Abstract
Nontransgenic and 3xTG transgenic mice, which express mutant transgenes encoding human amyloid precursor protein (hAPP) along with Alzheimer disease (AD)-associated versions of hTau and a presenilin mutation, acquired the Barnes Maze escape task equivalently at 3–9 months of age. Although nontransgenics retested at 6 and 9 months acquired the escape task more quickly than naïve mice, 3xTG mice did not. Deficits in Kalirin, a multidomain protein scaffold and guanine nucleotide exchange factor that regulates dendritic spines, has been proposed as a contributor to the cognitive decline observed in AD. To test whether deficits in Kalirin might amplify deficits in 3xTG mice, mice heterozygous/hemizygous for Kalirin and the 3xTG transgenes were generated. Mouse strain, age and sex affected cortical expression of key proteins. hAPP levels in 3xTG mice increased total APP levels at all ages. Kalirin expression showed strong sex-dependent expression in C57 but not B6129 mice. Decreasing Kalirin levels to half had no effect on Barnes Maze task acquisition or retraining in 3xTG hemizygous mice.
Keywords: 3xTG-AD, Rotarod, Western analysis, peptide, prohormone convertase, peptidylglycine alpha-amidating monooxygenase, Barnes Maze
Introduction
Alzheimer Disease (AD) is very age dependent and was initially described as a human disease (Carlsson et al., 2017; Gotz et al., 2018). Many experimental animal systems show several age-dependent symptoms similar to aspects of human aging, such as slower movement, loss of coordination, degradation of vision and hearing, plus Type II diabetes. However, the massive losses in cortical tissue, along with deposition of extracellular β-amyloid plaques and intracellular phosphorylated Tau tangles, are uniquely human (Carlsson et al., 2017; Carmona et al., 2018; Neuner et al., 2019). Understanding the underlying causes of AD is essential if the long string of failed therapeutic trials can ever achieve successful closure (Doig et al., 2017; Gotz et al., 2018; Lutz and Peng, 2018; Martini et al., 2018). Recent genetic linkage studies have expanded the list of proteins implicated in AD from a dozen to a few hundred, including apolipoprotein E isoforms, aggregatin (FAM222A) and members of neuroinflammation and membrane trafficking pathways (Carlsson et al., 2017; Carmona et al., 2018; Castillo et al., 2017; Gotz et al., 2018; Lutz and Peng, 2018; Neuner et al., 2019; Yan et al., 2020).
Because nontransgenic experimental animals generally do not develop neurocognitive or neuroanatomical AD symptoms up to at least one year of age (no amyloid plaques, no Tau tangles, little memory impairment) (Gotz et al., 2018), transgenic animal models of AD often incorporate AD-associated human or humanized mutant amyloid precursor protein (APP), mutant human TAU, and mutations in presenilin 1 (PSEN1). Two widely used models are the 3xTG mice (Oddo et al., 2003) and the 5xFAD mice (Oakley et al., 2006). The 3xTG mice express transgenes encoding hAPP (Swedish; KM670/671NL) and hTAU with a single mutation (P301L), plus a knock-in activating mutation into endogenous mouse Presenilin1 (M146V). The 5xFAD mice harbor two Thy1-promoter-driven plasmids expressing two proteins with a total of five mutations identified in different cases of familial AD: APP with the Swedish, Florida and London mutations; plus PSEN1 with two activating mutations, M146L and L286V (Oakley et al., 2006). The 5xFAD mice develop behavioral symptoms within 2–4 months after birth (Oakley et al., 2006), and the 3xTG do so at 4 months (Billings et al., 2005). The 3xTG mice express transgenic APP and TAU at levels clearly above endogenous levels (Oddo et al., 2003), but lower than the marked overexpression seen in 5xFAD mice (Oakley et al., 2006). As a result, the 3xTG mice show graded development of symptoms similar to human nonfamilial AD, while 5xFAD mice are a model of very severe, rapidly developing familial AD (Kim et al., 2018; Lutz and Peng, 2018; Martini et al., 2018; Neuner et al., 2019). Interestingly, neuroanatomical symptoms (enlarged ventricles, alterations in cortex and white matter) are detectable in the 3xTG mice by 40 days of age (Kong et al., 2018), well before behavioral deficits are reported. A recent report on human patients showed that subtle but measurable cognitive deficits precede and predict amyloid accumulation and neurodegeneration later in life (Thomas et al., 2020).
The progressive loss of Kalirin-7 (Kal7), one product of the multidomain scaffold and guanine nucleotide exchange factor gene KALRN, has repeatedly been implicated in AD (Cisse et al., 2017; Krivinko et al., 2017; Mandela et al., 2012; Murray et al., 2012; Penzes and Jones, 2008; Xie et al., 2019; Youn et al., 2007). Kalirin is a major regulator of the number and functionality of dendritic spines (Miller et al., 2013; Penzes et al., 2013). When neurons are exposed to APP peptide aggregates, their dendrites lose spines and a major fraction of the most abundant Kalirin isoform in the adult brain, Kal7, is lost (Cisse et al., 2017; Xie et al., 2019). Kal7 protein is substantially decreased in postmortem AD cortical extracts (Cisse et al., 2017; Penzes et al., 2013; Youn et al., 2007), and in late stage human AD, KALRN mRNA decreases significantly, while not dropping in healthy older adults (Kim et al., 2018; Neuner et al., 2019). Calpains are activated in AD (Ahmad et al., 2018; Ferreira, 2012), and Kal7 is particularly sensitive to calpain cleavage (Miller et al., 2017). To test the possible synergism of Kalirin loss with the presence of mutant forms of hAPP, hTAU and PSEN1, we examined mice from matings of 3xTG mice with Kalirin knockout mice (Mandela et al., 2012).
Kalirin was first identified as an interactor with peptidylglycine α-amidating monooxygenase (PAM), an enzyme essential for the biosynthesis of many of the neuropeptides whose levels are strikingly decreased in AD brains (Castillo et al., 2017; Do et al., 2018; Gatta et al., 2014; Ishii et al., 2014; Sterniczuk et al., 2010b; Ye et al., 2018). Since levels of the mRNAs encoding the major endoproteases essential for neuropeptide biosynthesis (prohormone convertase 1 (PC1; PCSK1) and prohormone convertase 2 (PC2; PCSK2) and PAM all decrease in AD, but not in normal aging (Castillo et al., 2017; Kim et al., 2018; Neuner et al., 2019), expression of these three enzymes was also evaluated.
Materials and Methods
Mice
All work with mice was approved by the University of Connecticut Health Center Institutional Animal Care and Use Committee, in accordance with National Institutes of Health guidelines for animal care and use and the ARRIVE guidelines. All efforts were made to minimize animal suffering and to reduce the number of animals used. The KalSRKO mice are on a pure C57BL/6 background (Mandela et al., 2012) (the parental line to JAX 031466). The 3xTG/3xTG mice (34830-JAX from The Jackson Laboratory [JAX]) are on the B6129SF2/J mixed background. Three nontransgenic lines (B6129SF2/J [JAX 101045, abbreviated B6129], 129 [JAX 002448] and C57BL/6J [JAX 000664, abbreviated C57]) were purchased from JAX and bred in our facility. The B6129SF2/J mice are the F2 hybrid (second familial generation) from mating C57BL/6J females with 129 males (grandparents of the mice used) (https://www.jax.org/strain/101045). Genotyping of KalSRKO was by polymerase chain reaction (PCR) from identifying ear punches as described (Mandela et al., 2012). Genotyping of the hAPP and hTAU transgenes and the PSEN1 knock-in mutation is described in Table 1 and Suppl.Fig.1. We will use simplified genetic nomenclature (Billings et al., 2005), with 3xTG-H for Homozygous 3xTG/3xTG mice on the B6129 background, and 3xTG-h for the hemizygous 3xTG/+ offspring from mating 3xTG-H mice with C57 mice. We only used a small number of B6129 mice as nontransgenic controls, because half of our first batch (9 of 17) of B6129 mice from JAX were also positive for the hAPP transgene and some for the hTAU transgene (but none for the PSEN1 knock-in mutation) (Suppl.Fig.1). Instead we used C57 mice as controls, as have many other studies (Li et al., 2018; Lipton et al., 2016; Liu et al., 2019; Nakashima et al., 2010; Pedrazzoli et al., 2019; Sterniczuk et al., 2010a; Sterniczuk et al., 2010b; Xu et al., 2014; Yu et al., 2018; Zhang et al., 2010). All 3xTG and B6129 mice used in this study had hAPP, hTAU and PSEN1(M146V) presence (3xTG) or absence (B6129) demonstrated by PCR analyses of identifying earclips; homozygosity of 3xTG-H mice was demonstrated by genotyping progeny after pairing with nontransgenic mice (Hirata-Fukae et al., 2008). Although not commonly reported in the literature, based on our experience, genotyping all mice purchased from commercial sources is essential. Where needed, we have compared C57BL/6, B6129 and 129 mice both behaviorally and biochemically. The mice used in this work are tabulated in Table 2. Behavioral analyses involved 66 males and 69 females, many at more than one age. Biochemical studies used 28 males and 34 females.
Table 1.
Genotyping primers and protocol
| gene | Forward primer | Reverse primer | Product size (nt) |
|---|---|---|---|
| hAPPspecific Set 1 | TCTCGTTCCTGACAAGTGCAATTCTTAC From Ishii et al (Ishii et al., 2014) | GCAAGTTGGTACTCTTCTCACTGCATG From Ishii et al (Ishii et al., 2014) | 116 |
| hAPPspecific set 2 | TTGCCCACTGGCTGAAGAAAGTGACAAT (gives a faint band with C57BL/6) | TTCCTCTACCTCATCACCATCCTCATCG | 211 |
| hAPPspecific Set 3 | GGGGTAGAGTTTGTGTGTTGCCCAC | CCTCTACCTCATCACCATCCTCATCG | 226 |
| hAPPspecific Set 4 | CAGCCGTGGCATTCTTTTGGGGCT | CACATCTTCTGCAAAGAACACCAATTTTTGATGATGA | 231 |
| hTauspecific | CACCAGCCGGGAGGCGGG | GACGTGGGTGATATTGTCCAGGGAC | 300 |
| Psen1-generic and M146V-specific | GGTGTTTTGTTTCCCTCTGTAGAATCTACAC | CACACAAGGACAACCCATAGGCAGG = generic and M146V-specific GACCACCAGGAGGATGGTCACC | 221 140 |
PCR conditions for all three reactions are the same:
94C, 2 min; 94C, 30 sec; 59C, 1 min; 72C, 32 sec; repeat 36x; 72C, 5min.
Primer melt temperatures are 60.4 – 61.7C (http://biotools.nubic.northwestern.edu/OligoCalc.html).
Routine screening used hAPP primer set 4.
Table 2.
Mice used for behavioral and biochemical analyses
| Purpose↓ | C57 male | C57 fem | 3xTG-H male | 3xTG-H fem | B6129 male | B6129 fem | 129 male | Kal SRko-h male | Kal SRko-h fem | 3xTG-H male | 3xTG-H fem | Kal SRko/3xTG male | Kal SRko/3xTG fem |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open field; Rotarod; Barnes | 14–3mo 7–6mo 7–9mo |
12–3mo 8–6mo 9–9mo |
21–3mo 13–6mo 6–9mo |
21–3mo 16–6mo 14–9mo |
4–6mo 4–9mo |
4–6mo 4–9mo |
3–6mo | 8–3mo 8–6mo 4–9mo |
9–3mo 9–6mo |
7–3mo 7–6mo 4–9mo |
9–3mo 9–6mo |
9–3mo 9–6mo |
7–3mo 7–6mo |
| Biochem | 4–3mo 3–9mo |
4–3mo 5–9mo |
3–3mo | 3–3mo 4–9mo |
3–3mo 4–9mo |
6–3mo 4–9mo |
4–9mo | 4–9mo | 3–3mo 4–9mo |
4–9mo |
Mobility testing
Behavioral testing extended over a 15 day period, with Open Field testing on day 1, Rotarod on days 1 to 3, Barnes Maze acclimation on day 9 and testing on days 10 to 12 and 15. Open Field was performed once for 15 minutes per mouse in a 38cm x 38cm Plexiglas chamber from San Diego Instruments while beam breaks were recorded by PAS Software, as described (Ma et al., 2008a). Mice were tested on a Rotarod apparatus (Med Associates) for 3 trials/day, 15–20 minutes apart. For each trial, the Rotarod was set to accelerate from 4–40 rpm linearly over the course of 5 minutes, and the longest time for the day for each mouse was recorded as the data point for the day. If an animal fell off before 60 sec in the first trial of the day, it was placed back on the wheel for learning purposes. All additional aspects of this test were performed as described (Mandela et al., 2012). When the RM-ANOVA was performed on males vs. females for each genotype, no significant differences by sex were seen, for both Open Field and Rotarod.
Memory testing
The Barnes Maze test of spatial learning and memory is universally regarded as less stressful (based on plasma corticosterone levels) than the Morris Water Maze, and more sensitive to early cognitive deficits (Barnes, 1979; Gawel et al., 2019; Hunsberger et al., 2014; Illouzm et al., 2016; Paul et al., 2009; Rosenfeld and Ferguson, 2014; Stover et al., 2015; Suzuki and Imayoshi, 2017; Varodayan et al., 2018). The Barnes Maze protocol used in this work was a composite of these published studies. For acclimatization, each mouse was placed, using an opaque plastic beaker, in the center of a 20-hole San Diego Instruments Barnes Maze apparatus (91 cm diameter) and allowed to explore freely for 2 minutes. The mouse was then gently ushered with a gloved hand into the single “escape hole” compartment for 1 minute. The mouse was then transported, in the escape box, back to the home cage.
The mice were trained for 3 days, 2 trials per day, 15–20 min apart. For each training trial, the mouse was placed, using the opaque plastic beaker, in the center of the Barnes Maze apparatus. The beam breaks in each hole were recorded using PAS software while the mouse was allowed to explore freely for 3 min, or until the first beam break in the “escape hole”. If unsuccessful, the mouse was gently ushered with a gloved hand into the escape hole for 1 min before being transported, in the escape box, back to the home cage. The latency to first beam break into the escape hole was recorded for each trial. After a 72h rest, a single probe trial was then conducted for each mouse. This trial was performed in the same way as the previous training trials and the latency to first beam break into the escape hole was recorded.
The Barnes Maze apparatus and escape box were thoroughly cleaned with 70% ethanol before and between trials. All training and probe trials were recorded on a video camera for later reference. Lighting was adjusted to 1300 lux at the center of the apparatus and between 300–530 lux around the periphery, with the brightest quadrant being centered on the escape hole. There were 4 large (18 cm diameter) black and white symbols at the edge of the platform for visual orientation, placed 45° from the escape hole and at 90° intervals.
Western blot analyses
Extracts of somatosensory cortex were prepared using SDS sample buffer with protease and phosphatase inhibitors with sonication and boiling (Powers et al., 2019). Proteins were electrophoretically transferred to polyvinylidene difluoride membranes, stained with Coomassie brilliant blue to facilitate cutting into strips, cleared, blocked in 5% nonfat milk in Tween-Tris Buffered Saline (TTBS), incubated overnight in mouse or rabbit primary antibody, rinsed and exposed to the appropriate horseradish peroxidase-tagged secondary antibody (Jackson ImmunoResearch Laboratories). Bound antibodies were visualized with ECL Plus (ThermoFisher Scientific) and exposures in the linear range were captured and quantified using GeneTools software (Syngene) (Powers et al., 2019). Primary antisera are listed in Table 3. Cortical samples from 62 mice were analyzed in this work.
Table 3.
Antibodies used for biochemical analyses
| Antibody | Description | Source | Research Resource Identifier (RRID) | Literature Citation |
|---|---|---|---|---|
| JH2958 | rabbit polyclonal to the COOH-terminal of Kal7 | This lab | AB_2801571 | (Penzes et al., 2000) |
| CT301 | rabbit polyclonal to the Sec14 domain of all major Kalirin isoforms | This lab | AB_2801573 | (Yan et al., 2015) |
| JH629 | rabbit polyclonal to the linker between the two enzymatic domains of PAM | This lab | AB_2721274 | (Powers et al., 2019) |
| CT267 | rabbit polyclonal against the COOH-terminal cytoplasmic domain of PAM | This lab | AB_2801640 | (Rajagopal et al., 2009) |
| JH1761 | rabbit polyclonal against the PHM monooxygenase domain of PAM | This lab | AB_2819148 | (El Meskini et al., 2000) |
| JH888 | rabbit polyclonal against prohormone convertase 1 (PC1) | This lab | AB_2802129 | (Zhou and Mains, 1994) |
| JH1159 | rabbit polyclonal against prohormone convertase 2 (PC2) | This lab | AB_2814973 | (Zhou and Mains, 1994) |
| hAPP Ab | human APP-specific mouse monoclonal | Biolegend #803001 | ||
| All-APP | rabbit polyclonal for mouse and human APP | Sigma #A8717 | ||
| TAU | human and mouse TAU indistinguishable, mouse monoclonal | Sigma #T9450 | ||
| β3-tubulin | mouse monoclonal Tuj1 | Biolegend #801201 | ||
| Gapdh | mouse monoclonal | EMD Millipore #MAB374 |
The all-APP, TAU and GAPDH antibodies were incubated with PVDF membranes in TTBS containing 5% milk.
Statistics
Barnes Maze, Open Field and Rotarod data were subjected to 2-way ANOVA analyses (Graphpad Prism 8.4.1) where appropriate. The biochemical studies were all analyzed using pairwise Student’s t-tests.
Results
General mobility and motor coordination
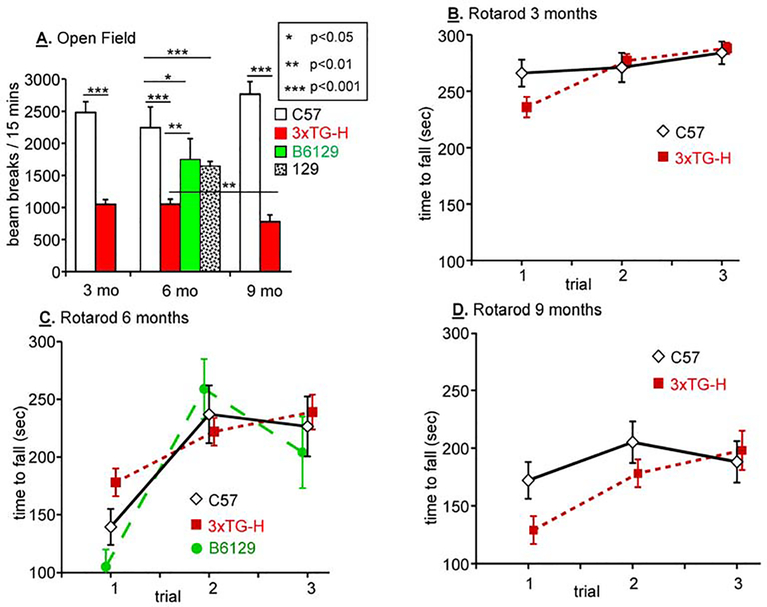
In preparation for neurocognitive testing, open field mobility and motor coordination on the Rotarod were tested (Fertan et al., 2019; Gawel et al., 2019; Hutton et al., 2018; Janczura et al., 2018) (Fig.1). Open field testing revealed that C57 mice produced far more beam breaks than 3xTG-H mice when tested at 3, 6 and 9 months of age (Fig.1A). While the open field behavior of C57 mice did not differ with age, 9 month old 3xTG mice produced fewer beam breaks than younger 3xTG mice. Since the set of transgenes and the knock-in allele that define 3xTG mice is maintained on the mixed C57BL/6J;129 background (“B6129”), B6129 mice and pure 129 mice were also tested in the open field. When tested at 6 months of age, the behavior of mice of both lines was intermediate between that of C57 and 3xTG-H mice.
Fig.1. Mobility and motor coordination in multiple mouse strains and genotypes.
A. Open field testing was performed for 15 min on sets of 3 nontransgenic strains plus 3xTG-H mice at 3, 6 and 9 months of age. B,C&D. Rotarod testing was performed on the same sets of mice as in A at the same ages. Open field and rotarod testing, followed by 5 days without handling, was always performed before animals were subjected to Barnes Maze acclimatization, training and testing. Results of significant paired t-tests are shown; NS, not significant; data are mean + s.e.m. Color code: C57, white diamonds; 3xTG-H, red boxes; B6129, green circles.
The rotarod was used to compare motor coordination in 3xTG-H and C57 mice at 3, 6 and 9 months of age (Fig.1B,C,D). A major age-dependent loss of motor performance was observed in 9 month old vs. 3 month and 6 month old 3xTG-H and C57 mice, but the performance of 3xTG-H and C57 mice did not differ from each other. Consistent with this observation, B6129 mice tested at 6 months of age performed similarly. For the mice used in this study, the only motor impairments were age-dependent, independent of sex, strain or genotype.
Barnes Maze spatial learning and memory at 3 and 9 months of age; effects of retesting
The Barnes Maze was chosen because it is considered the most sensitive test for mild cognitive deficits (Stover et al., 2015) and is significantly less stressful than the Morris Water Maze (Gawel et al., 2019; Paul et al., 2009). Published tests of different control (non-transgenic) strains of mice indicate that C57 mice are significantly faster than 129, BalbC or Swiss Webster mice at learning the Barnes Maze (Koopmans et al., 2003; Paul et al., 2009).
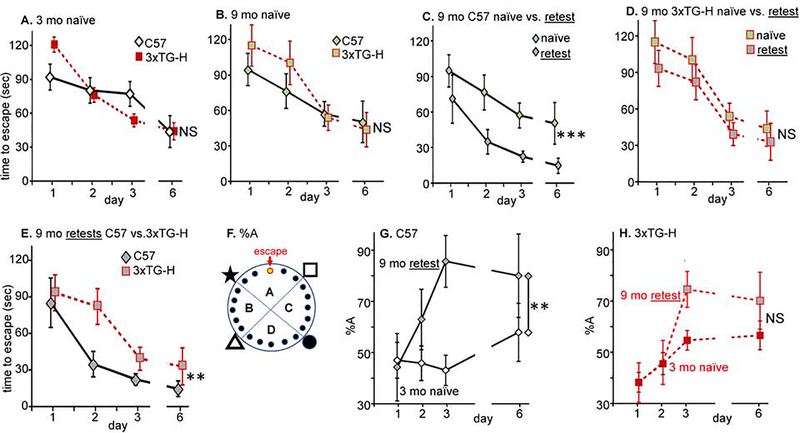
Based on time to escape (latency) measurements, at 3 months of age (Fig.2A), naïve C57 and 3xTG-H mice performed equivalently in the Barnes Maze; ‘naïve’ in this context means that the mice had been handled repeatedly and tested in open field and rotarod, but had never been trained or tested in the Barnes Maze. This result agrees well with the conclusions of Billings et al (Billings et al., 2005), namely that the 3xTG-H mice are not born with any cognitive impairments, but rather that they develop cognitive impairments as a function of age. When naïve C57 and 3xTG-H mice were tested at 9 months of age, their behavior in the Barnes Maze was again indistinguishable (Fig.2B) and was indistinguishable from the behavior of 3 month old naïve mice (Fig.2A). Alzheimer Disease in humans is most notable for the loss of memory (Carlsson et al., 2017), which in mice is represented by acquired abilities to perform spatially-cued tasks. To address this parameter, C57 mice and 3xTG-H mice introduced to the Barnes Maze at 3 months of age were tested again at 6 and 9 months (‘retest’). When tested again at 9 months of age, retested C57 mice substantially outperformed naive 9 month old C57 mice (Fig.2C). There was a main effect of prior training (F1,111 = 128.1, p<0.0001). By comparison, retested 9 month old 3xTG-H mice did not outperform naïve 9 month old 3xTG-H mice (Fig.2D). The AD model mice did not retain a functional memory of testing from 3 and 6 months prior, while the nontransgenic C57 mice retained a functional memory of prior testing (data replotted in Fig.2E). There was a main effect of genotype (F3,195 = 5.905, p=0.0007). ***, p<0.0001; **, p<0.001; NS, not significant (p>0.05).
Fig.2. Barnes Maze training and testing of C57 and 3xTG-H: 3 mo (naïve) vs. 9 mo (retested).
A. Naïve 3 month old C57 and 3xTG-H mice were trained for 3 days, two periods per day spaced by 20–30 min, then tested in a single trial after a 3 day period without training. The latency to the escape hole was averaged for the 2 trials on each training day. Males and females performed equivalently, so the data were pooled by genotype (26 C57, 42 3xTG-H). B. Procedure as in A, except the naïve mice were 9 months old (11 C57; 10 3xTG-H). C. 9 month old C57 mice were tested as in A, but one group had not previously been tested in the Barnes Maze (naïve, N=11) and one group had been trained and tested at 3 and 6 months of age (retest, N=6). All retesting involved the same 7 training-testing sessions as the initial training. D. Same procedure as in C except using 3xTG-H mice (10 naïve and 12 retest); no significant difference was detected. E. Replotting data for 9 mo C57 retest (N=6) from C and for 9 mo 3xTG-H retest (N=12). F. The percentage of beam breaks in the escape target quadrant (%A) was determined. G. The % beam breaks in quadrant A is plotted for 3 month naïve and 9 month retested C57 mice. H. Same as G except using 3xTG-H mice. Color code: C57, white diamonds; 3xTG-H, red boxes; 9 month naïve mice, light green center of symbol; 9 month retested mice, gray center of symbol.
One possible explanation of these findings could be that mice of different strains and ages employ different search strategies to find the escape hole. By tracking beam breaks that occur from nose poking into holes in each quadrant of the Barnes Maze, differences in the search strategy used to locate the escape hole can be discerned. Most mice progress from random searching to serial searching (moving around the circle) and then to direct approach (initially very close to the target) (Gawel et al., 2019; Illouzm et al., 2016). Based on measurement of the percentage of time spent in quadrant A, which contains the escape hole (Fig.2F), older, retested C57 mice were significantly more efficient at searching in the target quadrant for the escape hole than younger, naïve C57 mice (Fig.2G; p<0.0001). In contrast, prior exposure to the training and testing paradigms did not have a significant effect on the percentage of time spent in quadrant A by the older, retested 3xTG-H mice (Fig.2H; p=0.059). Naïve, 3 month old C57 and 3xTG mice exhibited no difference in %A (F1,275 = 0.7289, p=0.39) while a difference in %A was observed between retested 9 month C57 and 3xTG mice (p=0.0002). The next studies addressed possible biochemical explanations for this loss of ability to acquire and retain spatial information.
Nontransgenic B6129 and transgenic 3xTG-H mice differ in hAPP levels at 3 months of age
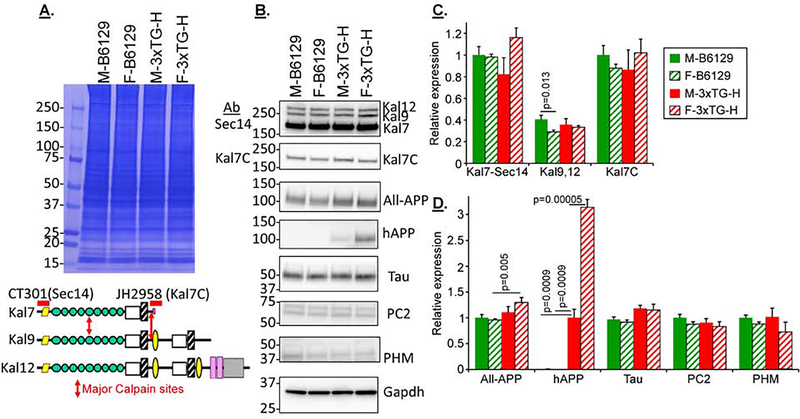
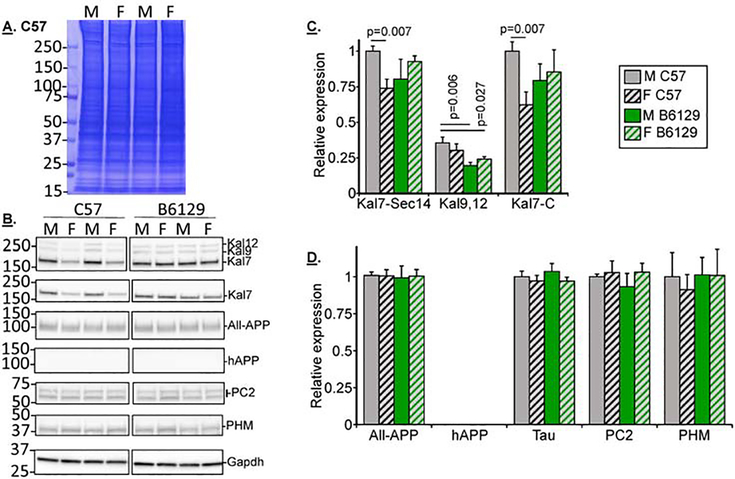
The 3xTG-H mice show neuroanatomical alterations within 6 weeks of birth (Kong et al., 2018). Understanding the causes of the anatomical and cognitive changes seen in 3xTG-H mice at 3, 6 and 9 months requires knowledge of the biochemical changes happening in each strain and genotype of mouse under study. Fig.3 shows the results of Western blot analyses of several sets of cortical extracts from male and female non-transgenic B6129 and transgenic 3xTG-H mice at 3 months of age. Since alternative splicing of the Kalrn gene generates functionally distinct isoforms, two different Kalirin antisera were used for these studies. The one directed to the N-terminal Sec14 domain detects the three major isoforms of Kalirin: Kal7, Kal9 and Kal12 (Johnson et al., 2000) (CT301: Fig.3A lower). The other Kalirin antibody is specific for the unique COOH-terminus of Kal7, which is absent from the larger isoforms, interacts with multiple PDZ-domain containing proteins and is very sensitive to removal by calpain (JH2958: Fig.3A, red arrows) (Miller et al., 2017).
Fig.3. Comparing 3 month old male and female B6129 and 3xTG-H mice.
A&B. Example gels from individual cortical extracts from 12 mice (3 each, B6129 male + female; 3xTG-H male + female; 3 months old) are shown; two identical gels were analyzed to allow all the Western blot analyses. C&D. Composite data from 6–9 mice in each category are shown (net 6 gels). For any one gel set, signals in the linear range were normalized to Male B6129 for all proteins except hAPP; since Kal7, Kal9 and Kal12 were all detected with a single antibody (CT301), Kal7 was chosen as the normalizer for all Kalirin isoforms. For hAPP, male 3xTG-H mice were treated as the normalizer. Means + s.e.m. are shown. For simplicity, pairings which were not significant are not marked. Color code: 3xTG-H, red bars; B6129, green bars; females, striped.
All three isoforms of Kalirin were detected, with levels of Kal7 exceeding those of Kal9 and Kal12 (Fig.3B). There was striking agreement between the patterns in the two western blots detecting Kal7 with N- and C-terminal specific antisera (Kal7-Sec14 and Kal7C antibodies, respectively) (Fig.3B,C), suggesting that there was no differential cleavage of the calpain-sensitive C-terminal portion of Kal7 based on sex or age (Miller et al., 2017). Antisera to the C-terminal domain of APP, which is identical in sequence in mouse and human APP (“All-APP”), detected endogenous APP plus the human APP transgene protein which was significantly increased in female transgenic (3xTG-H) mice, presaging the marked increase seen in 3 month old female over male 3xTG-H mice. The human APP-specific antiserum detected nothing in the nontransgenic extracts, as expected, while the signal for the transgenic hAPP was significantly higher in female mice compared to male mice; several studies have reported that females have more cortical hAPP than males, but the sex-dependent difference in hAPP is usually reported only in older mice (Belfiore et al., 2019; Carroll et al., 2010; Clinton et al., 2007; Hirata-Fukae et al., 2008; Kosaraju et al., 2017; Stimmell et al., 2019). The signals for Tau, PC2 and PHM did not differ with genotype or sex.
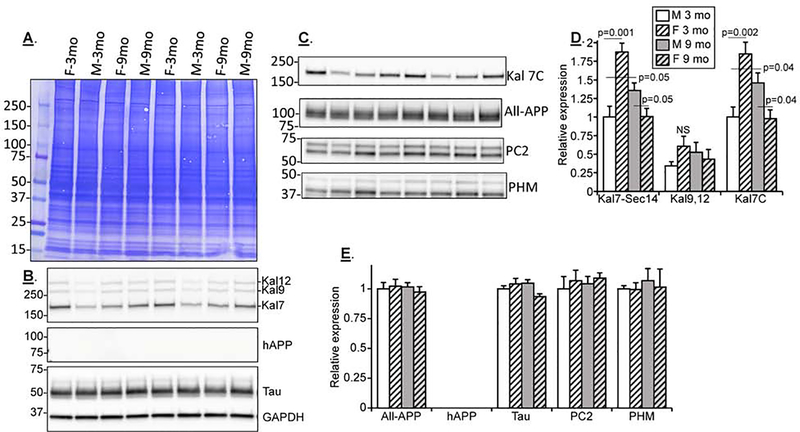
Nontransgenic B6129S and transgenic 3xTG-H female mice show increasing differences during aging
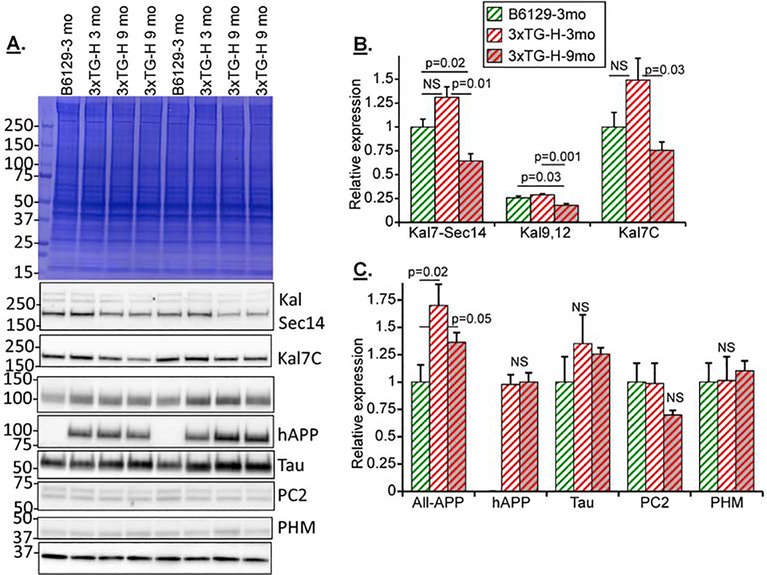
Three month old nontransgenic B6129 and 3 and 9 month old transgenic 3xTG-H female mice were studied biochemically (Fig.4); this comparison focused on older females, since females with the 3xTG genotype are more severely affected by aging than males (Belfiore et al., 2019; Carroll et al., 2010; Clinton et al., 2007; Hirata-Fukae et al., 2008; Kosaraju et al., 2017; Stimmell et al., 2019). A substantial decrease in Kal7, Kal9 and Kal12 levels in 3xTG mice was observed between 3 and 9 months of age (Figs.4A and B). The decreases in Kalirin isoform levels coincided with the expected appearance of hAPP (not detectable in nontransgenic B6129 mice) (Fig.4C) and a small but significant increase in the All-APP signal. Interestingly, hAPP levels did not increase with age from 3 to 9 months. The peptide biosynthetic processing endoprotease PC2 and the peptide biosynthetic α-amidating enzyme PHM were not significantly altered in the 9 vs. 3 month 3xTG-H mice.
Fig.4. Nontransgenic B6129S and transgenic 3xTG-H female mice show increasing differences with aging.
A. Example gels from individual cortical extracts from 12 mice (3 female B6129 and 3 female 3xTG-H, 3 months old; 6 female 3xTG-H, 9 months old) are shown; two identical gels were analyzed to enable all the Western blot analyses. B&C. Composite data for mice in each category. Signals in the linear range were normalized to 3 month old female B6129 for all antibodies except hAPP. For hAPP, 3 month old female 3xTG-H samples were treated as the normalizer. Means + s.e.m. are shown. For simplicity, pairings which were not significant are not marked. Color code: 3xTG-H, red bars; B6129, green bars; females are striped; 9 mo mice, gray background in bar.
Kalirin levels differ between nontransgenic C57 and B6129S mice at 9 months of age
Both C57and B6129 mice are used in published studies comparing nontransgenic to 3xTG-H mice, which are on the B6129 background (Clinton et al., 2007; Hirata-Fukae et al., 2008; Nakashima et al., 2010; Sterniczuk et al., 2010b); the Kalirin knockout line (KalSRKO) is on the C57 background. Sets of male and female mice from both background strains were compared at 9 months of age (Fig.5A,B). C57 mice showed a sex-dependent difference in Kal7 levels, with lower levels detected in females vs. males with both Kalirin antisera; this may reflect the estrogen-dependent expression of Kal7 observed in C57 mice (Ma et al., 2011; Ma et al., 2008b). In contrast, Kal7 levels did not differ in 9 month old male and female B6129 mice (Fig.5C). Kal9 and Kal12 levels were lower in B6129 mice than in C57 mice. There were no sex- or strain-dependent differences detected in these 9 month old mice using antibodies for APP (All-APP antibody), Tau, PC2 or PHM (Fig.5D). Since this study necessarily used both C57 and B6129 mice as nontransgenic controls for transgenic 3xTG and Kalirin strains, expression of these proteins was next examined in C57 mice as a function of age, without the presence of transgenes or knockouts.
Fig.5. Kalirin levels differ between nontransgenic C57 and B6129 mice at 9 months of age.
A&B. Example gels of 9 month old C57 and B6129 male and female extracts; two identical gels were analyzed for each set to enable all the Western blot analyses (net 8 gels). C&D. Composite data from 6–10 mice in each category are shown. For any one gel set, signals in the linear range were normalized to male C57 samples for all antibodies. Means + s.e.m. are shown. For simplicity, pairings which were not significant were not marked. Color code: C57, white bars; B6129, green bars; females, striped; 9 month mice, gray background in bar.
Kalirin protein changes with age and sex are distinct in nontransgenic C57 mice
Protein expression was compared in male and female C57 mice at 3 and 9 months of age (Fig. 6). At 3 months, Kal7 was twice as prevalent in C57 female cortical extracts as in male extracts (Fig.6B,C,D); data obtained for Kal7 using the Sec14 and Kal7-specific antibodies were identical. In contrast, 3 month old B6129 mice showed no sex-dependent difference in Kal7 levels (Fig.3C). Between 3 and 9 months, Kal7 expression in C57 females declined while Kal7 expression in C57 males rose (Fig.3D); the changes were of such a magnitude that Kal7 levels in 9 month old C57 males exceeded those in 9 month old C57 females (Fig.6B,D). None of the other proteins analyzed in lysates prepared from 3 and 9 month old C57 mice showed differences based on age or sex (Fig.6E). Again no human transgene hAPP was detected in nontransgenic samples. Changes in All-APP, Tau, PC2 and PHM were not apparent across sexes or nontransgenic strains at 3 or 9 months of age (Fig.4E).
Fig.6. Kalirin protein changes with age and sex are distinct in nontransgenic C57 mice.
A,B&C. Example gels of 3 and 9 month old male and female C57 extracts; two identical gels were analyzed for each set to allow all the Western blot analyses (net 6 gels). D&E. Composite data from 6–10 mice in each category are shown. For any one gel set, signals in the linear range were normalized to 3 month old male C57 for all proteins. Means + s.e.m. are shown. For simplicity, pairings which were not significant were not marked. Color code: C57, white bars; females, striped; 9 month mice, gray background in bar.
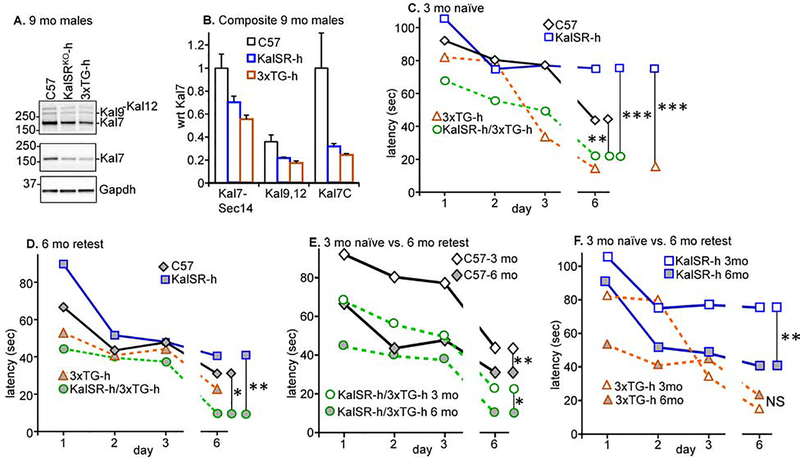
Kalirin heterozygosity and retesting improve Barnes Maze performance
To determine whether an age-related decline in Kalirin expression could affect neurocognitive functioning, we utilized KalSRKO-h mice, which have a single functional Kalrn allele on a pure C57 background and express Kal7, Kal9 and Kal12 at levels that are half those observed in C57 mice (Fig.7A,B). Interestingly, Kal7, Kal9 and Kal12 levels were also decreased in 3xTG-h mice, as seen earlier in the homozygous 3xTG-H mice (Fig.4). The performance of naïve 3 month old KalSRKO-h mice and C57 mice in the Barnes Maze was indistinguishable (Fig.7C, blue squares vs. black diamonds). To determine whether a reduction in Kalirin expression had an impact on the cognitive ability of 3xTG-h mice, naïve 3 month old 3xTG-h mice and KalSRKO-h/3xTG-h mice were tested (Fig.7C, orange triangles vs. green circles); their performances were indistinguishable from each other. Strikingly, 3xTG-h mice outperformed C57 mice (orange triangles vs. black diamonds) and KalSRKO-h/3xTG-h mice outperformed KalSRKO-h mice (green circles vs. blue squares) (Fig.7C). Comparing C57 to 3xTG-h mice, there was a main effect of genotype (F1,67 = 13.16, p=0.0006), and similarly comparing KalSRKO-h to KalSRKO-h/3xTG-h (F1,67 = 19.33, p<0.0001). The presence of hAPP/hTAU/hPSEN1 (on a mixed genetic background; half C57 and half B6129) improved memory acquisition by naïve 3 month old mice.
Fig.7. Kalirin heterozygosity and retesting improve Barnes Maze performance.
A&B. Example gels of 9 month old C57, KalSRKO-h and 3xTG-h male extracts; two identical gels were analyzed. All behavioral procedures were as in Fig.2. C. 3 month old naïve mice were tested (14 C57, 17 KalSRKO-h, 16 3xTG-h, 16 KalSRKO-h;3xTG-h). The first two groups were littermates from pairing a pure C57 mouse with a KalSRKO-h mouse (on a pure C57 background). The latter two groups were the littermates from pairing a 3xTG-H mouse with a KalSRKO-h mouse. D. Mice initially trained at 3 months were retested at 6 months of age (13 C57, 11 KalSRKO-h, 16 3xTG-h, 16 KalSRKO-h;3xTG-h). All retesting involved the same 7 training-testing sessions as the initial training. E,F. Data from C and D were replotted to compare naïve and retest data for nontransgenic C57 and the double heterozygote/hemizygote KalSRKO-h;3xTG-h mice. ***, p<0.0001; **, p<0.001; comparisons not indicated were not significant (p>0.05).
Mice of the same four genotypes were retested at 6 months of age (gray-filled symbols, Fig.7D). KalSRKO-h mice performed as well as C57 mice in the Barnes Maze spatial memory acquisition task (blue squares vs. black diamonds) and KalSRKO-h/3xTG-h mice performed as well as 3xTG-h mice (green circles vs. orange triangles). A two-fold decline in Kalirin levels was without effect on Barnes Maze performance in naïve 3 month old mice or in retested 6 month old mice. As observed with naïve 3 month old mice, retested 6 month old KalSRKO-h/3xTG-h mice performed better than KalSRKO-h mice (green circles vs. blue squares) (Fig.7D). The presence of hAPP/hTAU/hPSEN1 improved memory acquisition by KalSRKO-h mice on the mixed genetic background. When retested at 6 months of age, 3xTG-h mice no longer outperformed C57 mice (orange triangles vs. black diamonds) (Fig.7D).
When retested after 6 and 9 months, C57 mice exhibited markedly improved performance in the Barnes Maze (Fig.2C) while 3xTG-H mice did not (Fig.2D). To focus on the effects of Kalirin and hAPP/hTAU/hPSEN1 on memory retention, data for naïve 3 month old and retested 6 month old C57 mice and KalSRKO-h/3xTG-h mice were replotted (Fig.7E). Compared to naïve C57 mice at 3 months, C57 mice retested at 6 months exhibited improved Barnes Maze performance (black diamonds, gray vs. white fill). Unlike 3xTG-H mice (Fig.2D), retested KalSRKO-h/3xTG-h mice exhibited improved Barnes Maze performance when retested at 6 months (green circles, gray vs. white fill). There was a main effect of genotype between the C57 and KalSRKO-h;3xTG-h mice (F1,67 = 7.878, p=0.0065 and between the KalSRKO-h and the KalSRKO-h;3xTG-h mice (F1,67 =15.36, p=0.0002). The KalSRKO-h mice also improved from 3 months (naïve) to 6 months (retested) (blue squares, gray vs. white fill, Fig.7F), while the 3xTG-h mice were already so fast at acquiring the task at 3 months that little improvement was seen at 6 months in retesting (orange triangles, gray vs. white fill, Fig. 7F). There were significant effects of retesting for the C57 mice (F1,67 =14.73, p=0.0003), KalSRKO-h;3xTG-h mice (F1,67 = 9.791, p=0.0026) and KalSRKO-h mice (F1,67 = 14.15, p=0.0004), but not for the 3xTG-h mice (p=0.0535).
Discussion
Mobility testing
While some researchers have reported that 3xTG-H mice move more slowly than B6129 mice in the open field (Fertan et al., 2019; Nakajima et al., 2015; Torres-Lista et al., 2019), others have reported no differences in open field testing (Adler et al., 2019; Li et al., 2018; Lin et al., 2019; Nie et al., 2017; Sterniczuk et al., 2010a; Yu et al., 2015) or increased mobility in 3xTG-H mice (Krivinko et al., 2017; Stover et al., 2015). As in most similar studies, there were no sex differences in these open field and rotarod results (Gawel et al., 2019). Some previous reports found that 3xTG-H mice perform better than nontransgenic mice on the rotarod (Fertan et al., 2019; Garvock-deMontbrun et al., 2019; Yu et al., 2015) or equal to nontransgenic mice (Fertan et al., 2019; Sterniczuk et al., 2010a). The overall conclusion is that all the mice lose speed and motor coordination with age, but there is a lack of consensus whether the AD model 3xTG mice have motor impairments compared to age-matched controls, which must be considered when interpreting neurocognitive testing. While C57 mice moved faster than 3xTG-H, B6129 and 129 mice in the open field and did not lose speed with age to 9 months (whites bars, Fig.1A), mice of all four strains performed in a similar manner when tested on the rotarod (Fig.1B–D). Thus, the performance of 3xTG-H AD model mice in various tests of learning and memory should not be impaired by any lack of mobility.
Spatial memory acquisition
Good methods have been developed for measuring the acquisition and retention of spatial memory in rodents; these tests target some of the key characteristic of human AD, namely the loss of memory, in particular the loss of the ability to recall recent events (Carlsson et al., 2017). The Morris Water Maze has been used more often than the Barnes Maze, which we utilized because it is less stressful (Barnes, 1979; Gawel et al., 2019; Hunsberger et al., 2014; Illouzm et al., 2016; Paul et al., 2009; Rosenfeld and Ferguson, 2014; Stover et al., 2015; Suzuki and Imayoshi, 2017; Varodayan et al., 2018). Using the Barnes Maze to compare nontransgenic and 3xTG-H mice at various ages, some studies revealed deficits in age-matched 3xTG-H mice (Fertan et al., 2019; Vandal et al., 2014), while others showed no consistent differences by genotype (Stover et al., 2015; Virgili et al., 2018). Comparing 3xTG-H mice with C57 controls, Aloni et al. reported no difference in Barnes Maze performance (Aloni et al., 2019), in agreement with our results for naïve mice (Fig.2A,B). Interestingly, some studies with continued Barnes Maze training for long periods of time (e.g. 15 days) showed that control mice were faster at finding the escape hole in initial training trials, but after further training the difference was undetectable (Fertan et al., 2019). One study reported that 6.5 month old female 3xTG-H mice acquire the Barnes Maze spatial memory task faster than males of the same age and genotype and faster than B6129 mice of either sex (Stover et al., 2015).
Data obtained using the Morris Water Maze generally support the conclusion that wildtype mice perform better than 3xTG-H mice and that this difference becomes more dramatic with age (Kong et al., 2018; Kosaraju et al., 2017; Li et al., 2018; Lin et al., 2019; Liu et al., 2019; Nie et al., 2017; VanDerJeugd et al., 2018; Ying et al., 2017; Zhang et al., 2010). Interestingly, a subset of studies report data for only one sex, stating that data for the other sex was too variable for clear results (Belfiore et al., 2019). Several studies found no deficiencies in 3xTG-H mice vs. controls, even with mice as old as 23 months (Nakajima et al., 2015; Song et al., 2014; Yu et al., 2015).
Spatial memory retention
There is an abundance of clinical and epidemiological data with elderly human patients that active mental and physical pursuits are protective against general memory decline and the appearance of AD (Yeung et al., 2015). A series of studies compared naïve and experienced wildtype and 3xTG-H mice using the Morris Water Maze (Billings et al., 2007; Billings et al., 2005; Yeung et al., 2015). Control nontransgenic and transgenic 3xTG-H mice performed equally well at 2–3 months of age; 3xTG-H mice developed a deficit in retention of training beginning at 4 months, failing to retain training from one day to the next. The initial studies found no differences between naïve and experienced (retested) mice in Morris Water Maze performance at 6 months, but later studies showed that experienced mice outperformed naïve mice as early as the first retesting at 6 months of age (Billings et al., 2007; Billings et al., 2005; Clinton et al., 2007). The data established that mice experienced in the Morris Water Maze also outperformed naïve mice in the Barnes Maze, suggesting that spatial memory training, not merely handling and exercising the mice, is transferrable and can lead to long term improvement in spatial memory acquisition tasks.
Our retesting studies, which used the Barnes Maze (Fig.2C,D,E,F), showed that C57 mice, but not 3xTG-H mice, retained functional memory of Barnes Maze training for many months. This finding is distinct from that of Billings et al. (Billings et al., 2005), who concluded that the deficiency in 3xTG-H mice in the Morris Water Maze was caused by impairment of their ability to retain training day-to-day, rather than over a 3 month period as in this study using the Barnes Maze (Figs. 2B,C,D). The marked deficit in Morris Water Maze performance by naïve 3xTG-H mice, compared to nontransgenic mice (Billings et al., 2007) was not seen in the current studies at 3 and 9 months of age using the less stressful Barnes Maze test (Fig.2A,B); instead, these data demonstrate that previously tested C57 mice exhibited markedly improved Barnes Maze performance over many months (Fig.2C), while 3xTG-H mice did not benefit from prior Barnes Maze training (Fig.2D,E). The 3xTG-h mice were quite fast at spatial skill acquisition at 3 months but did not benefit from prior training when retested at 6 months (Fig.7F).
Changes in cortical protein patterns as a function of genotype, sex and age
Using an APP antiserum specific for hAPP, we could detect its presence in 3 month old 3xTG-H mice, with significantly higher levels of hAPP present in female cortices than in male (Fig.3B,D). Using an APP antiserum equally capable of detecting human and mouse APP, it was clear that the expression of hAPP in 3 month of females was sufficient to increase the total APP level (Fig.3B,D, All-APP). hAPP levels were not increased further in 9 mo 3x-TG-H mice (Fig.4C). A decline in Kal7 mRNA and protein levels in human AD brains, but not in cognitively normal brains, has been observed consistently (Cisse et al., 2017; Kim et al., 2018; Neuner et al., 2019; Xie et al., 2019; Youn et al., 2007). An important question is whether the loss of Kal7 is a causal factor in AD or occurs as a result of AD. The experimental observation that addition of APP peptide oligomers to cultured neurons causes a decrease in Kal7 and loss of dendritic spines (Cisse et al., 2017; Xie et al., 2019) suggests that the loss of Kal7 mRNA and protein is a result, not a cause, of AD. We saw no difference in Kal7, Kal9 or Kal12 levels between 3 month old 3xTG-H mice and age- and sex-matched B6129 nontransgenic controls (Figs.3 and 4). However, there was a significant drop in the levels of all three Kalirin isoforms in 9 month old 3xTG-H female mice (Fig.4). While this result indicated that some combination of the plaques, tangles and neuroanatomical degeneration seen in older 3xTG-H mice was coincident with the decline in Kalirin expression, our data do not establish causation.
The other notable differences in Kalirin isoform levels reflected sex and age. As expected (Ma et al., 2011), Kal7 levels were higher in 3 month old C57 female mice than in male mice of the same age (Fig.6); at 9 months of age, Kal7 levels in male C57 mice exceeded those in female C57 mice (Fig.5C,6C). While Kalirin expression in C57 mice was age and sex dependent, Kalirin expression in B6129 mice was not (Fig.3,5).
Effect of loss of Kal7 on spatial memory acquisition
Since Kal7 mRNA and protein levels decrease in extracts from AD patients compared to age-matched but cognitively normal patients (Cisse et al., 2017; Kim et al., 2018; Neuner et al., 2019; Penzes et al., 2013; Youn et al., 2007), we and others (Krivinko et al., 2017) investigated whether genetic deletion of one Kalirin allele would hamper the acquisition of spatial memory tasks. The conclusion from studies using a radial arm water maze (Krivinko et al., 2017) and the Barnes Maze (Fig.7) is that acquisition of spatial memory tasks is not harmed by deletion of one Kalirin allele, reducing the expression of all Kalirin isoforms to half of control, from the moment of conception. A study that used sufficient lentivirus encoding a short-hairpin RNA specific for Kal7 to reduce its expression after bilateral injection of the virus into the CA1 region of the adult mouse hippocampus came to a different conclusion (Cisse et al., 2017): Morris Water Maze performance was impaired, with platform-seeking behavior converted into marked platform avoidance. Clearly, further studies are needed to explain these differences, especially since possible therapeutic interventions for AD involving the manipulation of Kalirin function have been proposed (Penzes et al., 2013). This is important, because the endogenous levels of Kalirins 7, 9 and 12 mRNA and protein all decline dramatically in human AD patients (Cisse et al., 2017; Kim et al., 2018; Neuner et al., 2019; Penzes et al., 2013; Youn et al., 2007)..
Conclusions
When tested for spatial memory acquisition at 3, 6 or 9 months of age using the Barnes Maze, naïve 3xTG-H mice, a model of human AD, and nontransgenic control mice performed equally well. When retested at 9 months of age, nontransgenic mice retained memory of Barnes Maze training carried out 3 and 6 months earlier, as did mice with a single functional Kalirin allele. In contrast, hemizygous and homozygous 3xTG-AD mice were the only mice tested in which prior training produced no improvement in their performance when retested at 6 or 9 months of age. Levels of Kalirin, a cytoskeletal regulator with a key role in synaptic function, are known to decline in AD. Kalirin expression was found to be strongly sex- and age dependent in C57 but not in B6129 mice. Although identified as a candidate contributor to AD symptoms, inactivation of a single Kalirin allele had no effect on Barnes Maze performance by C57 mice or by 3X-TG-AD mice.
Supplementary Material
Suppl.Fig.1. Genotyping B6129SF2/J and 3xTG-AD mice. Mice purchased from Jackson Labs were individually identified using earclips and the earclip samples were used to examine the genotypes, using the primers and program in Table 1. Similar results were found for these samples using hAPP primer sets #1, 2, and 3. The hAPP primers on the JAX website (oIMR3610 and 3611) were not as helpful for genotyping, since oIMR3610 is identical in mouse and human APP. The hAPP bands from B6129 mice, produced with primer sets 3 and 4, were excised and sequenced by JAX staff, who verified that the bands have the expected sequences of human APP cDNA (4/30/2019). These B6129 tissue samples were all taken before the lab had received any 3xTG-H mice from JAX.
AD paper Highlights.
Barnes Maze performance by C57 and 3xTG-H mice (3 & 9 months) are indistinguishable
Retested C57 mice retain functional memory of earlier Barnes Maze testing (months)
Barnes Maze performance by 3xTG-H and 3xTG-h mice are not improved by prior testing
Acknowledgments
This work was supported by National Institutes of Health Grants 5R01 DK-032948, 3R01 DK032948S1 and the Daniel Schwartzberg Fund. We thank Kathryn G. Powers for help with Western analyses. The funding sources had no role in study design; collection, analysis or interpretation of data; writing; or decision to submit the article for publication.
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
Verification
1. All authors must disclose:
(a) Any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) their work. Examples of potential conflicts of interest which should be disclosed include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding. If there are no actual or potential conflicts of interest, please state this. Should a significant conflict of interest be present, the Editors reserve the right to reject the article on that basis. NONE
(b)Whether any author’s institution has contracts relating to this research through which it or any other organization may stand to gain financially now or in the future. NONE
(c) Any other agreements of authors or their institutions that could be seen as involving a financial interest in this work. NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Adler P, Mayne J, Walker K, Ning Z, and Figeys D (2019). Therapeutic Targeting of Casein Kinase 1δ/ε in an Alzheimer’s Disease Mouse Model. JProteomRes 18, 3383–3393. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Das D, Kommaddi RP, Diwakar L, Gowaikar R, Rupanagudi KV, Bennett DA, and Ravindranath V (2018). Isoform-specific hyperactivation of calpain-2 occurs presymptomatically at the synapse in Alzheimer’s disease mice and correlates with memory deficits in human subjects. Sci Rep 8, 13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni E, Oni-Biton E, Tsoory M, Moallem DH, and Segal M (2019). Synaptopodin Deficiency Ameliorates Symptoms in the 3xTg Mouse Model of Alzheimer’s Disease. JNeurosci 39, 3983–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA (1979). Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. JCompPhysiolPsychol 93, 74–104. [DOI] [PubMed] [Google Scholar]
- Belfiore R, Rodin A, Ferreira E, Velazquez R, Caccamo A, and Oddo S (2019). Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 18, e12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Green KN, McGaugh JL, and LaFerla FM (2007). Learning decreases A beta*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J Neurosci 27, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, and LaFerla FM (2005). Intraneuronal Abeta Causes the Onset of Early Alzheimer’s Disease-Related Cognitive Deficits in Transgenic Mice. Neuron 45, 675–688. [DOI] [PubMed] [Google Scholar]
- Carlsson CM, Gleason CE, Puglielli L, and Asthana S (2017). Dementia Including Alzheimer Disease In Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education; ). [Google Scholar]
- Carmona S, Hardy J, and Guerreiro R (2018). The genetic landscape of Alzheimer disease In Handbook of Clinical Neurology, Geschwind DH, Paulson HL, and Klein C, eds. (Elsevier; ), pp. 395–408. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Kreimer S, Villamagna A, Stanczyk FZ, and Pike CJ (2010). Sex differences in β-amyloid accumulation in 3xTg-AD mice: Role of neonatal sex steroid hormone exposure. Brain Res 1366, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo E, Leon J, Mazzei G, Abolhassani N, Haruyama N, and Nakabeppu Y (2017). Comparative profiling of cortical gene expression in Alzheimer’s disease patients and mouse models demonstrates a link between amyloidosis and neuroinflammation. Sci Rep 7, 17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M, Duplan E, Lorivel T, Dunys J, Bauer C, and Checler F (2017). The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model. MolPsych 22, 1562–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton LK, Billings LM, Green KN, Caccamo A, McGaugh JL, and LaFerla FM (2007). Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. NeurobiolDis 28, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K, Laing BT, Landry T, Bunner W, Mersaud N, and Huang H (2018). The effects of exercise on hypothalamic neurodegeneration of Alzheimer’s disease mouse model. PLoS ONE 13, e0190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig AJ, delCastillo-Frias MP, Berthoumieu O, Tarus B, Nasica-Labouze J, Sterpone F, Nguyen PH, Hooper NM, Faller P, and Derreumaux P (2017). Why Is Research on Amyloid-β Failing to Give New Drugs for Alzheimer’s Disease? ACS Chem Neurosci 8, 1435–1437. [DOI] [PubMed] [Google Scholar]
- El Meskini R, Mains RE, and Eipper BA (2000). Cell type-specific metabolism of peptidylglycine alpha-amidating monooxygenase in anterior pituitary. Endocrinology 141, 3020–3034. [DOI] [PubMed] [Google Scholar]
- Ferreira A (2012). Calpain Dysregulation in Alzheimer’s Disease. ISRN Biochem 2012, 728571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertan E, Stover KRJ, Brant MG, Stafford PM, Kelly B, and Brown RE (2019). Effects of the Novel IDO Inhibitor DWG-1036 on the Behavior of Male and Female 3xTg-AD Mice. FrontPharm 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvock-deMontbrun T, Fertan E, Stover KR, and Brown RE (2019). Motor deficits in 16-month-old male and female 3xTg-AD mice. BehavBrain Res 356, 305–313. [DOI] [PubMed] [Google Scholar]
- Gatta V, D’Aurora M, Granzotto A, Stuppia L, and Sensi SL (2014). Early and sustained altered expression of aging-related genes in young 3xTg-AD mice. Cell Death Dis 5, e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawel K, Gibula E, Marszalek-Grabska M, Filarowska J, and Kotlinska JH (2019). Assessment of spatial learning and memory in the Barnes maze task in rodents — methodological consideration. Naunyn-Schmiedeberg ArchPharmacol 392, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Bodea LG, and Goedert M (2018). Rodent models for Alzheimer disease. NatRevNeurosci 19, 583–598. [DOI] [PubMed] [Google Scholar]
- Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, LaFerla FM, and Matsuoka Y (2008). Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res 1216, 92–103. [DOI] [PubMed] [Google Scholar]
- Hunsberger HC, Rudy CC, Weitzner DS, Zhang C, Tosto DE, Knowlan K, Xub Y, and Reed MN (2014). Effect size of memory deficits in mice with adult-onset P301L tau expression. BehavBrain Res 272, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton CP, Lemon JA, Sakic B, Rollo CD, Boreham DR, and Becker S (2018). Early Intervention with a Multi-Ingredient Dietary Supplement Improves Mood and Spatial Memory in a Triple Transgenic Mouse Model of Alzheimer’s Disease. JAlzheimers Dis 64, 835–857. [DOI] [PubMed] [Google Scholar]
- Illouzm T, Madar R, Clague C, Griffioen KJ, Louzoun Y, and Okun E (2016). Unbiased classification of spatial strategies in the Barnes maze. Bioinformatics 32, 3314–3320. [DOI] [PubMed] [Google Scholar]
- Ishii M, Wang G, Racchumi G, Dyke JP, and Iadecola C (2014). Transgenic Mice Overexpressing Amyloid Precursor Protein Exhibit Early Metabolic Deficits and a Pathologically Low Leptin State Associated with Hypothalamic Dysfunction in Arcuate Neuropeptide Y Neurons. JNeurosci 34, 9096–9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczura KJ, Volmar CH, Sartor GC, Rao SJ, Ricciardi NR, and Wahlestedt C (2018). Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. ProcNatlAcadSciUSA 115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Johnson RC, Penzes P, Eipper BA, and Mains RE (2000). Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5’- and 3’-ends along with an internal translational initiation site. J Biol Chem 275, 19324–19333. [DOI] [PubMed] [Google Scholar]
- Kim BY, Lim HS, Kim Y, Kim YJ, Koo, and Jeong SJ (2018). Evaluation of Animal Models by Comparison with Human Late-Onset Alzheimer’s Disease. MolNeurobiol 55, 9234–9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong V, Devenyi GA, Gallino D, Ayranci G, Germann J, and Chakravarty MM (2018). Early-in-life neuroanatomical and behavioural trajectories in a triple transgenic model of Alzheimer’s disease. Brain StructFunc 223, 3365–3382. [DOI] [PubMed] [Google Scholar]
- Koopmans G, Blokland A, vanNieuwenhuijzen P, and Prickaerts J (2003). Assessment of spatial learning abilities of mice in a new circular maze. PhysiolBehav 79, 683–693. [DOI] [PubMed] [Google Scholar]
- Kosaraju J, Holsinger RMD, Guio L, and Tam KY (2017). Linagliptin, a Dipeptidyl Peptidase-4 Inhibitor, Mitigates Cognitive Deficits and Pathology in the 3xTg-AD Mouse Model of Alzheimer’s Disease. MolNeurobiol 54, 6074–6084. [DOI] [PubMed] [Google Scholar]
- Krivinko JM, Erickson SL, Abrahamson EE, Wills ZP, Penzes P, and Sweet RA (2017). Kalirin reduction rescues psychosis-associated behavioral deficits in APPswe/PSEN1dE9 transgenic mice. NeurobiolAging 54, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Jiao JJ, Holscher C, Wu MN, Zhang J, and Qi JS (2018). A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer’s disease. Hippocampus 28, 358–372. [DOI] [PubMed] [Google Scholar]
- Lin YS, Lin YF, Chen KC, Yang YK, and Hsiao YH (2019). Collapsin response mediator protein 5 (CRMP5) causes social deficits and accelerates memory loss in an animal model of Alzheimer’s disease. Neuropharmacology 157, 107673. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rezaie T, Nutter A, Lopez KM, Parker J, and Nakanishi N (2016). Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer’s disease models. Cell Death Dis 7, e2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang Y, Liu P, Bai H, Li X, and Wang Y (2019). MicroRNA-128 knockout inhibits the development of Alzheimer’s disease by targeting PPARγ in mouse models. EurJPharmacol 843, 134–144. [DOI] [PubMed] [Google Scholar]
- Lutz BM, and Peng J (2018). Deep Profiling of the Aggregated Proteome in Alzheimer’s Disease: From Pathology to Disease Mechanisms. Proteomes 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Huang JP, Kim EJ, Zhu Q, Kuchel GA, Mains RE, and Eipper BA (2011). Kalirin-7, an Important Component of Excitatory Synapses, is Regulated by Estradiol in Hippocampal Neurons. Hippocampus 21, 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, and Mains RE (2008a). Kalirin-7 Is Required for Synaptic Structure and Function. J Neurosci 28, 12368–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, and Eipper BA (2008b). Kalirin-7 Is an Essential Component of both Shaft and Spine Excitatory Synapses in Hippocampal Interneurons. J Neurosci 28, 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandela P, Yankova M, Conti LH, Ma XM, Grady J, Eipper BA, and Mains RE (2012). Kalrn plays key roles within and outside of the nervous system. BMC Neurosci 13, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini AC, Forner S, Trujillo-Estrada L, Baglietto-Vargas D, and LaFerla FM (2018). Past to Future: What Animal Models Have Taught Us About Alzheimer’s Disease. JAlzheimers Dis 64, S365–S378. [DOI] [PubMed] [Google Scholar]
- Miller MB, Yan Y, Eipper BA, and Mains RE (2013). Neuronal Rho GEFs in Synaptic Physiology and Behavior. Neuroscientist 19, 255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Yan Y, Machida K, Kiraly DD, Levy AD, Wu YI, Lam TL, Abbott T, Koleske AJ, Eipper BA, et al. (2017). Brain region and isoform-specific phosphorylation alters Kalirin SH2 domain interaction sites and calpain sensitivity. ACS Chem Neurosci 8, 1554–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, Kirkwood CM, Gray MC, Ikonomovic MD, Penzes P, and Sweet RA (2012). beta-Amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol Aging 33, 2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Aoyama Y, Shin EJ, Nam Y, Kim HC, and Yamada K (2015). Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Alevels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). BehavBrain Res 289, 69–77. [DOI] [PubMed] [Google Scholar]
- Nakashima AS, Oddo S, LaFerla FM, and Dyck RH (2010). Experience-dependent regulation of vesicular zinc in male and female 3xTg-AD mice. NeurobiolAging 31, 605–613. [DOI] [PubMed] [Google Scholar]
- Neuner SM, Heuer SE, Huentelman MJ, O’Connell KMS, and Kaczorowski CC (2019). Harnessing Genetic Complexity to Enhance Translatability of Alzheimer’s Disease Mouse Models: A Path toward Precision Medicine. Neuron 101, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie L, Wei G, Peng S, Qu Z, Yang Y, and Yang X (2017). Melatonin ameliorates anxiety and depression-like behaviors and modulates proteomic changes in triple transgenic mice of Alzheimer’s disease. Biofactors 43, 593–611. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, and Vassar R (2006). Intraneuronal beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. JNeurosci 26, 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, and LaFerla FM (2003). Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Abeta and Synaptic Dysfunction. Neuron 39, 409–421. [DOI] [PubMed] [Google Scholar]
- Paul CM, Magda G, and Abel S (2009). Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. BehavBrain Res 203, 151–164. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli M, Losurdo M, Paolone G, Medelin M, Jaupaj L, Cisterna B, Slanzi A, Malatesta M, Coco S, and Buffelli M (2019). Glucocorticoid receptors modulate dendritic spine plasticity and microglia activity in an animal model of Alzheimer’s disease. Neurobiol Dis 132, 104568. [DOI] [PubMed] [Google Scholar]
- Penzes P, Buonanno A, Passafaro M, Sala C, and Sweet RA (2013). Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. JNeurochem 126, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, and Eipper BA (2000). An Isoform of Kalirin, a Brain-Specific GDP/GTP Exchange Factor, Is Enriched in the Postsynaptic Density Fraction. jbc 275, 6395–6403. [DOI] [PubMed] [Google Scholar]
- Penzes P, and Jones KA (2008). Dendritic spine dynamics--a key role for kalirin-7. TINS 31, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KG, Ma XM, Eipper BA, and Mains RE (2019). Identifying roles for peptidergic signaling in mice. ProcNatlAcadSciUSA 116, 20169–20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal C, Stone KL, Francone VP, Mains RE, and Eipper BA (2009). Secretory Granule to the Nucleus: role of a multiply phosphorylated intrinsically unstructured domain. JBiolChem 284, 25723–25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, and Ferguson SA (2014). Barnes Maze Testing Strategies with Small and Large Rodent Models. JVosExp 84, e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, DiBattista AM, Sung YM, Ahn JM, Turner RS, and Hoe HS (2014). A tetra(ethylene glycol) derivative of benzothiazole aniline ameliorates dendritic spine density and cognitive function in a mouse model of Alzheimer’s disease. ExpNeurol 252, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R, Antle MC, LaFerla FM, and Dyck RH (2010a). Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res 1348, 149–155. [DOI] [PubMed] [Google Scholar]
- Sterniczuk R, Dyck RH, LaFerla FM, and Antle MC (2010b). Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: Part 1. Circadian changes. Brain Res 1348, 139–148. [DOI] [PubMed] [Google Scholar]
- Stimmell AC, Baglietto-Vargas D, Moseley SC, Lapointe V, Thompson LM, LaFerla FM, McNaughton BL, and Wilber AA (2019). Impaired Spatial Reorientation in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Sci Rep 9, 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover KR, Campbell MA, VanWinssen CM, and Brown RE (2015). Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. BehavBrain Res 289, 29–38. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, and Imayoshi I (2017). Network analysis of exploratory behaviors of mice in a spatial learning and memory task. PLoS ONE 12, e0180789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Bangen KJ, Weigand AJ, Edmonds EC, Wong CG, Cooper S, Delano-Wood L, and Bondi MW (2020). Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology 94, e397–e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Lista V, Lopez-Pousa S, and Gimenez-Liort L (2019). Impact of Chronic Risperidone Use on Behavior and Survival of 3xTg-AD Mice Model of Alzheimer’s Disease and Mice With Normal Aging. FrontPharmacol 10, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, and Calon F (2014). Insulin Reverses the High-Fat Diet–Induced Increase in Brain Ab and Improves Memory in an Animal Model of Alzheimer Disease. Diabetes 63, 4291–4301. [DOI] [PubMed] [Google Scholar]
- VanDerJeugd A, Parra-Damas A, Baeta-Corral R, Soto-Faguas CM, LaFerla FM, and Saura CA (2018). Reversal of memory and neuropsychiatric symptoms and reduced tau pathology by selenium in 3xTg-AD mice. Sci Rep 8, 6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Sidhu H, Kreifeldt M, Roberto M, and Contet C (2018). Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology 133, 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili J, Lebbadi M, Tremblay C, St-Amour I, Pierrisnard C, and Calon F (2018). Characterization of a 3xTg-AD mouse model of Alzheimer’s disease with the senescence accelerated mouse prone 8 (SAMP8) background. Synapse 72, e22025. [DOI] [PubMed] [Google Scholar]
- Xie Z, Shapiro LP, Cahill ME, Russell TA, Lacor PN, Klein WL, and Penzes P (2019). Kalirin-7 prevents dendritic spine dysgenesis induced by amyloid beta-derived oligomers. EurJNeurosci 49, 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chatterjee M, Baguley TD, Brouillette J, Nairn AC, Ellman JA, and Lombroso PJ (2014). Inhibitor of the Tyrosine Phosphatase STEP Reverses Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. PLoS Biology 12, e1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Liang J, Gao J, Wang L, Fujioka H, Zhu X, and Wang X (2020). FAM222A encodes a protein which accumulates in plaques in Alzheimer’s disease. Nat Commun 11, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Eipper BA, and Mains RE (2015). Kalirin-9 and Kalirin-12 play essential roles in dendritic outgrowth and branching. Cerebral Cortex 25, 3487–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Li H, and Gao Z (2018). Copper Binding Induces Nitration of NPY under Nitrative Stress: Complicating the Role of NPY in Alzheimer’s Disease. ChemResToxicol 31, 904–913. [DOI] [PubMed] [Google Scholar]
- Yeung ST, Martinez-Coria H, Ager RR, Rodriguez-Ortiz CJ, Baglietto-Vargas D, and LaFerla FM (2015). Repeated cognitive stimulation alleviates memory impairments in an Alzheimer’s disease mouse model. Brain Res Bull 117, 10–15. [DOI] [PubMed] [Google Scholar]
- Ying M, Sui X, Zhang Y, Sun Q, Qu Z, and Yang X (2017). Identification of Novel Key Molecules Involved in Spatial Memory Impairment in Triple Transgenic Mice of Alzheimer’s Disease. MolNeurobiol 54, 3843–3858. [DOI] [PubMed] [Google Scholar]
- Youn H, Jeoung M, Koo Y, Ji H, Markesbery WR, Ji I, and Ji TH (2007). Kalirin is under-expressed in Alzheimer’s disease hippocampus. J Alzheimers Dis 11, 385–397. [DOI] [PubMed] [Google Scholar]
- Yu Y, Li X, Blanchard J, Li Y, Iqbal K, Liu F, and Gong CX (2015). Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm 122, 593–606. [DOI] [PubMed] [Google Scholar]
- Yu YZ, Li QL, Wang HC, Liu S, Pang XB, Xu Q, Zhou XW, and Huang PT (2018). Improved synaptic and cognitive function in aged 3×Tg-AD mice with reduced amyloid-β after immunotherapy with a novel recombinant 6Aβ15-TF chimeric vaccine. ClinImmunol 193, 12–23. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Greengard P, Strittmatter SM, Nairn AC, and Lombroso PJ (2010). Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. ProcNatlAcadSciUSA 107, 19014–19019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, and Mains RE (1994). Endoproteolytic processing of POMC and the peptide biosynthetic endoproteases PC1 and PC2 in neuroendocrine cells overexpressing PC1 or PC2. J Biol Chem 269, 17440–17447. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl.Fig.1. Genotyping B6129SF2/J and 3xTG-AD mice. Mice purchased from Jackson Labs were individually identified using earclips and the earclip samples were used to examine the genotypes, using the primers and program in Table 1. Similar results were found for these samples using hAPP primer sets #1, 2, and 3. The hAPP primers on the JAX website (oIMR3610 and 3611) were not as helpful for genotyping, since oIMR3610 is identical in mouse and human APP. The hAPP bands from B6129 mice, produced with primer sets 3 and 4, were excised and sequenced by JAX staff, who verified that the bands have the expected sequences of human APP cDNA (4/30/2019). These B6129 tissue samples were all taken before the lab had received any 3xTG-H mice from JAX.