Abstract
Parkinson's disease (PD) is a neurodegenerative disease caused by the degeneration of substantia nigra neurons due to oxidative stress. Sesaminol has strong antioxidant and anti-cancer effects. We investigated the preventive effect on PD as a new physiological action of sesaminol produced from sesaminol glycoside using in vitro and in vivo PD models. To prepare an in vitro PD model, 6-hydroxydopamine (6-OHDA) was added to human neuroblastoma (SH-SY5Y cells). The viability of SH-SY5Y cells decreased dose-dependently following 6-OHDA treatment, but the addition of sesaminol restored viability to the control level. 6-OHDA increased intracellular reactive oxygen species production, and the addition of sesaminol significantly suppressed this increase. No Nrf2 expression in the nucleus was observed in the control group, but a slight increase was observed in the 6-OHDA group. The sesaminol group showed strong expression of Nrf2 in the cytoplasm and nucleus. NAD(P)H: quinone oxidoreductase (NQO1) activity was enhanced in the 6-OHDA group and further enhanced in the sesaminol group. Furthermore, the neurotoxine rotenone was orally administrated to mice to prepare an in vivo PD model. The motor function of rotenone-treated mice was shorter than that of the control group, but a small amount of sesaminol restored it to the control level. The intestinal motility in the rotenone group was significantly lower than that in the control group, but it remained at the control level in the sesaminol group. The expression of α-synuclein in the substantia nigra increased in the rotenone group but decreased in the sesaminol group. The rotenone group exhibited shortening and damage to the colonic mucosa, but these abnormalities of the colonic mucosa were scarcely observed in the sesaminol group. These results suggest that sesaminol has a preventative effect on PD.
Keywords: Neuroscience, Nutrition, Natural product, Oxidative stress, Antioxidant, Sesaminol, Parkinson's disease, 6-Hydroxydopamine, Rotenone, SH-SY5Y cells, Nrf2
Neuroscience; Nutrition; Natural product; Oxidative stress; Antioxidant; Sesaminol; Parkinson's disease; 6-Hydroxydopamine; Rotenone, SH-SY5Y cells; Nrf2
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disease with symptoms of bradykinesia, rigidity, tremor, and postural reflex disorder, due to a decrease in brain dopamine production resulting from the degeneration and loss of dopaminergic neurons in the substantia nigra. Previous studies showed the involvement of neurotoxins, oxidative stress, and mitochondrial disorders as causes of degeneration of substantia nigra cells [1, 2, 3, 4]. PD is the second most frequent neurodegenerative disease after Alzheimer's disease. Only symptomatic treatment is used for PD, and fundamental treatments or preventive methods are needed.
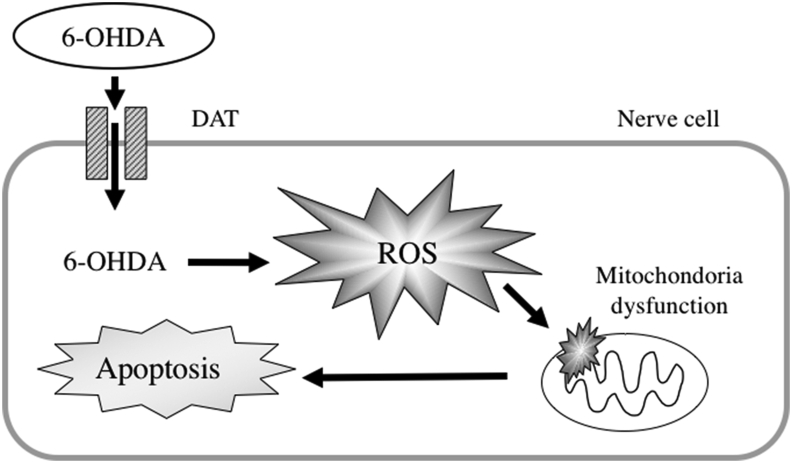
6-Hydroxydopamine (6-OHDA) is an oxidative analogue of dopamine that has a high affinity for catecholamine receptors due to its similar chemical structure to dopamine, and it is taken up into cells via the dopamine transporter (DAT) in neurons [5, 6]. 6-OHDA becomes quinone, which is a reactive oxygen species (ROS), via nonenzymatic autooxidation inside and outside cells, which produces hydrogen peroxide, superoxide radical and hydroxy radicals [7, 8]. Increased ROS deactivates biopolymers, such as nucleic acids and proteins, in neurons, disrupts organelle functions, such as mitochondria, and induces apoptosis [9] (Figure 1). Human neuroblastoma SH-SY5Y cells are most commonly used for in vitro PD studies. SH-SY5Y cells have nerve cell-like processes and express genes that are characteristic of dopaminergic neurons, such as DAT and vesicular monoamine transporter [10, 11]. To evaluate the protective effect of sesaminol against PD, SH-SY5Y cells were treated with 6-OHDA as an in vitro model of PD.
Figure 1.
The mechanism of inducing oxidative stress by 6-OHDA.
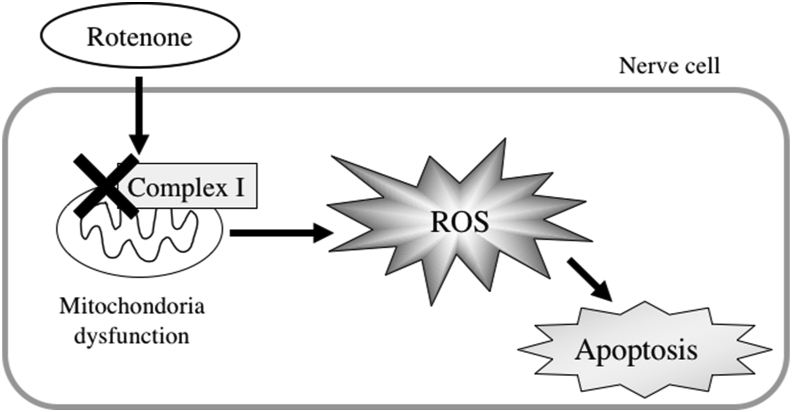
Rotenone is an active ingredient of the insecticide obtained from the roots of the legume Derris. Rotenone is lipophilic, readily crosses biological membranes, and specifically inhibits complex I in the mitochondrial electron transport chain [12]. Oral administration of rotenone causes motor and gastrointestinal dysfunction [13, 14] (Figure 2). Because of these properties, rotenone is used to establish PD-like animal models.
Figure 2.
The mechanism of inducing oxidative stress by Rotenone.
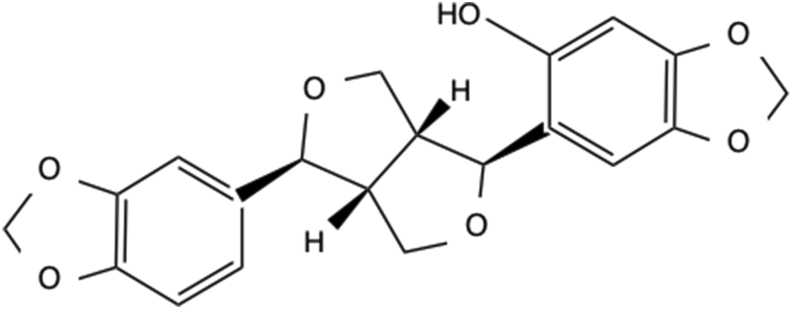
Sesaminol (3,4-methylenedioxy phenol) (Figure 3) is a sesame lignan found in sesame (Sesamum indicum L.) seeds, and it has a strong antioxidant effect. An anti-cancer effect of sesaminol was also reported [15, 16]. However, most sesaminol exists as a glycoside, and sesaminol remains in the form of glycoside in the defatted residue after the extraction of sesame oil. A recent method for purifying sesaminol from sesaminol glycoside was established, and the effective use of defatted sesame, which was treated as industrial waste, is expected [17].
Figure 3.
Structure of sesaminol.
Therefore, the present study investigated the preventive effect on PD as a new physiological action of sesaminol produced from sesaminol glycoside using in vitro and in vivo PD models. We also examined the mechanism of sesaminol protection in PD.
2. Materials and methods
2.1. Preparation of sesaminol
Paenibacillus sp. KB0549 strain was cultivated in a medium obtained by the addition of 1% tryptone, 0.5% yeast extract and 0.89% NaCl to a liquid extraction of sesame defatted debris with warm water. The obtained culture broth was added to heat-sterilized sesame defatted debris and fermented at 37 °C for 6 days using a solid fermenter under intermittent stirring and aeration conditions. The fermented sesame defatted debris was dried, 95% ethanol was added, and the mixture was stirred at 50 °C to extract sesaminol. The extract was filtered and concentrated in vacuo. Ethanol (99.5%) was added and concentrated in an evaporator to obtain a highly concentrated solution of sesaminol. The concentration of sesaminol was analyzed using HPLC. Then, the freeze-dried sesaminol preparation was dissolved in dimethyl sulfoxide (DMSO). The solution (72 mg/ml DMSO) was used as a sample in the experiment. In all experiments control cultures were made up of medium, DMSO and cells only.
2.2. Cell culture and measurement of cell viability
SH-SY5Y cells were seeded on a 96-well culture plate (Greiner Bio-One) at 1.0 × 10⁵ cells/ml using Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). Cells were cultured overnight and adhered to the plate. The medium was replaced with a culture medium containing sesaminol and/or 6-OHDA at various concentrations, and the cells were cultured in an incubator for various times. When sesaminol and 6-OHDA were used together, sesaminol was added 2 h before the addition of 6-OHDA. After the treatment, the medium was removed, and 100 μl of the culture medium containing 10% of a 5 mg/ml 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) solution was added to each well. The cells were left in the incubator for 2 h. The medium was removed, and 200 μl of DMSO was added to each well. The culture plate was stirred for 3 min on a plate mixer (Biotec, Tokyo, Japan), and the absorbance at a wavelength of 535 nm was measured using a microplate reader (Wallac 1420 ARVO sx).
2.3. Measurement of intracellular ROS production
A relatively specific probe for hydrogen peroxide, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), was used to analyze the formation of intracellular ROS. cells were incubated with 2.4 mM DCFH-DA (5 μL) for the final 30 min of the treatment. Then, cells were washed twice with PBS. For visualization of the intracellular fluorescence, the cells were observed with a FSX100 Bio Imaging Navigator, which is an all-in-one fluorescence imaging system (Olympus Corporation, Tokyo, Japan). The intracellular ROS production was evaluated via measurement of the fluorescence intensity.
2.4. Measurement of intracellular glutathione ratio (GSSG/GSH)
Cells treated with sesaminol and/or 6-OHDA were frozen and thawed twice with liquid nitrogen to disrupt the cell membrane. After the addition of 20 μl of a 5% 5-sulfosalicylic acid (SSA) solution and performing deproteinization, the supernatant was used as a measurement sample. The glutathione ratio was measured using the GSSG/GSH Quantification Kit (Dojindo, Kumamoto, Japan), and the absorbance at a wavelength of 420 nm was measured using a microplate reader (Wallac 1420 ARVO sx). The GSH concentration was calculated from the calculated total glutathione (GSH + GSSG) concentration and GSSG concentration using the following formula. GSH concentration = total glutathione concentration – [GSSG concentration] x 2 The value obtained by dividing the GSH concentration by the GSSG concentration was defined as the GSSG/GSH ratio.
2.5. Conformation of apoptotic cell death
Apoptotic cell death was confirmed using propidium iodide (PI). Cells were cultured for 24 h in 35 mm-dishes, washed twice with PBS and fixed with 70% ethanol at 4 °C for 30 min. After further washing twice with PBS, 400 μl of a PI staining solution was added at 37 °C for 1 h. The PI staining solution was removed, and the dish was covered with a cover glass (24 × 24 mm) for observation with a fluorescence microscope (Olympus, LSC101).
2.6. Identification of nuclear Nrf2 by immunofluorescence staining
SH-SY5Y cells (1.0 × 105 cells/ml) were cultured in a Lab-Tek Chamber Slides System (Thermo Fisher Scientific) overnight. Cells were exposed to 1 μg/ml sesaminol for 2 h and treated with 20 μM 6-OHDA for 3 h. Cells were rinsed with 0.5 ml PBS three times and permeabilized with 0.1% Triton-X (Sigma-Aldrich). The cells were blocked with 2 drops of Protein Block Serum-free for 30 min. Slides were exposed to an anti-Nrf2 antibody (Santa Cruz Biotechnology) overnight at 4 °C. The slides were washed with PBS and incubated with Alexa Fluor 488 goat anti-rabbit IgG (Life Technology) for 1 h. Following immunostaining, 4’,6-diamino-2-phenylindole dihydrochloride (DAPI) (Fujifilm Wako) was added. The slides were observed using a fluorescence microscope (Olympus, LSC101).
2.7. Measurement of NAD(P)H: quinone oxidoreductase (NQO1) activity
NQO1 activity was measured with reference to the method of Prochaska et al. [18]. SH-SY5Y cells (0.5 × 106 cells/ml) were cultured in 35-mm dishes overnight. Cells were exposed to 1 μg/ml sesaminol for 2 h and treated with 20 μM 6-OHDA for 6 h. The cells were sonicated on cooling with ice using a sonicator (Bio Ruptor, Cosmo Bio). After centrifugation (12000 rpm, 20 min 4 °C) in a centrifuge (TOMY MX-160 high speed refrigerated micro centrifuge), the supernatants were used for enzyme assays. To an assay tube, 3.85 ml of reaction mix was added. The reaction was started via the addition of 150 μl of the supernatant and stopped with a stop solution containing dicumarol. The MTT extinction coefficient e = 11.3 mM–1 cm–1 was used to calculate the amount of reduced MTT and defined as NQO1 activity.
2.8. Animal treatment
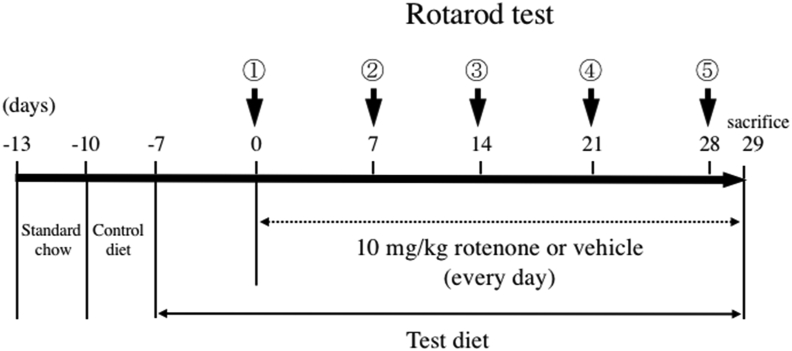
The study was approved by the Ethics Committee of laboratory animal and all analyses using laboratory animals complied with the regulations of the Osaka City University Laboratory Animal Committee (permission number: S0041 and S0073). Twenty-five 7-week-old male C57BL6/J mice were preliminarily fed standard chew for 3 days and a control diet for 3 days, then divided into 4 groups: (1) control group, (2) rotenone group, (3) Sesaminol (L) group, and (4) Sesaminol (H) group. Groups (2) to (4) received oral rotenone (10 mg/kg body weight) (Sigma-Aldrich) for 29 days via a gastric tube. The administration volume was 0.2 ml per animal. Rotenone was dissolved in 3% carboxysesaminolthyl cellulose sodium salt (CMC; Wako Pure Chemical Corporation) and 1.25% chloroform (Wako Pure Chemical Corporation). Only the solvent was administered to the control group. The diets were mixed at the ratios shown in Table 1. Diets of sesaminol (L) and sesaminol (H) contained 0.0008% sesaminol or 0.008% sesaminol, respectively. Mice were kept at 25 °C under a 12-hour light-dark cycle (lights from 8 am to 8 pm). Food and water were available ad libitum.
Table 1.
Composition of experimental diets.
| Components (g) | Control | 0.0008% sesaminol | 0.008% sesaminol |
|---|---|---|---|
| Casein | 140 | 140 | 140 |
| L-cystine | 1.8 | 1.8 | 1.8 |
| Cornstarch | 465.692 | 465.684 | 465.612 |
| α-cornstarch | 155 | 155 | 155 |
| Sucrose | 100 | 100 | 100 |
| Soybean Oil | 40 | 40 | 40 |
| Cellulose powder | 50 | 50 | 50 |
| AIN-93M mineral | 35 | 35 | 35 |
| AIN-93 vitamin | 10 | 10 | 10 |
| Choline Hydrogen Tartrate | 2.5 | 2.5 | 2.5 |
| tert-Butylhydroquinone | 0.008 | 0.008 | 0.008 |
| Sesaminol | 0 | 0.008 | 0.08 |
| Total | 1000 | 1000 | 1000 |
2.9. Motor function test
A preliminary test was performed one day before the start of the rotor rod main test. Mice were kept on the rod for 3 min in the preliminary test even after the mouse dropped to get accustomed to the test. As shown in Scheme 1, this test was performed 5 times in total every week from the start date of rotenone administration. The measurement was performed for a maximum of 5 min, which was repeated 3 times. The speed, time, and number of times for the rotor rod test are shown below (see Scheme 1).
| Preliminary test (6–10 rpm, maximum 3 mins) x 1 time, |
| (6–25 rpm, maximum 3 mins) x 1 time |
| Main test ① (6–25 rpm, maximum 5 min) × 3 times, |
| ② (6–30 rpm, maximum 5 min) × 3 times, |
| ③ ~ ⑤ 6–33 rpm (maximum 5 min) × 3 times |
Scheme 1.
The scheme of motor function test. The test was performed 5 times in total every week from the start date of rotenone administration. The measurement was performed for a maximum of 5 min, which was repeated 3 times. The test was per the speed, time, and number of times for the rotor rod test are shown below. Preliminary test (6–10 rpm, maximum 3 min) x 1 time, (6–25 rpm, maximum 3 min) x 1 time. Main test ① (6–25 rpm, maximum 5 min) × 3 times, ② (6–30 rpm, maximum 5 min) × 3 times, ③ ~ ⑤ 6–33 rpm (maximum 5 min) × 3 times.
2.10. Measurement of intestinal motor function
PD patients develop motor and nonmotor symptoms, such as gastrointestinal dysfunction [19, 20]. Therefore, the migration distance of Evans blue from the pylorus was measured to evaluate intestinal motility [21]. Five minutes before dissection, 0.3 mL of a 2.5% Evans blue solution was orally administered to each mouse. The distance from the pylorus to the furthest dye point was measured after dissection.
2.11. α-Synuclein staining in brain tissue
α-Synuclein is the main component of the Lewy bodies involved in PD development. Therefore, an anti-α-synuclein antibody was used to identify α-synuclein expression using the labeled streptavidin-biotinylated antibody (LSAB) method. The number of Lewy bodies expressing α-synuclein per visual field in the substantia nigra was measured.
2.12. Staining of tyrosine hydroxylase (TH) in brain tissue
TH is the rate-limiting enzyme for dopamine synthesis, and it catalyzes a reaction that adds a hydroxy group to tyrosine and biosynthesize dopa. Therefore, TH is a biomarker for dopamine neurons. TH expression was examined using the LSAB method and an anti-TH antibody (Sigma-Aldrich).
2.13. Statistical analysis
Values are expressed as the means ± standard error. After validating the differences between each group using one-way analysis of variance, the Tukey-Kramer method was used for multiple comparisons test. The significant difference test was performed at a 1 or 5% risk rate.
3. Results
3.1. Effects of sesaminol and/or 6-OHDA on cell viability
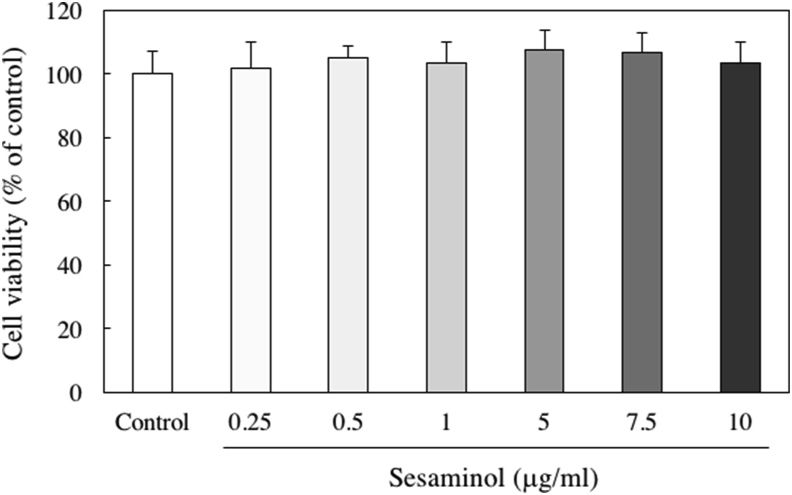
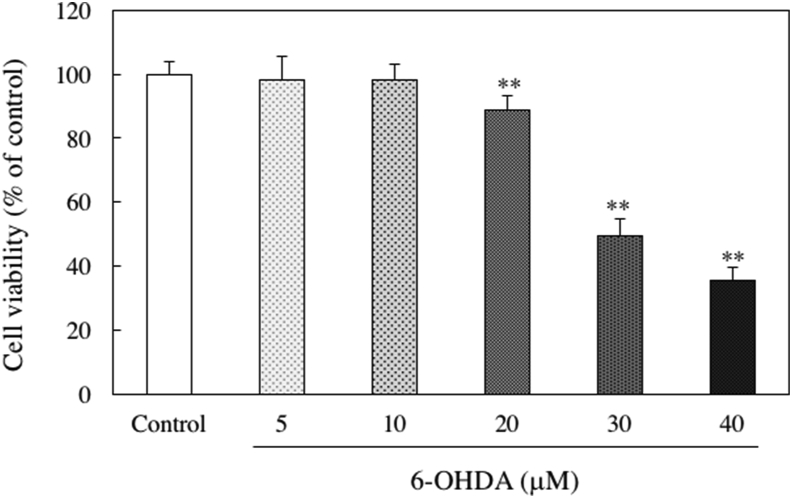
We evaluated the cytotoxicity of SH-SY5Y cells treated with sesaminol. Cell viability was measured using the MTT method. Sesaminol did not affect cell viability at any concentration from 0.25 to 10 μg/ml (Figure 4). To determine the most suitable concentration of 6-OHDA for an in vitro PD model, the cells were treated with various concentration of 6-OHDA. As shown in Figure 5, a significant decrease in cell viability was observed in the presence of 20 μM or higher 6-OHDA. Therefore, 20 μM 6-OHDA was used in subsequent experiments as a neuronal injury model in this study.
Figure 4.
Effect of sesaminol on the viability of SH-SY5Y cells. SH-SY5Y cells were incubated with 0.25–10 μg/ml sesaminol for 24 h. The cell viability was measured using the MTT assay. The results represent the means ± SD of 6 experiments.
Figure 5.
Effect of 6-OHDA on the viability of SH-SY5Y cells. SH-SY5Y cells were incubated with 5, 10, 20, 30 or 40 μM of 6-OHDA for 24 h. The cell viability was measured using the MTT assay. The results represent the means ± SD of 6 experiments. ∗∗p < 0.01 compared to control.
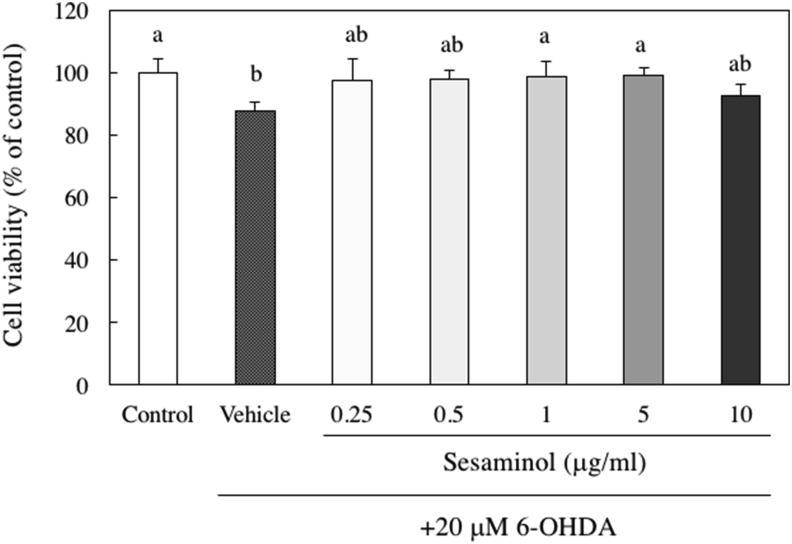
To evaluate the protective effect of sesaminol on 6-OHDA-induced cell death, the cells were exposed to various concentrations of sesaminol 2 h before the addition of 20 μM 6-OHDA. The reduced cell viability by 20 μM 6-OHDA was recovered with the pretreatment of 1 or 5 μg/ml sesaminol, which significantly restored cell viability to the same level as the control group (Figure 6). Based on these results, 1 μg/ml sesaminol was used in subsequent experiments.
Figure 6.
Effects of sesaminol and 6-OHDA on the viability of SH-SY5Y cells. SH-SY5Y cells were incubated with 0.25–10 μg/ml of sesaminol for 2 h, followed by treatment with 20 μM 6-OHDA for 24 h. The cell viability was measured using the MTT assay. The results represent the means ± SD 6 experiments. Values without a common letter are significantly different (p < 0.01).
3.2. Effect on intracellular ROS production
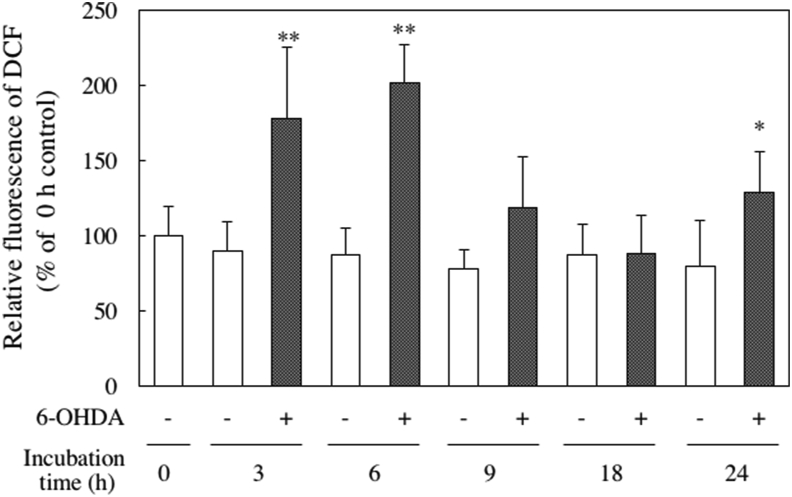
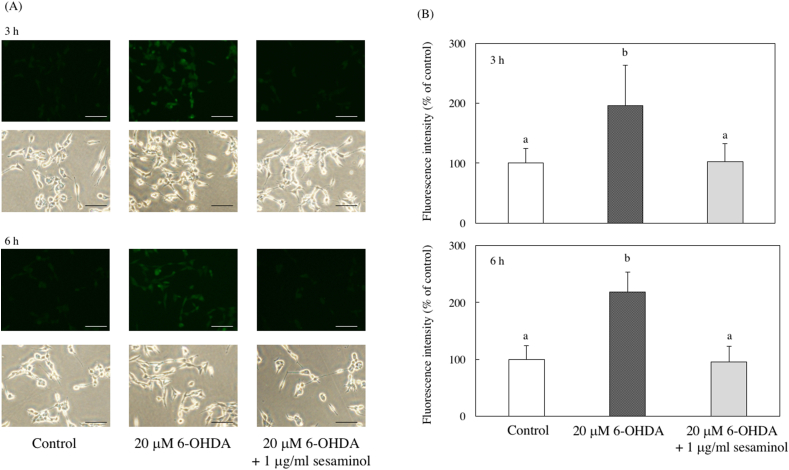
ROS induce oxidative stress, and it was fluorescently stained using the membrane-permeable probe DCFH-DA. Intracellular ROS production after the addition of 6-OHDA increased significantly 3 and 6 h after culture (Figure 7). As shown in Figure 8 (A, B), cells treated with 6-OHDA alone showed remarkably strong green fluorescence. However, the fluorescence of the cells pretreated with sesaminol for 2 h and cultured with 6-OHDA for 3 or 6 h was reduced to the control level. These results show that sesaminol pretreatment suppressed the 6-OHDA-induced increase in intracellular ROS production.
Figure 7.
Effect of 6-OHDA on intracellular ROS levels. SH-SY5Y cells were incubated with 20 μM 6-OHDA for 0, 3, 6, 9, 18 or 24 h. The results represent the means ± SD 6 experiments. ∗p < 0.05, ∗∗p < 0.01 compared to control of same incubation time.
Figure 8.
Effects of 6-OHDA and sesaminol on intracellular ROS levels. SH-SY5Y cells were incubated with 1 μg/ml of sesaminol for 2 h, followed by treatment with 20 μM 6-OHDA for 3 or 6 h. Intracellular ROS levels were measured using DCFH-DA. (A) The expression of intracellular ROS, (B) The fluorescence intensity of intracellular ROS. The results represent the means ± SD of 10 cells. Values without a common letter are significantly different (p < 0.05). Bar = 100 μm.
3.3. Protective effect of sesaminol against 6-OHDA-induced apoptotic cell death
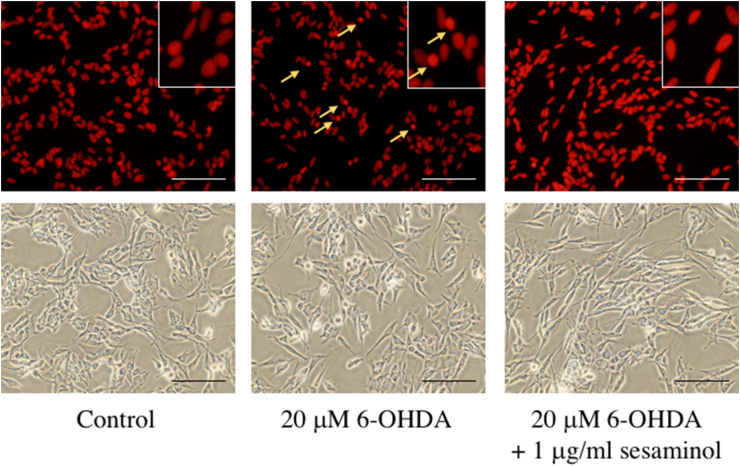
PI staining was performed to investigate whether the 6-OHDA-induced reduction in cell number was caused by apoptosis. As shown in Figure 9, morphological abnormalities, such as nuclear aggregation, were observed in cultures that only received 6-OHDA, but these morphological abnormalities were alleviated in the group pretreated with sesaminol for 2 h. These results suggest that sesaminol protected against 6-OHDA-induced apoptotic cell death.
Figure 9.
Effects of 6-OHDA and sesaminol on 6-OHDA-induced cell apoptosis. SH-SY5Y cells were incubated with 1 μg/ml of sesaminol for 2 h, followed by treatment with 20 μM 6-OHDA for 24 h. Cell apoptosis was measured using PI. Bar = 100 μm.
3.4. Effect of 6-OHDA and sesaminol on intracellular glutathione ratio
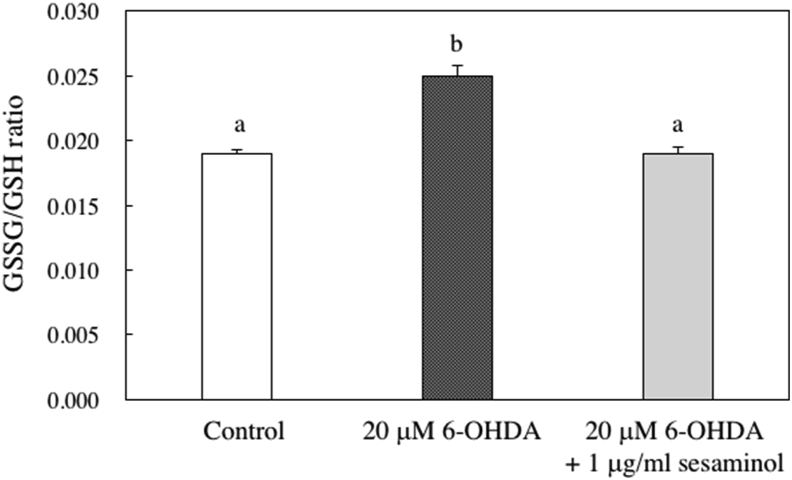
The intracellular glutathione ratio (GSSG/GSH) is an indicator of oxidative stress. After the addition of 6-OHDA and sesaminol and cultured for 6 h, the intracellular glutathione ratio was measured. The increase in the glutathione ratio (GSSG/GSH) by the addition of 6-OHDA was suppressed with the addition of sesaminol (Figure 10). These results indicate that the addition of sesaminol enhanced the intracellular antioxidant properties of SH-SY5Y cells.
Figure 10.
Levels of GSSG/GSH ratio in SH-SY5Y cells. SH-SY5Y cells were incubated with 1 μg/ml of sesaminol for 2 h, followed by treatment with 20 μM 6-OHDA for 6 h. Concentrations of GSSG and GSH were measured using a GSSG/GSH Quantification Kit. Data are presented as GSSG/GSH ratios, which were an average of three GSSG concentrations/GSH concentrations. The results represent the means ± SD of 5 experiments. Values without a common letter are significantly different (p < 0.01).
3.5. Effect of 6-OHDA and sesaminol on nuclear translocation of transcription factor Nrf2
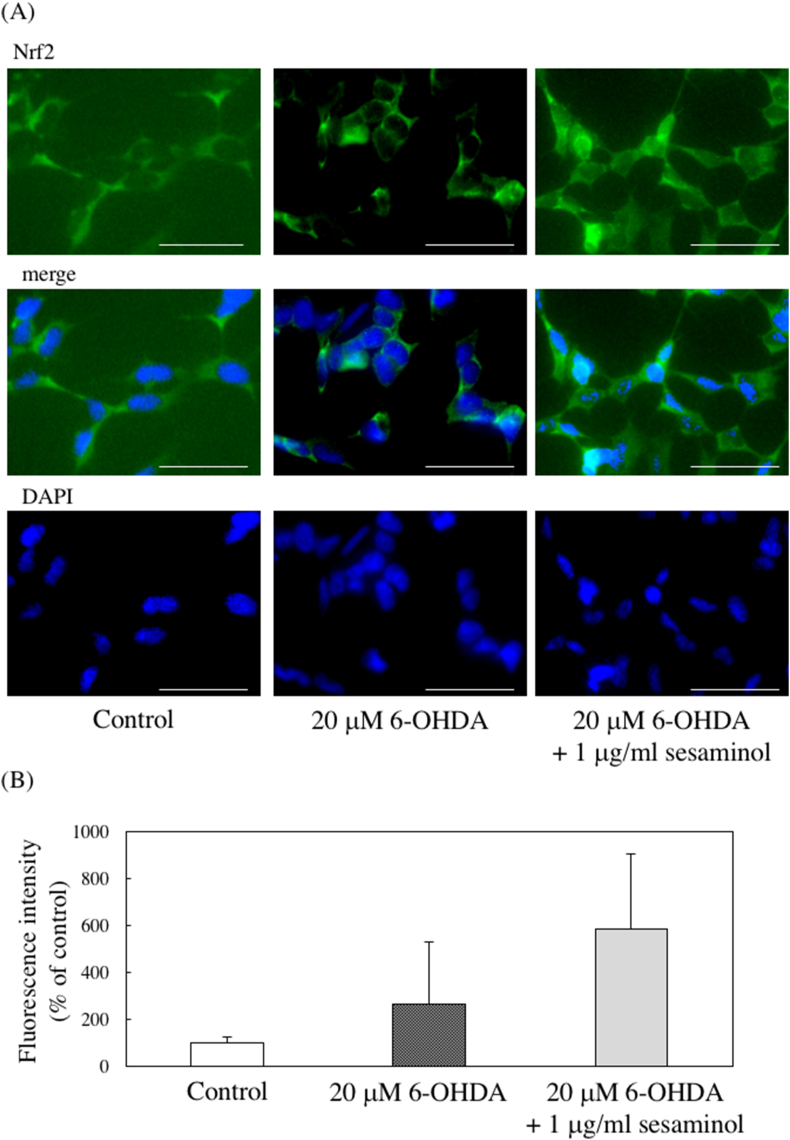
Translocation of the transcription factor nuclear factor-erythroid-2-related factor 2 (Nrf2) into the nucleus is important for activation of Nrf2-antioxidant response element (ARE) pathways, which play a major role in oxidative stress response. Nrf2 is normally localized in the cytoplasm and bound to the Keap1 protein, and it undergoes ubiquitination and promotes degradation in the proteasome system [22, 23]. However, conformational changes of Keap1 occur due to stimulation with electrophilic substances and ROS, and Nrf2 is dissociated from Keap1 and ubiquitin and translocated into the nucleus [24, 25, 26]. The translocate Nrf2 in the nucleus enhances the expression of antioxidant enzymes, such as NQO1 and γ-glutamyl cysteine synthetase (γ-GCS), via binding to the AREs on DNA. Therefore, immunofluorescent staining was performed to evaluate the effect of 6-OHDA and sesaminol on the nuclear translocation of Nrf2. Almost no expression of Nrf2 in the nucleus was observed in the control cells, and slight expression was observed in the cells treated with 6-OHDA alone. However, Nrf2 expression was observed in the cytoplasm and nuclei of cells pretreated with sesaminol (Figure 11). These results suggest that sesaminol promotes the nuclear translocation of Nrf2.
Figure 11.
Effects of 6-OHDA and sesaminol on nuclear translocation of Nrf2. SH-SY5Y cells were incubated with 1 μg/ml of sesaminol for 2 h, followed by treatment with 20 μM 6-OHDA for 3 h. Intracellular Nrf2 was stained using primary and secondary antibodies and nuclei were stained using DAPI. (A) The photos of the expression of Nrf2 and DAPI staining. Bar = 50 μm. (B) The fluorescence intensity of Nrf2 in nuclei. The results represent the means ± SD of 10 cells.
3.6. Effects of 6-OHDA and sesaminol on NQO1 activity
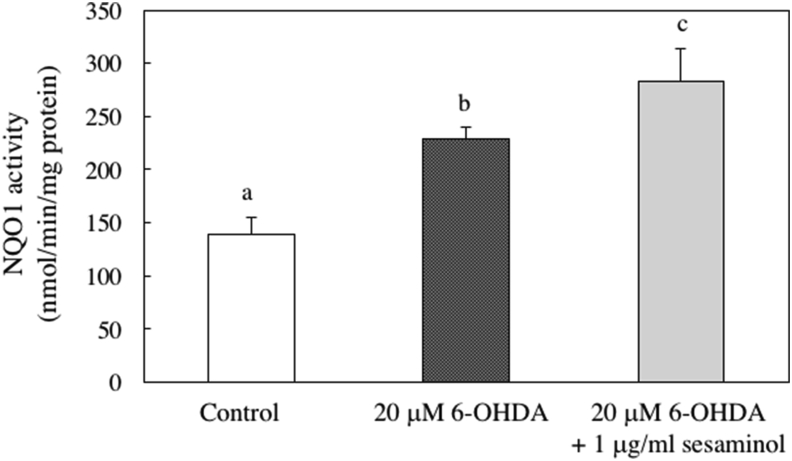
NQO1 is a typical enzyme that is expressed downstream of the Nrf2-ARE pathway, and it hydroxylates toxic quinones involved in production of ROS. NQO1 is involved in cell protection from ROS [27]. Therefore, we examined the effects of 6-OHDA and sesaminol on the activity of NQO1 and found that this activity increased in the cells treated with 6-OHDA alone, and it was markedly increased sesaminol pretreatment (Figure 12).
Figure 12.
Effect 6-OHDA and sesaminol on NQO1 activity. SH-SY5Y cells were incubated with 1 μg/ml of sesaminol for 2 h, followed by treatment with 20 μM 6-OHDA for 6 h. After incubation, NQO1 activity was measured using HJ Prochaska's method. The results represent the mean ± SD of 6 experiments. Values without a common letter are significantly different (p < 0.01).
3.7. Effects of rotenone and sesaminol on motor function in mice
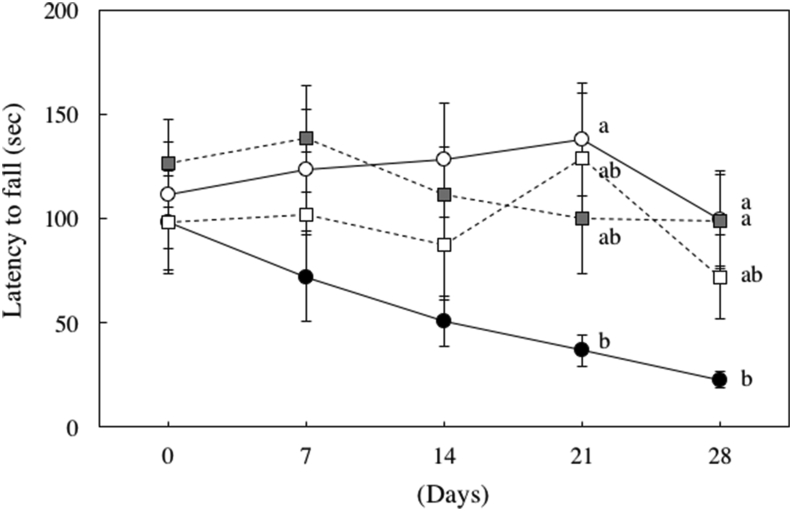
A rotor rod test was performed to evaluate motor function (Scheme 1). On day 28 after oral administration, the rod stay time of the rotenone group was significantly decreased compared to the control group, but the level of the group fed rotenone and a small amount of sesaminol (L) recovered to the level of the control group (Figure 13).
Figure 13.
Effect of rotenone on the latency to fall. Rotenone treatments induced motor dysfunction. ◯: Control group, ●: Rotenone group, ■: Sesaminol (L) group, □: Sesaminol (H) group. Values are means ± SEM. Values without a common letter are significantly different (p < 0.05).
3.8. Effects of rotenone and sesaminol on intestinal motility in mice
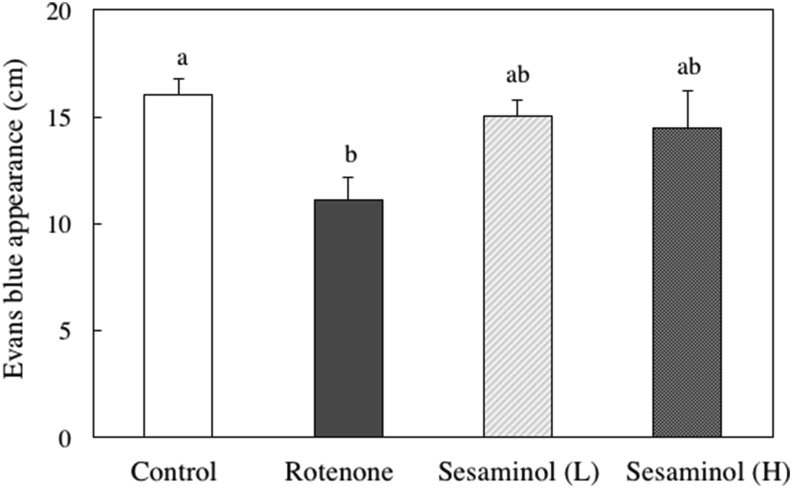
To evaluate intestinal motility, we measured the migration distance of Evans blue from the pylorus (Figure 14). The intestinal motility of the rotenone group was significantly decreased compared to the control group, but the intestinal motility function was restored with the feeding of a small amount of sesaminol with rotenone.
Figure 14.
Effects of sesaminol on intestinal transit indicated as the total distance traveled by Evans blue dye in the gastrointestinal tract 5 min after injection. Values are means ± SEM. Values without a common letter are significantly different (p < 0.05).
3.9. Effects of rotenone and sesaminol on α-synuclein expression in mouse brain
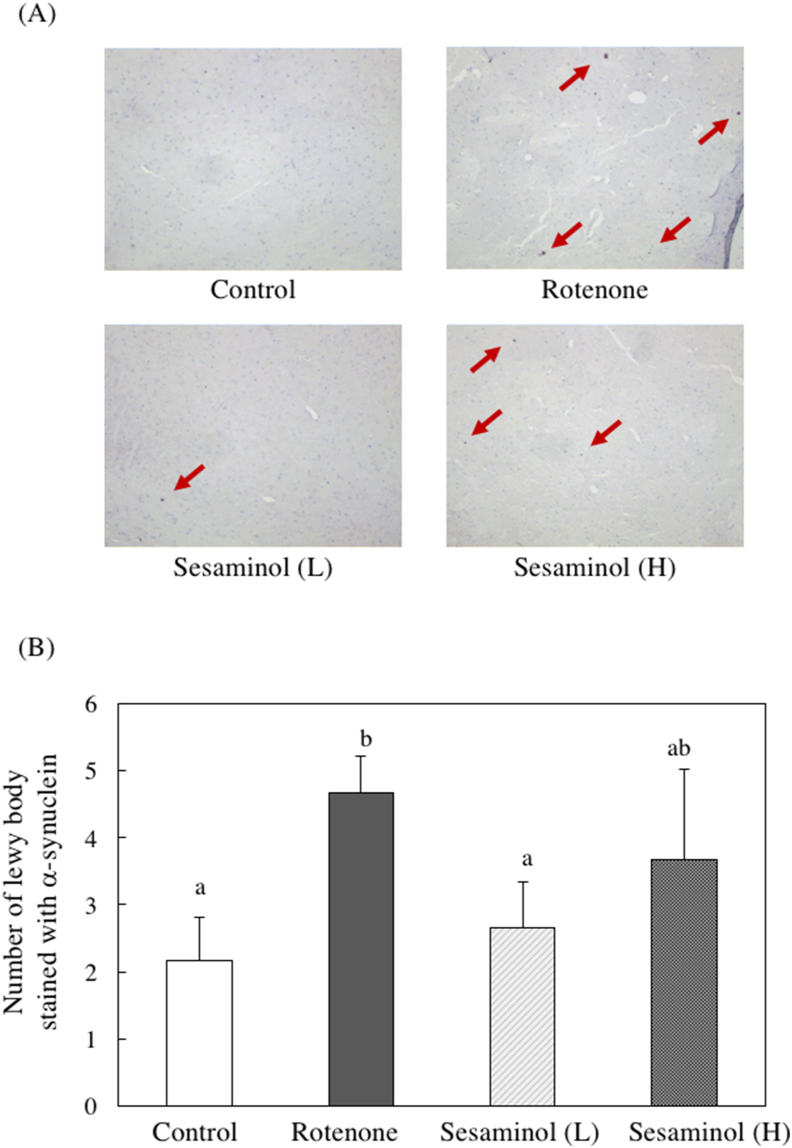
The onset of Parkinson's disease involves the aggregation of a protein called α-synuclein. Immunostaining of α-synuclein was performed (Figure 15A), and the number of Lewy bodies expressing α-synuclein in the substantia nigra area was counted (Figure 15B). α-Synuclein expression increased in the rotenone group compared to the control group, but it tended to decrease when sesaminol (L) was given with rotenone.
Figure 15.
Effects of sesaminol on (A) α-synuclein expression and (B) the number of Lewy body stained with α-synuclein in the substantia nigra. Values are means ± SEM. Values without a common letter are significantly different (p < 0.05).
3.10. Effects of rotenone and sesaminol on colonic mucosal morphology in mice
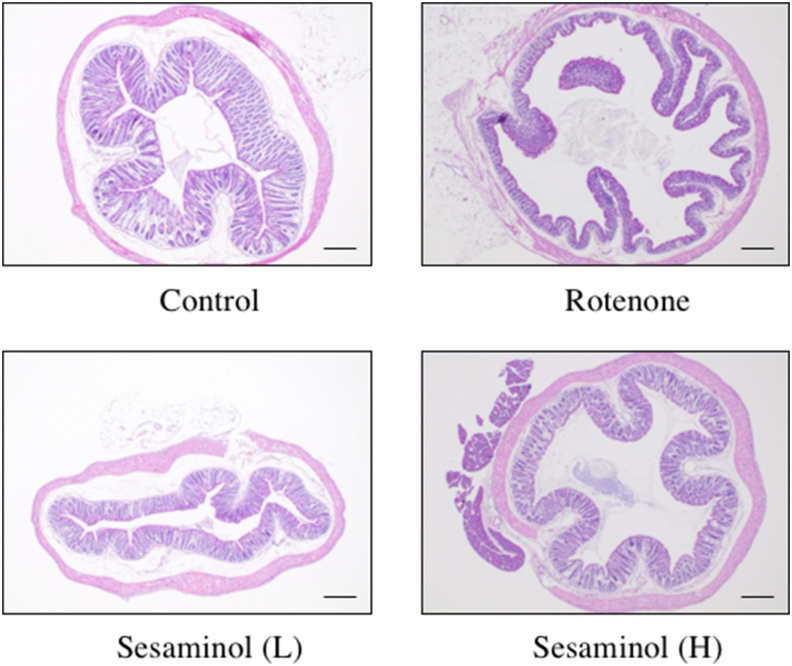
Parkinson's disease may start in the intestine rather than the brain. Therefore, the morphology of the mucosa in the colon stained with HE (hematoxylin-eosin) was observed (Figure 16). Compared to the control group, a shortening of the intestinal mucosal layer and damage to the mucosal surface were observed in the rotenone group. However, intestinal mucosal abnormalities were hardly observed in the group given rotenone and a small amount of sesaminol.
Figure 16.
Effects of sesaminol on colon morphology. Rotenone shortened the intestinal mucosal layer and caused abnormalities on the mucosal surface, but mice fed sesaminol showed the same colon morphology as the control. Bar = 200 μm.
3.11. Effects of rotenone and sesaminol on TH expression in the substantia nigra of mice
We performed the immunohistochemical staining of TH in the substantia nigra to investigate the neurodegeneration associated with movement disorders (Figure 17).
Figure 17.
Effects of sesaminol on TH expression in the substantia nigra. TH was stained using primary and secondary antibodies. Bar = 200 μm.
Administration of rotenone decreased TH-positive dopaminergic neurons compared to control. However, sesaminol tended to recover from s TH decrease.
4. Discussion
PD is a neurodegenerative disease caused by the degeneration of substantia nigra neurons. However, the treatment of PD is currently limited to symptomatic treatment, and there is an urgent need to establish a fundamental treatment and prevention method to prevent or delay neuronal cell death.
Oxidative stress is a primary cause of substantia nigra degeneration [28, 29, 30]. The substantia nigra has a high content of oxidizable substances, such as dopamine, and ROS is derived from dopamine, nerve melanin, highly unsaturated fatty acids and iron, and relatively low antioxidant substances. Therefore, oxidative stress easily controls the innate defense mechanism, and the resulting ROS sustains the oxidative stress state and induces the apoptosis of dopaminergic neurons [30].
Many studies revealed that 6-OHDA induced apoptosis via the application of oxidative stress to nerve cells, which caused mitochondrial damage from the byproducts that accompany autoxidation, such as H2O2 [31, 32] Therefore, ROS production was monitored during the culture time, and ROS production of SH-SY5Y cells increased 3–6 h after the addition of 6-OHDA. Measurement of intracellular ROS production revealed a significant increase in the intracellular ROS amount following the addition of 6-OHDA, and this increase was suppressed to the control level via 2 h of sesaminol pretreatment. The intracellular glutathione ratio (GSSG/GSH), which is an index of oxidative stress, was also measured. The addition of 6-OHDA increased the glutathione ratio, and the addition of sesaminol decreased the ratio. PI staining revealed an abnormal morphology, such as the aggregation of nuclei, which is a characteristic of apoptosis, after the addition of 6-OHDA alone, but not in the sesaminol and 6-OHDA group. These results show that sesaminol prevents apoptotic cell death via the suppression of oxidative stress associated with 6-OHDA-induced ROS production.
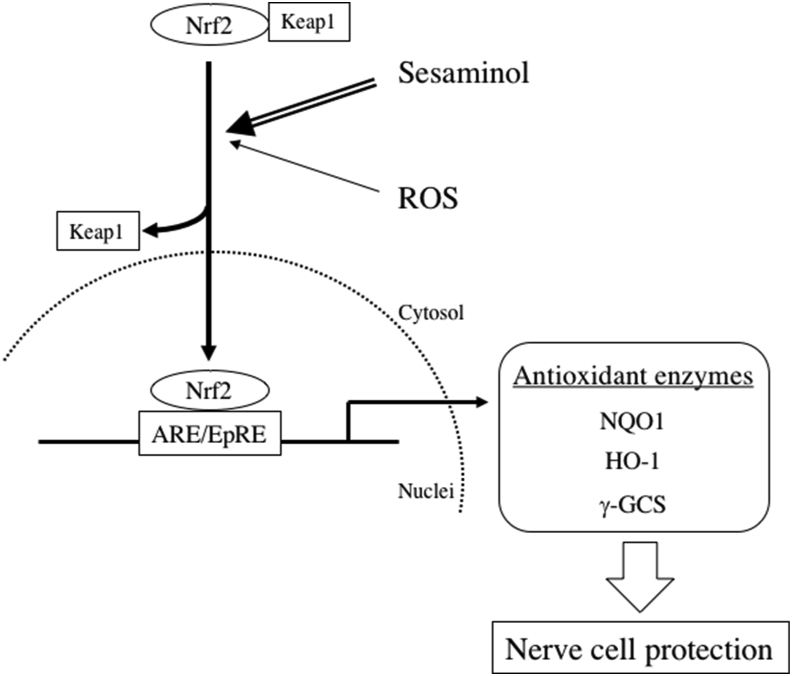
The Nrf2-ARE pathway is a potential defense system of cells, and it recent attracted much attention as a therapeutic target for neurodegenerative diseases because it is involved in the expression of numerous antioxidant enzyme systems [33, 34]. The present study suggested that sesaminol promoted the nuclear translocation of the transcription factor Nrf2 and enhanced NQO1 expression via activation of the Nrf2-ARE pathway to enhance the defense system against oxidative stress (Scheme 2).
Scheme 2.
The neuroprotection by sesaminol. ARE: antioxidant response element, EpRE: electrophile responsive element, NQO1: NAD(P)H: quinone oxidoreductase, HO-1: hemo oxygenase-1, γ-GCS: γ-glutamylcysteine synthetase.
We created PD model mice using rotenone and examined the preventive effect of sesaminol in PD. PD patients exhibit motor impairment as a symptom, and mice treated with rotenone exhibit motor impairment [13]. Therefore, a rotor rod test was performed to evaluate motor function. Rotenone significantly reduced rod stay time, and feeding a small amount of sesaminol (0.0008%) significantly restored stay times to the control level.
PD patients complain of constipation 10–20 years before the onset of movement disorder, and the transit time of feces in the intestine is more than twice as long as normal subjects [35, 36]. It is important to measure gastrointestinal motility because constipation adversely affects life and increases the risk of developing PD by a factor of 2.7–4.5 [36, 37]. Therefore, Evans blue was orally administered, and the gastrointestinal motility function was evaluated via measuring the migration distance of Evans blue from the pylorus. The intestinal motility function of the rotenone group decreased significantly compared to the control group, but sesaminol restored the intestinal motility.
The first Lewy bodies in PD likely appear in the substantia nigra because neuronal loss in the substantia nigra is observed. However, The various nonmotor symptoms appear before the onset of motor symptoms of PD, and the enteric nervous system (ENS) related to gastrointestinal symptoms in the onset of PD was recently investigated. The dual hit hypothesis proposed by Hawkes et al. [38] suggests that the path starts from the enteric nerve sac of the stomach to the dorsal motor nucleus of the vagus and spreads from the olfactory bulb to the substantia nigra and limbic system routes in the early stages of Lewy body pathology. Therefore, the morphology of the mucosa in the colon was observed using HE staining. Compared to the control group, a shortening of the intestinal mucosal layer and damage to the mucosal surface were observed in the rotenone group, but intestinal mucosal abnormalities were hardly observed in the sesaminol group. These results suggest that sesaminol prevents the progression of PD pathology from the intestine.
The results of the in vivo experimental system of the present study suggest that sesaminol prevents the development of PD pathology from the intestine and reduces α-synuclein expression in the substantia nigra, which suppresses motor dysfunction and the decline of intestinal motor function.
Whether sesaminol is able to across the blood-brain barrier (BBB) is an important problem. Therefore, we evaluated sesaminol by the following factors that determine the penetrability on the BBB. (1) High lipid solubility (water versus oil partition coefficient) [LogP] < 3, (2) number of hydrogen bonds <8, (3) a neutral charge or low degree of ionization (polar surface area) < 90 Å, (4) a less bulkey (number of rotable bonds) < 5 and (5) smaller molecular size <450 Da [39, 40]. The values of sesaminol for (1), (2), (3), (4) and (5) are 2.14, 7, 75.61 Å, 2 and 370.4, respectively. These values suggest that sesaminol may be able to across the BBB.
The present study also revealed that sesaminol had a neuroprotective effect in an in vitro experimental system and a PD preventive effect in an in vivo experimental system. Notably, the protective effect was observed with the feeding of a small amount of sesaminol. These results show that sesaminol is very suitable for use as a preventive treatment of PD. Further detailed elucidation of the mechanism of action will be necessary for practical application.
Declarations
Author contribution statement
Haruka Kaji: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Isao Matsui-Yuasa, Akiko Kojima-Yuasa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kayo Matsumoto: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ayano Omura, Kunio Kiyomoto: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Japan Society for the Promotion of Science (JSPAS) KAKENHI, Grant Number, JP15K00832.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Mizuno Y., Yoshino H., Ikebe S., Hattori N., Kobayashi T., Shimoda-Matsubayashi S., Matsumine H., Kondo T. Mitochondrial dysfunction in Parkinson’s disease. Ann. Neurol. 1998;44:S99–S109. doi: 10.1002/ana.410440715. [DOI] [PubMed] [Google Scholar]
- 2.Winklhofer K.F., Haass C. Mitochondorial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta 1802. 2010:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Patel M. Targeting oxidative stress in central nervous system disorders. Trends Pharmacol. Sci. 2016;37:768–778. doi: 10.1016/j.tips.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahrani M.A., Hoales S., Hargreaves I., Orford M. Oxidative stress: mechanistic insights into inherited mitochondrial disorders and Parkinson’s disease. J. Clin. Med. 2017;6:100. doi: 10.3390/jcm6110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen G. Oxy-radical toxicity in catecholamine neurons. Neulotoxicology. 1984;5:77–82. [PubMed] [Google Scholar]
- 6.Soto-Otero R., Mendez-Alvarez E., Hermida-Ameijeiras A., Munoz-Patino A.M., Labandeira-Garcia J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidant: potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem. 2000;74:1605–1612. doi: 10.1046/j.1471-4159.2000.0741605.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen G., Heikkila R.E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J. Biol. Chem. 1974;249:2447–2452. [PubMed] [Google Scholar]
- 8.Honrott K., Gudmunson L., O’Neill M.J. 6-Hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J. Biol. Chem. 2005;281:5373–5382. doi: 10.1074/jbc.M511560200. [DOI] [PubMed] [Google Scholar]
- 9.David B., Sakina T., Marie-France N., Alim-Louis B., Jean-Marc V. Extracellular toxicity of 6-hydroxydopamine on PC12 cells. Neurosci. Lett. 2000;283:193–196. doi: 10.1016/s0304-3940(00)00948-4. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T., Deng Y., Maruyama W., Dostert P., Kawai M., Naoi M. Uptake of a neurotoxin-candidate, (R)-1,2-dimethyl-6,7-dihygrooxy-1,2,3,4- tetrahydroisoquinoline into human dopaminergic neuroblastoma SH-SY5Y cells by dopamine transport system. J. Neural. Transm. 1994;98:107–118. doi: 10.1007/BF01277014. [DOI] [PubMed] [Google Scholar]
- 11.Pan T., Xie W., Jankovic J., Le W. Biological effects of pramipexole on dopaminergic neuron-associated genes; Relevance to neuroprotection. Neurosci. Lett. 2005;377:106–119. doi: 10.1016/j.neulet.2004.11.080. [DOI] [PubMed] [Google Scholar]
- 12.Valdez L.B., Zaobornyj T., Bandez M.J., Lopez-Cepero J.M., Boveris A., Navorro A. Complex syndrome in striatum and frontal in a rat model of Parkindon disease. Free Rad. Biol. Med. 2019;135:274–282. doi: 10.1016/j.freeradbiomed.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Inden M., Kitamura Y., Takeuchi H., Yanagida T., Takata K., Kobayashi Y., Taniguchi T., Yoshimoto K., Kaneko M., Okuma Y., Taira T., Ariga H., Shimohama S. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J. Neurochem. 2007;101:1491–1504. doi: 10.1111/j.1471-4159.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Pardo P., Doyiya H.B., Broersen L.M., Douna H., van Wijk N., Lopes da silva S., Garssen J., Keshavarzian A., Kraneveld A.P. Gut-brain and brain-gut axis in Parkinson’s disease models: effect of a uridine and fish oil diet. Nutr. Neurosci. 2018;21(6):391–402. doi: 10.1080/1028415X.2017.1294555. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M., Izumi Y., Iizuka-Ohashi M., Sowa Y., Sakai T. The pleiotropic regulation of cyclin D1 by newly identified sesaminol-binding protein. Oncogenesis. 2017;6:1–11. doi: 10.1038/oncsis.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyahara Y., Katsuzaki H., Imai K., Osawa T., Ina K., Komoya T. Sesaminol from sesame seed induced apoptosis in human lymphoid leukemia molt 4B cells. Int. J. Mol. Med. 2001;7:485–488. [PubMed] [Google Scholar]
- 17.Nair A., Kuwahara A., Nagase A., Yamaguchi H., Yamazaki T., Hosoya M., Omura A., Kiyomoto K., Yamaguchi M., Shimoyama T., Takahashi S., Nakayama T. Purification, gene cloning, and biochemical characterization of a beta-glucosidase capable of hydrolyzing sesaminol triglucoside from Paenibacillus sp KB0549. PloS One. 2013;8:4. doi: 10.1371/journal.pone.0060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prochaska H.J., Santamaria A.B. Direct measurement of NAD(P)H quinone oxidoreductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal. Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Pardo P., Kliest T., Dodiya H.B., Broersen L.M., Garssen J., Keshavarzian A., Kraneveld A.D. The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur. J. Pharmacol. 2017;817:86–95. doi: 10.1016/j.ejphar.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 20.Lubomski M., Davis R.L., Sue C.M. Gastrointestinal dysfunction in Parkinson’s disease. J. Neurol. 2020;267:1377–1388. doi: 10.1007/s00415-020-09723-5. [DOI] [PubMed] [Google Scholar]
- 21.Seerden T.C., De Winter B.Y., Van Den Bossche R.M., Herman A.G., Pelekmans P.A., De Man J.R. Regional differences in gastrointestinal motility disturbances during acute nectrotising pancreatitis. Neuro Gastroenterol. Motil. 2005;17:671–679. doi: 10.1111/j.1365-2982.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 22.Basso M., Giraudo S., Corpillo D., Bergamasso B., Lopiano L., Fasano M. Proteome analysis of human substantia nigra in Parkinson's disease. Proteomics. 2004;4:3943–3952. doi: 10.1002/pmic.200400848. [DOI] [PubMed] [Google Scholar]
- 23.Itoh K., Wakabayashi N., Katoh Y., Ishii T., O'Connor T., Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi A., Kang M., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.H., Huang J.U., Noh J.R., Gang G.T., Tadi S., Yim Y.H., Jeoung N.H., Kwak T.H., Lee S.H., Kweon G.R., Kim J.M., Shong M., Lee I.K., Lee G.H. Prevention of salt-induced renal injury by activation of NAD(P)H:quinone oxidoreductase, associated with NADPH oxidase. Free Rad. Biol. Med. 2012;52:880–888. doi: 10.1016/j.freeradbiomed.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Steffen V., Vizuete M.L., Machado A., Cano J. The effect of a vitamin-E-dependent diet on amino-acid levels in the substantia-nigra, striatum and hippocampus of rats. Life Sci. 1994;54:375–379. doi: 10.1016/0024-3205(94)00794-2. [DOI] [PubMed] [Google Scholar]
- 29.Kostic V., Gurney M.E., Deng H.X., Siddique T., Epstein C.J., Przedborski S. Midbrian dopaminergic neuronaldegeneration in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;41:497–504. doi: 10.1002/ana.410410413. [DOI] [PubMed] [Google Scholar]
- 30.Hald A., Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp. Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.S., Park W.J., Ko H.H., Han E.S. Differential involvement of mitochondrial permeability transition in cytotoxicity of 1-methyl-4-phenylpyridium and 6-hydroxydopamine. Mol. Cell. Biochem. 2006;289:193–200. doi: 10.1007/s11010-006-9164-0. [DOI] [PubMed] [Google Scholar]
- 32.Fonseca-Fonseca L.A., Nunez-Figueredo Y., Sanchez J.R., Guerra M.W., Ochoa-Rodrigues E., Verdecia-Reyes Y., Hernadez R.D., Menezes N.J., Costa T.C.S., A de Santana W., Oliveira J.L., Segura-Aguilar J., da Silva V.D.A., Costa S.L. KM-34, a novel antioxidant compound, protects against 6-hydrodopamine-induced mitochondrial damage and neurotoxicity. Neurotox. Res. 2019;36:279–291. doi: 10.1007/s12640-017-9851-5. [DOI] [PubMed] [Google Scholar]
- 33.Lim J.L., Wilhelmus M.M.M., de Vries H.E., Drukarch B., Hoozemans J.J.M., van Horssen J. Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspective in neurodegenerative diseases. Arch. Toxicol. 2014;88:1773–1786. doi: 10.1007/s00204-014-1338-z. [DOI] [PubMed] [Google Scholar]
- 34.Johnson D.A., Johnson J.A. Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Rad. Biol. Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jost W.H., Schimrigk K. Constipation in Parkinson’s disease. Klin. Wochenschr. 1991;69:906–909. doi: 10.1007/BF01798536. [DOI] [PubMed] [Google Scholar]
- 36.Abott R.D., Petrovitch H., White L.R., Masaki K.H., Tanner C.M., Curb J.D., Grandinetti A., Blanchette P.I., Popper J.S., Ross G.W. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 37.Uc E.Y., McDermott M.P., Marder K.S., Anderson S.W., Litvan I., Como P.G., Auinger P., Chou K.I., Growdon J.C. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohot. Neurology. 2009;73:1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkes C.H., Del Tredici K., Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakakibara R., Uchiyama T., Yamanishi T., Kishi M. Dementia and lower urinary dysfunction: with a reference to anticholinergic use in elderly population. Int. J. Urol. 2008;15:778–788. doi: 10.1111/j.1442-2042.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 40.Reichel A. The Role of Blood-Brain Barrier studies in the pharmaceutical industry. Curr. Drug Metabol. 2006;7:183–203. doi: 10.2174/138920006775541525. [DOI] [PubMed] [Google Scholar]