Abstract
Background:
Elabela (ELA) is a hormone that is secreted at high levels in the kidneys of a healthy adult. This study aims to investigate whether serum ELA levels of patients with Type 2 Diabetes vary with the severity of renal damage.
Methods:
Our study included 50 healthy control subjects and 100 diabetic patients, who were categorized into groups based on urine albumin/creatinine ratios (ACR). Patients included in the study were assigned to four groups: Group 1 (healthy control), Group 2 (ACR<29mg/g), Group 3 (ACR=30–299 mg/g), and Group 4 (ACR>300 mg/g normal or high serum creatinine). Physical examination findings, demographic characteristics of the study group were recorded, and serum ELA levels and other laboratory parameters were assessed using appropriate methods.
Results:
The results of the study indicated that ELA levels determined in healthy individuals gradually decreased through stages of normal albuminuria, microalbuminuria, and macroalbuminuria. Moreover, ELA had a significant negative correlation with LDL-C (r=−0.201, p=0.014), glucose (r=−0.437, P<0.001), retinopathy (r=−0.222, P=0.006), serum BUN (r=−0.161, P=0.049), and a positive correlation with eGFR (r=0.250, P=0.002).
Conclusions:
The fact that ELA levels are higher in healthy individuals compared to diabetic patients without microalbuminuria, and higher in diabetic patients without microalbuminuria compared to patients with advanced albuminuria and kidney damage, suggests that the ELA level can be an important clinical prognostic variable and even a promising agent for the treatment of diabetic nephropathy patients.
Keywords: Elabela, diabetes, diabetic kidney disease, albuminuria
Introduction
Diabetes mellitus is a chronic disease characterized by vascular and neuropathic complications related to insulin deficiency or problems induced by insulin1. Diabetes ranks first among factors that lead to end stage renal disease (ESRD) worldwide as a result of the significant increase in the prevalence of diabetes and associated complications. It is well-known that hyperglycemia triggers microvascular complications both directly and through local and systemic increases in certain cytokine, chemokine, and growth factors. As the disease may progress through various stages and reach end-stage renal disease (ESRD) via an uneventful course, yearly albuminuria tests are recommended for type 2 diabetic patients starting from the time of diagnosis, and for type 1 diabetic patients after five years on average. In the early stages of diabetes that precede morphological changes, changes such as an increase in renal plasma flow, intraglomerular hydrostatic pressure, and glomerular filtration rate are encountered2.
These changes appear due to numerous factors that result from the interactions of a series of metabolic and hormonal factors induced by elevated glucose. The loss of sulfated proteoglycans and anionic regions in the glomerular basement membrane and the mesangial matrix was shown to induce excess accumulation of proteoglycans such as chondroitin sulphate and dermatan sulphate in these areas3. This causes charge-dependent renal selectivity to decrease and the basement membrane to thicken. In tissues where glucose intake is independent of insulin, excess glucose is typically metabolized to sorbitol through the polyol pathway and this reaction is catalyzed by the aldose reductase enzyme. Many experimental diabetes models have shown that this pathway has anspan style=“font-family:‘Times New Roman’”> important role in the development of microvascular complications and can be intercepted by aldose reductase enzyme inhibitors4. Therefore, there is a need for novel albuminuria biomarkers and intervention approaches designed to reduce albuminuria that target the kidneys in order to delay the progression of diabetic kidney disease (DKD) and decrease the risk of cardiovascular diseases and associated mortality5,6,7.
Elabela (ELA), which is also known as Toddler and Apela, is a peptide of 54 amino acids recently discovered by two research groups that possesses a mature form of 32 amino acids and a secretory signal8,9. ELA is secreted in many tissues including the kidney, is an endogenous ligand of apelin receptor (APJ) and a G-protein-coupled receptor (GCPR). It becomes active during embryogenesis and circulates in adults as a secreted hormonal peptide9. A recent study has shown that ELA has protective effects against ischemia-reperfusion damage in the kidneys and cultured kidney cells through anti-inflammatory, antiapoptotic, and antifibrotic effects10.
Since ELA is predominantly expressed in the kidneys, we think that changes in serum ELA levels may reflect the severity of kidney damage, and therefore, could function as a marker of kidney function in patients with DKD. In this study, we aimed to determine the relationship of ELA levels with diabetic nephropathy and metabolic indices by measuring ELA levels in a healthy control group and type 2 diabetic (T2D) patients with and without diabetic nephropathy.
Materials and methods
This study was conducted in accordance with the ethical guidelines stated in the Helsinki Declaration and with an approval from Firat University Medical Faculty Ethics Committee (22.11.2018, 14). This observational study included a total of 150 subjects, of which 50 were healthy controls and 100 were type 2 diabetics who had presented to the internal diseases polyclinic between 2018–2019. Inclusion criteria were as follows: (1) A T2D diagnosis according to 1999 World Health Organization diagnostic and classification criteria; (2) a glycosylated hemoglobin (HbA1c) value between 6.0–13.0%; (3) age ≥ 30 years; and (4) a baseline urine albumin/creatinine ratio (ACR) level determined using two specimens. Exclusion criteria included: (1)A diagnosis of type 1 diabetes, (2) age below 30 years, (3) a diagnosis of chronic hypertensive and congenital anomaly, liver disease, acute infection, and/or hypothyroidism, and (4) presence of malignancy; also excluded were patients with end stage renal disease who undergo hemodialysis or peritoneal dialysis and those with mental retardation or any psychiatric problems. The first group was comprised of healthy individuals who had presented to the internal diseases polyclinic for routine check-ups and had no diseases. Diabetic patients were categorized into three groups based on their urinary albumin/creatinine ratios (ACR): Group 2 (n=40, no albuminuria, ACR ≤ 29 mg/g), Group 3 (n=30, microalbuminuria, ACR = 30–299 mg/g), Group 4 (n=30, macroalbuminuria and normal or elevated serum creatinine, ACR ≥ 300 mg/g)11.
Venous blood samples were collected for fasting plasma glucose (FPG), HbA1c, blood urea nitrogen (BUN), serum creatinine, total cholesterol (t-CHOL), low density lipoprotein cholesterol (LDL-C), and serum ELA measurements. Urine was collected over 7 days in 2 separate occasions and the ACR value was determined as the average of the two test results. Urinary albumin was measured using immunonephelometry (DCA 2000; Bayer, Leverkusen, Germany) and urine creatinine was determined using the alkaline picrate method. ACR was calculated as albumin (mg)/creatinine (g). Estimated GFR (eGFR) was computed using the MDRD equation 12. Glucose levels were determined in plasma samples by enzymatic methods. HbA1c was measured in EDTA whole blood samples (D10; Bio-Rad, Hercules, CA, USA) using high-performance liquid chromatography. Serum ELA levels were determined using an ELABELA (human) -EIA kit (Peninsula Laboratories International, Inc., San Carlos, CA, USA) according to manufacturer's instructions. This test has inter- and intra- assay coefficients of variation below 8% and 5%, respectively.
Statistical analysis
All statistical analyses were performed using a computer packaged software program (SPSS-22). In addition to descriptive statistical methods Mean (), Standard deviation (SD); quantitative data was analysed using the Student's t-test in testing parameters that show normal distribution and one-way variance analysis in comparisons across groups (One-way ANOVA). The Wilcoxon matched pairs test, which assesses the significance of the difference between pairs was utilized and the chisquare test was used for the comparison of qualitative data. Multiple linear regression and Pearson correlation analyses were used to assess the relationship among variables. The results were evaluated with a 95% confidence interval and a p<0.05 level of significance.
Results
The study group consisted of 71 were male and 79 were female participants. There were no differences between the groups with regard to age, body mass index (BMI), systolic blood pressure, diastolic blood pressure, hemoglobin, and platelet counts. However, the length of time with disease was higher in Group 4 compared to the other two diabetic groups. HbA1c levels were determined to be higher in Group 3 and Group 4 compared to Group 2, with statistical significance (p=0.026 and p=0.034, respectively). Diabetic retinopathy was encountered at a greater rate in Group 4 compared to Groups 2 and 3, with statistical significance. Simultaneous with increased serum creatinine and BUN levels, patients in Group 4 had significantly lower eGFR values compared to the other groups (p <0.001, p <0.001, and p = 0.046, versus Group 1, 2, and 3, respectively).
Table 1.
The demographic, clinical and biochemical characteristics of all subjects.
| Variables | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| gender (M/F) | 50(24/26) | 40(15/25) | 30(14/16) | 30(18/12) |
| Age (years) | 54,6 ± 6,3 | 52,9±7,9 | 56,3±8,2 | 56,8±8,5 |
| Dur (years) | 0 | 8,3±8,6 | 8,1±5,8 | 10,6±7,1 |
| DR (%) | 0 | 55a | 70a,b | 86,6a,b,c |
| BMI (kg/m2) | 25,3±3,3 | 27,4±4,9 | 26,8±4 | 27±4,6 |
| FPG (mg/dl) | 90,2±10,2 | 186,9±78,8a | 185,3±65,5a | 213,1±99,1a |
| HbA1c (%) | 4,5±1,1 | 9±2,7a | 10,9±3a,b | 10,8±2,8a,b |
| SBP (mmHg) | 112,4±4,8 | 116,5±8,6 | 117,5±8,4 | 117,6±8,4 |
| DBP (mmHg) | 70,4±4,8 | 72,6±5,8 | 72,1±5,6 | 72,1±6,5 |
| t-CHOL (mmol/L) | 172,5±29,9 | 209,5±43,4a | 190,3±44,9 | 179,1±58,8b |
| LDL-C (mmol/L) | 100,5±25,5 | 136,5±37,6a | 116,9±41,2 | 102,9±34,9b |
| Cre (umol/L) | 0,73±0,15 | 0,71±0,15 | 0,70±0,13 | 1,35±1,05a,b,c |
| BUN (mmol/L) | 13,3±3,35 | 14,4±3,18 | 16,7±5,15 | 25,5±17,52a,b,c |
| eGFR(mL/min/1.73 m2) | 138,1±24,5 | 133,3±46,7 | 113,1±20,2a | 87,6±40,06a,b,c |
| ACR (mg/g) | 5,8±3,6 | 16,02±5,2a | 175±52,2a,b | 1755,3±2160,6a,b,c |
| ELA (ng/mL) | 35,93±21,05 | 11,91±12,76a | 7,35±4,55a | 6,67±2,85a |
| Haemoglobin(g/dL) | 13,9±1,3 | 13,9±1,8 | 13,1±1,9 | 12±2,3 |
| Plt(x109/L) | 270,3±64,3 | 268,9±77,4 | 252,1±83,2 | 291,6±88 |
Note: Data are expressed as the mean ± SEM. Group1: healthy control; Group 2: diabetic patients without albuminuria; Group 3: diabetic patients with microalbuminuria; Group 4: diabetic patients with macroalbuminuria and normal or increased serum creatinine; M, male: F, female: Dur: duration of diabetes; DR: diabetic retinopathy; BMI: body mass index; FPG: fasting plasma glucose; HbA1c: glycosylated hemoglobin; SBP: systolic blood pressure; DBP: diastolic blood pressure; t-CHOL: total cholesterol; LDL-C: low-density lipoprotein cholesterol; BUN: blood urine nitrogen; Cre: serum creatinine; eGFR: estimated glomerular filtration rate; ACR: urinary albumin/creatinine ratio. The data are expressed as the mean ± SEM for normally distributed data with 25th and 75th quartiles for skewed data.
P < 0.05 vs. Group 1;
P < 0.05 vs. Group 2;
P < 0.05 vs. Group 3.
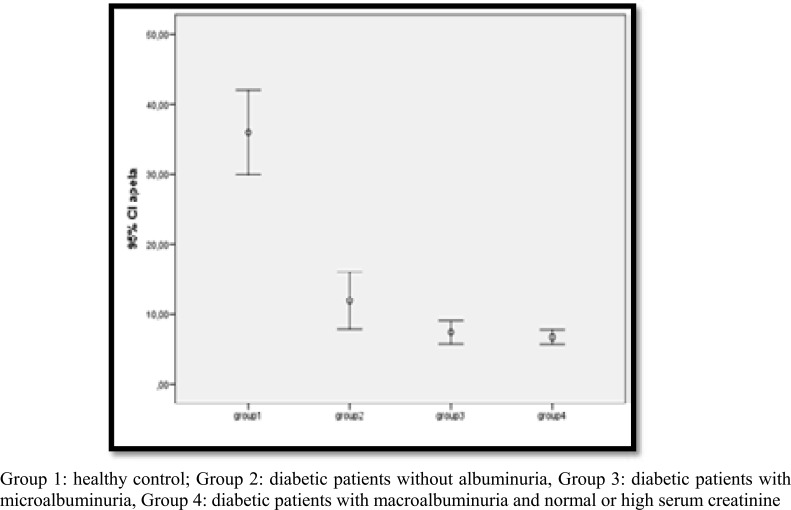
Comparisons of serum ELA levels across groups (as shown in Figure 1), revealed that serum ELA levels gradually decrease as one moves from the healthy control group (Group 1), to diabetic patients without albuminuria (Group 2), diabetic patients with microalbuminuria (Group 3), and diabetic patients with macroalbuminuria and normal or high serum creatinine, paralleling the increase in albuminuria. Pairwise comparisons determined a statistically significant difference between the healthy group and the other three groups (Group 2, 3, 4) in terms of ELA levels (p< 0.001, p< 0.001, p< 0.001, respectively). Although we detected a gradual decrease in serum ELA levels across Groups 2, 3, and 4, this progressive decrease was not statistically significant.
Figure 1.
Cross-group comparison of ELA levels.
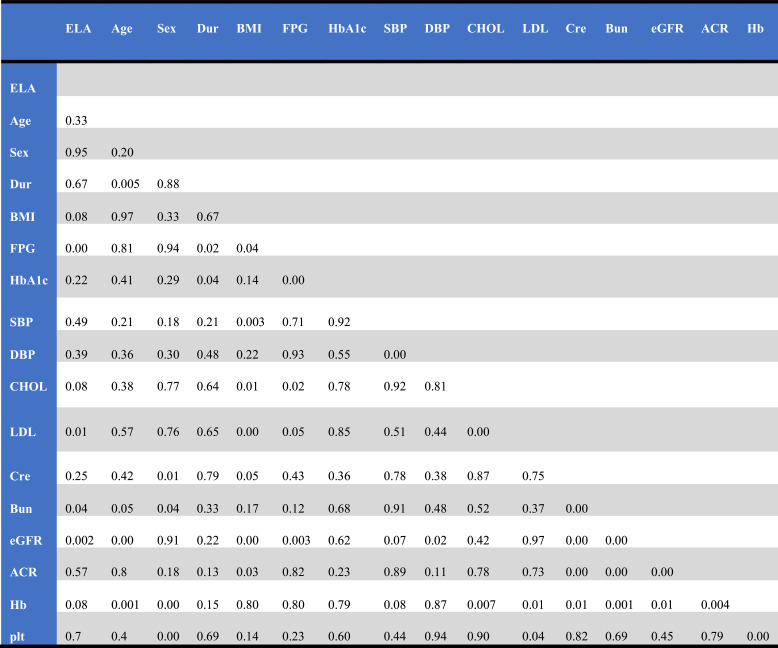
Table 2.
P-values between study variables
Table 3.
Relationship of serum ELA levels with other variables
| LDL-C | FBG | BUN | eGFR | DR | ||
|---|---|---|---|---|---|---|
| ELA | Pearson Correlation | −,201 | −,437 | −,161 | ,250 | −,222 |
| Sig.(2-tailed) | 0.014 | 0.00 | 0,049 | 0.002 | 0.006 |
We studied the relationship between serum ELA levels and other variables. In simple correlation analyses, as shown in Table 2 (P-values between variables) and table 3 (correlation coefficients between variables) ELA had a significantly negative correlation with LDL-C (r = −0.201, P <=0.014), retinopathy (r = −0.222, P = 0.006), serum BUN(r = −0.161, P = 0.049), serum FBG (r = −0.437, P < 0.001) and a positive correlation with eGFR(r = 0.250, P = 0.006).
Discussion
Diabetes ranks first among factors that lead to end stage renal disease (ESRD) worldwide due to the significant increase in the prevalence of diabetes and associated complications. It is well-known that hyperglycemia triggers microvascular complications both directly and through local and systemic increases in certain cytokine, chemokine, and growth factors. In the early stages of diabetes that precede morphological changes, changes such as an increase in renal plasma flow, intraglomerular hydrostatic pressure, and glomerular filtration rate are seen2. These changes are caused by numerous factors that result from the interactions of a series of metabolic and hormonal factors induced by elevated glucose. The loss of sulfated proteoglycans and anionic regions in the glomerular basement membrane and the mesangial matrix was shown to induce excess accumulation of proteoglycans such as chondroitin sulphate and dermatan sulphate in these areas3. This causes renal charge selectivity to decrease and the basement membrane to thicken. In tissues where glucose intake is independent of insulin, excess glucose is typically metabolized to sorbitol through the polyol pathway and this reaction is catalyzed by the aldose reductase enzyme. Many experimental diabetes models have shown that this pathway is important in the development of microvascular complications and can be intercepted by aldose reductase enzyme inhibitors4.
ELA is coded by gene AK092578, which is located at a region of the human genome that was previously defined as non-coding RNA. The cDNA that codes ELA is conserved to the utmost extent in vertebrates, indicating its essential physiological functions13,14. ELA shows similarities to apelin, which is another ligand of the apelin receptor. Being expressed widely in various organs including the heart, kidney, lung, brain, skeletal muscles, and the gastrointestinal tract, Apelin is a multi-functional protein related to angiogenesis, regulation of the energy metabolism, and fluid homeostasis15,16. Apelin was reported to have favorable effects by increasing glucose intake in diabetics, improving insulin sensitivity, controlling diabetes-induced kidney hypertrophy and albuminuria, and suppressing oxidative stress17–20. In this regard, the role of apelin in chronic kidney disease (CKD) is subject to discussion. Chng et al. showed that ELA is expressed earlier than apelin during development and is expressed simultaneously with APJ before the onset of gastrulation. As both ELA and apelin act on APJ, ELA may serve as a functionally selective ligand for the APJ 8.
We evaluated the relationship of serum ELA levels with diabetic nephropathy and metabolic indices by measuring serum ELA levels of a healthy, disease-free control group and type 2 diabetic patients with and without diabetic nephropathy. Our study determined that serum ELA levels were much higher in the healthy population compared to patients with diabetes. When we classified diabetic patients according to their albuminuria levels; diabetic patients without albuminuria were found to have higher ELA levels compared to patients with albuminuria. ELA levels showed a gradual decrease across patient groups as albuminuria increased and eGFR, which indicates impaired kidney function, decreased. Furthermore, we showed that serum ELA levels were negatively correlated with LDL-C, retinopathy, serum BUN, serum fasting plasma glucose (FPG) and positively correlated with eGFR, with statistical significance. Therefore, in light of the results of this study, we reason that ELA levels may be a novel marker variable candidate. Although our study sample included 59 healthy individuals and 100 diabetic patients, studies with larger and more comprehensive patient groups are warranted to confirm our findings.
Yang et al. 21 showed that ELA and apelin could be detected in healthy human plasma at subnanomolar levels and ELA was found at higher levels than apelin in circulation. Studies have determined that, differently from apelin, ELA transcripts are predominantly expressed in pluripotent stem cells, fetal and adult kidneys, prostate, and vascular endothelium, as well as in renal tubular epithelial cells in humans and rodents8,10,13,21. Chen et al.10 reported that ELA therapy markedly improved acute kidney damage through in vitro and in vivo inhibition of apoptosis, reduction of the inflammatory response, suppression of fibrosis and related markers (TGF-ß1, fibronectin, vimentin, and collagen 1) and reduction of autophagy and the DNA damage response. These findings collectively suggest that ELA may play a protective role in kidney diseases, including diabetic nephropathy. However, the relationship between serum ELA and DKD had not been characterized prior to this study.
This clinical study includes 50 healthy controls and 100 diabetic patients with comprehensive clinical characteristics, at all stages of diabetic kidney disease. In comparison to healthy individuals, we determined a statistically significant decrease in serum ELA levels in diabetic patients without microalbuminuria, a further decrease in diabetic patients with microalbuminuria, and a greater decrease in patients with macroalbuminuria and/or high serum creatinine. Therefore, serum ELA levels show a progressive decrease that parallels albuminuria severity and we infer that decreased ELA may serve as a new marker variable for diabetic kidney damage. Compared to the ELA levels of healthy individuals, we determined a marked decrease at early stages of diabetes that precede morphological changes. Our theoretical explanation for this finding is as follows; with the elevated serum glucose-induced interactions of a series of metabolic and hormonal factors, various factors emerge that cause an increase in renal plasma flow, intraglomerular hydrostatic pressure, and glomerular filtration rate.
Consequently, the loss of sulfated proteoglycans and anionic regions in the glomerular basement membrane and the mesangial matrix causes increased accumulation of proteoglycans such as chondroitin sulphate and dermatan sulphate in these areas. We think that this causes charge-dependent renal selectivity to decrease, reducing the synthesis and expression of ELA in the kidney. Remarkably, pairwise comparisons demonstrated that ELA levels were negatively correlated with LDL-C, FBG, retinopathy, serum BUN and positively correlated with eGFR. We can interpret this as serum ELA levels being linked to degraded glomerular structure and albuminuria due to high serum FBG and LDL-C in T2D patients. Considering the known functions of ELA, such as protection against tissue damage, increasing cell survival, and reducing blood pressure 8,9, it seems plausible that the decrease in renal ELA expression is not only a simple marker for kidney damage but also a factor that causes the secondary complications of diabetic kidney disease mentioned above, and this was our impression. More comprehensive studies with a larger sample size are warranted in order to explain the role of ELA in DKD and other kidney diseases. Additional experimental and interventional studies are also needed to investigate the mechanisms that bring about the relationship between ELA and DKD.
Conclusion
In conclusion, our study shows that serum ELA levels are lower at all stages of diabetic nephropathy compared to the healthy control group, that they are related to the severity of albuminuria, although without statistical significance, and that they can serve as a clinically significant and important biomarker in patients with DKD. ELA can be used as a new diagnostic marker and/or therapeutic agent.
Acknowledgments
None.
Funding
This research received no external funding.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
Study concept and design: Erhan O. and Ebru O. ; acquisition of data: Y.D. and I. B. ; data analysis, Erhan O. and N.G. ; writing-original draft preparation, Erhan O. ; writing-review and editing, Erhan O.; supervision, E.D. All authors consented to publish. All authors reviewed and approved manuscript.
References
- 1.Diagnosis, Classification and Screening in Glycemic Disorders, author. Diagnosis, Treatment and Follow-up of TEMD Diabetes Mellitus and Complications. 9th Edition. Turkish Endocrinology and Metabolism Association; 2017. [Google Scholar]
- 2.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to niolecular pathology. Eur J Clin Invest. 2004;34:785–96. doi: 10.1111/j.1365-2362.2004.01429.x. Indexed for MEDLINE. [DOI] [PubMed] [Google Scholar]
- 3.Van Dijk C, Beri T. Pathogenesis of diabetic nephropathy. Rev Endocr Metab Disord. 2004;5:237–48. doi: 10.1023/B:REMD.0000032412.91984.ec. Indexed for MEDLINE. [DOI] [PubMed] [Google Scholar]
- 4.Chang W, Dimitriadis E, Ailen T, Dunlop ME, Cooper M, Larkins RG. The effect of aldose reductase inhibitors on glomerular prostaglandin production and urinary albumin excretion in experimental diabetes mellitus. Diabetologia. 1991;34:225–31. doi: 10.1007/BF00405080. [DOI] [PubMed] [Google Scholar]
- 5.Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Shao X, Lu K, Zhou J, Ren M, Xie X, Liu J, Xu Y, Ding Y, Shen X, Zhu C. Urinary RBP and NGAL Levels are Associated with Nephropathy in Patients with Type 2 Diabetes. Cell Physiol Biochem. 2017;42:594–602. doi: 10.1159/000477860. Indexed for MEDLINE. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Zhang X, Hu C, Lu W. Exenatide reduces urinary transforming growth factor-beta1 and type IV collagen excretion in patients with type 2 diabetes and microalbuminuria. Kidney Blood Press Res. 2012;35:483–488. doi: 10.1159/000337929. [DOI] [PubMed] [Google Scholar]
- 8.Chng SC, Ho L, Tian J. Reversade B: ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, Mitchell A, Ma J, Dubrulle J, Reyon D, Tsai SQ, Joung JK, Saghatelian A, Schier AF. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Wang L, Wang W, Cheng C, Zhang Y, Zhou Y, Wang C, Miao X, Wang J, Wang C, Li J, Zheng L, Huang K. ELABELA and an ELABELA Fragment Protect against AKI. J Am Soc Nephrol. 2017;28:2694–2707. doi: 10.1681/ASN.2016111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Microvascular complications and foot care. Diabetes Care. 2015;38:S58–66. doi: 10.2337/dc15-S012. [DOI] [PubMed] [Google Scholar]
- 12.Fontela PC, Winkelmann ER, Ott JN, Uggeri DP. Estimated glomerular filtration rate in patients with type 2 diabetes mellitus. Rev Assoc Med Bras (1992) 2014;60:531–537. doi: 10.1590/1806-9282.60.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Yu D, Wang M, Wang Q, Kouznetsova J, Yang R, Qian K, Wu W, Shuldiner A, Sztalryd C, Zou M, Zheng W, Gong DW. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep. 2015;5:8170. doi: 10.1038/srep08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie F, Lv D, Chen L. ELABELA: a novel hormone in cardiac development acting as a new endogenous ligand for the APJ receptor. Acta Biochim Biophys Sin (Shanghai) 2014;46:620–622. doi: 10.1093/abbs/gmu032. [DOI] [PubMed] [Google Scholar]
- 15.Chapman NA, Dupre DJ, Rainey JK. The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem Cell Biol. 2014;92:431–440. doi: 10.1139/bcb-2014-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219:R13–35. doi: 10.1530/JOE-13-0227. Indexed for MEDLINE. [DOI] [PubMed] [Google Scholar]
- 17.Attane C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, Guzman-Ruiz R, Dray C, Bezaire V, Rancoule C, Kuba K, Ruiz-Gayo M, Levade T, Penninger J, Burcelin R, Penicaud L, Valet P, Castan-Laurell I. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–320. doi: 10.2337/db11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu H, He L, Li L, Chen L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol Genet Metab. 2016;119:20–27. doi: 10.1016/j.ymgme.2016.07.012. Indexed for MEDLINE. [DOI] [PubMed] [Google Scholar]
- 19.Day RT, Cavaglieri RC, Feliers D. Apelin retards the progression of diabetic nephropathy. Am J Physiol Renal Physiol. 2013;304:F788–800. doi: 10.1152/ajprenal.00306.2012. Indexed for MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng H, He X, Hou X, Li L, Chen JX. Apelin gene therapy increases myocardial vascular density and ameliorates diabetic cardiomyopathy via upregulation of sirtuin 3. Am J Physiol Heart Circ Physiol. 2014;306:H585–597. doi: 10.1152/ajpheart.00821.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, Upton PD, Crosby A, Sawiak SJ, Carpenter TA, Glen RC, Morrell NW, Maguire JJ, Davenport AP. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation. 2017;135:1160–1173. doi: 10.1161/CIRCULATIONAHA.116.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]