Significance Statement
There is intense interest in engineering new kidneys from embryonic stem cells and induced pluripotent stem cells as research models, and perhaps eventually for clinical transplant. Although protocols exist for producing renal organoids from stem cells, these organoids lack an essential component, the ureter. The authors describe the production of ureter-like tissue consisting of embryonic stem cell–derived ureteric buds that organize ex fetu mesenchyme around it to form smooth muscle layers. These muscles spontaneously contract with a periodicity that is a little slower than that of peristalsis in natural ureters. This work represents a step toward developing organoids with a ureter, although inducing the tissue to elongate into a tube and connect it to the kidney is a remaining challenge.
Keywords: stem cell, renal stem cell, ureteric bud, kidney development
Visual Abstract
Abstract
Background
There is intense interest in replacing kidneys from stem cells. It is now possible to produce, from embryonic or induced pluripotent stem cells, kidney organoids that represent immature kidneys and display some physiologic functions. However, current techniques have not yet resulted in renal tissue with a ureter, which would be needed for engineered kidneys to be clinically useful.
Methods
We used a published sequence of growth factors and drugs to induce mouse embryonic stem cells to differentiate into ureteric bud tissue. We characterized isolated engineered ureteric buds differentiated from embryonic stem cells in three-dimensional culture and grafted them into ex fetu mouse kidney rudiments.
Results
Engineered ureteric buds branched in three-dimensional culture and expressed Hoxb7, a transcription factor that is part of a developmental regulatory system and a ureteric bud marker. When grafted into the cortex of ex fetu kidney rudiments, engineered ureteric buds branched and induced nephron formation; when grafted into peri-Wolffian mesenchyme, still attached to a kidney rudiment or in isolation, they did not branch but instead differentiated into multilayer ureter-like epithelia displaying robust expression of the urothelial marker uroplakin. This engineered ureteric bud tissue also organized the mesenchyme into smooth muscle that spontaneously contracted, with a period a little slower than that of natural ureteric peristalsis.
Conclusions
Mouse embryonic stem cells can be differentiated into ureteric bud cells. Grafting those UB-like structures into peri-Wolffian mesenchyme of cultured kidney rudiments can induce production of urothelium and organize the mesenchyme to produce rhythmically contracting smooth muscle layers. This development may represent a significant step toward the goal of renal regeneration.
The past decade has seen significant advances toward the goal of generating kidneys from various types of stem cell. It is now possible to produce renal organoids, representing immature kidneys and showing some physiologic functions, from embryonic and induced pluripotent cells of mouse and human.1–6 So far, these organoids have not featured a ureter. This report describes a technique for differentiating mouse embryonic stem (ES) cells into urothelium that can organize fetal peri-Wolffian mesenchyme around it to produce contractile muscle layers.
Our experiments rest on two bodies of prior work. One is an effort to explore the self-organizing properties of cells, specifically renogenic stem cells from young fetal mouse kidney rudiments. It was shown in 2010 that these cells, disaggregated then reaggregated, could interact to produce what have since come to be called renal organoids, containing small collecting duct trees and immature nephrons.7 A series of technical advances,8–10 most of which break the symmetry of the system to create large-scale order, have improved the realism of the tissues produced, resulting in organoids with nephrons arranged around a single collecting duct tree with a single urothelial end.10
The other body of work aimed to produce kidneys from human induced pluripotent stem (iPS) cells.11,12 From early application of sequences of signaling molecules to differentiate mouse ES cells into renal epithelia that could integrate into developing kidneys,13 methods were established to produce complete renal organoids from human iPS and ES cells4–6 that are able to connect with host blood systems.14 Like the 2010 ex fetu mouse organoids, they lack proper large-scale anatomic organization. This issue has been partly addressed by Taguchi and Nishinakamura in 2017,4 who generated the first anatomically organized kidney organoid derived mainly from mouse ES cells, with some ex fetu material included. The organoids had a single collecting duct tree, but no ureter.
Here, we report the results of combining the Taguchi and Nishinakamura differentiation techniques4 with grafting into ex fetu peri-Wolffian mesenchyme to produce urothelial structures surrounded by smooth muscle coats showing spontaneous contractions.
Methods
Animals
Mice mated overnight, and the morning of vaginal plug discovery was considered embryonic day (E) 0.25. Pregnant mice were euthanized at E11.5 by trained UK Home Office license holders, by methods listed in Schedule One of the UK Animals (Scientific Procedures) Act. Embryos were decapitated and dissected to obtain “nephrogenic areas” (the metanephros, ureter, nearby Wolffian duct, and mesenchyme), or isolated metanephroi, according to the experiment.
Induction of Ureteric Bud Differentiation from Mesenchymal ES Cells
A Hoxb7-GFP mouse ES cell line was a gift from Professor Ryuichi Nishinakamura’s laboratory (Kumamoto University, Kumamoto, Japan). Cells were maintained in GMEM (G5154; Sigma) supplemented with 10% FBS, GlutaMAX (1×, Gibco), MEM-NEAA (1×, Gibco), sodium pyruvate (1 mM, Gibco), β-mercaptoethanol (0.1 mM), and leukemia inhibitory factor (sc-4989, LIF, 1 U/μl; Santa Cruz Biotechnology). The mouse embryonic stem (mES) cell line was differentiated into ureteric bud (UB) cells using a slight modification of a method previously described by Taguchi and Nishinakamura.4 Briefly, at 0 hours cells were dissociated with Accutase (Gibco); reaggregated at 2000 cells/aggregate in 96-well, U-bottomed, low cell–binding plates (650970; Greiner); and cultured to form embryonic bodies (EBs). At 48 hours, the medium was replaced by “base medium,” comprising 75% Iscove modified Dulbecco medium (12440–046; Gibco) and 25% Ham F12 (11765–054; Gibco), with 0.5× N2 (17502–048; Gibco), 0.5× B27 (12587–010; Gibco), 0.5× penicillin/streptomycin, 0.05% BSA (Sigma), 2 mM L-glutamine (Life Technologies), 0.5 mM ascorbic acid (Sigma), 450 μM 1-thioglycerol (Sigma), with the addition of 10 ng/ml human Activin A (338-AC; R&D Systems) as step 1. At 72 hours (all times are from 0 hours), the medium was changed for base medium containing 0.3 ng/ml human BMP4 (314-BP; R&D Systems) and 10 μM CHIR99021 (TOCRIS 4423) as step 2. At 108 hours, the medium was changed for base medium containing 0.1 μM retinoic acid (R-2625, RA; Sigma), 100 ng/ml human FGF9 (273-F9; R&D Systems), and 10 μM SB431542 (TOCRIS 1614) as step 3. At 132 hours, the medium was changed for base medium containing 0.1 μM RA, 100 ng/ml human FGF9, and 5 μM CHIR99021 as step 4. At 156 hours, the medium was changed for base medium containing 10 µM Y27632 (72302; Stem Cell Technologies), 0.1 µM RA, 1 µM CHIR99021, 5 ng/ml human FGF9, and 10% growth factor–reduced Matrigel (354230; Corning) as step 5. At 180 hours, 3 µm CHIR99021 and 1 ng/ml GDNF (212-GD; R&D Systems) were added to a fresh change of the medium used from 156 hours, as step 6. At 204 hours, this was changed to the same medium with 2 ng/ml GDNF and without FGF9, as step 7. After 24 hours of step 7, the EBs developed numerous ES cell–derived UB-like radiating tubules, which we refer to as engineered uteric buds (eUBs) in this report.
For culture in Matrigel,4 projecting eUB tubules were isolated by manual dissection from EBs produced using the method described above, and were suspended in 20% Matrigel in DMEM/F12 medium containing 10% FBS, 0.1 μM RA, 100 ng/ml human Rspondin1 (4645-RS; R&D Systems), 2 ng/ml human GDNF (212-GD; R&D Systems), and 100 ng/ml mouse FGF1 (450–33A; Pepro Tech) in U-bottomed, low cell–binding plates.
Grafting of eUBs into Cultured Kidney Rudiments
E11.5 kidneys were isolated from CD1 mouse embryos, and the rudiments were cultured on 24-mm, 0.4-μm-pore membranes (3450, Transwells; Corning) in kidney culture medium (KCM) comprising Minimum Eagle Medium with Earle salts (M5650, MEM; Sigma) with 10% FBS. Hoxb7-GFP eUBs were isolated manually from day 10 (approximately 230 hours) EBs using sharpened tungsten needles, and grafted into either the metanephric mesenchyme (Figure 1A) or the peri-Wolffian mesenchyme (Figure 3A) of E11.5 embryonic kidneys in culture as above. The kidneys and grafts were cultured for 5 or 9 days in KCM, with the medium being changed every 2 days.
Figure 1.
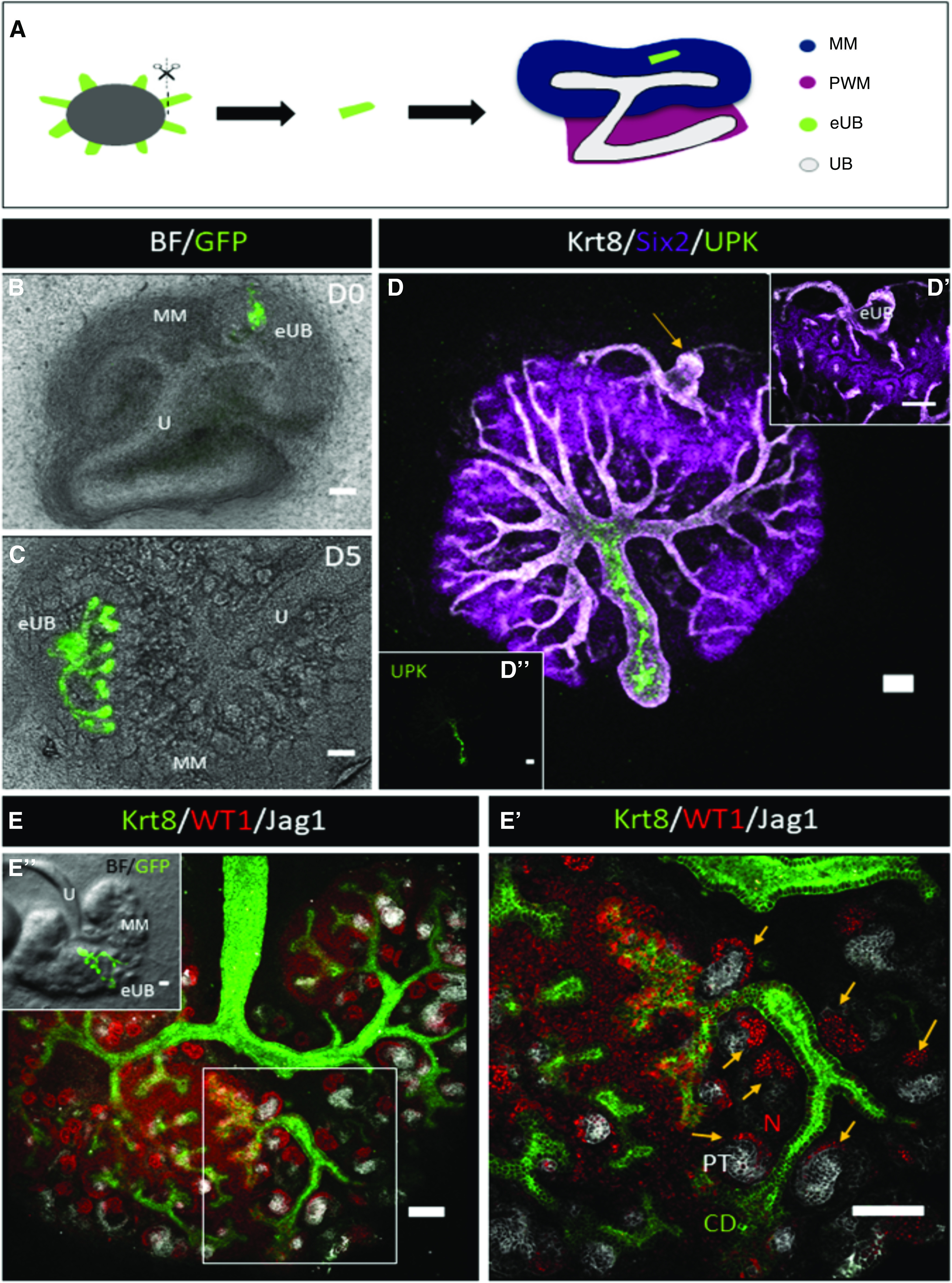
eUB branches and induced nephrogenesis in renal metanephric mesenchyme. (A) Steps of grafting eUB into MM. (B and C) Combined bright-field and GFP fluorescence image of a HoxB7-GFP mES cell–derived eUB grafted into the MM of an E11.5 kidney at (B) 0, and (C) 5 days of culture. (D) Immunofluorescence of (C); the grafted eUB is indicated by the arrow (the sample has been rotated by 90° because of the differences between microscopes); (D’) region of (D) showing the eUB tips surrounded by SIX2+ nephron progenitor cells, like those of the natural UB. (D’’) Image (D) with only the UPK channel, showing UPK expression in the host ureter but not the eUB graft. (E) Immunofluorescence image of an eUB graft in MM showing eUB branching and induction of JAG1+ early nephrons. (E’) Magnified image of the boxed area in (E) showing the early nephrons (arrows) associated with the eUB. (E’’) Combined bright-field and GFP image of (E) showing the location of the GFP-eUB graft. Scale bar for all images, 100 µm. CD, collecting duct; MM, metanephric mesenchyme; N, nephron; PT, proximal tubule; U, natural ureter.
Figure 3.
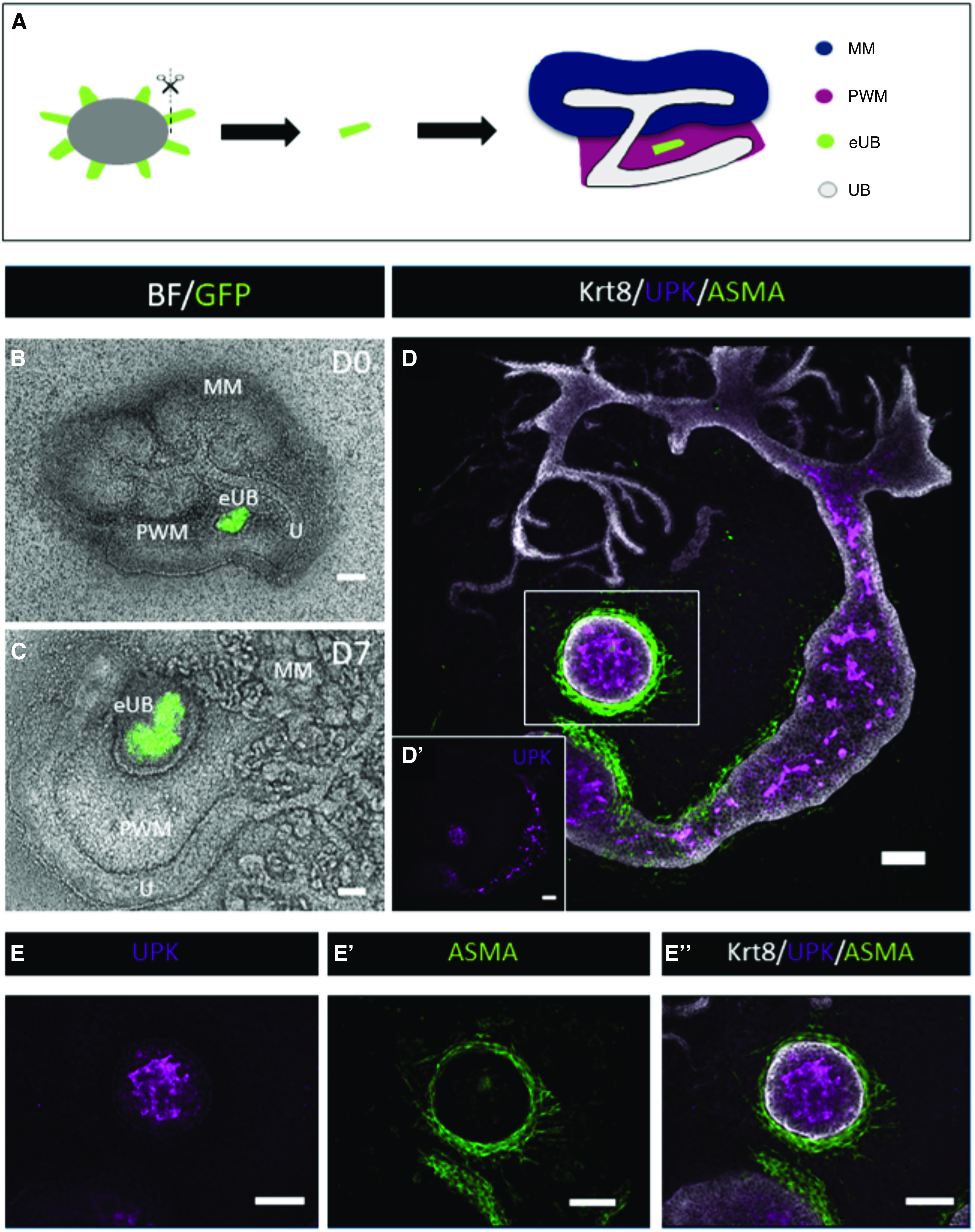
mES cell–derived eUBs differentiate into ureter tissue when grafted into peri-Wolffian mesenchyme. (A) Steps of grafting eUB into peri-Wolffian mesenchyme (PWM). (B) A combined bright-field (BF) and GFP image of a Hoxb7-GFP eUB grafted into PWM cells at the time of grafting, and (C) 7 days later. (D) Immunofluorescence stain of an eUB grafted into PWM showing expression of UPK in the epithelium, KRT8 in the epithelial layers, and smooth muscle actin (ASMA) around the epithelium. (D') Separate UPK channel of D, for clarity. (E-E'') Shows a magnification of the boxed area in D, individual UPK channel in E. ASMA channel in (E'). (E'') Shows the combined channels UPK, ASMA, and Krt8. MM, metanephric mesenchyme.
Combination of eUB with Peri-Wolffian Mesenchyme or Metanephric Mesenchyme
Peri-Wolffian mesenchyme was isolated by manual dissection from E11.5 nephrogenic areas using sharp tungsten needles, and dispersed by incubation in 1× trypsin/EDTA (T4174; Sigma) at 37°C for 2 minutes. Around 150,000 cells were suspended in 150 µl KCM, and centrifugation (3 minutes at 3000×g) was used to obtain a cell pellet. Pellets were transferred to wells in 96-well, low cell–binding, U-bottom plates. The eUBs were dissected from day 10 organoids, added to the mesenchymal cell pellet at 1 eUB per well, and incubated at 37°C and 5% CO2. After 24 hours, the combination had formed a compact spheroid, which was transferred to a 24-mm, 0.4 μm-pore Transwell membrane (3450) in a well containing 1.5 ml KCM (Figure 4A). The same method was used to combine eUB with isolated metanephric mesenchyme (Figure 2A), with the spheroids being cultured in KCM for 5 days, with the addition of 50 µl KCM containing 10% Matrigel on top.
Figure 4.
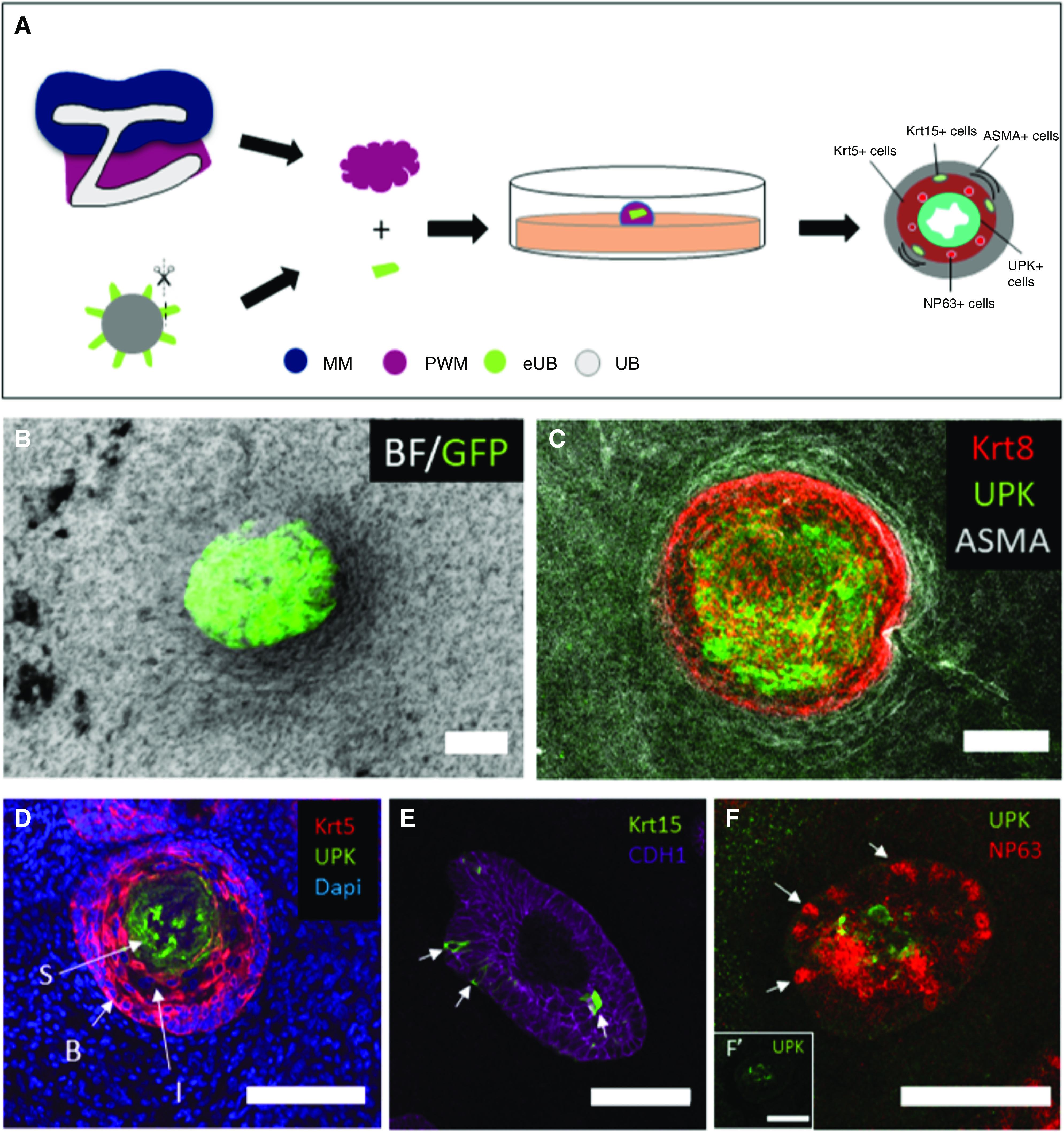
Urothelial differentiation in pure peri-Wolffian mesenchyme. (A) Steps of recombination of Hoxb7-GFP-eUB with PWM cells. (B) A combined bright-field (BF) and GFP image of a Hoxb7-GFP eUB recombined with PWM cells. (C) Immunofluorescence stain of an eUB recombined with PWM cells showing expression of UPK in the adluminal epithelium, KRT8 in the urothelium as a whole, and smooth muscle actin (ASMA) around the epithelium. (D) A 6-μm section of an eUB combined with PWM shows UPKIII expression in superficial (“S”) cells, KRT5 in basal (“B”) cells, and also the presence of KRT5- intermediate (“I”) cells in the less basal zone of the area otherwise dominated by B cells. (E) Krt15 is expressed by occasional cells in the B cell layer; the counterstain E-cadherin (Cdh1) marks all epithelial cells of the eUB graft. (F) Cells expressing the intermediate cell marker NP63 (arrows) and others expressing UPK; the insert (F') shows the UPK channel alone, for clarity. MM, metanephric mesenchyme; PWM, peri-Wolffian mesenchyme.
Figure 2.
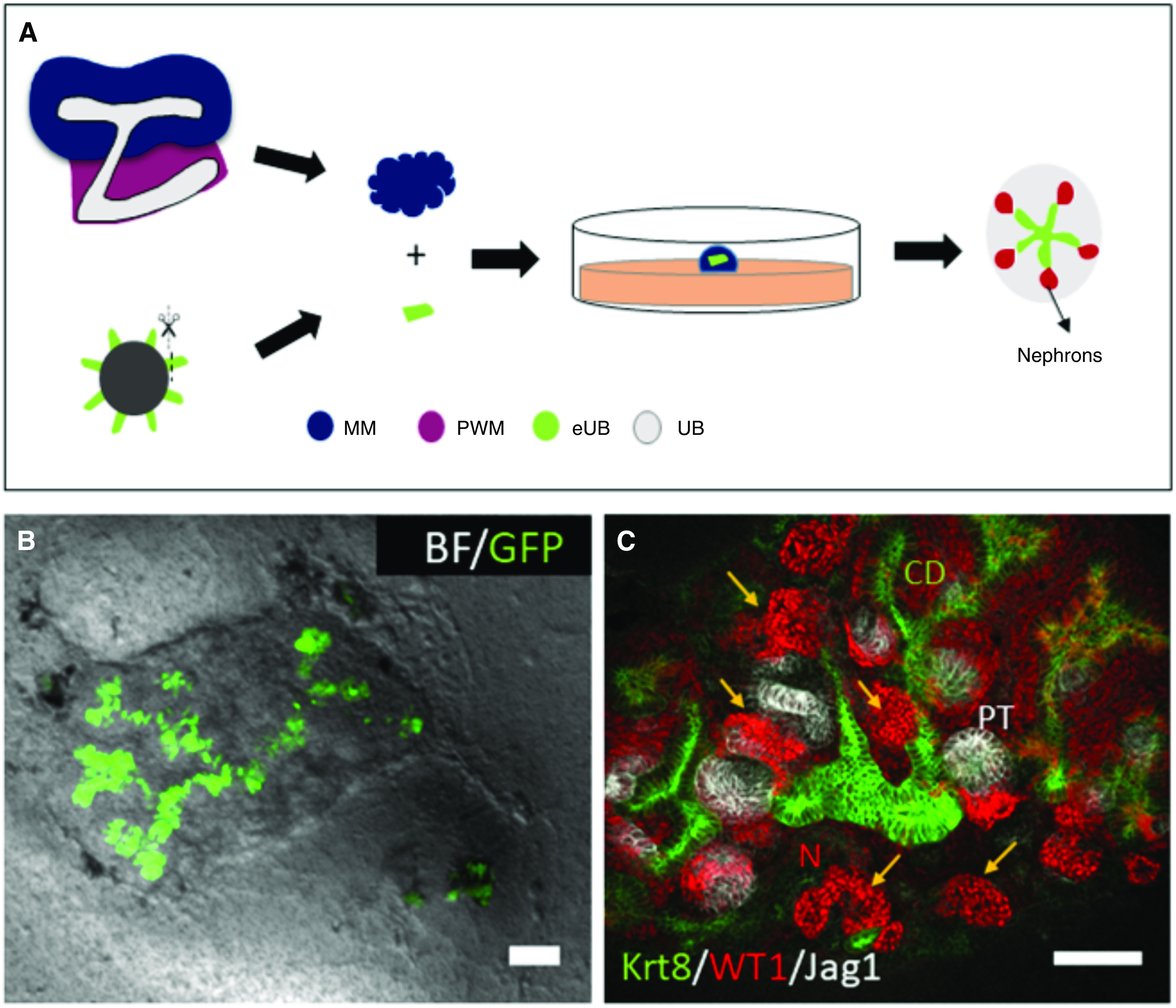
eUBs branch and induce nephrons when placed into isolated metanephric mesenchyme. (A) Steps of recombination of eUB with isolated MM cells. (B) Combined bright-field (BF) and GFP image of Hoxb7-GFP eUB recombined with MM, showing branching of the eUB, survival of the MM, and induction of nephron differentiation. (C) Immunofluorescence showing the eUB epithelial branching (KRT8 expression), nephrogenic condensates (indicated by arrows and WT1 expression), and early nephrons expressing strong WT1 at their glomerular poles and the proximal tubule marker JAG1, connecting to the eUB. Scale bar for all images, 100 µm. MM, metanephric mesenchyme; PWM, peri-Wolffian mesenchyme.
Immunofluorescence
Samples were fixed by immersion in cold methanol, and the immersed samples were allowed to warm to room temperature (19°C±5°C) over 30 minutes. They were washed with PBS and blocked in staining buffer, comprising 5% BSA in PBS, overnight at 4°C. For Krt15 and NP63 staining, samples were fixed in 4% PFA in PBS and blocked using 5% BSA and 0.2% Triton X-100 (Sigma) in PBS. After blocking, primary antibodies (Supplemental Table 1) diluted in staining buffer were applied to samples at 4°C for 24 hours. Unbound primary antibody was washed off in PBS (3×5 minutes), and secondary antibodies (Supplemental Table 1) in staining buffer were applied overnight at 4°C. Samples were washed (3×15 minutes PBS) and mounted onto a slide using Vectashield (H-1000; Vector Laboratories).
Paraffin Wax–Embedded Tissue Sectioning
Samples were fixed in methanol as above, and were then placed in an automatic wax processing machine (Sakura VIP E300; Sakura). Wax-infused samples were embedded in paraffin wax blocks, and 6-µm sections were cut using a Leica RM2245 microtome. Sections were floated out before mounting on slides (Superfrost plus; Thermo Fisher Scientific) and dried in a 37°C oven. Samples were dewaxed in xylene (30 minutes), rehydrated in an ethanol series (100%, 90%, 70%, for 5 minutes each), and then placed under running water. Antigen retrieval was carried out by microwaving the dewaxed slides in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for 3×15 minutes. Immunohistochemical staining was carried out as described above.
Statistical Analyses
For categorical (feature present/absent) data, 95% confidence intervals (95% CIs) were calculated using the binomial normal approximation interval, corrected for small sample sizes, as ±1.96[√(p(1−p)/n)]+1/2n.15
Results and Discussion
Engineering of GFP-Labeled UBs from mES Cells
We differentiated HoxB7-GFP mESC4,16 to UB cells using the method of Taguchi and Nishinakamura.4 In agreement with their findings, by day 2, mES cells formed Hoxb7-GFP− EBs (Supplemental Figure 1, A and B). By day 10, they developed numerous epithelial projections (Supplemental Figure 1C) expressing the UB marker HoxB7-GFP (Supplemental Figure 1D: six runs, at least six EBs in each, all showing these features). When isolated and cultured in three-dimensional Matrigel supplemented with GDNF, R-Spondin1, FGF1, and retinoic acid,4 the epithelial projections branched in a manner similar to natural UBs in gel culture (Supplemental Figure 1, F and G; three runs, four samples in each, all branching).17 Expression of HoxB7-GFP was maintained (Supplemental Figure 1H), suggesting retention of UB character, and the UB markers Calbindin D28k, pan-cytokeratin, Krt8, E-cadherin (Cdh1), Gata3, and Pax2 were present in all three samples tested (Supplemental Figure 1, I–L). Furthermore, the epithelia expressed the GNDF receptor c-Ret (Supplemental Figure 2, A–D; all five samples) and the “tip” marker Sox9 (Supplemental Figure 2, E and F; all six samples). They did not, however, bind the “stalk” maker Dolichos biflorus agglutinin18 (Supplemental Figure 2, G and H; zero out of three samples). Therefore, the whole structure had the character of ureteric tip, with no evidence of differentiation to stalk. We refer to tubular structures as eUBs.
eUBs Differentiate into Collecting Duct-Like Epithelial Trees in a Metanephric Mesenchymal Environment
Building on previous work,19–21 Mills and colleagues grafted isolated tips or stalks of natural UBs into either the metanephric mesenchyme or the peri-Wolffian mesenchyme of cultured kidney rudiments. They found that differentiation of the UB fragments was controlled by the identity of surrounding mesenchyme. Importantly for our study, they found that ureteric tips grafted into the peri-Wolffian mesenchyme expressed the urothelial marker, Uroplakin (UPK).10
We tested whether eUBs showed the same plasticity, beginning with grafting to the metanephric mesenchyme (Figure 1A). Grafted eUBs (Figure 1B) grew and branched to produce a tree (Figure 1C: all ten branched; 100%, 95% CI, 95% to 100%). As well as expressing Hoxb7-GFP (Figure 1C) and KRT8 (Figure 1D), they organized a nephrogenic response in the host metanephric mesenchyme. Their tips became surrounded by SIX2+ cap mesenchyme cells (Figure 1D; all three samples tested, 100%, 95% CI, 83% to 100%). Early-stage nephrons, with WT1+ glomerular poles and JAG1+ proximal tubules, formed near the grafted eUBs and eventually connected to them (Figure 1, E and E’; all eight samples tested 100%, 95% CI, 94% to 100%). All but one graft into metanephric mesenchyme were UPK−, with the UPK+ host ureter acting as a positive staining control (Figure 1D; five out of six tested). The exception was in a damaged host kidney that had lost its own ureter and had a torn mesenchyme. Across these experiments, UPK expression rate was therefore 17% (95% CI, 0% to 56%).
The host UB is not necessary for this response. When eUBs were grafted into isolated metanephric mesenchyme (Figure 2A), they still branched (Figure 2B) and induced the differentiation of nephrons with WT1+ glomerular poles and Jagged-1+ proximal tubules (Figure 2C; all three samples tested, 100%, 95% CI, 83% to 100%). Again, this observation confirms the prior work of Taguchi and colleagues.
eUBs Differentiate into Ureter-Like Epithelia in a Peri-Wolffian Mesenchyme Environment
When grafted instead into the peri-Wolffian mesenchyme of ex fetu kidney rudiments (Figure 3A), eUBs did not branch and did not induce nephrons, although they retained Hoxb7-GFP expression (Figure 3, B and C). They now showed robust expression of UPK (Figure 3D; all 12 samples examined; 100%; 95% CI, 96% to 100%, a range that does not overlap the 95% CI of grafts into metanephric mesenchyme described in the previous paragraph). In addition to expressing UPK, they acquired a smooth muscle layer expressing α-smooth muscle actin (Figure 3, D and E).
It is known that the ureteric stalk epithelium and the mesenchyme that surrounds it collaborate to produce a ureter via reciprocal inductive signaling. There is strong evidence that epithelium-derived SHH signaling to the mesenchyme is necessary for the mesenchyme to express BMP4 as a result of an internal FOXF1-dependent pathway and to become competent to differentiate into muscle.22 BMP4 from the mesenchyme signals to the epithelium to drive urothelial differentiation,10 whereas the epithelium signals to the mesenchyme to drive smooth muscle differentiation. This urothelium-to-mesenchyme communication involves β-catenin–mediated WNT signaling, probably by WNT7B and/or WNT9B, both present in the epithelium.23 In addition, retinoic acid signaling is required for a correct balance of differentiation in both compartments.24 Are local paracrine signals such as these sufficient to drive urothelial eUB differentiation, or are influences from the kidney or natural ureter needed? Testing this by combination of eUBs with isolated ex fetu peri-Wolffian mesenchyme (Figure 4A) again resulted in the eUBs remaining unbranched (Figure 4B), activating UPK expression and gaining a smooth muscle layer (Figure 4C; all three cases examined, 100%, 95% CI, 83% to 100%). This argues that local interactions between peri-Wolffian mesenchyme and the eUB are sufficient to induce differentiation.
When in the peri-Wolffian mesenchyme, either in the kidney or isolated, the form of these grafts was fully or oblately spherical, with no evidence of elongation into a tube. Within the structures, the eUB-derived urothelium differentiated to form the layered structure similar to a natural ureter. At the core were cells showing strong expression of UPK (Figure 4, C and D), a classic superficial (“S”) cell marker (UPKIII).25 In some samples, there was evidence of a lumen, albeit somewhat collapsed rather than inflated (Figure 4D). Between the superficial cells and the basement membrane were cells showing strong expression of KRT5 (Figure 4D; all three samples), a classic basal (“B”) cell marker.25–27 Within the B cell zone were occasional cells expressing KRT15 (Figure 4E; all five samples), as in natural ureter.28 In some places along the least-basal parts of the zone dominated by B cells were cells expressing no KRT5 and only very weak UPK (Figure 4D), and expressing strong NP63 (Figure 4F; all four samples), a pattern characteristic of intermediate (“I”) cells.25
Ureter-Like Tissues Made by Combination of eUBs with Peri-Wolffian Mesenchyme Show Spontaneous Contractions
By 7 days after combination, the ureter-like tissues formed by grafting eUBs into peri-Wolffian mesenchyme of host kidneys started to show rhythmic contractions. These became stronger and more frequent by day 9 and were detectable in all three of the samples filmed under time lapse (100%, 95% CI, 83% to 100%; Supplemental Video 1). To assess whether contractions of the graft were synchronized with those of the host ureter, this video was analyzed frame by frame and the times of peak contraction (minimum diameter) of the graft and host were recorded separately. Times of individual contractions are shown in Supplemental Figure 3. The period of contraction of the natural ureter, averaged over eight intervals between nine contractions in the recording, was 12 seconds (SEM 0.8 seconds), comparable with that in vivo29; the period of the graft, averaged over seven intervals between its eight contractions, was slightly slower at 15 seconds (SEM 0.6 seconds). There was no obvious relationship between the timings, the graft sometimes leading and sometimes lagging the host (Supplemental Figure 3). No contractility was detected in any eUB grafted in the metanephric mesenchyme.
The asynchronous contraction of graft and host implies that the contractions of the muscles formed around the graft are spontaneous and independent of activity in the nearby natural ureter. To verify this, we filmed combinations of eUBs and pure peri-Wolffian mesenchyme with no host ureter present. These still showed large spontaneous rhythmic contractions (Supplemental Figure 4, Supplemental Video 2), of period 11 seconds (SEM 0.8 seconds) in one video and 20 seconds (SEM 0.7 seconds) in another. This indicates not only that the muscles are functional, but that at least some have the “pacemaker” activity usually ascribed to atypical muscle cells normally found at the proximal end of the ureter or renal pelvis.30 Careful observation showed that, between these large contractions, there were very small contractions that, with the large contractions, formed a steady sequence with periods 6.4 seconds (SEM 0.4 seconds) and 7.5 seconds (SEM 0.5 seconds) in the same two videos. It is already known from electrical measurements that pacemaker activity in ureter smooth muscle cells runs at two to four times the frequency of gross peristaltic contraction because of the mechanism of muscle contraction having a refractory period29; the small contractions we observed between large ones may reflect this underlying clock. We did not observe small contractions in either host or grafted UBs in the whole-kidney samples described in the previous paragraph, perhaps because the more closely packed stroma in these prevented visible small movements.
This is not the first report of differentiation of ES and iPS cells into urothelial cells, but previous examples31 lacked three-dimensional structure, and both these and those of Santos et al.32 lacked smooth muscle. A recent publication by Mullenders et al.33 described ureter organoids made from bladder cancers and from adult human tissue. They adopted a cyst-like shape with a lumen, but with no organization of mesenchymal components or muscle and no evidence of contraction. Our approach is distinct in combining ES-derived ureters with ex fetu mesenchymal cells to generate multiple epithelial layers and smooth muscle coat that contract spontaneously. Important future goals are to develop techniques for differentiating peri-Wolffian mesenchyme from ES cells, and inducing the engineered tissue to elongate into a proper tube.
Disclosures
All authors have nothing to disclose.
Funding
Work in this report was funded by Medical Research Council grant MR/R026483/1 (to J.A. Davies) and Kidney Research UK grants RP_002_20160223 and ST_001_20161116 (to J.A. Davies). M. Sallam is funded by a scholarship from Newton-Mosharafa program between the Egyptian Cultural and Educational Bureau and the British Council in Egypt.
Supplementary Material
Acknowledgments
We thank Prof. Tung-Tien Sun (New York University) for the uroplakin antibody and Prof. Ryuichi Nishinakamura (Kumamato University) for the HoxB7-GFP cell line.
Dr. May Sallam conducted experiments and led manuscript writing. Dr. Anwar Palakkan helped in data analysis and paper editing, Dr. Christopher G. Mills helped in grafting experiments. Ms. Julia Tarnick helped in time-lapse video recording and paper editing. Dr. Mona Elhendawi assisted with thoughtful discussions. Prof. Lorna Marson cosupervised Dr. May Sallam’s work and made suggestions about the strategy for experiment. Prof. Jamie A. Davies is primary supervisor of Dr. May Sallam’s work, participated in overall project design and detailed experimental design, advised about methods of analysis, analyzed timing of contractions, and participated in writing the manuscript. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Patterning a Ureter Is All in the Stroma,” on pages 2231–2232.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/looSkup/suppl/doi:10.1681/ASN.2019101075/-/DCSupplemental.
Supplemental Table 1. Antibodies used for immunofluorescence analysis.
Supplemental Video 1. A GFP-expressing eUB grafted into the peri-Wolffian mesenchyme of an intact nephrogenic zone, showing regular smooth muscle contractions.
Supplemental Video 2. An eUB combined with peri-Wolffian mesenchyme in the absence of an associated kidney, also showing regular smooth muscle contractions.
Supplemental Figure 1. Production of eUBs from Hoxb7-GFP mES cells.
Supplemental Figure 2. ES cell–derived eUBs show tip markers.
Supplemental Figure 3. Contraction in grafted eUB-derived ureter-like tissue and in the natural ureter.
Supplemental Figure 4. Contraction in eUB-derived ureter-like tissue in isolated peri-Wolffian mesenchyme.
References
- 1.Morizane R, Miyoshi T, Bonventre JV: Concise review: Kidney generation with human pluripotent stem cells. Stem Cells 35: 2209–2217, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morizane R, Bonventre JV: Kidney organoids: A translational journey. Trends Mol Med 23: 246–263, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies JA, Chang CH, Lawrence ML, Mills CG, Mullins JJ: Engineered kidneys: Principles, progress, and prospects. Adv Regen Biol 1: 24990, 2014 [Google Scholar]
- 4.Taguchi A, Nishinakamura R: Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21: 730–746.e6, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. : Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Unbekandt M, Davies JA: Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 77: 407–416, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Ganeva V, Unbekandt M, Davies JA: An improved kidney dissociation and reaggregation culture system results in nephrons arranged organotypically around a single collecting duct system. Organogenesis 7: 83–87, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies JA, Chang CH: Engineering kidneys from simple cell suspensions: An exercise in self-organization. Pediatr Nephrol 29: 519–524, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills CG, Lawrence ML, Munro DAD, Elhendawi M, Mullins JJ, Davies JA: Asymmetric BMP4 signalling improves the realism of kidney organoids. Sci Rep 7: 14824, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al. : Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, et al. : Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16: 118–126, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Dressler GR: Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 16: 3527–3534, 2005. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al. : Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremer M, Doerge RW: Statistics at the Bench, NY, Cold Spring Harbor Laboratory Press, 2010 [Google Scholar]
- 16.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F: Expression of green fluorescent protein in the ureteric bud of transgenic mice: A new tool for the analysis of ureteric bud morphogenesis. Dev Genet 24: 241–251, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Qiao J, Sakurai H, Nigam SK: Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc Natl Acad Sci U S A 96: 7330–7335, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michael L, Sweeney DE, Davies JA: The lectin Dolichos biflorus agglutinin is a sensitive indicator of branching morphogenetic activity in the developing mouse metanephric collecting duct system. J Anat 210: 89–97, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michos O, Gonçalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, et al. : Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney D, Lindström N, Davies JA: Developmental plasticity and regenerative capacity in the renal ureteric bud/collecting duct system. Development 135: 2505–2510, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Bohnenpoll T, Bettenhausen E, Weiss AC, Foik AB, Trowe MO, Blank P, et al. : Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev Biol 380: 25–36, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Bohnenpoll T, Wittern AB, Mamo TM, Weiss AC, Rudat C, Kleppa MJ, et al. : A SHH-FOXF1-BMP4 signaling axis regulating growth and differentiation of epithelial and mesenchymal tissues in ureter development. PLoS Genet 13: e1006951, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trowe MO, Airik R, Weiss AC, Farin HF, Foik AB, Bettenhausen E, et al. : Canonical Wnt signaling regulates smooth muscle precursor development in the mouse ureter. Development 139: 3099–3108, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Bohnenpoll T, Weiss AC, Labuhn M, Lüdtke TH, Trowe MO, Kispert A: Retinoic acid signaling maintains epithelial and mesenchymal progenitors in the developing mouse ureter. Sci Rep 7: 14803, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohnenpoll T, Feraric S, Nattkemper M, Weiss AC, Rudat C, Meuser M, et al. : Diversification of cell lineages in ureter development. J Am Soc Nephrol 28: 1792–1801, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI: Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem 277: 7412–7419, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Wu XR, Manabe M, Yu J, Sun TT: Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem 265: 19170–19179, 1990. [PubMed] [Google Scholar]
- 28.Tai G, Ranjzad P, Marriage F, Rehman S, Denley H, Dixon J, et al. : Cytokeratin 15 marks basal epithelia in developing ureters and is upregulated in a subset of urothelial cell carcinomas. PLoS One 8: e81167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang RJ, Hashitani H, Tonta MA, Bourke JL, Parkington HC, Suzuki H: Spontaneous electrical and Ca2+ signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin Exp Pharmacol Physiol 37: 509–515, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Lang RJ, Hashitani H: Pacemaker mechanisms driving pyeloureteric peristalsis: Modulatory role of interstitial cells. Adv Exp Med Biol 1124: 77–101, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Osborn SL, Thangappan R, Luria A, Lee JH, Nolta J, Kurzrock EA: Induction of human embryonic and induced pluripotent stem cells into urothelium. Stem Cells Transl Med 3: 610–619, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos CP, Lapi E, de Villarreal JM, Álvaro-Espinosa L, Fernández-Barral A, Barbáchano A, et al. : Urothelial organoids originating from Cd49f high mouse stem cells display Notch-dependent differentiation capacity. Nat Commun 10: 4407, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullenders J, de Jongh E, Brousali A, Roosen M, Blom JPA, Begthel H, et al. : Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc Natl Acad Sci U S A 116: 4567–4574, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



