Abstract
Mycobacterium tuberculosis (Mtb) is an etiologic pathogen of human tuberculosis (TB), a serious infectious disease with high morbidity and mortality. In addition, the threat of drug resistance in anti-TB therapy is of global concern. Despite this, it remains urgent to research for understanding the molecular nature of dynamic interactions between host and pathogens during TB infection. While Mtb evasion from phagolysosomal acidification is a well-known virulence mechanism, the molecular events to promote intracellular parasitism remains elusive. To combat intracellular Mtb infection, several defensive processes, including autophagy and apoptosis, are activated. In addition, Mtb-ingested phagocytes trigger inflammation, and undergo necrotic cell death, potentially harmful responses in case of uncontrolled pathological condition. In this review, we focus on Mtb evasion from phagosomal acidification, and Mtb interaction with host autophagy, apoptosis, and necrosis. Elucidation of the molecular dialogue will shed light on Mtb pathogenesis, host defense, and development of new paradigms of therapeutics.
Keywords: Tuberculosis, Host-pathogen interactions, Autophagy, Apoptosis, Necrosis
INTRODUCTION
There is a need for new therapeutics for tuberculosis (TB), the leading cause of death by a single infectious agent worldwide. Although much effort has been focused on controlling TB, multidrug- and extensively drug-resistant TB threaten human health globally. In addition, approximately 25% of people are estimated to have latent TB, and so are at risk of active TB (World Health Organization, Global Tuberculosis Report 2018).
Mycobacterium tuberculosis (Mtb) is the major pathogen of human TB and can escape from host immunity and phagolysosomal fusion, surviving in phagosomes (1). Mtb cell-wall lipid components are coordinately expressed during different stages of infection (2). Mtb infection triggers intracellular signaling pathways, enhancing the inflammatory cytokine/chemokine responses that are crucial for controlling Mtb replication and the immunopathologic response (3). The host-Mtb interaction alters the host immune response and triggers cell death (4,5). The virulence factors of Mtb manipulate host immune, inflammatory, and cell death responses to facilitate intracellular growth (4).
Host protective immunity involves the control of inflammation and cell death, as well as the induction of antimicrobial factors (6). Autophagy is a cell-autonomous defense mechanism against a variety of stresses, including intracellular pathogens (7,8). Xenophagy is activated to target cytosolic Mtb for lysosomal delivery and degradation (9). However, Mtb evades xenophagy and LC3-associated phagocytosis via its virulence effectors and by modulating host factors (10). Mtb and its components regulate host cell death—i.e., apoptosis and necrosis, during infection (11,12). Generally, apoptosis is beneficial, whereas necrosis is detrimental, depending on the context. Mtb causes cell death by apoptosis and necrosis, contributing to the outcomes of infection.
This review provides insight into how Mtb manipulates host-cell autophagy, apoptosis, and necrosis. An appreciation of the host-Mtb interaction will enable the development of novel therapeutics and identification of the factors implicated in progression to active TB.
HOST REACTION TO Mtb INFECTION
Mtb is an obligate aerobic mycobacterium with a unique cell-wall structure and is capable of surviving within an immunocompetent host (13). As a primarily airborne disease, TB is transmitted person-to-person by aerosolized droplets containing Mtb (13,14). Mtb travels through the respiratory tract to the alveoli, where it is phagocytosed by alveolar macrophages, monocytes, and dendritic cells (14,15). Once phagocytosed, Mtb replicates within the macrophages, resulting in a robust inflammatory response followed by T-cell activation and recruitment of mononuclear cells and lymphocytes from neighboring blood vessels, forming granulomatous lesions (14,16,17). In this environment, macrophages differentiate into foamy macrophages filled with lipid droplets, multinucleate giant cells, and epithelial macrophages. Over time, the granuloma acquires a more organized structure with the formation of a fibrous sheath of extracellular matrix (17). Although most granulomas comprise a balance between Mtb and host-derived immune cells, under certain conditions, these structures progress into a more pathologic state characterized by diminished vascularization, increased necrosis, proliferation of foamy macrophages, and accumulation of caseous and hypoxic portions in the center (17). Rupture of these granulomas releases infectious bacilli into the airway (17,18). Our understanding of the granulomatous response to mycobacteria is in its infancy, and the mechanisms underlying the formation of protective and destructive granulomas are unclear.
The cell wall of Mtb is composed primarily of mycolic acids, which contribute to its acid resistance, along with smaller proportions of other lipids, including mannosyl-phosphatidyl-myo-inositol-based glycolipids, lipomannan, and lipoarabinomannan (19,20). To transport proteins across the cell wall, Mtb uses the 6-kDa early secretory antigenic target (ESAT-6) protein family secretion (ESX) system, which is encoded by the esx-1 locus. ESAT-6 (EsxA) is a key virulence factor (21). Upon infection with Mtb, phagocytes, particularly macrophages, respond to pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs), such as TLRs, C-type lectin receptors (e.g., Dectin-1), and Fc receptors (22,23,24). Recognition of Mtb PAMPs by PRRs triggers an intracellular signaling cascade resulting in activation of the NF-κB and mitogen-activated protein kinase pathways. This induces the production of proinflammatory cytokines and other antimicrobial effector molecules (24).
The mechanisms by which innate immune signaling activates inflammatory and effector pathways during mycobacterial infection are reviewed elsewhere (24,25,26,27). The details of the innate immune pathways subverted by virulent Mtb are beyond the scope of this review. To successfully respond to Mtb infection, it is important to minimize immune-related damage while maintaining the host's ability to mount a protective immune response.
Mtb ESCAPE FROM PHAGOLYSOSOMAL FUSION
Mtb has evolved multiple strategies to evade host immunity and overcome macrophage defenses. Mtb survives within macrophages primarily by blocking phagosomal maturation (28,29). Mtb phagosomal vacuoles do not acquire vesicular proton-adenosine triphosphatase (ATPase) (30). The ESAT-6-CFP-10 secretion system is implicated in the blockade of phagolysosomal fusion, contributing to intracellular survival of Mtb (31). In addition, the function of Mtb Rv3671c, which encodes a membrane-associated protein, is related to acid resistance and virulence; it also maintains intrabacterial pH (32). Furthermore, the most abundant Mtb lipid component, trehalose 6,6′-dimycolate (TDM), inhibits phagosomal acidification and promotes virulence (33). Notably, injection of TDM emulsion into mice resulted in a similar immunopathology in the lung as that observed in mice infected with Mtb (34). By interacting with the Mincle receptor, TDM inhibits phagosome maturation, contributing to TDM-induced virulence (35). Also, Mtb-derived TDM has been shown to induce severe tissue disruption, vascular occlusion, cell damage, and inflammation (36).
Phagosomal maturation by fusion with lysosomes depends on vacuolar ATPase (V-ATPase), which acidifies the phagosomal lumen by hydrolyzing ATP (37,38). Mtb inhibits host V-ATPase via mechanisms involving the mycobacterial phosphatase PtpA (39). As a secreted protein, PtpA interacts with V-ATPase to promote Mtb survival and pathogenicity (39). PtpA promotes escape from Mycobacterium-containing vacuoles by inhibiting the recruitment of V-ATPase (40). The Mtb SecA2 accessory system is required for arrest of phagosome maturation and the intracellular growth of Mtb (41). In addition, the phosphatase SapM and the kinase PknG, which are exported by SecA2, block Mtb delivery to autophagolysosomes (42).
Host factors are required for arresting phagosome acidification during Mtb infection. Mtb-induced STAT5-mediated expression of cytokine-inducible SH2-containing protein (CISH) promotes intracellular Mtb replication by inducing the degradation of V-ATPase catalytic subunit A (43). By contrast, disruption of Wiskott-Aldrich syndrome protein and SCAR homolog (WASH), a host actin nucleation-promoting factor, contributes to the accumulation of V-ATPase around the phagosomal vacuole by generating and associating with F-actin on the mycobacterial vacuole (44). Pathogenic mycobacteria subvert actin polymerization to escape phagosomal acidification. Cytokines are implicated in phagolysosomal acidification. For example, IL-12 exerted a positive, and IL-27 a negative, effect on phagosomal acquisition of V-ATPase and cathepsin D activity (Table 1) (45). Although arrest of phagosome maturation is a key virulence mechanism of Mtb, the effectors and their functions in vivo are unknown. Identification of the effectors that promote Mtb escape from phagolysosomal fusion will promote the development of therapeutics for TB.
Table 1. Mtb strategies for escaping phagolysosomal fusion.
| Effectors/factors | Strategy | Ref. | |
|---|---|---|---|
| Mtb effectors | |||
| ESAT-6-CFP-10 | Inhibition of phagolysosomal fusion | (31) | |
| Rv3671c | Maintenance of intrabacterial pH | (32) | |
| TDM | Inhibition of phagosomal acidification and promotion of virulence | (33) | |
| PtpA | Interaction with V-ATPase and inhibition the recruitment of V-ATPase to phagosome | (39) | |
| SapM & PknG | Blockade of Mtb delivery to autophagolysosomes | (42) | |
| Host factors | |||
| CISH | Degradation of V-ATPase catalytic subunit A | (43) | |
| WASH | Accumulation of V-ATPase of phagosomal vacuole to escape phagosomal acidification | (44) | |
| IL-27 | Neutralization of lysosomal acidification and cathepsin D activity | (45) | |
Mtb INTERACTIONS WITH HOST AUTOPHAGY
Autophagy is an intrinsic catabolic process of lysosomal degradation of damaged organelles or intracellular protein aggregates (46). There are three major types of autophagy—macro-autophagy, micro-autophagy, and chaperone-mediated autophagy (47). Although initially regarded as a nonspecific bulk degradation process, autophagy is required for selective degradation of protein aggregates, damaged mitochondria, and intracellular pathogens (48,49). Importantly, selective autophagy is mediated by autophagy receptors that recognize signals and bind to LC3/GABARAP proteins on the autophagosome membrane. Selective autophagy operates via ubiquitin (Ub)-dependent or -independent pathways (49,50,51). As an antibacterial defense, xenophagy of intracellular pathogens has been reported for Mtb and Salmonella typhimurium (2).
Mtb interactions with xenophagy
Direct contact between Mtb and the cytosol induces xenophagy, which promotes clearance of pathogens (52,53,54). The ESX-1 secretion system of Mtb facilitates phagosomal permeabilization, thus activating Ub-mediated, STING-dependent xenophagy and enhancing resistance to Mtb infection (53). The Ub ligases Parkin and Smurf1 are required for Ub-mediated autophagy of Mtb (54,55). The sensing of bacterial DNA by the cytosolic DNA sensor, cyclic GMP-AMP synthase (c-GAS), promotes the production of type I IFN (53), which is related to host susceptibility to chronic TB and impaired control of intracellular mycobacteria in human macrophages (56,57). In IFN-γ-activated macrophages, the host protein ubiquilin 1 is required for xenophagy activation and intracellular Mtb control, as it promotes accumulation of Ub, p62, and LC3 around Mtb bacilli (58). Mtb Ag-induced IFN-γ responses are correlated with the autophagy level in CD14-positive cells from healthy donors and patients with TB (59).
After ubiquitination of bacterial phagosomes, autophagy adaptors including p62 and NDP52 interact with Ub and deliver Mtb phagosomes to autophagosomes through LC3-interacting regions (53,54). In lysosomal and phagosomal damage models, TRIM16 controls ubiquitination and autophagy by recognizing endosomal/lysosomal damage and interacting with the cytosolic lectin, Galectin-3. Furthermore, TRIM16 interacts with the key autophagic core proteins ATG16L1, ULK1, and Beclin 1, and is required for the translocation of Mtb to autolysosomal compartments. Importantly, TRIM16 and Galectin-3 are required to control intracellular Mtb infection (60). Galectin-8 regulates xenophagy through interacting with mTOR complex in damaged endomembrane/lysosomal damage during Mtb infection (61).
Modulation of autophagy by Mtb
Mtb-derived effectors regulate host xenophagy, LC3-associated phagocytosis (LAP), and macroautophagy. The ESX-1 system, which includes ESAT-6 and EspB, intereferes with autophagy to inhibit microbial clearance from host cells (62,63). The Mtb enhanced intracellular survival (eis) gene is involved with the regulation of autophagic cell death and redox balance, but is not directly involved in host innate immunity in vivo (64). In addition, CpsA, a LytR-CpsA-Psr domain-containing protein of Mtb, promotes evasion of host defense mechanisms involving NADPH oxidase and LAP (65). The Mtb components required for escaping from autophagy are unclear. Further studies should characterize Mtb effectors that modulate autophagy.
Host factors that regulate xenophagy
Polymorphisms of GTPase family M protein (IRGM) modulate autophagy and antimicrobial effector function (66,67,68,69). IRGM interacts with core autophagy regulators including ULK1 and Beclin 1, and activates Beclin 1 to promote autophagy and host antimicrobial defense (70). Although xenophagy controls intracellular Mtb replication in vitro, its role in vivo is unclear. Mice with an Atg5 deficiency in monocyte-derived cells and neutrophils exhibited increased susceptibility to Mtb infection because of enhanced pathological inflammation (53,71,72). However, the lack of Smurf1, an E3 Ub ligase essential for xenophagy of Mtb, led to an increased bacterial load and accelerated mortality due to chronic Mtb infection. Therefore, autophagy may play a role in chronic infection in vivo (55). Galectin-8 is critical in the host defense against Mtb, because Galectin-8-knockout mice are susceptible to Mtb infection (61). Mechanistically, Galectin-8 is required for the activation of autophagy via the mTOR-AMP activated protein kinase pathway (61).
The C-type lectin receptor CLEC4E, which associates with TLR4, suppressed Mtb growth in mouse and guinea pig models (73). Importantly, combination therapy using agonists of both CLEC4E and TLR4 together with antibiotic treatment activated host defensive pathways at least partially through macroautophagy/autophagy (73). Mitochondrial sirtuin 3 promotes host antimycobacterial defense in macrophages and in vivo by enhancing antibacterial autophagy and controlling mitochondrial homeostasis (74). A variety of host factors modulate autophagy to enhance or inhibit the host antimicrobial response to Mtb infection (75). It is important to identify new therapeutic agents that restrict the survival of Mtb (Fig. 1).
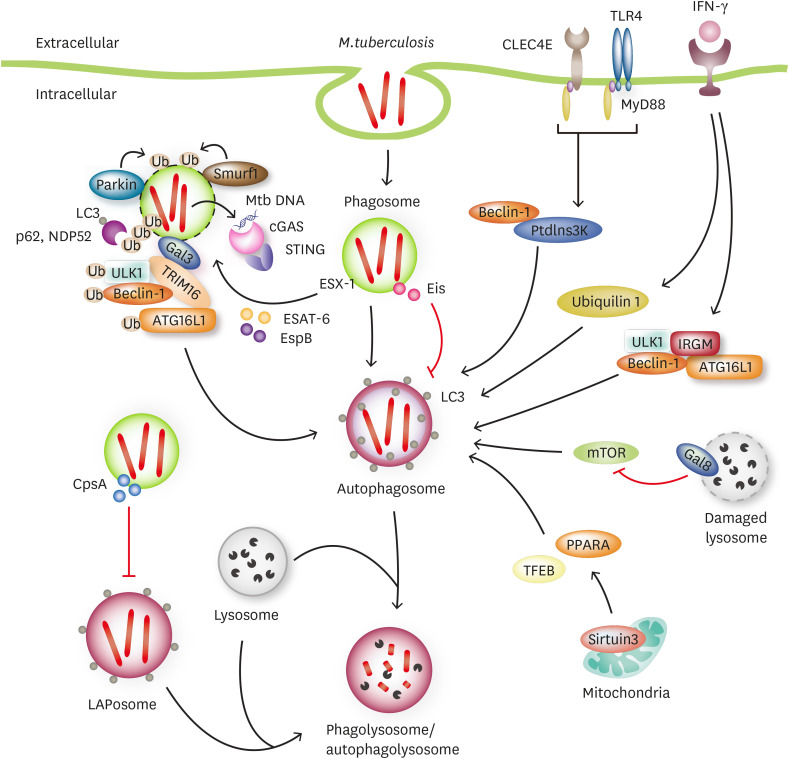
Figure 1. Crosstalk between Mtb and xenophagy.
ESX-1 secretion system damage Mtb-contained phagosome and resist from xenophagic pathway. Parkin1 and Smurf1 ubiuitinate damaged Mtb-contained phagosome to control Mtb survival. During Mtb infection, cGAS binds Mtb DNA and then activate type I interferons through STING pathway. In addition, damaged phagosome recruits Galectin-3, resulting in Galectin-3-TRIM16-ULK1-Beclin-1-ATG16L1 complex and initiation of autophagy. Galectin-8 suppresses mTOR activity in response to lysosomal damage. In IFN-γ-activated macrophages, ubiquilin 1 promotes ubiquitin, p62, and LC3 around Mtb and IFN-γ-induced IRGM interacts with autophagy machinery such as Beclin 1, ULK1, and ATG16L1. Mtb eis gene play role in regulating of autophagy. Furthermore, CpsA, protein of Mtb, evades from LAP and inhibits LAPosome formation during Mtb infection. In mouse and guinea pigs model, agonists for both CLEC4E and TLR4 activate autophagy, thereby reducing bacterial load. Sirtuin 3, enhances autophagy through TFEB and PPARA.
MODULATION OF APOPTOSIS BY Mtb
The survival of Mtb in host cells is promoted by its interactions with the apoptosis, necrosis, necroptosis, and autophagy pathways (76). Apoptosis is an important mechanism by which host cells suppress intracellular replication of Mtb (77,78). Virulent Mtb strains evade apoptosis (79,80). Several mycobacterial effectors inhibit apoptosis by various mechanisms, thus enhancing virulence (76,78). However, apoptosis during later stages of Mtb infection is implicated in dissemination (81).
Several Mtb proteins/Ags induce apoptosis. ESAT-6 induces apoptosis in macrophages by activating the intrinsic pathway and ROS signaling (82) and by targeting miRNA-155 and the SOCS1 pathway (83). In addition, ESAT-6, in cooperation with phthiocerol dimycocerosate, induced the rupture of phagosomal membranes and host cell apoptosis, thus contributing to virulence (84). The PGRS domain of Rv0297 (Rv0297PGRS), which is required for endoplasmic reticulum (ER) localization, is implicated in caspase-8-mediated apoptosis and ER stress-induced cell death (81). Wang et al. (85) reported that the Mtb Ag MPT83 (Rv2872), a secreted lipoprotein, activated apoptosis of macrophages via a mechanism involving the TLR2/p38 MAPK/cyclooxygenase (COX)-2 signaling pathway. The Mtb-derived TLR2 ligand, Rv1016c lipoprotein, induces macrophage apoptosis and inhibits MHC-II expression induced by IFN-γ, resulting in escape from immune surveillance and promoting chronic infection (86). The Mtb lipoprotein known as 38-kDa Ag induces MCP-1, which in turn induces MCP-1-induced protein and enhances the production of ROS and ER-stress-induced apoptosis (87).
Mannose-capped lipoarabinomannan inhibits host-cell apoptosis by inducing antiapoptotic B-cell CLL/lymphoma 2 family member A1 (88). Interestingly, the Mtb chaperone protein Cpn60.2 suppresses apoptosis by interacting with mitochondrial mortalin, thereby enhancing intracellular Mtb survival (89). The Mtb PE/PPE family protein PE_PGRS18 promotes pathogenesis by attenuating macrophage apoptosis (90). LpqT from Mtb inhibits TLR2-dependent inflammatory signaling and apoptosis (91). Mycobacterial acyl carrier protein (Rv2244), which is involved in mycolic acid biosynthesis, inhibits macrophage apoptosis by suppressing the ROS/JNK signaling pathway, contributing to Mtb virulence (92). SP110b, an IFN-induced nuclear protein, exerts a protective effect by modulating NF-κB-mediated inflammatory signaling and ameliorating cell death and necrotic lung lesions (93). The protective effect from Ag-mediated regulation of apoptosis is context dependent (Table 2). Further studies should clarify the role of Ags in antimycobacterial immunity. There is a strong relationship between apoptosis and autophagy in various biological responses. However, the roles of mycobacterial Ag(s) in regulating apoptosis and autophagy during Mtb infection are unclear.
Table 2. Ag-mediated regulation of host cell apoptosis.
| Proteins/antigen | Experimental model | Mechanism of action | Effects | Ref. | |
|---|---|---|---|---|---|
| Induction of host cell apoptosis | |||||
| ESAT-6 | Mouse BMDM | TLR2 mediated ROS-MAPK dependent caspase-9 and caspase 3 activation | Apoptosis | (82) | |
| Human PBMCs, RAW264.7 cells | Targeting the miRNA-155-SOCS1 interaction, where miRNA-155 expression is dependent on TLR2/NF-κB activation | Enhance protective immune response | (83) | ||
| DIM | THP-1 cells, human MDMs | DIM contributes, along with ESX-1, to induce phagosomal membrane damage and rupture | Host cell apoptosis | (84) | |
| Rv0297PGRS | RAW264.7 cells, HEK293T cells | TLR4 dependent ER-stress-mediated cell death, disrupted Ca2+ homeostasis and increased NO and ROS leading to caspase-8 activation | Host cell apoptosis | (81) | |
| MPT83 | Mouse BMDM, THP-1 cells, in vivo mouse model | TLR2 mediated p38 MAPK activation and COX-2 expression | Protection from mycobacterial infection | (85) | |
| Rv1016c | THP-1 cells, MDMs | Acts as a TLR2 ligand and inhibition IFN-γ induced expression of CIITA IV through TLR2 and MAPK signaling | Increased survival of mycobacteria | (86) | |
| 38-kDa antigen | Mouse BMDM, RAW264.7 cells | TLR-mediated MAPK activation leading to MCPIP activation, ROS production and ER stress induction | Host cell apoptosis | (87) | |
| Inhibition of host cell apoptosis | |||||
| ManLAM | Mouse BMDMs, J774A.1 cell line | Upregulation of Bcl2 family member A1, upregulation of STAT5α in a PPARγ-dependent manner | Attenuation of host cell apoptosis | (88) | |
| Cpn60.2 | THP-1, RAW264.7 cells | Interacts with mortalin, a member of HSP70 gene family | Anti-apoptotic action | (89) | |
| PE-PGRS18 | THP-1 cells | Modulation of cytokine production and attenuation of apoptosis | Enhance survival of M. smegmatis in macrophages | (90) | |
| LpqT | Mouse BMDMs, RAW264.7 cells, mouse in vivo study | Reduction of TLR2 mediated NF-κB and MAPK activation | Increase mycobacterial survival in macrophage and mice | (91) | |
| AcpM; Rv2244 | Mouse BMDM, RAW264.7 cells | Inhibition of ROS/JNK signaling | Enhance intracellular survival of Mtb | (92) | |
| SP110b | THP-1, U937, HEK293T, human MDMs, human subject study | Downregulation of NF-κB-induced TNF-α and upregulation of anti-apoptotic gene expression | Less severe necrotic lung lesion and reduced tuberculosis susceptibility | (93) | |
BMDM, bone marrow-derived macrophage; CIITA, class II transactivator; HSP70, 70 kilodalton heat shock protein; MCPIP, MCP-1-induced protein; MDM, monocyte-derived macrophages; NO, nitric oxide; PPAR, peroxisome proliferator-activated receptor; SOCS, suppressor of cytokine signaling.
REGULATION OF HOST CELL NECROSIS BY Mtb
The cells that are frequently observed to participate in inflammatory pathology in actively necrotic granulomas are thought to be neutrophils (94). Indeed, intra-alveolar neutrophil infiltration and an excessive inflammatory response play key roles in the progression to active TB, which is characterized by initial caseous and later liquefactive necrosis in the lung (95,96). Neutrophil necrosis induced by Mtb contributes to Mtb growth in host cells, thus sustaining the infection (97). Interestingly, this can be ameliorated by inhibiting ROS production (97). Mtb infection renders human macrophages necrotic, favoring Mtb replication (98). Also, Mtb rapidly proliferate as a clump inside dead cells rather than in live cells (99). Mtb induces neutrophil extracellular traps (NETs) that promote the recruitment and activation of effector cells (100).
Several Mtb proteins activate necrosis of host macrophages in vivo. ESAT-6 triggers intracellular Ca2+ influx, inducing neutrophil necrosis and production of NETs, ultimately contributing to necrotic pathology and TB transmission (94). Therefore, the virulence protein ESAT-6 is an important therapeutic target (101). In addition, PPE11 (Rv0453), which has been found in infected guinea pig lung, promoted mycobacterial survival under stressful conditions by enhancing inflammation, organ pathology, and host-cell death (102). Recombinant PE17 (Rv1646) inhibits the production of proinflammatory cytokines (IL-6, IL-12, and TNF-α) and enhances macrophage necrosis (Table 3) (103). The degree of tissue necrosis and lung inflammation may be strain-specific—TLR2-deficient mice infected with Mtb W-Beijing exhibited increased neutrophil infiltration (104). Characterization of the functions of Mtb proteins and lipids in inducing host cell necrosis will provide insight into its virulence mechanisms (105).
Table 3. Ag-mediated regulation of host cell necrosis.
| Proteins/Ag | Experimental model | Mechanism of action | Effects | Ref. |
|---|---|---|---|---|
| ESAT-6 | Human neutrophils | Ca2+ mediated calpain activation | NETosis | (94) |
| PPE11 | THP-1 cells, in vivo studies | Imbalance of pro-inflammatory and anti-inflammatory cytokines | Establishment of a persistent infection | (102) |
| PE17 | Mouse peritoneal macrophages, in vivo studies | Nuclear damage, loss of cytoplasmic membrane integrity, reduction of pro-inflammatory cytokines | Enhance bacterial survival and bacterial burden in mice | (103) |
The local expression of CXC chemokines such as CXCL5, primarily by epithelial cells, enhances the recruitment of polymorphonuclear leukocytes, promoting pulmonary inflammation and a defective host defense (106). Also, IL-17A expression by non-hematopoietic cells is involved in neutrophil infiltration during mycobacterial infection (107). Excessive pulmonary inflammatory responses, mainly induced by neutrophils, promote pathologic inflammation by increasing CXCL5 and TNF-α levels and suppressing host defenses during Mtb infection (74). The detrimental effects of type I IFN and its receptor (IFNAR1) in pulmonary TB is due in part to CXCL5/CXCL1-induced infiltration of neutrophils (108). The molecular mechanisms by which Mtb and its effectors aggravate host cell necrosis during TB are unclear. Understanding the mechanisms of induction of host cell necrosis would facilitate the development of novel therapeutic approaches (109).
CONCLUSIONS
Several Mtb effectors participate in escape from phagolysosomal acidification. Xenophagy is an important lysosomal degradation pathway that activates host antimicrobial defenses. Mtb has evolved several mechanisms to exploit autophagy. Several Mtb effectors modulate apoptosis and so influence host antimicrobial defenses in a context-dependent manner. The roles of autophagy and apoptosis in the host-Mtb interaction are unclear. Additionally, Mtb and its components induce inflammation, which is implicated in both protective immune responses and pathogenic necrosis. Neutrophils aggravate pathologic inflammation and necrosis, highlighting their involvement in the pathogenesis of TB.
Several questions remain to be addressed. What are the determinant(s) of the complexity of the interactions between Mtb and host cells? In addition, the mechanisms by which Mtb and its components inhibit autophagy and apoptosis are unclear. The in vivo functions of autophagy need to be clarified to enable the development of autophagy-targeted adjunctive therapies. Do host signaling factors orchestrate apoptosis and necrosis to promote antimicrobial defense? Also, the molecular mechanism that controls necrosis warrants further investigation. Our understanding of the immune, autophagic, and cell-death responses during TB is incomplete. Further studies will provide insight into the molecular dialogue between Mtb and host cells, and so facilitate the development of novel therapeutics for TB and other chronic intracellular infections.
ACKNOWLEDGEMENTS
We are indebted to current and past members of our laboratory for discussions and investigations that contributed to this article. This work was supported by the NRF grant funded by the Korea government (NRF-2019R1I1A1A01062086). We apologize to everyone whose work and publications could not be referenced due to space limitations.
Abbreviations
- ATPase
adenosine triphosphatase
- c-GAS
cyclic GMP-AMP synthase
- CISH
cytokine-inducible SH2-containing protein
- COX
cyclooxygenase
- ER
endoplasmic reticulum
- ESAT-6
6-kDa early secretory antigenic target
- ESX
early secretory antigenic target protein family secretion
- LAP
LC3-associated phagocytosis
- Mtb
Mycobacterium tuberculosis
- NET
neutrophil extracellular trap
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- TB
tuberculosis
- TDM
trehalose 6,6′-dimycolate
- Ub
ubiquitin
- V-ATPase
vacuolar adenosine triphosphatase
- WASH
Wiskott-Aldrich syndrome protein and SCAR homolog
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Validation: Silwal P.
- Writing - original draft: Kim JK, Jo EK.
References
- 1.Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14:963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Queiroz A, Riley LW. Bacterial immunostat: Mycobacterium tuberculosis lipids and their role in the host immune response. Rev Soc Bras Med Trop. 2017;50:9–18. doi: 10.1590/0037-8682-0230-2016. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stutz MD, Clark MP, Doerflinger M, Pellegrini M. Mycobacterium tuberculosis: rewiring host cell signaling to promote infection. J Leukoc Biol. 2018;103:259–268. doi: 10.1002/JLB.4MR0717-277R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirier V, Av-Gay Y. Mycobacterium tuberculosis modulators of the macrophage's cellular events. Microbes Infect. 2012;14:1211–1219. doi: 10.1016/j.micinf.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Brighenti S, Joosten SA. Friends and foes of tuberculosis: modulation of protective immunity. J Intern Med. 2018;284:125–144. doi: 10.1111/joim.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao Y, Sun J. Bacterial manipulation of autophagic responses in infection and inflammation. Front Immunol. 2019;10:2821. doi: 10.3389/fimmu.2019.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmey JM, Stallings CL. Bacterial pathogens versus autophagy: implications for therapeutic interventions. Trends Mol Med. 2016;22:1060–1076. doi: 10.1016/j.molmed.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhyay S, Mittal E, Philips JA. Tuberculosis and the art of macrophage manipulation. Pathog Dis. 2018;76:76. doi: 10.1093/femspd/fty037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krakauer T. Inflammasomes, autophagy, and cell death: the trinity of innate host defense against intracellular bacteria. Mediators Inflamm. 2019;2019:2471215. doi: 10.1155/2019/2471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam A, Prabhu R, Gross CM, Riesenberg LA, Singh V, Aggarwal S. Role of apoptosis and autophagy in tuberculosis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L218–L229. doi: 10.1152/ajplung.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. 2018;215:1135–1152. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra A, Surolia A. Mycobacterium tuberculosis: surviving and indulging in an unwelcoming host. IUBMB Life. 2018;70:917–925. doi: 10.1002/iub.1882. [DOI] [PubMed] [Google Scholar]
- 15.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 16.Pagán AJ, Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol. 2018;36:639–665. doi: 10.1146/annurev-immunol-032712-100022. [DOI] [PubMed] [Google Scholar]
- 17.Russell DG, VanderVen BC, Lee W, Abramovitch RB, Kim MJ, Homolka S, Niemann S, Rohde KH. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe. 2010;8:68–76. doi: 10.1016/j.chom.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, Mathema B, Ramaswamy SV, Walther G, Steyn LM, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daffé M, Crick DC, Jackson M. Genetics of capsular polysaccharides and cell envelope (glyco)lipids. Microbiol Spectr. 2014;2:MGM2–MGM0021. doi: 10.1128/microbiolspec.MGM2-0021-2013. [DOI] [PubMed] [Google Scholar]
- 20.Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis . Tuberculosis (Edinb) 2003;83:91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 21.Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol. 2016;14:677–691. doi: 10.1038/nrmicro.2016.131. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 24.Basu J, Shin DM, Jo EK. Mycobacterial signaling through Toll-like receptors. Front Cell Infect Microbiol. 2012;2:145. doi: 10.3389/fcimb.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schorey JS, Schlesinger LS. Innate immune responses to tuberculosis. Microbiol Spectr. 2016;4:4. doi: 10.1128/microbiolspec.TBTB2-0010-2016. [DOI] [PubMed] [Google Scholar]
- 26.Shi L, Eugenin EA, Subbian S. Immunometabolism in tuberculosis. Front Immunol. 2016;7:150. doi: 10.3389/fimmu.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh P, Subbian S. Harnessing the mTOR pathway for tuberculosis treatment. Front Microbiol. 2018;9:70. doi: 10.3389/fmicb.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hmama Z, Peña-Díaz S, Joseph S, Av-Gay Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis . Immunol Rev. 2015;264:220–232. doi: 10.1111/imr.12268. [DOI] [PubMed] [Google Scholar]
- 29.Meena LS, Rajni Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J. 2010;277:2416–2427. doi: 10.1111/j.1742-4658.2010.07666.x. [DOI] [PubMed] [Google Scholar]
- 30.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 31.Tan T, Lee WL, Alexander DC, Grinstein S, Liu J. The ESAT-6/CFP-10 secretion system of Mycobacterium marinum modulates phagosome maturation. Cell Microbiol. 2006;8:1417–1429. doi: 10.1111/j.1462-5822.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 32.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis . Nat Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kan-Sutton C, Jagannath C, Hunter RL., Jr Trehalose 6,6′-dimycolate on the surface of Mycobacterium tuberculosis modulates surface marker expression for antigen presentation and costimulation in murine macrophages. Microbes Infect. 2009;11:40–48. doi: 10.1016/j.micinf.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei L, Wu J, Liu H, Yang H, Rong M, Li D, Zhang P, Han J, Lai R. A mycobacteriophage-derived trehalose-6,6′-dimycolate-binding peptide containing both antimycobacterial and anti-inflammatory abilities. FASEB J. 2013;27:3067–3077. doi: 10.1096/fj.13-227454. [DOI] [PubMed] [Google Scholar]
- 35.Patin EC, Geffken AC, Willcocks S, Leschczyk C, Haas A, Nimmerjahn F, Lang R, Ward TH, Schaible UE. Trehalose dimycolate interferes with FcγR-mediated phagosome maturation through Mincle, SHP-1 and FcγRIIB signalling. PLoS One. 2017;12:e0174973. doi: 10.1371/journal.pone.0174973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMullen AM, Hwang SA, O'Shea K, Aliru ML, Actor JK. Evidence for a unique species-specific hypersensitive epitope in Mycobacterium tuberculosis derived cord factor. Tuberculosis (Edinb) 2013;93 Suppl:S88–S93. doi: 10.1016/S1472-9792(13)70017-9. [DOI] [PubMed] [Google Scholar]
- 37.Kissing S, Saftig P, Haas A. Vacuolar ATPase in phago(lyso)some biology. Int J Med Microbiol. 2018;308:58–67. doi: 10.1016/j.ijmm.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Kissing S, Hermsen C, Repnik U, Nesset CK, von Bargen K, Griffiths G, Ichihara A, Lee BS, Schwake M, De Brabander J, et al. Vacuolar ATPase in phagosome-lysosome fusion. J Biol Chem. 2015;290:14166–14180. doi: 10.1074/jbc.M114.628891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong D, Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc Natl Acad Sci U S A. 2011;108:19371–19376. doi: 10.1073/pnas.1109201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koliwer-Brandl H, Knobloch P, Barisch C, Welin A, Hanna N, Soldati T, Hilbi H. Distinct Mycobacterium marinum phosphatases determine pathogen vacuole phosphoinositide pattern, phagosome maturation, and escape to the cytosol. Cell Microbiol. 2019;21:e13008. doi: 10.1111/cmi.13008. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan JT, Young EF, McCann JR, Braunstein M. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect Immun. 2012;80:996–1006. doi: 10.1128/IAI.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zulauf KE, Sullivan JT, Braunstein M. The SecA2 pathway of Mycobacterium tuberculosis exports effectors that work in concert to arrest phagosome and autophagosome maturation. PLoS Pathog. 2018;14:e1007011. doi: 10.1371/journal.ppat.1007011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Queval CJ, Song OR, Carralot JP, Saliou JM, Bongiovanni A, Deloison G, Deboosère N, Jouny S, Iantomasi R, Delorme V, et al. Mycobacterium tuberculosis controls phagosomal acidification by targeting CISH-mediated signaling. Cell Reports. 2017;20:3188–3198. doi: 10.1016/j.celrep.2017.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolonko M, Geffken AC, Blumer T, Hagens K, Schaible UE, Hagedorn M. WASH-driven actin polymerization is required for efficient mycobacterial phagosome maturation arrest. Cell Microbiol. 2014;16:232–246. doi: 10.1111/cmi.12217. [DOI] [PubMed] [Google Scholar]
- 45.Jung JY, Robinson CM. IL-12 and IL-27 regulate the phagolysosomal pathway in mycobacteria-infected human macrophages. Cell Commun Signal. 2014;12:16. doi: 10.1186/1478-811X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells. 2018;7:278. doi: 10.3390/cells7120278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraft C, Reggiori F, Peter M. Selective types of autophagy in yeast. Biochim Biophys Acta. 2009;1793:1404–1412. doi: 10.1016/j.bbamcr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Khaminets A, Behl C, Dikic I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2016;26:6–16. doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Curr Biol. 2012;22:R540–R545. doi: 10.1016/j.cub.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Huang J, Brumell JH. Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol. 2014;12:101–114. doi: 10.1038/nrmicro3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins AC, Cai H, Li T, Franco LH, Li XD, Nair VR, Scharn CR, Stamm CE, Levine B, Chen ZJ, et al. Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis . Cell Host Microbe. 2015;17:820–828. doi: 10.1016/j.chom.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–516. doi: 10.1038/nature12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franco LH, Nair VR, Scharn CR, Xavier RJ, Torrealba JR, Shiloh MU, Levine B. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe. 2017;21:59–72. doi: 10.1016/j.chom.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, Komisopoulou E, Kelly-Scumpia K, Chun R, Iyer SS, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donovan ML, Schultz TE, Duke TJ, Blumenthal A. Type I interferons in the pathogenesis of tuberculosis: molecular drivers and immunological consequences. Front Immunol. 2017;8:1633. doi: 10.3389/fimmu.2017.01633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakowski ET, Koster S, Portal Celhay C, Park HS, Shrestha E, Hetzenecker SE, Maurer K, Cadwell K, Philips JA. Ubiquilin 1 promotes IFN-γ-induced xenophagy of Mycobacterium tuberculosis . PLoS Pathog. 2015;11:e1005076. doi: 10.1371/journal.ppat.1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rovetta AI, Peña D, Hernández Del Pino RE, Recalde GM, Pellegrini J, Bigi F, Musella RM, Palmero DJ, Gutierrez M, Colombo MI, et al. IFNG-mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active tuberculosis. Autophagy. 2014;10:2109–2121. doi: 10.4161/15548627.2014.981791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chauhan S, Kumar S, Jain A, Ponpuak M, Mudd MH, Kimura T, Choi SW, Peters R, Mandell M, Bruun JA, et al. Trims and Galectins globally cooperate and TRIM16 and Galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev Cell. 2016;39:13–27. doi: 10.1016/j.devcel.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, Peters R, Mudd MH, Allers L, Salemi M, et al. Galectins control mTOR in response to endomembrane damage. Mol Cell. 2018;70:120–135.e8. doi: 10.1016/j.molcel.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong KW. The role of ESX-1 in Mycobacterium tuberculosis pathogenesis. Microbiol Spectr. 2017;5:TBTB2-0001-2015. doi: 10.1128/microbiolspec.tbtb2-0001-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Behura A, Mishra A, Chugh S, Mawatwal S, Kumar A, Manna D, Mishra A, Singh R, Dhiman R. ESAT-6 modulates Calcimycin-induced autophagy through microRNA-30a in mycobacteria infected macrophages. J Infect. 2019;79:139–152. doi: 10.1016/j.jinf.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, Lee SH, Lee ZW, Cho SN, Kim JM, et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010;6:e1001230. doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Köster S, Upadhyay S, Chandra P, Papavinasasundaram K, Yang G, Hassan A, Grigsby SJ, Mittal E, Park HS, Jones V, et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc Natl Acad Sci U S A. 2017;114:E8711–E8720. doi: 10.1073/pnas.1707792114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wellcome Trust Case Control Consortium. Craddock N, Hurles ME, Cardin N, Pearson RD, Plagnol V, Robson S, Vukcevic D, Barnes C, Conrad DF, et al. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010;464:713–720. doi: 10.1038/nature08979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brest P, Lapaquette P, Souidi M, Lebrigand K, Cesaro A, Vouret-Craviari V, Mari B, Barbry P, Mosnier JF, Hébuterne X, et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn's disease. Nat Genet. 2011;43:242–245. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- 69.McCarroll SA, Huett A, Kuballa P, Chilewski SD, Landry A, Goyette P, Zody MC, Hall JL, Brant SR, Cho JH, et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chauhan S, Mandell MA, Deretic V. IRGM governs the core autophagy machinery to conduct antimicrobial defense. Mol Cell. 2015;58:507–521. doi: 10.1016/j.molcel.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimmey JM, Huynh JP, Weiss LA, Park S, Kambal A, Debnath J, Virgin HW, Stallings CL. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature. 2015;528:565–569. doi: 10.1038/nature16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S, Delgado-Vargas M, Timmins GS, Bhattacharya D, Yang H, et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci U S A. 2012;109:E3168–E3176. doi: 10.1073/pnas.1210500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pahari S, Negi S, Aqdas M, Arnett E, Schlesinger LS, Agrewala JN. Induction of autophagy through CLEC4E in combination with TLR4: an innovative strategy to restrict the survival of Mycobacterium tuberculosis . Autophagy. 2020;16:1021–1043. doi: 10.1080/15548627.2019.1658436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim TS, Jin YB, Kim YS, Kim S, Kim JK, Lee HM, Suh HW, Choe JH, Kim YJ, Koo BS, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15:1356–1375. doi: 10.1080/15548627.2019.1582743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruns H, Stenger S. New insights into the interaction of Mycobacterium tuberculosis and human macrophages. Future Microbiol. 2014;9:327–341. doi: 10.2217/fmb.13.164. [DOI] [PubMed] [Google Scholar]
- 76.Mohareer K, Asalla S, Banerjee S. Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2018;113:99–121. doi: 10.1016/j.tube.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Zhai W, Wu F, Zhang Y, Fu Y, Liu Z. The immune escape mechanisms of Mycobacterium tuberculosis . Int J Mol Sci. 2019;20:340. doi: 10.3390/ijms20020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Behar SM, Briken V. Apoptosis inhibition by intracellular bacteria and its consequence on host immunity. Curr Opin Immunol. 2019;60:103–110. doi: 10.1016/j.coi.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 80.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 81.Grover S, Sharma T, Singh Y, Kohli S, P M, Singh A, Semmler T, Wieler LH, Tedin K, Ehtesham NZ, et al. The PGRS domain of Mycobacterium tuberculosis PE_PGRS protein Rv0297 is involved in endoplasmic reticulum stress-mediated apoptosis through Toll-like receptor 4. MBio. 2018;9:e01017-18. doi: 10.1128/mBio.01017-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin J, Chang Q, Dai X, Liu D, Jiang Y, Dai Y. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis promotes caspase-9/caspase-3-mediated apoptosis in macrophages. Mol Cell Biochem. 2019;457:179–189. doi: 10.1007/s11010-019-03522-x. [DOI] [PubMed] [Google Scholar]
- 83.Yang S, Li F, Jia S, Zhang K, Jiang W, Shang Y, Chang K, Deng S, Chen M. Early secreted antigen ESAT-6 of Mycobacterium Tuberculosis promotes apoptosis of macrophages via targeting the microRNA155-SOCS1 interaction. Cell Physiol Biochem. 2015;35:1276–1288. doi: 10.1159/000373950. [DOI] [PubMed] [Google Scholar]
- 84.Augenstreich J, Arbues A, Simeone R, Haanappel E, Wegener A, Sayes F, Le Chevalier F, Chalut C, Malaga W, Guilhot C, et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol. 2017;19:e12726. doi: 10.1111/cmi.12726. [DOI] [PubMed] [Google Scholar]
- 85.Wang L, Zuo M, Chen H, Liu S, Wu X, Cui Z, Yang H, Liu H, Ge B. Mycobacterium tuberculosis lipoprotein MPT83 induces apoptosis of infected macrophages by activating the TLR2/p38/COX-2 signaling pathway. J Immunol. 2017;198:4772–4780. doi: 10.4049/jimmunol.1700030. [DOI] [PubMed] [Google Scholar]
- 86.Su H, Zhu S, Zhu L, Huang W, Wang H, Zhang Z, Xu Y. Recombinant lipoprotein rv1016c derived from Mycobacterium tuberculosis is a TLR-2 ligand that induces macrophages apoptosis and inhibits MHC II antigen processing. Front Cell Infect Microbiol. 2016;6:147. doi: 10.3389/fcimb.2016.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim YJ, Choi JA, Lee JH, Choi CH, Kim HJ, Song CH. Mycobacterium tuberculosis 38-kDa antigen induces endoplasmic reticulum stress-mediated apoptosis via toll-like receptor 2/4. Apoptosis. 2015;20:358–370. doi: 10.1007/s10495-014-1080-2. [DOI] [PubMed] [Google Scholar]
- 88.Halder P, Kumar R, Jana K, Chakraborty S, Ghosh Z, Kundu M, Basu J. Gene expression profiling of Mycobacterium tuberculosis Lipoarabinomannan-treated macrophages: a role of the Bcl-2 family member A1 in inhibition of apoptosis in mycobacteria-infected macrophages. IUBMB Life. 2015;67:726–736. doi: 10.1002/iub.1430. [DOI] [PubMed] [Google Scholar]
- 89.Joseph S, Yuen A, Singh V, Hmama Z. Mycobacterium tuberculosis Cpn60.2 (GroEL2) blocks macrophage apoptosis via interaction with mitochondrial mortalin. Biol Open. 2017;6:481–488. doi: 10.1242/bio.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang W, Deng W, Zeng J, Ren S, Ali MK, Gu Y, Li Y, Xie J. Mycobacterium tuberculosis PE_PGRS18 enhances the intracellular survival of M. smegmatis via altering host macrophage cytokine profiling and attenuating the cell apoptosis. Apoptosis. 2017;22:502–509. doi: 10.1007/s10495-016-1336-0. [DOI] [PubMed] [Google Scholar]
- 91.Li F, Feng L, Jin C, Wu X, Fan L, Xiong S, Dong Y. LpqT improves mycobacteria survival in macrophages by inhibiting TLR2 mediated inflammatory cytokine expression and cell apoptosis. Tuberculosis (Edinb) 2018;111:57–66. doi: 10.1016/j.tube.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Paik S, Choi S, Lee KI, Back YW, Son YJ, Jo EK, Kim HJ. Mycobacterium tuberculosis acyl carrier protein inhibits macrophage apoptotic death by modulating the reactive oxygen species/c-Jun N-terminal kinase pathway. Microbes Infect. 2019;21:40–49. doi: 10.1016/j.micinf.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 93.Leu JS, Chen ML, Chang SY, Yu SL, Lin CW, Wang H, Chen WC, Chang CH, Wang JY, Lee LN, et al. Sp110b controls host immunity and susceptibility to tuberculosis. Am J Respir Crit Care Med. 2017;195:369–382. doi: 10.1164/rccm.201601-0103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Francis RJ, Butler RE, Stewart GR. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014;5:e1474. doi: 10.1038/cddis.2014.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niazi MK, Dhulekar N, Schmidt D, Major S, Cooper R, Abeijon C, Gatti DM, Kramnik I, Yener B, Gurcan M, et al. Lung necrosis and neutrophils reflect common pathways of susceptibility to Mycobacterium tuberculosis in genetically diverse, immune-competent mice. Dis Model Mech. 2015;8:1141–1153. doi: 10.1242/dmm.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marzo E, Vilaplana C, Tapia G, Diaz J, Garcia V, Cardona PJ. Damaging role of neutrophilic infiltration in a mouse model of progressive tuberculosis. Tuberculosis (Edinb) 2014;94:55–64. doi: 10.1016/j.tube.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Dallenga T, Repnik U, Corleis B, Eich J, Reimer R, Griffiths GW, Schaible UE. M. tuberculosis-induced necrosis of infected neutrophils promotes bacterial growth following phagocytosis by macrophages. Cell Host Microbe. 2017;22:519–530.e3. doi: 10.1016/j.chom.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Lerner TR, Borel S, Greenwood DJ, Repnik U, Russell MR, Herbst S, Jones ML, Collinson LM, Griffiths G, Gutierrez MG. Mycobacterium tuberculosis replicates within necrotic human macrophages. J Cell Biol. 2017;216:583–594. doi: 10.1083/jcb.201603040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahamed D, Boulle M, Ganga Y, Mc Arthur C, Skroch S, Oom L, Catinas O, Pillay K, Naicker M, Rampersad S, et al. Correction: intracellular growth of Mycobacterium tuberculosis after macrophage cell death leads to serial killing of host cells. eLife. 2017;6:e28205. doi: 10.7554/eLife.28205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filio-Rodríguez G, Estrada-García I, Arce-Paredes P, Moreno-Altamirano MM, Islas-Trujillo S, Ponce-Regalado MD, Rojas-Espinosa O. In vivo induction of neutrophil extracellular traps by Mycobacterium tuberculosis in a guinea pig model. Innate Immun. 2017;23:625–637. doi: 10.1177/1753425917732406. [DOI] [PubMed] [Google Scholar]
- 101.Rybniker J, Chen JM, Sala C, Hartkoorn RC, Vocat A, Benjak A, Boy-Röttger S, Zhang M, Székely R, Greff Z, et al. Anticytolytic screen identifies inhibitors of mycobacterial virulence protein secretion. Cell Host Microbe. 2014;16:538–548. doi: 10.1016/j.chom.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 102.Peng X, Luo T, Zhai X, Zhang C, Suo J, Ma P, Wang C, Bao L. PPE11 of Mycobacterium tuberculosis can alter host inflammatory response and trigger cell death. Microb Pathog. 2019;126:45–55. doi: 10.1016/j.micpath.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Liu H, Li H, Dang G, Cui Z, Song N, Wang Q, Liu S, Chen L. PE17 protein from Mycobacterium tuberculosis enhances Mycobacterium smegmatis survival in macrophages and pathogenicity in mice. Microb Pathog. 2019;126:63–73. doi: 10.1016/j.micpath.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 104.Gopalakrishnan A, Dietzold J, Verma S, Bhagavathula M, Salgame P. Toll-like receptor 2 prevents neutrophil-driven immunopathology during infection with Mycobacterium tuberculosis by curtailing CXCL5 production. Infect Immun. 2019;87:e00760-18. doi: 10.1128/IAI.00760-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simeone R, Bottai D, Frigui W, Majlessi L, Brosch R. ESX/type VII secretion systems of mycobacteria: insights into evolution, pathogenicity and protection. Tuberculosis (Edinb) 2015;95 Suppl 1:S150–S154. doi: 10.1016/j.tube.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 106.Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J, 3rd, Faé KC, Arrey F, Kuhlmann S, Bandermann S, Loewe D, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124:1268–1282. doi: 10.1172/JCI72030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lombard R, Doz E, Carreras F, Epardaud M, Le Vern Y, Buzoni-Gatel D, Winter N. IL-17RA in non-hematopoietic cells controls CXCL-1 and 5 critical to recruit neutrophils to the lung of mycobacteria-infected mice during the adaptive immune response. PLoS One. 2016;11:e0149455. doi: 10.1371/journal.pone.0149455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dorhoi A, Yeremeev V, Nouailles G, Weiner J, 3rd, Jörg S, Heinemann E, Oberbeck-Müller D, Knaul JK, Vogelzang A, Reece ST, et al. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur J Immunol. 2014;44:2380–2393. doi: 10.1002/eji.201344219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pagán AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med. 2014;5:a018499. doi: 10.1101/cshperspect.a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]