Abstract
Background
Following initiation of MDR-TB treatment, patients have a choice to receive follow up DOT supervision at either the central initiating facility or at a peripheral facility.
Objectives
We describe the adherence patterns of MDR-TB patients undergoing DOT supervision at the two health facility categories during intensive phase of treatment.
Methods
We used a retrospective cohort of patients initiated on MDR TB treatment at Mulago National Referral Hospital between 2014 and 2016. We extracted data from the National Tuberculosis and Leprosy Program records and analysed these using STATA V14.
Result
Majority (84.01%) of the patients received their DOT supervision from the peripheral facilities. Males made up 62.1% of patients, and 91.2% had had their household contacts screened for MDR-TB. 26.5% of the patients on peripheral DOT supervision had good adherence to treatment protocol compared to 0% among patients on central initiating health facility DOT supervision. Among the patients with good adherence, 24.1% had contacts screened for MDR-TB as compared to 3.6% with poor adherence.
Conclusion
More patients preferred MDR-TB DOT supervision at peripheral facilities, which had better adherence to the treatment protocol compared to the central initiating facility. Younger people and those with household contacts screened had better adherence to the treatment protocol, highlighting areas for targeted interventional programs for MDR-TB in resource limited settingsMore patients preferred MDR-TB DOT supervision at peripheral facilities, which had better adherence to the treatment protocol compared to the central initiating facility. Younger people and those with household contacts screened had better adherence to the treatment protocol, highlighting areas for targeted interventional programs for MDR-TB in resource limited settings
Keywords: MDR-TB, adherence, central initiating, peripheral health facility, DOTS, SORT IT
Background
Multi-Drug Resistant Tuberculosis (MDR-TB) remains a public health threat worldwide with an estimated 558,000 MDR -TB cases registered globally in 20171. Poor adherence to TB treatment has been cited as a major con-tributor to the development of MDR-TB2–5. In addition to the spread of MDR-TB, poor adherence while on treatment can lead to poor treatment outcomes for the sick individual6.
The major mile stone in the management of MDR-TB is sputum conversion, mostly expected to occur while still in the intensive phase of treatment7. Adherence to treatment is cited among the factors associated with sputum conversion8. In order to enhance adherence, WHO recommends the use of Directly Observed Therapy (DOT) in managing TB especially MDR-TB9,10. DOT is part of the support package offered to all TB patients who include the MDR-TB patients11. This package is sensitive and supportive to the patient's needs, with a treatment supporter observing intake of every dose, and paying due attention to the dosing and dosage of the right drugs11. DOT among MDR-TB patients is at a health facility by a health worker10 and in the past patients have been hospitalized and managed till they achieved sputum conversion12. However, as a result of resource limitations, this aspect in TB management has changed over the years.
In Uganda, patients are diagnosed and initiated on MDR-TB treatment at central facilities, typically referral hospitals. These patients are then counselled and given one of two options for follow up of facility DOT supervision – either at the central initiating health facility or at a peripheral health facility; except when patients are very sick and need to be hospitalized till improvement when the two options are then presented to them. Despite the option chosen, the patients are expected to return to the central initiating facility monthly for a routine clinical check-up, evaluation of on-going care, and assessment for treatment side effects.
This strategy is not used in Uganda alone; other countries such as South Africa, Kenya, Peru and regions like Tomsk in Russia, among others13–16, are using a similar system in an effort to address resource limitations at the central initiating facilities while enabling patients to receive treatment from a convenient location17.
DOT was designed to address poor drug adherence; however, several others factors play a role in poor adherence to MDR-TB treatment. For example, long duration of treatment, coupled with drug toxicities, and unfavorable dosage formulations have been cited13. Poor adherence among MDR-TB patients increases the risk of disease transmission in the community as well as having a poor prognosis in the affected patient18.
While acknowledging that all factors that lead to poor adherence need to be addressed, the authors of this paper find that there is a need to evaluate the impact that choice of facility for DOT follow-up has on adherence to MDR-TB treatment, a factor on which there is a paucity of information. Previous comparison studies showed better treatment outcomes among individuals receiving treatment at peripheral rather than central health facilities, and poorer adherence in patients receiving DOT follow-up at central facilities as compared to peripheral facilities13–16.
We therefore aimed to describe the adherence patterns of MDR-TB patients undergoing DOT supervision following initiation at Mulago National Referral Hospital in central Uganda in 2014–2016. Furthermore, we aimed to describe characteristics associated with the adherence patterns seen at both the central initiating facility and the peripheral facilities.
Methods
This was a retrospective cohort study among MDR TB patients initiated on treatment between 1st January 2014 and 31st December 2016 at Mulago National Referral Hospital (MNRH). MNRH serves as a specialist treatment and diagnostic centre for MDR TB with a 39-bed in-patient capacity. There were 1,384 MDR-TB patients registered by NTLP country wide with 494 at the hospital between January 2014 and December 2016.
We included all patients diagnosed with pulmonary MDR-TB and initiated on treatment at MNRH between 1st January 2014 and 31st December 2016. We excluded those patients that were below the age of 15 because of the difficulties in detecting bacteria in their sputum even when actually present.
Upon confirmation of MDR-TB, patients are counselled and given the option of either receiving treatment from the central initiating facility (MNRH) or a peripheral facility (health centres that patients might feel are conveniently located near them). Although they would receive their daily treatment at these facilities, they would be expected to report back to the central facility at the end of every month, during which time, they would undergo clinical evaluation, including a check for sputum conversion.
Data collection
We extracted data from the electronic MDR-TB register which is maintained by the National Tuberculosis and Leprosy Program (NTLP). We extracted data on the following: date of initiation of MDR-TB treatment, site of DOT supervision, submitted sputum samples collected on a monthly basis, age, sex, baseline smears, HIV sero-status, di- agnostic category, treatment regimen one is started on, and type of patient (retreatment or new patient).
The availability of results from the monthly submitted sputum samples during the intensive phase of treatment was used as a proxy for adherence to the treatment protocol during the intensive phase of treatment. Adherence is defined as one having submitted ALL six sputum samples at the monthly visits during the intensive phase of treatment among ambulatory patients. All patients included in this study were ambulatory and all of them were counselled and offered a choice of follow up at either the central initiating facility or peripheral health facility, with the instruction to return every month to the central initiating facility to submit sputum samples.
Data management
The extracted data was checked for accuracy and was later double-entered into Epi Data.
Data analysis
Categorical variables were summarised using proportions and percentages. The continuous variables were summarised using means and standard deviations or medians and interquartile ranges depending on their distribution. To determine the statistical significance of the observed differences, a p-value of 0.05 or less was used. Pearson's Chi square (χ2) test for two independent proportions was used to test for significance. Power of 98% was calculated for Pearson χ2 test at P<0.05 when we took sample size of 268 at peripheral health facilities and 51 at central initiating facility and effect size of 30%.
Ethical considerations
Approval to conduct the study was obtained from Makerere University, School of Medicine Review Ethics Committee (SOMREC).
Results
Of 319 MDR-TB patients initiated on treatment at Mulago National Referral Hospital between 1st January and 31st December 2016, 268 opted for a DOT supervision programme at a peripheral health facility. The patients were evenly distributed in the different categories of age used in the study. Majority of them were male (62.5%) and had standard treatment for MDR-TB (90.5%). 54.1% of the study participants were HIV positive and 91.8% had had their household contacts screened for TB (table 1).
Table 1.
Baseline characteristics of the study population
| Peripheral-DOTs/ n (%) | Central-DOTs/ n (%) | Total/ n (%) | |
|---|---|---|---|
| AGE | |||
| 15–25 | 93 (91.20) | 9 (8.80) | 102 (31.9) |
| 26–35 | 85 (77.30) | 25 (22.70) | 110 (34.6) |
| >=36 | 90 (84.10) | 17 (15.90) | 107 (33.5) |
| Total | 268 (84.01) | 51 (15.99) | 319 (100) |
| SEX | |||
| Female | 103 (85.10) | 18 (14.90) | 121 (37.9) |
| Male | 165 (83.30) | 33 (16.70) | 198 (62.1) |
| TYPE OF PATIENT | |||
| New | 134 (82.70) | 28 (17.30) | 162(50.8) |
| Retreatment | 134 (85.40) | 23 (14.60) | 157 (49.2) |
| CONTACTS SCREENED? | |||
| No | 7 (25.00) | 21 (75.00) | 28 (8.8) |
| Yes | 261 (89.70) | 30 (10.30) | 291 (91.2) |
| REGIMEN TYPE | |||
| Empirical | 7 (100.00) | 0 (0.00) | 7 (2.2) |
| Standard | 240 (83.60) | 47 (16.40) | 287 (90.5) |
| Individualized | 21 (91.30) | 2 (8.70) | 23 (7.3) |
| DIAGNOSTIC CATEGORY | |||
| INH Mono | 5 (1.6) | ||
| resistant | 3 (60.00) | 2 (40.00) | |
| MDR | 167 (52.3) | ||
| Confirmed | 153 (91.60) | 14 (8.40) | |
| Pan Sensitive | 7 (70.00) | 3 (30.00) | 10 (3.1) |
| RIF-Mono | 137 (43.0) | ||
| resistant | 105 (76.64) | 32 (23.36) | |
| HIV STATUS | |||
| Positive | 133 (77.30) | 39 (22.70) | 172 (54.1) |
| Negative | 135 (92.50) | 11 (7.50) | 146 (45.9) |
Adherence to the intensive phase of the MDR-TB treatment protocol
There was poor adherence to the intensive phase of the MDR-TB treatment protocol with only 26.5% of the individuals at the peripheral sites adhering and 0% of those at the central initiating facility.
Within the overall poor adherence, individuals aged 15 to 25 years reported the highest adherence to the protocol (30.4% at peripheral facilities). Furthermore, only 3.6% of individuals who had not had their household contacts screened had good adherence in comparison to 24.1% of the individuals who had had their household contacts screened (See table 2).
Table 2.
Factors affecting adherence to intensive phase of MDR-TB treatment
| Adherence to policy during intensive phase | |||
| Good adherence/ n (%) | Poor adherence/ n (%) | p.value | |
|---|---|---|---|
| Facility DOT supervision | |||
| Peripheral- | |||
| DOTs | 71 (26.50) | 197 (73.50) | |
| Central-DOTs | 0 (0.00) | 51 (100.00) | <0.001* |
| RECODE of AGE | |||
| (AGE) | |||
| 15–25 | 31 (30.40) | 71 (69.60) | |
| 26–35 | 18 (16.40) | 92 (83.60) | |
| >=36 | 22 (20.60) | 85 (79.40) | 0.043* |
| SEX | |||
| Female | 28 (23.10) | 93 (76.90) | |
| Male | 43 (21.70) | 155 (78.30) | 0.767 |
| TYPE OF | |||
| PATIENT | |||
| New | 39 (24.10) | 123 (75.90) | |
| Retreatment | 32 (20.40) | 125 (79.60) | 0.428 |
| CONTACTS SCREENED? | |||
| No | 1 (3.60) | 27 (96.40) | |
| Yes | 70 (24.10) | 221 (75.90) | 0.013* |
| REGIMEN TYPE | |||
| Empirical | 2 (28.60) | 5 (71.40) | |
| Standard | 67 (23.30) | 220 (76.70) | |
| Individualized | 2 (8.70) | 21 (91.30) | 0.248 |
| HIV STATUS | |||
| Positive | 34 (19.80) | 138 (80.20) | |
| Negative | 37 (25.30) | 109 (74.70) | 0.234 |
Variable is significant using a p-value of . 0.05 for statistical significance
Smear results recorded at the monthly visits during the intensive phase of MDR-TB treatment
Fig 1 shows the percentages of patients who submitted their monthly sputum samples after the baseline visit.
Fig 1.
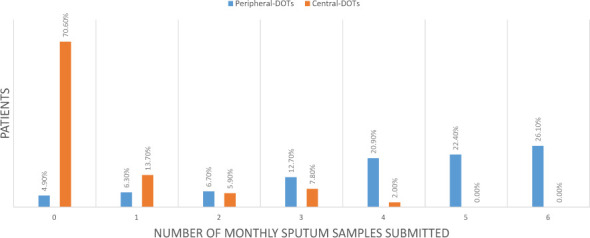
A bar graph of recorded monthly smear results at the peripheral and central DOT supervising facilities
The trend for the patients who received DOT supervision at the central initiating facilities shows over 70% of patients did not submit any monthly sputum samples after initiation on treatment. The remaining approximately 30% of patients submitted at least one sputum sample, with none submitting more than 4, that is, 5 or 6 monthly sputum samples after initiating treatment. Among the patients who received DOT supervision at the peripheral facilities, 26.1% of them submitted all the 6 monthly sputum samples after initiation of treatment during the intensive phase, 20.9% and 22.4% submitted 4 and 5 samples respectively while 4.9% did not submit any samples.
Discussion
In this study we aimed to describe the adherence patterns of MDR-TB patients undergoing DOT supervision in central or peripheral health facilities, following treatment initiation at a central one, in Uganda, in 2014–2016. Furthermore, we aimed to describe characteristics associated with the adherence patterns seen at both types of facilities. We found that of 319 MDR-TB patients initiated on treatment at Mulago National Referral Hospital between 1st January and 31st December 2016, 268 (84.01%) opted for a DOT supervision programme at a peripheral health facility. We also found that although adherence to the treatment protocol was generally poor, it was significantly better in those aged 15–25, those who had their household contacts screened and those using a peripheral facility for DOT supervision.
Furthermore, this study found that majority (84.01%) of patients preferred DOT supervision at peripheral health facilities. This might be explained by the fact that these peripheral facilities that are allowed to offer DOT supervision being spread out amongst different regions are closer to the patients than the central facility would be. This makes their access more convenient in comparison, unless the patient lives within reasonable distance of the central facility. This finding is different from what was observed in South Africa where less than half (47.5%) of patients chose to receive treatment from the peripheral health facilities13. This observed difference might be due to a difference in availability of resources at the different health facilities. For example, Mulago National Referral Hospital has a bed capacity of only 39 at the MDR-TB section of the TB clinic, which automatically limits the number of MDR-TB patients that can be hospitalised and be fully monitored at the central health facility. Coupling the two reasons could explain the observed higher percentages of individuals choosing to receive DOT supervision from the peripheral health facilities in Uganda.
The overall adherence to the intensive phase of the treatment protocol was poor. Only 26.5% of individuals who had DOT supervision at the peripheral health facilities had good adherence to the protocol while at the central facility, this proportion was actually 0%, representing an overall adherence percentage of 24.76%. The overall adherence to the protocol could have been affected by the long duration of MDR-TB treatment, the side effects of the drugs and the route of administration of some MDR-TB drugs13. However, the difference between the central and peripheral health facilities could be explained by the long distances to the central facility and possibly higher and more intimate support given to patients who receive DOT supervision at the peripheral sites. This group had more house hold members having been screened and thus more people were aware of their illness and the need for daily dosing and submission of sputum samples on a monthly basis. These possibly supported them more to adhere to the treatment protocol as compared to those who had DOT supervision at the central health facility. The study from South Africa refrred to earlier found a much higher overall adherence of 78.28% which may be because the study assessed adherence to treatment defining it as not missing treatment for more than two months13. This is different from the outcome used in our study of adherence to treatment protocol which defined adherence as submission and having results of the monthly sputum samples throughout the intensive phase of treatment.
Of the 291 patients who had had their household members screened for TB, 89.7% were from patients who were having DOT supervision at the peripheral health facilities. This may be because of a more intimate relationship with staff at the peripheral facility, as they most likely would be living in the same area. It may also be because of proximity to the health facility whereby it is easier to invite household folks to it than if they had to travel to the central facility in the city. This results in more aware and counselled family members, which in turn leads to a higher degree of support that is given to individuals receiving DOT supervision from the peripheral facilities from these family and friends. An individual having to go to the health facility for dosing daily or regularly would most likely raise a lot of questions amongst his peers and household members, which would be uncomfortable and even eventually lead to defaulting on the visits, if they had not been involved and/or did not have a good understanding of the process. Once the household members are in the know, they may be more supportive and encouraging for patients to adhere to treatment, and thus the observed higher percentages among individuals choosing peripheral facilities for their follow-up treatment.
Individuals aged 15–25 years of age had better adherence to the protocol (30.4%) as compared to 16.4% and 20.6% from 26–35 and above 35 years age groups. In Uganda, individuals below 25 years typically are still under care of their parents or guardians. The adults are in control of the health of this age group, and determine their health seeking behaviour. Individuals in this age group go to hospital for screening for TB in the company of an adult, and upon confirmation of TB, the parent or guardian is counselled into encouraging the patient to adhere to the treatment protocol. As a result of this
continuous support from the adult, patients 15 to 25 years might have better adherence as compared to other age groups where there is no external influence on adherence. However, we note that our results are different from those observed in Southern Ethiopia where age had no bearing on adherence19. This difference might be a result of the study in Ethiopia being among TB drug sensitive patients with shorter duration of treatment. In addition, there might be a differing social structure where individuals leave their parents' or guardians' care much earlier in Ethiopia as compared to Uganda.
The percentages of individuals who submitted monthly sputum samples during the intensive phase of treatment increased with time among individuals receiving DOT supervision from the peripheral facilities, while reducing with time among those at the central initiating facility. 4.9% of the individuals receiving DOT supervision from the peripheral facilities did not return any sputum samples after initiating on treatment, while this proportion was 70.6% among those at the central initiating facility. This high attrition rate at the central initiating facility could be attributed to the long distances the patients might have needed to travel to the central facility, the failure to build personal relationships with health workers at the central facility and less family and community involvement and support for patients to adhere to the treatment protocol. Taking DOT supervision to the peripheral facilities is more convenient to the patients, and the patients can build bonds with the health workers at the facilities in addition to the community and family support which accounted for the 26.1%, 22.4% and 20.9% of the patients submitting 6, 5 and 4 sputum samples respectively during the intensive phase of treatment in our study.
Strengths of this study
This is the first study that we know of looking at adherence to treatment protocols at the different facilities offering DOTS for MDR-TB in Uganda. In a bid to find lasting solutions to a problem like Drug Resistant TB, the findings are a critical step in improving the context of the disease and its solutions in the country. The study capitalised on routine NTLP records which reflect the actual settings in the program. Being retrospective, they also provide information independent of potential behavioural modifications by patients which is likely to arise if they were recruited for a prospective cohort study. In addition, this study also uses routine data to identify a local problem which would call for a tailored solution, away from ‘one-size-fits-all’ solutions that are not contextualised.
Limitations of the study
The study used routine data that is collected at the MDR-TB DOT supervision health facilities. This data is often prone to being incomplete and may thus affect the study results.
We made an assumption that the records of results equates directly to the fact that a sputum sample was submitted for examination. This might be an underestimation if there are persons who submitted a sample but for some reason did not get the sample examined or the results recorded.
Implications for policy
In this study, it is clear that peripheral health facilities are doing better with MDR-TB patients adhering to the treatment protocol during the intensive stage of treatment. This raises the confidence in using these for follow up of patients for DOTS and similar interventions. It should also be motivation for equipping these centres more, to enable them manage more numbers of these kinds of patients.
The study showed a generally low adherence overall, but especially in the central initiating facility. This calls for health managers to devise means to improve adherence overall but also target the central facilities and the particular groups like older individuals. It also calls for interventions that increase screening in household contacts. This would not only help identify those affected but also increase awareness that seems to be important for adherence of those already diagnosed.
Implications for research
It is not completely clear why there is poor adherence at the central facility to a very high level. This might not be entirely explained by distance from the facility. Therefore, there is need to carry out a better designed study probing the factors driving adherence at both central and peripheral facilities. This would provide a better understanding of the factors and other issues that can be addressed by particular interventions.
Conclusion
In this study we found that more patients prefer to receive their follow up MDR-TB DOT supervision from peripheral facilities, and in addition, show a better adherence to the treatment protocol compared to those at the central initiating facility. Younger people and those with household contacts screened also had better adherence to the treatment protocols. This highlights areas for targeted interventional programs for Drug Resistant TB in resource-limited settings. Furthermore, it highlights areas for further research, to understand why central
facilities might not be performing as expected. In general, a very low adherence rate was noted all round and therefore there is still a need to tackle non-adherence to treatment protocols more aggressively, otherwise this represents a potential public health risk to the general population.
Funding
The SORT IT program that supported this study was funded by the United Kingdom's Department for International Development (DFID), The Union, MSF and La Fondation Veuve Emile Metz- Tesch (Luxembourg). La Fondation Veuve Emile Metz- Tesch supported open access publications costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the man- uscript.
Conflict of interest
None declared.
Open access statement
In accordance with WHO's open-access publication policy for all work funded by WHO or authored / co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1.World Health Organization . Geneva: 2018. Global Tuberculosis Report 2018. Licence: CC BY-NC-SA 3.0 IGO. Report No.: 978-92-4-156564-6. [Google Scholar]
- 2.Davies PD. Drug-resistant tuberculosis. Journal of the Royal Society of Medicine. 2001;94(6):261–3. doi: 10.1177/014107680109400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wurie FB, Cooper V, Horne R, Hayward AC. Determinants of non-adherence to treatment for tuberculosis in high-income and middle-income settings: a systematic review protocol. BMJ Open. 2018;8(1):e019287. doi: 10.1136/bmjopen-2017-019287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moonan PK, Quitugua TN, Pogoda JM, Woo G, Drewyer G, Sahbazian B, et al. Does directly observed therapy (DOT) reduce drug resistant tuberculosis? BMC Public Health. 2011;11:19. doi: 10.1186/1471-2458-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumende CM. Risk Factors for Multidrug-resistant Tuberculosis. The Indonesian Journal of Internal Medicine. 2018;50(1):1–2. [PubMed] [Google Scholar]
- 6.Burman WJ, Cohn Dl Fau - Rietmeijer CA, Rietmeijer Ca Fau - Judson FN, Judson Fn Fau - Sbarbaro JA, Sbarbaro Ja Fau - Reves RR, Reves RR. Noncompliance with directly observed therapy for tuberculosis. Epidemiology and effect on the outcome of treatment. Chest Journal. 1997;115(5):1168–73. doi: 10.1378/chest.111.5.1168. [DOI] [PubMed] [Google Scholar]
- 7.Curry International Tuberculosis Center and California Department of Public Health. Chen Lisa, Schecter Gisela F.(editors) Third ed. 2016. Drug-Resistant Tuberculosis: A survival Guide for Clinicians. [Google Scholar]
- 8.Akinsola OJ, Yusuf OB, Ige OM, Okonji PE. Models for Predicting Time to Sputum Conversion Among Multi-Drug Resistant Tuberculosis Patients in Lagos, South-West Nigeria; pp. 2296–2565. (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annabel. Kanabus. DOTS and DOTS-PLus- failed Global Plans 2018Global Plans 2018. [Available from: https://www.tbfacts.org/dots-tb/.
- 10.World Health Organization . Guidelines for establishing DOTS-PLUS pilot projects for the management of Multidrug-resistant Tuberculosis (MDR-TB) In: Gupta Rajesh, Arnadottir T., editors. 20, avenue Appia, Geneva, Switzerland: WHO, Stop TB Department; 2000. [Google Scholar]
- 11.World Health Organization . Fourth Edition ed. Geneva 27, Switzerland: World Health Organisation; 2010. Treatment of Tuberculosis guidelines. 147 p. [Google Scholar]
- 12.Nathanson E, Lambregts-van Weezenbeek C Fau - Rich ML, Rich Ml Fau - Gupta R, Gupta R Fau - Bayona J, Bayona J Fau - Blondal K, Blondal K Fau - Caminero JA, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerging Infectious Diseases. 2006;12(9):1389–97. doi: 10.3201/eid1209.051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loveday M, Wallengren K, Brust J, Roberts J, Voce A, Margot B, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients, KwaZulu-Natal, South Africa. The international Journal of Tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(2):163–71. doi: 10.5588/ijtld.14.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyieng'o D, Park P, Gardner A, Kisang G, Diero L, Sitienei J, et al. Community-based treatment of multidrug-resistant tuberculosis: early experience and results from Western Kenya. Public Health Action. 2012;2(2):38–42. doi: 10.5588/pha.12.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitnick C, Bayona J Fau - Palacios E, Palacios E Fau - Shin S, Shin S Fau - Furin J, Furin J Fau - Alcantara F, Alcantara F Fau - Sanchez E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. New England Journal of Medicine. 2003;348(2):119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 16.Keshavjee S, Gelmanova IY, Pasechnikov AD, Mishustin SP, Andreev YG, Yedilbayev A, et al. Treating Multidrug-Resistant Tuberculosis in Tomsk, Russia. Annals of the New York Academy of Sciences. 2008;1136(1):1–11. doi: 10.1196/annals.1425.009. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh RD, Dhande DJ, Sachdeva KS, Sreenivas A, Kumar AM, Satyanarayana S, et al. Patient and Provider Reported Reasons for Lost to Follow Up in MDRTB Treatment: A Qualitative Study from a Drug Resistant TB Centre in India. PLoS Medicine. 2015;10(8) doi: 10.1371/journal.pone.0135802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke MF, Appleton Sc Fau - Bayona J, Bayona J Fau - Arteaga F, Arteaga F Fau - Palacios E, Palacios E Fau - Llaro K, Llaro K Fau - Shin SS, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment; pp. 1537–6591. (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woimo TT, Yimer WK, Bati T, Gesesew HA. The prevalence and factors associated for anti-tuberculosis treatment non-adherence among pulmonary tuberculosis patients in public health care facilities in South Ethiopia: a cross-sectional study. BMC Public Health. 2017;17(1):269. doi: 10.1186/s12889-017-4188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


