Abstract
To evaluate all simulation models for ophthalmology technical and non-technical skills training and the strength of evidence to support their validity and effectiveness. A systematic search was performed using PubMed and Embase for studies published from inception to 01/07/2019. Studies were analysed according to the training modality: virtual reality; wet-lab; dry-lab models; e-learning. The educational impact of studies was evaluated using Messick’s validity framework and McGaghie’s model of translational outcomes for evaluating effectiveness. One hundred and thirty-one studies were included in this review, with 93 different simulators described. Fifty-three studies were based on virtual reality tools; 47 on wet-lab models; 26 on dry-lab models; 5 on e-learning. Only two studies provided evidence for all five sources of validity assessment. Models with the strongest validity evidence were the Eyesi Surgical, Eyesi Direct Ophthalmoscope and Eye Surgical Skills Assessment Test. Effectiveness ratings for simulator models were mostly limited to level 2 (contained effects) with the exception of the Sophocle vitreoretinal surgery simulator, which was shown at level 3 (downstream effects), and the Eyesi at level 5 (target effects) for cataract surgery. A wide range of models have been described but only the Eyesi has undergone comprehensive investigation. The main weakness is in the poor quality of study design, with a predominance of descriptive reports showing limited validity evidence and few studies investigating the effects of simulation training on patient outcomes. More robust research is needed to enable effective implementation of simulation tools into current training curriculums.
Subject terms: Health care, Education, Medical research, Health occupations
摘要
本文旨在评估所有眼科技术与非技术仿真训练设备的优点, 以找到支持其有效性的证据。使用PubMed和Embase从开始研究至2019年7月1日进行文献检索。研究根据训练模式进行分类: 虚拟现实、实际操作实验室、教学实验室以及跟随电子设备学习。使用Messick有效性框架以及转化医学McGaghie模型评估其有效性。本文共纳入131项研究, 涉及 93种不同的模拟训练设备, 其中53项研究基于虚拟现实设备、47项基于实际操作的实验室模型、26项基于教学实验室模型和5项基于电子设备学习。只有2项研究涉及所有5种模拟设备。最有效的模型有Eyesi眼外科系统、Eyesi直接检眼镜和眼外科技能评估测试系统 (Eye Surgical Skills Assessment Test, ESSAT)。模拟训练设备刺激模型的有效性等级大多限制在2级 (包含有效), Sophocle玻璃体视网膜手术模拟设备显示为3级 (下游效应), 以及白内障手术5级 (靶向效应) 。本文针对大多数模型进行了阐述, 对Eyesi进行了深入探讨。本文的局限性在于纳入的研究实验设计质量差, 描述性报告显示有效性证据有限, 很少有研究调查模拟训练对患者结局的影响。因此需要更有力的研究证据, 以便在现有培训课程中有效地应用仿真训练设备。
Introduction
Historically training in ophthalmology, as in other surgical specialties, has been based on a Halstedian model of apprenticeship learning. Trainees are assumed to be competent upon completing a minimum number of surgical procedures. Changes to the clinical environment and professional values have forced a review of this approach [1]. One of the problems associated with this model is the inconsistency in levels of knowledge and skills gained due to variations in clinical exposure and educational opportunities [2]. Using the total number of procedures that a trainee has performed as a benchmark for skill is also problematic as quantity does not equate to quality and competency cannot be accurately discerned in this way. Reductions in training hours due to regulations such as the European Working Time Directive further limit potential training opportunities [3]. Furthermore, growing ethical concerns over the use of patients for training purposes [4] are also having major impacts on training particularly in the early stages of the learning curve. Studies have shown close correlation between experience and complication rate [5, 6].
These issues highlight the need for improved training programmes with the development and objective assessment of proficiency prior to treating patients. Simulation models offer a platform for trainees to improve their clinical and surgical skills, enabling focussed, competency-based training without putting patients at risk. The healthcare sector is continually making rapid technological advances and the development of simulator models as safe and effective tools for training and assessment has risen dramatically. This trend has been observed within the field of ophthalmology [7], but the extent to which simulation is used varies widely between different training programmes. Its role remains limited by a lack of formal, standardised integration into existing curricula.
The purpose of this systematic review is to comprehensively evaluate the effectiveness and validity of all simulator models developed for ophthalmic training to date.
Methods
This review was carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement and registered on the international prospective register of systematic reviews, “PROSPERO”, prior to conduction of this study (registration number: CRD42018087929).
Eligibility criteria
All original studies were included if they described simulation or e-learning for technical or non-technical skills development in ophthalmic training. Inclusion criteria for study participants were ophthalmologists of any grade and medical students who had completed or were completing their ophthalmology attachment. Studies were excluded if they did not provide original data; articles not specific to ophthalmology; and studies that did not use simulation for training or assessment purposes. We included all papers irrespective of the language.
Search methods
A systematic search of PubMed and Embase was carried out, using the terms “(simulat* OR virtual reality OR wet lab OR cadaver OR model OR e-learning) AND ophthalm* AND (training OR programme OR course)”. Search date was from inception to 01/07/2019. Reference lists from included articles and relevant reviews were hand searched for eligible studies.
Study selection
Two authors, RL and WYL, carried out independent, duplicate searches. All abstracts were reviewed and articles that were potentially eligible were read in full. A final list of studies meeting the eligibility criteria was compared and disagreements resolved by discussion (Fig. 1).
Fig. 1. PRISMA Flow Diagram.
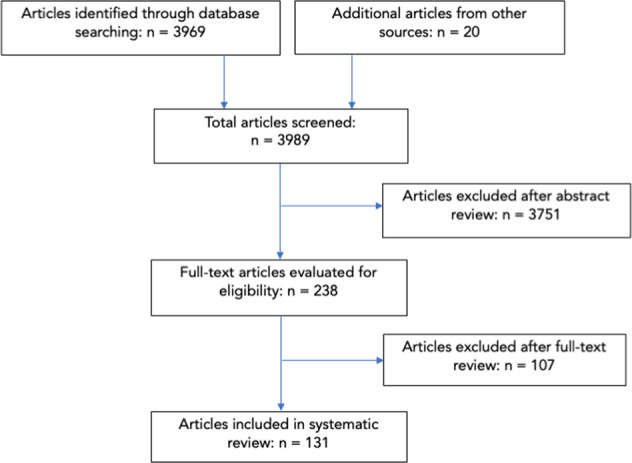
Flow diagram of study selection process.
Data collection
The same two authors extracted data for each study separately and differences were resolved through discussion. Data collected included details of the simulator model, type of study design, number of participants and their training level, training task(s) involved, duration of training, and outcome data addressing validity and effectiveness of the model.
Data analysis
Studies were grouped according to simulator type: virtual reality; wet lab (live or cadaveric animal models and human cadaveric models); dry lab (synthetic models); and e-learning models. Validity was evaluated based on Messick’s modern validity framework [8] and the strength of each source of validity evidence was measured using a validated rating scale [9]. Effectiveness was quantified using an adaptation of McGaghie’s proposed levels of simulation-based translational outcomes (Table 1) [10]. Qualitative analysis was carried out due to the heterogeneity of study designs.
Table 1.
Details of the frameworks used for evaluation of validity and educational impact.
| Framework | Parameter | Definition | Examples | Rating |
|---|---|---|---|---|
| Modern concept of validity—Messick | Content | Test items are relevant and representative of the intended construct | Using expert opinions to ensure all domains are accurately represented |
N = no discussion of source of validity evidence 0 = discussion of source of validity but no data presented 1 = data weakly supports source of validity or is limited 2 = data strongly supports source of validity |
| Response processes | Thought processes and actions of subjects and observers are made in accordance with the intended construct | Quality control of assessments, such as in standardising test administration and minimising examiner bias | ||
| Internal structure | Test scores across tasks can be reliably reproduced | Calculating inter-item reliability and test-retest reliability | ||
| Relations to other variables | Test scores correlate with external, independent measures which share a theoretical relationship | Comparing scores between groups with different levels of experience in the tested skill | ||
| Consequences | The impact of using the assessment | Determining the pass-fail score and considerations for the subject on obtaining a pass or fail | ||
| Translational outcomes of simulation-based learning (adapted)—McGaghie et al. | Internal acceptability | The trainee’s satisfaction with using the simulator | Favourable responses from feedback forms or post-training survey questionnaires | Level 1 |
| Contained effects | Changes in performance in the simulation context | Development of knowledge and/or skills as measured by the simulator tool | Level 2 | |
| Downstream effects | Behavioural changes in the clinical context | Transfer of knowledge/skills to clinical practice | Level 3 | |
| Target effects | Direct changes to patient outcomes | Reduced rates of surgical complications | Level 4 | |
| Collateral effects | Changes on a wider, systemic level | Cost saving; skill retention | Level 5 |
Results
A total of 3989 articles were screened, of which 3751 were excluded following abstract review. After reading the remaining 238 articles in full, a further 107 were excluded. A total of 131 original articles were included in this systematic review (Fig. 1). Details of findings are summarised in Tables 2–5 according to simulator type.
Table 3.
Wet-lab studies.
| Model | Description | Reference | Area of training | Training task | Study design | Participants | Training time | Validity | Effectiveness |
|---|---|---|---|---|---|---|---|---|---|
| Rabbit eyes + human cataracts | Human cataract removed in its capsule and implanted into a rabbit eye | Tolentino and Liu [79] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eye | External tissue of a post-mortem porcine eye removed then placed in a microwave oven to induce cataract | van Vreeswijk and Pameyer [80] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Pig eyes filled with cooked chestnuts of varying hardness as pseudonuclei | Mekada et al. [71] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 1 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Pig eyes | A range of formalin: alcohol ratios tested on pig eyes to simulate human lens | Sugiura et al. [78] | Cataract surgery | Not specified | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Goat eyes | Goat eyes injected with formalin and fixed on a stand | Dada and Sindhu [65] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Post-mortem pig eye injected with formalin and hydroxyethylcellulose to induce cataract | Hashimoto et al. [67] | Cataract surgery | Capsulorhexis | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Goat eyes | Goat eyes injected with formalin through the pars plana before capsulorhexis vs through a clear corneal side port into the nucleus after capsulorhexis | Sudan et al. [77] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Anterior chamber of pig eyes filled 75% with methylcellulose then injected with a formaldehyde-methanol solution to induce cataract | Saraiva and Casanova [75] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes + electronic sensor | Cup supporting an ex vivo human or porcine eye mounted on a 6 axis/torque sensor which detects direction and magnitude of forced applied by trainee | Leuschke et al. [69] | Cataract surgery | Not specified | Descriptive | n = 2 (ophthalmic surgeons) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Rabbit eyes | Lens from enucleated rabbit eyes fixed with varying concentrations of paraformaldehyde | Ruggiero et al. [74] | Cataract surgery | Capsulorhexis | Experimental | n = 6 (cataract surgeons) | Not specified |
Content: 2 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Goat eyes + human lens | Human cataractous nuclear implanted into a goat lens and mounted on rectangular polystyrene | Sengupta et al. [76] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Assessment of wet-lab performance using a modified surgical assessment tool (ICO- OSCAR) | Farooqui et al. [66] | Cataract surgery | Phacoemulsification | Pilot study | n = 12 (3rd year residents) | 5 days |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes | Post-mortem human eyes with Karnovsky solution to induce cataract | Pandey et al. [72] | Cataract surgery | Not specified | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes | Medical lubricating jelly injected into in a human cadaver eye | Liu et al. [70] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Sheep + human lens | Human cataractous lens nucleus implanted in a sheep eye lens | Kayikcioglu et al. [68] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Survey of wet-lab training with pig eyes on residents’ perceived preparedness and difficulty with cataract surgery | Puri et al. [73] | Cataract surgery | Unspecified | Retrospective cross-sectional study | n = 116 (residents) | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Rabbit eye | Rabbit eye used as a replacement for human eye | Abrams et al. [81] | Vitreoretinal surgery | Pars plana vitrectomy | Descriptive | N/A | 2 h |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human/artificial eyes + Marty the Surgical Simulator | 3 model systems: artificial eye with a plastic head model; human cadaver eye with the head model; and human cadaver eye without the model | Patel and Levin [84] | Glaucoma surgery | Goniotomy | Case series study | n = 4 (paediatric ophthalmology fellows) | 2 h |
Content: 0 Response processes: 2 Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Pig eyes | Pig eyes soaked in 10% formaldehyde then mounted on a dummy head | Lee et al. [83] | Glaucoma surgery | Trabeculectomy | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes | Human donor cadaveric eyes with contact lens inserted into a surgical model mannequin head | Patel and Sit [85] | Glaucoma surgery | Trabeculectomy | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes + artificial orbit | Enucleated pig eyes placed into the orbit of a styrofoam model head; microsphere-based canalograms used to measure extent of outflow tract access | Dang et al. [82] | Glaucoma surgery (microincisional) | Ab-interno trabeculectomy (trabectome) | Case series | n = 7 (ophthalmology trainees) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Human eyes | Human cadaveric corneoscleral rims used for angle surgery simulation | Arora et al. [86] | Glaucoma surgery | Microinvasive glaucoma surgery | Descriptive study | N/A | N/A |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes | Human cadaver corneoscleral rims fixated with a tack through the centre of the cornea to a styrofoam base | Nazarali et al. [87] | Glaucoma surgery | Bimanual skills with gonioscopy, microbypass stent insertion + removal, gonioscopy-assisted transluminal trabeculotomy | Experimental, feasibility study | n = 10 (ophthalmic residents) | Not specified |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
1 |
| Human eye + artificial anterior chamber | Human donor corneoscleral button placed over an artificial anterior chamber | Fontana et al. [89] | Corneal surgery | Deep Anterior Lamellar Keratoplasty using the big-bubble technique | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Pseudo-grafts created from lens capsule of enucleated porcine eyes and implanted into an intact globe | Droutsas et al. [88] | Corneal surgery | Descemet Membrane Endothelial Keratoplasty (DMEK) | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human cornea + artificial anterior chamber | One human cornea for donor graft preparation + one for practising graft insertion and unfolding in an artificial anterior chamber model | Vasquez Perez and Liu [90] | Corneal surgery | Descemet membrane endothelial keratoplasty (DMEK) | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes + artificial anterior chamber | Human corneas mounted on an artificial anterior chamber with a 3D-printed iris. Intraoperative OCT used to validate each step of the procedure. | Famery et al. [91] | Corneal surgery | Descemet membrane endothelial keratoplasty | Prospective, feasibility study | n = 5 (ophthalmic surgeons) | 2 sessions (duration unspecified) |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: 2 Consequences: N |
1 |
| Pig eyes | Cadaveric pig eyes with bacon as extraocular muscles | White et al. [92] | Strabismus surgery | Steps for strabismus surgery | Case series | 30 Residents | Not specified |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Pig eyes + chicken breast model | Wet-lab session using a chicken breast model for practice, followed by pig eyes | Vagge et al. [93] | Strabismus surgery | Partial-thickness scleral suture passes | Prospective cohort pilot study | n = 12 (8 first year and 4 second year residents) | 2 h |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Pig eyelid | A rubber ball used to simulate the globe; a board with 4 metal screws mimicking the canthal tendons and arcus marginalis. Corners of a pig eyelid then sutured to the screws. | Pfaff [95] | Oculoplastic surgery | Eyelid margin repair | Descriptive | Oculoplastic staff and fellow, residents (numbers not specified) | Not specified |
Content: 2 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig head | Pig head split in half and rested on a surface for practising lid procedures | Kersey [94] | Oculoplastic surgery | Unspecified | Descriptive | Ophthalmologists of varying grades (numbers not specified) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Pig eyelids | Pig eyelids with surgically induced ptosis | Zou et al. [96] | Oculoplastic surgery | Ptosis repair | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes | Lecture on lateral cantholysis followed by video-demonstration, live demonstration on human cadaver eyes by an oculoplastic surgeon and practice on the same eyes | Patel et al. [97] | Oculoplastic surgery | Lateral cantholysis | Prospective study + survey | n = 12 (residents) | Not specified |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
2 |
| Sheep cranium | Intracranial and ocular dissection of 1-week-old sheep cranium | Altunrende et al. [98] | Orbital surgery | Micro-surgical skills | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes | Iron particles placed on cadaver cornea for rust ring formation before mounting on slit lamps. Removal of rust ring photographed and analysed using open-source computer software programme | Mednick et al. [99] | Ocular trauma | Corneal rust ring removal | Prospective | n = 22 (8 medical students, 10 residents, 4 attending ophthalmologists) | Not specified |
Content: 1 Response processes: N Internal structure: N Relations to other variables: 0 Consequences: N |
1 |
| Goat eyes + artificial model head | Enucleated goats’ eyes are mounted on a model head. An incision is made using a scalpel along the corneoscleral limbus, simulating a full-thickness laceration. | Pujari et al. [100] | Ocular trauma | Corneoscleral perforation repair | Descriptive study | N/A | N/A |
Content: 0 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes + artificial orbit | Enucleated porcine eyes placed inside a metal orbit created using an adjustable eye support, cylinder and removable ring | Uhlig and Gerding [101] | Diagnostic examination | Direct and indirect ophthalmoscopy; gonioscopy | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes + formalin | Human autopsy eyes with cornea cleared with hyperosmotic dextran solution and fixed with formalin | Auffarth et al. [102] | General Ophthalmic Surgery | Not specified | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes + contact lens | Cyanoacrylate glue used to secure polymethylmethacrylate contact lens to the corneal rim of cadaver eyes | Lenart et al. [103] | General Ophthalmic Surgery | Not specified | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eyes with keratoprosthesis | Lander wide-field keratoprosthesis placed over cadaver eyes | Borirak-chanyavat et al. [104] | Anterior and posterior segment surgeries | Phacoemulsification, vitrectomy, panretinal laser | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Human eye + Spring-action Apparatus for Fixation of Eyeball (SAFE) | Hollow iron cylinder attached to a spring-action syringe forms a vacuum for fixation of human/animal cadaveric eyes | Ramakrishnan et al. [105] | Anterior and posterior segment surgeries | Various procedures (e.g., phacoemulsification, MSICS, LASIK, DALK, DSEK and trabeculectomy) | Descriptive | n = 2 (ophthalmic surgeons) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Sheep eyes | Sheep eyes mounted on an artificial orbit | Mohammadi et al. [106] | Anterior segment surgery | Range of anterior segment procedures (e.g., capsulorhexis; keratoplasty; trabeculectomy) | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes | Porcine eyes placed in an ocular bulb holder that is secured to a polyvinylchloride pillar on a modified polystyrene head | Porrello et al. [107] | Anterior and posterior segment procedures | Laser iridotomy, photocoagulation and all steps of cataract surgery | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes + SS Microscope-Integrated OCT (MI-OCT) | Real time 3D imaging to aid wet-lab microsurgery training | Todorich et al. [108] | Anterior segment surgery | Corneal suture passes and laceration repair | Randomised controlled study (with crossover) | n = 14 (6 first year, 4 second year and 4 third year residents) | Not specified |
Content: N Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Pig eyes | Micro-surgical skills course using pig eye models and a video-based scoring system for assessment | Ezra et al. [109] | General ophthalmic surgery | Micro-surgical skills | Prospective longitudinal cohort study | n = 14 (residents) | 1 day |
Content: N Response processes: N Internal structure: 2 Relations to other variables: 2 Consequences: N |
2 |
| Pig eyes and foot (ESSAT) | 3-station wet-lab course: pig’s foot inserted with red plastic tubing to simulate temporal artery biopsy; pig eyes for muscle recession; pig eyes for cataract procedures. | Fisher et al. [110] | Ophthalmic surgery (a range of different areas) | Temporal artery biopsy, muscle resection and phacoemulsification | Survey | n = 22 (content experts: residency programme directors and faculty members) | N/A |
Content: 2 Response processes: 1 Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pig eyes and foot (ESSAT) | Same as above | Taylor et al. [111] | Ophthalmic surgery (a range of different areas) | Temporal artery biopsy, muscle resection and phacoemulsification | Masked, prospective study | n = 29 (1 first year resident, 1 third year resident and 27 content experts) | N/A |
Content: N Response processes: N Internal structure: 2 Relations to other variables: 2 Consequences: 2 |
N/A |
Table 4.
Dry-lab studies.
| Model | Description | Reference | Area of training | Training task | Study design | Participants | Training time | Validity evidence | Effectiveness |
|---|---|---|---|---|---|---|---|---|---|
| Aluminium foil with methacrylate support | Methacrylate for hand support, PVC sheet and aluminium foil for performing capsulorhexis on | Abellan et al. [112] | Cataract surgery | Capsulorhexis | Randomised controlled trial | n = 65 (ophthalmologists) | 2 h |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Japanese quail eggs | Sharp end of a quail egg cut and fitted with a silicone sclerocorneal cap; the yolk and albumen simulate the vitreous body and the inner eggshell membrane simulates the internal limiting membrane | Hirata et al. [113] | Vitreoretinal surgery | Membrane peeling | Case series | n = 8 (3 experienced vitreous surgeons and 5 inexperienced surgeons) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| VitRet eye with fluid | An artificial eye model filled with vitreous-like fluid made of dairy creamer + balanced saline | Yeh et al. [114] | Vitreoretinal surgery | Three-port vitrectomy setup; intraocular tasks (e.g., core vitrectomy and membrane peel); wound closure | Case series | n = 13 (8 residents and 5 fellows) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
1 |
| Artificial orbit with diascleral illumination | Eye support made from transparent polymethylmethacrylate, fitted onto cylinder and fixed with a metal ring | Uhlig and Gerding [115] | Vitreoretinal surgery | Not specified | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Reusable rubber eye | Rubber globe with removable plastic anterior segment for access to posterior segment; rubber bands used to simulate the rectus muscles and a coat of liquid skin bandage applied to simulate the membrane | Iyer and Han [116] | Vitreoretinal surgery | Epiretinal membrane peeling | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Medium-fidelity model | Wooden frame to simulate patient’s forehead; a table tennis ball to simulate the globe; pre-equatorial holes created light source insertions; tasks performed using real instruments and foot-pedal-controlled microscope | Rice et al. [117] | Vitreoretinal surgery | Sets of exercises including training single hand and bimanual dexterity | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| ILM peeling simulator | Artificial eye model placed in an ocular surgery simulator and an artificial ILM made using a PVA hydrogel. ILM peeling can be simulated under water. | Omata et al. [118] | Vitreoretinal surgery | Inner limited membrane (ILM) peeling | Descriptive study | N/A | N/A |
Content: 0 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
N/A |
| Nonbiologic Strabismus Surgery Simulator | Components include a rubber ball mounted to on a wooden based simulate the globe. An elastic band is attached to the eyeball, simulating the rectus muscle and a small piece of latex is attached to the eyeball with a thumbtack to simulate the conjunctiva and cornea. | Adebayo et al. [119] | Strabismus surgery | Steps for strabismus surgery | Randomised controlled trial | n = 41 (1st and 2nd year medical students) | 1 week |
Content: 1 Response Process: 2 Internal Structure: 2 Relations to other variables: N Consequences: N |
2 |
| Simulator for practising laser procedures | A model eye with artificial tissues | Simpson et al. [122] | Laser procedures | Peripheral iridotomy, posterior capsulotomy and laser retinopexy | Case series | n = 13 (6 inexperienced and 7 experienced residents) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
N/A |
| Capsulotomy simulator | An adjustable artificial anterior chamber for fitting laser instrument; an intraocular lens coated with a crust at the posterior surface to simulate posterior capsule opacification | Moisseiev and Michaeli [121] | Laser procedures | Neodymium: YAG posterior capsulotomy | Descriptive | n = 3 (residents) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
2 |
| RETILAPP eye model | A ping pong ball simulating the globe is cut in half; a paper diagram of the lesion is cut out and placed between the two hemispheres of the globe; the eye is clamped to a slit lamp; contact lens is used over the model for practising lasers | Ganne et al. [120] | Laser procedures | Retinal laser photocoagulation | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| MIRA practice eye | Model eye stabilised onto a wooden mount; the optic nerve and fundal pattern are painted onto the globe for orientation | Weidenthal [123] | Laser procedures | Laser photocoagulation | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Child skull model | Anatomically correct model of the nasolacrimal duct drainage | Coats [124] | Oculoplastic surgery | Not specified | Descriptive | N/A | N/A |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| 3D-printed orbit models | Use of 3D printing to produce orbit models that replicate a patient’s bony anatomy for use in orbital surgical training | Scawn et al. [126] | Orbital surgery | Orbital decompression | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| 3D-printed copies of human cadaveric orbital dissections | Surface mesh of orbit prosections created, processed using 3D laser scan, then printed | Adams et al. [125] | Orbital surgery | Not specified | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Newport eye corneal foreign body training phantom | A polyvinyl and gelatine-based model with resin used to secure a craft eye inside a plastic container; ground black pepper used to simulate a foreign body | Marson and Sutton [127] | Ocular Trauma | Corneal foreign body removal | Case series | n = 23 (6 ophthalmologists, 11 ED physicians and 2 ophthalmology + 4 ED nurse practitioners) | N/A |
Content: 1 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
1 |
| EYE Exam Simulator (Kyoto Kagaku Co.) | A mannequin head with adjustable pupil sizes and a holder to place slides showing different retinal conditions; a standard ophthalmoscope is used to simulate fundoscopy examination | McCarthy et al. [132] | Diagnostic examination | Direct ophthalmoscopy | Case series | n = 43 (32 emergency medicine and 11 ophthalmology residents) | N/A |
Content: N Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| EYE Exam Simulator (Kyoto Kagaku Co.) | Same as above | Akaishi et al. [128] | Diagnostic examination | Direct ophthalmoscopy | Cross-sectional | n = 73 (3 medical students, 41 residents, 29 attending physicians) | Not specified |
Content: N Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
N/A |
| Toy model eyes | Toy eyes cut around the pupil edge and everted; partial-thickness cuts made to simulate retinal tears; eye re-inverted and mounted on a wooden base; a 90-dioptre lens is mounted in the pupil and fixed with tape | Chew and Gray [129] | Diagnostic examination | Indirect ophthalmoscopy with scleral indentation | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Rubber ball eye | Eye made from rubber ball is cut in half and retinal details drawn on a painted orange background before sticking the 2 halves together; eyeball is inserted into a paperpulp head model | Kumar and Shetty [130] | Diagnostic examination | Indirect ophthalmoscopy | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Glass vial | Screw-top glass vial filled with mouthwash and face powder to simulate presence of cells and flare in the anterior chamber; holding the vial at different angles and positions in front of a slit lamp simulates appearance of an optical section and variations in thickness of the cornea | Morris [134] | Diagnostic examination | Slit-lamp examination | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Mannequin head model | Vacuum tubes with glue applied to the curved ends inserted into styrofoam mannequins to imitate slit lamp appearance of the anterior segment, flare and cells, hypopyon, hyphema, red reflex, cataract and corneal epithelial defects | Romanchuk [135] | Diagnostic examination | Slit-lamp examination | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Glass eyeball | A glass marble eye set onto a small bottle cap for stabilisation; piece of paper with letters placed behind the marble to assess visualisation; a hole punched in a separate piece of paper to simulate the pupil | Lewallen [131] | Diagnostic examination | Indirect ophthalmoscopy | Descriptive | N/A | N/A |
Content: N Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Origami model | A sheet of letter paper with a retinal drawing or photograph on one side is folded into a box with a small aperture that acts as a pupil | Miller [133] | Diagnostic examination | Binocular indirect ophthalmoscopy | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Model for simulating indirect ophthalmoscopy and retinal photocoagulation | Model consists of a 60D lens, a bulb syringe to simulate the globe, card paper for the iris and a printed fundus photograph attached to the base | Kylstra and Diaz [136] | Diagnostic examination and laser procedures | Binocular indirect ophthalmoscopy and indirect laser retinal photocoagulation | Descriptive study | N/A | N/A |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
N/A |
Table 2.
Virtual reality studies.
| Model | Description | Reference | Area of training | Training task | Study design | Participants | Training time | Validity | Effectiveness |
|---|---|---|---|---|---|---|---|---|---|
| Eyesi Surgical |
Hardware: mannequin head; artificial eye with CCD camera; operative microscope; set of surgical instruments; foot pedals; touchscreen Software: VR platform; cataract and vitreoretinal modules; storage of performance metrics |
Feudner et al. [14] | Cataract surgery | Forceps, anti-tremor and capsulorhexis | Randomised controlled trial | n = 63 (32 residents, 31 medical students) | 3 weeks (±5 days) |
Content: 0 Response processes: 2 Internal structure: 2 Relations to other variables: 0 Consequences: N |
2 |
| Eyesi Surgical | Same as above | Solverson et al. [27] | Cataract surgery | Phacoemulsification | Uncontrolled | n = 25 (18 residents, 7 ophthalmic surgeons) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Eyesi Surgical | Same as above | Ahmed et al. [11] | Cataract surgery | Not specified | Cross-sectional survey | n = 56 (ophthalmology residency programme directors) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Eyesi Surgical | Same as above | Privett et al. [22] | Cataract surgery | Capsulorhexis | Prospective, comparative case series | n = 23 (7 surgeons, 16 medical students and residents) | Not specified |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: 1 Consequences: N |
1 |
| Eyesi Surgical | Same as above | Belyea et al. [12] | Cataract surgery | Phacoemulsification | Retrospective case-control study | n = 42 (3rd year residents) | Not specified |
Content: 0 Response processes: N Internal structure: 1 Relations to other variables: N Consequences: 1 |
4 |
| Eyesi Surgical | Same as above | Le et al. [16] | Cataract surgery | Forceps, anti-tremor and capsulorhexis | Multi-centre cross-sectional study | n = 65 (4 medical students, 4 technicians, 36 residents, 3 fellows, 18 staff ophthalmologists) | 20 min |
Content: N Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
N/A |
| Eyesi Surgical | Same as above | Nathoo et al. [20] | Cataract surgery | Forceps and anti-tremor | Retrospective cohort study | n = 10 (5 junior + 5 senior residents with no previous simulator use) | 14 months |
Content: N Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Eyesi Surgical | Same as above | Selvander and Asman [25] | Cataract surgery | Navigation and capsulorhexis | Randomised uncontrolled trial | n = 35 (medical students) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Eyesi Surgical + wet lab | Intensive training programme involving wet lab and Eyesi simulator experience | Baxter et al. [38] | Cataract surgery | Not specified | Case series | n = 3 (3rd year ophthalmology trainees) | Eyesi Surgical = 50 h; wet-lab not specified |
Content: 2 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
4 |
| Eyesi Surgical | Same as above | Daly et al. [13] | Cataract surgery | Capsulorhexis | Randomised controlled trial | n = 21 (residents—10 trained in the wet lab vs 11 on the simulator) | Not specified |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: 1 Consequences: 1 |
3 |
| Eyesi Surgical | Same as above | Li et al. [18] | Cataract surgery | N/A | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Eyesi Surgical | Same as above | Pokroy et al. [21] | Cataract surgery | Phacoemulsification | Retrospective cohort study | n = 20 (residents) | ≥6 h training (mean = 21.2 h) |
Content: N Response processes: N Internal structure: N Relations to other variables: 1 Consequences: 1 |
4 |
| Eyesi Surgical | Same as above | Saleh et al. [24] | Cataract surgery | Navigation, anti-tremor, bimanual, cracking + chopping, capsulorrhexis | Prospective | n = 18 (1st year ophthalmic trainees) | 3 repeats (session duration not specified) |
Content: N Response processes: N Internal structure: 2 Relations to other variables: 1 Consequences: 0 |
2 |
| Eyesi Surgical | Same as above | Selvander and Asman [26] | Cataract surgery | Capsulorhexis, hydromaneuvers and phacoemulsification | Uncontrolled | n = 24 (7 cataract surgeons, 17 medical students) | Not specified |
Content: 0 Response processes: N Internal structure: 0 Relations to other variables: 1 Consequences: N |
N/A |
| Eyesi Surgical | Same as above | Spiteri et al. [28] | Cataract surgery | Forceps, anti-tremor, capsulorhexis and phacoemulsification | Uncontrolled | n = 30 (10 novice, 10 intermediate, 10 experienced surgeons) | 2 sessions an hour apart |
Content: N Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
N/A |
| Eyesi Surgical | Same as above | Thomsen et al. [30] | Cataract surgery | Phacoemulsification (all modules except chopping) | Uncontrolled | n = 42 (26 ophthalmic trainees, 16 ophthalmic surgeons) | ≤2 h |
Content: 1 Response processes: N Internal structure: 2 Relations to other variables: 1 Consequences: 1 |
2 |
| Eyesi Surgical | Same as above | Gonzalez-Gonzalez et al. [15] | Cataract surgery | Capsulorhexis | Prospective, comparative case series | n = 14 (3 attending physicians, 11 trainees) | Not specified |
Content: N Response processes: N Internal structure: N Relations to other variables: 0 Consequences: N |
2 |
| Eyesi Surgical | Same as above | Li et al. [18] | Cataract surgery | N/A | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Eyesi Surgical | Same as above | McCannel [19] | Cataract surgery | Capsulorhexis | Retrospective case-control study | n = 38 | Within a span of 4 years |
Content: 0 Response processes: N Internal structure: 0 Relations to other variables: 1 Consequences: 1 |
3 |
| Eyesi Surgical | Same as above | Roohipoor et al. [23] | Cataract surgery | Anti-tremor, bimanual, capsulorhexis, forceps and navigation training | Retrospective cohort study | n = 30 (residents) | ≤3 months |
Content: 0 Response processes: N Internal structure: 0 Relations to other variables: 1 Consequences: 1 |
2 |
| Eyesi Surgical | Same as above | Thomsen et al. [31] | Cataract surgery | Navigation, anti-tremor, forceps, bimanual, capsulorhexis, divide and conquer | Cross-sectional study | n = 11 (surgeons) | 1 h warm up before assessment |
Content: 0 Response processes: N Internal structure: 0 Relations to other variables: 2 Consequences: N |
N/A |
| Eyesi Surgical | Same as above | Bozkurt et al. [37] | Cataract surgery | Navigation, forceps, bimanual, anti-tremor, capsulorhexis | Prospective cohort study | n = 16 (ophthalmic residents + faculty members) | Not specified |
Content: 1 Response Process: 1 Internal Structure: N Relations to other variables: 1 Consequences: 0 |
2 |
| Eyesi Surgical | Same as above | Staropoli et al. 29] | Cataract surgery | Phacoemulsification | Retrospective case series | n = 22 (3rd year residents) | Within span of 3 years |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: 2 |
4 |
| Eyesi Surgical | same as above | Ng et al. [33] | Cataract surgery | Navigation, anti-tremor, capsulorhexis, cracking + chopping | Cross-sectional, multi-centre study | n = 19 (ophthalmic trainees) | 4 weeks |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
3 |
| Eyesi Surgical | Same as above | Colné et al. [34] | Cataract surgery | Irrigation + aspiration, capsulorhexis, cracking | Prospective study | n = 18 (12 residents + 6 cataract surgeons) | Not specified |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
N/A |
| Eyesi Surgical | Same as above | Ferris et al. [35] | Cataract surgery | Cataract training modules (unspecified) | Retrospective cohort study | n = 265 (1st and 2nd year trainees) | N/A |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: 1 Consequences: 2 |
5 |
| Eyesi Surgical | Same as above | La Cour et al. [36] | Cataract surgery | Eyesi cataract modules | Prospective, uncontrolled study | n = 19 (cataract surgeons) | Mastery learning (time taken for the trainee to reach a pre-defined pass score) |
Content: 1 Response Process: 1 Internal Structure: 2 Relations to other variables: 1 Consequences: 1 |
3 |
| Eyesi Surgical | Same as above | Lucas et al. [32] | Cataract surgery | Cataract training modules (unspecified) | Retrospective cohort study | n = 14 (2nd year residents) | Not specified |
Content: 1 Response Process: N Internal Structure: N Relations to other variables: N Consequences: 2 |
4 |
| Eyesi Surgical | Same as above | Rossi et al. [41] | Vitreoretinal surgery | Navigation and membrane peeling | Prospective, comparative case series | n = 44 (6 medical student, 24 residents, 14 vitreoretinal surgeons) | Not specified |
Content: N Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Eyesi Surgical | Same as above | Park et al. [40] | Vitreoretinal surgery | Navigation, forceps, anti-tremor and vitrector | Prospective cohort study | n = 14 (12 residents, 1 medical retina fellow, 1 vitreoretinal surgeon) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Eyesi Surgical | same as above | Koch et al. [39] | Vitreoretinal surgery | Not specified | Cross-sectional survey | N = 156 (108 residents, 48 ophthalmologists with more experience) | Not specified |
Content: 0 Response processes: 1 Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Eyesi Surgical | Same as above | Vergmann et al. [42] | Vitreoretinal surgery | Navigation, forceps, bimanual, laser coagulation, posterior hyaloids, membrane peeling | Prospective | n = 35 (20 medical students, 10 residents, 5 surgeons) | 2 sessions with up to 2 weeks apart |
Content: 1 Response processes: 1 Internal structure: N Relations to other variables: 1 Consequences: N |
2 |
| Eyesi Surgical | Same as above | Cissé et al. [43] | Vitreoretinal surgery | Navigation, forceps, vitrector, epiretinal membrane peeling | Prospective study | n = 21 (15 residents + 6 VR surgeons) | 2 × 60-min sessions |
Content: 1 Response Process: 1 Internal Structure: N Relations to other variables: 1 Consequences: N |
N/A |
| MicroVisTouch |
Hardware: mannequin head; blunt-tipped handpiece; robotic arm; footpedals Software: VR platform; haptic feedback interface; cataract surgery modules |
Banerjee et al. [44] | Cataract surgery | Capsulorhexis | Prospective | n = 8 (4th year residents) | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
N/A |
| MicroVisTouch | Same as above | Sikder et al. [45] | Cataract surgery | Capsulorhexis | Prospective | n = 78 (residents) | 6 months |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
N/A |
| MicroVisTouch | Same as above | Kozak et al. [46] | Vitreoretinal surgery | Epiretinal membrane + internal limiting membrane peeling procedures | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| PhacoVision | A personal computer with 3D visual interface, phacoemulsification handpiece, a nucleus manipulator and foot pedals for control of the phacoemulsification procedure and microscope adjustments | Laurell et al. [54] | Cataract surgery | Phacoemulsification | Experimental | n = 7 (medical students + ophthalmic surgeons) | Not specified |
Content: 0 Response processes: 0 Internal structure: N Relations to other variables: N Consequences: N |
1 |
| Phantom Phaco-simulator | Simulator with Phantom haptic device | Agus et al. [52] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Cataract surgery stimulator | Low-cost simulator using computer-based algorithms for tissue deformation, surface cutting and volume sculpting; two-handed device with six degrees-of-freedom for human–computer interactions | Choi et al. [53] | Cataract surgery | Phacoemulsification | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Pars plana vitrectomy simulator | A vitrectomy probe and handpiece of an intraocular illumination probe tracked by CCD cameras within a mechanical eye, housed inside a mannequin head | Jonas et al. [56] | Vitreoretinal surgery | Pars plana vitrectomy | Descriptive | n = 14 (residents and medical students) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Sophocle | Binocular microscope with a slit lamp and 3D translation controlled by a swingle bar | Peugnet et al. [58] | Vitreoretinal surgery | Retinal photocoagulation | Randomised controlled trial | n = 10 (residents) | N/A |
Content: 0 Response processes: N Internal structure: 0 Relations to other variables: N Consequences: N |
3 |
| VR surgery simulator | 3D position tracking stylus, Pentium II desktop, Open GL and Microsoft Visual C + + languages to control the interaction and update the visual feedback tracking the instruments | Verma et al. [59] | Vitreoretinal surgery | Unspecified | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Vitrectomy simulator | Computer software with special hardware. | Neumann et al. [57] | Vitreoretinal surgery | Vitrectomy | Descriptive | N/A | N/A |
Content: 1 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Vitreous surgery simulator | High-resolution colour stereo binoculars, haptic devices, foot switches and a high-speed graphics computer | Hikichi et al. [55] | Vitreoretinal surgery | Vitrectomy | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Endoscopic Endonasal Surgery Simulator (EESS) | VR software to convert endoscope and surgical instrument to a video display that can be simultaneously seen by instructor and trainee. | Weiss et al. [60] | Endoscopic endonasal surgery | Endoscopic navigation, endonasal injection and middle turbinate medialization | Randomised controlled trial | n = 15 (residents) | 5 h |
Content: 0 Response processes: N Internal structure: 1 Relations to other variables: N Consequences: N |
2 |
| Eye surgery simulator | High-speed computer graphics workstation, a stereo operating system, a wrist rest and a position tracking stylus connected to force feedback motors | Sinclair et al. [62] | General ophthalmic surgery | Unspecified | Descriptive | N/A | N/A |
Content: 1 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Micro-surgical robot | A virtual environment; micro-surgical master and slave; mannequin | Hunter et al. [61] | General ophthalmic surgery | Unspecified | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Ophthalmic Retrobulbar Injection Simulator (ORIS) | Use of QuickTime to create digital video sequences for instructing residents on retrobulbar injection; the user can control the viewing angles and video sequence using controls on the screen. | Merril et al. [63] | Ophthalmic anaesthesia | Retrobulbar injection | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| Ocular ultrasound using VR software Blender | A 3D virtual model built using open-source software used to generate movie clips to simulate different movements and orientations of an ocular ultrasound scanner head. | Mustafa et al. [64] | Ocular Ultrasound | Imaging | Descriptive | N/A | N/A |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
N/A |
| EyeSi Direct Ophthalmoscope | Simulator consists of an ophthalmoscope handpiece with built-in display, a patient model head and a PC with touchscreen. Performance metrics for different components of the examination are calculated and recorded | Borgersen et al. [48] | Fundoscopy examination | Direct ophthalmoscopy | Prospective validation study | n = 21 (13 medical students; 8 ophthalmic consultants) | Not specified |
Content: 1 Response Process: 2 Internal Structure: 2 Relations to other variables: 2 Consequences: 1 |
N/A |
| EyeSi Direct Ophthalmoscope | Same as above | Boden et al. [49] | Fundoscopy examination | Direct ophthalmoscopy | Randomised, controlled study | n = 34 (medical students) | Not specified |
Content: 1 Response Process: 1 Internal Structure: N Relations to other variables: N Consequences: 0 |
2 |
| Eyesi Indirect Ophthlamoscope | Simulator consists of diagnostic lenses, a model patient head and an ophthalmoscope headband with mounted stereo display, showing a 3D virtual patient and virtual lens when the trainee’s hand is placed over the patient’s eyes. Software comes with a range of patient cases and pathologies | Chou et al. [50] | Fundoscopy examination | Indirect ophthalmoscopy | Prospective | n = 42 (25 medical students, 17 trainees) | Not specified |
Content: 0 Response processes: N Internal structure: N Relations to other variables: 1 Consequences: N |
1 |
| Eyesi Indirect Ophthlamoscope | Same as above | Loidl et al. [51] | Fundoscopy examination | Indirect ophthalmoscopy | Prospective study + survey | n = 292 (medical students) | 1 week |
Content: N Response Process: N Internal Structure: N Relations to other variables: N Consequences: N |
1 |
Table 5.
E-learning studies.
| Model | Description | Reference | Area of training | Training task | Study design | Participants | Training time | Validity | Effectiveness |
|---|---|---|---|---|---|---|---|---|---|
| Computer-Assisted Learning Ophthalmology Programme |
(1) A software programme delivering a multi-media tutorial for learning about the pupillary light reflex (2) A Macintosh-connected mannequin model housing motor-driven camera diaphragms to simulate pupil response to the swinging flashlight test (3) A multiple-choice quiz to test the user’s understanding of the material |
Kaufman and Lee [137] | Diagnostic examination | Swinging flashlight test | Evaluation study | n = 29 (medical students) | 2 weeks |
Content: 2 Response processes: 2 Internal structure: N Relations to other variables: N Consequences: N |
1 |
| E-learning modules | Case presentations based on an interactive Q&A game format | Stahl et al. [138] | Ophthalmology education | Ophthalmology-related patient cases | Prospective study | n = 272 (medical students) | 2 terms |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: 1 |
2 |
| Ophthalmic Operation Vienna |
(1) Surgical videos accompanied by 3D animations of 5 surgical procedures: cataract, glaucoma, retinal detachment, vitrectomy and ablative refractive surgery (2) A multiple-choice test on cataract and glaucoma surgery topics |
Prinz et al. [139] | Anterior and posterior segment surgery | Cataract and glaucoma surgery knowledge | Randomised controlled trial | n = 172 (medical students) | 2 weeks |
Content: 0 Response processes: N Internal structure: 2 Relations to other variables: N Consequences: N |
2 |
| 3D computer animations | Software and hardware systems used to create 3D animations (e.g., Cinema 4D XL Studio Bundle to create the ocular muscles) into interactive computer programmes to simulate eye movements, pathologies and neuro-surgical techniques | Glittenberg and Binder [140] | Ophthalmology education | Neuro-ophthalmology and oculomotor anatomy knowledge | Case-control comparative study | n = 40 (medical students) | ~45 min lecture presentations |
Content: 0 Response processes: N Internal structure: N Relations to other variables: N Consequences: N |
2 |
| Virtual Mentor | An interactive, computer-based simulation of the cognitive components of performing hydrodissection | Henderson et al. [141] | Cataract surgery | Hydrodissection | Single-masked, randomised controlled trial | n = 68 (residents) | Not specified |
Content: 2 Response processes: N Internal structure: 0 Relations to other variables: 1 Consequences: N |
2 |
Virtual reality
Eyesi Surgical
The Eyesi Surgical (VRmagic, Mannheim, Germany) is a high-fidelity virtual reality simulator designed for practising intraocular procedures. It consists of a mannequin head that houses a model eye connected to a computer interface and an operating microscope. The movements and positions of surgical instruments are tracked by internal sensors, producing a virtual image that is viewed through the microscope, as well as on separate touchscreen. The software contains training modules that simulate different steps in cataract and vitreoretinal surgeries. The system records performance metrics, enabling scores and feedback to be generated [11]. Of all virtual reality simulator models developed for use in ophthalmology training, the Eyesi has been the most extensively assessed, with a total of 33 validity studies.
Cataract surgery
[Summary: content = 2; response processes = 1; internal structure = 2; relations to other variables = 2; consequences = 2; translational outcomes = level 5].
Twenty-eight studies assessed the Eyesi cataract training modules, collectively demonstrating all five sources of validity evidence, with data strongly supporting each parameter (score = 2) except for response processes, which had more limited evidence (score = 1) [11–38]. A randomised controlled trial (RCT) by Feudner et al. showed that those who trained with the Eyesi achieved significant improvements in their capsulorhexis performance in the wet lab compared with the no-training, control group [14]. Another RCT suggested that virtual reality training was comparable to training using wet lab [13]. Residents were assessed on their first capsulorhexis in the operating room following either Eyesi or web-lab training. Overall technical scores were equivalent. The study also provided evidence of predictive validity with a direct correlation between time taken to complete the training modules on the Eyesi and true operating room time, as well as overall performance score.
Regarding patient outcomes, five studies demonstrated the transfer effects of Eyesi with reduced complications in live cataract surgery following training [12, 21, 29, 35, 38]. Of note, a multi-centre retrospective study involving 265 ophthalmology trainees across the UK showed that complication rates dropped from 4.2 to 2.6% (38% reduction) following the introduction of Eyesi simulators into training programmes [35]. Similarly, a study by Baxter et al. demonstrated that the use of a structured curriculum with wet lab and Eyesi training led to a considerable reduction in complication rates compared with reported figures for traditional training programmes [38]. However a recent study also testing transfer of skills showed some limitations to Eyesi training [36]. Performance during Eyesi training was comparing to subsequent performance in theatre. Results showed that improvements in OR performance was only observed for ophthalmologists who were less experienced and that the ability for Eyesi scores to discriminate between novice and experienced surgeons could only be seen in the first few training sessions.
Vitreoretinal surgery
[Summary: content = 1; response processes = 1; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes: level 2].
Only four studies have evaluated the vitreoretinal modules on the Eyesi Surgical Simulator [39–42]. These studies support the content validity for vitreoretinal surgery training, as well as response processes, and relations to other variables. Similar to cataract surgery training, scores on the vitreoretinal modules were able to discriminate between experienced and inexperienced surgeons. One study reported evidence for response processes through the standardisation of testing and assessment such as allocating set time periods for training, standardised instructions and using the same supervisor. This evidence remains limited at the best [43]. Studies on the vitreoretinal modules also demonstrated a learning curve with overall scores increasing and completion time decreasing with repeated attempts, indicating contained effects in using the Eyesi for vitreoretinal training. No evidence has been published to support internal structure and consequences or transfer of skills to the operating room.
MicroVisTouch
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes = N].
The MicroVisTouch (ImmersiveTouch, Inc, Chicago, USA) is another commercially available virtual reality simulator that was introduced after the Eyesi, with a report of the prototype published in 2012 [44]. Unlike the Eyesi, the MicroVisTouch features a single handpiece that is attached to a robotic arm and is used to control the appropriate instrument according to the procedure being simulated. It also differs from the Eyesi in that it has an integrated tactile feedback interface, reportedly the first ophthalmic simulator to have this feature [45]. Currently, simulation is limited to three key steps in cataract surgery (clear corneal incision, capsulorhexis and phacoemulsification), although further modules are being developed.
Compared with the Eyesi, fewer studies have assessed the MicroVisTouch. Two groups have reported, implicitly, that the simulator demonstrates content validity for simulating capsulorhexis and that there is evidence of relations to other variables [44, 45], but other sources of validity evidence are lacking. Evidence supporting the effectiveness of using the simulator is also lacking. A third group adapted the MicroVisTouch by customising the algorithm and integrating OCT (Optical Coherence Tomography) scans of varying vitreoretinal conditions to the simulator, enabling patient-specific simulation training of vitreoretinal procedures (epiretinal membrane and internal limiting membrane peeling) [46]. However, the validity and effectiveness of this model was not tested in the original study and no further reports have been found.
Eyesi Ophthalmoscopes
Direct
[Summary: content = 1; response processes = 2; internal structure = 2; relations to other variables = 2; consequences = 1; translational outcomes: level 2].
The Eyesi Direct Ophthalmoscope (VRmagic, Mannheim, Germany) is a virtual reality simulator that enables fundoscopy examination practice, consisting of an ophthalmoscope handpiece with built-in display and a patient model head connected to a touchscreen. A range of patient cases and pathologies can be selected from the programme and objective feedback is provided based on the trainee’s performance [47].
Although only two studies were found evaluating this simulator, there was strong evidence for its validity. Borgersen et al. published the only study in this review to assess validity using all five parameters in Messick’s framework, and showed that the consequences of using a set pass/fail score to accurately discriminate between inexperienced participants (medical students), who were given a fail compared with the experienced participants (ophthalmology consultants) who all passed [48]. The second study showed that participants who trained with the simulator achieved higher scores in an OSCE (Objective Structured Clinical Examination) assessment compared with a control group who only received classical training, thus demonstrating contained effects for translational outcomes [49].
Indirect
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes: level 1].
The Eyesi Indirect Ophthalmoscope (VRmagic, Mannheim, Germany) is similar to the Eyesi Direct, an ophthalmoscope headband that is connected to a display showing a 3D virtual patient and virtual lenses when physical, diagnostic lenses are placed over the model head. As with the Eyesi Direct, physiologic and pathologic functions for the virtual patient can be controlled and varied.
Only two studies were found for this simulator [50, 51]. In contrast to the Eyesi Direct, validity evidence was limited to relations to other variables as one study showed that the simulator could discriminate between medical students and ophthalmology trainees [50]. Effectiveness was limited to internal acceptability as participants gave positive feedback of their experience in using the simulator.
Others
A variety of different virtual reality simulators have also been described, including three models for cataract surgery [52–54]; five for vitreoretinal surgery [55–59]; one for endoscopic endonasal surgery [60]; two for general ophthalmic surgery [61, 62]; 1 for ophthalmic anaesthesia [63]; 1 on ocular ultrasound [64]; and 1 for indirect ophthalmoscopy [54]. However, these have all been stand-alone reports with limited evidence of content validity only (scores of 0 or 1). An exception is the Endoscopic Endonasal Surgery Simulator by Weiss et al., which was tested in an RCT and demonstrated good internal structure [60]. Effectiveness was only tested in four models, with the Sophocle retinal photocoagulation simulator shown to be the most effective (downstream effects) as live assessment on real patients showed that the simulator group performed similarly to the control group who had previously practised on patients [58]. As with the other descriptive study models, these simulators have not been further investigated.
Wet lab
A total of 47 studies on wet-lab models were found, of which 12 were mixed models used in conjunction with an inanimate device or artificial system. From the animal model studies, 22 used porcine-related specimens, 3 used sheep specimens, 4 used goat eyes and 3 rabbit eyes. The number of studies using human cadaveric eyes or isolated lens were 17, of which 3 were used in combination with animal tissue.
Cataract surgery
[Summary: content = 2; response processes = 0; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 2].
There were 16 studies describing the use of wet-lab models for cataract surgery [65–80]. These demonstrated content validity only, with no evidence for other validity parameters. Models which showed the strongest evidence for validity were pig eyes filled with cooked chestnuts for practising phacoemulsification [71] and rabbit eyes fixed with paraformaldehyde for simulating capsulorhexis [74]. These two models demonstrated contained effects and internal acceptability respectively.
Vitreoretinal surgery
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = N].
One study described the use of rabbit eyes for performing pars plana vitrectomy, from which content validity could be inferred [81]. However, all other sources of validity evidence and indications of effectiveness were lacking.
Glaucoma surgery
[Summary: content = 1; response processes = 2; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes = level 2].
Six studies were found for glaucoma surgery [82–87], with the majority lacking formal validity assessment. One study, which tested placement of human cadaveric eyes into a model head Marty the Surgical Simulator (Iatrotech Inc., Del Mar, USA) for goniotomy simulation, demonstrated good response processes and evidence of internal acceptability [84]. Dang et al. also showed that performing trabeculectomies on porcine eyes with added canalograms for outflow quantification had some evidence for relations to other variables and contained effects [82].
Corneal surgery
[Summary: content = 1; response processes = N; internal structure = N; relations to other variables = 2; consequences = N; translational outcomes = 1].
The use of wet-lab models for practising corneal surgery has been described in four studies [88–90]. Content validity and relations to other variables were demonstrated in one study [91], which tested the feasibility of simulating Descemet’s membrane endothelial keratoplasty on human corneas with an artificial anterior chamber with a 3D-printed iris. However, evidence of other validity parameters and effects were not demonstrated in the other studies.
Strabismus surgery
[Summary: content = 0; response processes = 0; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 2].
Two wet-lab models were found for strabismus surgery, both using porcine eyes. White et al. added bacon to the eyes to simulate extraocular muscles [92], whereas Vagge et al. asked residents to practice on a chicken breast model followed by the pig eyes [93]. Discussion of content validity and response processes was made in both studies but no data were reported. Internal acceptability and contained effects were demonstrated for the two models respectively.
Oculoplastic surgery
[Summary: content = 2; response processes = 0; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 2].
Four studies described the use of wet-lab oculoplastic simulators [94–97]. These all demonstrated content validity, with one study by Pfaff showing strongest evidence for this parameter [95]. One group showed that using a split pig head for practising lid procedures had good internal acceptability [94] and another group using human cadaver eyes showed that trainees had improved comfort, confidence and technical skills in performing canthotomy and cantholysis procedures [97].
Orbital surgery
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = N].
Altunrende et al. describe using a sheep cranium to practise ocular dissection for orbital surgery. Content validity was reported but any further effectiveness of the model was not testes [98].
Ocular trauma
[Summary: content = 1; response processes = N; internal structure = N; relations to other variables = 0; consequences = N; translational outcomes = level 1].
A recent study by Mednick et al. showed that placing iron particles on human cadaver eyes for corneal rust ring removal simulation had evidence of content validity and relations to other variables [99]. Internal acceptability was shown to be high. Another study on ocular trauma surgery described the use of goats’ eyes for practising corneoscleral perforation repair [100]. However, as the study was purely descriptive, it was not possible to assess its validity or effectiveness.
Diagnostic examination
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = N].
One Study by Uhlig and Gerding tested the use of porcine eyes placed inside an adjustable, artificial orbit for practising direct and indirect fundoscopy, as well as gonioscopy [101]. As this was a descriptive study, no evidence for validity or effectiveness was given.
Others
The remaining wet-lab models were either used to simulate a wide range of anterior and/or posterior segment surgeries or general micro-surgical skills [102–111].
Only two models, both using porcine eyes for micro-surgical skills assessment, provided data supporting their validity. Ezra et al. investigated the use of a video-based, modified Objective Structured Assessment of Technical Skill (OSATS) assessment tool. They demonstrated good internal structure, with high inter-rater reliability, and relations to other variables, with significant correlation between the OSATS scores and results from a separate motion-tracking device [109].
The Eye Surgical Skills Assessment Test (ESSAT), involving the use of porcine eyes and feet as part of a three-station assessment, demonstrated all five sources of validity evidence. One study showed, via a panel of ophthalmic surgery experts, that there was strong evidence of content validity [110]. A further masked study demonstrated that the ESSAT showed strong inter-rater reliability (internal structure) and that the senior resident in the study scored higher than the junior resident (relations to other variables) [111]. Unlike other models, the study authors also went on to discuss the potential consequences of using the ESSAT as an assessment tool, weighing up the benefits of setting a competence score that trainees would need to meet before performing on real patients, with the potential problems of the ESSAT becoming a stressful test preventing less confident residents from entering the operating room. The effectiveness of using this test, however, was not tested.
Altogether, the wet-lab studies, which assessed effectiveness only evaluated responses to participant surveys (internal acceptability) [105] and performance improvements on the models themselves (contained effects) [108, 109]; downstream effects were not demonstrated.
Dry lab
Twenty-six studies on synthetic models were identified, of which eight were developed for practising diagnostic examination techniques (slit lamp, direct and indirect ophthalmoscopy), six for vitreoretinal surgery, one for strabismus surgery, four for laser procedures, two for orbital surgery, one for cataract surgery, one for oculoplastic surgery, one for ocular trauma, one for general ophthalmic surgery and one for combined fundoscopy examination and laser procedures.
Cataract surgery
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 2].
Abellán et al. developed a low-cost cataract surgery simulator using a methacrylate support and aluminium foil for capsulorhexis simulation [112]. This was the only inanimate simulator to be tested in an RCT and demonstrated transfer effects as those who trained using the model achieved a higher percentage of satisfactory capsulorhexis in subsequent practice with animal eye models compared with those who had begun training with the animal eyes. Further validity evidence was lacking from the study.
Vitreoretinal surgery
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes = level 2].
For vitreoretinal surgery, two different validated models were found. Hirata et al. used quail eggs within a silicone cap to simulate membrane peeling. The model was shown to discriminate between experienced and inexperienced surgeons in terms of operating time and the success rate of membrane peeling (relations to other variables) [113]. This was similarly tested in a study by Yeh et al. where, using the artificial VitRet Eye Model (Phillips Studio, Bristol, UK) filled with vitreous-like fluid to simulate a variety of vitreoretinal surgery procedures, a positive correlation was observed between the trainees’ level of experience and total score [114]. In terms of effectiveness, the models by Yeh and Hirata showed internal acceptability and contained effects, respectively.
Other dry-lab models for vitreoretinal surgery included the use of an artificial orbit with diascleral illumination [115]; a modified rubber eye [116]; a medium-fidelity model constructed using a wooden frame and tennis ball to simulate the globe [117]; and an artificial eye with inner limited membrane made using hydrogel [118]. However, these models were only described, with no assessment of validity or effectiveness.
Strabismus
[Summary: content = 1; response processes = 2; internal structure = 2; relations to other variables = N; consequences = N; translational outcomes = level 2].
One study was found on the use of a low-fidelity, dry-lab model for strabismus surgery simulation [119]. The model consisted of a rubber ball simulating the globe; elastic band simulating the recti muscles, a piece of latex to simulate the conjunctiva and cornea. Results showed no significant differences between this model and a higher-fidelity, wet-lab model and this dry-lab model. The study showed strong evidence of valid response processes and internal structure as the authors performed a pre-randomisation test to determine baseline dexterity and ensured stratified randomisation of participants into two different groups with equal baseline dexterity. The process for evaluating the participants’ skills after training was also robust as their performance was evaluated by two independent ophthalmologists using three different validated assessment scales (ICO-OSCAR, OSATS and ASS).
Laser surgery
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes = level 2].
Out of the four laser simulators found [120–123], only the model designed by Simpson et al. showed evidence of validity through relations to other variables [103]. The effectiveness of training with this model, however, was not investigated. Conversely, a capsulotomy simulator by Moisseiev and Michaeli demonstrated contained effects but did not test for validity [121].
Oculoplastic surgery
[Summary: content = 0; response processes = 0; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = N].
One oculoplastic surgery dry-lab model was found, using an anatomically correct skull model for simulating nasolacrimal duct surgery [124]. However, this was descriptive only, with no assessment of validity or effectiveness.
Orbital surgery
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = N].
There were two studies using 3D-printed orbit models for simulating orbital surgery [125, 126]. However, as these were also descriptive only, evidence for their validity and effectiveness was not shown.
Trauma management
[Summary: content = 1; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 1].
The Newport Eye is a simple training phantom using a craft eye, resins and ground black pepper to simulate corneal foreign body removal [127]. This demonstrated evidence for content validity as experts agreed that the model was realistic in terms of its tissue colour, consistency and anatomy. Trainees also reported being more confident with the procedure after using the model, demonstrating internal acceptability. However, this simulator does not appear to have been used by other groups and no further reports were identified.
Diagnostic examination
[Summary: content = 0; response processes = 0; internal structure = N; relations to other variables = 1; consequences = N; translational outcomes = 0].
The largest proportion of dry-lab models were designed for practising examinations, including slit lamp and fundoscopy (direct and indirect) [128–136]. Two studies tested the validity and effectiveness of the EYE Exam Simulator (Kyoto Kagaku Co., Japan), a popular tool for fundoscopy practice. This consists of a head model with adjustable pupil sizes and changeable fundus slides to represent different retinal conditions. McCarthy et al. showed that response processes were generally poor as letters were added to the slides to check the participant’s field of vision through the ophthalmoscope and the majority were unable to identify the markers or pathology on the slides [132]. Survey responses also indicated that there was low user satisfaction with the model as trainees did not feel it was realistic or that the exercise improved their skills. On the other hand, Akaishi et al. showed that there was a strong correlation between accuracy of examination on the EYE simulator and previous experience of performing fundoscopy in the clinic, providing some evidence for its validity [128].
All other studies of ophthalmoscopy and slit-lamp simulators were descriptive, showing internal content validity only.
E-learning
Aside from training technical skills, tools have also been developed for improving cognitive and other non-technical skills such as teamwork and leadership. A total of five studies were found using this modality, with the majority testing its use amongst medical students. All studies incorporated both training and assessment as part of the course.
Computer-Assisted Learning Ophthalmology Program
[Summary: content = 2; response processes = 2; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 1].
The Computer-Assisted Learning Ophthalmology Program designed by Kaufman and Lee is a multi-media, interactive tutorial, which aims to help medical students learn about the pupillary light reflex [137]. Content validity was demonstrated extensively by experts and response processes were thoroughly assessed as each student’s experience and thought process during the training was evaluated by an external interviewer after the programme. Despite the positive responses from all groups showing that it was a valid and effective simulator (internal acceptability), it has not been used by other medical schools and no further reports have been found.
Case-based e-learning modules with Q&A games
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = 1; translational outcomes = level 2].
A study by Stahl et al. also tested the consequences of using e-learning modules as a part of ophthalmology teaching for a group of 272 medical students [138]. Although validity parameters were not formally tested, the authors found that students who used e-learning more frequently achieved better exam results.
Ophthalmic Operation Vienna
[Summary: content = 0; response processes = N; internal structure = 2; relations to other variables = N; consequences = N; translational outcomes = level 2].
An RCT by the Medical University of Vienna evaluated the use of a 3D animated programme for learning different steps in ophthalmic surgery [139]. This demonstrated strong internal structure as a reliability analysis of the multiple-choice questions used at the end of the programme showed a Cronbach’s α coefficient of 0.7, indicating high reliability. Those in the simulation group also outperformed the control group in the final test, showing contained effects.
3D computer animations for learning neuro-ophthalmology and anatomy
[Summary: content = 0; response processes = N; internal structure = N; relations to other variables = N; consequences = N; translational outcomes = level 2].
A similar study by Glittenberg and Binder was carried out, investigating the use of a combination of various 3D design software for teaching complex topics in neuro-ophthalmology [140]. No evidence was provided supporting its validity. However, effectiveness was demonstrated as students responded very positively to the programme in a satisfaction questionnaire (internal acceptability) and also achieved significantly better results in a post-lecture test compared with the control group (contained effects).
The Virtual Mentor
[Summary: content = 2; response processes = N; internal structure = 0; relations to other variables = 1; consequences = N; translational outcomes = level 2].
Whilst most e-learning studies were designed to help medical students, one model was developed for ophthalmology residents to develop non-technical skills. A multi-centre RCT tested the effects of using The Virtual Mentor, an interactive, computer-based programme teaching the cognitive aspects of performing hydrodissection in cataract surgery, including decision making and error recognition [141]. Test questions demonstrated good content validity as they were developed and modified by cataract surgery experts across nine academic institutions. Test scores also demonstrated relations to experience, with correlation between total marks and residency year of training. Despite the lack of data quantifying the reliability of this model, the study showed a degree of internal structure as residents were randomised using a stratified design according to their academic centre and residency year, factors which would likely have influenced the test scores. Internal acceptability was demonstrated by positive user feedback and contained effects through higher post-test scores and a greater mean increase in pre- to post-test results in the simulator group compared with the control group.
Discussion
This systematic review of simulation training in ophthalmology provides a comprehensive evaluation of all available simulation tools using the modern taxonomy. Virtual reality simulators were the most widely evaluated and the Eyesi Surgical Simulator in particular. For cataract surgery, evidence to support all aspects of content validity have been reported. Critically data support the collateral effects of using the Eyesi with training being shown to result in improve operating room performance and lower complications. In contrast, only a much more limited assessment of other ophthalmic simulation training tools has been undertaken including the vitreoretinal training modules for the Eyesi Surgical system. A wide variety of dry-lab and wet-lab training models were reported. Use of dry-lab models in ophthalmology was more limited compared with other surgical specialities [142] with no evidence to suggest any model was particularly effective. In contrast, a relatively high number of wet-lab models was reported. In general, acceptability was high with positive participant feedback and there was evidence, albeit limited, to support the educational impact of wet-lab training. Cadaveric animal tissue was most commonly used and no significant benefits of human over animal cadaveric models were reported. Only five studies reported the use of e-learning. These results do support its potential for ophthalmology training but further assessment needs to be undertaken before incorporation into the training curriculum. Lastly, there was a paucity of studies addressing non-technical skills training in this area. The impact of human factors on patient safety is well-recognised corresponding to the rapid increase in non-technical skills training in medicine [143]. One study in this review, the Virtual Mentor e-learning programme, included cognitive components of cataract surgery training [141]. A pilot study by Saleh et al. also demonstrated the feasibility of using high-fidelity, immersive simulation for cataract surgery, using scenarios based on previous patient safety incidents and evaluating the cross-validity and reliability of four established assessment tools (OTAS, NOTECHS, ANTS and NOTSS) [144].
Simulation tools are increasingly being used for assessment of technical and non-technical skills, both formative and summative. In this review, only one assessment tool, ESSAT, has been described. Strong validity evidence has been shown for the ESSAT but further research on the development of standards and application of the ESSAT tool has not yet been performed. Effective skills assessment is becoming increasingly important both to support competency-based training, as well as enable objective proficiency assessment. In response to growing calls for greater transparency and accountability, formal ongoing credentialing and certification are being considered to ensure doctors maintain the necessary skills and knowledge throughout their professional careers. Simulators are being used to provide objective skills assessment but especially in such high-stakes assessment, rigorous validation of the assessment tools is required before they can be implemented.
Overall, the majority of studies lacked a formal validation process, with 45% of studies (n = 59) being purely descriptive. Furthermore, most validity assessments used the outdated validity frameworks which greatly limits the value of these results. “Face validity” was commonly reported as validity evidence despite the recognition that such subjective assessment of the perceived realism of a simulator is largely irrelevant to its educational impact [145]. Likewise the concept of construct validity using expert–novice comparisons remains widely used but again offers little useful insight into a simulator’s educational impact. The lack of validation studies appears greater than in other specialties. Similar systematic reviews of otolaryngology and orthopaedic simulation training reported rates of descriptive studies of 23% and 38%, respectively [146, 147]. This resonates with findings from a recent review of simulation-based validation studies across all surgical specialities that reported that only 6.6% used Messick’s validity framework [148]. Evidence for a number of components were particularly deficient. Internal structure was rarely assessed, a fundamental area evaluating the reliability and generalisability of scores. For the wet-lab and dry-lab groups, a large number of authors have attempted to establish validity through feedback from study participants on whether the simulator was a valid representation of the surgical correlate. However, this is flawed since the majority of these participants are inexperienced and input should be made from those who have more expertise in the procedure of interest. Effectiveness and translational outcomes were also not extensively tested. In particularly wet-lab and dry-lab simulation studies predominantly reported evidence from user satisfaction surveys, with few assessing for skill improvement and none investigating the relationship to OR performance or patient-related outcomes. Although several studies have linked Eyesi training with reduced complication rates, the majority of these have been retrospective studies which did not control for important confounders such as participants undertaking other forms of training. A few studies explored the collateral effects of simulation training on a systemic level, such as cost saving or policy changes. Two separate preliminary analyses on cost effectiveness were carried out in 2013, both suggested that the cost to benefit ratio was unfavourable. One study predicted, on the basis of cost modelling, that residency programmes would not be able to recoup the costs of purchasing one Eyesi model within 10 years under the most optimistic scenario [149]. The other study suggested that, realistically, it would take 34 years to make a cost recovery [150]. In contrast, the most recent study by the Royal College of Ophthalmologists in the UK argued that the Eyesi was a cost-effective method if costs of complication were include. Access to an Eyesi simulator led to a 1.5% decrease in complication rates, which were inferred to result in an estimated 280 fewer cases of posterior capsular rupture complications alone per year. This would amount to a saving of roughly £560,000 per year and, using this figure, the authors calculated that the cost of purchasing 20 Eyesi simulators would be regained within 4 years. Due to the contrast in findings between these three studies and the implications for both patient safety and costs for healthcare providers, further attempts should be made to provide an updated reflection of current cost effectiveness of Eyesi simulators. In addition, there should be more studies to test whether the same potential benefits gained from Eyesi training can be achieved with a lower-cost model.
The limitations to this study are that, although a broad search criterion was applied using comprehensive search terms, it is possible that some reports using different terminology may have been missed. As discussed above, a large proportion of studies suffered from poor methodologies, utilising outdated concepts of validation and greatly limiting the conclusions that can be drawn. The heterogeneity in methodology and outcomes across the studies also prevented the use of quantitative analysis. In addition, the majority of e-learning studies included in this review recruited medical students rather than ophthalmic professionals, thus results obtained may not be reflective of specialised training.
Conclusion
The increasing importance of simulation training in ophthalmology is reflected by the number and variety of models described in the literature. The Eyesi Surgical remains the only model to have undergone extensive testing and the necessary evidence supporting its use has been reported. The main limitations of current research lie in the use of outdated validity frameworks, a lack of attempt made to establish the collateral, systemic effects of using simulator models and the low quality of validation study designs. Future studies need to follow current recommendations on the assessment and validation of educational tools to ensure that simulation-based training is successfully incorporated into current systems of training in ophthalmology, especially for high-stakes applications such as credentialing and assessment.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reznick RK, MacRae H. Teaching surgical skills-changes in the wind. N Engl J Med. 2006;355:2664–9. doi: 10.1056/NEJMra054785. [DOI] [PubMed] [Google Scholar]
- 2.Zhou AW, Noble J, Lam WC. Canadian ophthalmology residency training: an evaluation of resident satisfaction and comparison with international standards. Can J Ophthalmol. 2009;44:540–7. doi: 10.3129/i09-155. [DOI] [PubMed] [Google Scholar]
- 3.Chikwe J, de Souza AC, Pepper JR. No time to train the surgeons. BMJ. 2004;328:418–9. doi: 10.1136/bmj.328.7437.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher AG, Traynor O. Simulation in surgery: opportunity or threat? Ir J Med Sci. 2008;177:283–7. doi: 10.1007/s11845-008-0204-5. [DOI] [PubMed] [Google Scholar]
- 5.Tarbet KJ, Mamalis N, Theurer J, Jones BD, Olson RJ. Complications and results of phacoemulsification performed by residents. J Cataract Refract Surg. 1995;21:661–5. doi: 10.1016/s0886-3350(13)80562-7. [DOI] [PubMed] [Google Scholar]
- 6.Rutar T, Porco TC, Naseri A. Risk factors for intraoperative complications in resident-performed phacoemulsification surgery. Ophthalmology. 2009;116:431–6. doi: 10.1016/j.ophtha.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 7.McCannel CA. Simulation surgical teaching in ophthalmology. Ophthalmology. 2015;122:2371–2. doi: 10.1016/j.ophtha.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Messick S. Meaning and values in test validation: the science and ethics of assessment. Educ Res. 1989;18:5–11. [Google Scholar]
- 9.Beckman TJ, Cook DA, Mandrekar JN. What is the validity evidence for assessments of clinical teaching? J Gen Intern Med. 2005;20:1159–64. doi: 10.1111/j.1525-1497.2005.0258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48:375–85. doi: 10.1111/medu.12391. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed Y, Scott IU, Greenberg PB. A survey of the role of virtual surgery simulators in ophthalmic graduate medical education. Graefes Arch Clin Exp Ophthalmol. 2011;249:1263–5. doi: 10.1007/s00417-010-1537-0. [DOI] [PubMed] [Google Scholar]
- 12.Belyea DA, Brown SE, Rajjoub LZ. Influence of surgery simulator training on ophthalmology resident phacoemulsification performance. J Cataract Refract Surg. 2011;37:1756–61. doi: 10.1016/j.jcrs.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Daly MK, Gonzalez E, Siracuse-Lee D, Legutko PA. Efficacy of surgical simulator training versus traditional wet-lab training on operating room performance of ophthalmology residents during the capsulorhexis in cataract surgery. J Cataract Refract Surg. 2013;39:1734–41. doi: 10.1016/j.jcrs.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 14.Feudner EM, Engel C, Neuhann IM, Petermeier K, Bartz-Schmidt KU, Szurman P. Virtual reality training improves wet-lab performance of capsulorhexis: results of a randomized, controlled study. Graefes Arch Clin Exp Ophthalmol. 2009;247:955–63. doi: 10.1007/s00417-008-1029-7. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Gonzalez LA, Payal AR, Gonzalez-Monroy JE, Daly MK. Ophthalmic surgical simulation in training dexterity in dominant and nondominant hands: results from a pilot study. J Surg Educ. 2016;73:699–708. doi: 10.1016/j.jsurg.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Le TD, Adatia FA, Lam WC. Virtual reality ophthalmic surgical simulation as a feasible training and assessment tool: results of a multicentre study. Can J Ophthalmol. 2011;46:56–60. doi: 10.3129/i10-051. [DOI] [PubMed] [Google Scholar]
- 17.Li E, Fay P, Greenberg PB. A virtual cataract surgery course for ophthalmologists-in-training. R I Med J. 2013;96:18–9. [PubMed] [Google Scholar]
- 18.Li E, Paul AA, Greenberg PB. A revised simulation-based cataract surgery course for ophthalmology residents. R I Med J. 2016;99:26–7. [PubMed] [Google Scholar]
- 19.McCannel CA. Continuous curvilinear capsulorhexis training and non-rhexis related vitreous loss: the specificity of virtual reality simulator surgical training (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2017;115:T2. [PMC free article] [PubMed] [Google Scholar]
- 20.Nathoo N, Ng M, Ramstead CL, Johnson MC. Comparing performance of junior and senior ophthalmology residents on an intraocular surgical simulator. Can J Ophthalmol. 2011;46:87–8. doi: 10.3129/i10-065. [DOI] [PubMed] [Google Scholar]
- 21.Pokroy R, Du E, Alzaga A, Khodadadeh S, Steen D, Bachynski B, et al. Impact of simulator training on resident cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2013;251:777–81. doi: 10.1007/s00417-012-2160-z. [DOI] [PubMed] [Google Scholar]
- 22.Privett B, Greenlee E, Rogers G, Oetting TA. Construct validity of a surgical simulator as a valid model for capsulorhexis training. J Cataract Refract Surg. 2010;36:1835–8. doi: 10.1016/j.jcrs.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Roohipoor R, Yaseri M, Teymourpour A, Kloek C, Miller JB, Loewenstein JI. Early performance on an eye surgery simulator predicts subsequent resident surgical performance. J Surg Educ. 2017;74:1105–15. doi: 10.1016/j.jsurg.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Saleh GM, Theodoraki K, Gillan S, Sullivan P, O’Sullivan F, Hussain B, et al. The development of a virtual reality training programme for ophthalmology: repeatability and reproducibility (part of the International Forum for Ophthalmic Simulation Studies) Eye. 2013;27:1269–74. doi: 10.1038/eye.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvander M, Asman P. Virtual reality cataract surgery training: learning curves and concurrent validity. Acta Ophthalmol. 2012;90:412–7. doi: 10.1111/j.1755-3768.2010.02028.x. [DOI] [PubMed] [Google Scholar]
- 26.Selvander M, Asman P. Ready for OR or not? Human reader supplements Eyesi scoring in cataract surgical skills assessment. Clin Ophthalmol. 2013;7:1973–7. doi: 10.2147/OPTH.S48374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solverson DJ, Mazzoli RA, Raymond WR, Nelson ML, Hansen EA, Torres MF, et al. Virtual reality simulation in acquiring and differentiating basic ophthalmic microsurgical skills. Simul Healthc. 2009;4:98–103. doi: 10.1097/SIH.0b013e318195419e. [DOI] [PubMed] [Google Scholar]
- 28.Spiteri AV, Aggarwal R, Kersey TL, Sira M, Benjamin L, Darzi AW, et al. Development of a virtual reality training curriculum for phacoemulsification surgery. Eye. 2014;28:78–84. doi: 10.1038/eye.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staropoli PC, Gregori NZ, Junk AK, Galor A, Goldhardt R, Goldhagen BE, et al. Surgical simulation training reduces intraoperative cataract surgery complications among residents. Simul Healthc. 2018;13:11–5. doi: 10.1097/SIH.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen AS, Kiilgaard JF, Kjaerbo H, la Cour M, Konge L. Simulation-based certification for cataract surgery. Acta Ophthalmol. 2015;93:416–21. doi: 10.1111/aos.12691. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen AS, Smith P, Subhi Y, Cour M, Tang L, Saleh GM, et al. High correlation between performance on a virtual-reality simulator and real-life cataract surgery. Acta Ophthalmol. 2017;95:307–11. doi: 10.1111/aos.13275. [DOI] [PubMed] [Google Scholar]
- 32.Lucas L, Schellini SA, Lottelli AC. Complications in the first 10 phacoemulsification cataract surgeries with and without prior simulator training. Arq Bras Oftalmol. 2019;82:289–94. doi: 10.5935/0004-2749.20190057. [DOI] [PubMed] [Google Scholar]
- 33.Ng DS, Sun Z, Young AL, Ko ST, Lok JK, Lai TY, et al. Impact of virtual reality simulation on learning barriers of phacoemulsification perceived by residents. Clin Ophthalmol. 2018;12:885–93. doi: 10.2147/OPTH.S140411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colne J, Conart JB, Luc A, Perrenot C, Berrod JP, Angioi-Duprez K. EyeSi surgical simulator: construct validity of capsulorhexis, phacoemulsification and irrigation and aspiration modules. J Fr Ophtalmol. 2019;42:49–56. doi: 10.1016/j.jfo.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Ferris JD, Donachie PH, Johnston RL, Barnes B, Olaitan M, Sparrow JM. Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 6. The impact of EyeSi virtual reality training on complications rates of cataract surgery performed by first and second year trainees. Br J Ophthalmol. 2019;104:324–9. doi: 10.1136/bjophthalmol-2018-313817. [DOI] [PubMed] [Google Scholar]
- 36.la Cour M, Thomsen ASS, Alberti M, Konge L. Simulators in the training of surgeons: is it worth the investment in money and time? 2018 Jules Gonin lecture of the Retina Research Foundation. Graefes Arch Clin Exp Ophthalmol. 2019;257:877–81. doi: 10.1007/s00417-019-04244-y. [DOI] [PubMed] [Google Scholar]
- 37.Bozkurt Oflaz A, Ekinci Koktekir B, Okudan S. Does cataract surgery simulation correlate with real-life experience? Turk J Ophthalmol. 2018;48:122–6. doi: 10.4274/tjo.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter JM, Lee R, Sharp JA, Foss AJ. Intensive cataract training study G. Intensive cataract training: a novel approach. Eye. 2013;27:742–6. doi: 10.1038/eye.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch F, Koss MJ, Singh P, Naser H. Virtual reality in ophthalmology. Klin Monbl Augenheilkd. 2009;226:672–6. doi: 10.1055/s-0028-1109383. [DOI] [PubMed] [Google Scholar]
- 40.Park L, Song JJ, Dodick JM, Helveston EM. The EYESI 2.2 ophthalmosurgical simulator: is it a good teaching tool? Investig Ophthalmol Vis Sci. 2006;47:4421. [Google Scholar]
- 41.Rossi JV, Verma D, Fujii GY, Lakhanpal RR, Wu SL, Humayun MS, et al. Virtual vitreoretinal surgical simulator as a training tool. Retina. 2004;24:231–6. doi: 10.1097/00006982-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Vergmann AS, Vestergaard AH, Grauslund J. Virtual vitreoretinal surgery: validation of a training programme. Acta Ophthalmol. 2017;95:60–5. doi: 10.1111/aos.13209. [DOI] [PubMed] [Google Scholar]
- 43.Cisse C, Angioi K, Luc A, Berrod JP, Conart JB. EYESI surgical simulator: validity evidence of the vitreoretinal modules. Acta Ophthalmol. 2019;97:e277–e82. doi: 10.1111/aos.13910. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee PP, Edward DP, Liang S, Bouchard CS, Bryar PJ, Ahuja R, et al. Concurrent and face validity of a capsulorhexis simulation with respect to human patients. Stud Health Technol Inform. 2012;173:35–41. [PubMed] [Google Scholar]
- 45.Sikder S, Luo J, Banerjee PP, Luciano C, Kania P, Song JC, et al. The use of a virtual reality surgical simulator for cataract surgical skill assessment with 6 months of intervening operating room experience. Clin Ophthalmol. 2015;9:141–9. doi: 10.2147/OPTH.S69970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozak I, Banerjee P, Luo J, Luciano C. Virtual reality simulator for vitreoretinal surgery using integrated OCT data. Clin Ophthalmol. 2014;8:669–72. doi: 10.2147/OPTH.S58614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricci LH, Ferraz CA. Ophthalmoscopy simulation: advances in training and practice for medical students and young ophthalmologists. Adv Med Educ Pract. 2017;8:435–9. doi: 10.2147/AMEP.S108041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borgersen NJ, Skou Thomsen AS, Konge L, Sorensen TL, Subhi Y. Virtual reality-based proficiency test in direct ophthalmoscopy. Acta Ophthalmol. 2018;96:e259–e61. doi: 10.1111/aos.13546. [DOI] [PubMed] [Google Scholar]
- 49.Boden KT, Rickmann A, Fries FN, Xanthopoulou K, Alnaggar D, Januschowski K, et al. Evaluation of a virtual reality simulator for learning direct ophthalmoscopy in student teaching. Ophthalmologe. 2019;117:44–49. doi: 10.1007/s00347-019-0909-z. [DOI] [PubMed] [Google Scholar]
- 50.Chou J, Kosowsky T, Payal AR, Gonzalez Gonzalez LA, Daly MK. Construct and face validity of the Eyesi Indirect Ophthalmoscope Simulator. Retina. 2017;37:1967–76. doi: 10.1097/IAE.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 51.Loidl M, Schneider A, Keis O, Ochsner W, Grab-Kroll C, Lang GK, et al. Augmented reality in ophthalmology: technical innovation complements education for medical students. Klin Monbl Augenheilkd. 2019. [Epub ahead of print]. [DOI] [PubMed]
- 52.Agus M, Gobbetti E, Pintore G, Zanetti G, Zorcolo A. Real time simulation of phaco-emulsification for cataract surgery training. The third workshop on virtual reality interactions and physical simulations (VRIPHYS 2006). Madrid: The Eurographics Association; 2006. p. 10.
- 53.Choi KS, Soo S, Chung FL. A virtual training simulator for learning cataract surgery with phacoemulsification. Comput Biol Med. 2009;39:1020–31. doi: 10.1016/j.compbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Laurell CG, Soderberg P, Nordh L, Skarman E, Nordqvist P. Computer-simulated phacoemulsification. Ophthalmology. 2004;111:693–8. doi: 10.1016/j.ophtha.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 55.Hikichi T, Yoshida A, Igarashi S, Mukai N, Harada M, Muroi K, et al. Vitreous surgery simulator. Arch Ophthalmol. 2000;118:1679–81. doi: 10.1001/archopht.118.12.1679. [DOI] [PubMed] [Google Scholar]
- 56.Jonas JB, Rabethge S, Bender HJ. Computer-assisted training system for pars plana vitrectomy. Acta Ophthalmol Scand. 2003;81:600–4. doi: 10.1046/j.1395-3907.2003.0078.x. [DOI] [PubMed] [Google Scholar]
- 57.Neumann PF, Sadler LL, Gieser J, editors. Virtual reality vitrectomy simulator. Medical image computing and computer-assisted intervention—MICCAI’98. Berlin: Springer Berlin Heidelberg; 1998.
- 58.Peugnet F, Dubois P, Rouland JF. Virtual reality versus conventional training in retinal photocoagulation: a first clinical assessment. Comput Aided Surg. 1998;3:20–6. doi: 10.1002/(SICI)1097-0150(1998)3:1<20::AID-IGS3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Verma D, Wills D, Verma M. Virtual reality simulator for vitreoretinal surgery. Eye. 2003;17:71–3. doi: 10.1038/sj.eye.6700166. [DOI] [PubMed] [Google Scholar]
- 60.Weiss M, Lauer SA, Fried MP, Uribe J, Sadoughi B. Endoscopic endonasal surgery simulator as a training tool for ophthalmology residents. Ophthalmic Plast Reconstr Surg. 2008;24:460–4. doi: 10.1097/IOP.0b013e31818aaf80. [DOI] [PubMed] [Google Scholar]
- 61.Hunter IW, Jones LA, Sagar MA, Lafontaine SR, Hunter PJ. Ophthalmic microsurgical robot and associated virtual environment. Comput Biol Med. 1995;25:173–82. doi: 10.1016/0010-4825(94)00042-o. [DOI] [PubMed] [Google Scholar]
- 62.Sinclair MJ, Peifer JW, Haleblian R, Luxenberg MN, Green K, Hull DS. Computer-simulated eye surgery. A novel teaching method for residents and practitioners. Ophthalmology. 1995;102:517–21. doi: 10.1016/s0161-6420(95)30992-x. [DOI] [PubMed] [Google Scholar]
- 63.Merril JR, Notaroberto NF, Laby DM, Rabinowitz AM, Piemme TE. The Ophthalmic Retrobulbar Injection Simulator (ORIS): an application of virtual reality to medical education. Proc Annu Symp Comput Appl Med Care. 1992:702–6. [PMC free article] [PubMed]
- 64.Mustafa M, Montgomery J, Atta H. A novel educational tool for teaching ocular ultrasound. Clin Ophthalmol. 2011;5:857–60. doi: 10.2147/OPTH.S19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dada VK, Sindhu N. Cataract in enucleated goat eyes: training model for phacoemulsification. J Cataract Refract Surg. 2000;26:1114–6. doi: 10.1016/s0886-3350(00)00448-x. [DOI] [PubMed] [Google Scholar]
- 66.Farooqui JH, Jaramillo A, Sharma M, Gomaa A. Use of modified international council of ophthalmology- ophthalmology surgical competency assessment rubric (ICO-OSCAR) for phacoemulsification- wet lab training in residency program. Indian J Ophthalmol. 2017;65:898–9. doi: 10.4103/ijo.IJO_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hashimoto C, Kurosaka D, Uetsuki Y. Teaching continuous curvilinear capsulorhexis using a postmortem pig eye with simulated cataract(2)(2) J Cataract Refract Surg. 2001;27:814–6. doi: 10.1016/s0886-3350(00)00728-8. [DOI] [PubMed] [Google Scholar]
- 68.Kayikcioglu O, Egrilmez S, Emre S, Erakgun T. Human cataractous lens nucleus implanted in a sheep eye lens as a model for phacoemulsification training. J Cataract Refract Surg. 2004;30:555–7. doi: 10.1016/j.jcrs.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 69.Leuschke R, Bhandari A, Sires B, Hannaford B. Low cost eye surgery simulator with skill assessment component. Stud Health Technol Inform. 2007;125:286–91. [PubMed] [Google Scholar]
- 70.Liu ES, Eng KT, Braga-Mele R. Using medical lubricating jelly in human cadaver eyes to teach ophthalmic surgery. J Cataract Refract Surg. 2001;27:1545–7. doi: 10.1016/s0886-3350(01)00898-7. [DOI] [PubMed] [Google Scholar]
- 71.Mekada A, Nakajima J, Nakamura J, Hirata H, Kishi T, Kani K. Cataract surgery training using pig eyes filled with chestnuts of various hardness. J Cataract Refract Surg. 1999;25:622–5. doi: 10.1016/s0886-3350(99)00003-6. [DOI] [PubMed] [Google Scholar]
- 72.Pandey SK, Werner L, Vasavada AR, Apple DJ. Induction of cataracts of varying degrees of hardness in human eyes obtained postmortem for cataract surgeon training. Am J Ophthalmol. 2000;129:557–8. doi: 10.1016/s0002-9394(99)00437-7. [DOI] [PubMed] [Google Scholar]
- 73.Puri S, Srikumaran D, Prescott C, Tian J, Sikder S. Assessment of resident training and preparedness for cataract surgery. J Cataract Refract Surg. 2017;43:364–8. doi: 10.1016/j.jcrs.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 74.Ruggiero J, Keller C, Porco T, Naseri A, Sretavan DW. Rabbit models for continuous curvilinear capsulorhexis instruction. J Cataract Refract Surg. 2012;38:1266–70. doi: 10.1016/j.jcrs.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saraiva VS, Casanova FH. Cataract induction in pig eyes using viscoelastic endothelial protection and a formaldehyde-methanol mixture. J Cataract Refract Surg. 2003;29:1479–81. doi: 10.1016/s0886-3350(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 76.Sengupta S, Dhanapal P, Nath M, Haripriya A, Venkatesh R. Goat’s eye integrated with a human cataractous lens: a training model for phacoemulsification. Indian J Ophthalmol. 2015;63:275–7. doi: 10.4103/0301-4738.156937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudan R, Titiyal JS, Rai H, Chandra P. Formalin-induced cataract in goat eyes as a surgical training model for phacoemulsification. J Cataract Refract Surg. 2002;28:1904–6. doi: 10.1016/s0886-3350(02)01327-5. [DOI] [PubMed] [Google Scholar]
- 78.Sugiura T, Kurosaka D, Uezuki Y, Eguchi S, Obata H, Takahashi T. Creating cataract in a pig eye. J Cataract Refract Surg. 1999;25:615–21. doi: 10.1016/s0886-3350(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 79.Tolentino FI, Liu HS. A laboratory animal model for phacoemulsification practice. Am J Ophthalmol. 1975;80:545–6. doi: 10.1016/0002-9394(75)90225-1. [DOI] [PubMed] [Google Scholar]
- 80.van Vreeswijk H, Pameyer JH. Inducing cataract in postmortem pig eyes for cataract surgery training purposes. J Cataract Refract Surg. 1998;24:17–8. doi: 10.1016/s0886-3350(98)80068-0. [DOI] [PubMed] [Google Scholar]
- 81.Abrams GW, Topping T, Machemer R. An improved method for practice vitrectomy. Arch Ophthalmol. 1978;96:521–5. doi: 10.1001/archopht.1978.03910050289022. [DOI] [PubMed] [Google Scholar]
- 82.Dang Y, Waxman S, Wang C, Parikh HA, Bussel II, Loewen RT, et al. Rapid learning curve assessment in an ex vivo training system for microincisional glaucoma surgery. Sci Rep. 2017;7:1605. doi: 10.1038/s41598-017-01815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee GA, Chiang MY, Shah P. Pig eye trabeculectomy-a wet-lab teaching model. Eye. 2006;20:32–7. doi: 10.1038/sj.eye.6701784. [DOI] [PubMed] [Google Scholar]
- 84.Patel HI, Levin AV. Developing a model system for teaching goniotomy. Ophthalmology. 2005;112:968–73. doi: 10.1016/j.ophtha.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 85.Patel SP, Sit AJ. A practice model for trabecular meshwork surgery. Arch Ophthalmol. 2009;127:311–3. doi: 10.1001/archophthalmol.2008.536. [DOI] [PubMed] [Google Scholar]
- 86.Arora A, Nazarali S, Sawatzky L, Gooi M, Schlenker M, Ahmed IK, et al. K-RIM (Corneal Rim) angle surgery training model. J Glaucoma. 2019;28:146–9. doi: 10.1097/IJG.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 87.Nazarali S, Arora A, Ford B, Schlenker M, Ahmed IK, Poulis B, et al. Cadaver corneoscleral model for angle surgery training. J Cataract Refract Surg. 2019;45:76–9. doi: 10.1016/j.jcrs.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 88.Droutsas K, Petrak M, Melles GR, Koutsandrea C, Georgalas I, Sekundo W. A simple ex vivo model for teaching Descemet membrane endothelial keratoplasty. Acta Ophthalmol. 2014;92:e362–5. doi: 10.1111/aos.12371. [DOI] [PubMed] [Google Scholar]
- 89.Fontana L, Dal Pizzol MM, Tassinari G. Experimental model for learning and practicing big-bubble deep anterior lamellar keratoplasty. J Cataract Refract Surg. 2008;34:710–1. doi: 10.1016/j.jcrs.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 90.Vasquez Perez A, Liu C. Human ex vivo artificial anterior chamber model for practice DMEK surgery. Cornea. 2017;36:394–7. doi: 10.1097/ICO.0000000000001112. [DOI] [PubMed] [Google Scholar]
- 91.Famery N, Abdelmassih Y, El-Khoury S, Guindolet D, Cochereau I, Gabison EE. Artificial chamber and 3D printed iris: a new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol. 2019;97:e179–e83. doi: 10.1111/aos.13852. [DOI] [PubMed] [Google Scholar]
- 92.White CA, Wrzosek JA, Chesnutt DA, Enyedi LB, Cabrera MT. A novel method for teaching key steps of strabismus surgery in the wet lab. J AAPOS. 2015;19:468–70.e1. doi: 10.1016/j.jaapos.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 93.Vagge A, Gunton K, Schnall B. Impact of a strabismus surgery suture course for first- and second-year ophthalmology residents. J Pediatr Ophthalmol Strabismus. 2017;54:339–45. doi: 10.3928/01913913-20170703-17. [DOI] [PubMed] [Google Scholar]
- 94.Kersey TL. Split pig head as a teaching model for basic oculoplastic procedures. Ophthalmic Plast Reconstr Surg. 2009;25:253. doi: 10.1097/IOP.0b013e3181a394c1. [DOI] [PubMed] [Google Scholar]
- 95.Pfaff AJ. Pig eyelid as a teaching model for eyelid margin repair. Ophthalmic Plast Reconstr Surg. 2004;20:383–4. doi: 10.1097/01.iop.0000134269.82082.a8. [DOI] [PubMed] [Google Scholar]
- 96.Zou C, Wang JQ, Guo X, Wang TL. Pig eyelid as a teaching model for severe ptosis repair. Ophthalmic Plast Reconstr Surg. 2012;28:472–4. doi: 10.1097/IOP.0b013e31826a5146. [DOI] [PubMed] [Google Scholar]
- 97.Patel SR, Mishall P, Barmettler A. A human cadaveric model for effective instruction of lateral canthotomy and cantholysis. Orbit. 2020;39:87–92. doi: 10.1080/01676830.2019.1600151. [DOI] [PubMed] [Google Scholar]
- 98.Altunrende ME, Hamamcioglu MK, Hicdonmez T, Akcakaya MO, Birgili B, Cobanoglu S. Microsurgical training model for residents to approach to the orbit and the optic nerve in fresh cadaveric sheep cranium. J Neurosci Rural Pract. 2014;5:151–4. doi: 10.4103/0976-3147.131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mednick Z, Tabanfar R, Alexander A, Simpson S, Baxter S. Creation and validation of a simulator for corneal rust ring removal. Can J Ophthalmol. 2017;52:447–52. doi: 10.1016/j.jcjo.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 100.Pujari A, Kumar S, Markan A, Chawla R, Damodaran S, Kumar A. Buckling surgery on a goat’s eye: A simple technique to enhance residents’ surgical skill. Indian J Ophthalmol. 2019;67:1327–8. doi: 10.4103/ijo.IJO_1779_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uhlig CE, Gerding H. A dummy orbit for training in diagnostic procedures and laser surgery with enucleated eyes. Am J Ophthalmol. 1998;126:464–6. doi: 10.1016/s0002-9394(98)00111-1. [DOI] [PubMed] [Google Scholar]
- 102.Auffarth GU, Wesendahl TA, Solomon KD, Brown SJ, Apple DJ. A modified preparation technique for closed-system ocular surgery of human eyes obtained postmortem: an improved research and teaching tool. Ophthalmology. 1996;103:977–82. doi: 10.1016/s0161-6420(96)30576-9. [DOI] [PubMed] [Google Scholar]
- 103.Lenart TD, McCannel CA, Baratz KH, Robertson DM. A contact lens as an artificial cornea for improved visualization during practice surgery on cadaver eyes. Arch Ophthalmol. 2003;121:16–9. doi: 10.1001/archopht.121.1.16. [DOI] [PubMed] [Google Scholar]
- 104.Borirak-chanyavat S, Lindquist TD, Kaplan HJ. A cadaveric eye model for practicing anterior and posterior segment surgeries. Ophthalmology. 1995;102:1932–5. doi: 10.1016/s0161-6420(95)30773-7. [DOI] [PubMed] [Google Scholar]
- 105.Ramakrishnan S, Baskaran P, Fazal R, Sulaiman SM, Krishnan T, Venkatesh R. Spring-action Apparatus for Fixation of Eyeball (SAFE): a novel, cost-effective yet simple device for ophthalmic wet-lab training. Br J Ophthalmol. 2016;100:1317–21. doi: 10.1136/bjophthalmol-2015-308330. [DOI] [PubMed] [Google Scholar]
- 106.Mohammadi SF, Mazouri A, Jabbarvand M, Rahman AN, Mohammadi A. Sheep practice eye for ophthalmic surgery training in skills laboratory. J Cataract Refract Surg. 2011;37:987–91. doi: 10.1016/j.jcrs.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 107.Porrello G, Giudiceandrea A, Salgarello T, Tamburrelli C, Scullica L. A new device for ocular surgical training on enucleated eyes. Ophthalmology. 1999;106:1210–3. doi: 10.1016/S0161-6420(99)90253-1. [DOI] [PubMed] [Google Scholar]
- 108.Todorich B, Shieh C, DeSouza PJ, Carrasco-Zevallos OM, Cunefare DL, Stinnett SS, et al. Impact of microscope-integrated OCT on ophthalmology resident performance of anterior segment surgical maneuvers in model eyes. Investig Ophthalmol Vis Sci. 2016;57:146–53. doi: 10.1167/iovs.15-18818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ezra DG, Aggarwal R, Michaelides M, Okhravi N, Verma S, Benjamin L, et al. Skills acquisition and assessment after a microsurgical skills course for ophthalmology residents. Ophthalmology. 2009;116:257–62. doi: 10.1016/j.ophtha.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 110.Fisher JB, Binenbaum G, Tapino P, Volpe NJ. Development and face and content validity of an eye surgical skills assessment test for ophthalmology residents. Ophthalmology. 2006;113:2364–70. doi: 10.1016/j.ophtha.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 111.Taylor JB, Binenbaum G, Tapino P, Volpe NJ. Microsurgical lab testing is a reliable method for assessing ophthalmology residents’ surgical skills. Br J Ophthalmol. 2007;91:1691–4. doi: 10.1136/bjo.2007.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abellan E, Calles-Vazquez MC, Cadarso L, Sanchez FM, Uson J. Design and validation of a simulator for training in continuous circular capsulotomy for phacoemulsification. Arch Soc Esp Oftalmol. 2013;88:387–92. doi: 10.1016/j.oftal.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 113.Hirata A, Iwakiri R, Okinami S. A simulated eye for vitreous surgery using Japanese quail eggs. Graefes Arch Clin Exp Ophthalmol. 2013;251:1621–4. doi: 10.1007/s00417-012-2247-6. [DOI] [PubMed] [Google Scholar]
- 114.Yeh S, Chan-Kai BT, Lauer AK. Basic training module for vitreoretinal surgery and the Casey Eye Institute Vitrectomy Indices Tool for Skills Assessment. Clin Ophthalmol. 2011;5:1249–56. doi: 10.2147/OPTH.S23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uhlig CE, Gerding H. Illuminated artificial orbit for the training of vitreoretinal surgery in vitro. Eye. 2004;18:183–7. doi: 10.1038/sj.eye.6700596. [DOI] [PubMed] [Google Scholar]
- 116.Iyer MN, Han DP. An eye model for practicing vitreoretinal membrane peeling. Arch Ophthalmol. 2006;124:108–10. doi: 10.1001/archopht.124.1.108. [DOI] [PubMed] [Google Scholar]
- 117.Rice JC, Steffen J, du Toit L. Simulation training in vitreoretinal surgery: a low-cost, medium-fidelity model. Retina. 2017;37:409–12. doi: 10.1097/IAE.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 118.Omata S, Someya Y, Adachi S, Masuda T, Hayakawa T, Harada K, et al. A surgical simulator for peeling the inner limiting membrane during wet conditions. PLoS ONE. 2018;13:e0196131. doi: 10.1371/journal.pone.0196131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adebayo T, Abendroth M, Elera GG, Kunselman A, Sinz E, Ely A, et al. Developing and validating a simple and cost-effective strabismus surgery simulator. J AAPOS. 2018;22:85–8 e2. doi: 10.1016/j.jaapos.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 120.Ganne P, Krishnappa NC, Baskaran P, Sulaiman SM, Venkatesh R. Retinal laser and photography practice eye model: a cost-effective innovation to improve training through simulation. Retina. 2018;38:207–10. doi: 10.1097/IAE.0000000000001885. [DOI] [PubMed] [Google Scholar]
- 121.Moisseiev E, Michaeli A. Simulation of neodymium:YAG posterior capsulotomy for ophthalmologists in training. J Cataract Refract Surg. 2014;40:175–8. doi: 10.1016/j.jcrs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 122.Simpson SM, Schweitzer KD, Johnson DE. Design and validation of a training simulator for laser capsulotomy, peripheral iridotomy, and retinopexy. Ophthalmic Surg Lasers Imaging Retina. 2017;48:56–61. doi: 10.3928/23258160-20161219-08. [DOI] [PubMed] [Google Scholar]
- 123.Weidenthal DT. The use of a model eye to gain endophotocoagulation skills. Arch Ophthalmol. 1987;105:1020. doi: 10.1001/archopht.1987.01060080018011. [DOI] [PubMed] [Google Scholar]
- 124.Coats DK. A simple model for practicing surgery on the nasolacrimal drainage system. J AAPOS. 2004;8:509–10. doi: 10.1016/j.jaapos.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 125.Adams JW, Paxton L, Dawes K, Burlak K, Quayle M, McMenamin PG. 3D printed reproductions of orbital dissections: a novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br J Ophthalmol. 2015;99:1162–7. doi: 10.1136/bjophthalmol-2014-306189. [DOI] [PubMed] [Google Scholar]
- 126.Scawn RL, Foster A, Lee BW, Kikkawa DO, Korn BS. Customised 3D printing: an innovative training tool for the next generation of orbital surgeons. Orbit. 2015;34:216–9. doi: 10.3109/01676830.2015.1049367. [DOI] [PubMed] [Google Scholar]
- 127.Marson BA, Sutton LJ. The Newport eye: design and initial evaluation of a novel foreign body training phantom. Emerg Med J. 2014;31:329–30. doi: 10.1136/emermed-2012-202230. [DOI] [PubMed] [Google Scholar]
- 128.Akaishi Y, Otaki J, Takahashi O, Breugelmans R, Kojima K, Seki M, et al. Validity of direct ophthalmoscopy skill evaluation with ocular fundus examination simulators. Can J Ophthalmol. 2014;49:377–81. doi: 10.1016/j.jcjo.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 129.Chew C, Gray RH. A model eye to practice indentation during indirect ophthalmoscopy. Eye. 1993;7:599–600. doi: 10.1038/eye.1993.133. [DOI] [PubMed] [Google Scholar]
- 130.Kumar KS, Shetty KB. A new model eye system for practicing indirect ophthalmoscopy. Indian J Ophthalmol. 1996;44:233–4. [PubMed] [Google Scholar]
- 131.Lewallen S. A simple model for teaching indirect ophthalmoscopy. Br J Ophthalmol. 2006;90:1328–9. doi: 10.1136/bjo.2006.096784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McCarthy DM, Leonard HR, Vozenilek JA. A new tool for testing and training ophthalmoscopic skills. J Grad Med Educ. 2012;4:92–6. doi: 10.4300/JGME-D-11-00052.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller KE. Origami model for teaching binocular indirect ophthalmoscopy. Retina. 2015;35:1711–2. doi: 10.1097/IAE.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 134.Morris WR. A simple model for demonstrating abnormal slitlamp findings. Arch Ophthalmol. 1998;116:93–4. doi: 10.1001/archopht.116.1.93. [DOI] [PubMed] [Google Scholar]
- 135.Romanchuk KG. Enhanced models for teaching slit-lamp skills. Can J Ophthalmol. 2003;38:507–11. doi: 10.1016/s0008-4182(03)80032-7. [DOI] [PubMed] [Google Scholar]
- 136.Kylstra JA, Diaz JD. A simple eye model for practicing indirect ophthalmoscopy and retinal laser photocoagulation. Digit J Ophthalmol. 2019;25:1–4. doi: 10.5693/djo.01.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaufman D, Lee S. Formative evaluation of a multimedia CAL program in an ophthalmology clerkship. Med Teach. 1993;15:327–40. doi: 10.3109/01421599309006655. [DOI] [PubMed] [Google Scholar]
- 138.Stahl A, Boeker M, Ehlken C, Agostini H, Reinhard T. Evaluation of an internet-based e-learning ophthalmology module for medical students. Ophthalmologe. 2009;106:999–1005. doi: 10.1007/s00347-009-1916-2. [DOI] [PubMed] [Google Scholar]
- 139.Prinz A, Bolz M, Findl O. Advantage of three dimensional animated teaching over traditional surgical videos for teaching ophthalmic surgery: a randomised study. Br J Ophthalmol. 2005;89:1495–9. doi: 10.1136/bjo.2005.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Glittenberg C, Binder S. Using 3D computer simulations to enhance ophthalmic training. Ophthalmic Physiol Opt. 2006;26:40–9. doi: 10.1111/j.1475-1313.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 141.Henderson BA, Kim JY, Golnik KC, Oetting TA, Lee AG, Volpe NJ, et al. Evaluation of the virtual mentor cataract training program. Ophthalmology. 2010;117:253–8. doi: 10.1016/j.ophtha.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 142.Cook DA, Brydges R, Zendejas B, Hamstra SJ, Hatala R. Technology-enhanced simulation to assess health professionals: a systematic review of validity evidence, research methods, and reporting quality. Acad Med. 2013;88:872–83. doi: 10.1097/ACM.0b013e31828ffdcf. [DOI] [PubMed] [Google Scholar]
- 143.Wood TC, Raison N, Haldar S, Brunckhorst O, McIlhenny C, Dasgupta P, et al. Training tools for nontechnical skills for surgeons—a systematic review. J Surg Educ. 2017;74:548–78. doi: 10.1016/j.jsurg.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 144.Saleh GM, Wawrzynski JR, Saha K, Smith P, Flanagan D, Hingorani M, et al. Feasibility of human factors immersive simulation training in ophthalmology: the London Pilot. JAMA Ophthalmol. 2016;134:905–11. doi: 10.1001/jamaophthalmol.2016.1769. [DOI] [PubMed] [Google Scholar]
- 145.Downing SM. Face validity of assessments: faith-based interpretations or evidence-based science? Med Educ. 2006;40:7–8. doi: 10.1111/j.1365-2929.2005.02361.x. [DOI] [PubMed] [Google Scholar]
- 146.Musbahi O, Aydin A, Al Omran Y, Skilbeck CJ, Ahmed K. Current status of simulation in otolaryngology: a systematic review. J Surg Educ. 2017;74:203–15. doi: 10.1016/j.jsurg.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 147.Morgan M, Aydin A, Salih A, Robati S, Ahmed K. Current status of simulation-based training tools in orthopedic surgery: a systematic review. J Surg Educ. 2017;74:698–716. doi: 10.1016/j.jsurg.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 148.Borgersen NJ, Naur TMH, Sorensen SMD, Bjerrum F, Konge L, Subhi Y, et al. Gathering validity evidence for surgical simulation: a systematic review. Ann Surg. 2018;267:1063–8. doi: 10.1097/SLA.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 149.Lowry EA, Porco TC, Naseri A. Cost analysis of virtual-reality phacoemulsification simulation in ophthalmology training programs. J Cataract Refract Surg. 2013;39:1616–7. doi: 10.1016/j.jcrs.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 150.Young BK, Greenberg PB. Is virtual reality training for resident cataract surgeons cost effective? Graefes Arch Clin Exp Ophthalmol. 2013;251:2295–6. doi: 10.1007/s00417-013-2317-4. [DOI] [PubMed] [Google Scholar]


