Abstract
Ephrin B2 is critical for endochondral bone development. In this study, we investigated its role in fracture repair by deleting ephrin B2 in type II collagen (Col.2) expressing cells. We used a nonstable tibia fracture model to evaluate fracture repair at 3 sites: intramembranous bone formation, endochondral bone formation, and intramedullary bone formation. We observed that during fracture repair, deletion of ephrin B2 impaired periosteal stem cell activation, inhibited their proliferation, decreased their survival, and blocked their differentiation into osteoblasts and chondrocytes. In addition, deletion of ephrin B2 decreased vascular endothelial growth factor production as well as vascular invasion into the fracture site. These changes led to reduced cartilage to bone conversion in the callus with decreased new bone formation, resulting in impaired fracture repair. Our data indicate that ephrin B2 in Col2-expressing cells is a critical regulator of fracture repair, pointing to a new and potentially targetable mechanism to enhance fracture repair.
Keywords: ephrin b2, fracture repair, intramembranous bone formation, endochondral bone formation, intramedullary bone formation, transdifferentiation
Fractures are one of the most frequent injuries of the musculoskeletal system. A fracture leads to surrounding soft-tissue trauma, damage to local blood vessels, and disruption of the bone marrow structure. Fracture repair is a complex process to restore the original structure and biomechanical functions of bone (1-3). Bone regeneration during fracture repair recapitulates the process that occurs during embryonic development. It involves three interrelated mechanisms—intramembranous bone formation (IBF), endochondral bone formation, and the more recently described intramedullary bone formation (IMBF) (4, 5). IBF occurs along the periosteal surface at the proximal and distal edges of the callus, in which progenitors differentiate into osteoblasts directly to form the bony hard callus. Endochondral bone formation occurs in regions that are mechanically less stable, immediately adjacent to the fracture site, in which the progenitors differentiate initially into chondrocytes to form the cartilage soft callus, followed by vascular invasion, mineralization of the cartilage callus, and differentiation of the hypertrophic chondrocytes into osteoblasts to form bone. The newly formed bone is remodeled into cortical bone through the coupling actions of osteoblasts and osteoclasts to bridge the fracture gap (6-8). Recently, we described a third site, IMBF, in which the progenitors appear to come from cortical bone, osteocytes in appearance, that have the unique characteristics of expressing DMP1, type II collagen (Col.2), and Sox 2 (9). These cells appear to migrate into the intramedullary region where they transdifferentiate directly into osteoblasts to form intramedullary bone during the initial stages of fracture repair.
Most fractures progress to union. However, 10% to 20% of fractures are complicated by delayed union or persistent nonunion, resulting in prolonged disability, impairing the quality of life, and inflating health care costs (9-11). Although the overall process of fracture repair has been well characterized, the molecular pathways underlying fracture repair remain unclear. In this regard, it is important to examine pathways that influence fracture repair to develop therapeutic agents that efficiently stimulate the repair process to shorten healing time and prevent nonunion.
In recent years, the Eph family of receptor tyrosine kinases has been implicated in an increasing number of physiological and pathological processes in many cell types and different organs (12, 13). In previous studies, we (14) and others (15, 16) demonstrated that EphB4 and its ligand, ephrin B2 (Efnb2) play important roles in bone homeostasis. In the skeleton, Efnb2 and EphB4 are expressed in mesenchymal stem cells, chondrocytes, osteoblasts, and osteocytes (14, 17-19). In addition, Efnb2 is also expressed in osteoclasts (18). During bone development, Efnb2 stimulates osteoblast differentiation as well as IBF in calvaria organ cultures (20). Moreover, Efnb2-EphB4 regulates endochondral bone formation by promoting chondrocyte differentiation and mediates the communication between chondrocytes and osteoclasts regulated by IGF-1 (14). Furthermore, deletion of Efnb2 in osterix expressing cells, including prehypertrophic chondrocytes, causes defects in osteoclast attachments to the cartilage and bone surface, leading to abnormal endochondral bone formation (21). On the other hand, during bone remodeling, Efnb2-EphB4 constitutes a bidirectional signaling pathway (22). The reverse signaling through ephrinB2 into osteoclast precursors has been reported to suppress osteoclastogenesis by inhibiting the osteoclastogenic c-Fos-NFATc1 cascade, whereas the forward signaling through EphB4 into osteoblasts has been reported to enhance osteoblast differentiation (17, 23). However, our data in which Efnb2-EphB4 signaling was disrupted in either osteoblasts or chondrocytes indicate that Efnb2-EphB4 signaling promotes both osteoclastogenesis and osteoblast differentiation (14). Disrupting Efnb2-EphB4 interaction either by deletion of Efnb2 in osteoblasts (24) or by the treatment of soluble Ephb4 blunts the anabolic effects of PTH on bone (18, 25) in vivo, and inhibits human mesenchymal stem cell activation, migration, and cartilage matrix formation in vitro (19). In addition, Efnb2-EphB4 signaling is critical in various bone diseases and trauma. Overexpression of EphB4 in osteoblasts in mice enhances endochondral bone formation during fracture repair (26) and reduces articular cartilage degradation and subchondral bone sclerosis in osteoarthritis models (27, 28). Treatment of cells from osteoarthritis joints with Efnb2 inhibits their production of catabolic factors, suggesting the therapeutic potential of Efnb2 (29, 30). Thus, Efnb2-EphB4 is a key pathway for intramembranous and endochondral bone formation as well as bone remodeling, which are all critical stages during fracture repair. This makes Efnb2 a candidate to regulate fracture repair.
To investigate the role of Efnb2 in regulating fracture repair, in this study, we deleted Efnb2 in Col.2-expressing cells then created a nonstabilized fracture at the mid-shaft of the tibia. We evaluated the effects of Efnb2 on fracture repair at the 3 sites of bone formation, intramembranous, endochondral, and intramedullary, on days 5, 10, and 28 of fracture repair.
Methods
Experimental animals
To delete Efnb2 in Col.2-expressing cells (col2Efnb2KO), female floxed-Efnb2 (Efnb2flox/flox) mice (The Jackson Laboratory, stock number:006042) that carry loxP sequences flanking exon 1 of the gene (31) were bred with male transgenic mice expressing the Cre recombinase under the control of a Col.2 promoter (Col.2cre) (The Jackson Laboratory, stock number: 003554) as well as the floxed Efnb2. Because no skeletal phenotype in the floxed Efnb2 mice compared with the wild-type was apparent, we used these mice (littermates without cre) as controls. In addition, to trace how Efnb2 affects cell fate of Col.2-expressing cells, the female Efnb2flox/flox mice were bred with male Col2CreERT mice (gift from Dr. Susan Mackem, National Institutes of Health; The Jackson Laboratory, stock number: 006774) to obtain tamoxifen-regulated Efnb2KO (tamcol2Efnb2KO). The tamcol2Efnb2KO mice were further bred with the mice homozygous for the ROSA tomato transgene (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze) (Ai9/tdT mice, Jackson Laboratories, stock number: 007909). The Ai9/tdT mice carried a Cre reporter allele with a loxP-flanked STOP cassette preventing transcription of a CAG promoter-driven tdTomato, all inserted into the Gt (ROSA)26Sor locus. When bred with the tamcol2Efnb2KO, Col.2-expressing cells and their progeny (tamcol2Efnb2KO/tdT) expressed robust tdT following Col.2 Cre mediated recombination. In these experiments, the mice lacking the floxed Efnb2 (tamcol2tdT), but expressing the ROSA tomato transgene enabling them to be activated by tamoxifen and thus express robust tdT, served as controls for these lineage tracing experiments (see Results). All mice were housed in a barrier facility with a 12-hour light-dark cycle and maintained on standard chow. For this report, only skeletons from male col2Efnb2KO and their control littermates were analyzed. All animal studies were approved by the Animal Use Committee of the San Francisco Veterans Affairs Medical Center, where the animals were raised and studied. Mice were anesthetized with approved anesthetics (isoflurane) before procedures. For euthanasia, animals were exposed to isoflurane before cervical dislocation.
Genotyping
Genomic DNA was extracted from tail snips of the mice using REDTaq ReadyMix PCR Reaction Mix (Sigma-Aldrich, St. Louis, MO). PCR analyses of the DNA were performed to detect cre, tdT, and floxed-Efnb2 alleles using corresponding primer sets as described previously (31, 32) or following the manufacturer’s instructions (tdT). The primer sets are shown in Table 1.
Table 1.
Primers for genotyping
| Name | Sequences | |
|---|---|---|
| Efnb2 | WT | Forward: 5′- GCTGCCCGCGGCCGGTCCCAACG-3′ Reverse: 5′- CCGTTAGTGGCAACGTCCTCCGTC CTC-3′ |
| Floxed allele | Forward: 5′-AAGTTATAAGCT TCAACGCGTCC-3′ Reverse: 5′- GAGCCCCAGGTTCTAGAATAA-3′ |
|
| Cre | Forward: 5′-GCAAAACAG GCTCTAGCGTTCG-3′ Reverse: 5′-CTGTTTCACTATCCAGGTTACGG-3′ |
|
| tdT | WT | Forward: 5′-AAGGGAGCTGCAGTGGAGTA-3′ Reverse: 5′-CCGAAA ATCTGTGGGAGGTC-3′ |
| Transgene | Forward: 5′-CTGTTCCTGTACGGCATGG-3′ Reverse: 5′-GGCATTAAAGCAGCGTATCC-3′ |
Abbreviations: Efn2, ephrin b2; tdT, tdTomato; WT, wild-type.
Nonstabilized fracture model
A closed tibia fracture was created by 3-point bending using a Bose Electroforce 3200 mechanical instrument (Eden Prairie, MN, USA) as described (33, 34). Briefly, 3-month-old mice were anesthetized with 1% to 3% isoflurane using an anesthesia machine. The right hind limb was placed on the lateral side, and an impactor was placed against the skin at the midpoint of the medial side of the lower leg. Then a preload of 1.0 N was applied before reaching a fracture impact at a constant speed of 0.2 mm/s, and the bone breaking force was recorded. The fracture site occurred in the upper-middle portion of the right diaphysis. Mice received analgesics after fracture and were returned to their cages and allowed to ambulate freely after awakening. Fractured and normal tibias were harvested for analysis at 5, 10, and 28 days after fracture according to the experimental protocol.
Microcomputed Tomography
The fractured tibias were scanned at day 10 after fracture using a Scanco VivaCT-40 microcomputed tomography (µCT) system (Bruttisellen, Switzerland) with an X-ray energy of 55 kV, a voxel size of 10.5 mm, and an integration time of 1000 ms. Each scan comprised 1000 slices encompassing the full fracture callus (33). For fracture callus analysis, we segregated newly formed bone by manually delineating a mask to exclude preexisting cortical bone. A thresholding algorithm protocol was used to segment mineralized tissue. Each callus was determined with a fixed, global threshold of 250 (sigma ¼ 0.8, support ¼ 1, corresponding to 400 mg hydroxyapatite/cm3). In addition, a threshold of 350 (sigma ¼ 0.8, support ¼ 1; corresponding to 625 mg hydroxyapatite/cm3) was used to quantify the higher density bone component within the same region of interest. The following measurements of callus structure and composition were evaluated: bone volume fraction (BV/TV, %), trabecular thickness (mm), trabecular number (Tb.N, 1/mm), and connective density (Conn.D) (35).
Histology
Normal and fractured tibias were fixed with 4% paraformaldehyde (PFA) in PBS (4% PFA/PBS) overnight at 4 C and decalcified by 10% EDTA. For tracing experiments, decalcified bones were embedded in optimal cutting temperature compound and cut into 10-µm sections. For other histologic measurements, decalcified bones were embedded in paraffin and cut into 5-µm sections. The sections were stained by hematoxylin and eosin (H&E) and Safranin O/Fast Green following standard procedures or subjected to immunohistochemistry for proliferating cell nuclear antigen (PCNA) (36), vascular endothelial growth factor (VEGF) (37), SRY-box2 (Sox2) (38) osteocalcin (39), Sox9 (40), and ephrin B2 (41), and counterstained with hematoxylin. To detect apoptotic periosteal cells, we used the deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using TUNEL Apoptosis Detection Kit (EMD Millipore, Billerica, MA) following the manufacturer’s instructions with slight modification. Briefly, after treating with 10 μg/mL proteinase K, sections were reacted with deoxynucleotidyl transferase (TdT) cocktail, including TdT and biotin-dUTP. The reaction was stopped by TB buffer, and biotin-dUTP labeled DNA cleavage inside the apoptotic cells (TUNEL-positive cells) was detected by Avidin-FITC (green) and counterstained with propidium iodide (red). Sections from at least 3 animals of each group at each time point were analyzed.
Statistics
Results were presented as mean ± SD and compared using unpaired Student t test. Significance was assigned for P < 0.05. Samples of at least 4 animals were analyzed for each experimental and control group.
Results
Effects of deletion of Efnb2 in Col.2-expressing cells during bone development
To investigate the role of Efnb2 in fracture repair, we generated mice with Efnb2 ablation in Col2-expressing cells (Col.2.Efnb2KO). We first tested the effects of deletion of Efnb2 during postnatal bone development (Fig. 1). The deletion of Efnb2 was confirmed by immunohistochemistry in the proliferating chondrocytes (Fig. 1A; GP, 5% of control) in the Col.2.Efnb2KO. Interestingly, the expression of Enfb2 in the osteoblasts of the trabecular bone surface within the primary spongiosa (metaphysis) was also dramatically decreased in the Col.2.Efnb2KO (27% of control) when compared with the controls (Fig. 1B, Tb; Fig. 1D). In contrast, the deletion of Efnb2 in chondrocytes did not affect the periosteum (Efn+/total, 21% in KO vs 23% in control) (Fig. 1C-P). At P7, H&E staining revealed a trend toward an increased resting zone (P = 0.06), a subtle but significantly shorter proliferating zone (74% of control) and a reduction in the hypertrophic zone (63% of control) in the Col.2.Efnb2KOs (Fig. 1E and F), although the bone lengths were comparable (femur: 7.96 ± 0.30 in control vs 7.84 ± .033 in KO, P = 0.67; tibia: 10.64 ± .054 in control vs. 10.67 ± 1.14 in KO, P = 0.85). Immunohistochemistry using a PCNA antibody revealed no significant difference in number of PCNA-positive cells in the growth plates between the 2 genotypes (Fig. 1G and H; GP), but fewer PCNA-positive cells in the cells lining the trabecular bone surface in the Col.2.Efnb2KOs when compared with the controls (40% of control, Fig. 1G and H; Tb). As determined by µCT, at 6 weeks, trabecular BV/TV (30%), Tb.N (28%), and Conn.D (52%) were significantly lower in the Col.2.Efnb2KOs than in the controls (Fig. 1I and J). To determine whether deletion of Efnb2 affects cell fate of Col.2-expressing cells, 2 doses of tam or vehicle (non-tam-treated control) beginning on day of tam administration were given to tamcol2Efnb2KO/tdT and control (tamcol2tdT) mice (Fig. 2A) to delete Efnb2 and activate tdTomato expression. As shown in Fig. 2B, at P21, no col2tdT-labeled cells were observed in the non-tam-treated control, indicating no leakage of the Cre. In the tam-treated tamcol2tdT mice, at 1 day post-tam administration, col2tdT-labeled cells appeared at the upper growth plate (Fig. 2C, GP). At this time point very a few (1-2 tdT-positive cells per bone) labeled cells were seen along the trabecular bone surface at the primary spongiosa (Fig. 2C, PS), and none were observed in the secondary ossification center (Fig. 2C, SOC), or cortical bone at the mid-diaphysis (Fig. 2D). At 7 days after tam administration, col2tdT-labeled cells were observed not only in the growth plate, but were now found in the bone surface of PS, the SOC (Fig. 2E), and mid-diaphysis of cortical bone (cortex, Fig. 2F) in both controls and col2Efnb2KOs. But the number of labeled cells was lower in the Col.2.Efnb2KOs compared with the controls in the SOC (16% of control, Fig. 2E and G), growth plate (43% of control, Fig. 2E and H), PS (25% of control, Fig. 2E and I), and in the mid-diaphysis of the cortical bone (70% of control, Fig. 2F and J). These data indicate the migration and transdifferentiation of these labeled chondrocytes at day 1 to osteoblasts in the PS and SOC and to osteocytes in the mid cortical region within 7 days after labeling. Moreover, the reduction in labeled cells in the Efnb2KO mice in the PS, SOC, and cortical bone suggests that deletion of Efnb2 in Col.2-expressing cells induces only minor effects on growth plate formation, but dramatically impairs cell distribution/chondrocyte-osteoblast transdifferentiation and bone formation in the metaphysis and cortical bone during postnatal development. With these observations in normal bone development we turned our attention to fracture repair.
Figure 1.
Impact of Efnb2 deletion (col2Efnb2KO) on endochondral bone formation during bone development. (A-C) Immunohistochemistry (IHC) for the expression of Efnb2 (brown) at the tibia growth plate (GP, A), trabecular bone (Tb) at the primary spongiosa (PS, B), and periosteum (P, C) in the col2Efnb2KO (KO) and control (Con). (D) Efnb2-positive cells in the Tb surface at the PS in KO and Con were measured. E-F: Morphology (H&E staining) of the growth plate (E) and measurement (F). RZ: resting zone; PZ: proliferating zone; HZ: hypertrophic zone. G-H: IHC for PCNA-positive cells (brown) (G) and calculation of cell number (H) in the GP and in the Tb surface at the PS of the KO and Con. 5× in C, bar = 100 µm, 20× in A &D, bars = 50 µm. n = 4 in each group. I-J: Bone structure was determined by µCT in distal femurs of the Con (n = 5) and KO, (n = 6), bar = 1 mm. In all bar graphs, results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. Con (solid bars). H&E, hematoxylin and eosin; KO, knockout.
Figure 2.
Deletion of Efnb2 affects chondrocyte-osteoblast transdifferentiation. (A) Strategy to generate tamcol2Efnb2KO/tdT mice and controls (tamcol2tdT). (B) Section from vehicle-treated (no tamoxifen) tamcol2tdT (P21, 7 days postinjection). (C-D) Section from tamcol2tdT, 1 day after tamoxifen administration (P15). (E-F) Sections from tamcol2Efnb2KO/tdT (KO in E and F) and tamcol2tdT (Con in E and F) (P21, 7 days postinjection). Frozen sections were counterstained by DAPI (blue dots). White dotted lines indicate the boundaries of the growth plate (GP, B and C) or cortical bone and periosteum (D and F). BM, bone marrow cavity side; P, periosteum; PS, primary spongiosa; SOC, secondary ossification center. 5× in B, C, and E, bars = 100 µm, 10× in D and F, bars = 50 µm. G-J: percentage of tdT-labelled cells (red dots, tdT+/total cells) at (G) SOC, (H) GP, and (J) cortex in the KO (open bars, n = 4) and Con (solid bars, n = 3) mice were calculated. In the PS (I), to exclude the DAPI-positive cells at BM, the tdT labelled cells at bone surface (BS) were measured and calculated as tdT+cells/BS. Results are expressed as mean ± SD. *P < 0.05 KO vs Con.
Deletion of Efnb2 affects early callus formation
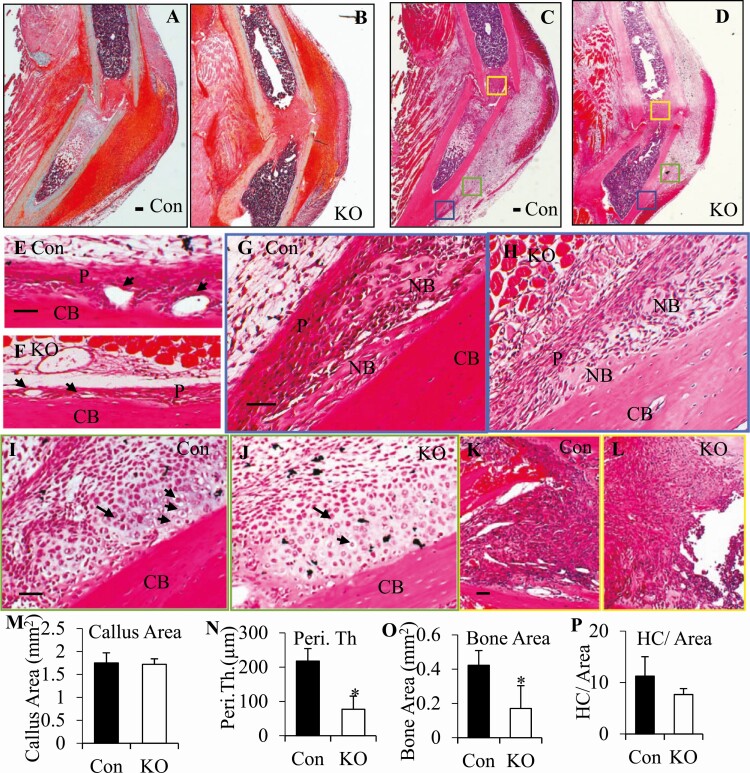
We next investigated the effects of Efnb2 deletion during fracture repair. To evaluate the effects Efnb2 deletion on all 3 sites of bone formation during the repair process, we used a nonstabilized diaphysis fracture model. In this model, callus formation partially overlaps with the inflammatory phase starting with IBF as early as 3 to 7 days postfracture (8). By day 5 postfracture (Fig. 3), deletion of Efnb2 did not affect callus size (Fig. 3A-D), as indicated by the total callus area (Fig. 3M), but altered morphology of the callus. Figure 3A-B depicts safranin O/fast green staining, whereas Fig. 3C-D are H&E-stained sections in which the 3 sites of fracture repair are marked with colored boxes. In response to the injury, the inter layer of adjacent periosteum of the controls became thickened and highly vascular (Fig. 3E), whereas in the Col.2.Efnb2KOs, the periosteum was thinner (33% of control, Fig. 3F and N) with smaller vessels (28% of control, Fig. 3F) as indicated by the average vessel size (347.36 ± 135.78 µm2 in KO vs 1236.48 ± 363.37 µm2 in Con, P = 0.003, n = 4 in each group). In the distal or proximal edges of the callus (blue frames in Fig. 3C and D), woven bone formed via IBF in both controls and Col.2.Efnb2KOs, but 41% less woven bone formed in the Col.2.Efnb2KOs (Fig. 3H and O) when compared with the controls (Fig. 3G and O). In the area adjacent to the injury site (green frames in Fig. 3C and D), cartilage started to form in both genotypes of the mice, proliferating and hypertrophic chondrocytes were observed inside the callus, but fewer hypertrophic chondrocytes were observed in the Col.2.Efnb2KOs (59% of control, Fig. 3J and P) than in the controls (Fig. 2I and P). In the intramedullary area, within the fractured ends of the bone (yellow frames in Fig. 3C and D), neither bone nor cartilage had yet formed in the controls (Fig. 3K) or in the Col.2.Efnb2KOs (Fig. 3L). Immunohistochemistry showed strong Sox2 staining in periosteal cells (Fig. 4A) in the controls but these Sox2-expressing cells were significantly fewer in the periosteum of the Col.2.Efnb2KOs (14% of control, Fig. 4B and G), suggesting that deletion of Efnb2 inhibited periosteal progenitor activation (Fig. 4G). Moreover, compared with the controls (Fig. 4C), deletion of Efnb2 (Fig. 4D) decreased the number of Sox2-positive cells in the sites of IBF and endochondral bone formation (EndoBF) (Fig. 4H, 16.4% of control). In addition, very few Sox2-positive cells were observed in the bone marrow at site of IMBF in the controls (Fig. 4E) and Col.2.Efnb2KOs (Fig. 4F) at this time point. These data suggest that deletion of Efnb2 impairs intramembranous and endochondral bone formation at day 5, but IMBF had not yet started in either controls or Col.2.Efnb2KOs at this early stage of callus formation.
Figure 3.
Deletion of Efnb2 delayed the onset of fracture repair at day 5 post fracture. (A-B) Safranin O/fast green staining of the callus of the col2Efnb2KO (B, KO) and their littermate controls (A, Con). (C-L) H&E staining revealed abnormal morphology in the KO callus (D, F, H, J, L) compared with the Con callus (C, E, G, I, K). (C) Low magnification of H&E staining of the Con and (D) KO callus. (E-L) High magnification. (E-F) The periosteum of the (E) control and (F) KO in response of the fracture. P, periosteum. Arrowheads, vessels under the periosteum. (G-H) Intramembranous bone formation sites (IBF), high magnification of blue frame areas in (G) C and (H) D. (I-J) Endochondral bone formation sites (EndoBF)—green frame areas in (I) C and (J) D. (K-L) Intramedullary bone formation sites (IMBF)—yellow frame areas in (K) C and (L) D. 2.5× in A-D, bars = 100 µm, 10× in E-L bars = 50 µm. CB, cortical bone; NB, newly formed bone; P, periosteum. (M-P) The callus area in (M) A-B, periosteal thickness (N, Peri, Th) in E-F, (O) newly formed bone area in G-H and hypertrophic chondrocyte number (HC)/EndoBF area (P) in I-J were measured. The results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. Con (solid bars). H&E, hematoxylin and eosin; KO, knockout.
Figure 4.
Deletion of Efnb2 reduced progenitor activation during fracture repair. At day 5 postfracture, immunohistochemistry (IHC) using anti-Sox2 antibody in control (Con, A, C, E) and KO (B, D, F) callus. (A-B) Periosteum. (C-D) Intramembranous (IBF) and endochondral bone formation (EndoBF) sites. (E-F) Intramedullary areas. BM, bone marrow; CB, cortical bone; P, periosteum. 10× in C-D, 20× in A-B and E-F, bars = 50 µm. (G-H) Percentage of Sox2-positive cells per (G) total cells in the periosteum (A-B) and IBF and (C-D) EndoBF site (H) were measured. The results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. Con (solid bars). KO, knockout.
Deletion of Efnb2 alters the structure and morphology of the callus
At day 10 after fracture, µCT showed that BV/TV (24%), Tb.N (26%), and Conn.D (14%) were significantly reduced in the Col.2.Efnb2KOs callus compared with the control callus (Fig. 5A), indicating that Efnb2 deletion impaired trabecular bone formation during callus formation. Consistent with µCT measurements, at D10, Safranin O/fast green staining showed that compared with the control callus, more cartilage tissue remained in the Col.2.Efnb2KO callus (30% in KO vs 18% in control) at the endochondral bone formation site, although the total callus sizes were comparable (Fig. 5B).
Figure 5.
Impact of Efnb2 deletion on structure and histology of the callus at day 10 postfracture. (A) Callus structure of controls (Con, solid bars, n = 4) and col2Efnb2KOs (KO, open bars, n = 5) was determined by µCT. (B) Safranin O/fast green staining revealed more cartilage (Cart. Area, red) in the KO (open bar) compared with the controls (con, solid bar). In all bar graphs, results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. con (solid bars). µCT, microcomputed tomography; KO, knockout.
Histological assessment was used to determine the morphological changes in the callus of col2Efnb2KOs and the controls at the 3 different sites of bone repair marked in Fig. 6 with colored boxes. H&E staining showed that, compared with the D5 callus (Fig. 3), at D10, more woven bone had formed at the IBF (blue boxes in Fig. 6A) site in the control callus. More blood vessels and marrow space were observed at D10 (Fig. 6A and B) than D5. Similarly, more cartilage had formed at the EndoBF site through endochondral bone formation (Fig. 6A and B) with hypertrophic chondrocytes inside. Vessels were observed in the boundary of bone and cartilage, suggesting cartilage was being replaced by bone in this area of vascular invasion (Fig. 6B). Compared with the controls, in the Col.2.Efnb2KOs, less (65% of control) woven bone (BV/TV, Fig. 6C and H) with fewer vessels (60% of control, vessels/TV) and marrow space were observed at the IBF site (Fig. 6C and I). The boundary between woven bone and cartilage was not as clear cut as in the controls, and more cartilage remained in this area with less (33% of control, indicated by vessel number/boundary between woven bone and cartilage, P = 0.06) vascular invasion (Fig. 6C and J), suggesting that the replacement of cartilage by bone was delayed. In addition, more proliferating (2-fold of control), but fewer hypertrophic chondrocytes (60% of control) were observed inside the callus in the Col.2.Efnb2KOs than in the controls (Fig. 6A, C and K). Granulation tissue and woven bone formed to bridge the gap, with less cartilage remaining (0.35% per gap area) in the controls (Fig. 6D, L), whereas in the Col.2.Efnb2KO, no woven bone was observed but cartilage remained (17% per gap area) in this region (Fig. 6E, L). At D10, bone had formed in the IMBF, accompanied by vascular invasion and marrow space formation within the newly formed bone in both control (Fig. 6F) and col2Efnb2KO (Fig. 6G) callus, although with modest reductions in both in the col2Efnb2KO callus (BV/TV 18%) compared with the control callus (BV/TV 21%). These data indicate impaired intramembranous and endochondral bone formation in the Col.2.Efnb2KOs during fracture repair but with less effect on IMBF consistent with the lesser impact of Efnb2 deletion on the Col.2-expressing cells in the mid-diaphysis compared with that in the trabecular bone (PS and SOC) (Fig. 2).
Figure 6.
Effects of deletion of Efnb2 on callus morphology at the 3 bone formation sites marked by the colored boxes. (A) Morphology (H&E staining) of the KO and Con callus. (B-G) High magnification pictures of A. (B-C) Blue frame areas in A, intramembranous bone formation site (IBF); (D-E) yellow frame areas in A, endochondral bone formation site (EndoBF); (F-G) green frame areas in A, intramedullary bone formation site (IMBF). 2.5× in A, bars = 100 µm. 10× in B-G, bars = 50 µm. Cart, cartilage; M, bone marrow space; WB, woven bone. Arrows: blood vessels. (H) BV/TV (H), (I) vessel number/TV, (J) vessel number along the boundary of bone and cartilage (BBC, dotted lines in C and D) in IBF, percentage of proliferating chondrocytes (PC), and (K) hypertrophic chondrocytes (HC) per total chondrocytes and (L) cartilage remaining in the bridge area in the Con (solid bars) and KO (open bars) were measured. Results are expressed as mean ± SD. *P < 0.05 KO vs. Con; n = 4 in each group. H&E, hematoxylin and eosin; KO, knockout.
Deletion of Efnb2 inhibited periosteal stem cell activation and function
Previous studies demonstrated that the periosteum is the main source of progenitor cells during fracture repair at least for intramembranous and endochondral bone formation. To further investigate the mechanism of how deletion of Enfb2 impairs fracture repair, we first determined periosteal stem cell activation and function by morphology and immunohistochemistry. At D10, mesenchymal progenitors from the periosteum migrate into the callus to form an invasion front (IF) (Fig. 6A, black frame areas). As shown in Fig. 7, compared with the controls, in the col2Efnb2KOs, the cambium layer of the periosteum close to or surrounding the callus was thinner and contained fewer Sox2-labeled progenitor cells. Fewer cells and vessels migrated into the callus from the periosteum (IF) in the col2Efnb2KOs (Fig. 7B) than in the controls (Fig. 7A). Immunohistochemistry revealed that in the control callus, activated progenitors, defined as Sox2-expressing cells in the invasion front (IF) (Fig. 7C) and the periosteum adjacent to or surrounding the callus (Fig. 7E) in the controls, were markedly increased during fracture repair but dramatically reduced to 22% of control in the invasion front (Fig. 7D and G; IF) and to 34% of control in the periosteum (Fig. 7F and G-periosteum) of the col2Efnb2KOs in these areas. Moreover, PCNA-positive cells in the invasion front and periosteum were fewer (Fig. 7J, 16% of control) in the col2Efnb2KOs callus (Fig. 7I) compared with the control callus (Fig. 7H). In contrast, more apoptotic cells (3-fold that of controls, Fig. 7M) were observed in the periosteum and the invasion front by TUNEL assay in the col2Efnb2KOs (Fig. 7L) than in the controls (Fig. 7K). These data indicate that during fracture repair, Efnb2 in Col.2-expressing cells stimulates fracture repair by promoting periosteal stem cell activation, growth, and survival.
Figure 7.
Impact of Efnb2 deletion on periosteal progenitor activation, proliferation, and survival during fracture repair. (A-B) High magnification of black frame areas of Fig. 6A. H&E staining of the periosteal invasion front (IF) in the col2Efnb2KO (B, KO,) and the controls (A, Con). Arrows indicate vessels. (C-J) immunohistochemistry (IHC) of Sox2 (C-F) and PCNA (H-I) in the IF (C and D, left panels in H-I) and periosteum overlying the callus (E and F, right panels in H-I) of the Con (C, E, and H), and KO (D, F, and I) sections. Signals were indicated by brown DAB stain. Sections for IHC were counterstained with hematoxylin. TUNEL staining of the IF (left panel) and periosteum (right panel) in the (L) KO and the (K) Con. Apoptotic cells (green, indicated by arrows). Propidium iodide labeled the cell nuclei (red). 10× in A-L. Bars = 50 µm. In E-F: c: cortical bone, arrows in E and F indicate the direction of the image from the distal periosteum toward the fracture site. (G) Percentage of Sox2, (J) PCNA, and (M) TUNEL positive cells in total cells were measured. Results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. con (solid bars), n = 4 in each group. H&E, hematoxylin and eosin; KO, knockout; TUNEL, deoxynucleotidyl transferase dUTP nick end labeling.
Impaired cell activation, proliferation, and VEGF production in the col2Efnb2KO mice during fracture repair
To evaluate the effects of deletion of Efnb2 on the vasculature during fracture repair, we performed immunohistochemistry using antibodies against CD31 and VEGF. Consistent with Fig. 7A, we observed that CD31-labeled vessels in the invasion front in the col2Efnb2KOs (Fig. 8B and E) callus were 50% less than in the control callus (Fig. 8A and E). Similarly, less VEGF production was observed in the periosteum (20% of control, Fig. 8D; F-Peri), invasion front (25% of control, Fig. 8D and F-IF) and cartilage in the col2Efnb2KOs callus compared with the control callus (Fig. 8C), indicating that deletion of Efnb2 decreased the vasculature and VEGF production in the periosteum. We then evaluated the impact of deletion of Efnb2 on cell proliferation and VEGF production in the 3 bone formation sites during fracture repair. In addition to the periosteum and invasion front, Sox2-positive cells were also observed in the IBF site (Fig. 9A; Con) and the granulation tissue in the bridge region (EndoBF) (Fig. 9B; Con), but not in the newly formed cartilage (Fig. 9C; Con) in the control callus. Consistent with the decrease in Sox2-expressing cells in the periosteum and invasion front, Sox2-positive cells were decreased by 57% at IBF (Fig. 9A; graph IBF) and 78% at EndoBF (Fig. 9B; graph EndoBF) in the col2Efnb2KO callus (Fig. 9A and B; KO). Interestingly, no Sox2-positive cells were observed in the intramedullary site in either control or col2Efnb2KO (Fig. 9C; IMBF) callus, again pointing out the differences in these different sites of fracture repair. Apparently Efnb2 enables the activation and migration of periosteal progenitors in response to injury at IBF and EndoBF sites, but the activation and migration of the progenitors for the IMBF site seem to be under a different control mechanism. The number of proliferating cells (PCNA positive) in the IBF (Fig. 9D), EndoBF (Fig. 9E), and IMBF (Fig. 9F) sites of the col2Efnb2KOs were significantly reduced when compared with the controls (70%, 63%, and 80%, respectively).
Figure 8.
Impact of Efnb2 deletion on the vasculature during fracture repair (day 10 postfracture). Immunohistochemistry (IHC) using (A-B) antibodies against CD31 and (C-D) VEGF was performed in the controls (Con, A,C) and the col2Efnb2KOs (KO, B,D) in the invasion front (IF) and periosteum overlaying the callus. Arrows indicate vessels. Cart.: cartilage. 10× in A-D. Bars = 50 µm. (E-F) The number of CD31-labeled vessels (brown) in the (E) IF and percentage of (F) VEGF positive cells (brown) per total cells in the IF and periosteum (Peri.) was measured and shown in the bar graphs. Results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs Con (solid bars), n = 4 in each group.
Figure 9.
Deletion of Efnb2 affects progenitor activation and cell growth at the 3 bone formation sites during fracture repair. At day 10 postfracture (D10), immunohistochemistry (IHC) using antibodies against (A-C) Sox2 and (D-F) PCNA, were performed in the callus of the controls (Con) and the col2Efnb2KOs (KO). The impact of deletion of Efnb2 was evaluated at the intramembranous bone formation site (IBF, A and D), bridge region (EndoBF) (B and E), and intramedullary area (IMBF, C and F). Signals were indicated by brown DAB stain (brown). Sections for IHC were counterstained with hematoxylin. 10× in A-F and bars = 50 µm. Cart.: cartilage, WB: woven bone. Arrowheads in F: PCNA-positive cells. Percentage of Sox2-positive cells and PCNA-positive cells in total cells in each site were measured and showed in graphs in the panel to the right of the images. Results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. Con (solid bars).
To test whether and how Efnb2 regulates vessel formation during fracture repair, we evaluated CD31 labeled vessels and VEGF, a target gene of ephrin B2, by immunohistochemistry in the col2Efnb2KOs and controls. As shown in Fig. 10, consistent with H&E staining, CD31 labeled vessels were reduced in the col2Efnb2Kos (36% of control) when compared with the controls at IBF (Fig. 10D), but not in EndoBF (Fig. 10E) and IMBF sites (Fig. 10F). Similarly, VEGF staining revealed that compared with the control callus, fewer vessels were formed in the col2Efnb2KO callus, especially at the boundary of bone and cartilage (Fig. 10A-C; Con). VEGF was expressed in the hypertrophic chondrocytes, osteoblasts along the woven bone surface, and endothelial cells of the vessels in both control and col2Efnb2KO (Fig. 10A-C KO), but the expression of VEGF in the col2Efnb2KO callus was decreased when compared with the control callus at IBF (Fig. 10A KO) and EndoBF sites (Fig. 10B KO), but with less impact at the IMBF site (Fig. 10C KO). Thus, deletion of Efnb2 in chondrocytes impaired vasculature development during fracture repair primarily at sites of intramembranous and endochondral bone formation.
Figure 10.
Deletion of Efnb2 affects the vasculature at the 3 bone formation sites during fracture repair. At day 10 postfracture (D10), immunohistochemistry (IHC) using antibodies against (A-C) VEGF and (D-F) CD31 were performed in the callus of the controls (Con) and KOs. The impact of deletion of Efnb2 was evaluated at the intramembranous bone formation site (IBF, A and D), endochondral bone formation site (EndoBF, B and E), and intramedullary bone formation site (IMBF, C and F). Signals were indicated by brown DAB stain. Sections for IHC were counterstained with hematoxylin. 10× in A-F and bars = 50 µm. Arrows: vessels. CD31-labeled cells/mm2 were measured and shown in the graphs next to the pictures. Results are expressed as expressed as mean ± SD. *P < 0.05 KO (open bars) vs. Con (solid bars).
Deletion of Efnb2 decreased chondrocyte and osteoblast differentiation
To investigate whether deletion of Efnb2 affects chondrocyte and osteoblast differentiation during fracture repair, we evaluated Sox9 and osteocalcin expression at the different sites. No Sox9 was expressed at either the IBF site or IBF site or in the periosteum and endosteum surrounding the fracture callus in either control or col2Efnb2KO mice. On the other hand, at the endochondral bone formation site, Sox9 was expressed in chondrocytes and newly formed bone in the transition zone in the controls (Fig. 11A), but Sox 9 expression was markedly reduced in the col2Efnb2KO (Fig. 11B) callus (22% of control, Fig. 11E). In contrast, osteocalcin-positive cells were localized in the bone surface at the IBF site in both control (Fig. 11C) and col2Efnb2KO (Fig. 11D) callus. However, fewer osteocalcin-positive cells were observed in the col2Efnb2KO callus compared with the control callus (67% of control, Fig. 11F). Similar data were obtained at the IMBF site (ie, osteocalcin positive cells lining the bone surface, but more abundant in control that KO [data not shown]). No osteocalcin-positive cells were observed in endochondral bone formation site in either genotype. These data indicate that deletion of Efnb2 impaired chondrocyte and osteoblast differentiation, leading to delayed IBF and endochondral bone formation during fracture repair.
Figure 11.
Deletion of Efnb2 impairs chondrocyte and osteoblast differentiation during fracture repair. At day 10 post fracture, immunohistochemistry was performed using antibodies against Sox9 (red, A and B) and osteocalcin (OCN, green, C and D) in the callus sections of the controls (Con, A and C) and the col2Efnb2KO (KO, B and D). Sections were counterstained with DAPI (blue). 10× in A -D, bars = 50 µm. Cart., cartilage compartments; CB, cortical bone; Tb, trabecular bone. White dotted lines in A and B indicate the boundary of cartilage and trabecular bone. (E-F) Percentage of (E) Sox9- or (F) OCN-positive cells per total cells was measured. Results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. con (solid bars).
Deletion of Efnb2 affects late stage fracture repair
Finally, we evaluated the effects of deletion of Efnb2 at the later stages of fracture repair. At 28 days after fracture, histology showed that in the control (Fig. 12A and C) and in the col2Efnb2KOs (Fig. 12B and D), cartilage was replaced by bone inside the callus such that the fracture gap was bridged by newly formed trabecular bone. However, less trabecular bone was formed in the col2Efnb2KO callus compared with the control callus at the IBF site (Fig. 12E vs F) and bridge area (Fig. 12G vs H), as indicated by the 50% reduction of BV/TV in the col2Efnb2KO callus (Fig. 12I). In addition, the newly formed cortical bone that bridged the gap was thinner in the col2Efnb2KOs (c; Fig. 12F) than in the controls (c; Fig. 12E) (66% of control; Fig. 12J), indicating that deletion of Efnb2 in chondrocytes led to a persistently impaired bone formation during fracture repair. No trabecular bone was observed at the intramedullary site at this time, indicating the transient nature of IMBF during fracture repair.
Figure 12.
Impaired bone formation in col2Efnb2KOs at day 28 postfracture (D28). (A-B) Safranin O staining of the callus of (A) control and (B) col2Efnb2KO at D28. (D-H) H&E staining of callus of control (C, E, and G) and col2Efnb2KO (D, F, and H). (E and F) High magnification of the green frame area in C and D; (G and H): high magnification of the yellow frame area in C and D, respectively. 2.5× in A-D, bars = 100 µm. 10× in E-H, bars = 50 µm. c in E and F, newly formed cortical bone; b in E-H, newly formed trabecular bone; BM in E-H, bone marrow. (I-J) Measurements of trabecular bone volume (BV) per total volume (TV) (BV/TV, I) and cortical thickness (C.Th, J). Results are expressed as mean ± SD. *P < 0.05 KO (open bars) vs. Con (solid bars). H&E, hematoxylin and eosin.
Discussion
In the current study, we demonstrate that during bone development, deletion of Efnb2 in Col.2-expressing cells inhibits chondrocyte-osteoblast transdifferentiation, leading to decreased bone formation in both the primary spongiosa and the secondary ossification center. During fracture repair, in some sense a recapitulation of bone development, deletion of Efnb2 in Col.2 expressing cells impaired mesenchymal progenitor activation and proliferation, increased progenitor cell apoptosis, decreased VEGF production and vascular invasion, and inhibited chondrocyte and osteoblast differentiation, thus impairing both intramembranous and endochondral bone formation, leading to delayed fracture repair. Thus, Efnb2 plays a critical role during the fracture repair process as it does during bone development.
Efnb2 in Col.2-expressing cells stimulates endochondral bone formation during bone development
In previous studies, we and others demonstrated that Efnb2 was expressed in chondrocytes (14, 21). To our surprise, we found that ablation of Efnb2 in Col.2-expressing cells did not have a profound effect on chondrocytes in the growth plate but showed significantly decreased cell proliferation and decreased trabecular bone in the metaphysis (PS) and epiphysis (SOC), suggesting that Efnb2 in Col.2-expressing cells plays a major role in stimulating trabecular bone formation, although a previous study (42) with a different Efnb2flox/flox mouse also found growth plate abnormalities. Recent studies demonstrated that in addition to the traditional concept in which the hypertrophic chondrocytes undergo programmed cell death with osteoprogenitor cells entering the PS and SOC only from the perichondrium with the invading vasculature to replace the cartilage with bone (43), some chondrocytes are able to transdifferentiate into osteoblasts, contributing to bone formation in these ossification centers (44-46). Consistent with these previous studies, our data indicate the transdifferentiation of Col.2-expressing cells into osteoblasts in the controls, but that this was markedly reduced in the tamcol2Efnb2KO/tdT mice. These data suggest that during bone development, deficiency of Efnb2 in Col.2-expressing cells, at least in part, blocks the Col.2-expressing chondrocytes from transdifferentiating into osteoblasts leading to decreased trabecular bone formation, a point that will reappear when the impact of Efnb2 deletion on fracture repair is evaluated. In our study, in which the cells were labeled at 2 weeks of age, we did not observe tdT-labeled cells in the perichondrium, periarticular region, or periosteum, but only in the growth plate 1 day after labeling, and these cells then contribute to the metaphysis during the subsequent 7 days but not the SOC. However, a recent study (47) demonstrated that when cells were labeled at birth Col2CreERT also labels multipotent mesenchymal progenitors at the periarticular region that contribute to formation of the SOC. Moreover, Col2Cre and Col2CreERT appear to target somewhat different cell populations in the knee joint (48), so we recognize that age of the animals and the timing and type of Cre may influence the results. But the main conclusion of our studies in bone development in the postnatal period is that Efnb2 in chondrocytes is more directed at maintaining their ability to transdifferentiate into osteoblasts rather than regulating their proliferation and differentiation. This understanding of its role in the growth plate is applicable to its role in fracture repair.
Efnb2 enhances IBF during fracture repair
During fracture repair, decreased soft cartilage callus and hard bony callus formation were observed in the Col.2.Efnb2KO, resulting in impaired fracture repair. However, the impact of Efnb2 deletion varied according to the site of repair. First, we consider IBF. This process is initiated by periosteal progenitor activation and migration which requires Efnb2-EphB4 interaction (19). These progenitors do not pass through a chondrocyte stage before their differentiation into osteoblasts at this site (5). In the Col.2.Efnb2KO, we observed reduced periosteal progenitor activation, as indicated by reduced numbers of Sox2- and PCNA-positive cells, and reduced migration into the IBF site. Osteocalcin expression was also reduced in the Col.2.Efnb2KO, indicative of the reduction in osteoblast differentiation in the context of decreased IBF. Thus, we conclude that Efnb2 promotes IBF (20) as part of its regulation of fracture repair.
Efnb2 stimulates endochondral bone formation during fracture repair
During endochondral bone formation, progenitors from the periosteum first differentiate into chondrocytes before transdifferentiating into osteoblasts (5). Compared with IBF, the effects of Efnb2 in regulating endochondral bone formation are more complicated. Similar to the IBF, Efnb2 deficiency decreased the number of Sox2- and PCNA-positive cells because of decreased proliferation and increased apoptosis of the progenitor cells in the periosteum and the invasion front, similar to that found with deletion of Efnb2 in osteoblasts (21) in a different model. Thus, there were fewer numbers of progenitors entering the fracture gap to differentiate into chondrocytes in the Col.2.Efnb2KO. Moreover, lack of Efnb2-inhibited chondrocyte differentiation (reduced Sox9 expression) contributing to decreased cartilage formation and its subsequent conversion to bone. In addition, Efnb2 is a key regulator of VEGF-induced angiogenesis (49). Deletion of Efnb2 reduced VEGF production in the periosteum and invasion front as well as in the hypertrophic chondrocytes (42) of the cartilage callus, impairing vessel formation in the Col.2.Efnb2KO. In the Col.2.Efnb2KO, although the chondrocyte differentiation was decreased, more cartilage callus remained in these mice than in the controls. We suggest this is due to 1 or both of the following reasons: (1) impaired vascular invasion. During endochondral bone formation at the growth plate, hypertrophic chondrocytes produce VEGF-inducing vascular invasion leading to bone/cartilage resorption by osteoclasts and bone formation by osteoblasts, resulting in the replacement of cartilage by bone (43). In the Col.2.Efnb2KO, decreased VEGF production and vascular invasion result in delayed conversion of cartilage to bone. (2) Blunted transdifferentiation of chondrocytes to osteoblasts. Similar to endochondral bone formation during bone development, recent studies demonstrate that during endochondral fracture repair, in a region defined as the transition zone and at the site of vascular invasion, a number of hypertrophic chondrocytes regain some stem cell-like properties by expressing the pluripotent transcription factors OCT4, Sox2, and NANOG. These cells reenter the cell cycle, divide, and then transdifferentiate into osteoblasts (50). In our study, Efnb2 ablation blocked the transdifferentiation of chondrocytes to osteoblasts during bone development and may do so to decrease the transdifferentiation of chondrocytes to osteoblasts at the endochondral bone formation site delaying the conversion of cartilage to bone during fracture repair. Thus, at the endochondral bone formation site, Efnb2 deficiency decreased progenitor activation and proliferation, increased progenitor apoptosis, decreased vasculature formation, and blocked chondrocyte differentiation and conversion to osteoblasts impairing fracture repair at this site.
Deletion of Efnb2 in chondrocytes has limited impact on IMBF during fracture repair
Compared with the well-characterized intramembranous and endochondral bone formation, less is known about bone formation at the intramedullary area during fracture repair. Consistent with a previous study (4), no cartilage was observed in the intramedullary area in either controls or Col.2.Efnb2KO, and in this sense resembles IBF. However, IMBF differs from IBF that occurs along the periosteal surface at the proximal and distal edges of the callus. First of all, the origin of the progenitors appears to come from a unique group of osteocytes in the mid-diaphyseal cortex, and not from the periosteum (or endosteum) (5, 51). Second, it is initiated later; no bone was observed at D5, and is transient, disappearing by D28. Third, unlike intramembranous and endochondral bone formation, Sox2-positive cells were not found in the intramedullary bone itself, although as will be discussed Sox2 expression was found in the osteocytes that appear to contribute to bone formation at this site. Our previous studies demonstrated that periosteal prx-1 cre/tdTomato-labeled cells did not contribute to IMBF as they do to intramembranous and endochondral bone formation, but Col.2-expressing osteocytes in the cortex did (5). These Col.2-expressing cells coexpressed DMP-1 as well as Sox 2, leading us to hypothesize that the progenitors for IMBF are from the Col.2-expressing osteocytes in the mid-diaphysis of cortical bone, but lose Sox 2 expression during new IMBF. In the current study, deletion of Efnb2 in chondrocytes modestly decreased the number of Col.2-expressing osteocytes in the cortical bone, but this was not as pronounced as in the metaphysis. We propose that the subtle reduction of progenitor number and cell proliferation at the IMBF site and the only modest decrease in VEGF production and vascularity in the Col.2.Efnb2KO, compared with the other sites, resulted in less of an impact on IMBF compared with that in intramembranous and endochondral bone formation. Why this is the case remains under investigation, but further demonstrates the uniqueness of this region of fracture repair.
In summary, we have demonstrated an important role for Efnb2 in regulating fracture repair. However, we also found that this role varies according to the site of fracture repair within the forming callus, with endochondral bone formation being more affected than IBF, and IMBF being affected even less than either of these other sites. Whether this relates to the deletion of Efnb2 only in chondrocytes, although this deletion profoundly affected periosteal stem cell activation, proliferation, and differentiation (periosteal stem cells do not express Col.2), or to as yet unexplored actions of Efnb2 within Col.2-expressing cells affecting both periosteal stem cells and osteoblasts remains for future investigation.
Acknowledgments
Financial Support: This work was supported by a National Institutes of Health grant RO1AR055924 (D.D.B.) and a CTSI Pilot Award from University of California, San Francisco (Y.W.).
Author Contribution: Study design: Y.W., S.H., and D.D.B.; experimental conduct: Y.W., L.L., F.T., and S.H.W.K.; data analysis, histology, and immunohistochemistry: Y.W.; and manuscript preparation: Y.W. and D.D.B.
Glossary
Abbreviations
- µCT
microcomputed tomography
- BV/TV
bone volume fraction
- Col.2
type II collagen
- Conn.D
connective density
- Efnb2
ephrin B2
- EndoBF
endochondral bone formation
- GP
growth plate
- H&E
hematoxylin and eosin
- IBF
intramembranous bone formation
- IF
invasion front
- IMBF
intramedullary bone formation
- PCNA
proliferating cell nuclear antigen
- PS
primary spongiosa
- SOC
secondary ossification center
- Sox2
SRY-box2
- Tb.N
trabecular number
- TdT
tdTomato
- TUNEL
deoxynucleotidyl transferase
- dUTP
nick end labeling
- VEGF
vascular endothelial growth factor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in the references.
References
- 1. Hadjiargyrou M, O’Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res. 2014;29(11): 2307-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Keefe RJ. Fibrinolysis as a target to enhance fracture healing. N Engl J Med. 2015;373(18):1776-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Chen L, Kang M, et al. The fracture callus is formed by progenitors of different skeletal origins in a site-specific manner. JBMR Plus. 2019;3(9):e10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392-1404. [DOI] [PubMed] [Google Scholar]
- 7. Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87(2):107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133-143. [DOI] [PubMed] [Google Scholar]
- 9. Mabry TM, Prpa B, Haidukewych GJ, Harmsen WS, Berry DJ. Long-term results of total hip arthroplasty for femoral neck fracture nonunion. J Bone Joint Surg Am. 2004;86(10):2263-2267. [DOI] [PubMed] [Google Scholar]
- 10. Antonova E, Le TK, Burge R, Mershon J. Tibia shaft fractures: costly burden of nonunions. BMC Musculoskelet Disord. 2013;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hankenson KD, Zimmerman G, Marcucio R. Biological perspectives of delayed fracture healing. Injury. 2014;45(Suppl 2):S8-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38-52. [DOI] [PubMed] [Google Scholar]
- 13. Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Menendez A, Fong C, ElAlieh HZ, Chang W, Bikle DD. Ephrin B2/EphB4 mediates the actions of IGF-I signaling in regulating endochondral bone formation. J Bone Miner Res. 2014;29(8):1900-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin TJ, Allan EH, Ho PW, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51-60. [DOI] [PubMed] [Google Scholar]
- 16. Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012;6(2):148-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111-121. [DOI] [PubMed] [Google Scholar]
- 18. Allan EH, Häusler KD, Wei T, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23(8):1170-1181. [DOI] [PubMed] [Google Scholar]
- 19. Arthur A, Zannettino A, Panagopoulos R, et al. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48(3):533-542. [DOI] [PubMed] [Google Scholar]
- 20. Benson MD, Opperman LA, Westerlund J, et al. Ephrin-B stimulation of calvarial bone formation. Dev Dyn. 2013;241(12):1901-1910. [DOI] [PubMed] [Google Scholar]
- 21. Tonna S, Poulton IJ, Taykar F, et al. Chondrocytic ephrin B2 promotes cartilage destruction by osteoclasts in endochondral ossification. Development. 2016;143(4):648-657. [DOI] [PubMed] [Google Scholar]
- 22. Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci. 2008;5(5):263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126(3):441-443. [DOI] [PubMed] [Google Scholar]
- 24. Tonna S, Takyar FM, Vrahnas C, et al. EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. Faseb J. 2014;28(10):4482-4496. [DOI] [PubMed] [Google Scholar]
- 25. Takyar FM, Tonna S, Ho PW, et al. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28(4):912-925. [DOI] [PubMed] [Google Scholar]
- 26. Arthur A, Panagopoulos RA, Cooper L, et al. EphB4 enhances the process of endochondral ossification and inhibits remodeling during bone fracture repair. J Bone Miner Res. 2013;28(4):926-935. [DOI] [PubMed] [Google Scholar]
- 27. Valverde-Franco G, Pelletier JP, Fahmi H, et al. In vivo bone-specific EphB4 overexpression in mice protects both subchondral bone and cartilage during osteoarthritis. Arthritis Rheum. 2012;64(11):3614-3625. [DOI] [PubMed] [Google Scholar]
- 28. Valverde-Franco G, Hum D, Matsuo K, et al. The in vivo effect of prophylactic subchondral bone protection of osteoarthritic synovial membrane in bone-specific Ephb4-overexpressing mice. Am J Pathol. 2015;185(2):335-346. [DOI] [PubMed] [Google Scholar]
- 29. Kwan Tat S, Pelletier JP, Amiable N, et al. Activation of the receptor EphB4 by its specific ligand ephrin B2 in human osteoarthritic subchondral bone osteoblasts. Arthritis Rheum. 2008;58(12):3820-3830. [DOI] [PubMed] [Google Scholar]
- 30. Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lavigne M, Martel-Pelletier J. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Res Ther. 2009;11(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129(6):1397-1410. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Cheng Z, Elalieh HZ, et al. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res. 2011;26(7):1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Hsiao EC, Lieu S, et al. Loss of Gi G-Protein-coupled receptor signaling in osteoblasts accelerates bone fracture healing. J Bone Miner Res. 2015;30(10):1896-1904. [DOI] [PubMed] [Google Scholar]
- 34. Wang T, Wang Y, Menendez A, et al. Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res. 2015;30(9):1572-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468-1486. [DOI] [PubMed] [Google Scholar]
- 36. RRID:AB_2814950, https://scicrunch.org/resolver/AB_2814950.
- 37. RRID:AB_2212642, https://scicrunch.org/resolver/RRID:AB_2212642.
- 38. RRID:AB_2302581, https://scicrunch.org/resolver/AB_2302581.
- 39. RRID:AB_10675660, https://scicrunch.org/resolver/RRID:AB_10675660.
- 40. RRID: AB_778028, https://scicrunch.org/resolver/RRID:AB_778028.
- 41. RRID:AB_2095700, https://scicrunch.org/resolver/RRID:AB_2095700.
- 42. Valverde-Franco G, Lussier B, Hum D, et al. Cartilage-specific deletion of ephrin-B2 in mice results in early developmental defects and an osteoarthritis-like phenotype during aging in vivo. Arthritis Res Ther. 2016;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332-336. [DOI] [PubMed] [Google Scholar]
- 44. Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111(33):12097-12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. Plos Genet. 2014;10(12):e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16(12):1157-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tong W, Tower RJ, Chen C, et al. Periarticular mesenchymal progenitors initiate and contribute to secondary ossification center formation during mouse long bone development. Stem Cells. 2019;37(5):677-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagao M, Cheong CW, Olsen BR. Col2-Cre and tamoxifen-inducible Col2-CreER target different cell populations in the knee joint. Osteoarth Cartil. 2016;24(1):188-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y, Nakayama M, Pitulescu ME, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483-486. [DOI] [PubMed] [Google Scholar]
- 50. Hu DP, Ferro F, Yang F, et al. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development. 2017;144(2):221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torreggiani E, Matthews BG, Pejda S, et al. Preosteocytes/osteocytes have the potential to dedifferentiate becoming a source of osteoblasts. Plos One. 2013;8(9):e75204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in the references.