Abstract
Extracellular vesicles (EVs), membrane-bound vesicles that are naturally released by cells, have emerged as new therapeutic opportunities. EVs, particularly exosomes and microvesicles, can transfer effector molecules and elicit potent responses in recipient cells, making them attractive therapeutic targets and drug delivery platforms. Furthermore, containing predictive biomarkers and often being dysregulated in various disease settings, these EVs are being exploited for diagnostic purposes. In contrast, the therapeutic application of apoptotic bodies (ApoBDs), a distinct type of EVs released by cells undergoing a form of programmed cell death called apoptosis, has been largely unexplored. Recent studies have shed light on ApoBD biogenesis and functions, promisingly implicating their therapeutic potential. In this review, we discuss many strategies to develop ApoBD-based therapies as well as highlight their advantages and challenges, thereby positioning ApoBD for potential EV-based therapy.
Keywords: apoptosis, apoptotic bodies, extracellular vesicles, therapeutics
Introduction
Extracellular vesicles (EVs) are cell-derived membrane-bound vesicles, traditionally classified as exosomes (30 − 100 nm, endosomal origin), microvesicles (50 − 1000 nm, plasma membrane origin) and apoptotic bodies (ApoBDs, 1000 − 5000 nm, released exclusively by apoptotic cells) based on sizes and molecular markers (Figure 1) [1,2]. Through the transfer of effector molecules such as protein, RNA and DNA, EVs act as important mediators of intercellular communication in various physiological and pathological contexts. In particular, levels and contents of exosomes and microvesicles, are dysregulated in cancer, infection and neurodegenerative diseases, critically contributing to disease development and progression. Due to such pathological roles, many exosome- and microvesicle-targeting approaches have been devised through inhibiting EV formation and/or neutralising circulating EVs [3–5]. For example, pharmacological blockade of ceramide-mediated exosome production and release by GW4869, which acts on neutral sphingomyelinase — a ceramide-metabolising enzyme, reduces gemcitabine resistance and pancreatic tumour growth in vivo[5]. Treatment with anti-CD9 or anti-CD63 (two of the most enriched proteins on the surface of exosomes) also substantially diminishes breast cancer metastasis in animal models [3]. Furthermore, in recent years, exosomes and microvesicles are being increasingly recognised as promising therapeutic agents. In fact, naïve and engineered EVs may offer certain favourable outcomes for anticancer therapy, pathogen vaccination, immunotherapy and regenerative therapy [2,6]. EVs also pose unique properties as highly effective and low-toxicity drug delivery platforms [6,7]. For instance, mesenchymal stem cell (MSC)-derived exosomes loaded with siRNA against KrasG12D mutation (known as iExosomes) are undergoing phase I clinical trial in treating pancreatic cancer [8]. Moreover, as circulating messenger vessels often dysregulated in pathological settings, exosomes and microvesicles are being investigated for a minimally invasive diagnostic strategy, by detecting the packaged disease biomarkers and/or disease-cell-derived EVs. Among current clinical trials involving exosomes (54 studies) and microvesicles (84 studies) listed in www.clinicaltrials.gov, many focus on translating preclinical demonstrations of predictive biomarkers to viable diagnosis or prognosis of therapy outcome for different diseases. The development of exosome- and microvesicle-based therapy and diagnosis is undoubtedly a fast-growing and promising research area, attributable to their clinically desirable characteristics, namely (i) diverse cell-derived biomolecule cargos, (ii) the ability to elicit potent cellular responses, (iii) the ability to surmount biological barriers, (iii) availability, (iv) bioengineerability and (v) scalability [2,9].
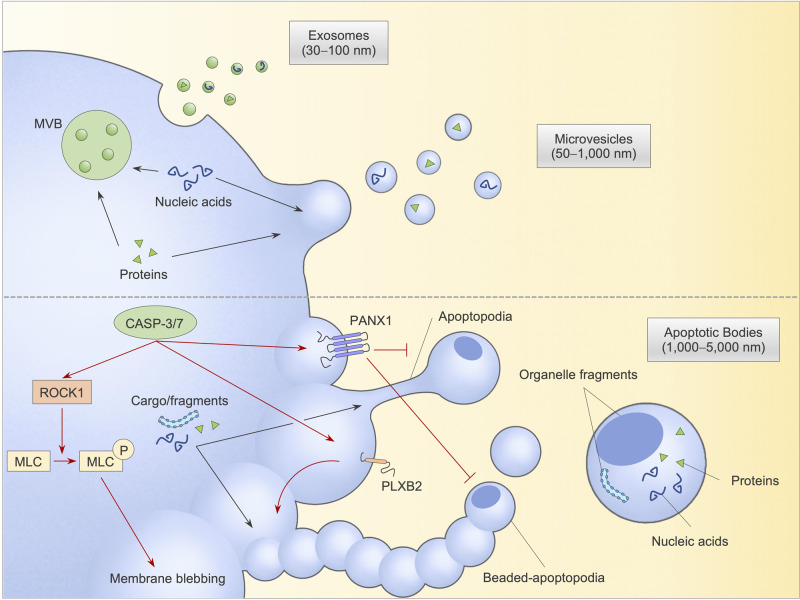
Figure 1. Extracellular vesicle (EV) formation and cargo packaging.
Healthy cells release two major EV subtypes, namely exosomes through exocytosis of multivesicular bodies and microvesicles through plasma membrane shedding (upper panel). Apoptotic cells generate apoptotic bodies (ApoBDs) via apoptotic cell disassembly with three distinct morphological changes: (i) membrane blebbing, (ii) formation of thin membrane protrusion including apoptopodia and beaded-apoptopodia and (iii) fragmentation of membrane protrusion to form distinct ApoBDs. ApoBD biogenesis is regulated by caspase-cleaved substrates, including ROCK1, which phosphorylates and activates blebbing-regulating myosin light-chain (MLC), and PLXB2 (positive regulators) as well as ATP channel PANX1 (negative regulator). Various cellular biomolecules, including nucleic acids and proteins, can be packed into ApoBDs, exosomes and microvesicles, which aid intercellular communications. CASP-3/7, caspases 3 and 7; ROCK1, Rho-associated kinase 1; MLC, myosin light-chain; MLC-P, phosphorylated myosin light-chain; PANX1, pannexin 1; PLXB2, plexin B2.
Whilst exosomes and microvesicles, specifically those released by healthy cells, have been extensively tested as pharmacological targets and cell-free therapeutic agents (as noted above), ApoBDs, a relatively large, dying cell-originated EV type, are generally less defined and their therapeutic applicability remains underexplored. To date, there are only a modest number of ApoBD studies, and no clinical trial has been reported. Nevertheless, recent efforts in establishing ApoBD characterisation criteria and developing ApoBD research tools have led to further insights into ApoBDs biogenesis and function [10,11]. It is becoming apparent that ApoBDs are key messengers released by dying cells to regulate processes including cell clearance, tissue homeostasis, pathogen dissemination and immunity, thus implicating its therapeutic potential [1,12–15]. In this review, we discuss this exciting, yet underappreciated, aspect of ApoBDs, thereby positioning ApoBDs as another candidate in EV's race to therapeutic application.
Targeting apoptotic cell disassembly to therapeutically modulate ApoBD formation
In contrast with previously thought, ApoBD formation is a highly co-ordinated process downstream of apoptosis induction, coined as apoptotic cell disassembly [14,16]. This knowledge has since implied the exploitability of ApoBD biogenesis, similar to other EVs’, in therapeutic targeting as emerging evidence links ApoBD formation with many pathological conditions. For example, billions of cells undergo apoptosis daily as part of normal development and homeostasis; however, apoptotic cells are rarely seen under these physiological conditions, suggesting fast-rate apoptotic cell clearance in the steady state. Of many mechanisms that aid the cell clearance process [17,18], the disassembly of apoptotic cells into ‘bite-sized’ ApoBDs could mediate the rapid and efficient debris removal via efferocytosis, by tissue-resident professional phagocytes (e.g. macrophages and immature dendritic cells) or by neighbouring non-professional phagocytes [12,13,19]. Impaired ApoBD release and consequential defective apoptotic cell clearance have been associated with inflammation and autoimmunity due to subsequent cell/ApoBD lysis as well as exposure of autoantigens (Figure 2) [13,17,20,21]. In atherosclerosis, a condition in which plaques (fatty deposits) build up inside arteries, secondary necrosis by uncleared apoptotic foam cells can promote necrotic core formation, thus accelerating plaque rupture. In addition, previous studies have suggested that ApoBDs that are often enriched with calcium may initiate pathological calcium deposition, leading to vascular calcification in atherosclerosis [22,23]. In systemic lupus erythematosus, an autoimmune disease, clinically isolated antibodies can recognise histone H3 autoantigen enriched in ApoBDs and trigger autoimmune T helper response [24]. In certain settings, active formation of ApoBD by infected or transformed cells can promote disease progression and worsen disease burden. For instance, ApoBDs derived from influenza A virus-infected monocytes can propagate viral infection in bystander cells through the transfer of accompanying virions [25]. Intriguingly, apoptosis of hepatitis C virus-infected hepatocytes induced by alcohol also accelerates viral spread via the similar ApoBD-assisted dissemination [26]. Tumour-derived ApoBDs can horizontally transfer oncogenes h-ras and c-myc to p53-deficient recipient cells, facilitating tumour formation [27].
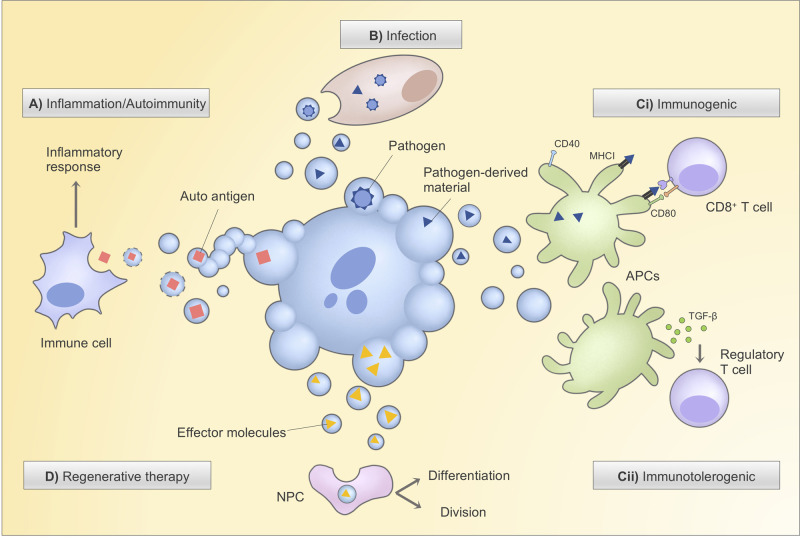
Figure 2. Strategies to leverage ApoBDs for therapeutic development.
ApoBDs are rapidly and efficiently engulfed by tissue-resident professional phagocytes (e.g. macrophages and immature dendritic cells) or by neighbouring non-professional phagocytes. (A) Defective ApoBD and apoptotic cell clearance have been linked to inflammation and autoimmunity due to subsequent secondary necrosis and ApoBD lysis, causing leakage of autoantigens. Pharmacological promotion of ApoBD formation, e.g. via PANX1 inhibition, to aid cell clearance may provide therapeutic relief for clearance-associated diseases. (B) ApoBDs derived from infected cells may contain infectious agents for infection propagation, which could be pharmacologically blocked through the inhibition of the positive regulator of ApoBD formation (e.g. ROCK1). (C) Depending on the cargo materials (e.g. pathogen-derived materials, oncogenes, autoantigens), ApoBDs may or may not induce maturation of engulfing dendritic cells (through the expression of maturation markers CD40, CD80, etc.), respective leading to (i) immunogenic response via antigen presentation-mediated T cell activation or (ii) immunotolerogenic response through the secretion of TGF-β and activation of regulatory T cells. Therefore, ApoBDs and cargo packaging can potentially be manipulated to deliver desired immunotherapeutic outcomes. (D) ApoBDs, such as those derived from stem cells, carry signalling effectors that can promote survival, proliferation and differentiation in engulfing non-professional phagocytes, paving ways to the promising exploitation of stem cell-derived ApoBDs for regenerative therapies. CD40, cluster of differentiation 40; CD80, cluster of differentiation 80; TGF-β, transforming growth factor β; MHCI, major histocompatibility complex I; APC, antigen-presenting cell; NPC, non-professional phagocyte.
The diverse pathological roles of ApoBDs, therefore, suggests that ApoBD formation could potentially be modulated to combat specific diseases. Intriguingly, based on current mechanistic understanding of the apoptotic cell disassembly process, it poses as a versatilely harnessable target that can be either inhibited or promoted for desirable therapeutic outcomes. Notably, ApoBD generation through apoptotic cell disassembly is thought to consist of three characteristic morphological steps regulated by distinct molecular factors (Figure 1) [14]. Firstly, co-ordinated by several protein kinases including caspase-activated Rho-associated kinase 1 (ROCK1), apoptotic cells form membrane blebs on the cell surface [13,28,29]. The dying cells’ membrane then extends, resulting in long membrane protrusions to radiate the blebs. This step is typically negatively regulated by the caspase-activated membrane channel pannexin 1 (PANX1) protein and, in certain cell types, positively controlled by the caspase-cleaved membrane receptor plexin B2 (PLXB2) [12,30]. Afterwards, numerous ApoBDs are released through the fragmentation of the membrane protrusions and/or apoptotic cells [31]. With PANX1 being a key negative regulator of the apoptotic cell disassembly process, PANX1 channel disruption promotes ApoBD formation and, as a result, enhances engulfment efficiency by macrophages [13,30], thus representing an interesting strategy to compensate for clearance defects in conditions such as atherosclerosis and autoimmunity (as mentioned above). Therefore, PANX1 inhibitors, such as FDA-approved probenecid and carbenoxolone, as well as trovafloxacin, could be potentially repurposed for the cell clearance-associated diseases [30]. In contrast, inhibition of ApoBD formation may be beneficial in treating infection and cancer. Recently, haloperidol, an antipsychotic drug, has been reported to inhibit apoptotic monocyte disassembly, effectively reducing the propagation of influenza A virus in in vitro models and lessen disease severity in mouse models [25]. Speculatively, sertraline, a commonly prescribed antidepressant and recently demonstrated ApoBD formation blocker, may be repurposed to achieve similar antiviral dissemination effect. Likewise, inhibitors of ROCK1 and PLXB2, two currently known positive regulators of ApoBD biogenesis would also be of interest for therapeutic design. While it is reasonable to suggest apoptosis inhibition to arrest apoptotic cell disassembly altogether, in certain circumstances such as chemotherapy, blocking ApoBD formation specifically would be preferred to minimise oncogene transfer by treatment-induced formation of tumour cell-derived ApoBDs (as discussed above).
Leveraging ApoBDs as therapeutic agents and diagnostic tools: opportunities and current challenges
Despite current modest knowledge of ApoBD functions, it is becoming clear that ApoBDs also possess a few similar therapeutically exploitable properties compared with other EV types. Particularly, ApoBDs are readily available and easily purified from bodily fluids [11,32]. ApoBDs can also facilitate intercellular communication through biomolecule trafficking and trigger responses in recipient cells [14,15]. Due to the rapid clearance of apoptotic cells by phagocytes, ApoBDs are a crucial messenger vessel between damaged cells, healthy cells and immune cells in processes such as tissue regeneration and disease-fighting immunity. It is, therefore, possible that ApoBDs can be leveraged for therapeutic and diagnostic purposes. In fact, one could infer the potential effectiveness and safety profile of ApoBDs in delivering therapeutic outcomes based on several ApoBD studies to date, albeit cautiously because a few studies may require revalidation with purified ApoBD samples (discussed further below).
ApoBDs for vaccine development and immunotherapy
Under pathological conditions, such as cancer and infection, ApoBDs reportedly contain effective tumour and pathogen-derived antigens that can be recognised by the immune system. Since ApoBDs are readily efferocytosed by antigen-presenting phagocytes like dendritic cells [12,13,19], they could promote adaptive T cell response via the cross-antigen presentation process (Figure 2). For instance, once infected with certain mycobacterial strains, macrophages undergo apoptosis and release pathogen antigen-containing ApoBDs, which can trigger dendritic cell-mediated cross-presentation and CD8+ T cell activation through MHC-I and CD1b [33]. This phenomenon is abolished by the inhibition of apoptosis and hence ApoBD formation, not only suggesting the potent ability of ApoBDs to mount adaptive immune response, but also their potential application for vaccine development and immunotherapy. Furthermore, ApoBDs possess two unique properties that may offer less off-target effect and potent response over healthy cell-derived EVs and other antigen-pulsing approaches: (i) the specific molecular machinery for ApoBD recognition and efferocytosis, and (ii) phagocytosed cellular fragments generate MHC-antigen complex more efficiently than pre-processed peptides and dendritic cell-tumour cell hybrid [17,34]. In fact, a few studies have claimed the effectiveness of tumour ApoBD-efferocytosed antigen-presenting cells in triggering antitumour responses and reducing disease burdens in preclinical mouse models and human patients [35–38]. Henry et al. [35] reported that antigen-presenting cells that pre-efferocytosed ApoBDs from apoptotic murine colorectal cancer cells, increased the survival rate of tumour-bearing mice by 80% . As a control, antigen-presenting cells exposed to nonapoptotic tumour extracts did not show any protective effect. Similarly, dendritic cells pulsed with putative leukemic B-cell ApoBDs stimulated greater T cell activation, proliferation and interferon γ release than dendritic cell-tumour cell hybrid [36]. In two following independent vaccination studies, autologous tumour ApoBD-pulsed dendritic cells appeared to elicit leukaemia-specific CD8+ T cell response in 40–60% of patient cohorts, T cell-tumour cell clustering, and reduced regulatory T cell level without dose-limiting toxicity and autoimmunity [37,38]. Nevertheless, one should be cautious upon interpreting these promising findings and implications on ApoBD-based vaccines because, based on sample preparations, the authors might have used whole apoptotic mixture despite claiming as ApoBDs. Furthermore, the apparent lack of clinical efficacy in these vaccination trials needs to be addressed, possibly by deploying autologous antigen-presenting cells, adding adjuvants or additional boosters.
It is, however, important to note that not all ApoBDs are immunogenic. Depending on a range of factors (e.g. apoptosis stimuli, cell types of origin, rate of clearance, absence of antigenic contents), ApoBDs can be immunologically silent to maintain homeostasis and immune tolerance [39]. In this case, autoantigens contained within ApoBDs are important to establish immune tolerance rather than autoimmunity (Figure 2). In fact, homeostatic engulfment of apoptotic materials does not lead to dendritic cell maturation, a process essential for antigen cross-presentation, but instead induce the release of anti-inflammatory mediators [39,40]. Apoptotic material-efferocytosed tissue-resident dendritic cells also migrate towards the lymph node and peripherally tolerise T cells through TGF-β secretion and regulatory T cell promotion [41,42]. The acquired tolerogenic effects on dendritic cells through apoptotic cells and ApoBDs uptake is thus essential for the induction of immunological tolerance to maintain tissue homeostasis. These observations open an invaluable avenue to translate antigen/allergen-containing ApoBDs for tolerogenic immunotherapy, particularly for allergy and autoimmune diseases such as type 1 diabetes (T1D), which is characterised by the self-attack against insulin-producing β-cells. Reportedly, accelerated autoimmune diabetic mice, once immunised with immature dendritic cells pulsed with β-cell-derived ApoBDs showed significant reduction in diabetes incidence and insulitis as well as T1D relapse [43]. However, like previously mentioned ApoBD vaccine reports, these studies did not appear to use purified ApoBD samples, but, rather, whole apoptotic mixtures. Whilst verification with cell-free ApoBD preparations is essential, these findings have provided evidence to further examine the potential of harnessing ApoBDs for immunotherapy. Furthermore, through advances in the understanding of ApoBD biology and cargo packaging, antigen engineering into ApoBDs could be developed to achieve desirable therapeutic responses, which has been promisingly demonstrated for other types of EVs [44,45].
ApoBD-based regenerative therapies
With the growing interest of stem cell therapies, particularly those based on MSCs, exploiting MSC-derived EVs, which display comparable tissue regenerative properties, are also becoming an attractive therapeutic strategy [9]. Many clinical trials involving MSC-exosomes and MSC-microvesicles have been conducted, with some yielding promising results [9]. Though yet to be clinically tested, the preclinically established importance of stem cell-derived ApoBDs in wound healing and tissue regeneration has implicated their use as regenerative medicine.
It is becoming evident that ApoBDs released by stem cells or differentiated cells contain signalling cues and recyclable materials for survival, proliferation and differentiation in recipient non-professional phagocytes (Figure 2). For instance, hepatocyte-derived ApoBDs can induce JAK/STAT-mediated up-regulation of anti-apoptotic protein Mcl-1 and stress-related activation of pro-survival PI3K/Akt/NF-κB cascade in hepatic stellate cells, resulting in improved cell survival [46]. Using the zebrafish model, Brock et al. [19] elegantly visually captured the formation of basal stem cell-derived ApoBDs in vivo as well as their uptake and ability to induce cell division in neighbouring stem cells via Wnt8a signalling . This local apoptosis-induced proliferation is pivotal to sustain tissue-wide cell number and epithelial homeostasis. Similarly, mature osteoclast-derived ApoBDs also promoted viability and differentiation in preosteoblastic cells, reportedly through receptor activator of NF-κB (RANK)-mediated PI3K/Akt/mTOR/protein S6 kinase signalling for survival and osteoblast differentiation [47]. Together these findings not only uncover the key roles of ApoBDs in both locally and distantly regulating tissue homeostasis, but also highlight the potential of stem cell-derived ApoBD treatment as a novel regenerative therapy. In addition, ApoBDs may hold unrivalled potency and safety-related advantages, compared with other EV- and cell-based approaches. Strikingly, compared with exosome, microvesicle and healthy cell counterparts, the aforementioned osteoclast-derived ApoBDs had the highest RANK level and osteogenic potency [47]. Furthermore, the use of ApoBDs in regenerative therapy, particularly organ transplantation, may offer better safety advantage as they did not an induce inflammatory response and graft rejection as observed by other EVs [48].
ApoBDs as a drug delivery platform
EVs, particularly exosomes and microvesicles, display desirable properties for cell-free delivery of biomolecules into recipient cells, including cargo versatility as well as a lipid composition and clearance-decelerating molecular machinery that improve EV stability and bioavailability. Importantly, cargoes can be loaded in- or onto EVs in vitro and ex vivo through direct loading (e.g. electroporation), passive loading (e.g. overexpressing in producer cells) or active loading (e.g. chimeric expression of cargos and native EV-resident proteins) [7]. These advantages promisingly compensate the limitations of synthetic delivery approaches (e.g. liposome- and nanoparticle-based delivery), thus gathering tremendous interest and efforts amongst EV researchers. Well-characterised preclinically exosome- and microvesicle-based drug delivery technology rapidly enters clinical trials with promising initial safety and efficacy profiles [7]. In contrast with those smaller EVs, ApoBD-based drug loading and delivery remains poorly explored. However, from available literature, one could infer and anticipate the emerging potential of developing ApoBDs as a therapeutic delivery vehicle. In the previously mentioned studies, ApoBDs were shown to transfer different molecules (DNA, miRNA and protein) and effectively regulating various aspects of the phagocytosed/recipient cells [46–50]. Other studies have also reported the efficiency of ApoBD contents in mounting cellular responses such as vascular protection and cell migration [51,52]. This evidence of direct, potent cargo-mediated functional consequences highlights the potential of ApoBDs as a drug delivery system.
A few other technicality and feasibility concerns should nevertheless be raised regarding the use of ApoBDs as a drug delivery platform. Firstly, whether therapeutic agents could be incorporated in ApoBDs is currently undetermined since majority of ApoBD studies to date primarily based on properties of naïve vesicles. Although it was shown that ApoBDs derived from c-myc-transfected murine fibroblasts appeared to transfer the oncogene to recipient cells, leading to the tumorigenic phenotype in vivo [27], more evidence is needed, especially for other biomolecules and pharmaceutical agents. Secondly, unlike the smaller EVs, the size and rapid phagocytic uptake of ApoBDs may hinder biodistribution and bioavailability. Whilst this concern requires further investigation, the fact that systemic administration of mature osteoclast-derived ApoBDs successfully delivered the anticipated functional outcomes in the preosteoclast cells [47] suggests the possibility of ApoBD-based delivery system for certain tissues. Furthermore, the inherent phagocyte targeting property of ApoBDs should be strategically exploited for the phagocyte-specific drug targeting. Finally, the third concern involves the apparent short-lived stability of ApoBDs (∼3–6 h in culture at 37°C [10]), which could overshadow their therapeutic potential. Thorough investigation of storage condition and formulation will possibly help to uncover new approaches to prolong ApoBDs’ shelf-life.
ApoBDs as a diagnostic tool
Being a hallmark of cell death and the carrier of dying cells’ materials, ApoBDs could be an invaluable tell-tale of cell death-prevalent diseases or conditions, such as drug-induced injury, brain injury, transplant rejection, cancer treatments, infection and immune disorders. However, the use of ApoBDs in disease diagnosis is currently limited to apoptotic colopathy, a histological test of gastrointestinal biopsies. Traditionally, an increase in ApoBD number is indicative of gastrointestinal injury [53]. Nonetheless, this visualisation technique for diagnosis requires high-resolution microscopy, well-trained pathologists and thorough assessments of multiple histological sections [53]. A recently developed flow cytometry-based approach [11,54] to quantify ApoBDs may improve the diagnostic accuracy and efficiency in place of the histological examination. Likewise, this flow cytometry-based approach can be applied for celiac disease, an immune disorder characterised by inflammation and villous atrophy due to epithelial apoptosis. In a recent histological study, celiac patients had markedly elevated level of ApoBDs in their duodenal biopsies [55]. Furthermore, coupled with cell surface markers, ApoBDs of different cell origins can be readily detected, quantified and isolated for cell-specific diagnosis [25,32], for example for graft-versus-host disease using crypt cell markers [56]. Since ApoBDs are abundantly circulating in bodily fluids [32,57], this cutting-edge technology would, therefore, allow for analyses of particular disease-associated apoptotic cells. Together with better understanding of ApoBD-contained disease biomarkers, ApoBDs have a great potential as a rapid, accurate and minimally invasive diagnosis.
Future perspectives and concluding remarks
It is becoming clear that ApoBDs are more than ‘garbage bags’ for disposal of dying cells’ materials. With recent mechanistic and functional findings, ApoBDs are emerging as a therapeutically exploitable EV subtype. Here, different strategies to pharmacologically target ApoBD biogenesis as well as to leverage ApoBDs for clinical use are discussed, along with the highlights of ApoBD-specific opportunities and challenges. Notably, future designs of ApoBD-based therapies could strategically focus on ApoBDs’ unique link to cell death and recognition by phagocytes. In doing so, we can maximise their therapeutic efficacy and specificity whilst overcome ApoBDs’ limitations. In addition to future insights into ApoBD biology (e.g. cargo sorting, phagocyte targeting, uptake mechanism), it is desirable to improve ApoBDs’ physiological properties, particularly stability, to optimally utilise ApoBDs for therapeutic purposes. Furthermore, better understanding of ApoBD biology as well as developing novel approaches to engineer and/or modify ApoBDs would also be beneficial in achieving desired therapeutic responses.
Perspectives
EVs have emerged as new therapeutic opportunities and are rapidly progressing toward clinical translation.
ApoBDs, despite being an underexplored type of EVs in preclinical and clinical settings, have recently been shown to play important roles in homeostasis and pathogenesis, implying their tremendous therapeutic potential.
Compared with other EV types, ApoBDs’ unique link to cell death and natural recognition by phagocytes provide distinctive opportunities for future therapeutic design as vaccine, immunotherapies, regenerative medicine, drug delivery and disease diagnosis. However, further understanding of ApoBD biology is certainly required.
Acknowledgements
We thank Dr. Georgia Atkin-Smith for providing helpful suggestions for this work.
Abbreviations
- ApoBDs
apoptotic bodies
- EVs
extracellular vesicles
- MSC
mesenchymal stem cell
- MSC
mesenchymal stem cell
- PANX1
pannexin 1
- PLXB2
plexin B2
- ROCK1
Rho-associated kinase 1
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was funded by the National Health and Medical Research Council [GNT1125033, GNT1140187].
Open Access
Open access for this article was enabled by the participation of La Trobe University in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
Author Contribution
T.K.P. and I.K.H.P. wrote the manuscript with input from D.C.O. D.C.O. designed and illustrated all the figures.
References
- 1.Caruso S. and Poon I.K.H. (2018) Apoptotic cell-derived extracellular vesicles: more than just debris. Front. Immunol. 9, 1486 10.3389/fimmu.2018.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiklander O.P.B., Brennan M., Lötvall J., Breakefield X.O. and Andaloussi S.E.L. (2019) Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 11, eaav8521 10.1126/scitranslmed.aav8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida-Aoki N., Tominaga N., Takeshita F., Sonoda H., Yoshioka Y. and Ochiya T. (2017) Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol. Ther. 25, 181–191 10.1016/j.ymthe.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalano M. and O'Driscoll L. (2020) Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 9, 1703244 10.1080/20013078.2019.1703244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E. and Hill R. (2017) Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 36, 1770–1778 10.1038/onc.2016.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek G., Choi H., Kim Y., Lee H.C. and Choi C. (2019) Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl. Med. 8, 880–886 10.1002/sctm.18-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.György B., Hung M.E., Breakefield X.O. and Leonard J.N. (2015) Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 55, 439–464 10.1146/annurev-pharmtox-010814-12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamerkar S., Lebleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A. et al. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendt M., Rezvani K. and Shpall E. (2019) Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 54, 789–792 10.1038/s41409-019-0616-z [DOI] [PubMed] [Google Scholar]
- 10.Poon I.K.H., Parkes M.A.F., Jiang L., Atkin-Smith G.K., Tixeira R., Gregory C.D. et al. (2019) Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J. Extracell. Vesicles 8, 1608786 10.1080/20013078.2019.1608786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan T.K., Poon I.K.H. and Atkin-Smith G.K. (2018) Detection and isolation of apoptotic bodies to high purity. J. Vis. Exp. 2018, 58317 10.3791/58317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles A.-S., Tixeira M.A., Lay R., Duan F.T., Hawkins M., Phan C.J., et al. (2019) Plexin B2 Is a regulator of monocyte apoptotic cell disassembly. Cell Rep. 29, 1821–1831 10.1016/j.celrep.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 13.Tixeira R., Phan T.K.T.K., Caruso S., Shi B., Atkin-Smith G.K.G.K., Nedeva C. et al. (2020) ROCK1 but not LIMK1 or PAK2 is a key regulator of apoptotic membrane blebbing and cell disassembly. Cell Death Differ. 27, 102–116 10.1038/s41418-019-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkin-Smith G.K. and Poon I.K.H. (2017) Disassembly of the dying: mechanisms and functions. Trends Cell Biol. 27, 151–162 10.1016/j.tcb.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 15.Xu X., Lai Y. and Hua Z.C. (2019) Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 39, BSR20180992 10.1042/BSR20180992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkin-Smith G.K., Tixeira R., Paone S., Mathivanan S., Collins C., Liem M. et al. (2015) A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 6, 7439 10.1038/ncomms8439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon I.K.H., Lucas C.D., Rossi A.G. and Ravichandran K.S. (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14, 166–180 10.1038/nri3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata S. (2018) Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 36, 489–517 10.1146/annurev-immunol-042617-053010 [DOI] [PubMed] [Google Scholar]
- 19.Brock C.K., Wallin S.T., Ruiz O.E., Samms K.M., Mandal A., Sumner E.A. et al. (2019) Stem cell proliferation is induced by apoptotic bodies from dying cells during epithelial tissue maintenance. Nat. Commun. 10, 1044 10.1038/s41467-019-09010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata S., Hanayama R. and Kawane K. (2010) Autoimmunity and the clearance of dead cells. Cell 140, 619–630 10.1016/j.cell.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 21.Cunin P., Beauvillain C., Miot C., Augusto J.F., Preisser L., Blanchard S. et al. (2016) Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses. Cell Death Dis. 7, e2215 10.1038/cddis.2016.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita H., Yamamoto M., Ogino T., Kobuchi H., Ohmoto N., Aoyama E. et al. (2014) Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochem. Funct. 32, 77–86 10.1002/cbf.2974 [DOI] [PubMed] [Google Scholar]
- 23.Proudfoot D., Skepper J.N., Hegyi L., Bennett M.R., Shanahan C.M. and Weissberg P.L. (2000) Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 87, 1055–1062 10.1161/01.res.87.11.1055 [DOI] [PubMed] [Google Scholar]
- 24.Fournel S., Neichel S., Dali H., Farci S., Maillère B., Briand J.-P. et al. (2003) CD4 + T cells from (New Zealand Black × New Zealand White)F 1 lupus mice and normal mice immunized against apoptotic nucleosomes recognize similar th cell epitopes in the C terminus of histone H3. J. Immunol. 171, 636–644 10.4049/jimmunol.171.2.636 [DOI] [PubMed] [Google Scholar]
- 25.Atkin-Smith G.K., Duan M., Zanker D.J., Loh L., Nguyen T.H.O., Koutsakos M. et al. (2020) Monocyte apoptotic bodies are a novel vehicle for influenza virus propagation. Commun Biol. 3, 223 10.1038/s42003-020-0955-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesan M., Natarajan S.K., Zhang J., Mott J.L., Poluektova L.I., McVicker B.L. et al. (2016) Role of apoptotic hepatocytes in HCV dissemination: regulation by acetaldehyde. Am. J. Physiol. - Gastrointest. Liver Physiol. 310, G930–G940 10.1152/ajpgi.00021.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergsmedh A., Szeles A., Henriksson M., Bratt A., Folkman M.J., Spetz A.L. et al. (2001) Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. U.S.A. 98, 6407–6411 10.1073/pnas.101129998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman M.L., Sahai E.A., Yeo M., Bosch M., Dewar A. and Olson M.F. (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 3, 339–345 10.1038/35070009 [DOI] [PubMed] [Google Scholar]
- 29.Sebbagh M., Renvoizé C., Hamelin J., Riché N., Bertoglio J. and Bréard J. (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 3, 346–352 10.1038/35070019 [DOI] [PubMed] [Google Scholar]
- 30.Poon I.K., Chiu Y.H., Armstrong A.J., Kinchen J.M., Juncadella I.J., Bayliss D.A. et al. (2014) Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507, 329–334 10.1038/nature13147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss D.K., Betin V.M., Malesinski S.D. and Lane J.D. (2006) A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 119(Pt 11), 2362–2374 10.1242/jcs.02959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkin-Smith G.K., Paone S., Zanker D.J., Duan M., Phan T.K., Chen W. et al. (2017) Isolation of cell type-specific apoptotic bodies by fluorescence-activated cell sorting. Sci. Rep. 7, 39846 10.1038/srep39846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaible U.E., Winau F., Sieling P.A., Fischer K., Collins H.L., Hagens K. et al. (2003) Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9, 1039–1046 10.1038/nm906 [DOI] [PubMed] [Google Scholar]
- 34.Inaba K., Turley S., Yamaide F., Iyoda T., Mahnke K., Inaba M. et al. (1998) Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188, 2163–2173 10.1084/jem.188.11.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry F., Boisteau O., Bretaudeau L., Lieubeau B., Meflah K. and Grégoire M. (1999) Antigen-presenting cells that phagocytose apoptotic tumor-derived cells are potent tumor vaccines. Cancer Res. 59, 3329–3332 PMID: [PubMed] [Google Scholar]
- 36.Kokhaei P., Rezvany M.R., Virving L., Choudhury A., Rabbani H., Österborg A. et al. (2003) Dendritic cells loaded with apoptotic tumour cells induce a stronger T-cell response than dendritic cell-tumour hybrids in B-CLL. Leukemia 17, 894–899 10.1038/sj.leu.2402913 [DOI] [PubMed] [Google Scholar]
- 37.Palma M., Hansson L., Choudhury A., Näsman-Glaser B., Eriksson I., Adamson L. et al. (2012) Vaccination with dendritic cells loaded with tumor apoptotic bodies (Apo-DC) in patients with chronic lymphocytic leukemia: effects of various adjuvants and definition of immune response criteria. Cancer Immunol. Immunother. 61, 865–879 10.1007/s00262-011-1149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hus I., Roliński J., Tabarkiewicz J., Wojas K., Bojarska-Junak A., Greiner J. et al. (2005) Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia 19, 1621–1627 10.1038/sj.leu.2403860 [DOI] [PubMed] [Google Scholar]
- 39.Vives-Pi M., Rodríguez-Fernández S. and Pujol-Autonell I. (2015) How apoptotic β-cells direct immune response to tolerance or to autoimmune diabetes: a review. Apoptosis 20, 263–272 10.1007/s10495-015-1090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauter B., Albert M.L., Francisco L., Larsson M., Somersan S. and Bhardwaj N. (2000) Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–434 10.1084/jem.191.3.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang F.P., Platt N., Wykes M., Major J.R., Powell T.J., Jenkins C.D. et al. (2000) A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 10.1084/jem.191.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kushwah R., Wu J., Oliver J.R., Jiang G., Zhang J., Siminovitch K.A. et al. (2010) Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3 + Treg. Eur. J. Immunol. 40, 1022–1035 10.1002/eji.200939782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin-Gallen S., Clemente-Casares X., Planas R., Pujol-Autonell I., Carrascal J., Carrillo J. et al. (2010) Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin. Exp. Immunol. 160, 207–214 10.1111/j.1365-2249.2009.04082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bliss C.M., Parsons A.J., Nachbagauer R., Hamilton J.R., Cappuccini F., Ulaszewska M. et al. (2020) Targeting antigen to the surface of EVs improves the in vivo immunogenicity of human and non-human adenoviral vaccines in mice. Mol. Ther. - Methods Clin. Dev. 16, 108–125 10.1016/j.omtm.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anticoli S., Falcone E., Ruggieri A. and Federico M. (2016) Engineered exosomes boost the HCV NS3-specific CD8+ T lymphocyte immunity in humans. Trials Vaccinol. 5, 105–110 10.1016/j.trivac.2016.05.001. [DOI] [Google Scholar]
- 46.Jiang J.X., Mikami K., Venugopal S., Li Y. and Török N.J. (2009) Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-κB-dependent pathways. J. Hepatol. 51, 139–148 10.1016/j.jhep.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Q., Liang M., Wu Y., Ding N., Duan L., Yu T. et al. (2019) Mature osteoclast- derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J. Biol. Chem. 294, 11240–11247 10.1074/jbc.RA119.007625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dieudé M., Bell C., Turgeon J., Beillevaire D., Pomerleau L., Yang B. et al. (2015) The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 7, 318ra200 10.1126/scitranslmed.aac9816 [DOI] [PubMed] [Google Scholar]
- 49.Liu D., Kou X., Chen C., Liu S., Liu Y., Yu W. et al. (2018) Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 28, 918–933 10.1038/s41422-018-0070-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H., Liu S., Qiu X., Yang X., Bao L., Pu F. et al. (2020) Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy 16, 1–16 10.1080/15548627.2020.1717128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zweemer A.J.M., French C.B., Mesfin J., Gordonov S., Meyer A.S. and Lauffenburger D.A. (2017) Apoptotic bodies elicit gas6-mediated migration of axl-expressing tumor cells. Mol. Cancer Res. 15, 1656–1666 10.1158/1541-7786.MCR-17-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B. et al. (2009) Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2, ra81 10.1126/scisignal.2000610 [DOI] [PubMed] [Google Scholar]
- 53.Karamchandani D.M. and Review R.C. (2018) Apoptotic colopathy: a pragmatic approach to diagnosis. J. Clin. Pathol. 71, 1033–1040 10.1136/jclinpath-2018-205388 [DOI] [PubMed] [Google Scholar]
- 54.Jiang L., Tixeira R., Caruso S., Aktin-Smith G.K., Baxter A.A., Hulett M.D. et al. (2016) Monitoring the progression of cell death and disassembly of dying cells by flow cytometry. Nat. Protoc. 4, 655–663 10.1038/nprot.2016.028 [DOI] [PubMed] [Google Scholar]
- 55.Lee M., Betman S., Iuga A., Yang H.M., Fleming J., Green P.H.R. et al. (2019) An association between crypt apoptotic bodies and mucosal flattening in celiac disease patients exposed to dietary gluten. Diagn. Pathol. 14, 98 10.1186/s13000-019-0878-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akpek G., Chinratanalab W., Lee L.A., Torbenson M., Hallick J.P., Anders V. et al. (2003) Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol. Blood Marrow Transplant. 9, 46–51 10.1053/bbmt.2003.49999 [DOI] [PubMed] [Google Scholar]
- 57.Lázaro-Ibáñez E., Sanz-Garcia A., Visakorpi T., Escobedo-Lucea C., Siljander P., Ayuso-Sacido Á. et al. (2014) Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate 74, 1379–1390 10.1002/pros.22853 [DOI] [PMC free article] [PubMed] [Google Scholar]