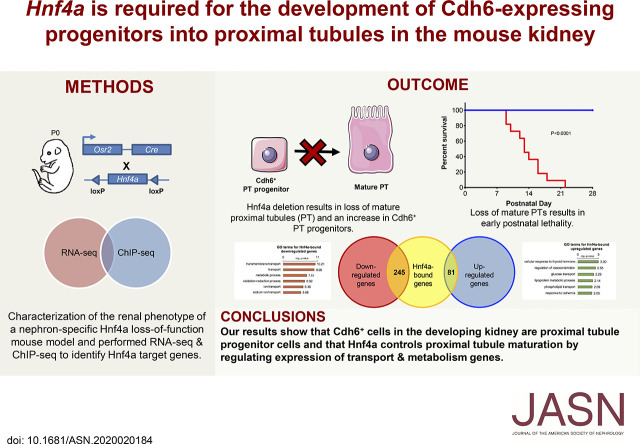
Significance Statement
Proximal tubule cells are the most abundant cell type in the mammalian kidney, and they perform the bulk of the renal reabsorption function. Despite the importance of these cells in kidney function, the molecular mechanisms of proximal tubule development and maturation are not well understood. Experiments reveal that, in the developing mouse kidney, Cadherin-6-expressing cells act as proximal tubule progenitors and they require Hnf4a to further develop into mature proximal tubules. Genomic analyses show that Hnf4a directly regulates the expression of genes required for reabsorption, such as transmembrane transporter genes and metabolism genes. This study advances understanding of how kidney proximal tubule cells form during development.
Keywords: proximal tubule, Hnf4a, kidney development, Fanconi renotubular syndrome
Visual Abstract
Abstract
Background
Hepatocyte NF 4α (Hnf4a) is a major regulator of renal proximal tubule (PT) development. In humans, a mutation in HNF4A impairs PT functions and is associated with Fanconi renotubular syndrome (FRTS). In mice, mosaic deletion of Hnf4a in the developing kidney reduces the population of PT cells, leading to FRTS-like symptoms. The molecular mechanisms underlying the role of Hnf4a in PT development remain unclear.
Methods
The gene deletion tool Osr2Cre removed Hnf4a in developing nephrons in mice, generating a novel model for FRTS. Immunofluorescence analysis characterized the mutant phenotype, and lineage analysis tested whether Cadherin-6 (Cdh6)–expressing cells are PT progenitors. Genome-wide mapping of Hnf4a binding sites and differential gene analysis of Hnf4a mutant kidneys identified direct target genes of Hnf4a.
Results
Deletion of Hnf4a with Osr2Cre led to the complete loss of mature PT cells, lethal to the Hnf4a mutant mice. Cdh6high, lotus tetragonolobus lectin-low (LTLlow) cells serve as PT progenitors and demonstrate higher proliferation than Cdh6low, LTLhigh differentiated PT cells. Additionally, Hnf4a is required for PT progenitors to differentiate into mature PT cells. Genomic analyses revealed that Hnf4a directly regulates the expression of genes involved in transmembrane transport and metabolism.
Conclusions
Hnf4a promotes the differentiation of PT progenitors into mature PT cells by regulating the expression of genes associated with reabsorption, the major function of PT cells.
The kidneys filter the blood, regulate osmotic levels, maintain electrolyte balance, and metabolize drugs. The functional unit of the kidney is the nephron, which is composed of the glomerulus, the proximal tubule, the loop of Henle, and the distal tubule.1 Each segment of the nephron has distinct physiologic functions and morphology. The proximal tubule cells are the most populous cell type in the kidney, and they carry out the bulk of reabsorption in the nephron.2–4 Under physiologic conditions, proximal tubules reabsorb approximately two thirds of glomerular-filtered water and sodium chloride as well as most of the filtered glucose and phosphate.5 Proximal tubular reabsorption of water and metabolites is essential in the regulation of body fluid composition and volume. Numerous transporter and metabolism genes are expressed in the proximal tubules in order to facilitate the function and energy demands of these highly active renal epithelial cells.6–12 Despite their importance in kidney function, the molecular mechanisms of proximal tubule development and maturation are not well understood.
Fanconi renotubular syndrome (FRTS) is defined as generalized proximal tubule dysfunction.13,14 Symptoms of FRTS include glucosuria, phosphaturia, proteinuria, polyuria, and polydipsia.14,15 These symptoms are consistent with a failure of the proximal tubules to reabsorb and transport filtered molecules, causing urinary wasting.16,17 In humans, the heterozygous mutation R76W in the gene encoding Hepatocyte NF4α (HNF4A) causes FRTS with nephrocalcinosis.18 Because this mutation is located in the DNA binding domain, it has been speculated that the mutation affects the interactions of HNF4A with regulatory DNA.18 A recent study of the FRTS HNF4A mutation in Drosophila nephrocytes confirmed that the mutation reduced binding of Hnf4a to DNA and caused nuclear depletion of Hnf4a in a dominant-negative manner, leading to mitochondrial defects and lipid accumulation.19
We have previously shown that Hnf4a is expressed in developing proximal tubules in the mouse kidney and that Hnf4a is important for proximal tubule formation.20 Mosaic loss of Hnf4a in the murine nephron lineage caused FRTS–like symptoms, including polyuria, polydipsia, glucosuria, and phosphaturia.20 Because of the mosaic expression of Six2GFPCre in mesenchymal nephron progenitor cells, the Hnf4a mutant kidney by Six2GFPCre was a chimera of wild-type and mutant cells.20 This made it difficult to perform more rigorous differential gene expression analyses. In this study, we generated a new mouse model with thorough deletion of Hnf4a in the proximal segments of the nephron using Osr2IresCre and investigated the requirement of mature proximal tubules for postnatal survival.21 We also performed lineage tracing to identify proximal tubule progenitor cells in the developing kidney. To further elucidate the role of Hnf4a in proximal tubule development, we performed genome-wide mapping of Hnf4a binding sites in the murine neonate kidney and transcriptomic analysis of the Hnf4a mutant kidney. We found that Hnf4a is required for terminal differentiation of proximal tubule cells and that mature proximal tubules are required for postnatal survival. Cadherin-6-expressing (Cdh6high), lotus tetragonolobus lectin-low (LTLlow) cells in the developing kidney are proximal tubule progenitor cells, and loss of Hnf4a causes their developmental arrest. Our genomic analyses revealed that Hnf4a directly regulates expression of many mature proximal tubule genes, including transporter and metabolism genes, consistent with the fact that active reabsorption is the major function of proximal tubules.
Methods
Mice
All mouse alleles used in this study have been previously published: Osr2tm2(cre)Jian (Osr2IresCre),22 Hnf4atm1Sad (Hnf4ac),23 Cdh6tm1.1(cre/ERT2)Jrs (Cdh6CreER),24 and Gt(ROSA)26Sortm3(CAG-EYFP)Hze (Rosa26Ai3).25 All mice were maintained in the Cincinnati Children’s Hospital Medical Center (CCHMC) animal facility according to animal care regulations. All experiments were performed in accordance with animal care guidelines, and the protocol was approved by the Institutional Animal Care and Use Committee of the CCHMC (IACUC2017–0037). We adhere to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tamoxifen Treatment
Tamoxifen (Sigma; T5648) was dissolved in corn oil (Sigma; C8267) at a concentration of 20 mg/ml. Pregnant female mice were injected with tamoxifen intraperitoneally (4 mg/40 g body wt).
Immunofluorescence Staining
Embryonic, neonatal, and adult murine kidneys were fixed in 4% paraformaldehyde in PBS, incubated overnight in 10% sucrose/PBS at 4°C, and imbedded in OCT (Fisher Scientific). Cryosections (8–9 μm) were incubated overnight with primary antibodies in 5% heat-inactivated sheep serum/PBS with 0.1% Triton X-100. We used primary antibodies for GFP (1:500; Aves GFP-1020), Jag1 (1:20; DSHB TS1.15H), Wt1 (sc-7385, 1:100; Santa Cruz), Biotin-LTL (B-1325, 1:500; Vector Labs), FITC-LTL (FL-1321, 1:200; Vector Labs), Hnf4a (ab41898, 1:500; Abcam), Hnf4a (sc-8987, 1:500; Santa Cruz), LDL-related protein 2 (Lrp2; sc-515772, 1:100; Santa Cruz), Ki67 (652402, 1:500; BioLegend), Slc12a1 (18970–1-AP, 1:500; Proteintech), Slc12a3 (HPA028748, 1:300; Sigma), Ass1 (16210–1-AP, 1:500; Proteintech), Slc5a2 (HPA041603, 1:100; Sigma), Miox (HPA039562, 1:500; Sigma), Cdh6 (MAB2715-SP, 1:100; R&D Systems), and Cdh6 (HPA007047, 1:200; Sigma). Fluorophore-labeled secondary antibodies were used for indirect visualization of the target. Images were taken with a Nikon Ti-E wide-field microscope equipped with an Andor Zyla camera and Lumencor SpectraX light source housed at the Confocal Imaging Core (CIC) at CCHMC.
Histology
Mouse kidneys were harvested and fixed in 4% paraformaldehyde in PBS overnight. Paraffin sections (5 μm) were stained with hematoxylin and eosin or periodic acid–Schiff reagent (American MasterTech KTPAS). Images were taken with a Nikon Ti-E wide-field microscope equipped with an Andor Zyla camera and Lumencor SpectraX light source housed at the CIC at CCHMC.
Cell Counts
The quantification of Ki67 staining was performed on Hnf4ac/c and Hnf4ac/+;Osr2IresCre postnatal day 0 (P0) control kidneys. Immunostained samples were imaged as described above. Three 250,000-μm2 sections from each pair of kidneys (four pairs of kidneys total) were quantified manually using the ImageJ multipoint tool.26 For the quantification of Cdh6+, LTL+ cells, immunostained sections from Hnf4ac/+;Osr2IresCre (control) and Hnf4ac/c;Osr2IresCre (mutant) P0 kidneys were analyzed. Three 250,000-μm2 sections from each pair of kidneys (three pairs of kidneys from each genotype) were quantified manually using ImageJ multipoint tool.26
Chromatin Immunoprecipitation Sequencing
Chromatin immunoprecipitation sequencing (ChIP-seq) was performed as previously described.27 Briefly, kidneys from newborn mice were crosslinked with 1% paraformaldehyde, sonicated, and incubated with Hnf4a antibody (ab41898)–coupled Dynabeads Protein G (ThermoFisher). Nonimmunoprecipitated chromatin (starting material) was used as the control. Eluted DNA was used for constructing sequencing libraries using the ThruPLEX DNA-seq kit (Takara). Libraries were sequenced on an Illumina HiSeq 2500 by the DNA Sequencing and Genotyping Core at CCHMC. ChIP-seq reads were mapped to mm9 using Bowtie.28 We performed peak calling and motif analysis using HOMER.29 Data are available at Gene Expression Omnibus under accession number GSE144824.
RNA Sequencing
RNA sequencing (RNA-seq) was performed as previously described.20 Briefly, 1 μg total RNA was isolated from P0 Hnf4a mutant and control kidneys using the RNeasy Plus Micro kit (74034; Qiagen) followed by mRNA isolation with NEBNext Poly(A) mRNA Magnetic Isolation Module (E7490; NEB). Fragmentation of mRNA followed by reverse transcription and second-strand cDNA synthesis was done using NEBNext Ultra RNA Library Prep Kit for Illumina (E7530; NEB). Sequencing libraries were constructed using ThruPLEX DNA-seq kit (R400428; Takara). Sequencing was performed as described above. RNA-seq reads were mapped to mm9 using TopHat, and normalized gene expression values were calculated using Cufflinks.30 Genes that showed at least a 1.5-fold change in expression with a P<0.05 were considered differentially expressed. Data are available at Gene Expression Omnibus under accession number GSE144772.
Genomic Regions Enrichment of Annotations Tool Analyses
Genomic Regions Enrichment of Annotations Tool (GREAT) analysis was performed using the online program, version 3 (great.stanford.edu).31 To associate genomic regions with genes, gene regulatory domains were defined as minimum 5.0 kb upstream and 1.0 kb downstream of the transcription start site and distally up to 1000 kb to the nearest gene’s basal domain (“basal plus extension” option); 10,417 genomic regions from the Hnf4a ChIP-seq dataset were entered into the GREAT online program, and Mouse Genome Informatics (MGI) Expression terms of genes associated with the genomic regions were assessed.
Gene Ontology Analyses
Gene ontology (GO) analysis was performed using DAVID Bioinformatics Resources (david.ncifcrf.gov) on differentially expressed genes identified from the RNA-seq analysis.32
Statistical Analyses
Statistical analysis of Kaplan–Meier survival curve was performed using GraphPad 8 Prism software (n=11 Hnf4a mutants and n=14 controls).33 The log-rank test was used for survival analysis. The t test was performed using GraphPad 8 Prism software. P<0.05 was considered to be significant.
Results
Hnf4a Is Required for Mature Proximal Tubule Formation
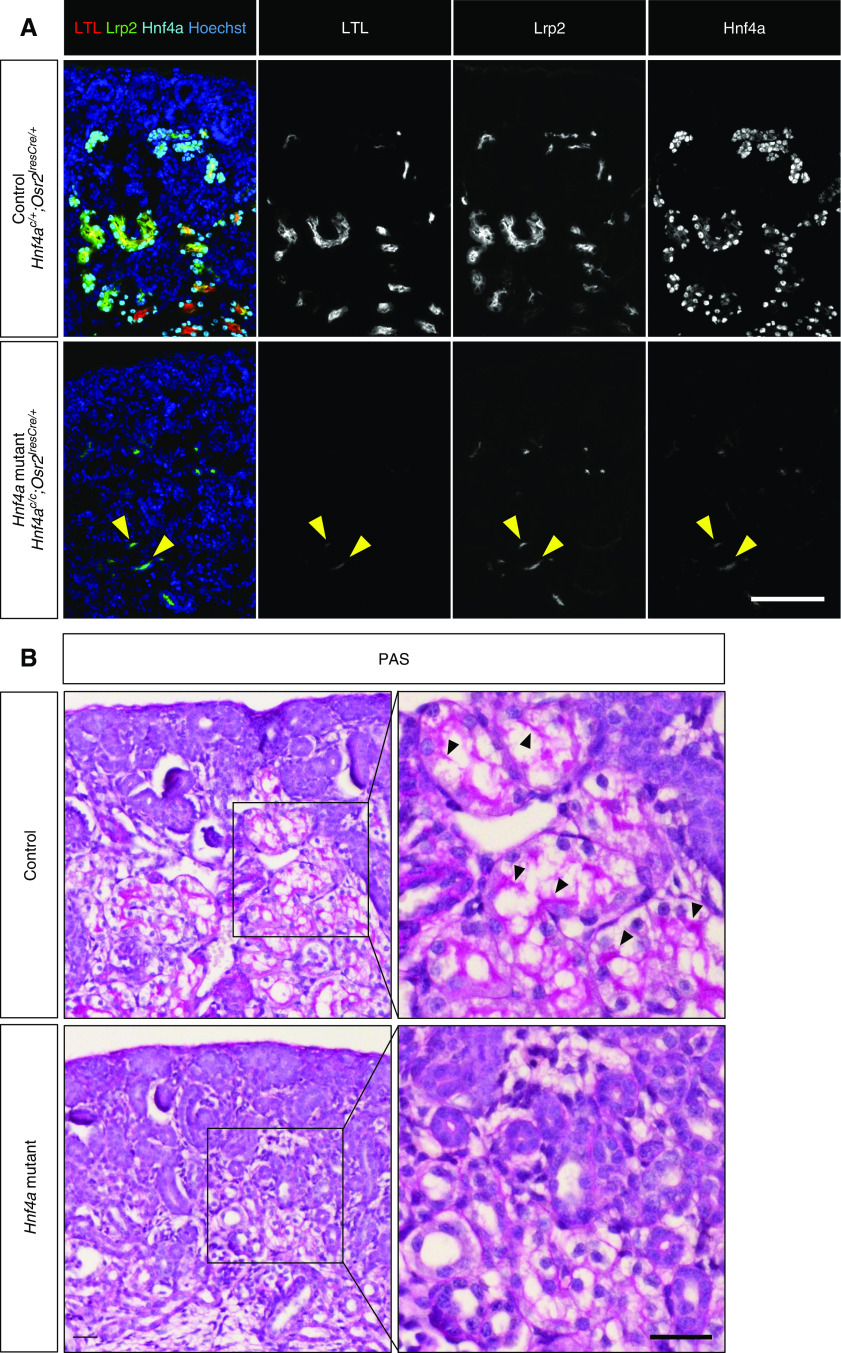
In our previous study, we used Hnf4a floxed alleles and Six2GFPCre to generate a mouse model with kidney-specific deletion of Hnf4a. However, Six2GFPCre displayed mosaic expression in nephron progenitor cells and allowed a subset of nephron progenitors to escape Cre-mediated recombination.20,34,35 Therefore, our previous Hnf4a mutant kidney was a chimera of wild-type and mutant cells leading to the FRTS-like phenotype we observed. In order to thoroughly investigate the Hnf4a loss-of-function phenotype, we used a less mosaic Cre that specifically targets the proximal segments of the nephron. We generated a new mouse model with nephron-specific deletion of Hnf4a using a mouse line expressing Cre recombinase under the Osr2 promoter (Osr2IresCre)22 bred with Hnf4a floxed mice (Hnf4ac/c).23 We have recently shown that Osr2IresCre is expressed in the proximal and medial segments of the S-shaped body (SSB) of the developing nephron and that the medial segment of SSB develops into proximal tubules and loops of Henle.21 This Cre, therefore, targets all nephron segments except for the distal tubule. Hnf4a deletion was apparent as early as the SSB stage in the Hnf4a mutant kidney, and loss of Hnf4a did not seem to affect SSB formation (Supplemental Figure 1). Osr2IresCre achieved almost complete deletion of Hnf4a in the kidney (Figure 1A). LTL is known to bind to glycoproteins on the surface of the proximal tubules specifically.36,37 Deletion of Hnf4a in the nephron led to the loss of differentiated proximal tubule cells with high LTL staining (LTLhigh) in P0 kidneys (Figure 1A), but it did not affect the formation of other nephron segments (Supplemental Figure 2A). Consistent with this, our transcriptomic analysis also showed that only proximal tubule genes were significantly downregulated in the Hnf4a mutant (Supplemental Figure 2B).
Figure 1.
Hnf4a deletion by Osr2Cre leads to loss of mature proximal tubule (PT) cells. (A) Loss of Hnf4a in the nephron inhibits formation of LTLhigh, mature PT cells and causes a decrease in expression of Lrp2, a PT-specific gene in the newborn (P0) kidney. Yellow arrowheads mark LTLlow, Lrp2low cells that persist in the mutant. Image is representative of n=3. Scale bar, 100 μm. (B) Periodic acid–Schiff (PAS) staining of control and Hnf4a mutant kidneys at P0 shows that Hnf4a mutants lack brush border. Black arrowheads mark brush border. Image is representative of n=3. Scale bar, 50 μm.
The Hnf4a mutant kidneys showed a decrease in the level of Lrp2, a proximal tubule–specific endocytic receptor protein also known as Megalin (Figure 1A).20,38 A few LTLlow, Lrp2low cells persisted in the Hnf4a mutant kidney (Figure 1A, yellow arrowheads). We reasoned that these cells might represent immature proximal tubules or proximal tubule progenitor cells and that the Hnf4a mutant kidney lacks mature proximal tubules. From previously published single-cell RNA-seq data,8 we identified Ass1, Slc5a2, and Miox as genes that are highly expressed in mature proximal tubules and examined their expression in the Hnf4a mutant kidney. We found that the Hnf4a mutant kidney showed little to no staining of Slc5a2, Ass1, and Miox, indicating decreased expression of mature proximal tubule genes in the Hnf4a mutant kidney (Supplemental Figure 3). A distinctive feature of the mature proximal tubules is the apical brush border. The brush border is composed of microvilli, which increase the surface area of the proximal tubule to facilitate reabsorption.9,10,39 The absence of the proximal tubule brush border has been associated with proximal tubule dysfunction in patients, highlighting the importance of brush border formation.40 We analyzed brush border formation using the periodic acid–Schiff stain41 and found that Hnf4a mutants showed a lack of brush border formation (Figure 1B). Considering that brush border formation only occurs in postmitotic, differentiated cells,42 our result suggests that the Hnf4a mutant kidney lacks terminally differentiated proximal tubule cells.
Because of Hnf4a mutant kidneys lacking mature proximal tubules, we examined whether the nephron tubules were still connected to the glomerulus in the mutant kidney. Immunofluorescence staining showed that the glomerulus was connected to Cdh6+ cells in both the control and Hnf4a mutant kidneys but that LTL staining was visible only in the control kidney (Supplemental Figure 4), suggesting that the glomerulus is connected to proximal tubule progenitors in the Hnf4a mutant kidney.
Loss of Hnf4a in the Nephron Leads to Postnatal Lethality
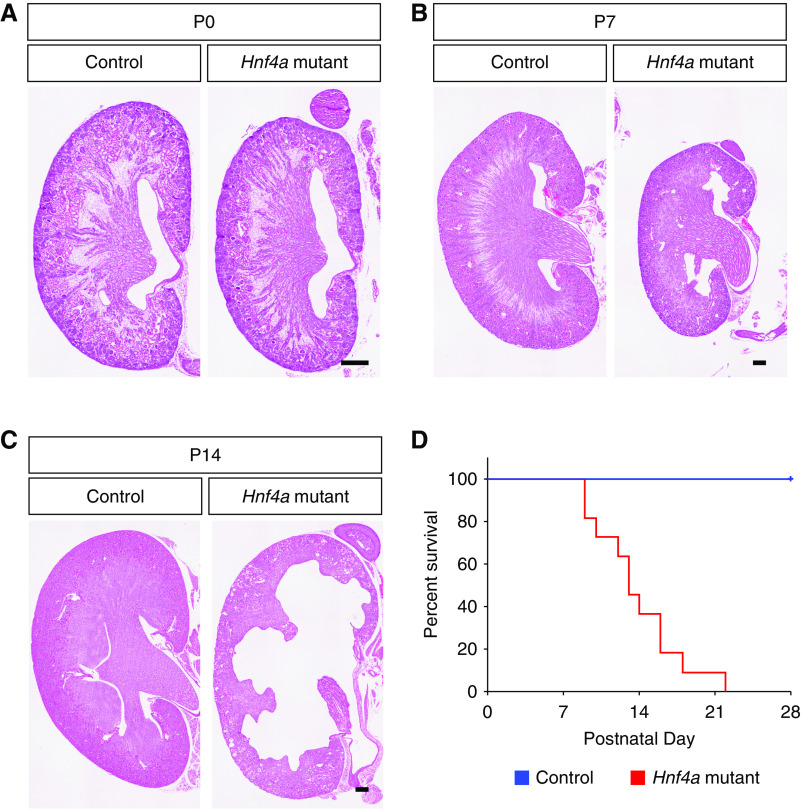
To examine the effects of loss of LTLhigh differentiated proximal tubules on postnatal kidney development, we analyzed the histology of Hnf4a mutant kidneys at P0, P7, and P14. At P0, the Hnf4a mutant kidney was similar in size to the control (Figure 2A). At P7, the Hnf4a mutant kidney was slightly smaller with a thinner cortex than the control kidney (Figure 2B). There was no apparent defect in glomerulus, loop of Henle, or distal tubule formation in the Hnf4a mutant at P7 (Supplemental Figure 5). At P14, the medullary region of the Hnf4a mutant kidney was severely damaged, cysts formed in the cortical region, and hydronephrosis was apparent (Figure 2C). Increased filtrate flow through the renal tubules can lead to renal pelvic dilation and nonobstructive hydronephrosis in nephrogenic diabetes insipidus.43–45 It is likely that hydronephrosis seen in the Hnf4a mutant kidney is caused by increased filtrate flow through the nephron tubules due to lack of reabsorption in the proximal tubule. Survival analysis of the Hnf4a mutant mice showed that approximately 60% of Hnf4a mutants were deceased by P14, likely due to kidney dysfunction (Figure 2D). No Hnf4a mutants survived to weaning age (P28). These results show that the lack of mature proximal tubules causes postnatal lethality, highlighting the importance of the mature proximal tubule function for survival.
Figure 2.
Loss of mature proximal tubules leads to postnatal lethality in Hnf4a mutant mice. (A–C) Hematoxylin and eosin staining of Hnf4a mutant kidneys at birth (P0), P7, and P14. Images are representative of n=4. Scale bar, 100 μm. (D) Kaplan–Meier survival analysis of the Hnf4a mutants (n=11) with heterozygous controls (n=14). P<0.001 determined by log-rank test.
Cdh6high, LTLlow Cells in the Developing Kidney Are Proximal Tubule Progenitor Cells
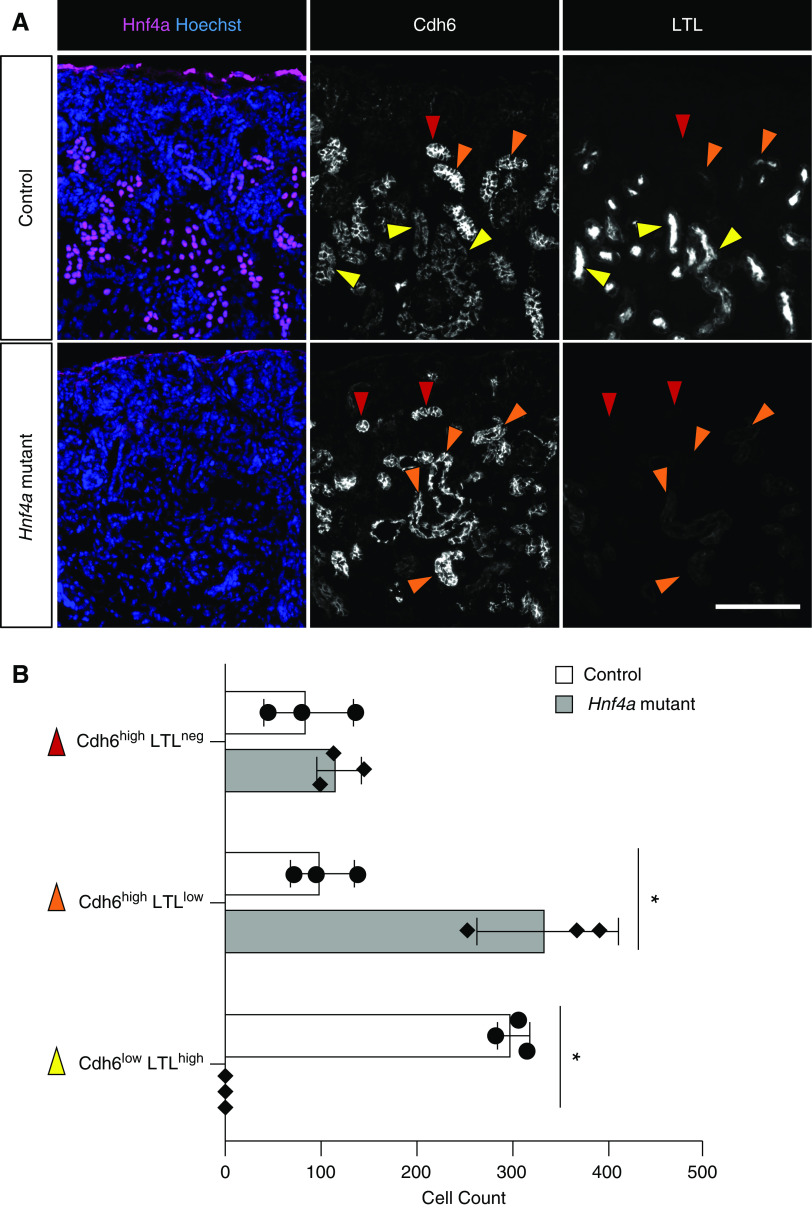
It has been previously suggested that Cdh6-expressing cells in the developing murine kidney are presumptive proximal tubule cells.46 It was reported that, in the mouse embryonic kidney, Cdh6 was expressed in the medial segment of the SSB and that LTL+ proximal tubules were still positive for Cdh6, although its expression was downregulated compared with Cdh6+ cells in the nephrogenic zone. On the basis of these observations, it was proposed that Cdh6-expressing cells in the nephrogenic zone were destined to become proximal tubules.46 Consistent with this, we found that there were two distinct populations of Cdh6+ cells in the wild-type developing murine kidney: Cdh6high and Cdh6low cells (Figure 3A). The majority of Cdh6high cells were Hnf4a+ and had no or low LTL staining (red arrowheads and orange arrowheads, respectively, in Figure 3A), suggesting that these Cdh6high, LTLlow cells are prospective, immature proximal tubule cells. Cdh6low cells were also positive for Hnf4a and had strong LTL staining (yellow arrowheads in Figure 3A), suggesting that these Cdh6low, LTLhigh cells are differentiated proximal tubule cells. We found that, in the Hnf4a mutant kidney, Cdh6low, LTLhigh cells were absent, and the number of Cdh6high, LTLlow cells was increased, suggesting that the loss of Hnf4a prevents Cdh6high, LTLlow cells from developing into Cdh6low, LTLhigh cells (Figure 3B). We found that Cdh6 expression in the kidneys persisted postnatally, suggesting continued proximal tubule development and maturation (Supplemental Figure 6).
Figure 3.
High Cdh6 expression is persistent in the Hnf4a mutant kidney. (A) In the P0 control kidney, Cdh6 expression is high in LTLneg and LTLlow, presumptive proximal tubule (PT) cells (red and orange arrowheads, respectively), and Cdh6 expression decreases as PT cells develop into LTLhigh, mature PT cells (yellow arrowheads). In the Hnf4a mutant kidney, Cdh6high, LTLlow cells are more abundant compared with the control, and there are no Cdh6low, LTLhigh cells to be found. Image is representative of n=3. Scale bar, 100 μm. (B) Quantification of Cdh6high and Cdh6low cells in the Hnf4a mutant and control kidney. *P<0.01 determined by t test.
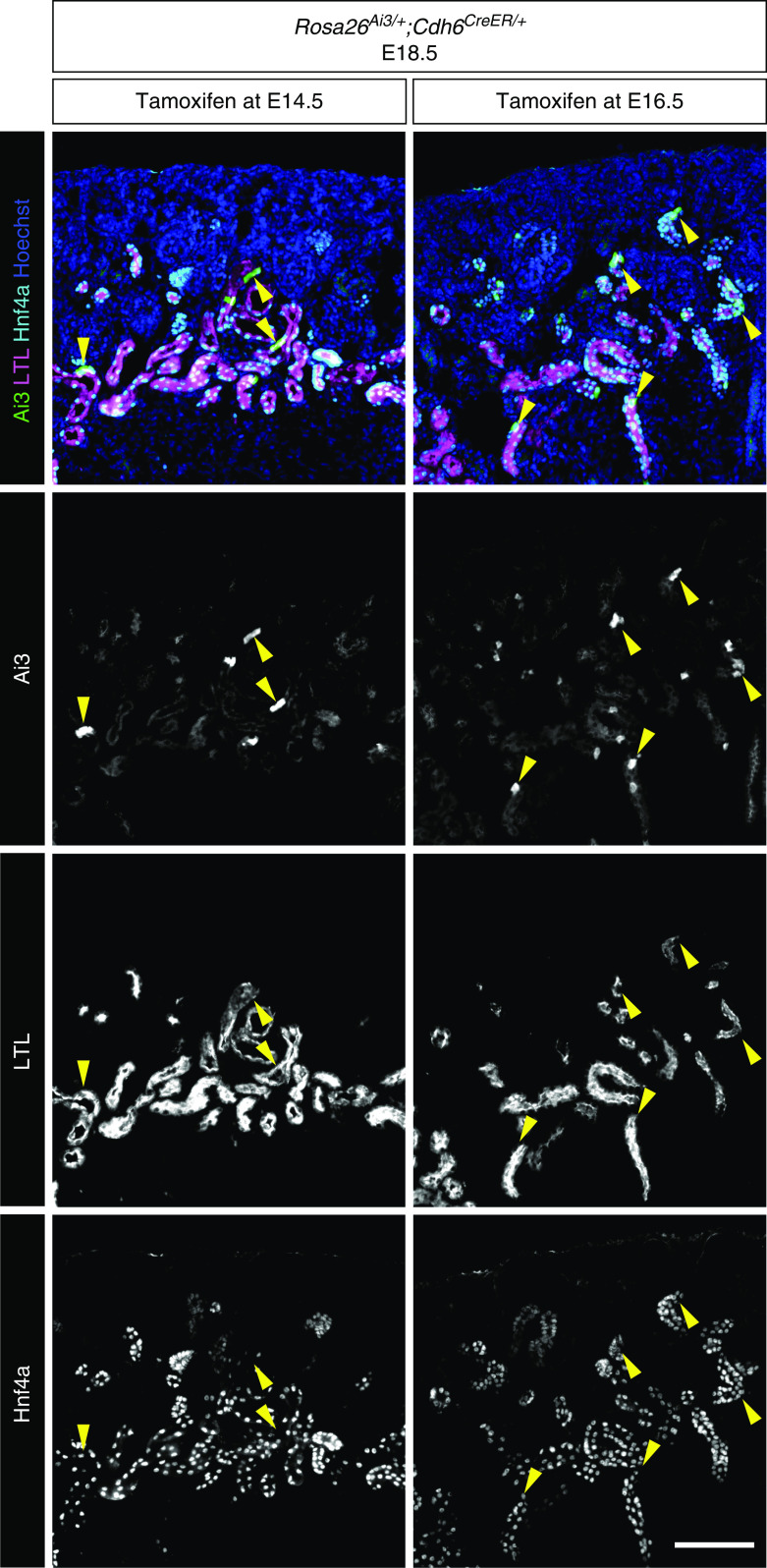
In order to definitively test if Cdh6high cells are proximal tubule progenitor cells, we performed lineage analysis using a tamoxifen-inducible Cre recombinase under the Cdh6 promoter (Cdh6CreER) and a Cre-inducible Rosa26Ai3 reporter.24,25 If Cre-mediated activation of the Rosa reporter is restricted to the proximal tubules and not detected in other nephron segments, it would suggest that Cdh6+ cells are proximal tubule progenitors. Pregnant dams were injected with tamoxifen at E14.5 or E16.5 to label Cdh6high cells and their descendant cells with the Rosa26Ai3 reporter. Embryos were harvested at E18.5. We found that all Rosa26Ai3-labeled cells were Hnf4a+ and that most were also LTL+. As expected, transient activation of Cre activity by tamoxifen was not able to produce a robust labeling of proximal tubules with the Rosa reporter, partly due to the fact that the formation of nephrons is an asynchronous process in the rapidly developing kidney. Nonetheless, our observation that the descendants of Cdh6+ cells differentiate into proximal tubules exclusively indicates that Cdh6high cells in the developing kidney are proximal tubule progenitor cells (Figure 4).
Figure 4.
Cdh6 lineage tracing shows that Cdh6+ cells are proximal tubule progenitor cells. Lineage labeling of Cdh6+ cells with Ai3 after tamoxifen injection into pregnant dams at embryonic day 14.5 (E14.5) or E16.5. All Ai3+ cells are Hnf4a+ and most are also LTL+ (yellow arrowheads) at E18.5. Images are representative of n=3. Scale bar, 100 μm.
Hnf4a has been shown to inhibit proliferation in hepatocytes and promote terminal differentiation.47 Many models of cellular differentiation show an inverse relationship between proliferation and differentiation.48–51 Terminal differentiation commonly involves exiting the cell cycle and entering a postmitotic state.48,52 To determine whether the transition from Cdh6high, LTLlow proximal tubule progenitors to Cdh6low, LTLhigh differentiated proximal tubule cells coincides with cell cycle exit, we examined Ki67 expression in Cdh6high and Cdh6low cell populations (Figure 5). Ki67 is present in actively proliferating cells and absent in resting cells.53–56 We found that Cdh6high proximal tubule progenitor cells were highly proliferative, whereas only few Cdh6low cells showed Ki67 expression (Figure 5A). When quantified, Cdh6high cells had a tenfold higher proliferative rate than Cdh6low cells, indicating an expansion of the progenitor cell population before they exit the cell cycle and undergo terminal differentiation into mature proximal tubule cells (Figure 5B). This suggests that the number of proximal tubule cells in the newborn kidney is largely determined by the proliferation of Cdh6high proximal tubule progenitor cells.
Figure 5.
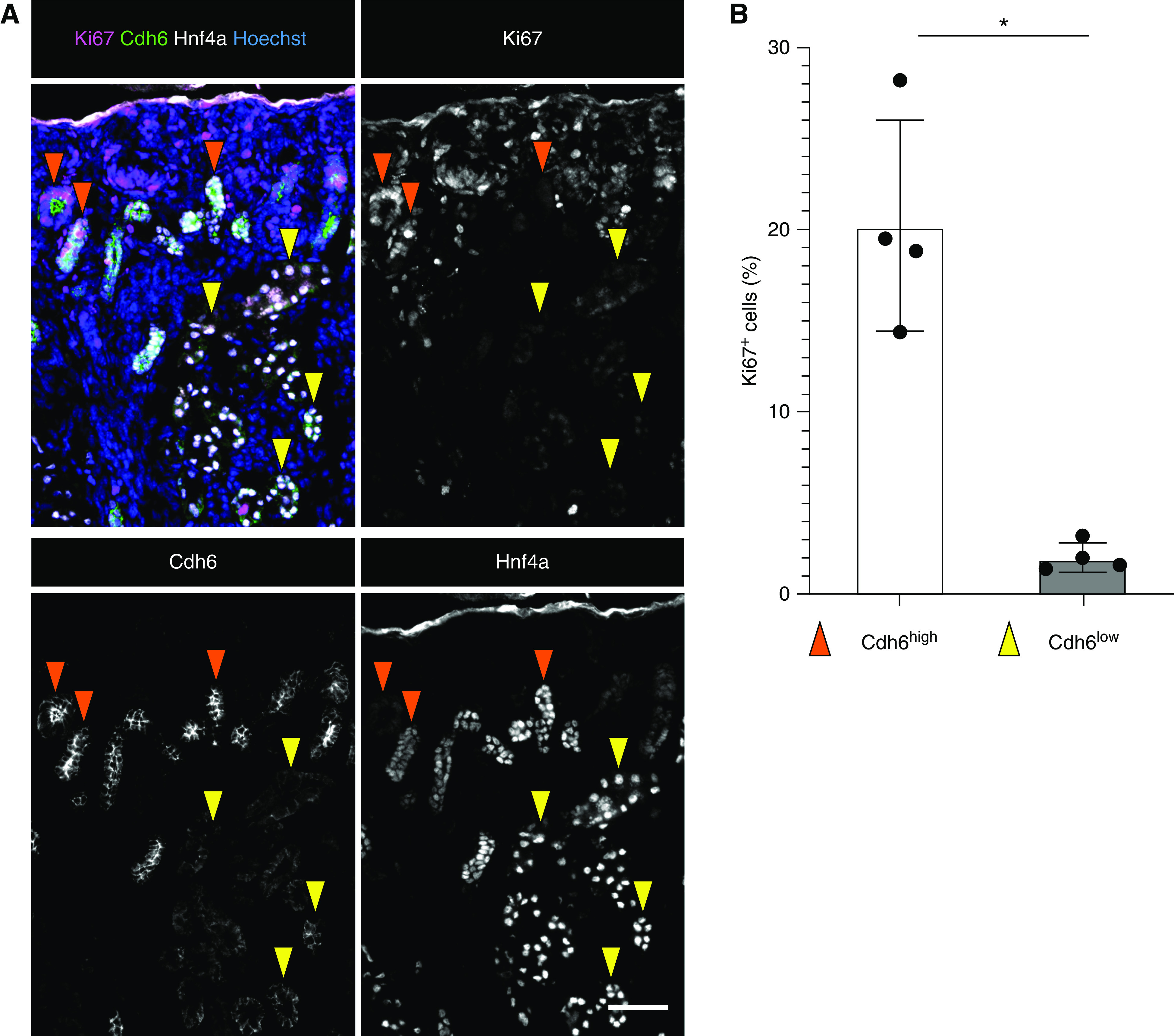
Cdh6high proximal tubule (PT) progenitor cells have a higher proliferation rate than Cdh6low mature PT cells. (A) Representative immunostains for Ki67, Cdh6, and Hnf4a in the P0 kidney (n=4). Cdh6high, orange arrowheads; Cdh6low, yellow arrowheads. Scale bar, 50 μm. (B) Quantification of Ki67-positive cells (n=4). *P<0.01 determined by t test.
Hnf4a Gene Regulatory Network Reveals the Roles of Hnf4a in Regulating Proximal Tubule Development
To further elucidate the role of Hnf4a in the proximal tubule transcriptional program, we performed chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) with P0 murine kidneys to identify Hnf4a-bound genomic regions. Two Hnf4a ChIP-seq replicates yielded 10,417 reproducible binding sites (peaks) (Supplemental Table 1). Our motif analysis showed high enrichment of the canonical Hnf4a binding DNA sequence within these Hnf4a peaks (Figure 6A), indicating that Hnf4a-bound genomic regions were successfully enriched in our ChIP-seq samples. We also found that DNA motifs for other nuclear receptors, including the estrogen-related receptor-α (ESRRA), the retinoid X receptor (RXR), the peroxisome proliferator-activated receptor (PPAR), and hepatocyte NF 1β (HNF1B), were also enriched within the Hnf4a-bound genomic regions, suggesting that these nuclear receptors share common target genes with Hnf4a to regulate proximal tubule development (Figure 6A). The majority of the Hnf4a peaks were found within 50 kb of transcription start sites (Figure 6B), and 30% of the peaks were located in promoter regions (Figure 6C). GREAT analysis of MGI expression annotations of genes associated with Hnf4a binding sites showed enrichment within the developing renal proximal tubules (Figure 6D),31 consistent with the fact that Hnf4a is specifically expressed in proximal tubules in the kidney. Multiple peaks were identified near the promoters of proximal tubule genes (Supplemental Table 1). In particular, we found Hnf4a peaks near genes such as Slc34a1 and Ehhadh, genes linked to FRTS in human patients (Figure 6E).57,58
Figure 6.
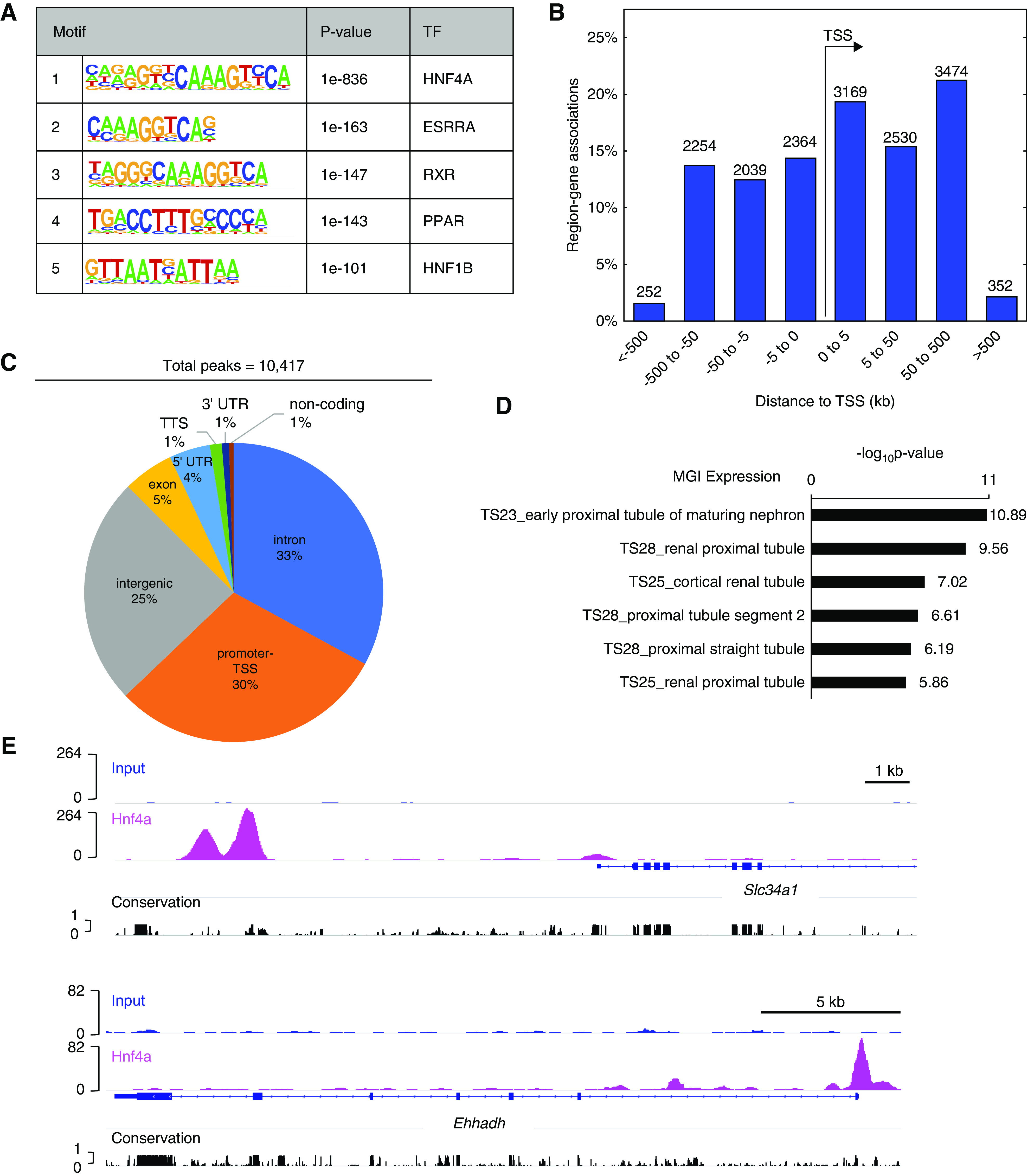
Genome-wide mapping of Hnf4a binding sites implicates its roles in proximal tubules. (A) Analysis of known transcription factor (TF) motifs from Hnf4a ChIP-seq. (B) Bar graph showing the percentage of region-gene associations according to genomic regions’ distance to the transcription start site (TSS) computed by GREAT (http://bejerano.stanford.edu/great/public/html/). (C) Pie chart representing the distribution of Hnf4a peaks within the annotated genome. (D) GREAT MGI Expression annotations of Hnf4a peaks showing the top six enriched terms. (E) Genome browser view of Hnf4a ChIP-seq peaks near the TSS of proximal tubule genes. UTR, untranslated region; TTS, transcription termination site.
We conducted transcriptomic analysis (RNA-seq) of P0 Hnf4a mutant kidneys to complement our candidate target gene list with differential gene expression data (Supplemental Table 2). In the Hnf4a mutant kidney, 442 genes showed a significant decrease in expression (fold change ≥1.5; P<0.05), including Slc34a1, Ehhadh, and Ass1, which are highly expressed in proximal tubules (Figure 7A, Supplemental Table 2).7,8 As previously mentioned, mutations in SLC34A1 and EHHADH are associated with FRTS in patients.57,58 GO analysis of these 442 downregulated genes showed enrichment of genes associated with metabolism and transport (Figure 7B). We found that 196 genes were significantly upregulated in the Hnf4a mutant kidneys (fold change ≥1.5; P<0.05), including Cdh6, the gene marking proximal tubule progenitors (Figure 7A, Supplemental Table 2). GO analysis of the 196 upregulated genes showed enrichment of genes associated with phospholipid homeostasis and cholesterol transport (Figure 7C). In order to determine which genes are directly regulated by Hnf4a, we compared the differentially expressed genes in the Hnf4a mutant with the 7823 genes associated with Hnf4a binding sites to find overlapping genes (Figure 7D, Supplemental Table 3). There were 245 genes in common between the significantly downregulated genes and the Hnf4a binding sites and 81 common genes between significantly upregulated genes and the Hnf4a binding sites (Figure 7D, Supplemental Table 3). GO analysis of the 245 downregulated genes showed enrichment of genes associated with transport and metabolism (Figure 7E). Of the downregulated genes, Hnf4a has previously been shown to activate the expression of Lrp2, Pdzk1, Slc22a1, and Pck1 in vitro, a validation that some of the downregulated genes from our data are directly regulated by Hnf4a.59–62 When we compared our 245 downregulated genes with proximal tubule genes identified from previously published single-cell RNA-seq,8 we found that there were 149 genes in common. These 149 genes were expressed mainly in mature proximal tubule populations rather than in progenitor cells (Supplemental Figure 7), implying that Hnf4a may be more active in mature proximal tubules. GO analysis of the 81 upregulated genes showed enrichment of genes associated with transport, metabolism, and response to thyroid hormone and ischemia (Figure 7F). Our genomic and transcriptomic analyses suggest that Hnf4a regulates proximal tubule maturation via activation of transporter and metabolism genes, consistent with the functions of the proximal tubule.
Figure 7.
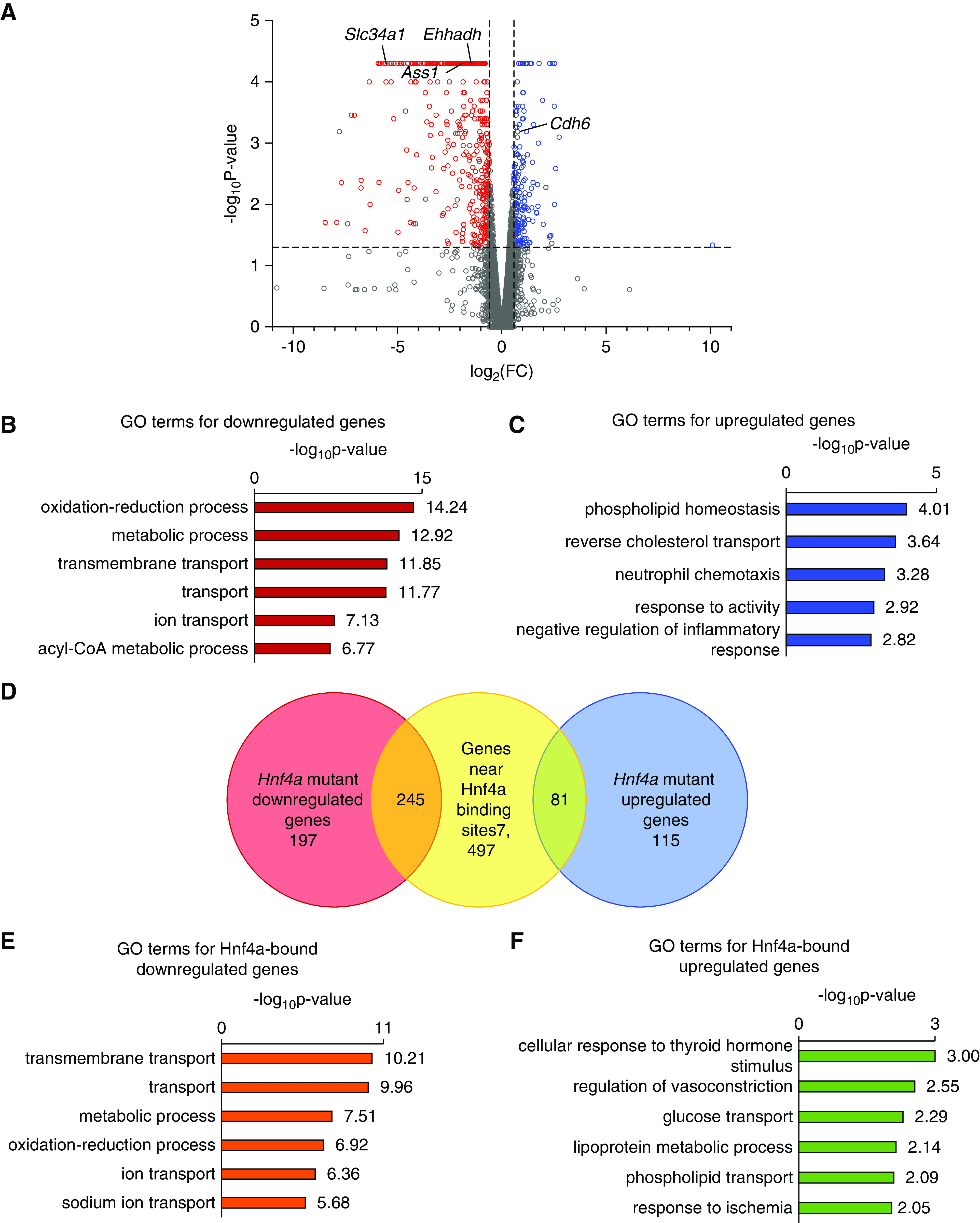
Intersection of Hnf4a ChIP-seq peaks with differentially expressed genes in the Hnf4a mutant kidney identified direct target genes of Hnf4a. (A) Differential expression analysis in the Hnf4a mutant versus the Hnf4a control kidney at P0. Red and blue points in the volcano plot mark genes with significantly decreased or increased expression, respectively, in the Hnf4a mutant. Vertical dashed lines (x axis) mark log2(1.5). The horizontal dashed line (y axis) marks −log10(0.05). (B) GO analysis of significantly downregulated genes in the Hnf4a mutant kidney showing the top six enriched terms. (C) GO analysis of significantly upregulated genes in the Hnf4a mutant kidney showing the top five enriched terms. (D) The Venn diagram shows the overlap of genes associated with Hnf4a binding sites and differentially expressed genes in the Hnf4a mutant kidney. (E) GO analysis of Hnf4a-bound, downregulated genes showing the top six enriched terms. (F) GO analysis of Hnf4a-bound, upregulated genes showing the top six enriched terms. FC, fold change.
Discussion
In our previous study, we identified two populations of LTL+ cells in the developing mouse kidney: LTLlow and LTLhigh. On the basis of our results, we concluded that these two populations represent presumptive proximal tubules and differentiated proximal tubules, respectively.20 In order to further examine the presumptive proximal tubule population, we sought to identify a marker of proximal tubule progenitors. Previously, Cdh6 had been proposed as a marker for prospective proximal tubule cells.46 However, it had not been definitively shown that Cdh6+ cells develop into proximal tubule cells.46 To address this, we performed lineage analysis of Cdh6+ cells in the developing kidney and found that these cells all became Hnf4a+ proximal tubule cells (Figure 4). This experiment provides strong evidence that Cdh6+ cells are proximal tubule progenitors in the developing kidney. Cdh6+ proximal tubule progenitors are highly proliferative, whereas LTLhigh, Hnf4a+ proximal tubule cells proliferate less frequently (Figure 5). This suggests that expansion of proximal tubule progenitors determines the number of proximal tubule cells. Identification of proximal tubule progenitors will allow us to further investigate the developmental mechanisms of proximal tubule development.
We have previously reported that mosaic deletion of Hnf4a by Six2GFPCre in the developing mouse kidney causes a significant reduction in proximal tubule cells, phenocopying FRTS.20 The paucity of proximal tubules is consistent with reduced expression of proximal tubule genes, including the genes encoding glucose and phosphate transporters. However, it was unknown which genes were directly regulated by Hnf4a in proximal tubules. In this study, we performed Hnf4a ChIP-seq on newborn mouse kidneys and RNA-seq analysis of Hnf4a mutant kidneys by Osr2IresCre. From the intersection of the ChIP-seq and RNA-seq datasets, we identified 245 Hnf4a direct target genes that were downregulated in the Hnf4a mutant during kidney development (Figure 7D). Among these 245 targets, the most enriched were genes associated with transmembrane transport, suggesting that the role of Hnf4a in proximal tubule development correlates with active reabsorption, the major function of the proximal tubule (Figure 7E, Table 1, genes associated with transmembrane transport). The genes associated with fatty acid metabolism were also enriched in these 245 direct target genes (Table 1, genes associated with lipid and fatty acid metabolism). Taking into account that proximal tubule cells are highly active in metabolism and that their energy demands are primarily met by fatty acid oxidation,11,63–65 our results suggest that Hnf4a regulates metabolic reprogramming during proximal tubule development. Consistent with this, it has been shown that Hnf4a controls lipid metabolism in Drosophila nephrocytes.19 Additional exploration of Hnf4a target genes will advance our understanding of proximal tubule development. Interestingly, only 3% of the Hnf4a-bound genes showed differential expression in the Hnf4a mutant kidney. Because many proximal tubule genes are upregulated postnatally,66,67 it is possible that Hnf4a alone is not sufficient to induce expression of the majority of its target genes and that cofactors are required.
Table 1.
Hnf4a target genes that were downregulated in the Hnf4a mutant kidney
| Gene Symbol | Gene Name |
|---|---|
| Genes associated with transmembrane transport | |
| Sfxn1 | Sideroflexin 1 |
| Slc13a1 | Solute carrier family 13 (sodium/sulfate symporters), member 1 |
| Slc16a4 | Solute carrier family 16 (monocarboxylic acid transporters), member 4 |
| Slc16a9 | Solute carrier family 16 (monocarboxylic acid transporters), member 9 |
| Slc2a2 | Solute carrier family 2 (facilitated glucose transporter), member 2 |
| Slc2a5 | Solute carrier family 2 (facilitated glucose transporter), member 5 |
| Slc22a6 | Solute carrier family 22 (organic anion transporter), member 6 |
| Slc22a8 | Solute carrier family 22 (organic anion transporter), member 8 |
| Slc22a12 | Solute carrier family 22 (organic anion/cation transporter), member 12 |
| Slc22a1 | Solute carrier family 22 (organic cation transporter), member 1 |
| Slc22a13 | Solute carrier family 22 (organic cation transporter), member 13 |
| Slc22a2 | Solute carrier family 22 (organic cation transporter), member 2 |
| Slc23a1 | Solute carrier family 23 (nucleobase transporters), member 1 |
| Slc47a1 | Solute carrier family 47, member 1 |
| Slc5a8 | Solute carrier family 5 (iodide transporter), member 8 |
| Slc5a1 | Solute carrier family 5 (sodium/glucose cotransporter), member 1 |
| Slc5a12 | Solute carrier family 5 (sodium/glucose cotransporter), member 12 |
| Genes associated with lipid and fatty acid metabolism | |
| Hmgcs2 | 3-Hydroxy-3-methylglutaryl-CoA synthase 2 |
| Gm2a | GM2 ganglioside activator protein |
| Acaa1b | Acetyl-CoA acyltransferase 1B |
| Acsm1 | Acyl-CoA synthetase medium-chain family member 1 |
| Dgat2 | Diacylglycerol O-acyltransferase 2 |
| Elovl2 | Elongation of very long–chain fatty acids-like 2 |
| Ehhadh | Enoyl-CoA, hydratase/3-hydroxyacyl CoA dehydrogenase |
| Fads3 | Fatty acid desaturase 3 |
| Hsd17b2 | Hydroxysteroid (17-β) dehydrogenase 2 |
| Nceh1 | Neutral cholesterol ester hydrolase 1 |
| Pck1 | Phosphoenolpyruvate carboxykinase 1, cytosolic |
| Pcx | Pyruvate carboxylase |
| Slc27a2 | Solute carrier family 27 (fatty acid transporter), member 2 |
| Amacr | α-Methylacyl-CoA racemase |
| Cryl1 | Crystallin, λ1 |
Motif analysis of genomic regions bound by a given transcription factor provides a list of other transcription factors that physically or genetically interact with the target transcription factor, sharing common target genes. Known motif analysis of our Hnf4a ChIP-seq datasets revealed that the DNA motifs for ESRRA, RXR, PPAR, and Hnf1b were enriched within the Hnf4a-bound genomic regions in the developing mouse kidney. The binding motifs of ESRRA and RXR are quite similar to the Hnf4a binding motif (Figure 6A), which could suggest that there is cooperative binding among these nuclear receptor transcription factors to activate a proximal tubule–specific transcriptional program. Alternatively, similar binding motifs could imply competitive binding because overlapping DNA motifs can lead to competition between transcription factors to activate or repress context-specific transcriptional programs.68 Recent studies in zebrafish have implicated retinoic acid signaling in the formation of proximal tubules, further supporting RXR as a potential coregulator of proximal tubule development.69,70 Both PPARa/g and Hnf1a/b have been implicated in proximal tubule development and function, indicating that they are good candidate coregulators of proximal tubule development.71–73 PPAR transcription factors are known binding partners of RXR, and one study predicted 17 common targets between Hnf4a and PPARa.67 Hnf1b is expressed in all nephron segments,74–78 and Hnf1b deficiency in the nephron lineage of the mouse kidney leads to defects in nephron formation, particularly the proximal tubules, loops of Henle, and distal tubules.78,79 Hnf1b is known to interact with Hnf4a and regulate common target genes.67,75,80 It has also been shown that expression of Hnf1b and Hnf4a, along with Emx2 and Pax8, can convert fibroblasts into renal tubular epithelial cells, strongly suggesting that Hnf1b is a coregulator of proximal tubule development.81 Further investigation is needed to elucidate the interactions among these transcription factors and their roles in proximal tubule development.
A number of protocols have been developed for in vitro differentiation of induced pluripotent stem cells into kidney organoids, and transdifferentiation protocols have been developed to produce renal epithelial cells for disease modeling, drug toxicity testing, and possible cell-based therapies for CKD.81–85 One of the challenges of kidney organoid differentiation is achieving cellular maturity.86 It is known that proximal tubule cells in kidney organoids fail to undergo the maturation process.87 Considering our finding that Hnf4a plays a critical role in proximal tubule development, the application of Hnf4a ligands, such as linoleic acid,88 may be useful for the generation of mature proximal tubules in kidney organoids.
In conclusion, we examined the molecular mechanisms of Hnf4a-regulated proximal tubule development. We found that, in the Hnf4a mutant kidney, proximal progenitors were present but that mature proximal tubules were absent, suggesting that Hnf4a is required for proximal tubule maturation, rather than specification. Loss of mature proximal tubule cells in the Hnf4a mutant mice caused postnatal lethality, highlighting the importance of functional proximal tubules for survival. In the Hnf4a mutant kidney, there is an increase in Cdh6high, LTLlow presumptive proximal tubule cells. We definitively showed that Cdh6+ cells in the developing kidney are proximal tubule progenitors. These results suggest that Hnf4a is required for proximal tubule progenitors to differentiate into mature proximal tubule cells. Genome-wide analysis of Hnf4a binding sites in the kidney and transcriptomic analysis of the Hnf4a mutant kidney indicate that Hnf4a directly regulates expression of multiple genes involved in transmembrane transport and metabolic processes in the proximal tubule.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK120164 (to S. Marable), DK120847 (to J.-S. Park), and DK125577 (to J.-S. Park).
Supplementary Material
Acknowledgments
The authors thank Dr. S. Steven Potter and Mr. Mike Adam for help with single-cell RNA-seq data. They also thank the CIC and the DNA Sequencing and Genotyping Core at CCHMC.
Dr. Sierra S. Marable performed mouse experiments; Dr. Eunah Chung and Dr. Sierra S. Marable performed ChIP-seq; Dr. Eunah Chung performed RNA-seq.; Dr. Sierra S. Marable and Dr. Joo-Seop Park designed the experiments, analyzed the data, and cowrote the manuscript; Dr. Sierra S. Marable made the figures; and all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020020184/-/DCSupplemental.
Supplemental Figure 1. Deletion of Hnf4a does not affect the formation of the S-shaped body.
Supplemental Figure 2. Deletion of Hnf4a does not affect the formation of other nephron segments in the newborn kidney.
Supplemental Figure 3. Mature proximal tubule markers are absent in the Hnf4a mutant kidney.
Supplemental Figure 4. The glomerulus is connected to Cdh6+ tubules in the Hnf4a mutant and control kidneys.
Supplemental Figure 5. Deletion of Hnf4a does not affect the formation of other nephron segments in the postnatal kidney.
Supplemental Figure 6. Cdh6 expression persists postnatally in the Hnf4a mutant and control kidneys.
Supplemental Figure 7. Expression of top marker genes that are enriched in early and mature proximal tubules.
Supplemental Table 1. Genome-wide mapping of Hnf4a binding sites in the mouse kidney at P0 (ChIP-seq).
Supplemental Table 2. Differential gene analysis of the Hnf4a mutant kidney at P0 (RNA-seq).
Supplemental Table 3. Intersection of ChIP-seq and RNA-seq.
References
- 1.McMahon AP: Development of the mammalian kidney. Curr Top Dev Biol 117: 31–64, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boron WF: Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura M, Shirai A, Yamazaki O, Satoh N, Suzuki M, Horita S, et al. : Roles of renal proximal tubule transport in acid/base balance and blood pressure regulation. BioMed Res Int 2014: 504808, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpern RJ: Cell mechanisms of proximal tubule acidification. Physiol Rev 70: 79–114, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Baum M, Quigley R: Proximal tubule water transport-lessons from aquaporin knockout mice. Am J Physiol Renal Physiol 289: F1193–F1194, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka M, Imai E, Kaneko T, Ito T, Moriyama T, Yamauchi A, et al. : Isolation of genes identified in mouse renal proximal tubule by comparing different gene expression profiles. Kidney Int 53: 562–572, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam M, Potter AS, Potter SS: Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: A molecular atlas of kidney development. Development 144: 3625–3632, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curthoys NP, Moe OW: Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9: 1627–1638, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo JL, Li XC: Proximal nephron. Compr Physiol 3: 1079–1123, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. : Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M: Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55: 2502–2509, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SB, Unwin RJ: Renal tubular disorders. Clin Med (Lond) 12: 476–479, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi T: Pediatric Fanconi syndrome In: Pediatric Nephrology, edited by Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL, Berlin, Springer, 2016, pp 1355–1388 [Google Scholar]
- 15.Klootwijk ED, Reichold M, Unwin RJ, Kleta R, Warth R, Bockenhauer D: Renal Fanconi syndrome: Taking a proximal look at the nephron. Nephrol Dial Transplant 30: 1456–1460, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Gonick HC: Pathophysiology of human proximal tubular transport defects. Klin Wochenschr 60: 1201–1211, 1982. [DOI] [PubMed] [Google Scholar]
- 17.Sirac C, Bridoux F, Essig M, Devuyst O, Touchard G, Cogné M: Toward understanding renal Fanconi syndrome: Step by step advances through experimental models. Contrib Nephrol 169: 247–261, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton AJ, Bingham C, McDonald TJ, Cook PR, Caswell RC, Weedon MN, et al. : The HNF4A R76W mutation causes atypical dominant Fanconi syndrome in addition to a β cell phenotype. J Med Genet 51: 165–169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchesin V, Perez-Marti A, Le Meur G, Pichler R, Grand K, Klootwijk ED, et al. : Molecular basis for autosomal-dominant renal Fanconi syndrome caused by HNF4A. Cell Rep 29: 4407–4421.e5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marable SS, Chung E, Adam M, Potter SS, Park JS: Hnf4a deletion in the mouse kidney phenocopies Fanconi renotubular syndrome. JCI Insight 3: e97497, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deacon P, Concodora CW, Chung E, Park J-S: β-catenin regulates the formation of multiple nephron segments in the mouse kidney. Sci Rep 9: 15915, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan Y, Wang Q, Ovitt CE, Jiang R: A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis 45: 618–624, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parviz F, Li J, Kaestner KH, Duncan SA: Generation of a conditionally null allele of hnf4alpha. Genesis 32: 130–133, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, et al. : Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31: 7753–7762, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. : A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 Years of image analysis. Nat Methods 9: 671–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, et al. : Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell 23: 637–651, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langmead B, Trapnell C, Pop M, Salzberg SL: Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. : Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L: Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31: 46–53, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, et al. : GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28: 495–501, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481, 1958 [Google Scholar]
- 34.Chung E, Deacon P, Marable S, Shin J, Park JS: Notch signaling promotes nephrogenesis by downregulating Six2. Development 143: 3907–3913, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surendran K, Boyle S, Barak H, Kim M, Stomberski C, McCright B, et al. : The contribution of Notch1 to nephron segmentation in the developing kidney is revealed in a sensitized Notch2 background and can be augmented by reducing Mint dosage. Dev Biol 337: 386–395, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte BA, Spicer SS: Histochemical evaluation of mouse and rat kidneys with lectin-horseradish peroxidase conjugates. Am J Anat 168: 345–362, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Hennigar RA, Schulte BA, Spicer SS: Heterogeneous distribution of glycoconjugates in human kidney tubules. Anat Rec 211: 376–390, 1985. [DOI] [PubMed] [Google Scholar]
- 38.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R: Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 27: 223–236, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Chevalier RL: The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manz F, Waldherr R, Fritz HP, Lutz P, Nützenadel W, Reitter B, et al. : Idiopathic de Toni-Debré-Fanconi syndrome with absence of proximal tubular brush border. Clin Nephrol 22: 149–157, 1984. [PubMed] [Google Scholar]
- 41.Longley JB, Fisher ER: Alkaline phosphatase and the periodic acid Schiff reaction in the proximal tubule of the vertebrate kidney; a study in segmental differentiation. Anat Rec 120: 1–21, 1954. [DOI] [PubMed] [Google Scholar]
- 42.Wessely O, Cerqueira DM, Tran U, Kumar V, Hassey JM, Romaker D: The bigger the better: Determining nephron size in kidney. Pediatr Nephrol 29: 525–530, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng K, Xie Y, Li H: Congenital nephrogenic diabetes insipidus presented with bilateral hydronephrosis and urinary infection: A case report. Medicine (Baltimore) 95: e3464, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung CC, Lin SH: Images in clinical medicine. Nonobstructive hydronephrosis with secondary polycythemia. N Engl J Med 365: e1, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Jin XD, Chen ZD, Cai SL, Chen SW: Nephrogenic diabetes insipidus with dilatation of bilateral renal pelvis, ureter and bladder. Scand J Urol Nephrol 43: 73–75, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR: Differential expression and function of cadherin-6 during renal epithelium development. Development 125: 803–812, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Walesky C, Apte U: Role of hepatocyte nuclear factor 4α (HNF4α) in cell proliferation and cancer. Gene Expr 16: 101–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruijtenberg S, van den Heuvel S: Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 15: 196–212, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachs L: Constitutive uncoupling of the controls for growth and differentiation in myeloid leukemia and the development of cancer. J Natl Cancer Inst 65: 675–679, 1980. [DOI] [PubMed] [Google Scholar]
- 50.Maione R, Amati P: Interdependence between muscle differentiation and cell-cycle control. Biochim Biophys Acta 1332: M19–M30, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Walsh K, Perlman H: Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev 7: 597–602, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Marx J: Cell biology. Cell cycle inhibitors may help brake growth as cells develop. Science 267: 963–964, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Gerdes J, Schwab U, Lemke H, Stein H: Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31: 13–20, 1983. [DOI] [PubMed] [Google Scholar]
- 54.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133: 1710–1715, 1984. [PubMed] [Google Scholar]
- 55.Alison MR: Assessing cellular proliferation: What’s worth measuring? Hum Exp Toxicol 14: 935–944, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Scholzen T, Gerdes J: The Ki-67 protein: From the known and the unknown. J Cell Physiol 182: 311–322, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, et al. : A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med 362: 1102–1109, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Klootwijk ED, Reichold M, Helip-Wooley A, Tolaymat A, Broeker C, Robinette SL, et al. : Mistargeting of peroxisomal EHHADH and inherited renal Fanconi’s syndrome. N Engl J Med 370: 129–138, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki S, Hara A, Sakaguchi M, Nangaku M, Inoue Y: Hepatocyte nuclear factor 4α regulates megalin expression in proximal tubular cells. Biochem Biophys Rep 17: 87–92, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ketharnathan S, Leask M, Boocock J, Phipps-Green AJ, Antony J, O’Sullivan JM, et al. : A non-coding genetic variant maximally associated with serum urate levels is functionally linked to HNF4A-dependent PDZK1 expression. Hum Mol Genet 27: 3964–3973, 2018. [DOI] [PubMed] [Google Scholar]
- 61.Kajiwara M, Terada T, Asaka J, Aoki M, Katsura T, Ikai I, et al. : Regulation of basal core promoter activity of human organic cation transporter 1 (OCT1/SLC22A1). Am J Physiol Gastrointest Liver Physiol 295: G1211–G1216, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto T, Shimano H, Nakagawa Y, Ide T, Yahagi N, Matsuzaka T, et al. : SREBP-1 interacts with hepatocyte nuclear factor-4 alpha and interferes with PGC-1 recruitment to suppress hepatic gluconeogenic genes. J Biol Chem 279: 12027–12035, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Balaban RS, Mandel LJ: Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol 254: F407–F416, 1988. [DOI] [PubMed] [Google Scholar]
- 64.Elhamri M, Martin M, Ferrier B, Baverel G: Substrate uptake and utilization by the kidney of fed and starved rats in vivo. Ren Physiol Biochem 16: 311–324, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Weidemann MJ, Krebs HA: The fuel of respiration of rat kidney cortex. Biochem J 112: 149–166, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunskill EW, Park JS, Chung E, Chen F, Magella B, Potter SS: Single cell dissection of early kidney development: Multilineage priming. Development 141: 3093–3101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martovetsky G, Tee JB, Nigam SK: Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol Pharmacol 84: 808–823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermsen R, Tans S, ten Wolde PR: Transcriptional regulation by competing transcription factor modules. PLoS Comput Biol 2: e164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, et al. : The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet 3: 1922–1938, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Cheng CN, Verdun VA, Wingert RA: Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and notch signaling. Dev Biol 386: 111–122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casemayou A, Fournel A, Bagattin A, Schanstra J, Belliere J, Decramer S, et al. : Hepatocyte nuclear factor-1β controls mitochondrial respiration in renal tubular cells. J Am Soc Nephrol 28: 3205–3217, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrè S, Igarashi P: New insights into the role of HNF-1β in kidney (patho)physiology. Pediatr Nephrol 34: 1325–1335, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Izzedine H, Launay-Vacher V, Baumelou A, Deray G: Renal effects of PPARalpha-agonists. Minerva Urol Nefrol 56: 339–342, 2004. [PubMed] [Google Scholar]
- 74.Lazzaro D, De Simone V, De Magistris L, Lehtonen E, Cortese R: LFB1 and LFB3 homeoproteins are sequentially expressed during kidney development. Development 114: 469–479, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Lau HH, Ng NHJ, Loo LSW, Jasmen JB, Teo AKK: The molecular functions of hepatocyte nuclear factors—in and beyond the liver. J Hepatol 68: 1033–1048, 2018. [DOI] [PubMed] [Google Scholar]
- 76.Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, et al. : Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 84: 575–585, 1996. [DOI] [PubMed] [Google Scholar]
- 77.Pontoglio M, Prié D, Cheret C, Doyen A, Leroy C, Froguel P, et al. : HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep 1: 359–365, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heliot C, Desgrange A, Buisson I, Prunskaite-Hyyryläinen R, Shan J, Vainio S, et al. : HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development 140: 873–885, 2013. [DOI] [PubMed] [Google Scholar]
- 79.Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler MC, et al. : Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140: 886–896, 2013. [DOI] [PubMed] [Google Scholar]
- 80.Hatzis P, Talianidis I: Regulatory mechanisms controlling human hepatocyte nuclear factor 4alpha gene expression. Mol Cell Biol 21: 7320–7330, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaminski MM, Tosic J, Kresbach C, Engel H, Klockenbusch J, Müller AL, et al. : Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol 18: 1269–1280, 2016. [DOI] [PubMed] [Google Scholar]
- 82.Little MH, Takasato M: Generating a self-organizing kidney from pluripotent cells. Curr Opin Organ Transplant 20: 178–186, 2015. [DOI] [PubMed] [Google Scholar]
- 83.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. : Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 536: 238, 2016. [DOI] [PubMed] [Google Scholar]
- 85.Rota C, Morigi M, Imberti B: Stem cell therapies in kidney diseases: Progress and challenges. Int J Mol Sci 20: 2790, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishinakamura R: Human kidney organoids: Progress and remaining challenges. Nat Rev Nephrol 15: 613–624, 2019. [DOI] [PubMed] [Google Scholar]
- 87.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD: Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan X, Ta TC, Lin M, Evans JR, Dong Y, Bolotin E, et al. : Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS One 4: e5609, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.