Abstract
Introduction
Small cell carcinoma of urinary bladder is rare and has an aggressive malignant behavior and poor prognosis. Advanced bladder cancers are treated with immune checkpoint inhibitors, however, its efficacy for small cell carcinoma of urinary bladder is unclear.
Case presentation
A 54‐year‐old female, diagnosed with clinical stage T2N0M0 small cell carcinoma of urinary bladder, underwent radical cystectomy after three cycles of etoposide‐cisplatin neoadjuvant chemotherapy. Despite the fact that pathological examination revealed no residual carcinoma in bladder in her cystectomy specimen, local recurrence of a 60‐mm mass detected in the follow‐up investigation 7.5 months later. This was completely treated by pembrolizumab without any adverse effects. Immunohistochemical staining revealed that the tumor had no programmed death ligand 1 expression but it showed CD8‐positive T‐lymphocyte infiltration into the tumor.
Conclusion
Immune checkpoint inhibitors might have curative potentials for treatment of small cell carcinoma of urinary bladder.
Keywords: bladder cancer, immune checkpoint inhibitor, immunotherapy, small cell carcinoma of urinary bladder, urothelial carcinoma
Abbreviations & Acronyms
- CT
computed tomography
- ICI
immune checkpoint inhibitor
- LRC
laparoscopic radical cystectomy
- MRI
magnetic resonance imaging
- NAC
neoadjuvant chemotherapy
- NSE
neuron specific enolase
- ORR
overall response rate
- PD‐1
programmed death 1
- PD‐L1
programmed death ligand 1
- SCCB
small cell carcinoma of urinary bladder
- SCLC
small cell lung cancer
- TURBT
transurethral resection of bladder tumor
- UC
urothelial carcinoma
Keynote message.
SCCB has an aggressive malignant behavior and has been usually treated with chemotherapy extrapolated from SCLC. We reported a successfully treated case which showed ICI may be considered as a novel therapeutic option for the treatment of SCCB.
Introduction
SCCB is a rare disease with an aggressive malignant behavior and poor prognosis. 1 , 2 , 3 Because of limited number of SCCB cases, standard treatment for SCCB has not been established yet and most of them have been treated with chemotherapy extrapolated from treatment of SCLC with or without surgery or radiotherapy. 4 However, the efficacy of treatment is unclear and further studies are needed to find the best treatment option. 2
Pembrolizumab is an ICI medication which targets the PD‐1 receptor and has been approved for treatment of patients with advanced stage UC and SCLC. However, the evidence for its efficiency in patients with SCCB is lacking. In this report, we describe a case of SCCB who had local recurrence after LRC with NAC which completely responded to pembrolizumab.
Case presentation
A 54‐year‐old female with gross hematuria and dysuria was referred to our hospital with the suspicion of bladder cancer. Urine analysis confirmed hematuria with pyuria and urine cytology showed class 5. Ultrasonography and cystoscopy revealed the presence of a non‐papillary bladder tumor adjacent to the right ureteric orifice (Fig. 1a ). CT scans and MRI showed a 20‐mm bladder tumor at the right posterior bladder wall suspected for muscle invasion and grade 1 right hydronephrosis (Fig. 1b–d). There was no evidence of distant or lymph node metastases.
Fig. 1.
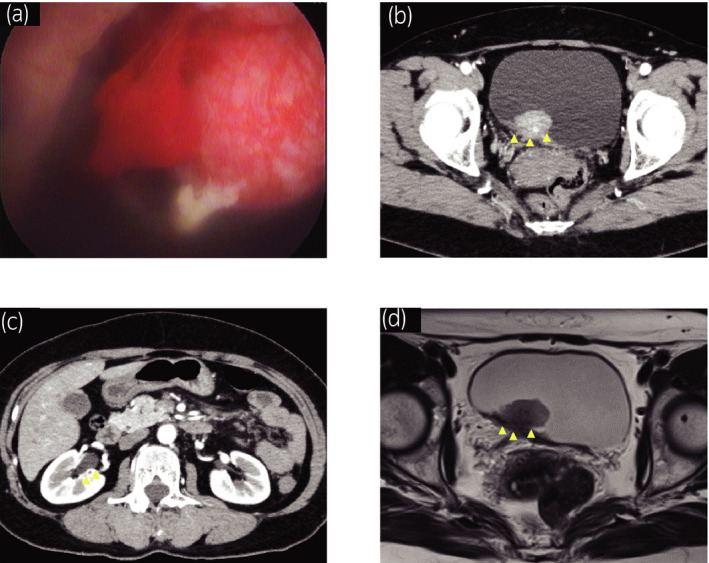
Clinical and radiological findings before TURBT. (a) Cystoscopy showed a sessile non‐papillary bladder tumor adjacent to the right ureteric orifice. (b) Abdominal CT with contrast showed a 20‐mm bladder tumor at the right posterior bladder wall and (c) Grade 1 right hydronephrosis. (d) Abdominal MRI showed a 20‐mm bladder tumor suspected for invasion to the muscular layer at the right posterior bladder wall on T2 weighted image.
TURBT was performed and pathological examination showed evidence of muscle invasion with UC accompanied by small cell carcinoma (Fig. 2a,b). Immunohistochemical staining showed that CD56 is partially positive (Fig. 2c) while Chromogranin A and Synaptophysin were negative on the specimen. TURBT, serum NSE level was within the normal level (6.1 ng/ml).
Fig. 2.
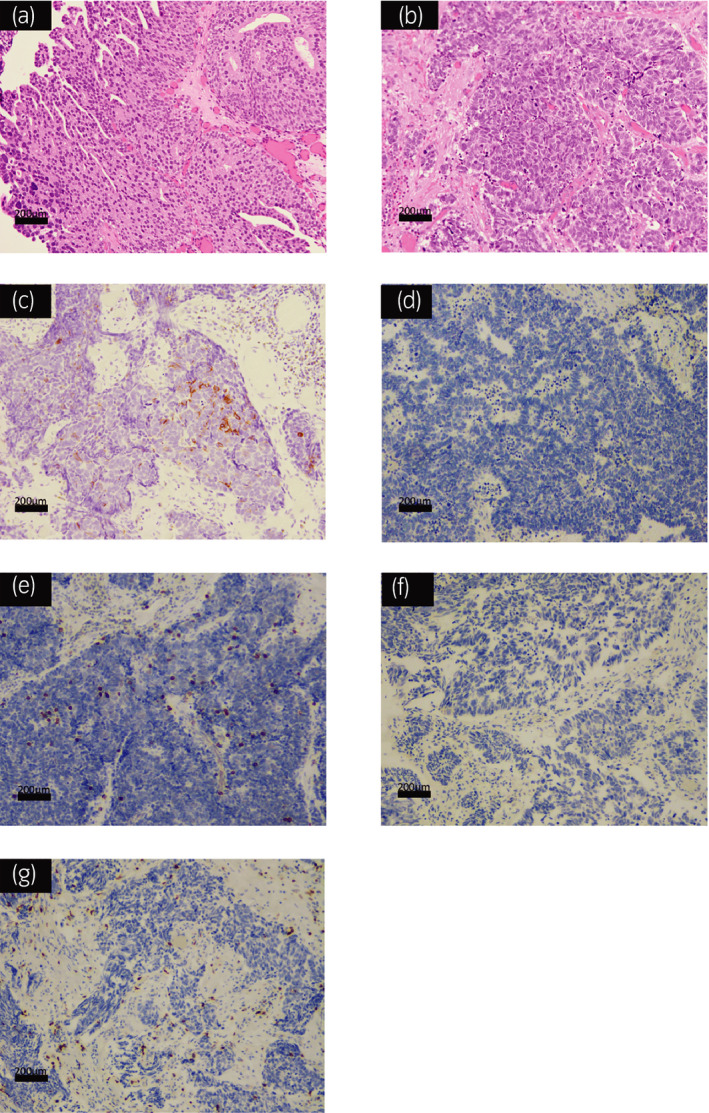
Pathological findings of hematoxylin and eosin staining and immunohistochemical special staining. (a) Hematoxylin and eosin staining of the bladder tumor showed both the urothelial cell carcinoma component and (b) small cell carcinoma component. (c) Immunohistochemical staining of the small cell carcinoma component showed that CD56 is partially positive, (d) PD‐L1 is negative. (e) Tumor infiltration of CD8+ T lymphocytes into tumor was observed. (f) PD‐L1 is also negative and (g) tumor infiltration of CD8+ T lymphocytes into tumor was observed in UC components.
Given her clinical staging (T2N0M0), we planned for three cycles of etoposide‐cisplatin NAC‐based treatment of SCLC. After NAC, CT scans showed decreasing size of bladder tumor and once again we did not detect any distant and lymph node metastases. Then, LRC with extended pelvic lymph node dissection and ileal conduit diversion was performed. Pathological finding revealed no residual carcinoma in the bladder with no lymph node metastases, pT0N0M0.
In the follow‐up imaging 4 months after LRC, there was no evidence of disease recurrence (Fig. 3a,b). However, 7.5 months after LRC, serum NSE level increased up to 45.7 ng/ml. CT scans showed a 60‐mm mass at the pelvis with possible invasion to the left distal ureter (Fig. 3c,d). This local recurrence of SCCB was discussed with the patient, she decided to proceed with pembrolizumab. After receiving two cycles of pembrolizumab therapy, CT scans demonstrated disappearance of recurrent tumor and left hydronephrosis (Fig. 3e,f). After completing five cycles of pembrolizumab therapy, local recurrent tumor and left hydronephrosis remained undetectable on CT scans (Fig. 3g,h). We evaluated efficacy of pembrolizumab therapy as complete response by the Response Evaluation Criteria in Solid Tumors 1.1 5 and serum NSE level decreased to the normal level (10.2 ng/mL). Twelve cycles of pembrolizumab therapy are ongoing and she has no adverse events.
Fig. 3.
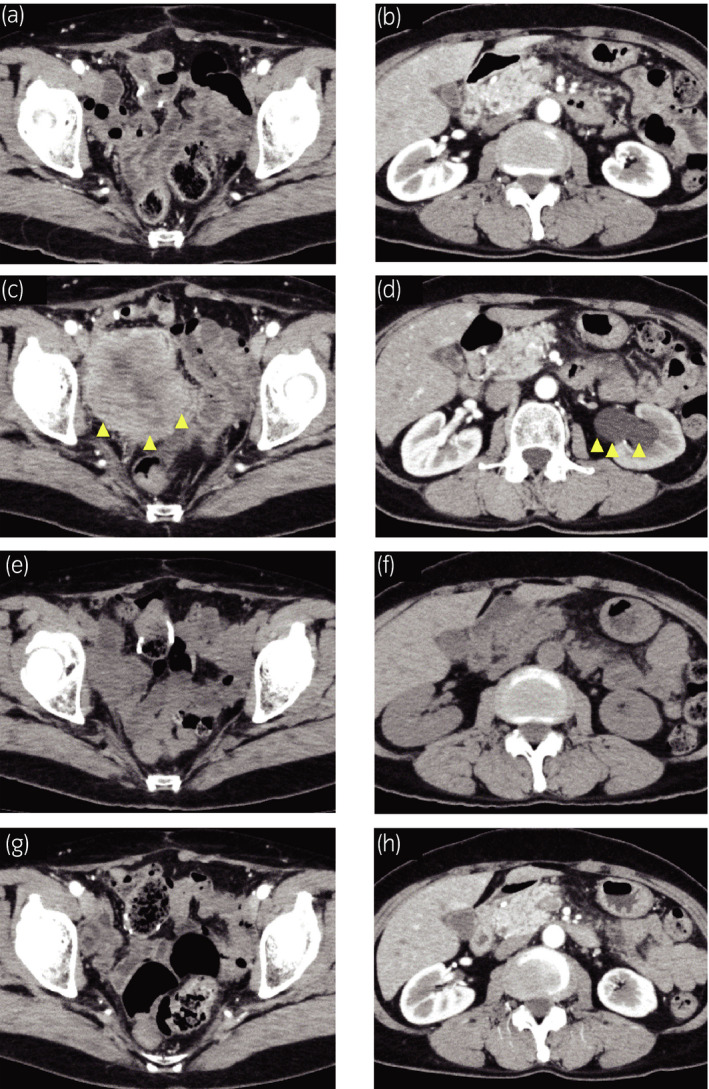
Radiological findings after LRC. (a) Abdominal CT showed no recurrent tumor 4 months after LRC. (b) No evidence of left hydronephrosis 4 months after LRC. (c) Abdominal CT with contrast showed a 60‐mm mass at the pelvis and (d) left hydronephrosis 7.5 months after LRC. (e) Abdominal CT showed disappearance of recurrent tumor at the pelvis and (f) left hydronephrosis after two cycles of pembrolizumab therapy. (g, h) Abdominal CT with contrast showed no tumor recurrence after five cycles of pembrolizumab therapy.
Discussion
SCCB accounts for less than 1% of all the bladder cancers and predominantly occurs in men (3:1 male‐to‐female ratio) at the 6th decade of their lives. In most cases, they initially present with gross hematuria and have muscle invasive stage T2 or higher at the time of diagnosis. 6 , 7 The standard treatment protocol is lacking and the prognosis of SCCB is poor, and 5‐year survival rate of all comers are somewhere between 16 and 25%. 8 , 9
According to the 2019 National Comprehensive Cancer Network guidelines in bladder cancer, initial chemotherapy followed by radiotherapy or cystectomy is recommended for patients with SCCB with localized disease regardless of stage. 10 A recent systematic review also showed that NAC and radical cystectomy could have some benefits for patients with early‐stage SCCB. 11 Although systematic chemotherapy with the agents used for SCLC are recommended for patients with SCCB with distant or lymph node metastases in the National Comprehensive Cancer Network guidelines, the 5‐year survival rates for patients with stage IV were 10.5% despite appropriate recommended therapy in a large series. 8
Two studies looked at the ORR of ICIs for patients with stage IV UC with neuronal subtypes including SCCB (Table 1). 12 , 13 One of them reported relatively high ORR to Atezolizumab. 12 The other case report showed reasonable response to pembrolizumab in a patient with chemotherapy refractory SCCB. 14 The complete response to immunotherapy in our case after relapse is consistent with previously described results in those studies.
Table 1.
Overall response rate of ICIs for patients with stage 4 neuronal subtype UC including SCCB
| Author | Patients | Treatment | Number of patients | Response | |
|---|---|---|---|---|---|
| ORR (%) | Best response | ||||
| Kim et al. 12 | Patients with platinum‐refactory or cisplatin‐ineligible neuronal subtype UC | Atezolizumab | 11 | 72% | Complete response 2 |
| Partial response 6 | |||||
| Not able to assess 3 | |||||
| Miller et al. 13 | Patients who received anti‐PD‐1 or PD‐L1 for UC with neuroendcrine feature | Atezolizumab | 6 | 25% | complete response + partial response 2 |
| Durvalumab | 1 | No response 6 | |||
| Nivolumab | 1 | Not able to assess 1 | |||
| Pembrolizumab | 1 | ||||
Some previous studies looking into pathologic features of responders to immunotherapy suggested that PD‐L1 expression, tumor infiltration with CD8+ T lymphocytes and tumor mutational burden are predictive biomarkers in ICI therapy. 15 , 16 PD‐L1 expression and tumor mutational burden have been also suggested as predictive biomarkers of response to immunotherapy for patients with SCLC. 17 It is however interesting to note that immunohistochemical staining of neuroendocrine bladder tumor including SCCB has shown less expression of PD‐L1 and density of CD8+ T lymphocyte infiltration. 18 In our case, we also performed immunohistochemical staining of specimens resected by TURBT. We found that PD‐L1 expression was negative in both SCCB and UC components; however, tumor infiltration with CD8+ T lymphocytes was observed in both components (Fig. 2d–g). Tumor infiltration with CD8+ T lymphocytes may be the reason that pembrolizumab had responded in our case.
Using our case and mentioned small series, we can conclude that cancer microenvironment needs more future attention and deserves performing prospective trials to test the ability of immunotherapy in SCCB. Recently, a phase II study (NCT03430895) planned to evaluate ICIs therapy in patients advanced non‐pure UC including SCCB and will hopefully answer more questions regarding this novel therapeutic option. 19
Conclusion
We presented a case of SCCB with local recurrence after NAC followed by LRC and responded totally to pembrolizumab. Treatment protocol for SCCB and this successfully treated case suggest that immunotherapy can be considered as a possible effective treatment option for patients with SCCB.
Ethics
We obtained written informed consent from the patient.
Conflict of interest
The authors declare no conflict of interest.
Hatayama T, Hayashi T, Matsuzaki S et al Successful treatment of recurrent small cell carcinoma of urinary bladder with pembrolizumab. IJU Case Rep. 2020; 3: 252–256.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Niu Q, Lu Y, Xu S et al Clinicopathological characteristics and survival outcomes of bladder neuroendocrine carcinomas: a population‐based study. Cancer Manag. Res. 2018; 10: 4479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of tumours of the urinary system and male genital organs‐part B: prostate and bladder tumours. Eur. Urol. 2016; 70: 106–19. [DOI] [PubMed] [Google Scholar]
- 4. Kouba EJ, Cheng L. Understanding the genetic landscape of small cell carcinoma of the urinary bladder and implications for diagnosis, prognosis, and treatment: a review. JAMA Oncol. 2017; 3: 1570–8. [DOI] [PubMed] [Google Scholar]
- 5. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 6. Erdem GU, Özdemir NY, Demirci NS, Şahin S, Bozkaya Y, Zengin N. Small cell carcinoma of the urinary bladder: changing trends in the current literature. Curr. Med. Res. Opin. 2016; 32: 1013–21. [DOI] [PubMed] [Google Scholar]
- 7. Schreiber D, Rineer J, Weiss J et al Characterization and outcomes of small cell carcinoma of the bladder using the surveillance, epidemiology, and end results database. Am. J. Clin. Oncol. 2013; 36: 126–31. [DOI] [PubMed] [Google Scholar]
- 8. Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer 2005; 103: 1172–8. [DOI] [PubMed] [Google Scholar]
- 9. Cheng L, Pan CX, Yang XJ et al Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer 2004; 101: 957–62. [DOI] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network . Bladder Cancer (Version 4.2019). 2019. [Cited 10 Aug 2019.] Available from URL: https://www2.tri‐kobe.org/nccn/guideline/urological/english/bladder.pdf
- 11. Veskimäe E, Espinos EL, Bruins HM et al What is the prognostic and clinical importance of urothelial and nonurothelial histological variants of bladder cancer in predicting oncological outcomes in patients with muscle‐invasive and metastatic bladder cancer? A European Association of Urology muscle invasive and metastatic bladder cancer guidelines panel systematic review. Eur. Urol. Oncol. 2019; 2: 625–42. [DOI] [PubMed] [Google Scholar]
- 12. Kim J, Kwiatkowski D, McConkey DJ et al The cancer genome atlas expression subtypes stratify response to checkpoint inhibition in advanced urothelial cancer and identify a subset of patients with high survival probability. Eur. Urol. 2019; 75: 961–4. [DOI] [PubMed] [Google Scholar]
- 13. Miller NJ, Khaki AR, Diamantopoulos LN et al Histologic subtypes and response to PD‐1/PD‐L1 blockade in advanced urothelial cancer: a retrospective study. J. Urol. 2020; 204: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilde L, Ali SM, Solomides CC, Ross JS, Trabulsi E, Hoffman‐Censits J. Response to pembrolizumab in a patient with chemotherapy refractory bladder cancer with small cell variant histology: a case report and review of the literature. Clin. Genitourin. Cancer 2017; 15: e521–e524. [DOI] [PubMed] [Google Scholar]
- 15. Tumeh PC, Harview CL, Yearley JH et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N. Engl. J. Med. 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gelsomino F, Lamberti G, Parisi C et al The evolving landscape of immunotherapy in small‐cell lung cancer: A focus on predictive biomarkers. Cancer Treat. Rev. 2019; 79: 101887. [DOI] [PubMed] [Google Scholar]
- 18. Mandelkow T, Blessin NC, Lueerss E et al Immune exclusion is frequent in small‐cell carcinoma of the bladder. Dis. Markers 2019; 2019: 2532518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ClinicalTrials.gov . Evaluating Immune Therapy, Durvalumab (MEDI4736) With Tremelimumab for Metastatic, Non‐transitional Cell Carcinoma of the Urinary Tract. [Cited 20 Feb 2020.] Available from URL: https://clinicaltrials.gov/ct2/show/NCT03430895.


