Abstract
This paper describes how the endosomal organization of immuno-stimulatory nanoconstructs can tune the in vitro activation of macrophages. Nanoconstructs composed of gold nanoparticles conjugated with unmethylated cytosine-phosphate-guanine (CpG) oligonucleotides have distinct endosomal distributions depending on surface curvature. Mixed-curvature constructs produce a relatively high percentage of hollow endosomes, where constructs accumulated primarily along the interior edges. These constructs achieved a higher level of toll-like receptor (TLR) 9 activation along with enhanced secretion of proinflammatory cytokines and chemokines compared to constant-curvature constructs that aggregated mostly in the center of the endosomes. Our results underscore the importance of intra-endosomal interactions in regulating immune responses and targeted delivery.
Keywords: immunostimulation, CpG, intracellular targeting, nanoparticle, surface curvature
Graphical Abstract
INTRODUCTION
The delivery of biomolecules into cells plays a key role in the manipulation of cell function1 for numerous cancer therapies.2–4 Nanoconstructs consisting of inorganic nanoparticles (NPs) with a shell of biomolecular ligands show improved delivery efficiency because of increased cellular uptake and multi-valent interactions with surface receptors.5–15 The physicochemical properties of nanoconstructs can influence intracellular trafficking16 and the distribution within different cellular compartments such as the cytoplasm,17 lysosomes,12 and nucleus.18, 19 During subcellular transport, constructs are concentrated in vesicular compartments, which often results in aggregation because of the high concentration of enzymes, acids, and salts.20–24 As a result, a significant fraction of the ligands are precluded from binding targeted receptors23, 25 and also NP aggregates are not readily cleared from cells.16, 26, 27
To correlate how the intracellular arrangement of nanoconstructs affects downstream cell signaling, we focused on targeting toll-like receptor (TLR) 9 in the endosome of macrophages as a model system. TLR9 recognizes unmethylated cytosine-phosphate-guanine (CpG) moieties of oligodeoxynucleotide (ODN) sequences that induce an immunostimulatory (IS) response from macrophages that clear microbial pathogens28, 29 or induce anti-tumor immunity.30 TLR9 signaling requires the dimerization of two CpG-bound TLR9 receptors.31, 32 Therefore, we hypothesized that the spatial organization of the CpG-constructs is critical to facilitate TLR9 clustering and hence to stimulate IS activity. Previous studies have only correlated the amount of endocytosed CpG ODNs with IS response.33–35 Elucidating effects of endosomal organization of nanoconstructs on TLR9-mediated IS responses is important to enhance IS activity and receptor-targeted delivery.
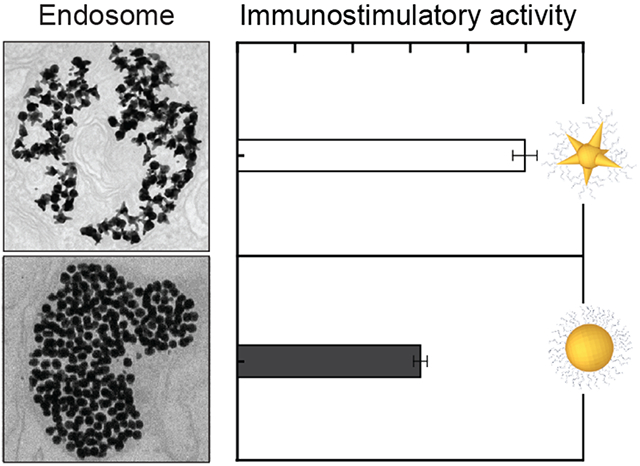
Here we show how the spatial organization of CpG-nanoconstructs in the endosome increases access and binding to TLR9, resulting in enhanced IS responses of macrophages. Two types of nanoconstructs with different surface curvatures were investigated: spiky NPs (mixed curvature) and spherical NPs (constant curvature). Mixed-curvature constructs resulted in hollow endosomes (ca. 60%) across the macrophage population, where constructs accumulated primarily along the interior edges of the endosomes. In contrast, constant-curvature constructs resulted in a lower percentage of hollow endosomes (ca. 10%). The mixed-curvature constructs that produced a higher percentage of hollow endosomes also achieved stronger IS activity based on alkaline phosphatase secretion, which correlates with higher levels of proinflammatory cytokines. These results indicate that regulating the endosomal organization of constructs via differences in surface curvature can impact intracellular delivery and cell responses.
RESULTS AND DISCUSSION
We synthesized CpG-constructs by loading thiolated CpG ODNs on gold NPs to target endosomal TLR9 in macrophages based on published protocols (Scheme 1a).34, 36 We focused on mixed-curvature and constant-curvature constructs of similar sizes and physicochemical properties (Figure S1 and Table S1) to compare how surface curvature affects binding to TLR9 (Scheme 1b), endosomal organization (Scheme 1c), and hence TLR9 activation. We tuned the loading of CpG on the NPs by modifying the pH of the citrate buffer or adjusting the ratio of NPs to CpG ODNs. After ODN functionalization, the constructs were back-filled with mercaptoundecyl hexa(ethylene glycol) (MUHEG) to prevent aggregation in solution by steric repulsion.34, 36 The protein adsorption profiles of the two differently shaped constructs were also similar; only twenty additional proteins were observed on mixed-curvature constructs and at extremely low concentrations (Table S2 and S3).
Scheme 1. Comparison of Surface Curvature Effects on In Vitro Macrophage Activation of CpG-constructs.
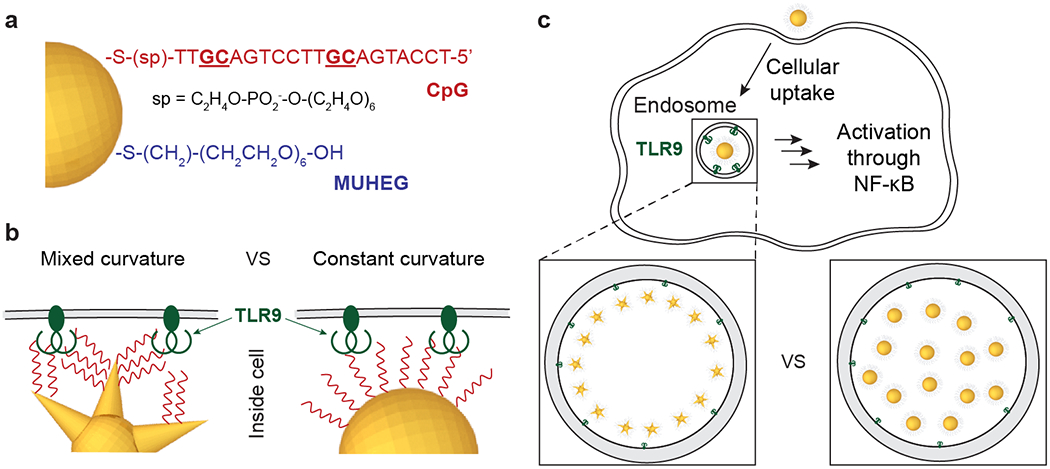
(a) Surface composition, (b) CpG ODN presentation, and (c) endosomal organization of CpG constructs can affect TLR9 signaling. After endocytosis, CpG-constructs that bind TLR9 activate macrophages through the transcription factor NF-κB. Different arrangements of CpG-constructs with different surface curvatures affect IS responses of macrophages.
We visualized the spatial organization of CpG-constructs in the endosomes using transmission electron microscopy (TEM) after treating macrophages with 200 nM CpG (250 CpG / NP) for 24 h (Supporting Information, METHODS). Mixed-curvature constructs resulted in hollow endosomes, where constructs accumulated along the interior surface of the endosome, which we refer to as the endosomal edge (Figure 1). Constant-curvature constructs yielded dense endosomes, where constructs aggregated in the center. When the two types of constructs were combined in a single treatment, the mixed-curvature constructs were again located along the endosomal edge while the constant-curvature constructs were in the center (Figure S2). This notable, segregated distribution of the two types of constructs was not affected by their order of incubation with macrophages. Incubation with constant-curvature constructs followed by mixed-curvature ones or the reverse sequence resulted in more mixed-curvature constructs along the endosomal edge (Figure S2d–f). One explanation for these results is that the surface curvature of the CpG-constructs affects the local chemical environment,37 which influences the separation of the two types of CpG-constructs in the endosomes.
Figure 1. Mixed-curvature constructs accumulate along the interior edge of endosomes.
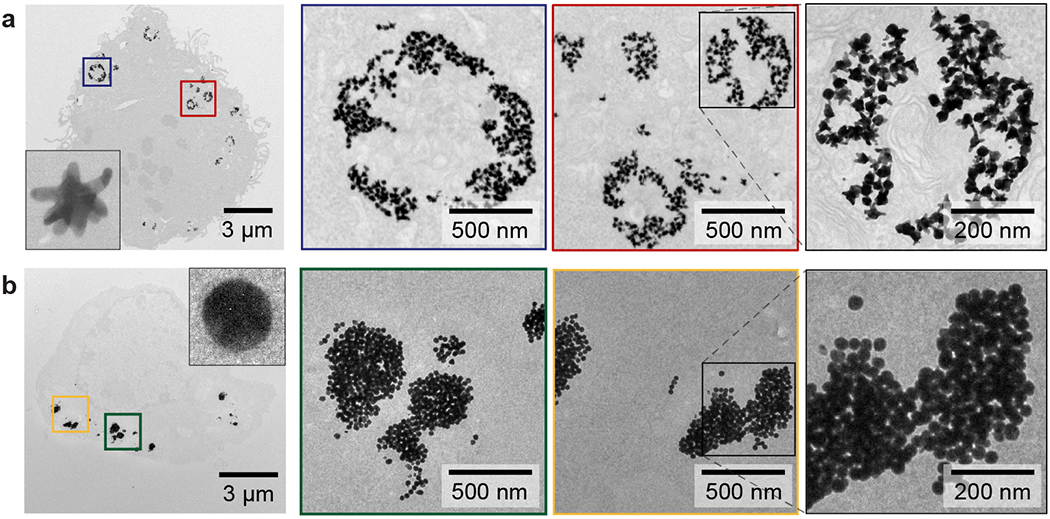
TEM images of macrophages after treatment with (a) mixed-curvature and (b) constant-curvature CpG-constructs. Images in boxes indicate zoomed-in views. The two types of CpG-constructs were incubated at the same concentration of CpG (200 nM). The representative TEM images were collected from three independent treatments.
To understand the impact of the different endosomal organizations of the CpG-constructs, we classified the endosomes by construct distribution; we quantified percentages of constructs along the endosomal edge and the sizes of endosomes after different incubation times (4, 8, 16, and 24 h). A higher percentage of hollow endosomes (ca. 60%) was observed with mixed-curvature constructs compared to constant-curvature ones (ca. 10%) after 4 h (Figure 2a). Prolonged incubation (8, 16, and 24 h) caused small increases in the percentage of hollow and dense endosomes (ca. 6-10%) while decreasing the percentage of endosomes with a small number of NPs (ca. 9%; scattered). More mixed-curvature constructs were located along the endosomal edge compared to constant-curvature constructs at all incubation times (Figure 2b; p < 0.001). After 4 h, the percentage of mixed-curvature constructs at the endosomal edge (55%) was higher than that of constant-curvature constructs (36%) (p < 0.0001). After 24 h, the percentage of mixed-curvature constructs along the endosomal edge decreased (34%) but was still higher than that of the constant-curvature ones (26%) (p < 0.0001). Hence, mixed-curvature constructs can produce hollow endosomes that provide better access and binding to TLR9 on the endosomal membrane compared to constant-curvature constructs.
Figure 2. Hollow endosomes have a higher percentage of CpG-constructs along the endosomal edge and are larger than dense endosomes.
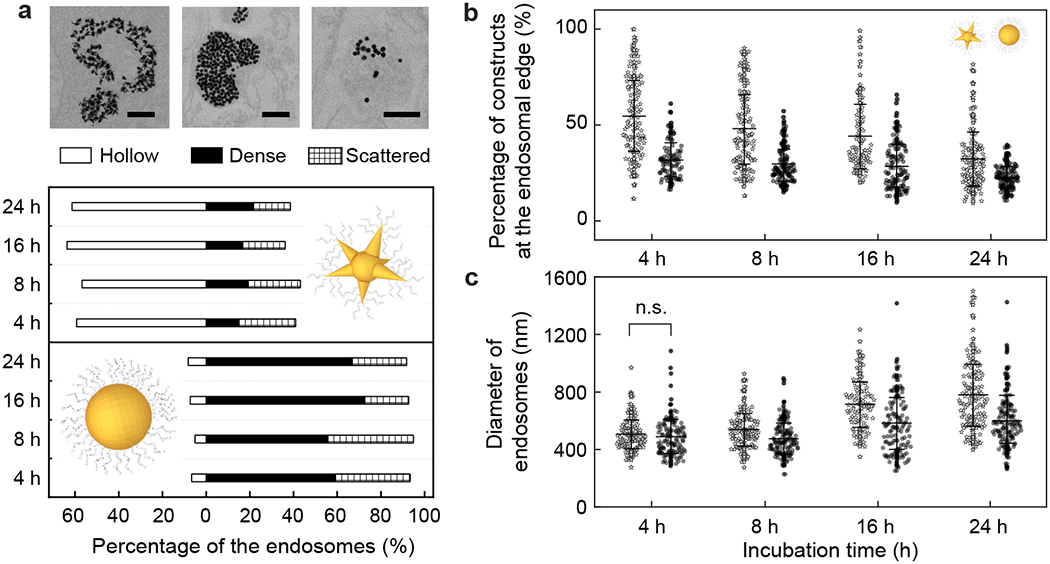
(a) Endosomes are classified by the CpG-construct distributions within them (hollow, dense, and scattered) after different incubation times (4, 8, 16, and 24 h; [CpG] = 200 nM). Scale bars = 200 nm. (b) Percentage of constructs along the endosomal edge and (c) diameter of endosomes were quantified. These characteristics of endosomes with different types of CpG-constructs show statistically significant differences (p < 0.0001, student’s t test) – otherwise marked with “n.s.” (not significant). Error bars in (b) and (c) indicate one standard deviation. All experiments were repeated at least twice.
We also quantified the diameter of the endosomes because large endosome sizes correlate with endosome maturation,38 and maturation is essential for TLR9-triggered macrophage activation.39, 40 The average diameters of the endosomes at 4 h were similar for both types of CpG-constructs (Figure 2c; p = 0.37). From 4 to 24 h, the average diameters of the endosomes with mixed-curvature constructs increased faster and ended up 30% larger (ca. 780 nm) than those treated with constant-curvature constructs (ca. 600 nm) after 24 h (Figure 2c). The larger endosomes containing mixed-curvature constructs suggest that these endosomes are more mature, which is beneficial for macrophage activation.
To identify the factors that control the formation of hollow endosomes, we tested the influence of: (1) multi-valent binding by CpG-constructs with varying surface-densities; (2) non-specific binding by GpC (a non-immune active) constructs; and (3) inhibiting TLR9 binding by CpG-constructs with added TLR9 antagonists. At high CpG-densities (250 CpG or 500 CpG / NP), mixed-curvature constructs produced a higher percentage of hollow endosomes (ca. 60%); at a lower CpG-density (150 CpG / NP), they produced a lower percentage (ca. 25%) of hollow endosomes (Figure 3a). Even when mixed-curvature constructs with high ODN surface-density (250 ODNs / NP) were applied, the non-specific and inhibited TLR9 bindings of these constructs yielded a low percentage of hollow endosomes (17% and 28%, respectively). For constant-curvature constructs, changes in CpG-density (400, 250, and 160 CpG / NP) did not drastically affect the percentage of hollow endosomes that were formed; less than 13% of hollow endosomes were formed in all cases (Figure S3). The smaller effect of CpG-surface density on the endosomal distribution of constant-curvature constructs is because positive curvature facilitates dense packing of ODNs that results in similar ODN presentations at various surface densities.41, 42
Figure 3. Formation of hollow endosomes requires specific and strong multi-valent binding between mixed-curvature constructs and TLR9 proteins.
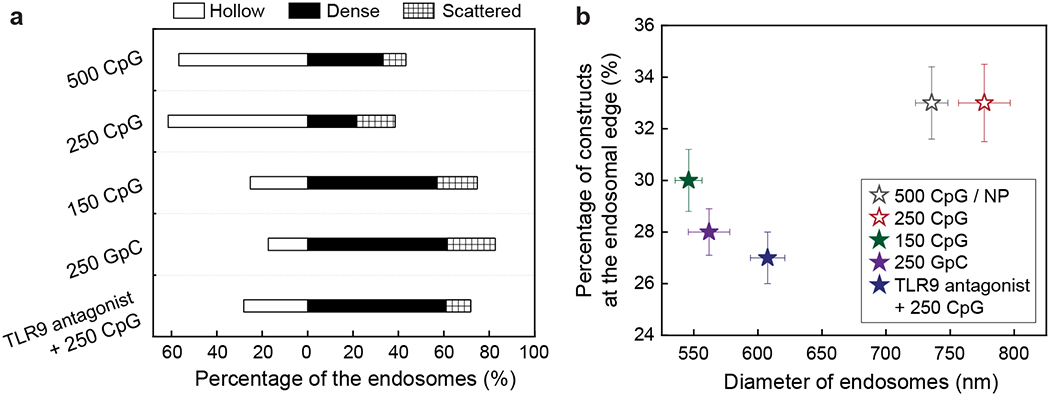
(a) Endosomal distributions of CpG-constructs were quantified after 24 h incubation under various conditions. The number in each row represents the number of ODNs per nanoparticle. (b) The percentage of constructs at the endosomal edge was plotted against the diameter of endosomes ([CpG] = 200 nM, 24 h incubation). Error bars represent standard error of the mean. All experiments were repeated twice.
Figure 3b indicates how both the endosomal distribution of CpG-constructs and endosome sizes are affected by binding between CpG-constructs and TLR9 proteins. Endosomes containing mixed-curvature constructs with higher CpG-densities (500 CpG / NP and 250 CpG / NP; open symbols) displayed a higher percentage of constructs along the endosomal edge (ca. 33%) as well as a larger average diameter (ca. 750 nm). In contrast, endosomes containing mixed-curvature constructs with lower CpG-density (150 CpG / NP), non-specific GpC sequence, or added TLR9 antagonists displayed a lower percentage of constructs along the endosomal edge (< 30%) and a smaller average diameter (< 650 nm) (Figure 3b; solid symbols). These results imply that the formation of hollow endosomes requires specific and strong multi-valent binding between the CpG-constructs and TLR9.
Because binding of CpG to TLR9 promotes an IS response,43 we hypothesized that macrophage populations with a higher proportion of hollow endosomes would show enhanced IS activity. To test our hypothesis, we characterized alkaline phosphatase (AP) and cytokine secretions from macrophages treated with constructs having different surface curvatures and CpG-densities. AP and cytokine secretion levels represent the way macrophages communicate to clear pathogens28, 29 or to induce anti-cancer immunity30 and provide a quantitative readout of IS activity. We observed that mixed-curvature constructs resulted in significantly higher AP secretion levels (ca. 1.0) at higher CpG-densities (500 CpG and 250 CpG / NP) but a lower level (ca. 0.4) at a lower CpG-density (150 CpG / NP) (Figure 4a). Constant-curvature constructs resulted in intermediate AP secretion levels (ca. 0.7) with only small changes observed at different CpG-densities (400, 250, and 160 CpG / NP). These data are consistent with previous work where IS activities of constant-curvature constructs saturated at lower CpG densities (5% CpG in a mixture of CpG and GpC).36
Figure 4. CpG-constructs that form a high percentage of hollow endosomes also result in enhanced IS activity.
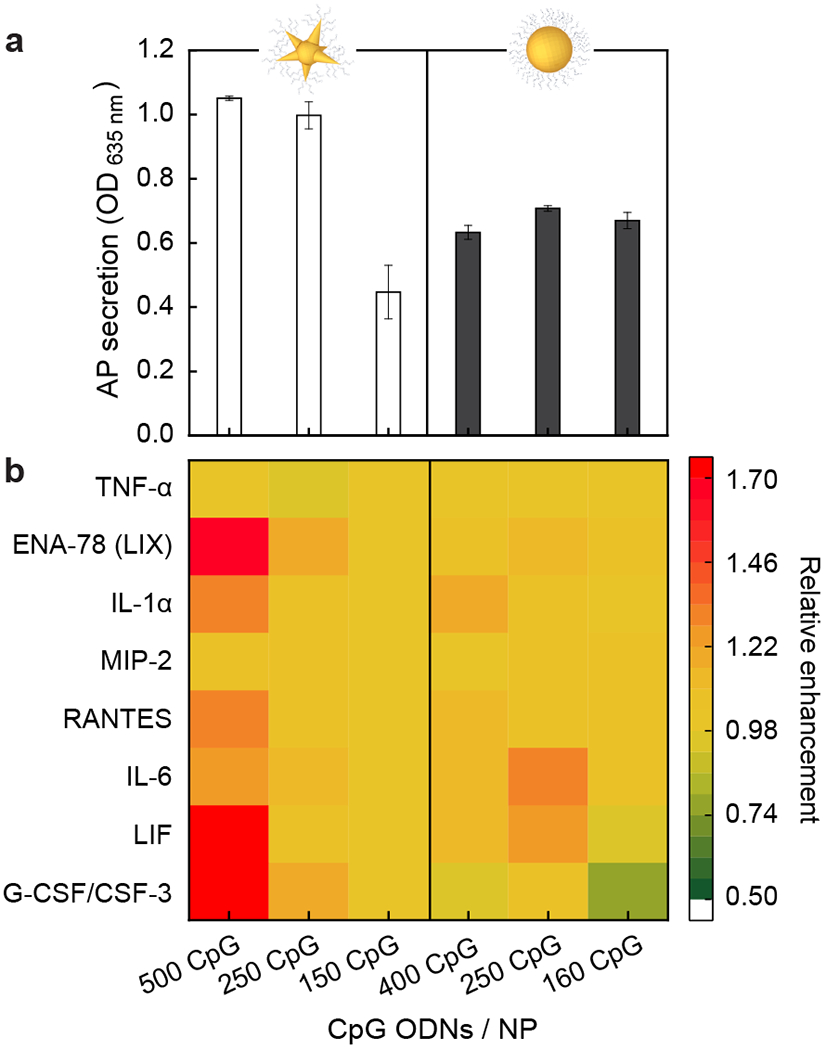
(a) Alkaline phosphatase (AP) and (b) cytokine secretion of macrophages after a 24 h treatment with CpG-constructs at varied CpG densities and surface curvatures. The results indicate that CpG-density changes the IS activity of constructs with mixed curvatures, but not with constant curvature. Error bars were from six independent experiments. ([CpG] = 200 nM, 24 h incubation)
We also measured cytokine secretion with different constructs and estimated the relative enhancement compared to mixed-curvature constructs at 150 CpG / NP, where the AP secretion level was the lowest. Figure 4b shows that only mixed-curvature constructs at 500 CpG / NP resulted in a significant relative enhancement of proinflammatory cytokines (2 times for G-CSF, 1.3 times for IL-6, and 1.3 times for IL-1α), anti-inflammatory cytokines (1.9 times for LIF), chemokines (1.3 times for RANTES), and ENA-78, but no enhancements of TNF-α and MIP-2. Mixed-curvature constructs at 250 CpG / NP resulted in small enhancements of G-CSF and ENA-78. Enhanced levels of proinflammatory cytokines and chemokines suggest stronger IS responses. We note enhanced secretion of an anti-inflammatory cytokine (LIF) at 500 CpG / NP, but LIF has only a minor effect on the IS response because the amount (ca. 50 pg / mL) was much smaller than other proinflammatory cytokines (> 1000 pg / mL) (Figure S4).44 Constant-curvature constructs showed small changes in cytokine levels at different CpG densities. Our results indicate that cytokine secretion levels are enhanced only with mixed-curvature constructs at higher CpG densities, which is consistent with AP secretion levels (Figure 4a). A higher CpG-density threshold was observed for enhanced cytokine secretions (500 CpG / NP) compared to AP secretion (250 CpG / NP) because of their different pathways.39 Taken together, mixed-curvature constructs resulted in the formation of hollow endosomes, and the location of the constructs facilitated more efficient access to TLR9, which increased IS activity.
In summary, we identified NP surface curvature as a key structural feature that determines the endosomal organization of CpG-constructs as well as the in vitro IS responses of macrophages. Mixed-curvature constructs resulted in a higher percentage of hollow endosomes and a higher IS response at higher CpG-densities (> 250 CpG / NP). Constant-curvature constructs resulted in a lower percentage of hollow endosomes and a lower IS response at all tested CpG-densities. One disadvantage of the mixed-curvature structures is their preparation, which requires an additional synthetic step. Nevertheless, our results highlight that such structures offer a means to control their local organization inside endosomes, which can improve immune cell responses and intracellular delivery. Both the local chemical environment and ODN presentation contribute to the endosomal distribution of CpG-constructs. In future work, we aim to understand how these effects influence the accumulation process and final nanoconstruct organization.
Supplementary Material
Acknowledgments.
This material is based on research sponsored by the National Cancer Institute of the National Institutes of Health under Award Number U54CA199091 (K.L., T.W.O., Z.N.H., C.A.M) and R01CA208783 (Z.N.H., C.A.M). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project was also supported by the Prostate Cancer Foundation and the Movember Foundation under award 17CHAL08 (Z.N.H., C.A.M). We thank Charlene Wilke for processing TEM samples. Z.N.H. acknowledges support by the Northwestern University Graduate School Cluster in Biotechnology, Systems, and Synthetic Biology, which is affiliated with the Biotechnology Training Program funded by NIGMS grant T32 GM008449. Fluorescence and absorbance measurements were carried out in the High Throughput Analysis Laboratory. TEM imaging was performed at the Biological Imaging Facility. Gold analysis was conducted at the Northwestern University Quantitative Bio-elemental Imaging Center generously supported by NASA Ames Research Center NNA06CB93G. Proteomics services were performed by the Northwestern Proteomics Core Facility, generously supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569.
Footnotes
Supporting Information Available: Experimental methods; characterization and protein corona profiles of CpG-constructs; TEM images of CpG-constructs in the endosomes when mixed-curvature and constant-curvature constructs were combined in a single treatment; characteristics of endosomes under various treatment conditions; cytokine produced by mixed-curvature constructs (150 CpG / NP). This materials is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Stewart MP; Sharei A; Ding XY; Sahay G; Langer R; Jensen KF, In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538 (7624), 183–192. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I; Coukos G; Dranoff G, Cancer immunotherapy comes of age. Nature 2011, 480 (7378), 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley RS; June CH; Langer R; Mitchell MJ, Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discovery 2019, 18 (3), 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SY; Qin L; Yamankurt G; Skakuj K; Huang ZY; Chen PC; Dominguez D; Lee A; Zhang B; Mirkin CA, Rational vaccinology with spherical nucleic acids. Proc. Natl. Acad. Sci. USA 2019, 116 (21), 10473–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirkin CA; Letsinger RL; Mucic RC; Storhoff JJ, A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382 (6592), 607–609. [DOI] [PubMed] [Google Scholar]
- 6.Albanese A; Tang PS; Chan WC, The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng 2012, 14, 1–16. [DOI] [PubMed] [Google Scholar]
- 7.Jain PK; Huang XH; El-Sayed IH; El-Sayed MA, Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res 2008, 41 (12), 1578–1586. [DOI] [PubMed] [Google Scholar]
- 8.Choi CHJ; Alabi CA; Webster P; Davis ME, Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107 (3), 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne JD; Betancourt T; Brannon-Peppas L, Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliver. Rev 2008, 60 (15), 1615–1626. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME; Chen Z; Shin DM, Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discovery 2008, 7 (9), 771–782. [DOI] [PubMed] [Google Scholar]
- 11.Dam DHM; Lee RC; Odom TW, Improved in Vitro Efficacy of Gold Nanoconstructs by Increased Loading of G-quadruplex Aptamer. Nano Lett. 2014, 14 (5), 2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H; Dam DH; Ha JW; Yue J; Odom TW, Enhanced Human Epidermal Growth Factor Receptor 2 Degradation in Breast Cancer Cells by Lysosome-Targeting Gold Nanoconstructs. ACS Nano 2015, 9 (10), 9859–9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasting C; Schalley CA; Weber M; Seitz O; Hecht S; Koksch B; Dernedde J; Graf C; Knapp EW; Haag R, Multivalency as a Chemical Organization and Action Principle. Angew. Chem., Int. Ed 2012, 51 (42), 10472–10498. [DOI] [PubMed] [Google Scholar]
- 14.Rosi NL; Giljohann DA; Thaxton CS; Lytton-Jean AKR; Han MS; Mirkin CA, Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 2006, 312 (5776), 1027–1030. [DOI] [PubMed] [Google Scholar]
- 15.Choi CHJ; Hao LL; Narayan SP; Auyeung E; Mirkin CA, Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc. Natl. Acad. Sci. USA 2013, 110 (19), 7625–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahue ND; Acar H; Wilhelm S, Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliver. Rev 2019, 143, 68–96. [DOI] [PubMed] [Google Scholar]
- 17.Yue J; Feliciano TJ; Li WL; Lee A; Odom TW, Gold Nanoparticle Size and Shape Effects on Cellular Uptake and Intracellular Distribution of siRNA Nanoconstructs. Bioconjugate Chem. 2017, 28 (6), 1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dam DH; Lee JH; Sisco PN; Co DT; Zhang M; Wasielewski MR; Odom TW, Direct observation of nanoparticle-cancer cell nucleus interactions. ACS Nano 2012, 6 (4), 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkachenko AG; Xie H; Coleman D; Glomm W; Ryan J; Anderson MF; Franzen S; Feldheim DL, Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc 2003, 125 (16), 4700–4701. [DOI] [PubMed] [Google Scholar]
- 20.Soenen SJ; Parak WJ; Rejman J; Manshian B, (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev 2015, 115 (5), 2109–2135. [DOI] [PubMed] [Google Scholar]
- 21.Moore TL; Rodriguez-Lorenzo L; Hirsch V; Balog S; Urban D; Jud C; Rothen-Rutishauser B; Lattuada M; Petri-Fink A, Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev 2015, 44 (17), 6287–6305. [DOI] [PubMed] [Google Scholar]
- 22.Spicer CD; Jumeaux C; Gupta B; Stevens MM, Peptide and protein nanoparticle conjugates: versatile platforms for biomedical applications. Chem. Soc. Rev 2018, 47 (10), 3574–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malcolm DW; Varghese JY; Sorrells JE; Ovitt CE; Benoit DSW, The Effects of Biological Fluids on Colloidal Stability and siRNA Delivery of a pH-Responsive Micellar Nanoparticle Delivery System. ACS Nano 2018, 12 (1), 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kneipp J; Kneipp H; McLaughlin M; Brown D; Kneipp K, In vivo molecular probing of cellular compartments with gold nanoparticles and nanoaggregates. Nano Lett. 2006, 6 (10), 2225–2231. [DOI] [PubMed] [Google Scholar]
- 25.Drescher D; Guttmann P; Buchner T; Werner S; Laube G; Hornemann A; Tarek B; Schneider G; Kneipp J, Specific biomolecule corona is associated with ring-shaped organization of silver nanoparticles in cells. Nanoscale 2013, 5 (19), 9193–9198. [DOI] [PubMed] [Google Scholar]
- 26.Chanana M; Gil PR; Correa-Duarte MA; Liz-Marzan LM; Parak WJ, Physicochemical Properties of Protein-Coated Gold Nanoparticles in Biological Fluids and Cells before and after Proteolytic Digestion. Angew. Chem., Int. Ed 2013, 52 (15), 4179–4183. [DOI] [PubMed] [Google Scholar]
- 27.Borkowska M; Siek M; Kolygina DV; Sobolev YI; Lach S; Kumar S; Cho YK; Kandere-Grzybowska K; Grzybowski BA, Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells. Nat. Nanotechnol 2020, 15, 331–341. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K; Akira S, Toll-like receptors in innate immunity. Int. Immunol 2005, 17 (1), 1–14. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi O; Akira S, Pattern Recognition Receptors and Inflammation. Cell 2010, 140 (6), 805–820. [DOI] [PubMed] [Google Scholar]
- 30.Arango Duque G; Descoteaux A, Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol 2014, 5, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohto U; Ishida H; Shibata T; Sato R; Miyake K; Shimizu T, Toll-like Receptor 9 Contains Two DNA Binding Sites that Function Cooperatively to Promote Receptor Dimerization and Activation. Immunity 2018, 48 (4), 649–658. [DOI] [PubMed] [Google Scholar]
- 32.Ohto U; Shibata T; Tanji H; Ishida H; Krayukhina E; Uchiyama S; Miyake K; Shimizu T, Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 2015, 520 (7549), 702–705. [DOI] [PubMed] [Google Scholar]
- 33.Radovic-Moreno AF; Chernyak N; Mader CC; Nallagatla S; Kang RS; Hao L; Walker DA; Halo TL; Merkel TJ; Rische CH; Anantatmula S; Burkhart M; Mirkin CA; Gryaznov SM, Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. U.S.A 2015, 112 (13), 3892–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yue J; Pallares RM; Cole LE; Coughlin EE; Mirkin CA; Lee A; Odom TW, Smaller CpG-Conjugated Gold Nanoconstructs Achieve Higher Targeting Specificity of Immune Activation. ACS Appl. Mater. Interfaces 2018, 10 (26), 21920–21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leleux JA; Pradhan P; Roy K, Biophysical Attributes of CpG Presentation Control TLR9 Signaling to Differentially Polarize Systemic Immune Responses. Cell Reports 2017, 18 (3), 700–710. [DOI] [PubMed] [Google Scholar]
- 36.Pallares RM; Choo P; Cole LE; Mirkin CA; Lee A; Odom TW, Manipulating Immune Activation of Macrophages by Tuning the Oligonucleotide Composition of Gold Nanoparticles. Bioconjugate Chem. 2019, 30 (7), 2032–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotz MW; Culver KSB; Parigi G; MacRenaris KW; Luchinat C; Odom TW; Meade TJ, High Relaxivity Gd(III) - DNA Gold Nanostars: Investigation of Shape Effects on Proton Relaxation. ACS Nano 2015, 9 (3), 3385–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rink J; Ghigo E; Kalaidzidis Y; Zerial M, Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122 (5), 735–749. [DOI] [PubMed] [Google Scholar]
- 39.Lee BL; Barton GM, Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014, 24 (6), 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacker H; Mischak H; Miethke T; Liptay S; Schmid R; Sparwasser T; Heeg K; Lipford GB; Wagner H, CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998, 17 (21), 6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eller MJ; Chandra K; Coughlin EE; Odom TW; Schweikert EA, Label Free Particle-by-Particle Quantification of DNA Loading on Sorted Gold Nanostars. Anal. Chem 2019, 91 (9), 5566–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones MR; Macfarlane RJ; Prigodich AE; Patel PC; Mirkin CA, Nanoparticle Shape Anisotropy Dictates the Collective Behavior of Surface-Bound Ligands. J. Am. Chem. Soc 2011, 133 (46), 18865–18869. [DOI] [PubMed] [Google Scholar]
- 43.Takeshita F; Gursel I; Ishii KJ; Suzuki K; Gursel M; Klinman DM, Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin. Immunol 2004, 16 (1), 17–22. [DOI] [PubMed] [Google Scholar]
- 44.Liu SC; Tsang NM; Chiang WC; Chang KP; Hsueh C; Liang Y; Juang JL; Chow KP; Chang YS, Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J. Clin. Invest 2013, 123 (12), 5269–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


