Highlights
-
•
Aging is the largest risk factors for severity and mortality in adult COVID-19.
-
•
Severe cases of COVID-19 are related to vascular damage with evidence of direct viral infection in the endothelial cells.
-
•
Increase risk of COVID-19 death are also highly related to disease with lower vascular Nitric Oxide (NO) level.
-
•
Vascular viral defence by endothelial nitric oxide synthase (eNOS) derive NO may be the protecting factor for the young.
-
•
eNOS polymorphism could potentially explain the disparity of COVID-19 mortality between Asian and non-Asian countries.
Keywords: Endothelial nitric oxide synthase (eNOS), Nitric oxide (NO), Bradykinin (BK), COVID-19, Endothelial cells (ECs)
Abstract
The COVID-19 pandemic poses an imminent threat to humanity, especially to the elderly. The molecular mechanisms underpinning the age-dependent disparity for disease progression is not clear. COVID-19 is both a respiratory and a vascular disease in severe patients. The damage endothelial system provides a good explanation for the various complications seen in COVID-19 patients. These observations lead us to suspect that endothelial cells are a barrier that must be breached before progression to severe disease. Endothelial intracellular defences are largely dependent of the activation of the interferon (IFN) system. Nevertheless, low type I and III IFNs are generally observed in COVID-19 patients suggesting that other intracellular viral defence systems are also activated to protect the young. Intriguingly, Nitric oxide (NO), which is the main intracellular antiviral defence, has been shown to inhibit a wide array of viruses, including SARS-CoV-1. Additionally, the increased risk of death with diseases that have underlying endothelial dysfunction suggest that endothelial NOS-derived nitric oxide could be the main defence mechanism. NO decreases dramatically in the elderly, the hyperglycaemic and the patients with low levels of vitamin D. However, eNOS derived NO occurs at low levels, unless it is during inflammation and co-stimulated by bradykinin. Regrettably, the bradykinin-induced vasodilation also progressively declines with age, thereby decreasing anti-viral NO production as well. Intriguingly, the inverse correlation between the percentage of WT eNOS haplotype and death per 100K population could potentially explain the disparity of COVID-19 mortality between Asian and non-Asian countries. These changes with age, low bradykinin and NO, may be the fundamental reasons that intracellular innate immunity declines with age leading to more severe COVID-19 complications.
COVID-19 is a devastating disease for the elderly, but relatively mild disease for the majority of younger people (Banerjee et al., 2020; Gandhi et al., 2020). The molecular mechanisms underpinning the age-dependent disparity for disease progression has not been determined. However, a careful interrogation of potential protective factor(s) in young individuals may provide a means to treat the older, more vulnerable population. Based on recently published clinical data, we hypothesize that the endothelial viral defence may play a prominent role in mitigating SARS-CoV-2 complications.
COVID-19 is both a respiratory and vascular disease, especially for those with severe symptoms. The layer of endothelial cells that separates blood from tissues forms an anatomic barrier to viruses within the body. Permeable viruses, especially viremic SARS-CoV-2 (with a size of 80–100 nm), must first infect and replicate in capillary endothelial cells (ECs) before infecting the underlying local tissue (Monteil et al., 2020). Patients who develop rapid clinical deterioration and death from multi-organ failure exhibit widespread endotheliitis, with accompanying cell death and direct viral infection (Ackermann et al., 2020; Varga et al., 2020). This indicates that the endothelial barrier has been breached, permitting systemic viral invasion. Moreover, mounting evidence suggests the altered vessel barrier integrity and a pro-coagulative state leads to pulmonary EC damage and, in turn, contributes to the initiation and propagation of acute respiratory distress syndrome (ARDS) (Varga et al., 2020), which is the main cause of death in patients with COVID-19 (Wu and McGoogan, 2020). High levels of the fibrin breakdown products, D-dimers, due to an EC death-induced clotting cascade (Nachman and Rafii, 2008) are predictive of poor COVID-19 patient outcome. In contrast, patients with mild signs and symptoms may have their infection resolved without thrombotic complications. These observations suggest that the integrity of endothelial cells is critical to prevent the development of severe COVID-19 (Teuwen et al., 2020).
The host defence against infection, which can be subdivided into systemic and cell-autonomous immunity, plays a critical role in protecting EC integrity. Systemic immunity detects and clears the infection, destroying the pathogens and the infected cells they inhabit. However, spontaneous EC regeneration is a slow and possibly insufficient process in the context of acute infection (Hirase and Node, 2012). Hence, the ability to counter infection and to evade host cell death at the individual cell level, termed cell-autonomous immunity (Beutler et al., 2006), is critical to prevent the spread of the virus. In cell autonomous immunity, the invading viruses trigger production of antiviral agents, including type I and III interferons, β-defensins and nitric oxide that lead to rapid virus clearance. However, viruses have also evolved various strategies to evade these antiviral mechanisms. Serum analysis of COVID-19 patients reveals an elevated pro-inflammatory cytokine and chemokine profile without detectable levels of type I and III IFNs in serum of both the China and USA cohorts (Blanco-Melo et al., 2020; Huang et al., 2020). Nevertheless, in the absent of these type of interferon, host cells could also mount an antiviral response through the nitric oxide (NO) pathway. Mehta et al. (Mehta et al., 2012) showed that in primary fibroblasts lacking interferon genes, NO production leads to expression of interferon stimulated genes (ISG), as well as other cytokines and chemokines to generate a vigorous antiviral response against both DNA and RNA viruses following poly IC stimulation. Endothelial NOS-derived NO is also a physiological vasodilator, an inhibitor of platelet aggregation and is considered to be a main antiviral defence mechanism. Replication of various RNA and DNA viruses, particularly SARS-CoV (Akerstrom et al., 2005), is inhibited by NO, which may indirectly decrease viral replication and protein production in host cells by directly inactivating or modifying viral replicating proteins (Abdul-Cader et al., 2016).
In addition, inadequate production of endothelial Nitric Oxide Synthase (eNOS)-derived NO is a major cause of EC dysfunction, which can lead to disruption of vascular integrity. Established risk factors for COVID-19 mortality, which include old age, hypertension and diabetes mellitus are all characterized by pre-existing endothelial dysfunction (Li et al., 2019). Endothelial dysfunction leads to vasoconstrictive, proinflammatory and pro-coagulant states, with ensuing organ ischemia and tissue oedema (Bonetti et al., 2003). Therefore, it is not surprising that patients with pre-existing endothelial dysfunction are also at high risk for adverse outcomes in COVID-19. More critically, newly diagnosed diabetes patients, which have uncontrolled hyperglycaemia, are at much higher risk of COVID-19 mortality than diabetes patients who have taken medication to control glucose levels (Li et al., 2020). This finding may be explained by the observation that high glucose is known to decrease endothelial cell NO bioavailability (Hoshiyama et al., 2003). Similarly, levels of vitamin D are correlated negatively with mortality in a large study of patients in 20 European Countries. For instance, low vitamin D levels in the aging population of Spain, Italy and Switzerland is associated with increasing vulnerability for COVID-19 mortality (Ilie et al., 2020). While the pathological mechanisms underlying this observation remains poorly understood, the known role of Vitamin D in upregulating eNOS and NO bioavailability may provide a mechanistic explanation and support a primary role for endothelial viral defence during SARS-CoV-2 infection (Andrukhova et al., 2014).
Among risk factors, old age is the biggest risk factor for mortality for COVID-19. Severe SARS-CoV-2 infections that require hospitalization are rare in young people, and the risk increases dramatically with age (Docherty et al., 2020). The reason why SARS-CoV-19 has mostly spared children is not clear, but the marked age-dependent decrease of NO bioavailability level (Cernadas et al., 1998; Lauer et al., 2008; Smith et al., 2006; Yoon et al., 2010) with age could be a major underlying factor sensitizing elders to SARS-CoV-2. Particularly, the activity of eNOS (Cernadas et al., 1998) and endothelium-dependent vasodilatation (Delp et al., 2008; Rodriguez-Manas et al., 2009) progressively declines with age. The reduced bioavailability of NO may thus significantly reduce EC autonomous defences in COVID-19 patients of advanced age.
While the beneficial effects of inhaled NO in ARDS (Rossaint et al., 1995) has been demonstrated and various COVID-19 clinical trials to deliver inhale NO are currently being examined, the importance of eNOS in endothelial cells is not generally considered, as eNOS-derived NO is traditionally perceived as transient and at lower levels than NO derived from inducible Nitric Oxide Synthase (iNOS) (Reiss and Komatsu, 1998). However, recent studies show that eNOS can also produce prolonged and elevated NO in human ECs under inflammatory conditions and bradykinin co-stimulation (Lowry et al., 2013). Bradykinin is an important pro-inflammatory and vasodilatory peptide that could be locally induced during tissue damage (Dray and Perkins, 1993). Bradykinin mediates its vascular actions via 2 types of receptors, bradykinin receptor type 1 (BK1R) and type 2 (BK2R) (Siltari et al., 2016). Bradykinin-stimulated BK2R in cytokine-treated human ECs intensifies the binding of eNOS to calcium-calmodulin (CaM) at basal Ca2+ levels, hence inducing the iNOS like NO output (Lowry et al., 2013). Additionally, eNOS-derived NO also inhibits microbial (e.g. Mycobacterium tuberculosis) growth when the ECs represent the site of microbial invasion (Konradt and Hunter, 2018), suggesting that eNOS-derived NO may be sufficient to play a critical role in ECs autonomous immunity. Regrettably, bradykinin-induced vasorelaxation drops progressively with age (Siltari et al., 2016), a phenomenon related to the reduced B2R expression in the aged vasculature, therefore establishing the link between aging and COVID-19 sensitivity.
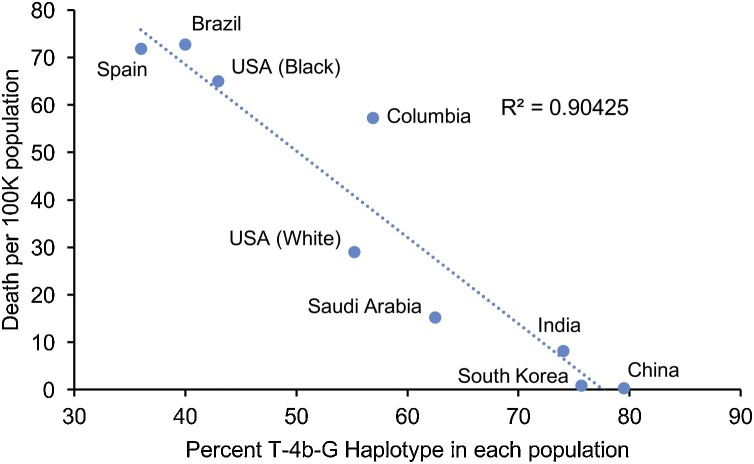
Given the critical role of eNOS in the cell autonomous defence against SARS-CoV-2, eNOS polymorphisms may be directly associated with COVID-19 severity. eNOS is encoded by NOS3 gene, a highly polymorphic and well-studied gene (Cooke et al., 2007). Some of the NOS3 polymorphisms, such as the promoter single nucleotide polymorphisms (SNPs) g.−786T>C (rs2070744), missense Glu298Asp polymorphism within exon 7 (rs1799983), and a variable number of tandem repeats (VNTRs) in intron 4 (4b/4a), are considered functional; because they affect NOS3 expression or NO formation activities (Oliveira-Paula et al., 2016). As impairment of endogenous NO production is highly implicated in various diseases, many studies have evaluated the clinical implications of NOS3 polymorphisms either individually or in combination (Oliveira-Paula et al., 2016). Haplotype analysis is often performed as SNPs may affect one another and are often inherited as a unit (Crawford and Nickerson, 2005). Although the relevance of individual NOS3 polymorphisms to various diseases have been discovered, the findings are often conflicting (Cooke et al., 2007; Pereira et al., 2007). These discrepancies may be related to the analysis of single genetic polymorphisms that neglect the interactions between other polymorphisms within NOS3 (Crawford and Nickerson, 2005; Tanus-Santos and Casella-Filho, 2007). For example, while individual NOS3 polymorphisms did not affect plasma nitrite/nitrate (NOx) levels, the C-4b-Glu haplotype was associated with decreased NOx levels (Metzger et al., 2011, 2005), suggesting that haplotype analysis may provide improved predictivity and inform better about the risks of specific genotypes (Crawford and Nickerson, 2005). In terms of haplotype analysis, the WT haplotype, which combines the wild-type variants for all three polymorphisms (T-4b-Glu) is the most common haplotype found in all populations studied (Luizon et al., 2009; Marroni et al., 2005; Tanus-Santos et al., 2001; Thomas et al., 2013), and has been least associated with all diseases examined (Kitsios and Zintzaras, 2010). In fact, it has been generally linked to be a protective phenotype (Kumar et al., 2009). Nonetheless, there is notable disparity in the distribution of WT haplotypes between different ethnic groups, with the frequency of the WT haplotype was much higher in Asians than in all other ethnic groups (Qi et al., 2020; Tanus-Santos et al., 2001). These findings could partially explain the lower incidence of cardiovascular diseases in Asians (Tanus-Santos et al., 2001). While COVID-19 morbidity and mortality are influenced by age, specific pre-existing health conditions and longstanding discriminatory societal and historical factors, the contribution of underlying biological factors to the burden of COVID-19 cannot be dismissed as many countries are witnessing differential health outcomes according to ethnicity. A recent study on the global clinical characteristics and mortality for COVID-19 discovered that hospital mortality was significantly higher in America and Europe than to Asia (Goel et al., 2020). More strikingly, there was a direct negative correlation between COVID-19 mortality per 100 K people when compared to the percentage of NOS3 WT (T-4b-Glu) haplotype based on available data (Fig. 1 ).
Fig. 1.
Correlation between COVID-19 death per 100 K population and the percent of NOS3 WT haplotype. The death per 100k was based on John Hopkins University Coronavirus Resources Centre and the US data was based on CDC COVID data tracker and U.S. Census Bureau. data (as of Oct 16, 2020). The reference for the percent of WT NOS3 haplotype was based on reference shown below: China (Han Chinese) (Qi et al., 2020), South Korea (Shim et al., 2010), India (Nishank et al., 2013), Saudi Arabia (Alkharfy et al., 2012), Columbian (Average of Black and White) (Serrano et al., 2010), US (White, Black) (Thomas et al., 2013), Brazil (Sandrim et al., 2006), Spain (Amoli et al., 2003).
This finding may explain the disparity in global mortality among Asians and other continents, especially when comparing countries with cases (as of October 2020), such as the US and India, where the death per million is significantly lower in India despite lower access to medical resources among infected individuals. This striking association further strengthens the hypothesis that eNOS levels provide robust defence against COVID-19, and warrants further clinical trials in the development of treatment strategies that enhance endothelial NO levels.
Taken together, all the connecting points strongly suggest that the progression of COVID-19 can be hindered by a strong endothelial cellular defence system through induction of NO. This hypothesis provides a rationale for therapies to increase endothelial NO as a means to treat hospitalized moderate-to-severe patients, particularly for older patients that have low NO levels, through drugs that upregulate NO bioavailability, such as nitrite anions, nitric oxide agonist (minoxidil), steroid hormones (dehydroepiandrosterone and estrogen), HMG-CoA reductase inhibitors (statins), resveratrol, and folic acid (Cau et al., 2012). In order to prove the validity of this hypothesis, further studies and clinical trials are warranted.
References
- Abdul-Cader M.S., Amarasinghe A., Abdul-Careem M.F. Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses. Arch. Virol. 2016;161:2075–2086. doi: 10.1007/s00705-016-2904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstrom S., Mousavi-Jazi M., Klingstrom J., Leijon M., Lundkvist A., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkharfy K.M., Al-Daghri N.M., Al-Attas O.S., Alokail M.S., Mohammed A.K., Vinodson B., Clerici M., Kazmi U., Hussain T., Draz H.M. Variants of endothelial nitric oxide synthase gene are associated with components of metabolic syndrome in an Arab population. Endocr. J. 2012;59:253–263. doi: 10.1507/endocrj.ej11-0278. [DOI] [PubMed] [Google Scholar]
- Amoli M.M., Garcia-Porrua C., Llorca J., Ollier W.E., Gonzalez-Gay M.A. Endothelial nitric oxide synthase haplotype associations in biopsy-proven giant cell arteritis. J. Rheumatol. 2003;30:2019–2022. [PubMed] [Google Scholar]
- Andrukhova O., Slavic S., Zeitz U., Riesen S.C., Heppelmann M.S., Ambrisko T.D., Markovic M., Kuebler W.M., Erben R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014;28:53–64. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L., Noursadeghi M., Pillay D., Sebire N., Holmes C., Pagel C., Wong W.K., Langenberg C., Williams B., Denaxas S., Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395:1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Jiang Z., Georgel P., Crozat K., Croker B., Rutschmann S., Du X., Hoebe K. Genetic analysis of host resistance: toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(1036-1045):e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti P.O., Lerman L.O., Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Cau S.B., Carneiro F.S., Tostes R.C. Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front. Physiol. 2012;3:218. doi: 10.3389/fphys.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernadas M.R., Sanchez de Miguel L., Garcia-Duran M., Gonzalez-Fernandez F., Millas I., Monton M., Rodrigo J., Rico L., Fernandez P., de Frutos T., Rodriguez-Feo J.A., Guerra J., Caramelo C., Casado S., Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ. Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Cooke G.E., Doshi A., Binkley P.F. Endothelial nitric oxide synthase gene: prospects for treatment of heart disease. Pharmacogenomics. 2007;8:1723–1734. doi: 10.2217/14622416.8.12.1723. [DOI] [PubMed] [Google Scholar]
- Crawford D.C., Nickerson D.A. Definition and clinical importance of haplotypes. Annu. Rev. Med. 2005;56:303–320. doi: 10.1146/annurev.med.56.082103.104540. [DOI] [PubMed] [Google Scholar]
- Delp M.D., Behnke B.J., Spier S.A., Wu G., Muller-Delp J.M. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J. Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G., investigators I.C. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- Goel S., Jain T., Hooda A., Malhotra R., Johal G., Masoomi R., Kamran H., Krishnamoorthy P.M., Senguttuvan N.B., Sharma A., Gidwani U. Clinical characteristics and in-hospital mortality for COVID-19 across the globe. Cardiol. Ther. 2020 doi: 10.1007/s40119-020-00189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T., Node K. Endothelial dysfunction as a cellular mechanism for vascular failure. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H499–505. doi: 10.1152/ajpheart.00325.2011. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M., Li B., Yao J., Harada T., Morioka T., Oite T. Effect of high glucose on nitric oxide production and endothelial nitric oxide synthase protein expression in human glomerular endothelial cells. Nephron Exp. Nephrol. 2003;95:e62–68. doi: 10.1159/000073673. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020 doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsios G.D., Zintzaras E. An NOS3 haplotype is protective against hypertension in a caucasian population. Int. J. Hypertens. 2010;2010 doi: 10.4061/2010/865031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradt C., Hunter C.A. Pathogen interactions with endothelial cells and the induction of innate and adaptive immunity. Eur. J. Immunol. 2018;48:1607–1620. doi: 10.1002/eji.201646789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Nejatizadeh A., Arif E., Akhtar S., Gupta M., Tyagi S., Goyal A.K., Jain S.K., Qadar Pasha M.A. Multi-locus interactions of vascular homeostasis genes in essential hypertension: a gender-based study. Clin. Chim. Acta. 2009;405:87–93. doi: 10.1016/j.cca.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Lauer T., Heiss C., Balzer J., Kehmeier E., Mangold S., Leyendecker T., Rottler J., Meyer C., Merx M.W., Kelm M., Rassaf T. Age-dependent endothelial dysfunction is associated with failure to increase plasma nitrite in response to exercise. Basic Res. Cardiol. 2008;103:291–297. doi: 10.1007/s00395-008-0714-3. [DOI] [PubMed] [Google Scholar]
- Li X., Sun X., Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019;30:414–433. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X., Qiu K., Zhang J., Zeng T., Chen L., Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes. Metab. 2020 doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry J.L., Brovkovych V., Zhang Y., Skidgel R.A. Endothelial nitric-oxide synthase activation generates an inducible nitric-oxide synthase-like output of nitric oxide in inflamed endothelium. J. Biol. Chem. 2013;288:4174–4193. doi: 10.1074/jbc.M112.436022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luizon M.R., Izidoro-Toledo T.C., Simoes A.L., Tanus-Santos J.E. Endothelial nitric oxide synthase polymorphisms and haplotypes in Amerindians. DNA Cell Biol. 2009;28:329–334. doi: 10.1089/dna.2009.0878. [DOI] [PubMed] [Google Scholar]
- Marroni A.S., Metzger I.F., Souza-Costa D.C., Nagassaki S., Sandrim V.C., Correa R.X., Rios-Santos F., Tanus-Santos J.E. Consistent interethnic differences in the distribution of clinically relevant endothelial nitric oxide synthase genetic polymorphisms. Nitric Oxide. 2005;12:177–182. doi: 10.1016/j.niox.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Mehta D.R., Ashkar A.A., Mossman K.L. The nitric oxide pathway provides innate antiviral protection in conjunction with the type I interferon pathway in fibroblasts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger I.F., Souza-Costa D.C., Marroni A.S., Nagassaki S., Desta Z., Flockhart D.A., Tanus-Santos J.E. Endothelial nitric oxide synthase gene haplotypes associated with circulating concentrations of nitric oxide products in healthy men. Pharmacogenet. Genomics. 2005;15:565–570. doi: 10.1097/01.fpc.0000167328.85163.44. [DOI] [PubMed] [Google Scholar]
- Metzger I.F., Ishizawa M.H., Rios-Santos F., Carvalho W.A., Tanus-Santos J.E. Endothelial nitric oxide synthase gene haplotypes affect nitrite levels in black subjects. Pharmacogenomics J. 2011;11:393–399. doi: 10.1038/tpj.2010.52. [DOI] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(905-913):e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R.L., Rafii S. Platelets, petechiae, and preservation of the vascular wall. N. Engl. J. Med. 2008;359:1261–1270. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishank S.S., Singh M.P., Yadav R., Gupta R.B., Gadge V.S., Gwal A. Endothelial nitric oxide synthase gene polymorphism is associated with sickle cell disease patients in India. J. Hum. Genet. 2013;58:775–779. doi: 10.1038/jhg.2013.99. [DOI] [PubMed] [Google Scholar]
- Oliveira-Paula G.H., Lacchini R., Tanus-Santos J.E. Endothelial nitric oxide synthase: from biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene. 2016;575:584–599. doi: 10.1016/j.gene.2015.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira T.V., Rudnicki M., Cheung B.M., Baum L., Yamada Y., Oliveira P.S., Pereira A.C., Krieger J.E. Three endothelial nitric oxide (NOS3) gene polymorphisms in hypertensive and normotensive individuals: meta-analysis of 53 studies reveals evidence of publication bias. J. Hypertens. 2007;25:1763–1774. doi: 10.1097/HJH.0b013e3281de740d. [DOI] [PubMed] [Google Scholar]
- Qi G., Yin S., Zhang G., Wang X. Genetic and epigenetic polymorphisms of eNOS and CYP2D6 in mainland Chinese Tibetan, Mongolian, Uygur, and Han populations. Pharmacogenomics J. 2020;20:114–125. doi: 10.1038/s41397-019-0104-2. [DOI] [PubMed] [Google Scholar]
- Reiss C.S., Komatsu T. Does nitric oxide play a critical role in viral infections? J. Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manas L., El-Assar M., Vallejo S., Lopez-Doriga P., Solis J., Petidier R., Montes M., Nevado J., Castro M., Gomez-Guerrero C., Peiro C., Sanchez-Ferrer C.F. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Rossaint R., Gerlach H., Schmidt-Ruhnke H., Pappert D., Lewandowski K., Steudel W., Falke K. Efficacy of inhaled nitric oxide in patients with severe ARDS. Chest. 1995;107:1107–1115. doi: 10.1378/chest.107.4.1107. [DOI] [PubMed] [Google Scholar]
- Sandrim V.C., Yugar-Toledo J.C., Desta Z., Flockhart D.A., Moreno H., Jr., Tanus-Santos J.E. Endothelial nitric oxide synthase haplotypes are related to blood pressure elevation, but not to resistance to antihypertensive drug therapy. J. Hypertens. 2006;24:2393–2397. doi: 10.1097/01.hjh.0000251899.47626.4f. [DOI] [PubMed] [Google Scholar]
- Serrano N.C., Diaz L.A., Casas J.P., Hingorani A.D., Moreno de Lucca D., Paez M.C. Frequency of eNOS polymorphisms in the Colombian general population. BMC Genet. 2010;11:54. doi: 10.1186/1471-2156-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S.H., Yoon T.K., Cha D.H., Han W.B., Choi D.H., Kim N.K. Endothelial Nitric Oxide Synthase (eNOS) gene polymorphisms in spontaneously aborted embryos. Genes Genomics. 2010;32:283–288. [Google Scholar]
- Siltari A., Korpela R., Vapaatalo H. Bradykinin -induced vasodilatation: role of age, ACE1-inhibitory peptide, mas- and bradykinin receptors. Peptides. 2016;85:46–55. doi: 10.1016/j.peptides.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Visioli F., Frei B., Hagen T.M. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell. 2006;5:391–400. doi: 10.1111/j.1474-9726.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- Tanus-Santos J.E., Casella-Filho A. Endothelial nitric oxide synthase polymorphisms and susceptibility to hypertension: genotype versus haplotype analysis. Hypertension. 2007;49 doi: 10.1161/01.HYP.0000251106.80955.38. E1; author reply E2. [DOI] [PubMed] [Google Scholar]
- Tanus-Santos J.E., Desai M., Flockhart D.A. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics. 2001;11:719–725. doi: 10.1097/00008571-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.N., Thakur T.J., Yi L., Guindo A., Diallo D.A., Ott J. Extensive ethnogenomic diversity of endothelial nitric oxide synthase (eNOS) polymorphisms. Gene Regul. Syst. Bio. 2013;7:1–10. doi: 10.4137/GRSB.S10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yoon H.J., Cho S.W., Ahn B.W., Yang S.Y. Alterations in the activity and expression of endothelial NO synthase in aged human endothelial cells. Mech. Ageing Dev. 2010;131:119–123. doi: 10.1016/j.mad.2009.12.010. [DOI] [PubMed] [Google Scholar]