Abstract
Long-lasting post-switched plasma cells (PCs) arise mainly from germinal center (GC) reactions, but little is known about the mechanism by which GC B cells differentiate into PCs. Based on our observation that the expression of the transcription factor CCAAT/enhancer binding protein β (C/EPBβ) is associated with the emergence of post-switched PCs, we enquired whether a cell-autonomous function of C/EPBβ is involved in the program for PC development. To address this, we generated C/EPBβ-deficient mice in which the Cebpb locus was specifically deleted in B cells after transcription of the Ig γ1 constant gene segment (Cγ1). In response to in vitro stimulation, B cells from these Cebpbfl/flCγ1Cre/+ mice had defects in the induction of B lymphocyte-induced maturation protein 1 (Blimp1) and the formation of IgG1+ PCs, but not in proliferation and survival. At steady state, the Cebpbfl/flCγ1Cre/+ mice had reduced serum IgG1 titers but normal IgG2c and IgM titers. Moreover, upon immunization with T-dependent Ag, the mice produced reduced levels of Ag-specific IgG1 Ab, and were defective in the production of Ag-specific IgG1 Ab-secreting cells. These results suggest that a cell-autonomous function of C/EPBβ is crucial for differentiation of post-switched GC B cells into PCs through a Blimp1-dependent pathway.
Keywords: Plasma cells, Immunoglobulin class switching, C/EBPβ
INTRODUCTION
Plasma cells (PCs) are terminally differentiated effector cells responsible for the production of Abs. Mature PCs that secrete class-switched high affinity Abs are central to establishing long-lasting Ab responses (1). The mature PCs mainly arise through germinal center (GC) reactions, although in a few mouse models such cells are generated via an extrafollicular pathway (2,3,4). In the GC—the transient structure formed in lymphoid follicles upon exposure to T-dependent (TD) Ags—primed CD4+ T cells provide co-stimulation and cytokine signals to their cognate B cells (5,6). As a result, the latter undergo somatic hypermutation and class-switch recombination (CSR) of Ig genes. The post-switched cells ultimately give rise to either PCs or memory cells.
Conversion of GC B cells into PCs requires silencing of the GC B cell transcriptional program, so allowing induction of the PC transcriptome. Transcription factors participating in this transition include paired box gene 5 (Pax5) and B cell lymphoma 6 (BCL6) as GC B cell identity factors, and B lymphocyte-induced maturation protein 1 (Blimp1) and interferon regulatory factor 4 (IRF4) as the main inducers of the PC transcriptional program (7,8). Unlike GC B cells, PCs lack both Pax5 and BCL6 but contain large amounts of IRF4 and Blimp1. Mutual exclusion between the genetic programs of GC B cells and PCs is evident: BCL6 inhibits the transcription of PR domain containing 1, with ZNF domain (Prdm1), the gene that encodes Blimp1, and Blimp1 represses transcription of the genes that encode Pax5 and BCL6 (9,10). Although the presence of this double negative feedback loop is relatively well reported, less is known about the signaling events that control the expression of the relevant transcription factors. In particular, it is not clear what other factors are needed to achieve PC differentiation after maturation of GC B cells.
CCAAT/enhancer binding protein β (C/EBPβ), also known as NF-IL6, is a transcription factor containing a basic leucine zipper domain (11). C/EBPβ is expressed in many types of cell, including hepatocytes, macrophages and B cells (12). By targeting a variety of genes associated with the acute phase response, inflammation, and hematopoiesis, C/EBPβ is able to exert diverse effects on fundamental cellular processes including proliferation, survival and differentiation in a cell type-specific manner (13,14,15). The functional versatility of C/EBPβ is in part attributable to the presence of 3 isoforms differing in their N-terminal domains: liver-enriched activator protein (LAP), LAP* (both are transcriptional activators), and liver-enriched inhibitory protein (a trans-dominant repressor), each having unique roles in various cellular processes (16). Previous studies suggest that C/EBPβ is also implicated in the development, survival and/or tumorigenesis of B-lineage cells. For example, mice deficient in C/EBPβ exhibit defects in the development and homeostatic expansion of B cells early in the pre-B cell stage in a cell-extrinsic manner (17). An in vitro study revealed that C/EBPβ regulates both proliferation and survival of multiple myeloma cells, which are neoplastic PCs, by regulating the expression of IRF4, Blimp1, and BCL2 (18). This result suggests that C/EBPβ is involved in the network of transcription factors critical for PC differentiation and survival. However, this idea has not been directly addressed.
In the present study, we aimed to determine whether C/EBPβ plays a role in the differentiation of activated GC B cells into PCs during humoral immune responses. C/EBPβ knockout mice would not be useful for this purpose, because they have an early B-cell lymphopenia. Since the program of PC development follows closely on the execution of CSR in the GC, we generated C/EBPβ conditional knockout (cKO) mice in which the gene encoding C/EBPβ could be specifically deleted in B cells after transcription of the Ig γ1 constant gene segment (Cγ1). We found that the emergence of PCs at the post-switched stage was impaired in the C/EBPβ-deficient B cells, and this was associated with reduced induction of Blimp1 but not of IRF4. Our data provide evidence that C/EBPβ is involved in the genetic regulatory network controlling the differentiation of post-switched B cells into PCs.
MATERIALS AND METHODS
Mice
B6.129P2(Cg)-Ighg1tm1(IRES-cre)Cgn/J (Cγ1Cre/+) and B6.129S1-Cebpbtm1Es/Mmnc (Cebpbfl/+) mice (14,19) were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and the Mutant Mouse Regional Resource Center at the University of North Carolina (Chapel Hill, NC, USA), respectively, and their inbred descendants were crossed to generate Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice. Mice were maintained in a specific pathogen-free barrier facility at Hanyang University. Sex-matched mice at 8–12-wk of age were used for experiments. This study was approved by the Institutional Animal Care and Use Committee (HY-IACUC-12-003). All animal experiments were carried out in strict accordance with guidelines and regulations.
Primary mouse cell culture, FACS and immunoblotting assays
Single-cell suspensions were prepared from spleen as described previously (20). B220+ B cells were sorted by positive selection using MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of B cells routinely exceeded 97%. Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and 5×10−5 M 2-mercaptoethanol (all from Gibco-Life Technologies Corporation, Grand Island, NY, USA) under the stimulation with 10 μg/ml LPS (Sigma-Aldrich, St. Louis, MO, USA) and 10 ng/ml IL-4 (Peprotech, Cranbury, NJ, USA) in the presence or absence of 10 ng/ml IL-21 (Peprotech). In some experiments they were stained with 3 μM cell proliferation dye eFluor 670 (eBioscience, San Diego, CA, USA) before culture. After growth for 96 h, cells were assayed by FACS and immunoblotting, as described previously (21,22). For FACS, cells were treated with FcR block reagent and surface or intracellular stained with anti-IgG1-PE (A85-1), anti-B220-PerCP (RA3-6B2), and/or anti-CD138-APC (281-2) mAbs (all from BD Biosciences, San Jose, CA, USA) in PBS containing 0.1% sodium azide and 0.5% BSA. For intracellular staining, cells were fixed and permeabilized using a Cytofix/Cytoperm solution kit (BD Biosciences) according to manufacturer's instructions. Data were acquired by FACS canto II, with more than 200,000 events per sample, and analyzed using Flowjo v10.6.2 (all from BD Biosciences). For immunoblotting, Abs to C/EBPβ (1H7; Biolegend, San Diego, CA, USA), Blimp1 (6D3; eBioscience), IRF4 (D9P5H; Cell Signaling, Danvers, MA, USA), GAPDH (0411; Santa Cruz, Dallas, TX, USA) and β-actin (C4; Sanra Cruz) were used. The intensities of protein bands were quantitated using ImageJ software (NIH, Bethesda, MD, USA).
Quantitative RT-PCR
B cells stimulated with LPS and IL-4 for 96 h were sorted into surface IgG1+ and IgG1− cells with a FACSAriaIII flow cytometer (BD Biosciences). Total RNA was purified from the sorted cells and assayed by quantitative RT-PCR, as described previously (20). Primer sequences used were as follows: C/EBPβ (5′ CAA GCT GAG CGA CGA GTA CA 3′ and 5′ GAC AGC TGC TCC ACC TTC TT 3′) and β2 microglobulin (5′ TGA CCG GCC TGT ATG CTA TC 3′ and 5′ CAG TGT GAG CCA GGA TAT AG 3′). Relative amounts of C/EBPβ transcripts were normalized to the amounts of β2 microglobulin transcripts.
Mouse immunization
Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice were injected intraperitoneally with 100 μg of 4-hydroxy-3-nitrophenylacetyl (NP) conjugated with keyhole limpet hemocyanin (KLH) (Biosearch Technologies, Novato, CA, USA) adsorbed on 100 μl alum (Thermo Scientific, Waltham, MA, USA). Serum, spleen and bone marrow (BM) were collected post mortem at days 14 and 28 post-immunization and assayed by ELISA and ELISPOT methods.
ELISA and ELISPOT assay
Serum titers of total and NP-specific Abs were determined by standard sandwich ELISA (4). In brief, immunosorbent plates were coated with 10 μg/ml goat anti-mouse IgG Fc fragment Ab (Bethyl Laboratories, Montgomery, TX, USA) or 10 μg/ml NP8-BSA (Biosearch Technologies). Sera were diluted 1:10,000-50,000 in PBS. Biotinylated Abs to mouse IgG1 (A85-1; BD Biosciences), IgG2b (R12-3; BD Biosciences), IgG2c (Abcam, Cambridge, UK), and IgM (Southern Biotech, Birmingham, AL, USA) were used as detection Abs. A serum sample containing high titer of each Ab was serially diluted and used as the reference measurement of the Ab. The amount of Ab in each serum sample was quantified as an arbitrary unit (AU) that defined the ratio of amount of the Ab to a reference measurement. The data are presented as the means±SEMs (AU/ml). Ab-secreting cells (ASCs) were enumerated by standard ELISPOT assay (4).
RESULTS AND DISCUSSION
Production of cKO mice with C/EBPβ selectively deleted in Cγ1-expressing B cells
To determine if C/EBPβ expressed in post-switched B cells plays a cell-autonomous role in the differentiation into PCs, we used the Cγ1Cre/+ knock-in system to specifically delete the floxed Cebpb locus in B cells after Cγ1 transcription, because Cγ1Cre/+ mice were previously proven to execute Cre-mediated recombination only in IgG1-switched GC B cells (19,23).
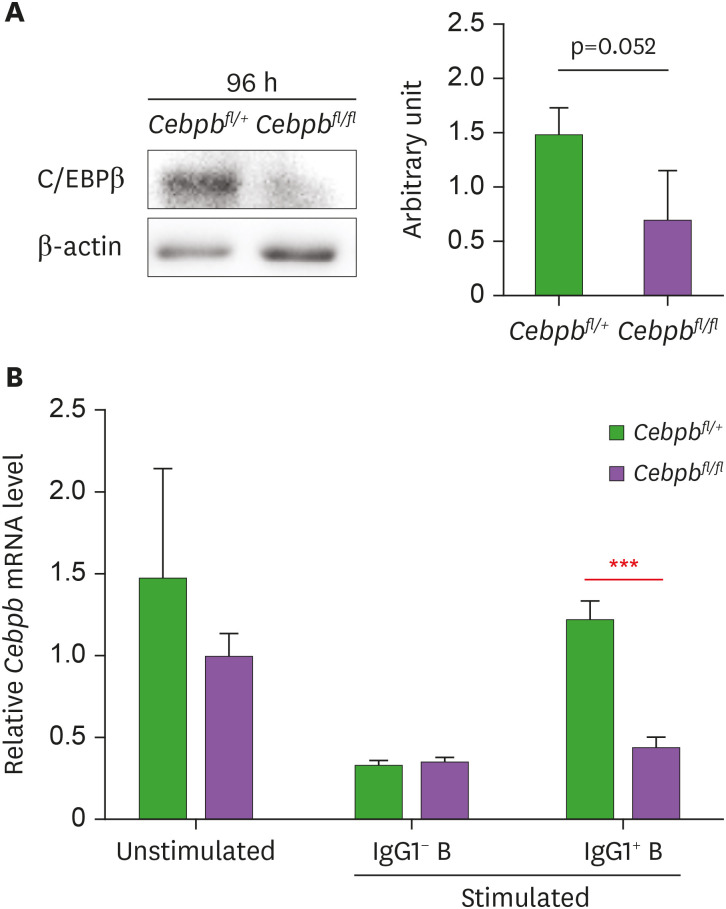
Unlike C/EBPβ whole KO mice that have B lymphopenia (17), the Cebpbfl/flCγ1Cre/+ mice had normal frequencies of B cells as well as T cells in central and peripheral organs at steady state (Supplementary Fig. 1). To evaluate if the cKO system was functional, we sorted splenic B cells from Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice and stimulated with LPS and IL-4 for 96 h to polarize them mainly into IgG1 class-switched cells. We found that the Cebpbfl/flCγ1Cre/+ B cells expressed significantly lower amounts of C/EBPβ protein than the Cebpbfl/+Cγ1Cre/+ B cells (Fig. 1A).
Figure 1. Selective deficiency of C/EBPβ in IgG1+ B cells of Cebpbfl/flCγ1Cre/+ mice. (A) B220+ B cells sorted from Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice were cultured with LPS and IL-4 for 96 h and assayed by immunoblotting. A representative blot and quantitated results of 3 independent experiments are shown. (B) The cultured cells were fractionated into surface IgG1+ and IgG1− cells by FACS, and each fraction was assayed by quantitative RT-PCR. Unstimulated cells were used as a control. The data are representative of 3 independent experiments.
***p<0.001 by unpaired 2-tailed Student's t-test.
To confirm the selective ablation of C/EBPβ in the Cγ1-expressing cells, we sorted surface IgG1+ and IgG1− cells after 96 h and conducted quantitative RT-PCR. We found that the level of C/EBPβ mRNA was reduced by approximately 60% in the IgG1 class-switched cells from the Cebpbfl/flCγ1Cre/+ mice, but not in the IgG1− and unstimulated B cells (Fig. 1B). This result clearly demonstrates specific ablation of C/EBPβ in those B cells that underwent CSR to IgG1. Despite its specificity, the reduction of C/EBPβ expression was not 100%, presumably due to allelic exclusion in heterozygotes for wild-type Cγ1 and mutant Cγ1-Cre (24).
C/EBPβ deficiency impairs development of PCs into post-switched B cells
To see whether the absence of C/EBPβ affected the activation and differentiation of B cells at the post-switched stage, we cultured B cells from Cebpbfl/flCγ1Cre/+ mice and their control littermates in the presence of LPS, IL-4, and IL-21 for 96 h, and assayed the phenotypes of the resultant cells. Unlike the situation in multiple myeloma cells (18), the B cells from Cebpbfl/flCγ1Cre/+ mice proliferated and survived in response to stimulation just like those from Cebpbfl/+Cγ1Cre/+ mice (Fig. 2A and B). In contrast, the intracellular IgG1+ Cebpbfl/flCγ1Cre/+ B cells gave rise to significantly fewer cells expressing CD138, a marker of PCs, than the intracellular IgG1+ Cebpbfl/+Cγ1Cre/+ B cells, and this phenomenon was restricted to IgG1+ cells only (Supplementary Fig. 2 and Fig. 2C). Consistent with this, whereas Blimp1 expression was induced in the Cebpbfl/+Cγ1Cre/+ B cells in response to the 96-h stimulation, it failed to be induced in the Cebpbfl/flCγ1Cre/+ B cells (Fig. 2D). However, the presence or absence of C/EBPβ did not significantly affect the expression of IRF4. These results demonstrate that C/EBPβ expression in post-switched B cells plays a cell-autonomous role in the differentiation of PCs but not in their proliferation and survival. This effect is associated with the induction of Blimp1 but not IRF4, suggesting that C/EBPβ act as an upstream regulator of Blimp-1 but IRF4 is not.
Figure 2. The effect of C/EBPβ ablation on post-switched B cells in vitro. B220+ B cells sorted from Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice were stained with eFluor 670 (A) or left unstained (B-D) and stimulated with LPS, IL-4, and IL-21 for 3 (A) or 4 (B-D) days. They were then assayed by FACS (A, C), trypan blue exclusion (B) and immunoblotting (D) methods. Representative FACS profiles gated on lymphocytes (A) and either intracellular IgG1+ or IgG1− cells (C), with percentages of cells in the indicated areas, are shown. A representative immunoblot with quantitated results is shown (D). The data are representative of 2 independent experiments.
**p<0.01 by unpaired 2-tailed Student's t-test.
Blimp1 is a master regulator that promotes the terminal differentiation of B cells. Indeed, within the B cell lineage, Blimp1 is exclusively expressed in ASCs, with its concentration dependent on the maturation state (25). The proposed functions of Blimp1 include repression of key regulators of the B cell program such as Pax5 and BCL6. Consistent with a previous study used multiple myeloma cell lines (18), our results provide evidence that the C/EBPβ–Blimp1 axis does indeed operate in post-GC B cells. However, our results differ from that study in terms of the effect of C/EBPβ on IRF4 induction. The discrepancy may stem from differences in the effect of IRF4 depending on its concentration. At low levels IRF4 promotes the GC fate through activation of Aicda, Pou2af1 and Bcl6, while at high levels it promotes the PC fate though repression of Bcl6 and activation of Prdm1 and Zbtb20 (26). Therefore, the expression level of IRF4 is critical for determining cell fate at the GC B cell stage, and our results suggest that C/EBPβ does not influence the level of IRF4 at that stage.
The presence of C/EBPβ in post-switched B cells is required to maintain the natural Ab pool at steady state
Sera from normal mice contain a substantial level of Abs, which arise naturally. The size of the natural Ab pool is important as are the isotypes that are present, because the Abs play isotype-specific roles, such as protection against infectious agents (27). To determine whether a cell-autonomous role of C/EBPβ in post-switched B cells contributes to Ab production in mice at steady state, we measured the serum titers of Abs and numbers of ASCs in untreated mice. Compared with their control littermates, Cebpbfl/flCγ1Cre/+ mice contained normal titers of serum IgM and IgG2c, but a significantly reduced titer of serum IgG1 (Fig. 3A). IgG1 ASCs were also significantly less numerous in the spleens of Cebpbfl/flCγ1Cre/+ mice, but numbers of IgG2c- and IgM ASCs were unaltered (Fig. 3B and C), as were numbers of ASCs in the BM, regardless of isotypes. These data demonstrate that deficiency of C/EBPβ at the post-switched stage impairs the emergence of ASCs in the spleen, thereby leading to reduced serum titers of class-switched Abs. This result thus suggests that C/EBPβ is crucial for maintenance of the class-switched natural Ab pool.
Figure 3. Cebpbfl/flCγ1Cre/+ mice have lower IgG1 titers and less numerous IgG1-secreting cells at steady state. (A) Sera were collected from naive Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice (n=12 per group) and assayed by ELISA to measure titers of total IgG1, IgG2c and IgM Abs. (B, C) Spleens and BM were collected from naive Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice and assayed by ELISPOT to enumerate ASCs. Representative ELISPOT wells with spleen cells are shown (B). The plots present the means±SEM of ASCs per 3×105 cells in spleens and BM. Each symbol represents the value of an individual mouse. The data are representative of 3 independent experiments (B, C).
*p<0.05, **p<0.01 by unpaired 2-tailed Student's t-test.
A cell-autonomous role of C/EBPβ in post-switched B cells is necessary for long-lasting TD Ab responses
Affinity-matured class-switched Abs are mostly produced as a result of the TD GC reaction. To determine whether a cell-autonomous function of C/EBPβ in post-switched GC B cells is critical for eliciting TD Ab responses, we immunized mice with a mixture of NP-KLH and alum, and measured Ab titers and ASCs specific for NP. On day 14 post-immunization, sera from Cebpbfl/flCγ1Cre/+ mice contained a significantly lower level of NP-specific IgG1 Ab than those from their control littermates but a similar level of NP-specific IgG2b Ab (Fig. 4A). NP-specific IgG1 ASCs were less abundant in the spleens of the Cebpbfl/flCγ1Cre/+ mice on day 14 post-immunization and this difference was maintained to day 28 (Fig. 4B and C). Numbers of NP-specific IgG2b ASCs did not differ between the 2 strains at either time. This IgG1-specific decrease was also seen in the BM but only on day 28. These results demonstrate that Cebpbfl/flCγ1Cre/+ mice have a defect in generating IgG1 class-switched Ab-secreting PCs in the spleen and in maintaining them in the BM. Thus, these results suggest that C/EBPβ plays a post-switched GC B cell-intrinsic role in differentiation of the B cells into PCs in the spleen and their maintenance in the BM as a long-lived PC compartment.
Figure 4. The effect of C/EBPβ ablation in post-switched B cells on TD Ab responses. Cebpbfl/flCγ1Cre/+ and Cebpbfl/+Cγ1Cre/+ mice were immunized with NP-KLH in alum, and sera, spleens and BM were collected on days 14 and 28 after injection. (A) Sera were assayed by ELISA to determine levels of NP-specific IgG1 and IgG2b. (B, C) Spleens and BM were assayed by ELISPOT to enumerate NP-specific ASCs. Representative ELISPOT wells with spleen cells at day 28 post-immunization are shown (B). The plots show means±SEM of ASCs per 1×106 cells in spleens and per 5×106 cells in BM. Each symbol represents the value of an individual mouse.
*p<0.05, **p<0.01, ***p<0.001 by unpaired 2-tailed Student's t-test.
In summary, our current study demonstrates that C/EPBβ is crucial for execution of the PC program at the post-GC stage. By promoting the differentiation of post-switched B cells into PCs, it functions not only in maintaining the pool of natural Abs at steady state but also in eliciting effective humoral immunity involving the production of long-lasting Ag-specific isotype-switched Ab in response to immunization. Such a cell-autonomous function of C/EBPβ appears to be associated with its ability to induce Blimp1 expression through an IRF4-independent pathway. Thus, we have identified a novel function of C/EPBβ in the PC program operating in post-switched GC B cells.
ACKNOWLEDGMENTS
We thank Mr. Changyeon Kim for technical assistance and the Analytical Instrumentation Center (Seoul) at Hanyang University for technical support. This work was supported by a grant from National Research Foundation of Korea (NRF-2018R1A2B6004853).
Abbreviations
- ASC
Ab-secreting cell
- AU
arbitrary unit
- BCL6
B cell lymphoma 6
- Blimp1
B lymphocyte-induced maturation protein 1
- BM
bone marrow
- C/EBPβ
CCAAT/enhancer binding protein β
- cKO
conditional knockout
- Cγ1
Ig γ1 constant gene segment
- CSR
class-switch recombination
- GC
germinal center
- IRF4
interferon regulatory factor
- KLH
keyhole limpet hemocyanin
- LAP
liver-enriched activator protein
- NP
4-hydroxy-3-nitrophenylacetyl
- Pax5
paired box gene 5
- PC
plasma cell
- Prdm1
PR domain containing 1, with ZNF domain
- TD
T-dependent
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Youn J.
- Data curation: Jang E, Youn J.
- Formal analysis: Lee G.
- Funding acquisition: Youn J.
- Investigation: Lee G, Jang E.
- Methodology: Lee G.
- Supervision: Youn J.
- Validation: Jang E.
- Visualization: Lee G.
- Writing - original draft: Lee G.
- Writing - review & editing: Youn J.
SUPPLEMENTARY MATERIALS
Normal development of B and T cells in Cebpbfl/flCγ1Cre/+ mice. Cells from the indicated lymphoid organs of Cebpbfl/flCγ1Cre/+ mice and their control littermates were analyzed by FACS. Representative FACS profiles gated on live lymphocytes are shown.
The emergence of IgG1+ cells in Cebpbfl/flCγ1Cre/+ mice. B220+ B cells sorted from Cebpbfl/flCγ1Cre/+ and Cebpbfl/flCγ1Cre/+ mice were stimulated with LPS, IL-4, and IL-21 for 4 days, stained to detect intracellular IgG1, and analyzed by FACS. Representative FACS profiles gated on live lymphocytes are shown.
References
- 1.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 2.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 3.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 4.Jang E, Kim UK, Jang K, Song YS, Cha JY, Yi H, Youn J. Bach2 deficiency leads autoreactive B cells to produce IgG autoantibodies and induce lupus through a T cell-dependent extrafollicular pathway. Exp Mol Med. 2019;51:1–13. doi: 10.1038/s12276-019-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HJ, Kim DH, Lim SH, Kim WJ, Youn J, Choi YS, Choi JM. Insights into the role of follicular helper T cells in autoimmunity. Immune Netw. 2014;14:21–29. doi: 10.4110/in.2014.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity. 2007;27:49–63. doi: 10.1016/j.immuni.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi K, Ochiai K, Itoh-Nakadai A, Muto A. Orchestration of plasma cell differentiation by Bach2 and its gene regulatory network. Immunol Rev. 2014;261:116–125. doi: 10.1111/imr.12201. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 11.Tsukada J, Yoshida Y, Kominato Y, Auron PE. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine. 2011;54:6–19. doi: 10.1016/j.cyto.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. Conditional ablation of C/EBP β demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene. 2006;25:1272–1276. doi: 10.1038/sj.onc.1209144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda T, Kido Y, Asahara S, Kaisho T, Tanaka T, Hashimoto N, Shigeyama Y, Takeda A, Inoue T, Shibutani Y, et al. Ablation of C/EBPbeta alleviates ER stress and pancreatic β cell failure through the GRP78 chaperone in mice. J Clin Invest. 2010;120:115–126. doi: 10.1172/JCI39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu X, Aiken KJ, Chokas AL, Beachy DE, Nick HS. Distinct functions of CCAAT enhancer-binding protein isoforms in the regulation of manganese superoxide dismutase during interleukin-1β stimulation. J Biol Chem. 2008;283:25774–25785. doi: 10.1074/jbc.M801178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka S, Miura Y, Yao H, Satake S, Hayashi Y, Tamura A, Hishita T, Ichinohe T, Hirai H, Takaor-Kondo A, et al. CCAAT/enhancer-binding protein β expressed by bone marrow mesenchymal stromal cells regulates early B-cell lymphopoiesis. Stem Cells. 2014;32:730–740. doi: 10.1002/stem.1555. [DOI] [PubMed] [Google Scholar]
- 18.Pal R, Janz M, Galson DL, Gries M, Li S, Jöhrens K, Anagnostopoulos I, Dörken B, Mapara MY, Borghesi L, et al. C/EBPbeta regulates transcription factors critical for proliferation and survival of multiple myeloma cells. Blood. 2009;114:3890–3898. doi: 10.1182/blood-2009-01-201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casola S, Cattoretti G, Uyttersprot N, Koralov SB, Seagal J, Hao Z, Waisman A, Egert A, Ghitza D, Rajewsky K. Tracking germinal center B cells expressing germ-line immunoglobulin γ1 transcripts by conditional gene targeting. Proc Natl Acad Sci U S A. 2006;103:7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang E, Cho WS, Oh YK, Cho ML, Kim JM, Paik DJ, Youn J. Splenic long-lived plasma cells promote the development of follicular helper T cells during autoimmune responses. J Immunol. 2016;196:1026–1035. doi: 10.4049/jimmunol.1401059. [DOI] [PubMed] [Google Scholar]
- 21.Choi SS, Jang E, Jang K, Jung SJ, Hwang KG, Youn J. Autoantibody-mediated dysfunction of salivary glands leads to xerostomia in SKG mice. Immune Netw. 2019;19:e44. doi: 10.4110/in.2019.19.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong M, Jang E, Choi SS, Ji C, Lee K, Youn J. The function of FK506-binding protein 13 in protein quality control protects plasma cells from endoplasmic reticulum stress-associated apoptosis. Front Immunol. 2017;8:222. doi: 10.3389/fimmu.2017.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda T, Kometani K, Takahashi N, Imai Y, Aiba Y, Kurosaki T. ERKs induce expression of the transcriptional repressor Blimp-1 and subsequent plasma cell differentiation. Sci Signal. 2011;4:ra25. doi: 10.1126/scisignal.2001592. [DOI] [PubMed] [Google Scholar]
- 24.Pone EJ, Lam T, Lou Z, Wang R, Chen Y, Liu D, Edinger AL, Xu Z, Casali P. B cell Rab7 mediates induction of activation-induced cytidine deaminase expression and class-switching in T-dependent and T-independent antibody responses. J Immunol. 2015;194:3065–3078. doi: 10.4049/jimmunol.1401896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, Chong AS, Klein U, Dinner AR, Singh H, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013;38:918–929. doi: 10.1016/j.immuni.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zorn E, See SB. Polyreactive natural antibodies in transplantation. Curr Opin Organ Transplant. 2017;22:8–13. doi: 10.1097/MOT.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normal development of B and T cells in Cebpbfl/flCγ1Cre/+ mice. Cells from the indicated lymphoid organs of Cebpbfl/flCγ1Cre/+ mice and their control littermates were analyzed by FACS. Representative FACS profiles gated on live lymphocytes are shown.
The emergence of IgG1+ cells in Cebpbfl/flCγ1Cre/+ mice. B220+ B cells sorted from Cebpbfl/flCγ1Cre/+ and Cebpbfl/flCγ1Cre/+ mice were stimulated with LPS, IL-4, and IL-21 for 4 days, stained to detect intracellular IgG1, and analyzed by FACS. Representative FACS profiles gated on live lymphocytes are shown.