of our findings to human immune complex-mediated GN. Further studies are needed to determine whether the immune response we have identified here occurs in other models of crescentic GN, e.g., experimental autoimmune GN, in which there is no glomerular deposition of heterologous Ig.
Third, although our live imaging data suggest that intravascular monocytes recruited to the glomerulus during NTN do not extravasate at the time points studied, this cannot be completely excluded because of the technical limitations of time-lapse imaging, i.e., although we imaged each glomerulus for as long as possible (up to 45 minutes), kept the time interval between consecutive imaging frames as short as possible (15–30 seconds), and analyzed many hours of imaging in total, it is possible that examples of extravasation were missed during image acquisition. Further work is now needed to determine whether intravascular monocytes extravasate into Bowman’s space during crescent formation, and the mechanisms by which this might occur.
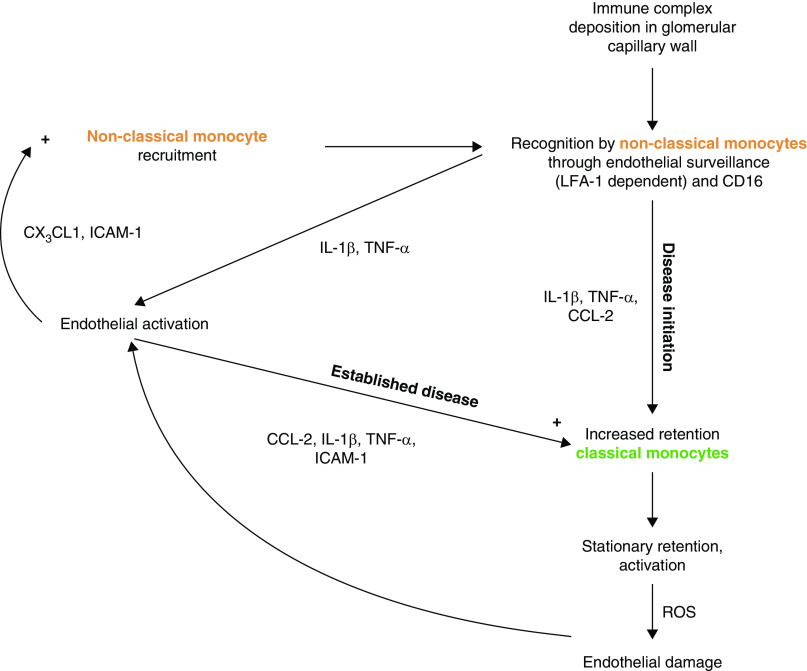
In summary, our data suggest that intravascular monocyte subpopulations with distinct effector functions may drive inflammation in immune complex-mediated GN. We propose in our working model (Figure 7) that immune complexes formed within the glomerular capillary wall are detected by intravascular non-classical monocytes through LFA-1–dependent patrolling and FcγRIII (CD16). These cells appear not to extravasate, but orchestrate the inflammatory response through a cytokine/chemokine axis that recruits further non-classical monocytes from the blood and increases the retention of classical monocytes on the endothelium. The latter generates a sustained inflammatory response and glomerular damage, leading to proteinuria and crescent formation. Further work is now needed to progress this working model and, ultimately, to develop more effective and targeted therapies for patients with GN.
Figure 7.
Working models proposes that non-classical monocytes orchestrate the inflammatory response to immune complex formation within the glomerulus. In summary, our data suggest non-classical monocytes survey glomerular endothelium via LFA-1 and during NTN, encounter immune complexes via CD16. Non-classical monocytes become activated and secrete inflammatory mediators (e.g., TNFα), which we propose then activates the endothelium to increase adhesion molecule (e.g., ICAM-1) and chemokine (e.g., CX3CL1) expression, which in turn recruits more non-classical monocytes. A subsequent wave of classical monocyte retention occurs at day 6 NTN, which we propose may cause endothelial damage through the production of ROS. As classical monocyte infiltration into the glomerulus coincides with the onset of proteinuria, we propose this subset is important in mediating a sustained inflammatory response within the glomerulus that ultimately results in glomerular damage, crescent formation, and disease progression.
Disclosures
K. Woollard is now an employee for AstraZeneca (BioPharmaceuticals R&D, Cambridge, UK). All of this work was performed at Imperial College London. No funding or support was received from AstraZeneca. All remaining authors have nothing to disclose.
Funding
This work was funded by Medical Research Council grant MR/M003159/1 (to T. Turner-Stokes). We acknowledge a contribution from the National Institute for Health Research Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Supplementary Material
Acknowledgments
We thank Ms. Jessica Schlatter for help with immunohistochemical staining of tissue sections, and Dr. Marie-Anne Mawhin for help with immunohistochemical staining analysis. We also thank Dr. Elizabeth Angus (Biomedical Imaging Unit, University of Southampton) and Dr. Liam Rasch for their help with electron microscopy; the London Institue of Medical Sciences/National Institue for Health Research Imperial Biomedical Research Centre Flow Cytometry Facility for support; and Dr. John Morris, Dr. Ela Biegun-Laroy, and Dr. Olivia Chan (biomedical scientists in the Clinical Biochemistry Laboratory, Hammersmith Hospital, Imperial College Healthcare National Health Service Trust) for their analysis of serum samples for creatinine measurement.
Dr. Candice Roufosse reports personal fees from Union Chimique Belge Biopharma, personal fees from Achillion Pharmaceutical, personal fees from Rigel Pharmaceuticals, outside the submitted work. Dr. Charles D. Pusey reports personal fees from CJASN, outside the submitted work. The K. Woollard laboratory is supported by Kidney Research UK (grants RP_019_20160303 and RP_002_20170914) and British Heart Foundation (grant PG/18/41/33813), who have supplied funds outside of the submitted work.
Dr. Tabitha Turner-Stokes performed experiments, analyzed data, generated figures, and prepared the manuscript. Ms. Ana Garcia Diaz performed experiments, analyzed data, and contributed to manuscript preparation. Dr. Damilola Pinheiro and Dr. Candice Roufosse performed experiments and analyzed data. Dr. Maria Prendecki performed experiments. Dr. Stephen P. McAdoo performed experiments. Prof. Charles D. Pusey and Prof. H. Terence Cook cosupervised the project and contributed to manuscript preparation. Dr. Kevin J. Woollard conceived and supervised the project performed experiments, analyzed data, and prepared the manuscript. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019121326/-/DCSupplemental.
Supplemental Table 1. Primers used for qPCR.
Supplemental Figure 1. Monocyte/macrophage specific GFP expression in novel WKY-hCD68-GFP transgenic reporter rat.
Supplemental Figure 2. hCD68-GFP trangsene insertion did not alter NTN phenotype.
Supplemental Figure 3. Baseline histology and NTN phenotype was not altered in hydronephrotic kidneys subjected to intravital microscopy.
Supplemental Figure 4. Monocyte subpopulations identified in WKY-hCD68-GFP rats are homologous to those identified in humans and mice.
Supplemental Figure 5. Ex vivo analysis of glomerular leukocyte subpopulations and glomerular endothelial cells during NTN.
Supplemental Figure 6. Ex vivo analysis of blood and glomerular leukocyte populations in Lewis rats with NTN.
Supplemental Figure 7. Glomerular deposition of heterologous rabbit and autologous rat IgG in WKY and Lewis rats with NTN.
Supplemental Video 1. Representative example of live glomerular imaging using intravital confocal microscopy of hydronephrotic kidney in WKY‐hCD68-GFP rat on day 6 NTN. Example of CD68pos intravascular monocyte (green) recruitment and behavior within glomerulus demonstrated. Scale bar=20μm. Green signal=CD68–GFP, red signal=150kDa dextran–TRITC (delineates glomerular vasculature).
Supplemental Video 2. Representative examples of live glomerular imaging using intravital confocal microscopy of hydronephrotic kidney in WKY-hCD68–GFP rats in, (a) steady state, (b) day 2 NTN, (c) day 6 NTN, and (d) day 8 NTN. In vivo antibody labelling by intravenous injection of anti-rat CD43-AlexaFluor647 during imaging in WKY–hCD68-GFP transgenic rats enables three distinct leukocyte subpopulations to be labeled and tracked in real time. Examples of CD68pos and CD43pos intravascular myeloid cell recruitment and behavior demonstrated. Green cells are CD68posCD43neg classical monocytes. Orange cells are CD68posCD43pos non-classical monocytes. Red cells are CD68negCD43pos myeloid cells (predominately neutrophils). Scale bar=20μm. Green signal=CD68-GFP, red signal=CD43-AlexaFluor647, blue signal=150kDa dextran-TRITC (delineates glomerular vasculature).
Supplemental Video 3.Representative example of intravital glomerular imaging using in vivo HIS48 antibody labeling in a WKY-hCD68‐GFP rat with NTN. The majority of glomerular CD68negCD43pos cells are HIS48pos, suggesting neutrophil phenotype. Scale bar=20μm. Green signal=CD68‐GFP, red signal=CD43-AlexaFluor647, blue signal=HIS48‐AlexaFluor555.
Supplemental Video 4. Examples of reactive oxygen species (ROS) expression in glomerular intravascular leukocytes using CellRox Deep Red Reagent injected intravenously during intravital imaging (a) on day 2 NTN, and (b) on day 8 NTN, in WKY–hCD68–GFP rats. Scale bar=20μm. Green signal=CD68-GFP. Red signal=ROS expression, blue signal=150 kDa dextran–TRITC (delineates glomerular vasculature).
Supplemental Video 5. Two examples (a-b) of CD68pos and CD43pos intravascular myeloid cell recruitment and behaviour on day 10 NTN in WKY‐hCD68-GFP rats. Green cells are CD68posCD43neg classical monocytes. Orange cells are CD68posCD43pos non-classical monocytes. Red cells are CD68negCD43pos myeloid cells (predominately neutrophils). Scale bar=20μm. Green signal=CD68GFP, red signal=CD43-AlexaFluor647, blue signal=150 kDa dextran (delineates glomerular vasculature).
Supplemental Appendix 1. Supplemental references.
References
- 1.Soares MF, Genitsch V, Chakera A, Smith A, MacEwen C, Bellur SS, et al. : Relationship between renal CD68+ infiltrates and the oxford classification of IgA nephropathy. Histopathology 74: 629–637, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Holdsworth SR, Neale TJ, Wilson CB: Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest 68: 686–698, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang XR, Kitching AR, Tipping PG, Holdsworth SR: Interleukin-10 inhibits macrophage-induced glomerular injury. J Am Soc Nephrol 11: 262–269, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ: Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int 63: 83–95, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki K, Yaoita E, Yamamoto T, Tamatani T, Miyasaka M, Kihara I: Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol 150: 1074–1083, 1993. [PubMed] [Google Scholar]
- 6.Schreiner GF, Cotran RS, Pardo V, Unanue ER: A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med 147: 369–384, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang WW, Qi M, Warren JS: Monocyte chemoattractant protein 1 mediates glomerular macrophage infiltration in anti-GBM Ab GN. Kidney Int 50: 665–671, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Zernecke A, Weber KS, Erwig LP, Kluth DC, Schröppel B, Rees AJ, et al. : Combinatorial model of chemokine involvement in glomerular monocyte recruitment: Role of CXC chemokine receptor 2 in infiltration during nephrotoxic nephritis. J Immunol 166: 5755–5762, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. : Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Cros J, Cagnard N, Woollard K, Patey N, Zhang S-Y, Senechal B, et al. : Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33: 375–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR: Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal AJ, McNeill E, Kapellos TS, Regan-komito D, Norman S, Burd S, et al. : Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 124: e33–e45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. : Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153: 362–375, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devi S, Li A, Westhorpe CLV, Lo CY, Abeynaike LD, Snelgrove SL, et al. : Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus [published correction appears in Nat Med 22: 446, 2016]. Nat Med 19: 107–112, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Finsterbusch M, Hall P, Li A, Devi S, Westhorpe CL, Kitching AR, et al. : Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc Natl Acad Sci U S A 113: E5172–E5181, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam FW, Smith J, Morel D, Karkar AM, Thompson EM, Cook HT, et al. : Development of scarring and renal failure in a rat model of crescentic glomerulonephritis. Nephrol Dial Transplant 14: 1658–1666, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Garcia Diaz AI, Moyon B, Coan PM, Alfazema N, Venda L, Woollard K, et al. : New Wistar Kyoto and spontaneously hypertensive rat transgenic models with ubiquitous expression of green fluorescent protein. Dis Model Mech 9: 463–471, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasaki K, Yaoita E, Yamamoto T, Kihara I: Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int 41: 1517–1526, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Baker F, Silverton R, Luckcock E: An Introduction to Medical Laboratory Technology, London, Butterworths, 1966 [Google Scholar]
- 21.Hickey MJ, Westhorpe CLV: Imaging inflammatory leukocyte recruitment in kidney, lung and liver--challenges to the multi-step paradigm. Immunol Cell Biol 91: 281–289, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Kitching AR, Kuligowski MP, Hickey MJ: In vivo imaging of leukocyte recruitment to glomeruli in mice using intravital microscopy. Methods Mol Biol 466: 109–117, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Kuligowski MP, Kitching AR, Hickey MJ: Leukocyte recruitment to the inflamed glomerulus: A critical role for platelet-derived P-selectin in the absence of rolling. J immunol 176: 6991–6999, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Cook HT, Smith J, Cattell V: Isolation and characterization of inflammatory leukocytes from glomeruli in an in situ model of glomerulonephritis in the rat. Am J Pathol 126: 126–136, 1987. [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiner GF, Kiely JM, Cotran RS, Unanue ER: Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest 68: 920–931, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett-Vanes A, Sharrock A, Birrell MA, Rankin S: A single 9-colour flow cytometric method to characterise major leukocyte populations in the rat: Validation in a model of LPS-induced pulmonary inflammation. PLoS One 11: e0142520, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irvine KM, Caruso M, Cestari MF, Davis GM, Keshvari S, Sehgal A, et al. : Analysis of the impact of CSF-1 administration in adult rats using a novel Csf1r-mApple reporter gene. J Leukoc Biol 107: 221–235, 2020. [DOI] [PubMed] [Google Scholar]
- 28.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. : Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172: 4410–4417, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler-Heitbrock L: The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol 81: 584–592, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Karkar AM, Koshino Y, Cashman SJ, Dash AC, Bonnefoy J, Meager A, et al. : Passive immunization against tumour necrosis factor-alpha (TNF-alpha) and IL-1 beta protects from LPS enhancing glomerular injury in nephrotoxic nephritis in rats. Clin Exp Immunol 90: 312–318, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karkar AM, Smith J, Pusey CD: Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant 16: 518–524, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Khan SB, Cook HT, Bhangal G, Smith J, Tam FW, Pusey CD: Antibody blockade of TNF-alpha reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int 67: 1812–1820, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Kluger MA, Zahner G, Paust HJ, Schaper M, Magnus T, Panzer U, et al. : Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney Int 83: 865–877, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Sheryanna A, Bhangal G, McDaid J, Smith J, Manning A, Foxwell BM, et al. : Inhibition of p38 mitogen-activated protein kinase is effective in the treatment of experimental crescentic glomerulonephritis and suppresses monocyte chemoattractant protein-1 but not IL-1beta or IL-6. J Am Soc Nephrol 18: 1167–1179, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Tam FW, Karkar AM, Smith J, Yoshimura T, Steinkasserer A, Kurrle R, et al. : Differential expression of macrophage inflammatory protein-2 and monocyte chemoattractant protein-1 in experimental glomerulonephritis. Kidney Int 49: 715–721, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Tam FW, Smith J, Agarwal S, Karkar AM, Morel D, Thompson EM, et al. : Type IV phosphodiesterase inhibitor is effective in prevention and treatment of experimental crescentic glomerulonephritis. Nephron 84: 58–66, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, et al. : A new anti-inflammatory compound, FR167653, ameliorates crescentic glomerulonephritis in Wistar-Kyoto rats. J Am Soc Nephrol 11: 1534–1541, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn F, Ravetch JV: Fc-receptors as regulators of immunity. Adv Immunol 96: 179–204, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. ; Immunological Genome Consortium : Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auffray C, Sieweke MH, Geissmann F: Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 27: 669–692, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Clarke BE, Ham KN, Tange JD, Ryan GB: Origin of glomerular crescents in rabbit nephrotoxic nephritis. J Pathol 139: 247–258, 1983. [DOI] [PubMed] [Google Scholar]
- 42.Magil AB: Histogenesis of glomerular crescents. Immunohistochemical demonstration of cytokeratin in crescent cells. Am J Pathol 120: 222–229, 1985. [PMC free article] [PubMed] [Google Scholar]
- 43.Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, et al. : Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Behmoaras J, Smith J, D’Souza Z, Bhangal G, Chawanasuntoropoj R, Tam FWK, et al. : Genetic loci modulate macrophage activity and glomerular damage in experimental glomerulonephritis. J Am Soc Nephrol 21: 1136–1144, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Souza Z, McAdoo SP, Smith J, Pusey CD, Cook HT, Behmoaras J, et al. : Experimental crescentic glomerulonephritis: A new bicongenic rat model. Dis Model Mech 6: 1477–1486, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuriakose J, Redecke V, Guy C, Zhou J, Wu R, Ippagunta SK, et al. : Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J Clin Invest 129: 2251–2265, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarzi RM, Davies KA, Robson MG, Fossati-Jimack L, Saito T, Walport MJ, et al. : Nephrotoxic nephritis is mediated by Fcgamma receptors on circulating leukocytes and not intrinsic renal cells. Kidney Int 62: 2087–2096, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Tarzi RM, Davies KA, Claassens JW, Verbeek JS, Walport MJ, Cook HT: Both Fcgamma receptor I and Fcgamma receptor III mediate disease in accelerated nephrotoxic nephritis. Am J Pathol 162: 1677–1683, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovalenko P, Fujinaka H, Yoshida Y, Kawamura H, Qu Z, El-Shemi AG, et al. : Fc receptor-mediated accumulation of macrophages in crescentic glomerulonephritis induced by anti-glomerular basement membrane antibody administration in WKY rats. Int Immunol 16: 625–634, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Nakatani K, Yoshimoto S, Iwano M, Asai O, Samejima K, Sakan H, et al. : Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: Association with their severity and diversity in lupus models. Am J Physiol Renal Physiol 299: F207–F216, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimoto S, Nakatani K, Iwano M, Asai O, Samejima K, Sakan H, et al. : Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am J Kidney Dis 50: 47–58, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Olaru F, Döbel T, Lonsdorf AS, Oehrl S, Maas M, Enk AH, et al. : Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis. JCI Insight 3: e96492, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.