Abstract
Background:
Activation of immunological and systemic inflammation markers are common in obesity and asthma.
Objective:
The target of this study was to assess impact of weight reduction on immunological and systemic inflammation markers in obese asthma patients.
Material and methods:
Eighty asthmatic patients of both sex; their age and body mass index (BMI) mean were 38.72 ± 7.14 year and 32.65 ± 3.18 Kg/m2 respectively. Exclusion criteria included smokers, infections, vaccinations, cancer, surgery, immune system disorders and medications that may influence immune system function as anti-inflammatory medications, analgesics and anti-depressant. All subjects were randomly enrolled in weight reduction group (group A) or control group (group B).
Results:
The main findings in the present study indicated that weight reducing program in group (A) was associated with significant reduction in the mean values of IL6, TNF-α, and IL8 in addition to significant increase in the mean values of CD4 and CD8 cell count . However, findings of group (B) showed no significant changes. Moreover, Comparison between both groups at the end of the study revealed significant differences.
Conclusion:
Weight reduction improved immunological and systemic inflammation markers in obese asthma patients.
Keywords: Bronchial asthma, cytokines, obesity, immune system, weight reduction
Introduction
Recently, bronchial asthma affects about 300 million subjects and this number will reach 400 million of worldwide subjects by 20251. Asthma characterized with attacks of obstruction, chronic inflammation and airway hyper-responsiveness of airways2. However, obesity is a the most common medical problem worldwide3,4. Several studies confirm association between the degree of adiposity and asthma5–7.
Both obesity and asthma prevalence is increasing concomitantly8. Obesity elevates the severity of asthmatic symptoms9 and reduce their response to medications10,11, due to many mechanisms include mechanical, anatomical12–14 or inflammatory causes15,16. Obesity is considered as a pro-inflammatory state as it is usually associated with persistent low-grade systemic inflammation17.
Several studies reported impaired immune system response and high susceptibility for infections and many disorders related to the degree of obesity18–21. In addition, there is close relation between asthma and obesity22. Obese adipose tissue is the site of marked accumulation of immune cells23–25.
Weight reduction intervention is the most recent management policy for control of obesity via exercise, diet regimen and life style modification26. The purpose of this research was to measure response of systemic inflammation and immunological parameters to weight loss in obese asthmatic patients.
Subjects and methods
Subjects
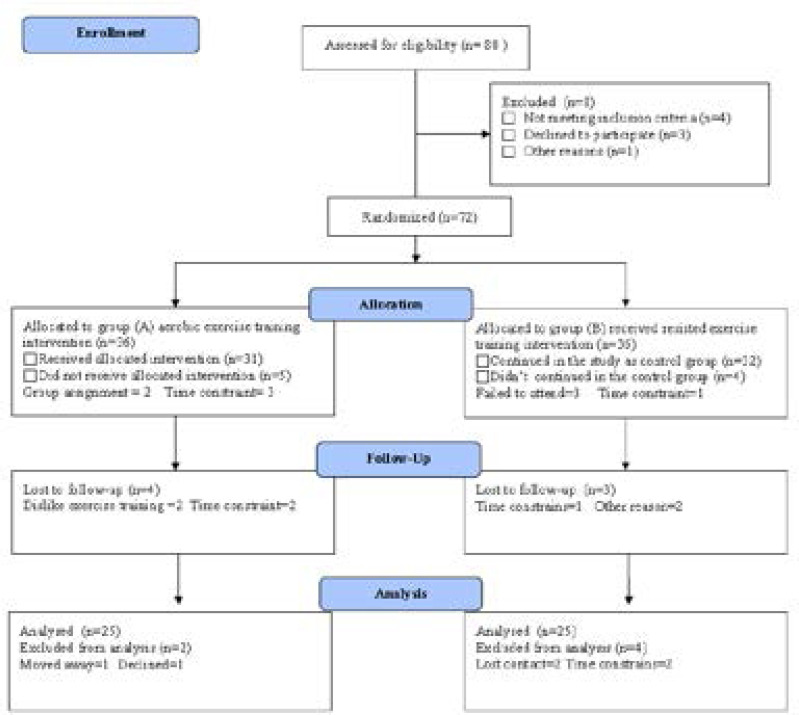
Eighty asthmatic patients of both sex; their age and body mass index (BMI) mean were 38.72 ± 7.14 year and 32.65 ± 3.18 Kg/m2 respectively. Exclusion criteria included smokers, infections, vaccinations, cancer, surgery, immune system disorders and medications that may influence immune system function as anti-inflammatory medications, analgesics and anti-depressant. All subjects were randomly enrolled in weight reduction group (group A) or control group (group B) according to the CONSORT diagram that outline the details of the screening and randomization (figure 1). All participants signed the consent before joining the study. In addition, ethical clearance was obtained from the concerned committee of King Abdulaziz University.
Figure (1):
Subjects screening and recruitment CONSORT diagram.
Measurements
A. Inflammatory cytokines: Overnight fasting venous blood sample will be drain and will be centrifuged at + 4 °C to measure interleukin -6 (IL-6) by “Immulite 2000” immunassay analyzer (Siemens Healthcare Diagnostics, Deerfield, USA). While, tumor necrosis factor-alpha (TNF-α) and interleukin- 8 (IL-8) were measured using ELISA.
B. Flow cytometry analysis: Immunological parameters CD4 and CD8 were measured by flow cytometry using Cytomics FC 500 and CXP software (Beckman Coulter).
Procedures
1. The training group (Group A) received aerobic treadmill exercise training for 12 weeks according to the standard recommendation of exercise training. Training session included warm up for 5 minutes, thirty minutes of 60–70% of maximum heart rate aerobic exercise training that followed by 10 minutes cooling down. Participants had 3 training sessions weekly for 3 months. Also, a dietician supervised diet regimen which provided 1200 Kilocalories/day for 3 months.
2. The control group (Group B) received no training intervention or diet control.
Statistical analysis
The statistical analysis was conducted using SPSS version 21 , where comparison between mean values of parameters in both groups was assessed with unpaired t- test .However, paired t-test used to compare the differences between mean values in the same group ( level of significance P<0.05).
Results
The descriptive statistics proved that weight reduction group (group A) or control group (group B) were homogenous as there were no significant differences between both groups regarding the baseline criteria (table 1).
Table 1:
Characteristics of participants in both groups.
| Characteristic | Group (A) | Group (B) | Significance | |
|---|---|---|---|---|
| Age (year) | 39.57 ± 7.34 | 38.26 ± 6.81 | 0.021 | |
| BMI (kg/m2) | 31.72 ± 2.78 | 31.51 ± 3.23 | 0.018 | |
| SBP (mm Hg) | 147.36 ± 10.14 | 145.17 ± 9.92 | 0.025 | |
| DBP (mm Hg) | 86.64 ± 8.72 | 85.13 ± 7.25 | 0.009 | |
| Hemoglobin (gm/dl) | 12.26 ± 2.83 | 12.48 ± 2.74 | 0.027 | |
| FVC (L) | 2.82 ± 0.97 | 2.97 ± 0.83 | 0.013 | |
| FEV1(L) | 1.65 ± 0.86 | 1.83 ± 0.79 | 0.028 | |
| FEV1/FVC (%) | 57.91 ± 8.11 | 59.87 ± 7.32 | 0.011 |
BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FVC= forced vital capacity; FEV1= forced expiratory volume in the first second; FEV1/FVC= Ratio between forced expiratory volume in the first second and forced vital capacity.
The main findings in the present study indicated that weight reducing program in group (A) was associated with significant reduction in the mean values of IL6, TNF-α, and IL8 in addition to significant increase in the mean values of CD4 and CD8 cell count (Tables 2). However, findings of group (B) showed no significant changes(table 3). Moreover, Comparison between both groups at the end of the study revealed significant differences (table 4).
Table 2:
Mean value and significance of immune and systemic inflammation markers in group (A) before and after the study.
| Mean + SD | t-value | Significance | ||
|---|---|---|---|---|
| Pre | Post | |||
| BMI (kg/m2) | 31.72 ± 2.78 | 27.86 ± 2.42* | 6.83 | 0.004 |
| TNF-α (pg/mL) | 11.97 ± 2.42 | 10.11 ± 1.93* | 5. | 0.016 |
| IL-6 (pg/mL) | 4.78 ± 1.31 | 2.96 ± 1.12* | 5.24 | 0.008 |
| IL-8 (pg/mL) | 16.52 ± 2.64 | 13.14 ± 2.15* | 6.53 | 0.002 |
| CD4 count (109/L) | 1.32 ± 0.53 | 1.73 ± 0.64* | 4.12 | 0.017 |
| CD8 count (109/L) | 0.61 ± 0.28 | 0.85 ± 0.39* | 4.84 | 0.013 |
BMI: Body Mass Index; TNF-α: tumor necrosis factor - alpha; IL-6: Interleukin-6; IL-8: Interleukin-8; (*) indicates a significant difference, P < 0.05.
Table 3:
Mean value and significance of immune and systemic inflammation markers in group (B) before and after the study.
| Mean + SD | t-value | Significance | ||
|---|---|---|---|---|
| Pre | Post | |||
| BMI (kg/m2) | 31.51 ± 2.85 | 32.75 ± 2.83 | 1.28 | 0.131 |
| TNF-α (pg/mL) | 11.64 ± 2.16 | 11.73 ± 2.24 | 0.93 | 0.084 |
| IL-6 (pg/mL) | 4.62 ± 1.23 | 4.85 ± 1.31 | 1.12 | 0.161 |
| IL-8 (pg/mL) | 16.48 ± 2.75 | 16.71 ± 2.88 | 1.25 | 0.214 |
| CD4 count (109/L) | 1.47 ± 0.56 | 1.32 ± 0.51 | 0.92 | 0.161 |
| CD8 count (109/L) | 0.66 ± 0.25 | 0.61 ± 0.23 | 0.85 | 0.245 |
BMI: Body Mass Index; TNF-α: tumor necrosis factor - alpha; IL-6: Interleukin-6; IL-8: Interleukin-8.
Table 4:
Mean value and significance of immune and systemic inflammation markers in group (A) and group (B) after the study.
| Mean + SD | t-value | Significance | ||
|---|---|---|---|---|
| Pre | Post | |||
| BMI (kg/m2) | 27.86 ± 2.42* | 32.75 ± 2.83 | 6.71 | 0.013 |
| TNF- α (pg/mL) | 10.11 ± 1.93* | 11.73 ± 2.24 | 5.93 | 0.018 |
| IL-6 (pg/mL) | 2.96 ± 1.12* | 4.85 ± 1.31 | 4.55 | 0.009 |
| IL-8 (pg/mL) | 13.14 ± 2.15* | 16.71 ± 2.88 | 5.76 | 0.006 |
| CD4 count (109/L) | 1.73 ± 0.64* | 1.32 ± 0.51 | 4.74 | 0.015 |
| CD8 count (109/L) | 0.85 ± 0.39* | 0.61 ± 0.23 | 4.65 | 0.024 |
BMI: Body Mass Index; TNF-α: tumor necrosis factor - alpha; IL-6: Interleukin-6; IL-8: Interleukin-8; (*) indicates a significant difference between the two groups, P < 0.05.
Discussion
Recently, millions of subjects are affected with asthma and obesity, therefore life style intervention is essential for clinical management of these population28,29. The present study aimed to detect to response of systemic inflammation and immunological parameters to weight loss in obese asthmatic patients. The main findings of this study indicated that weight reducing program resulted in modulation of immune system and inflammatory cytokines in asthma patients, our results agrred with manprevious researches30–44.
A study conducted by Dandona et al. approved that TNF-α significantly reduced in obese subjects as result of weight reduction30. In addition, Sandoval and Davis found that approved that IL-6 reduced and insulin sensitivity improved following bariatric surgery31. While, Loria-Kohen et al. stated that weight reducing program resulted in reduced TNF-α and C-reactive protein (CRP)32. However, Balagopal et al. confirmed that three month life style intervention resulted in reduced level of IL-6, insulin resistance33. Moreover, an exercise program for 3 years resulted in reduction of body weight and TNF-α34. Similarly, You and Nicklas & Nicklas et al. weight loss by liposuction and life style intervention led to low level of CRP, TNF-α and IL-635,36. Reduced mass of visceral fat and pro-inflammatory monocytes and increased number of regulatory T cells are the possible anti-inflammatory mechanisms of weight reduction as result of exercise training37–39.
Another main finding of our study, weight reduction was associated improved immunological parameters, these results agreed with Wasinski et al. reported that weight loss associated with low macrophage and greater number of CD8+ T and CD4+ T cells after exercise and diet control in mice40. In addition, Lamas et al. confirmed that one month of diet control significantly reduced body weight improved immunological parameters in overweight rats41. Reduction of serum level of pro-inflammatory cytokines as TNF-α , IL-6 and CRP42–44, in addition to increased anti-inflammatory cytokines as IL-10 may be the mechanism for improved immunological parameters with weight reduction43.
The main points of strength in current study were that all exercise sessions were supervised and the randomization of this study . In the other hand, the small sample size in both groups which limit the ability to generalize the findings of this study. Finally, it is recommended to have further studies to detect the impact of life style intervention in another biochemical parameters and quality of life in asthma patients.
Conclusion
Weight reduction improved immunological and systemic inflammation markers in obese asthma patients.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (G-219-142-40). The authors, therefore, acknowledge with thanks DSR technical and financial support.
References
- 1.Global Initiative for asthma (GINA): Global Burden of asthma. 2014. In http://www.ginasthma.org/. Accessed in February, 2015.
- 2.Global Initiative for Asthma (GINA): Global Strategy for Asthma Management and Prevention. 2014. In http://www.ginasthma.org/. Accessed in February, 2015.
- 3.World Health Organization (WHO), author Obesity and Overweight. Fact Sheet No. 311, 2016. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 5 July 2017).
- 4.World Health Organization (WHO), author Obesity and Overweight. In http:www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed in November, 2014.
- 5.Yiallours K.P., Lamnisos D., Kolokotroni O., Moustaki M., Middleton N. Associations of body fat percent and body mass index with childhood asthma by age and gender. Obesity. 2013;21:E474–E482. doi: 10.1002/oby.20284. [DOI] [PubMed] [Google Scholar]
- 6.Fenger R.V., Gonzalez-Quintela A., Vidal C., Husemoen L.L., Skaaby T., Thuesen B.H., Aadahl M., Madsen F., Linneberg A. The longitudinal relationship of changes of adiposity to changes in pulmonary function and risk of asthma in a general adult population. BMC Pulmonary Med. 2014;14:208. doi: 10.1186/1471-2466-14-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulgheri G, Sypniewska G. Is there a link between asthma and obesity? Folia Medica Copernicana. 2013;1:1–4. [Google Scholar]
- 8.Fulgheri G, Sypniewska G. Is there a link between asthma and obesity? Folia Medica Copernicana. 2013;1:1–4. [Google Scholar]
- 9.Akerman M, Calacanis C, Madsen M. Relationship between asthma severity and obesity. J Asthma. 2004;41:521–526. doi: 10.1081/jas-120037651. [DOI] [PubMed] [Google Scholar]
- 10.Camargo J, Boulet L, Sutherland E, Busse W, Yancey S, Emmett A. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47:76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 11.Telenga E, Tideman S, Kerstjens H, Hacken N, Timens W, Postma D. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67:1060–1068. doi: 10.1111/j.1398-9995.2012.02855.x. [DOI] [PubMed] [Google Scholar]
- 12.Shore S, Fredberg J. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005;115:925–927. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 13.Poulain M, Doucet M, Major G, Drapeau V, Sériès F, Boulet L. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ. 2006;174:1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Cerny F, Kufel J, Grant B. Simulated Obesity-Related Changes in Lung Volume Increases Airway Responsiveness in Lean, Non-asthmatic Subjects. Chest. 2006;130:834–840. doi: 10.1378/chest.130.3.834. [DOI] [PubMed] [Google Scholar]
- 15.Newson RB, Jones M, Forsberg B, Janson C, Bossios A, Dahlen SE, et al. The association of asthma, nasal allergies, and positive skin prick tests with obesity, leptin, and adiponectin. Clin Exp Allergy. 2014;44:250–260. doi: 10.1111/cea.12221. PubMed . [DOI] [PubMed] [Google Scholar]
- 16.Telenga E, Tideman S, Kerstjens H, Hacken N, Timens W, Postma D. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67:1060–1068. doi: 10.1111/j.1398-9995.2012.02855.x. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, Bouter L, McQuillan G. Lowgrade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 18.Chandra R, Au B. Spleen hemolytic plaque forming cell response and generation of cytotoxic cells in genetically obese (C57Bl/6J ob/ob) mice. Int Arch Allergy Appl Immunol. 1980;6:294–298. doi: 10.1159/000232498. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka S, Isoda F, Yamakawa T, Ishihara M, Sekihara H. T lymphopenia in genetically obese rats. Clin Immunol Immunopathol. 1998;86:219–225. doi: 10.1006/clin.1997.4467. [DOI] [PubMed] [Google Scholar]
- 20.Bade G., Khan M. A., Srivastava A. K., et al. Serum cytokine profiling and enrichment analysis reveal the involvement of immunological and inflammatory pathways in stable patients with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9(5):759–773. doi: 10.2147/COPD.S61347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denisenko Y. K., Lobanova E.G., Novgorodtseva T.P., et al. The role of arachidonic acid metabolites (endocannabinoids and eicosanoids) in the immune processes: a review. International Journal of Chemical and Biomedical Science. 2015;1(3):70–78. [Google Scholar]
- 22.Hersoug L, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–1213. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson E, Gutierrez D, Hasty A. Adipose tissue recruitment of leukocytes. Curr Opin Lipidol. 2010;21:172–177. doi: 10.1097/MOL.0b013e3283393867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity- driven type 2 diabetes. Semin Immunol. 2012;24:436–42. doi: 10.1016/j.smim.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denisenko Y. K., Lobanova E.G., Novgorodtseva T.P., et al. The role of arachidonic acid metabolites (endocannabinoids and eicosanoids) in the immune processes: a review. International Journal of Chemical and Biomedical Science. 2015;1(3):70–78. [Google Scholar]
- 26.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight andobesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Global Initiative for Asthma (GINA), author Global Strategy for Asthma Management and Prevention (Updated 2016) Global Initiative for Asthma (GINA); 2016. [Google Scholar]
- 28.Gomez-Llorente MA, Romero R, Chueca N, Martinez-Cañavate A, Gomez-Llorente C. Obesity and Asthma: A Missing Link. Int J Mol Sci. 2017 Jul 11;18(7) doi: 10.3390/ijms18071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters U, Suratt BT, Bates JHT, Dixon AE. Beyond BMI: Obesity and Lung Disease. Chest. 2017;(17):31260–6. doi: 10.1016/j.chest.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-α in serum of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83(8):2907–10. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 31.Sandoval D, Davis S. Leptin, Metabolic control and regulation. J. Diabetes Complications. 2003;17(2):108–13. doi: 10.1016/s1056-8727(02)00167-8. [DOI] [PubMed] [Google Scholar]
- 32.Loria-Kohen V, Fernández-Fernández C, Bermejo LM, Morencos E, Romero-Moraleda B, Gómez-Candela C. Effect of different exercise modalities plus a hypocaloric diet on inflammation markers in overweight patients: A randomised trial. Clinical Nutrition. 2013;32:511–518. doi: 10.1016/j.clnu.2012.10.015. PubMed . [DOI] [PubMed] [Google Scholar]
- 33.Balagopal P, George D, Patton N, Yarandi H, Roberts WL, Bayne E, Gidding S. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. Journal of Pediatrics. 2005;146(3):342–348. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Journal of the American Medical Association. 2003;289(14):1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 35.You T, Nicklas B. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr. Diabetes Rev. 2006;2:29–37. doi: 10.2174/157339906775473626. PubMed . [DOI] [PubMed] [Google Scholar]
- 36.Nicklas B, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. PubMed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathur M, Pedersen B. Exercise as a mean to control low-grade inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmerman K, Flynn M, Coen P, Markofski M, Pence B. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y, Ma Y. Effect of exercise training intensity on murine T regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22(5):643–52. doi: 10.1111/j.1600-0838.2010.01288.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 40.Wasinski F, Bacurau RF, Moraes MR, Haro AS, Moraes-Vieira PM, Estrela GR, Paredes-Gamero EJ, Barros CC, Almeida SS, Câmara NO, Araujo RC. Exercise and Caloric Restriction Alter the Immune System of Mice Submitted to a High-Fat Diet. Mediators Inflamm. 2013;2013:395672. doi: 10.1155/2013/395672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamas O, Marti'nez J, Marti A. Energy restriction restores the impaired immune response in overweight (cafeteria) rats. Journal of Nutritional Biochemistry. 2004;15:418–425. doi: 10.1016/j.jnutbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Starkie R, Ostrowski S, Jauffred S, Febbraio M, Pedersen B. Exercise and IL-6 infusion inhibit endotoxin induced TNF-alpha production in humans. The FASEB Journal. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 43.Jankord R, Jemiolo B. Influence of physical activity on serumIL-6 and IL-10 levels in healthy older men. Medicine and Science in Sports and Exercise. 2004;36(6):960–964. doi: 10.1249/01.mss.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- 44.Kasapis C, Thompson P. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. Journal of the American College of Cardiology. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]