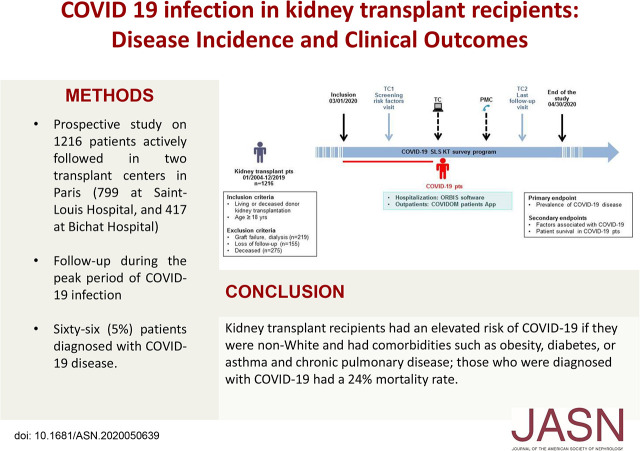
Significance Statement
Although studies have found coronavirus disease 2019 (COVID-19) to be associated with high morbidity and mortality among kidney transplant recipients, risk factors for COVID-19 among kidney transplant patients remain poorly defined. In this prospective cohort study in France, the authors enrolled 1216 kidney transplant patients, 66 (5%) of whom were diagnosed with COVID-19. The mortality rate associated with COVID-19 for the overall study population was 1% and 24% among COVID-19–positive patients. Factors that were independently associated with COVID-19 included non-White race and comorbidities, including obesity, diabetes, and asthma and chronic pulmonary disease. In the context of COVID-19, it is imperative that policy makers integrate information about risk factors to help clinicians balance benefits and risks and better advise patients about potential risks.
Keywords: COVID-19, coronavirus, pandemic, kidney transplantation, SARS-CoV-2
Visual Abstract
Abstract
Background
COVID-19 has been associated with high morbidity and mortality in kidney transplant recipients. However, risk factors for COVID-19 disease in patients with kidney transplants remain poorly defined.
Methods
We enrolled patients who underwent kidney transplantation and were actively followed up in two hospitals in Paris on March 1st, 2020. Patients were screened for baseline and transplant characteristics, functional parameters, comorbidities, and immunosuppressive therapies. COVID-19 disease was assessed. Patients were followed up during the pandemic until April 30th, 2020 by the COVID-19 SLS KT survey program, including teleconsulting, at-home monitoring for patients with COVID-19, and a dedicated phone hotline platform.
Results
Among 1216 patients with kidney transplants enrolled, 66 (5%) patients were identified with COVID-19 disease, which is higher than the incidence observed in the general population in France (0.3%). Their mean age was 56.4±12.5 years, and 37 (56%) patients were men. The following factors were independently associated with COVID-19 disease: non-White ethnicity (adjusted odds ratio [OR], 2.17; 95% confidence interval [95% CI], 1.23 to 3.78; P=0.007), obesity (OR, 2.19; 95% CI, 1.19 to 4.05; P=0.01), asthma and chronic pulmonary disease (OR, 3.09; 95% CI, 1.49 to 6.41; P=0.002), and diabetes (OR, 3.33; 95% CI, 1.92 to 5.77; P<0.001). The mortality rate related to COVID-19 disease was 1% in the overall study population and 24% in COVID-19–positive patients.
Conclusions
Patients with kidney transplants display a high risk of mortality. Non-White ethnicity and comorbidities such as obesity, diabetes, asthma, and chronic pulmonary disease were associated with higher risk of developing COVID-19 disease. It is imperative that policy makers urgently ensure the integration of such risk factors on response operations against COVID-19.
Since its onset in Wuhan, China,1 the epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide, and on March 11th, 2020, it was declared a pandemic by the World Health Organization (WHO).2 As the coronavirus disease 2019 (COVID-19) pandemic progresses, evidence of high morbidity is emerging daily, reflected by elevated hospitalization rates in intensive care units (ICUs) resulting in excessive mortality rates. As of May 1st, 2020, >247,000 people have died of COVID-19 worldwide, with over 85% of the deaths in Europe and the United States.3
Patients with kidney transplants seem to be at particularly high risk for severe COVID-19 disease. Two case series of patients with kidney transplants were reported in New York: 36 patients by Montfiore Medical Center4 and 15 patients by the Columbia University.5 Three additional series came from Europe: seven patients from St. George’s University Hospital National Health Service Foundation Trust in London, United Kindom6; 20 patients originating from Spedali Civili University Hospital in Brescia, Italy7; and eight patients from Instituto de Investigación Sanitaria Hospital “12 de Octubre” (imas12), Madrid, Spain.8 These reports have shown an unusually high mortality rate among kidney transplant recipients with COVID-19, fluctuating between 20% and 28% as compared with the report of 1%–5% mortality among patients with COVID-19 in the general population.
The general consensus regarding viral susceptibility in transplant recipients is that immune-compromised individuals are at greater risk of severe infection due to their impaired immune responses, particularly in the presence of concurrent comorbidities, which are widely common in patients with CKD. Yet, although early Chinese and Italian reports have associated age, men, smoking, and cardiometabolic comorbidities with adverse outcomes in the general population,9 specific investigations into the prevalence and the risk factors related to COVID-19 disease in the kidney transplant population are lacking. Shedding light on these factors has been identified as a priority in public health research and represents a potential response to pandemic control.10
The virus was confirmed to have reached France on January 24th, 2020, with the first reported death confirmed on February 14th. The number of patients has, since then, exponentially risen all over the country, and by May 1st, 2020, 24,594 deaths related to COVID-19 infection were recorded in France.11 As Paris emerged as an epicenter, the epidemic brought new major challenges into the transplantation activity, including the halt of the living and deceased donors transplant programs ordered by the National Agency of Organ Repartition (Agence de Biomédecine) and the important reduction of the post-transplant outpatient activity. Under the aegis of the recommendations enacted by the French government, medical and nonmedical staff combined their efforts to face the outbreak; most notably, the COVID-19 SLS Kidney Transplant survey program was built to minimize the risk of intrahospital disease transmission using teleconsultation and to maximize the identification and follow-up of COVID-19–positive patients with kidney transplants.12
In this exceptional setting, between March 1st and April 30th, 2020, concurrently with the ascending and peak stages of the epidemic, we undertook a prospective cohort study in Saint-Louis and Bichat hospitals in Paris (Public Hospitals of Paris Organization: Assistance Publique des Hôpitaux de Paris [APHP]), capitalizing on the abovementioned survey program, with the aim to evaluate the prevalence of COVID-19 disease in a nonselected population of 1216 kidney recipients. We also aimed to identify the demographics, comorbidities, and clinical and functional parameters associated with COVID-19 disease in this specific population.
Moreover and most importantly, by detecting modifiable and nonmodifiable determinants associated with the susceptibility and severity of COVID-19 infection, our study seeks to provide imperative information for the reshaping of renal transplantation in this new context, particularly in a crucial period when some countries—France among them—are questioning the reorganization of renal transplantation after the peak.
Methods
Study Population
This prospective study considered all consecutive patients who underwent kidney transplantation at Saint-Louis Hospital (Paris, France) between January 2004 and December 2019 (n=1865).
All patients with active follow-up in the two referral transplant centers (Saint-Louis and Bichat hospitals) located in the north of Paris, France (as shown in Supplemental Figure 1) on March 1st, 2020 were included in the COVID-19 SLS KT survey program. We excluded patients (1) with graft failure and return to dialysis (n=219), (2) lost to follow-up (n=155), and (3) who were deceased (n=275) before March 1st.
Study Design and Clinical Data
The study was performed and patients were included between March 1st and April 30th, 2020.
The COVID-19 SLS KT program is illustrated in Figure 1 and was composed of three units: (1) a unit dedicated to check, through a phone teleconsultation (TC), whether patients presented any symptoms and whether they had been diagnosed with COVID-19 disease. Specifically, we performed a first TC between March 1st and March 15th, where patients were contacted, following an alphabetical order, for inclusion in the study and screening of risk factors (TC1, n=1216) as well as to inform them about the survey program. Furthermore, we performed clinical indication TCs between March 16th and April 14th to assess kidney graft survival according to clinical indication (iTC, n=646). Finally, we performed a last follow-up visit (TC2, n=1172), between April 15th and April 30th, where patients were contacted again following an alphabetical order to detect subjects with a previously confirmed COVID-19 disease and possible actual symptoms, addressing them to ER departments to get tested. Additionally, a medical hotline platform was available, throughout the duration of the study, to answer to any request of the patients concerning the epidemics, and to direct every suspect case to nasal swab performing. Other units included (2) a web platform unit dedicated to educate and inform patients with kidney transplants (www.docmadi.net) and (3) a COVID-19 unit dedicated to register and follow COVID-19–positive patients.
Figure 1.
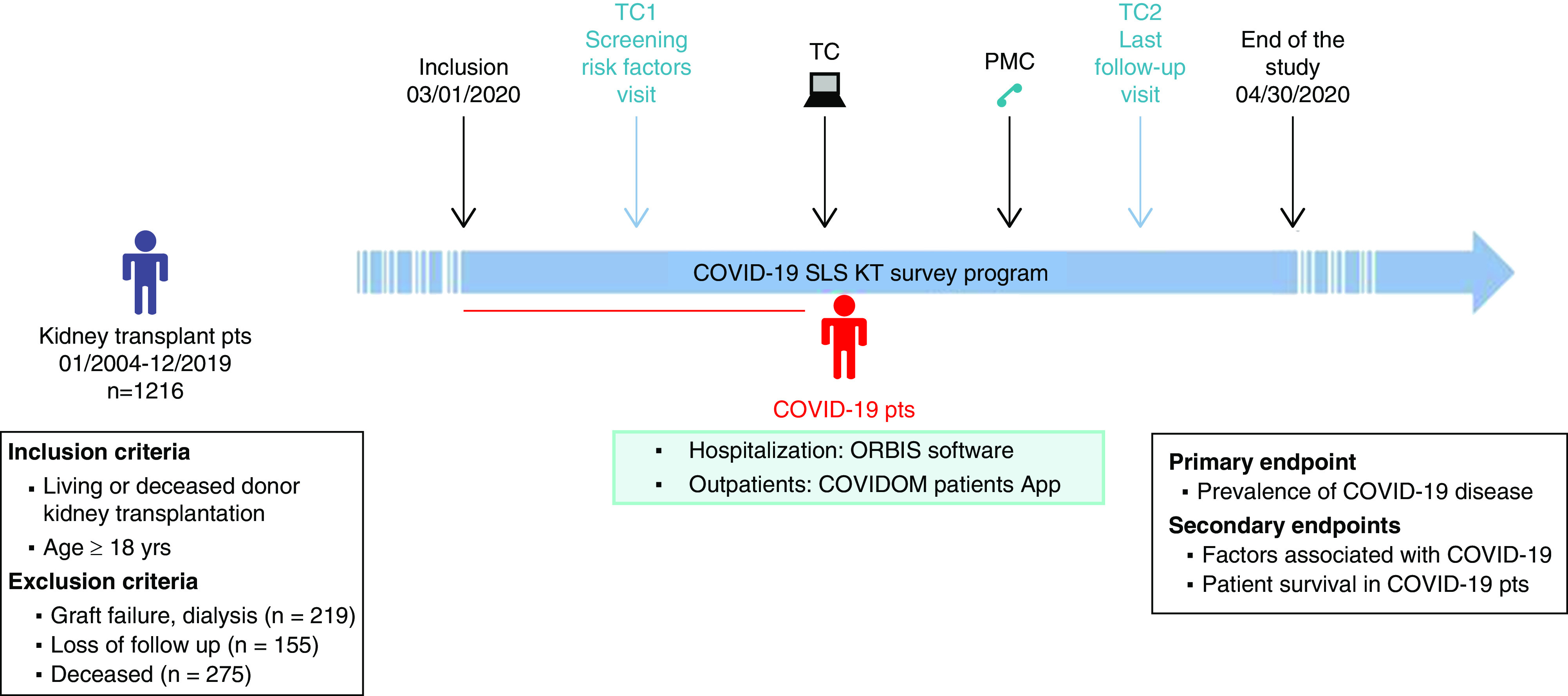
The figure illustates the design of this observational prospective cohort study performed in 1216 kidney transplant recipients with active follow-up in two referral transplant centers. COVIDOM, commercial name of patients' application; ORBIS, commercial name of medical software; PMC, medical hotline platform; pts, patients.
TC1 systematically screened patients for (1) potential clinical and biologic parameters associated with COVID-19 disease: age, sex, ethnicity, time after transplantation, body mass index, tobacco consumption, and existing comorbidities (asthma and chronic respiratory diseases, diabetes, hypertension, and cardiovascular diseases); (2) post-transplant rejection episodes and current graft function (serum creatinine); and (3) current immunosuppressive treatment.
For patients with COVID-19 disease, data collection included initial clinical manifestations, laboratory tests, computed tomographic scans of the chest, concomitant diagnoses during the hospital course, patient medications (including antiviral therapy, immunosuppression modification, oxygen support, invasive mechanical ventilation, and RRT), and clinical outcomes (discharge, readmission, and mortality).
Clinical data were registered in the prospective database: Données Informatiques Validées en Transplantation (official website: https://www.divat.fr). These data are computerized in real time as well as at each transplant anniversary and are submitted for an annual audit. Anonymized data from the registry were prospectively entered at specific time points for each patient (at the time of transplantation and 6 months and 1 year after transplantation), and they were updated annually thereafter, at the time of the TC1, and at the time of COVID-19 detection. Data were retrieved from the database on May 1, 2020. The institutional review board approved this study using data collected for routine clinical practice in the prospective cohort of kidney recipients from Saint-Louis and Bichat hospitals (French data protection authority registration no. 891735).
Principals of Patient’s Management
In the Study Population
In response to the COVID-19 pandemic, national and local policies and strategies have been implemented during the study period, relevant for our population of kidney transplant recipients.
At a national level, the government made general recommendations of social distancing and proclaimed a national health emergency. Paris and Ile-de-France were locked down on March 16th, 2020. The national agency (Agence de Biomédecine) ordered the suspension of the kidney transplant program. The Public Hospitals of Paris Organization (APHP) made major reorganization steps in order to increase hospital capacities for the ICU and to create COVID-19 units and areas.
For the monitoring of patients with kidney transplants in Saint-Louis and Bichat hospitals, we applied the strategy described in the Study Design and Clinical Data section (COVID-19 SLS KT program). During each of the different visits (TC1, iTCs, and TC2), patients presenting symptoms compatible with COVID-19 disease were addressed to the ER department in order to be tested.
In COVID-19–Positive Patients with Kidney Transplants
We recorded patients with COVID-19 disease represented by kidney transplant recipients with clinical manifestations and positive SARS-CoV-2 PCR. Screening for COVID-19 was performed only for symptomatic patients, with clinical manifestations such as fever or respiratory symptoms. According to the WHO guidance,13 the suspected patients diagnosed as COVID-19 cases were only those with positive result of real-time quantitative RT-PCR for SARS-CoV-2 of nasal or pharyngeal swabs. In the case of a first negative quantitative RT-PCR, if the patient had a clinical presentation or radiologic images compatible with COVID-19 infection, a second nasopharyngeal swab was performed.
Patients diagnosed with COVID-19 disease were registered and monitored by the COVID-19 SLS KT platform. Depending on the severity of the clinical presentation, patients were either hospitalized in COVID-19 units in Saint-Louis, Bichat, or the surrounding hospitals or were managed as outpatients. Clinical data on the disease evolution were collected from ORBIS software (a software deployed and routinely used in 39 hospitals in the Ile-de-France region) for hospitalized patients and from the COVIDOM patients’ application (www.covidom.fr) developed for the monitoring of outpatients.
Main Outcomes and Measures
The primary outcome was to measure the prevalence of COVID-19 disease among patients with kidney transplants. The secondary outcomes were to analyze (1) the factors associated with COVID-19 symptomatic infection and (2) the patient survival during the course of COVID-19 disease. These outcomes were prospectively assessed until May 1st, 2020.
Statistical Analyses
Mean ± SD described continuous variables, and categorical variables are expressed as number of patients (percentage). Percentages of available data for the overall population are on the basis of the total number of patients included in the study. Sample size varied because of missing data (summarized in Tables 1–3). We compared means and proportions using t test and the chi-squared test (or Wilcoxon and Fisher exact test if appropriate). All statistical tests were two tailed, and statistical significance was defined as P=0.05.
Table 1.
Baseline characteristics of the study population according to the presence of COVID-19 disease after kidney transplantation
| Demographic Characteristics | All Patients, n=1216 | COVID-19–Positive Patients, n=66 | COVID-19–Negative Patients, n=1150 | P Valuea |
|---|---|---|---|---|
| Age, yr | 54.1±13.4 | 56.4±12.5 | 54.0±13.4 | 0.14 |
| Men, no. (%) | 777 (64) | 37 (56) | 740 (64) | 0.17 |
| Non-White, no. (%) | 222 (24) | 24 (36) | 198 (17) | <0.001 |
| Blood group, no. (%) | 0.90 | |||
| A | 463 (38) | 23 (35) | 440 (38) | |
| B | 194 (16) | 12 (18) | 182 (16) | |
| O | 489 (40) | 28 (42) | 461 (40) | |
| AB | 70 (6) | 3 (5) | 67 (6) | |
| Cause of ESKD, no. (%) | 0.13 | |||
| Glomerulopathies | 254/1206 (21) | 7/66 (11) | 247/1140 (22) | |
| Diabetes | 195/1206 (16) | 12/66 (18) | 183/1140 (16) | |
| Vascular | 209/1206 (17) | 16/66 (24) | 196/1140 (17) | |
| Other | 548/1206 (46) | 31/66 (47) | 514/1140 (45) | |
| Deceased donor, no. (%) | 1053 (87) | 64 (97) | 989 (86) | <0.001 |
| Prior kidney transplant, no. (%) | 132 (11) | 8 (12) | 124 (11) | 0.69 |
| Time on dialysis prior to transplantation, mob | 49.4±42 | 57.8±46.2 | 48.8±41.8 | 0.09 |
| Multiorgan recipients, no. (%) | 96 (8) | 4 (6) | 92 (8) | 0.57 |
Plus-minus values are means ± SD.
P values are for the comparisons between COVID-19–positive and COVID-19–negative patients.
Time on dialysis prior to transplantation was available in 1078 patients.
Table 3.
Factors assessed at time of post-transplant risk evaluation associated with COVID-19 disease in patients with kidney transplants: univariate and multivariate analysis
| Factors Associated with COVID-19 Disease in Kidney Transplant Patients | No. of Patients | No. of COVID-19–Positive Patients | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Factors associated with COVID-19 disease: univariate analysis | ||||
| Recipient characteristics | ||||
| Age, per 1-yr increment | 1216 | 66 | 1.01 (1.00 to 1.04) | 0.15 |
| Sex | ||||
| Women | 439 | 29 | 1 | |
| Men | 777 | 37 | 0.71 (0.43 to 1.17) | 0.17 |
| Ethnicity | ||||
| White | 994 | 42 | 1 | |
| Non-White | 222 | 24 | 2.75 (1.63 to 4.64) | <0.001 |
| Transplant characteristics | ||||
| Donor type | ||||
| Deceased donor | 1053 | 64 | 1 | |
| Living donor | 163 | 2 | 0.19 (0.05 to 0.79) | <0.001 |
| Prior kidney transplant | ||||
| No | 1084 | 58 | 1 | |
| Yes | 132 | 8 | 0.88 (0.41 to 1.88) | 0.69 |
| Time on dialysis before graft per 1-mo increment | 1078 | 66 | 1.00 (1.00 to 1.01) | 0.09 |
| Multiorgan recipient | ||||
| No | 1120 | 62 | 1 | |
| Yes | 96 | 4 | 1.35 (0.48 to 3.79) | 0.57 |
| Time of risk evaluation | ||||
| Time from transplant to evaluation per 1-mo increment | 1216 | 66 | 0.99 (0.99 to 1.00) | 0.02 |
| Functional allograft parameters | ||||
| Recent episode of rejection | ||||
| No | 971 | 54 | 1 | |
| Yes | 245 | 12 | 0.87 (0.46 to 1.66) | 0.68 |
| Serum creatinine per μmol/L increment | 1185 | 66 | 1.00 (1.00 to 1.01) | 0.11 |
| Comorbidities | ||||
| Smoker | ||||
| No | 1060 | 60 | 1 | |
| Yes | 156 | 6 | 0.67 (0.28 to 1.57) | 0.35 |
| Obesity (BMI≥30) | ||||
| No | 1043 | 46 | 1 | |
| Yes | 151 | 20 | 3.31 (1.90 to 5.77) | <0.001 |
| Diabetes | ||||
| No | 926 | 35 | 1 | |
| Yes | 290 | 31 | 3.05 (1.84 to 5.04) | <0.001 |
| Hypertension | ||||
| No | 287 | 8 | 1 | |
| Yes | 929 | 58 | 2.32 (1.10 to 4.92) | 0.02 |
| Asthma and chronic pulmonary disease | ||||
| No | 1138 | 53 | 1 | |
| Yes | 78 | 13 | 4.09 (2.12 to 7.89) | <0.001 |
| Cardiovascular disease | ||||
| No | 1146 | 65 | 1 | |
| Yes | 70 | 1 | 0.24 (0.03 to 1.76) | 0.17 |
| Maintenance immunosuppressive therapies | ||||
| Prednisone | ||||
| No | 368 | 11 | 1 | |
| Yes | 829 | 55 | 2.31 (1.19 to 4.46) | 0.01 |
| Tacrolimus | ||||
| No | 510 | 29 | 1 | |
| Yes | 691 | 37 | 0.94 (0.57 to 1.55) | 0.80 |
| Cyclosporin | ||||
| No | 841 | 46 | 1 | |
| Yes | 360 | 20 | 1.02 (0.59 to 1.74) | 0.95 |
| Mycophenolate mofetil or mycophenolic acid | ||||
| No | 201 | 13 | 1 | |
| Yes | 996 | 53 | 0.81 (0.43 to 1.52) | 0.51 |
| Belatacept | ||||
| No (CNI regimen)a | 1051 | 57 | 0.61 (0.25 to 1.47) | 0.28 |
| Yes | 70 | 6 | 1 | |
| Azathioprine | ||||
| No | 1047 | 58 | 1 | |
| Yes | 150 | 8 | 0.96 (0.45 to 2.05) | 0.92 |
| Factors associated with COVID-19 disease: multivariate model | ||||
| Prednisoneb | ||||
| No | 293 | 11 | 1 | |
| Yes | 741 | 55 | 1.67 (0.87 to 3.47) | 0.15 |
| Ethnicity | ||||
| White | 829 | 42 | 1 | |
| Non-White | 205 | 24 | 2.17 (1.23 to 3.78) | 0.007 |
| Cardiovascular disease | ||||
| No | 970 | 65 | 1 | |
| Yes | 64 | 1 | 0.20 (0.03 to 1.50) | 0.12 |
| Obesity (BMI≥30) | ||||
| No | 900 | 46 | 1 | |
| Yes | 134 | 20 | 2.19 (1.19 to 4.05) | 0.01 |
| Asthma and chronic pulmonary disease | ||||
| No | 964 | 53 | 1 | |
| Yes | 70 | 13 | 3.09 (1.49 to 6.41) | 0.002 |
| Diabetes | ||||
| No | 785 | 35 | 1 | |
| Yes | 249 | 31 | 3.33 (1.92 to 5.77) | <0.001 |
| Time on dialysis before graft per 1-mo increment | 1034 | 66 | 1.01 (0.99 to 1.01) | 0.07 |
OR, odds ratio; BMI, body mass index; CNI, calcineurin inhibitor (cyclosporin or tacrolimus).
Three patients did not take either CNI or belatacept.
The multivariable analysis was restricted to 1034 patients without any missing data for each of the variables included in the final model by the stepwise backward elimination.
The association of potential factors with COVID-19 disease was assessed in separate simple logistic regression analysis. The factors identified in the univariate analysis (the P value threshold of 0.20) were therefore included in a final multivariable model and then restricted via stepwise backward elimination. Analyses were performed using R version 3.1.3 (R Development Core Team, Vienna, Austria).
Results
Baseline Characteristics of the Kidney Transplant Recipients
In total, 1216 patients who underwent kidney transplantation and were actively followed up in two transplant centers in Paris (799 at Saint-Louis Hospital and 417 at Bichat Hospital) were included in the study. Sixty-six (5%) patients were diagnosed with COVID-19 disease between March 1st and April 30th, 2020. The characteristics of the study population at the time of transplantation, according to the absence or presence of COVID-19 disease, are listed in Table 1.
Thirty-seven (56%) were men, with a similar sex ratio similar with that of patients without COVID-19 disease (740 of 1150; 64%). Their mean age was 56.4±12.5 years compared with 54±13.4 years in patients without COVID-19 disease. Their time on dialysis before transplantation was 57.8±46.2 months compared with 48.8±41.8 in patients without COVID-19. Eight (12%) COVID-19–positive patients had a prior kidney transplant, and four (6%) received a combined kidney graft with liver or pancreas transplant, which were not significantly different from COVID-19–negative patients (11% and 8%, respectively). COVID-19–positive patients were more often recipients from deceased donors (64 of 66 patients versus 989 of 1150; P<0.001) and were more frequently non-Whites as compared with patients who were COVID-19 negative (24 of 66 patients versus 198 of 1150; P<0.001).
Clinical Manifestations and Outcome of Kidney Recipients with COVID-19 Disease
Clinical manifestations and outcomes of COVID-19–positive patients are described herein, and their main characteristics are summarized in Table 2.
Table 2.
Clinical features and outcomes of kidney transplant recipients with COVID-19
| Clinical Features and Outcomes | All Patients, n=66 | Invasive Mechanical Ventilation, n=15 | No Invasive Mechanical Ventilation, n=51 |
|---|---|---|---|
| Features at diagnosis | |||
| Clinical presentation, no. (%) | |||
| Fever | 51 (77) | 12 (80) | 39 (76) |
| Cough | 38 (58) | 11 (73) | 27 (53) |
| Dyspnea (exertional or rest) | 26 (39) | 12 (80) | 14 (27) |
| Diarrhea | 17 (26) | 2 (13) | 15 (29) |
| Anosmia/ageusia | 7 (11) | 0 (0) | 7 (14) |
| Laboratory testsa | |||
| White blood cell count, 1000/µl, median (range)a | 5.9 (1.6–14.2) | 7.4 (2.6–14.2) | 5.3 (1.6–13.2) |
| Absolute lymphocyte count, /µl | 528 (80–1300) | 473 (80–1180) | 548 (169–1300) |
| C-reactive protein, mg/L | 115 (0–335) | 159 (18–316) | 96 (0–335) |
| Chest x-ray/pulmonary CT scan,a no. (%) | |||
| Multifocal/bilateral patchy opacities | 38/56 (68) | 12/13 (92) | 26/43 (60) |
| Lobar opacities | 3/56 (5) | 1/13 (8) | 2/43 (5) |
| No acute findings | 15/56 (27) | 0/13 (0) | 15/43 (35) |
| Immunosuppressive treatment, no. (%) | |||
| MMF/MPA/AZA | 61 (92) | 13 (87) | 48 (94) |
| CNI | 57 (86) | 14 (93) | 43 (84) |
| Belatacept | 6 (9) | 1 (7) | 5 (10) |
| Steroids | 55 (83) | 13 (87) | 42 (82) |
| Treatment and outcome | |||
| Change in immunosuppression, no. (%) | |||
| Discontinued only MMF/MPA/AZA | 38/61 (62) | 13 (100) | 25/48 (52) |
| Discontinued only CNI | 2/57 (4) | 2/14 (14) | 0 |
| Belatacept infusion postpone | 1/6 (17) | 0 | 1/5 (20) |
| No change | 24 (36) | 2 (13) | 22 (43) |
| Discontinued all immunosuppression | 1 (2) | 1 (7) | 0 |
| Anti–COVID-19 therapies, no. (%) | |||
| Hydroxychloroquine | 7 (11) | 4 (27) | 3 (6) |
| Tocilizumab | 1 (2) | 0 (l0) | 1 (2) |
| Eculizumab | 2 (3) | 0 (0) | 2 (4) |
| Outcomes, no. (%) | |||
| AKI | 28 (42) | 13 (87) | 15 (29) |
| RRT required | 7 (11) | 7 (47) | 0 (0) |
| Died | 16 (24) | 11 (73) | 5 (10) |
| Recovery | 50 (76) | 4 (27) | 46 (90) |
Data are displayed as n (%) or median (range). CT, computed tomography; MMF, mycophenolate mofetil; MPA, mycophenolic acid; AZA, azathioprine; CNI, calcineurin inhibitor.
Data recorded in the 60 hospitalized patients, with missing data for two patients in the invasive mechanical ventilation group and two patients in the noninvasive mechanical ventilation group, for patients admitted to other hospitals.
No cases were detected by TC1; a total of 35 patients were diagnosed after being referred by the hotline (the first patient contacted the hotline on March 13th), with fever being the most frequent complaint reported (33 of 35 patients); 12 patients were diagnosed by the following iTCs and five were diagnosed by the last phone call (TC2). The remaining 14 patients were either addressed by other colleagues or directly diagnosed in the ER department.
The most common presenting symptom was fever, which was reported in 51 of 66 (77%) patients, followed by cough, which was present in 38 (58%) patients. Depending on the severity of the initial presentation, patients were either hospitalized or managed as outpatients. Sixty of 66 (91%) patients needed hospitalization, whereas six (9%) patients were stable in the weeks following COVID-19 diagnosis and were managed as outpatients (biologic and pulmonary imaging was not performed in outpatients unless clinical deterioration).
Fifteen of 66 (22%) COVID-19–positive patients needed intubation and ventilation and were transferred to ICU. Dyspnea was the main clinical presentation of patients admitted to the ICU, and it was observed in 12 of 15 (80%) patients in the invasive mechanical group compared with 27% in the no invasive mechanical group. Anosmia and ageusia were restricted to the less severe group. The vast majority of patients who required invasive mechanical ventilation displayed bilateral/multifocal opacities on chest x-ray or CT scan. Only two patients in the noninvasive mechanical ventilation group received other forms of ventilation support (namely C-PAP).
Twenty-nine of 66 patients underwent a reduction in the immunosuppression regimen. Immunosuppression reduction was more frequently done in the invasive mechanical ventilation group (87%) than in the no invasive mechanical ventilation group (57%). The primary change in immunosuppression regimen in the majority of patients was the complete cessation of antimetabolites (mycophenolate mofetil, mycophenolic acid, or azathioprine [38 of 61; 62%]), especially in the invasive mechanical group (antimetabolites were stopped in all patients whose initial treatment included antimetabolites), while continuing tacrolimus (with a goal trough of 4–6 ng/ml) or cyclosporin (with a goal trough of 400–600 ng/ml) and the baseline prednisone in those individuals who were on maintenance prednisone. In two patients only, calcineurin inhibitors were interrupted. Six patients were also on maintenance belatacept, and in one patient, the perfusion of belatacept was delayed. Only two patients had all of their treatment discontinued, as both were severe patients. In addition to the reduction of immunosuppression, seven (11%) patients received hydroxychloroquine. A single dose of tocilizumab was given to one patient, and two patients received eculizumab.
Mortality rate related to COVID-19 disease in our kidney transplant population was of 1%. Sixteen of the 66 (24%) patients who were COVID-19 positive died during the pandemic period. The characteristics of deceased patients are presented in Supplemental Table 1. Mortality was higher in the invasive mechanical ventilation group: 11 of 15 (73%) in the invasive mechanical ventilation group and five of 51 (10%) in the no invasive ventilation group. At the end of follow-up, 50 (76%) patients had recovered.
Twenty-eight (42%) patients with COVID-19 developed AKI: ten patients (15%) with Kidney Disease Improving Global Outcomes (KDIGO) stage 1, five patients with KDIGO stage 2 (8%), and 13 patients with KDIGO stage 3 (20%), among whom seven patients required RRT (all of them where in the invasive mechanical ventilation group). Apart from the deceased patients, all of the others regained their baseline renal function before being discharged from the hospital.
Factors Assessed at Time of Post-Transplant Risk Evaluation Associated with COVID-19 Disease in Patients with Kidney Transplants
We first investigated the parameters measured at the time of post-transplant risk evaluation (TC1) that were associated with COVID-19 disease in univariable analysis (Table 3, univariate analysis).
The following factors identified in this analysis were thereafter tested in a final multivariable model: patient age, sex, ethnicity, donor type, time on dialysis before graft, time from transplant to evaluation, serum creatinine, obesity, diabetes, hypertension, asthma and chronic pulmonary disease, cardiovascular disease, and prednisone treatment.
The factors independently associated with COVID-19 disease were the following: non-White ethnicity (adjusted odds ratio, 2.17; 95% confidence interval [95% CI], 1.23 to 3.78; P=0.007), obesity (adjusted odds ratio, 2.19; 95% CI, 1.19 to 4.05; P=0.01), asthma and chronic pulmonary disease (adjusted odds ratio, 3.09; 95% CI, 1.49 to 6.41; P=0.002), and diabetes (adjusted odds ratio, 3.33; 95% CI, 1.92 to 5.77; P<0.001) (Table 3, multivariate analysis).
Discussion
This prospective study provides a comprehensive insight into COVID-19 disease in a large and nonselected population of immunocompromised patients. With a total number of 1216 kidney transplant recipients, we were able to estimate the clinical effect of SARS-CoV-2 over 8 weeks, which corresponded to the ascending and peak phases of the epidemic.
In our study, 5% of patients developed COVID-19 disease, and their mortality was of 1%. These two parameters do not merely capture the susceptibility and the fragility of our population to viral infection but also, reflect the capacity of medical and nonmedical actions, deployed at national and local levels, to efficiently combat this pandemic. Taking as a reference the end date of our study (April 30th, 2020), the cumulated number of patients with COVID-19 (defined according to the positivity of nasal swab) in Ile-de-France region (which includes the proper city of Paris and its conglomerations) was 40,239 (including 10,901 effective hospitalizations, 17,570 recovering, and 6008 deaths).14 Hence, a rough estimation of COVID-19 prevalence in Ile de France (according to a total population of 12,253,547 in 2017)3 is about 0.3%, which is far lower than the roughly 5% prevalence we found in our selected transplanted population.
The disease prevalence and mortality related to COVID-19 infection observed in our study are higher than those reported even in the general population in France15 and in other countries.3,9,16 Regarding specifically the mortality, it remains elevated (24%) in patients with COVID-19 in our study but comparable with those reported in the United States, the United Kingdom, and Italy groups, supporting the idea that kidney transplantation recipients should be considered a population at risk.4–6
Given the previous pandemic experience, it is imperative that policy makers urgently ensure the integration of risk factors in the SARS-CoV-2 fight measures.17 The identification of modifiable and nonmodifiable factors related to COVID-19 infection thus becomes pivotal information for the remold of health systems in the following weeks and months. Pragmatically, for the transplantation domain, the main call is how to organize the follow-up of patients with transplants during the period of outbreak. In France, as well as in other countries, we need to timely inform decision, as we face the crossroad between countries that have overcome the peak of the epidemic and those that are entering it.
Our study demonstrates the greater role of two nonmodifiable elements in the SARS-CoV-2–related disease in patients with kidney transplants: comorbidities and ethnic groups.
As in the general population,16 our study identifies obesity, diabetes, and asthma/chronic pulmonary diseases as factors independently associated with COVID-19 disease in patients with kidney transplants. Interestingly, in comparison with other studies,18 no specific role was found for age, sex, ABO blood group, and tobacco consumption.
On the other hand, the epidemics have unraveled another crucial factor: ethnic groups. The fact that infectious diseases are more frequently found in under-represented minorities has already been pointed out both in previous19 and in the actual pandemics.20 In New York City, a disproportionate burden has emerged especially within Black and Hispanic communities, which have accounted for 28% and 34% of deaths (population representation: 22% and 29%, respectively).20 The Johns Hopkins University and the American Community Survey indicate that to date, of 131 predominantly Black counties in the United States, the infection rate is 137.5 of 100,000, and the death rate is 6.3 of 100,000.21
With regard to Paris itself, a higher mortality (conceivably related to COVID-19) was reported, during the peak of the epidemic, in the Seine-Saint-Denis department,22 situated in the northern outskirts of Paris and known for its higher immigration rates (26% of total population according to the Institut national de la statistique et des études économiques, Paris Census 2015).23 The causes of this reported high mortality have been the objective of a preliminary analysis carried out by Observatoire régional de la Santé, Ile de France, published online in April 2020.24 This study pointed out the possible role of three different factors: those related to the urban tissue, notably largest families, homes with lower surface, higher density urban tissue; those related to employment, notably the higher presence of “key workers” who did not halt their activities during the quarantine; and those related to health system: the higher prevalence of comorbidities and the concurrent deficiency of health structures.
Eventually, the unfavorable conjunction of precarious health status, overcrowding, and overexposure to COVID-19 virus reflected by the impossibility of teleworking might be the explication for the higher infection rates among the northern suburban Paris population. Because of their geographical positions, Saint Louis and Bichat hospitals gather a significant population of patients with transplants who live in these areas of the Parisian conurbation. In this sense, the fact that non-White ethnicity is, in our model, an independent determinant of COVID-19 infection is not surprising, as it would be the expression of a certain social context responsible for an enhanced risk of contracting the disease. So, our study reinforces the importance of ethnicity in COVID-19 disease, confirming United States data in a European population.
Additionally, our analysis also investigated potential risk factors specifically related to transplantation. We observed that patients with COVID-19 are more frequently recipients from deceased donors and that infection occurs more commonly closer to the date of transplantation, suggesting a role of the induction depleting agents used at the time of graft. Similarly, patients with steroid-based regimens presented an increased risk of COVID-19 disease. In our centers, steroid-based regimens are used for ABO-incompatible transplantation in patients with post-transplant HLA donor-specific antibodies “de novo” or in patients who experienced a rejection episode. We did not find significant differences between the other immunosuppressive strategies.
Moreover, managing immunosuppression in patients with COVID-19 disease is arduous. In our experience, no changes were made in patients with moderate forms (outpatients or hospitalized patients with low need for oxygen). In more severe scenarios, the most frequent strategy was the withdrawal of antimetabolites; in particular, antimetabolites were suspended in all patients requiring invasive mechanical ventilation, although mortality rate was still 53%, with two (13%) patients still hospitalized by the end of the study. The duration of suspension still needs to be determined. In addition, the ideal treatment for kidney transplant recipients with COVID-19 disease remains uncertain. Insights about the interaction between the host and the virus, cytokine storm, and inflammation due to antiviral immune response, all of which are potential targets for future therapies, have been described. However, we still need trials aiming to define the role of these inflammatory pathways.25 Up to date, the use of immunosuppressive treatments, such as hydroxychloroquine, tocilizumab, and eculizumab (used in our cohort in seven, one, and two patients, respectively), remains to be demonstrated, and our limited data prevent any reliable conclusion about their clinical utility in patients with kidney transplants.
Our study has several limitations. The critical situation in Paris did not allow us to broaden this prospective study to other centers. Second, the follow-up time was relatively short and therefore, could not depict the complete course of the disease. Third, the therapeutic interventions used in this study were only descriptive. Fourth, by the time the study was conducted, large-scale screening for asymptomatic patients was not routinely carried out in France. So, the fact we included only symptomatic patients (with COVID-19–positive swab), on one hand, impedes any conclusion regarding the prevalence of COVID-19 in patients without clinical manifestations and on the other, can lead to possibly missing falsely negative patients.
In conclusion, the findings of our study represent an easily exploitable tool in the actual contest. They allow the identification of well-defined risk factors as well as a precise description of the burden of the COVID-19 disease in kidney recipients, on the basis of which physicians and public health agencies may offer advice and concrete measures regarding prevention and management.
As risk factors associated with COVID-19 were better described in the kidney transplantation population, with a better control of the pandemic, reopening the transplant program in France was possible. Scientific societies26 recommended a balance between benefits and risks on one hand and to inform patients about the potential risks. They also recommended establishing circuits with low risk of contamination context as well as tailored actions (such as social distancing, reinforced teleconsultation follow-up, and control of comorbidities) with regard to the risks identified in specific groups of individuals among kidney transplant recipients.
Disclosures
All authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors would like to thank all of the physicians and paramedics who, with their precious and restless job, took care of our patients in this emergent context.
Dr. Michelle Elias, Prof. Carmen Lefaucheur, and Dr. Daniele Pievani designed the study; Dr. Imad Abboud, Dr. Corinne Antoine, Dr. Alexandra Delion, Dr. Blandine Denis, Dr. Clarisse Greze, Dr. Oceane Le Goff, Dr. Evangéline Pillebout, and Dr. Christine Randoux collected data; Dr. Daniele Pievani analyzed the data; Dr. Michelle Elias and Dr. Daniele Pievani made the figures; Prof. Eric Daugas, Dr. Michelle Elias, Prof. Denis Glotz, Prof. Carmen Lefaucheur, Dr. Kevin Louis, and Dr. Daniele Pievani drafted and revised the paper; and all authors approved the final version of the manuscript. Prof. Eric Daugas reports personal fees from Amgen, non-financial support from Amgen, and non-financial support from Sanofi, outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050639/-/DCSupplemental.
Supplemental Figure 1. Ile de France area.
Supplemental Table 1. Demographic, comorbidity, and treatment characteristics of deceased patients.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization: Coronavirus Disease (COVID-19)—events as they happen, 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed May 8, 2020
- 3.Johns Hopkins University of Medicine Coronavirus Resource Center: COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), 2020. Available at: https://coronavirus.jhu.edu/map.html. Accessed May 8, 2020
- 4.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. : Covid-19 and kidney transplantation. N Engl J Med 382: 2475–2477, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Columbia University Kidney Transplant Program : Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol 31: 1150–1156, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M: COVID-19 infection in kidney transplant recipients. Kidney Int 97: 1076–1082, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates PT, Wong G, Drueke T, Rovin B, Ronco P; Associate Editors, for the Entire Editorial Team : Early experience with COVID-19 in kidney transplantation. Kidney Int 97: 1074–1075, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, et al. : COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant 20: 1849–1858, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [published correction appears in Lancet 395: 1038, 2020]. Lancet 395: 1054–1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pareek M, Bangash MN, Pareek N, Pan D, Sze S, Minhas JS, et al. : Ethnicity and COVID-19: An urgent public health research priority. Lancet 395: 1421–1422, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Government of France: Carte et donnees covid 19 en France, 2020. Available at: https://www.gouvernement.fr/info-coronavirus/carte-et-donnees. Accessed June 19, 2020
- 12.Haug C: French pandemic resistance. N Engl J Med 382: e51, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization: Coronavirus disease (COVID-19) advice for the public, 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed May 8, 2020
- 14.France et Monde : Infection au nouveau Coronavirus (SARS-CoV-2), COVID-19, 2020. Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/articles/infection-au-nouveau-coronavirus-sars-cov-2-covid-19-france-et-monde. Accessed June 19, 2020
- 15.Ministère des Solidarités et de la Santé : COVID-19 coronavirus situation points, 2020. Available at: https://solidarites-sante.gouv.fr/soins-et-maladies/maladies/maladies-infectieuses/coronavirus/etat-des-lieux-et-actualites/article/points-de-situation-coronavirus-covid-19. Accessed May 9, 2020
- 16.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. ; and the Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 323: 2052–2059, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Horby PW, Hayden FG, Gao GF: A novel coronavirus outbreak of global health concern [published correction appears in Lancet 395: 496, 2020]. Lancet 395: 470–473, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Sullivan JM, Ward S, Fogarty H, O’Donnell JS: More on ‘Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia.’ BJHaem 190: 27–28, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Harris RJ, Ellis J, Pebody RG: Ethnicity, deprivation and mortality due to 2009 pandemic influenza A(H1N1) in England during the 2009/2010 pandemic and the first post-pandemic season. Epidemiol Infect 143: 3375–3383, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yancy CW: COVID-19 and African Americans. JAMA 323: 1891–1892, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Pratt G: Chicago’s coronavirus disparity: Black Chicagoans still dying disproportionately in city as Mayor Lightfoot creates response teams for hard-hit neighborhoods. Chicago Tribune, 2020. Available at: https://www.chicagotribune.com/coronavirus/ct-coronavirus-chicago-african-american-population-20200420-c2nyvnq4vrcundwdfvhdldtlfe-story.html. Accessed May 8, 2020
- 22.Le Monde: Coronavirus: Very high excess mortality in Seine-Saint-Denis, 2020. Available at: https://www.lemonde.fr/societe/article/2020/05/17/coronavirus-une-surmortalite-tres-elevee-en-seine-saint-denis_6039910_3224.html. Accessed May 17, 2020
- 23.Institut national de la statistique et des études économiques: Housing, individuals, activity, educational and professional mobility, residential migration in 2015: Census of population—detail file, 2020. Available at: https://www.insee.fr/fr/statistiques/3565914?sommaire=3558417. Accessed June 19, 2020
- 24.L’Institut Paris Region : Excess mortality during the Covid-19 epidemic in the Paris region, 2020. Available at: https://www.ors-idf.org/nos-travaux/publications/la-surmortalite-durant-lepidemie-de-covid-19-dans-les-departements-franciliens.html. Accessed June 19, 2020
- 25.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. : A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583: 459–468, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Francophone Society of Transplantation: COvid-19, 2020. Available at: https://www.transplantation-francophone.org/covid-19. Accessed June 21, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.