Abstract
The aim of the study was to investigate the effects of estrogen receptors (ESR1 and ESR2) on the expression of the proteins involved with proliferation (CCND1) and differentiation (CDKN1B and CTNNB) of Sertoli cells from rat in different stages of development. ESR1-selective agonist PPT, but not ESR2-selective agonist DPN, increased CCND1 expression in Sertoli cells from 5- and 15-day old rats. PPT did not have any effect on CCND1 expression in Sertoli cells from 20- and 30-day-old rats. DPN, but not PPT, increased CDKN1B expression in Sertoli cells from 15-, 20-, 30-day-old rats. DPN did not have any effect on Sertoli cells from 5-day-old rats. 17β-estradiol (E2) and PPT enhanced the [Methyl-3H] thymidine incorporation in Sertoli cells from 15-day-old rats, whereas the treatment did not have any effect in 20-day-old rats. E2 and DPN, but not PPT, increased non-phosphorylated CTNNB expression in Sertoli cells from 20-day-old rats. This upregulation was blocked by ESR2-selective antagonist PHTPP. The activation of ESR1 and ESR2, respectively, plays a role in the proliferation and differentiation of Sertoli cells in a critical period of testicular development. Furthermore, in Sertoli cells from 20-day-old rats, upregulation of non-phosphorylated CTNNB by E2/ESR2, via c-SRC/ERK1/2 and PI3K/AKT, may play a role in the interaction between Sertoli cells and/or in cell-germ cell adhesion and/or in the stabilization and accumulation of CTNNB in the cytosol. CTNNB could be translocated to the nucleus and modulate the transcriptional activity of specific target genes. The present study reinforces the important role of estrogen in normal testis development.
Keywords: Cell biology, Reproductive medicine, Cell culture, Cell differentiation, Systems biology, Reproductive hormone, Steroid hormones, 17β-estradiol, Estrogen receptors, Sertoli cells
Cell biology; Reproductive medicine; Cell culture; Cell differentiation; Systems biology; Reproductive hormone; Steroid hormones; 17β-estradiol; Estrogen receptors,; Sertoli cells.
1. Introduction
Sertoli cells provide an environment for the development of germ cells (reviewed by Sharpe et al., 2003; Griswold 2018). A reduction in the proliferation of Sertoli cells from neonatal rat causes a change in the number of Sertoli cells and spermatids in the adult (Orth et al., 1988). The rise of the proliferation in the Sertoli cells in the neonatal period of the rat or mouse increases spermatids number and maintains the spermatid/Sertoli cell ratio (Simorangkir et al., 1995; Meachem et al., 1996; Auharek et al., 2011). The number of Sertoli cells defines and predicts population sizes of germ and Leydig cells in the adult mouse testis (Rebourcet et al., 2017). Thus, changes in the number/proliferation of Sertoli cells have consequent effects on fertility and health.
The differentiation of Sertoli cells is associated with a cessation of proliferation, establishment of the blood-testis barrier, changes in the expression of proteins, and the capacity to sustain spermatogenesis (reviewed by Sharpe et al., 2003; Tarulli et al., 2012; Griswold 2018).
The dynamics of the proliferation and differentiation of Sertoli cells seem to be controlled by a complex arrangement of hormones and growth factors (reviewed by Lucas et al., 2014a; Meroni et al., 2019). Among them, actions of FSH (follicle stimulating hormone) (Griswold et al. 1975, 1977), insulin family of growth factors (Villalpando et al., 2008), activin (Matzuk et al., 1995), androgens (Buzzard et al., 2003; Hazra et al., 2013), thyroid hormones (Buzzard et al., 2003), retinoic acid (Buzzard et al., 2003; Nicholls et al., 2013) and estrogens (Lucas et al., 2014b) must be highlighted (reviewed by Lucas et al., 2014a; Meroni et al., 2019). The main signal transduction pathways such as cyclic-AMP/PKA (cyclic adenosine monophosphate/protein kinase A), ERK1/2 (extracellular signal-regulated kinase 1/2) (Crépieux et al., 2001; Lucas et al., 2008), PI3K/AKT (phosphatidylinositol 3-kinase/AKT serine/threonine kinase) (Musnier et al., 2009), and mTORC1/p70SK6 (CREB-regulated transcription coactivator/protein70-S6-kinase-1) (Riera et al., 2012) are involved in the proliferation of the Sertoli cell (reviewed by Lucas et al., 2014a; Meroni et al., 2019).
Concerning the role of estrogen and estrogen receptors (ERs), ESR1 (ERα) and ESR2 (ERβ), in male reproduction, Esr1KO rats or mice are infertile with reductions in testis weight and cauda epididymal sperm (Rumi et al., 2014). However, Esr1KO rat had lower testosterone concentration, in contrast to Esr1KO mice, despite showing elevated LH levels. The Esr2KO rats were fertile and lacked reproductive tract abnormalities (Rumi et al., 2017), which is consistent with most observations from Esr2KO mice (Couse et al., 2000; Krege et al., 1998; Antal et al., 2008). Studies, using knockout or transgenic animal models, have shown that ESR1 is essential for male fertility, and that loss of the extranuclear pool of the ESR1 is sufficient to induce extensive male reproductive abnormalities and infertility (reviewed by Hess and Cooke, 2018).
In fact, in Sertoli cells from 15-day-old rats, our laboratory has shown that the interaction of 17β-estradiol (E2) with ESR1 promotes cell proliferation, through the activation of NF-kB (nuclear factor-kB) in PI3K- and ERK1/2-dependent manner, and increase of the CCND1 (Cyclin D1). In contrast, the interaction of E2 with ESR2 promotes cell cycle arrest and cell differentiation, through the activation of CREB (cAMP response element-binding protein) in PI3K-dependent manner, and this leads to an increase of CDKN1B (p27kip1), GATA1 (zinc finger transcription factor) and DMRT1 (doublesex and mab-3 related transcription factor-1) (Lucas et al., 2014b), which are proteins related with cell cycle arrest and cell differentiation. However, the ratio of ESR1/ESR2 expression in the basal level (control, without treatment) favors a predominant expression of ESR1 (Lucas et al., 2014b). Furthermore, E2 and ESR1-selective agonist PPT enhanced the [Methyl-3H] thymidine incorporation in these cells, indicating a proliferative effect of E2 through ESR1 at this stage of development (15-day-old rats) (Lucas et al. 2008, 2014b).
It is important to understand the role of estrogen on the proliferation and differentiation of the Sertoli cells during the development of the animals. The aim of the present study was to investigate the role of each estrogen receptor in the regulation of the proteins involved with proliferation CCND1 and differentiation CDKN1B and CTNNB (β-catenin) of Sertoli cells from rat in different stages of development.
2. Materials and methods
2.1. Sertoli cell culture
All experimental procedures performed in these studies were conducted according to guidelines for the care and use of laboratory animals as approved by the Research Ethical Committee from Escola Paulista de Medicina-Universidade Federal de São Paulo (#3723240815/2015). In these studies, were used 280 rats at different stages of development. The testes from 5-, 15-, 20- and 30-day-old rats were removed and decapsulated, and Sertoli cells were prepared as previously described by Skinner and Fritz (1985) with slight modification (Grima et al., 1997; Lucas et al. 2008, 2010), using serial enzymatic digestions and a hypotonic shock to eliminate germ cell contamination (Lucas et al., 2008). Previous studies from our laboratory evaluated the purity of rat Sertoli cell primary cultures using a combination of the morphological and immunocytochemistry analyses and confirmed the absolute predominance of Sertoli cells (94–98%) (Lucas et al., 2008). For CTNNB analysis, freshly isolated Sertoli cells from 20-day-old rats were plated on Matrigel (BD Biosciences, San Jose, CA, USA)-coated 6-well plate at 0.5 × 106 cells/cm2 (Li et al., 2009). The cells were 90%–95% confluent, and the number of viable cells in each culture was more than 90%, as determined by trypan blue exclusion. Each assay, using the cells from rats at different stages of development, was performed on the same day.
2.2. Western blot analysis for detection of CCND1, CDKN1B, ERK1/2 and AKT
Primary Sertoli cell cultures from 5-, 15-, 20- and 30-day-old rats were incubated in the absence (vehicle; control, C) and presence of E2 (0,1 nM, Sigma Chemical Co.), ESR1-selective agonist PPT (4,4′,4″-(4-propyl)-(1H)-pyrazole-1,3,5-triyl) trisphenol; 10 nM; Tocris Bioscience, Bristol, UK), or ESR2-selective agonist DPN (2,3-bis(4-hydroxyphenyl)-propionitrile; 10 nM; (Tocris Bioscience) for 24 h at 35 °C (Lucas et al. 2008, 2014b). The agonists are highly selective at these concentrations, as previously reported (Stauffer et al., 2000; Meyers et al., 2001; Lucas et al., 2008; Pisolato et al., 2016). Western blot assays for CCND1, CDKN1B and ACT (actin) were performed as previously described by Lucas et al. (2014b). Briefly, total cell lysates (40 μg of protein/lane) were resolved in 12% or 15% SDS/PAGE, transferred to PVDF membrane and probed with rabbit polyclonal antibody raised against a synthetic peptide conjugated to keyhole limpet hemocyanin derived from residues near the C-terminus of CCND1 (#2922, Cell Signaling Technology, 1:500); rabbit polyclonal antibody raised against a synthetic peptide derived from residues near the N-terminus of CDKN1B of human origin (N-20, Santa Cruz, 1:300); rabbit antibody raised against a synthetic peptide derived from residues 20–33 of actin with N-terminus added lysine (A5060, Sigma Aldrich, Co.). The results were normalized based on the expression of ACT and plotted (mean ± SEM) in relation to control, C (= 1). Western blot assays for total and phosphorylated ERK1/2 and AKT were performed as previously described (Lucas et al., 2008; Royer et al., 2012). Total cell lysates (40 μg of protein/lane) were resolved in 10% SDS/PAGE, transferred to PVDF membrane and probed with rabbit polyclonal antibody raised against a synthetic peptide derived from the sequence of rat MAPK3/1 (p44/p42 MAP kinase, ERK1/ERK2, #9102; Cell Signaling Technology, 1:1000) or anti-phospho-MAPK3/1 antibody (Thr202/Tyr204, #9101; Cell Signaling Technology, 1:2000); rabbit monoclonal antibody produced by immunizing animals with a synthetic peptide corresponding to residues in the C-terminal sequence of mouse AKT (AKT [pan], Cell Signaling Technology, 1:3000) or rabbit polyclonal antibody by immunizing animals with a synthetic phospho-peptide (KLH-coupled) corresponding to residues surrounding Ser473 of mouse AKT (p-AKT, Ser473, Cell Signaling Technology, 1:1000). The results were normalized based on the expression of total ERK1/2 or total AKT in each sample and plotted (mean ± SEM) in relation to control (C = 1).
2.3. Incorporation of [Methyl-3H] thymidine assays
Primary Sertoli cell cultures from 15- and 20-day-old rats were initially incubated with 2 μCi/ml [Methyl-3H] thymidine for 6 h at 35 °C. Afterward, the cells were incubated in the absence (vehicle; control, C) and presence of E2 (0.1 nM; Sigma Chemical Co.), PPT (10 nM; Tocris Bioscience), or DPN (10 nM; Tocris Bioscience) for 24 h at 35 °C. Incorporation of [Methyl-3H] thymidine into cellular DNA was estimated as previously described (Lucas et al. 2008, 2014b). Results (mean ± SEM) were expressed in relation to control (basal levels of [Methyl-3H] thymidine incorporation, C = 100%).
2.4. Immunofluorescence analysis for detection of non-phosphorylated CTNNB
Primary Sertoli cell cultures from 20-day-old rats were grown as described above on coverslips coated with matrigel (1:10) and placed into 6-well plates. Sertoli cells were incubated in the absence (vehicle; control, C) and presence of E2 (0.1 nM, Sigma Chemical Co.), PPT (10 nM; Tocris Bioscience), or DPN (10 nM; Tocris Bioscience) for 24 h at 35 °C. The cells were also untreated or pretreated with ESR2-selective antagonist PHTPP (4-(2-phenyl-5,7-bis(trifluoromethyl)pyrazolo(1,5-a)pyrimidin-3-yl)phenol; 10 nM; Tocris Bioscience), the selective inhibitor of the SRC family of protein tyrosine kinases PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d]pyrimidine; 5 nM; Calbiochem, Darmstadt, Germany), MEK1/2 inhibitor U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2- aminophenylthio] butadiene; 20 μM; Cell Signaling Technology), PI3K inhibitor Wortmannin (11-(acetyloxy)-1S,6bR,7,8,9aS,10,11R,11bR-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-3H-furo[4,3,2-de]indeno[4,5-h]-2- benzopyran-3,6,9-trione; 100 nM; Sigma Chemical Co.) or AKT inhibitor MK-2206 (8-(4-(1-aminocyclobutyl)phenyl)-9-phenyl-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one; 200 nM; Selleckchem, Houston, TX, USA) for 30 min. Afterward, the cells were incubated in the absence and presence of DPN (10 nM, 24 h). The agonists, antagonist and inhibitors are highly selective, at these concentrations, as previously reported (Stauffer et al., 2000; Meyers et al., 2001; Lucas et al., 2008; Royer et al., 2012; Pisolato et al., 2016; Lombardi et al., 2016). The medium was removed; the cells were washed with PBS (phosphate buffered saline, 137 mM NaCl, 2.68 mM KCl, 6.03 mM Na2HPO4 and 1,47 mM KH2PO4; pH 7.4; Sigma Chemical Co.), fixed in 2% paraformaldehyde for 20 min at room temperature and washed with PBS containing 0.1M glycine. The immunofluorescence assays were performed as previously described (Lombardi et al., 2016), using polyclonal antibody produced in rabbits by immunization with a synthetic peptide corresponding to the region around Ser37 (Ser33/37/Thr41) of human β-catenin (non-phosphorylated β-catenin) (#4270, Cell Signaling Technology; 1:200). Cells were also incubated with Alexa Fluor 488-labeled secondary antibody (Molecular Probe, Life Technologies; 1:300). Nuclear staining was performed with DAPI (4′,6-diamidino-2-phenylindole, Sigma Chemical Co.). Negative control was performed using normal rabbit serum at the same dilution of antibody. Immunostaining of non-phosphorylated CTNNB was visualized under a confocal microscope Leica Microsystems TCSSP8 (Leica Inc. Wetzlar, Germany), objective lenses of 63x. Images of the five random microscope fields were captured, in duplicate, in each assay (three independent experiments) and analyzed using the software LAS-AF.
2.5. Protein assays
Protein concentration was determined by the Bio-Rad protein assay, using bovine serum albumin as the standard (Bio Rad Laboratories Inc., Richmond, CA, USA).
2.6. Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was carried out by ANOVA (Prism software, version 5.00; GraphPad) followed by the Newman-Keuls test for multiple comparisons or by Student t-test to compare the differences between two data. P values <0.05 were accepted as significant.
3. Results
3.1. Activation of ESR1 upregulates the expression of CCND1 and proliferation of Sertoli cells from 5- and 15-day-old rats
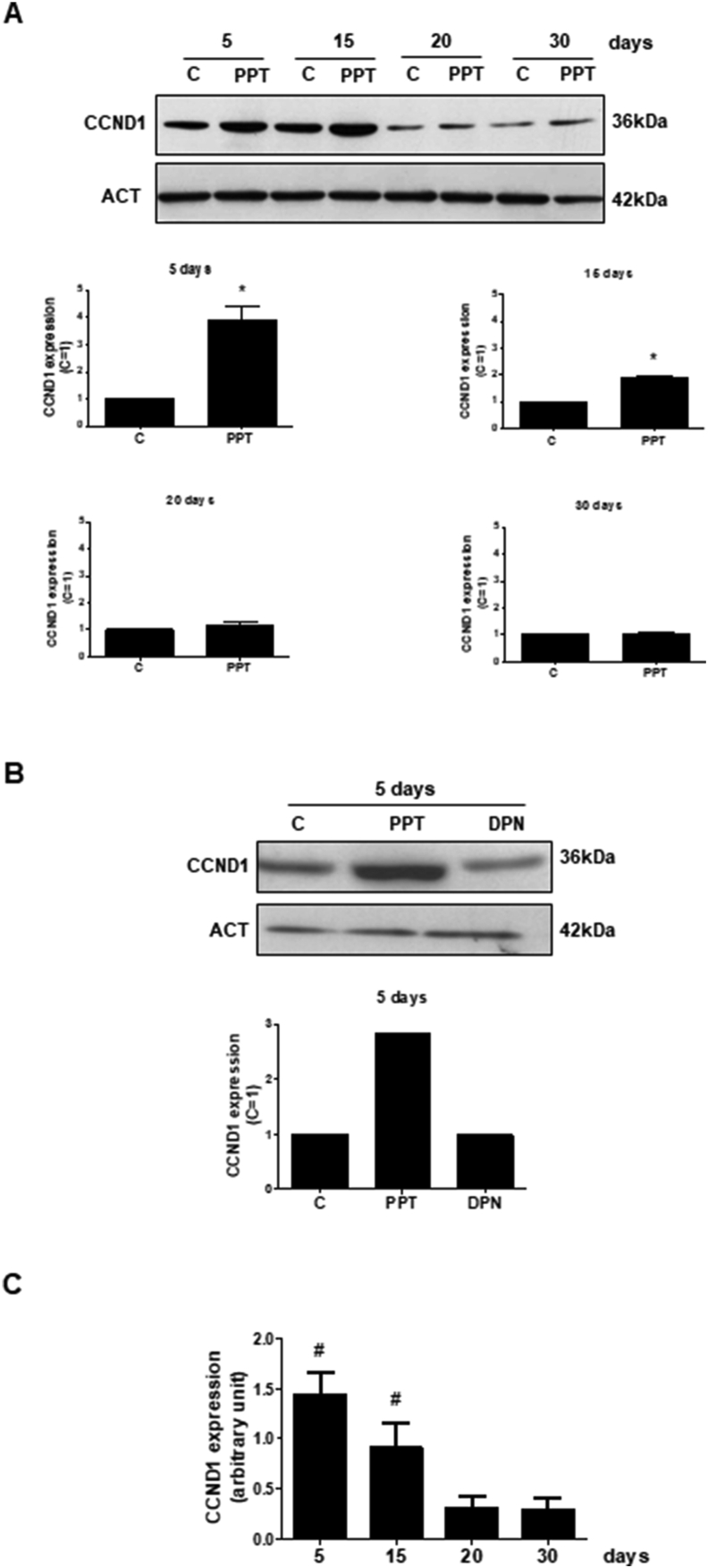
The expression of CCND1 was higher in Sertoli cells (control, C) obtained from 5- and 15-day-old rats than in 20- and 30-day old rats (Figure 1A, top panel, Figure 1C and Supplemental Figure S1A, top panel).
Figure 1.
Effects of ESR1-selective agonist PPT and ESR2-selective agonist DPN on the expression of CCND1 in the Sertoli cells from5-, 15-, 20- and 30-day-old rats. A. Sertoli cells from 5-, 15-, 20- and 30-day-old rats were incubated in the absence (C, control) and presence of PPT (10 nM) for 24 h. The relative positions of CCND1 (top panel) and ACT (bottom panel) proteins are shown at the right. The data shown are representative of four independent experiments (top and bottom panels). See, full image in Supplemental Fig S1. Bars represent the densitometric analysis of four independent experiments. Results were normalized to ACT expression in each sample and plotted (mean ± SEM) in relation to control, C (=1). ∗Significantly different from control (P < 0.05, Student t-test, n = 4). B. Sertoli cells from 5-day-old rats were incubated in the absence (C, control) and presence of PPT (10 nM) or DPN (10 nM) for 24 h. The data shown are representative of two independent experiments. See, full image in Supplemental Fig S1. C. Sertoli cells from 5-, 15-, 20- and 30-day-old rats were incubated in the absence of agonists (C, control, basal levels). Bars represent the densitometric analysis of four independent experiments. Results were normalized to ACT expression in each sample and plotted (mean ± SEM). # Significantly different from 20- or 30-day-od rats (P < 0.05, Newman-Keuls test).
The activation of ESR1 by PPT (10 nM, 24 h) increased about 4- and 2-fold, respectively, the expression of CCND1 in Sertoli cells from 5- and 15-day old rats. PPT did not have any effect on CCND1 expression in Sertoli cells from 20- and 30-day-old rats (Figure 1A and Supplemental Figure S1A, top panel).
The activation of ESR2 by DPN did not have any effect on CCND1 expression in Sertoli cells from 5-day-old rats (Figure 1B and Supplemental Figure S1B) and, also in Sertoli cells from 15-day old rats (Royer et al., 2012).
Taken together, these results indicate the involvement of ESR1 in the expression of CCND1 in Sertoli cells from 5- and 15-day-old rats.
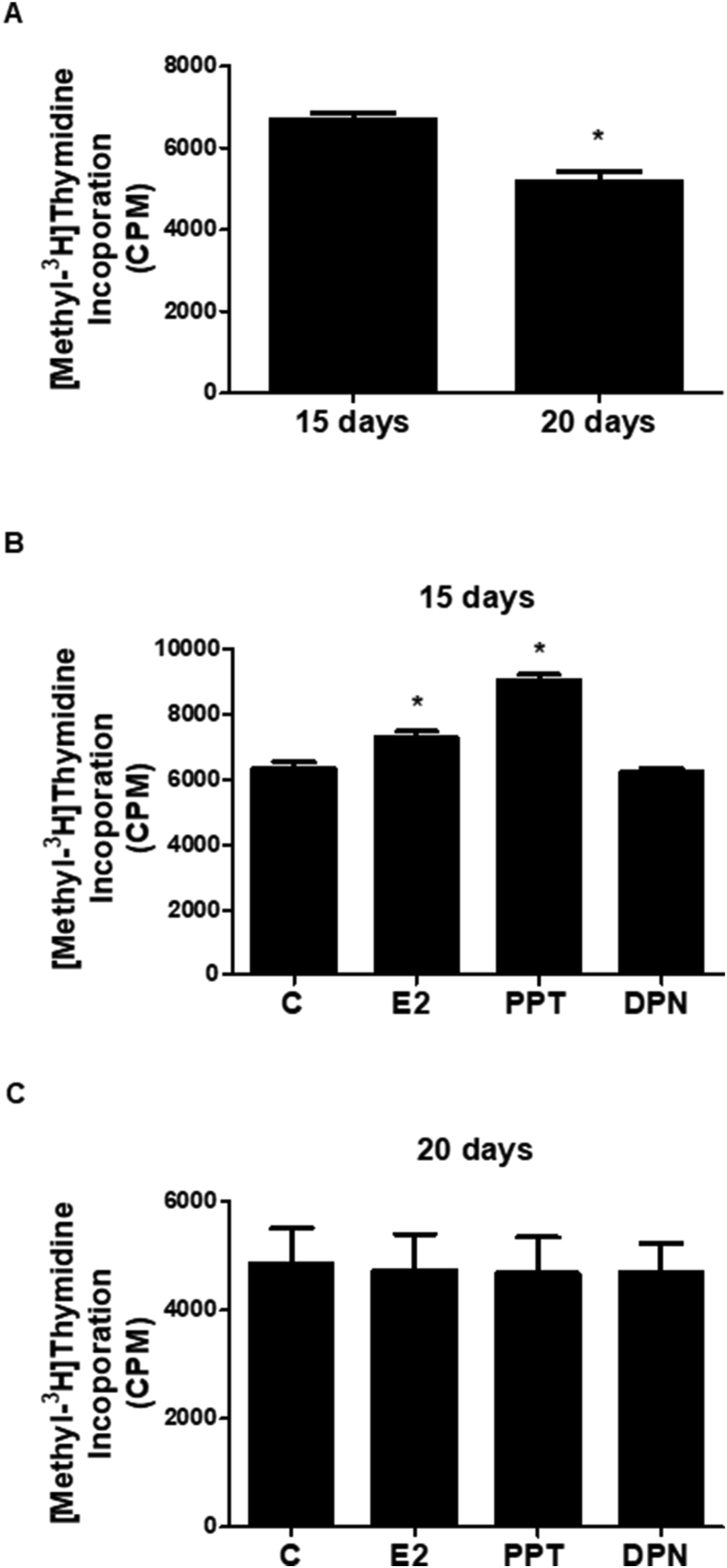
To confirm the involvement of ESR1 on the proliferation of Sertoli cells, [Methyl-3H] thymidine incorporation assays were performed. The basal incorporation of [methyl-3H] thymidine was higher in Sertoli cells obtained from 15-day-old rats than in 20-day old rats (Figure 2A). In Sertoli cells from 15-day-old rats, the treatment with E2 (0.1 nM, 24 h) and PPT (10 nM, 24 h) enhanced the [Methyl-3H] thymidine incorporation compared with control cells (C), whereas treatment with DPN (10 nM, 24 h) did not have any effect (Figure 2B), confirming the previous study from our laboratory (Lucas et al., 2014b). On the other hand, the treatment with E2, PPT or DPN did not have any effect on [Methyl-3H] thymidine incorporation in Sertoli cells from 20-day-old rats (Figure 2C). Taken together, these results confirm that the activation of ESR1 induces the proliferation of the Sertoli cell from 15-day-old rats. Cell cycle arrest was observed in Sertoli cells from 20-day-old rats (present study).
Figure 2.
Effects of 17β-estradiol (E2), ESR1-selective agonist PPT and ESR2-selective agonist DPN on the incorporation of [Methyl-3H thymidine in the Sertoli cells from 15- and 20-day-old rats. Sertoli cells from 15- and 20-day-old rats were initially incubated with 2 μCi/ml [Methyl-3H]thymidine for 6 h. After this incubation, A. cells were incubated in the absence of the agonists for 24 h (basal [Methyl-3H] thymidine incorporation). B and C. cells were incubated in the absence (C, control, basal [Methyl-3H] thymidine incorporation) and presence of E2 (0.1 nM), PPT (10 nM) or DPN (10 nM) for 24 h. Bound radioactivity was determined and the results plotted (mean ± SEM, n = 3 independent experiments). ∗Significantly different from control (P < 0.05, Student t-test or Newman-Keuls test).
3.2. Activation of ESR2 upregulates the expression of CDKN1B in Sertoli cells from 20- and 30-day-old rats
The basal levels of CDKN1B were higher in Sertoli cells from 20- and 30-day-old than from 5- and 15-day-old rats (Figure 3A and B, top panel, Figure 3C and Supplemental Figure S3A and B, top panel).
Figure 3.
Effects of ESR1-selective agonist PPT and ESR2-selective agonist DPN on the expression of CDKN1B in the Sertoli cells from 5-, 15-, 20- and 30-day-old rats. A. Sertoli cells from 30-, 20- and 15-day-old rats were incubated in the absence (C, control) and presence of DPN (10 nM) for 24 h. The relative positions of CDKN1B (top panel) and ACT (bottom panel) proteins are shown at the right. The data shown are representative of four independent experiments (top and bottom panels). See, full image in Supplemental Fig S3. Bars represent the densitometric analysis of four independent experiments. Results were normalized to ACT expression in each sample and plotted (mean ± SEM) in relation to control (C = 1). ∗Significantly different from control (P < 0.05, Student t-test, n = 4). B. Sertoli cells from 30- and 5-day-old rats were incubated in the absence (C, control) and presence of PPT (10 nM) or DPN (10 nM) for 24 h. The data shown are representative of two independent experiments. See, full image in Supplemental Fig S3. C. Sertoli cells from 5-, 15-, 20- and 30-day-old rats were incubated in the absence of agonists (C, control). Bars represent the densitometric analysis of four independent experiments. Results were normalized to ACT expression in each sample and plotted (mean ± SEM). # Significantly different from 20- or 30-day-od rats (P < 0.05, Newman-Keuls test).
The activation of ESR2 by DPN (10 nM, 24 h) increased about 2-, 3- and 1.5-fold, respectively, the expression of CDKN1B in Sertoli cells from 30-, 20- and 15-day old rats (Figure 3A and Supplemental Figure S3A, top panel). DPN did not have any effect on CDKN1B expression in Sertoli cells from 5-day-old rats (Figure 3B and Supplemental Figure S3B, top panel).
The activation of ESR1 by PPT (10 nM, 24 h) did not have any effect on the expression of CDKN1B in Sertoli cells from 30- and 5-day-old rats (Figure 3B and Supplemental Figure S3B) and also in Sertoli cells from 15-day old rats (Lucas et al., 2014b).
Taken together, these results indicate the involvement of ESR2 in the expression of CDKN1B in Sertoli cells from 15-, 20- and 30-day-old rats, suggesting the role of ESR2 at the end of the proliferation of Sertoli cells and the start of differentiation.
3.3. Activation of ESR2 upregulates the expression of non-phosphorylated CTNNB in Sertoli cells from 20-day-old rats
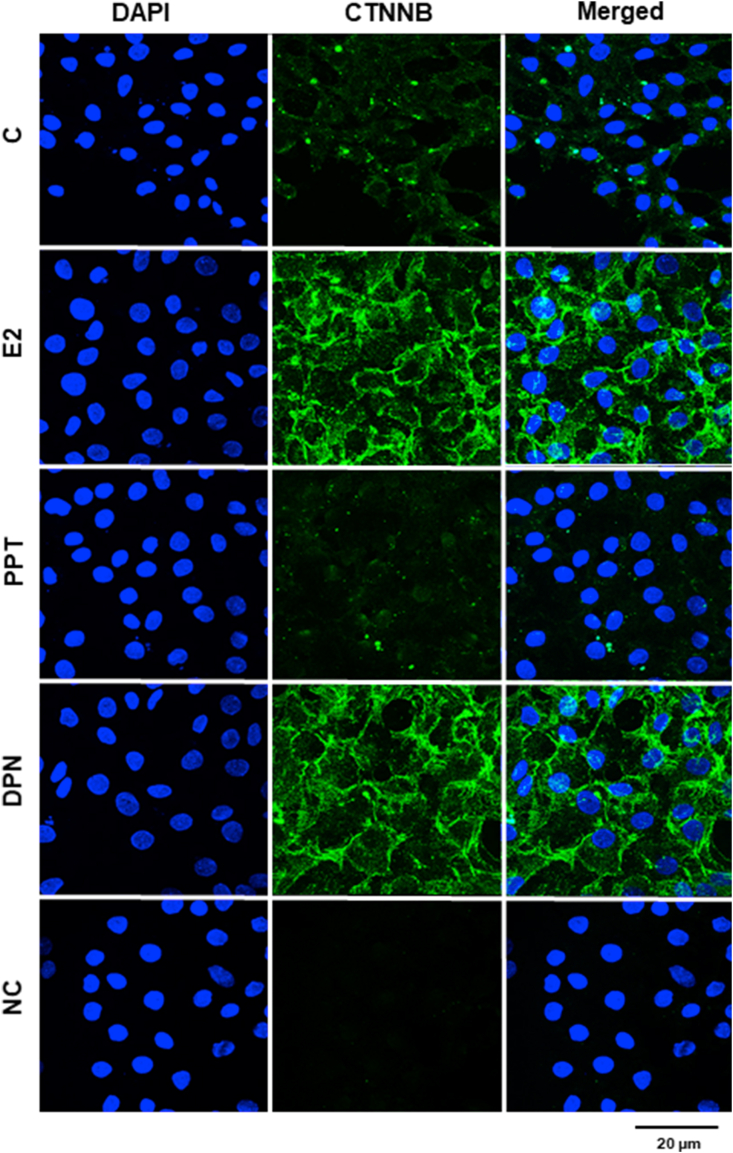
In the rat, the Sertoli cell barrier (blood-testis barrier, BTB) assembles on postnatal days 16–21 (Bergmann and Dierichs 1983), and it is associated with Sertoli cell differentiation. In this study, in nonstimulated Sertoli cells from 20-day-old rats (control, C), non-phosphorylated CTNNB was detected near the plasma membrane region and in the cytoplasm (Figure 4). The treatment with E2 (0.1 nM, 24 h) and DPN (10 nM, 24 h), but not PPT (10 nM, 24 h), induced an increase in the expression of non-phosphorylated CTNNB in these cells (Figure 4). Intense non-phosphorylated CTNNB immunostaining was found in the plasma membrane region and the cytoplasm of Sertoli cells from 20-day-old rats (Figure 4). No immunostaining was observed in the negative control (Figure 4).
Figure 4.
Effects of 17β-estradiol (E2), ESR1-selective agonist PPT and ESR2-selective agonist DPN on the expression of non-phosphorylated CTNNB in the Sertoli cells from 20-day-old rats. Sertoli cells from 20-day-old rats were incubated in the absence (C, control) and presence of E2 (0.1 nM), PPT (10 nM) or DPN (10 nM) for 24 h. Immunostaining for CTNNB (green) was detected using an antibody specific for non-phosphorylated CTNNB and Alexa Fluor 488-labeled secondary antibody. Nuclei were stained with DAPI (blue). Negative control (NC) was performed using normal rabbit serum at the same dilution of antibody. Scale bar as indicated. The data shown are representative of three independent experiments.
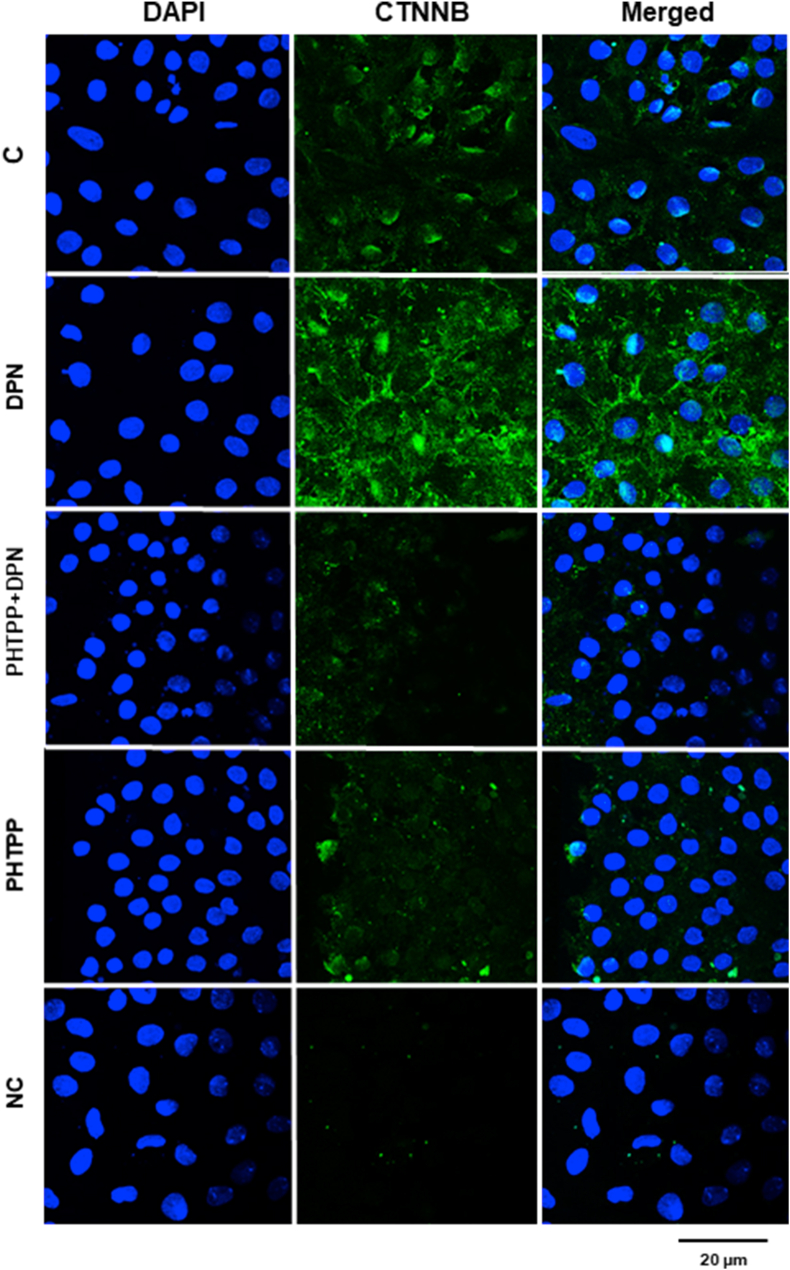
The upregulation of non-phosphorylated CTNNB induced by DPN (10 nM, 24h) was blocked by pretreatment with PHTPP, indicating that ESR2 is an upstream receptor involved in this process (Figure 5). PHTPP alone did not have any effect (Figure 5).
Figure 5.
Effects of ESR2-selective antagonist PHTPP on the expression of non-phosphorylated CTNNB induced by DPN in the Sertoli cells from 20-day-old rats. Sertoli cells from 20-day-old rats were incubated in the absence (C, control) and presence of DPN (10 nM) for 24 h. The cells were also untreated or pretreated with ESR2-selective antagonist PHTPP (10 nM) for 30 min. Afterward, the cells were stimulated with DPN (10 nM) 24 h. Immunostaining for CTNNB (green) was detected using an antibody specific for non-phosphorylated CTNNB and Alexa Fluor 488-labeled secondary antibody. Nuclei were stained with DAPI (blue). Negative control (NC) was performed using normal rabbit serum at the same dilution of antibody. Scale bar as indicated. The data shown are representative of three independent experiments.
The upregulation of non-phosphorylated CTNNB induced by DPN was blocked by PP2, an inhibitor of the SRC family of protein tyrosine kinases; U0126, an inhibitor of MEK1/2; Wortmannin, an inhibitor of PI3K and MK-2206, an inhibitor of AKT (Figure 6). PP2, U0126, Wortmannin or MK-2206 alone did not have any effect (data not shown). Taken together, our results indicate that in Sertoli cells from 20-day-old rats, E2- and DPN-ESR2, through activation of SRC/ERK1/2 and PIK3/AKT pathways, are involved in the upregulation of non-phosphorylated CTNNB expression.
Figure 6.
Effects of proteins kinases inhibitors on the expression of non-phosphorylated CTNNB induced by DPN in the Sertoli cells from 20-day-old rats. Sertoli cells from 20-day-old rats were incubated in the absence (C, control) and presence of DPN (10 nM) for 24 h. The cells were also untreated or pretreated with PP2, an inhibitor of the SRC family of protein tyrosine kinases; U0126, an inhibitor of MEK1/2; Wortmannin, an inhibitor of PI3K and MK-2206, an inhibitor of AKT for 30 min. Afterward, the cells were stimulated with DPN (10 nM) 24h. Immunostaining for CTNNB (green) was detected using an antibody specific for non-phosphorylated CTNNB and Alexa Fluor 488-labeled secondary antibody. Nuclei were stained with DAPI (blue). Negative control was performed using normal rabbit serum at the same dilution of antibody. Scale bar as indicated. The data shown are representative of three independent experiments. Note: Control, C, and DPN are the same results of the Figure 5. All treatments were performed on the same day (Figures 5 and 6).
It is important to mention that activation of estrogen receptors by E2 or DPN induced ERK and AKT phosphorylation in Sertoli cells from 20-day-old rats (Supplemental Figure S4 and S5).
4. Discussion
The regulation of the proliferation and differentiation of Sertoli cells in neonatal, peripubertal, and pubertal life remains one of the most critical questions in testicular biology and remain to be better explored (reviewed by Lucas et al., 2014a).
CCND1-Cyclin-dependent kinase 4/6 (CDK4/6) complex upon specific signals, among them the E2, promotes cell cycle progression and transformation in different cells (reviewed by Fang et al., 2018). The activity of the CCND1-CDK4/6 complex is inhibited by the CDK inhibitors p21Cip1/WAF1 (CDKN1A), p27Kip1 (CDKN1B) and p57Kip2 (CDKN1C) (reviewed by Murray 2004; Chu et al., 2008; Starostina and Kipreos 2012). In the present study, the expression of the CCND1 is higher in cultured Sertoli cells obtained from 5- and 15-day-old rats than in 20- and 30-day-old rats. On the other hand, the expression of the CDKN1B is higher in Sertoli cells from 20- and 30-day-old than in 5- and 15-day-old rats. Kang et al. (1997) also showed that the basal level of CCND1 is high in the testis from 7- and 14-day-old rats and decreases during testicular development. While the expression of CDKN1B is minimal in rapidly proliferating neonatal mice (3 and 4-day-old) and maximal in postmitotic adult Sertoli cells (Beumer et al., 1999). Taken together, these results suggest the regulated expression of CCND1 and CDKN1B in Sertoli cells during a specific phase of the rat and mouse development.
Several hormones, growth factors and their receptors, including E2-ERs, may be involved in the regulation of the expression of CCND1 and CDKN1B (reviewed by Lucas et al., 2014a; Meroni et al., 2019). In fact, an increase in the expression of CCND1 induced by PPT, but not DPN, was detected in the Sertoli cells from 5- and 15-day-old rats, indicating the involvement of ESR1 on the proliferation of these cells in rats with 5 and 15 days of age. Furthermore, the expression of the CDK inhibitor (CDKN1B) enhanced 2- and 3-fold by DPN, but not by PPT, in the Sertoli cells from 30- and 20-day-old rats, respectively. The activation of ERs by E2, PPT or DPN did not change the incorporation of [Methyl-3H] thymidine in Sertoli cells from 30-day-old rats. Taken together, these results indicate the role of ESR2 in the cell cycle arrest and/or Sertoli cell differentiation.
The differentiation of Sertoli cell is also associated with the establishment of the blood-testis barrier (BTB), and the capacity to sustain spermatogenesis (reviewed by Sharpe et al., 2003; Tarulli et al., 2012; Cheng and Mruk 2012; Lucas et al., 2014a). In the rat, the Sertoli cell barrier (BTB) assembles on postnatal days 16–21 (Bergmann and Dierichs 1983). In the present study, Sertoli cells were cultured in vitro with matrigel to allow the establishment of a tight junction (TJ) barrier that mimicked the BTB in vivo (Siu et al., 2005; Li et al., 2009; Gao et al., 2017). CTNNB is present in this barrier and plays a role in spermatogenesis. CTNNB is found in the ectoplasmic specialization (ES), a testis-specific adherens junction formed between Sertoli cells in the basal compartment (basal ES), site of the blood-testis barrier, as well as between Sertoli and germ cells in the adluminal compartment (apical ES) of the seminiferous epithelium (reviewed by Cheng and Mruk 2012). In addition to its role in cell–cell junctions, CTNNB is a key mediator in the canonical Wnt signaling pathway. The involvement of Wnt/CTNNB signaling in spermatogenesis has also been explored (Kerr et al., 2014).
The treatment with E2 and DPN, but not PPT, induced an increase in the expression of the non-phosphorylated CTNNB in Sertoli cells from 20-day-old rats. This upregulation of the non-phosphorylated CTNNB was blocked by pretreatment with ESR2-selective antagonist PHTPP, indicating that ESR2 is the upstream receptor involved in this process. The precise mechanism for Ctnnb gene regulation by ESR2 is not fully understood. No estrogen-responsive element (ERE)-related sequence has been identified in the proximal Ctnnb promoter. We identified consensus regions for specific protein transcription factor (Sp-1), c-Fos, c-Jun and zinc finger transcription factor (GATA-3) (analyzed by TRANFAC) (Lombardi et al., 2016), potential elements that may be involved in the transcriptional regulation of the Ctnnb gene by ERs.
The role of signaling pathways of the ER present in the membrane has been shown in many organs (reviewed by Levin, 2018) and also in Sertoli cells (reviewed by Lucas et al., 2014a; Meroni et al., 2019; Ni et al., 2020). The link between the activation of ER and protein kinases (ERK1/2 and AKT) has been shown in Sertoli cells from 15-day old rats (Lucas et al. 2008, 2014b; Royer et al., 2012) and from 20-day-old rats (present study). Furthermore, nonreceptor protein tyrosine kinase c-SRC (Lee and Cheng, 2005; Xiao et al., 2012), ERK1/2 (reviewed by Wong and Cheng 2005) and AKT1/2 (Gao et al., 2017) are present in the Sertoli cell barrier. Thus, these proteins may be involved in the regulation of the expression of non-phosphorylated CTNNB induced by the complex E2-ESR2-transcription factors. In fact, the upregulation of the non-phosphorylated CTNNB induced by DPN-ESR2 was blocked by PP2, an inhibitor of the SRC family of protein tyrosine kinases; U0126, an inhibitor of MEK1/2; Wortmannin, an inhibitor of PI3K and MK-2206, an inhibitor of AKT. Taken together, E2- or DPN-ESR2 complex, through activation of SRC/ERK1/2 and PIK3/AKT pathways, is involved in the upregulation of the expression of non-phosphorylated CTNNB.
Currently, studies rarely focus on the relationship between hormones and their signaling pathways in Sertoli cells, spermatogenesis, and clinical cases. However, the signaling pathways, especially the ER signaling pathways, promise a great future in the diagnosis and therapy of male diseases, as well as drug development.
In conclusion, the activation of estrogen receptors ESR1 and ESR2, respectively, play a role in the proliferation and differentiation of Sertoli cells in a critical period in testicular development (see Graphical abstract). The regulation of CTNNB by 17β-estradiol/ESR2, via c-SRC/ERK1/2 and PI3K/AKT, may play a role in the interaction between Sertoli cells and/or in cell-germ cell adhesion and/or in the stabilization and accumulation of CTNNB in the cytosol. CTNNB could be translocated to the nucleus and modulate the transcriptional activity of specific target genes. Our findings are important to clarify the role of estrogen in a critical period of testicular development.
Author contribution statement
C. Porto: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
C. Macheroni: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
T. Lucas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (grants numbers 2010/52306-8 and 2014/05292-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (grant number 302191/2017-8).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Gui Mi Ko, Priscila Veronica Sartorio, Elizabeth Kanashiro, Caroline Zito Romera and Carolina Melone Vicente for technical assistance; Financiadora de Estudos e Projetos (FINEP) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for facilities in the Instituto Nacional de Farmacologia e Biologia Molecular (Confocal microscope Leica Microsystems TCSSP8) and post-doctoral fellowship to Thais F. G. Lucas; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for master fellowship to Carla Macheroni.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Effects of ESR1-selective agonist PPT and ESR2-selective agonist DPN on the expression of CCND1 in the Sertoli cells from 5-, 15-, 20- and 30-day-old rats. A. Sertoli cells from 5-, 15-, 20- and 30-day-old rats were incubated in the absence (C, control) and presence of PPT (10 nM) for 24 h. The relative positions of CCND1 (top panel) and ACT (bottom panel) proteins are shown at the right. The data shown are representative of four independent experiments (top and bottom panels). B. Sertoli cells from 5-day-old rats were incubated in the absence (C, control) and presence of PPT (10 nM) or DPN (10 nM) for 24 h. The data shown are representative of two independent experiments.
Fig. S3.
Effects of ESR1-selective agonist PPT and ESR2-selective agonist DPN on the expression of CDKN1B in the Sertoli cells from 5-, 15-, 20- and 30-day-old rats. A. Sertoli cells from 30-, 20- and 15-day-old rats were incubated in the absence (C, control) and presence of DPN (10 nM) for 24 h. The relative positions of CDKN1B (top panel) and ACT (bottom panel) proteins are shown at the right. The data shown are representative of four independent experiments (top and bottom panels). B. Sertoli cells from 30- and 5-day-old rats were incubated in the absence (C, control) and presence of PPT (10 nM) or DPN (10 nM) for 24 h. The data shown are representative of two independent experiments.
Fig. S4.
Effects of 17β-estradiol (E2) and ESR2-selective agonist DPN on the phosphorylation of ERK1/2 in the Sertoli cells from 20-day-old rats. Cells were incubated in the absence (C, control) and presence of E2 (0.1 nM) (A) or DPN (10 nM) (B) for different times. The relative positions of phosphorylated ERK1/2 (top panel) and total ERK1/2 (bottom panel) proteins are shown at the right. The data shown are representative of four independent experiments. Bars represent the densitometric analysis of four independent experiments. Results were normalized to total ERK1/2 expression in each sample and plotted (mean ± SEM) in relation to control (C = 1). Open bars ERK1 and closed bars ERK2. ∗Significantly different from control (P < 0.05, Student t-test, n = 4).
Fig. S5.
Effects of 17β-estradiol (E2) and ESR2-selective agonist DPN on the phosphorylation of ERK1/2 in the Sertoli cells from 20-day-old rats. Cells were incubated in the absence (C, control) and presence of E2 (0.1 nM) (A) or DPN (10 nM) (B) for different times. The relative positions of phosphorylated ERK1/2 (top panel) and total ERK1/2 (bottom panel) proteins are shown at the right. The data shown are representative of four independent experiments. Bars represent the densitometric analysis of four independent experiments. Results were normalized to total AKT expression in each sample and plotted (mean ± SEM) in relation to control (C = 1). ∗Significantly different from control (P < 0.05, Student t-test, n = 4).
References
- Antal M.C., Krust A., Chambon P., Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ER-null mutant. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auharek S.A., Avelar G.F., Lara N.L., Sharpe R.M., França L.R. Sertoli cell numbers and spermatogenic efficiency are increased in inducible nitric oxide synthase mutant mice. Int. J. Androl. 2011;34:e621. doi: 10.1111/j.1365-2605.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Dierichs R. Postnatal formation of the blood-testis barrier in the rat with special reference to the initiation of meiosis. Anat. Embryol. 1983;168:269–275. doi: 10.1007/BF00315821. [DOI] [PubMed] [Google Scholar]
- Beumer T.L., Kiyokawa H., Roepers-Gajadien H.L., van den Bos L.A., Lock T.M., Gademan I.S., Rutgers D.H., Koff A., de Rooij D.G. Regulatory role of CDKN1B in the mouse and human testis. Endocrinology. 1999;140:1834–1840. doi: 10.1210/endo.140.4.6638. [DOI] [PubMed] [Google Scholar]
- Buzzard J.J., Wreford N.G., Morrison J.R. Thyroid hormone, retinoic acid, and testosterone suppress proliferation and induce markers of differentiation in cultured rat Sertoli cells. Endocrinology. 2003;144:3722–3731. doi: 10.1210/en.2003-0379. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y., Mruk D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu I.M., Hengst L., Slingerland J.M. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Canc. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Couse J.F., Curtis Hewitt S., Korach K.S. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J. Steroid Biochem. Mol. Biol. 2000;74:287–296. doi: 10.1016/s0960-0760(00)00105-9. [DOI] [PubMed] [Google Scholar]
- Crépieux P., Marion S., Martinat N., Fafeur V., Vern Y.L., Kerboeuf D., Guillou F., Reiter E. The ERK-dependent signaling is stage-specifically modulated by FSH during primary Sertoli cell maturation. Oncogene. 2001;20:4696–4709. doi: 10.1038/sj.onc.1204632. [DOI] [PubMed] [Google Scholar]
- Fang H., Huang D., Yang F., Guan X. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Canc. Res. Treat. 2018;168:287–297. doi: 10.1007/s10549-017-4612-y. [DOI] [PubMed] [Google Scholar]
- Gao Y., Chen H., Lui W.Y., Lee W.M., Cheng C.Y. Basement membrane laminin α2 regulation of BTB dynamics via its effects on F-actin and microtubule cytoskeletons is mediated through mTORC1 signaling. Endocrinology. 2017;158:963–978. doi: 10.1210/en.2016-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima J., Zhu L., Cheng C.Y. Testin is tightly associated with testicular cell membrane upon its secretion by sertoli cells whose steady-state mRNA level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. J. Biol. Chem. 1997;272:6499–6509. doi: 10.1074/jbc.272.10.6499. [DOI] [PubMed] [Google Scholar]
- Griswold M., Mably E., Fritz I.B. Stimulation by follicle stimulating hormone and dibutyryl cyclic AMP of incorporation of 3H-thymidine into nuclear DNA of cultured Sertoli cell-enriched preparations from immature rats. Curr. Top. Mol. Endocrinol. 1975;2:413–420. doi: 10.1007/978-1-4613-4440-7_29. [DOI] [PubMed] [Google Scholar]
- Griswold M.D. 50 years of spermatogenesis: sertoli cells and their interactions with germ cells. Biol. Reprod. 2018;99:87–100. doi: 10.1093/biolre/ioy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold M.D., Solari A., Tung P.S., Fritz I.B. Stimulation by follicle-stimulating hormone of DNA synthesis and of mitosis in cultured Sertoli cells prepared from testes of immature rats. Mol. Cell. Endocrinol. 1977;7:151–165. doi: 10.1016/0303-7207(77)90064-8. [DOI] [PubMed] [Google Scholar]
- Hazra R., Corcoran L., Robson M., McTavish K.J., Upton D., Handelsman D.J., Allan C.M. Temporal role of Sertoli cell androgen receptor expression in spermatogenic development. Mol. Endocrinol. 2013;27:12–24. doi: 10.1210/me.2012-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R.A., Cooke Paul S. Estrogen in the male: a historical perspective. Biol. Reprod. 2018;99:27–44. doi: 10.1093/biolre/ioy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.J., Kim M.K., Terhune A., Park J.K., Kim Y.H., Koh G.Y. Cytoplasmic localization of cyclin D3 in seminiferous tubules during testicular development. Exp. Cell Res. 1997;234:27–36. doi: 10.1006/excr.1997.3590. [DOI] [PubMed] [Google Scholar]
- Kerr G.E., Young J.C., Horvay K., Abud H.E., Loveland K.L. Regulated Wnt/beta-catenin signaling sustains adult spermatogenesis in mice. Biol. Reprod. 2014;90:3. doi: 10.1095/biolreprod.112.105809. [DOI] [PubMed] [Google Scholar]
- Krege J.H., Hodgin J.B., Couse J.F., Enmark E., Warner M., Mahler J.F., Sar M., Korach K.S., Gustafsson J.A., Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.R. Membrane estrogen receptors signal to determine transcription factor function. Steroids. 2018;132:1–4. doi: 10.1016/j.steroids.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Lee N.P., Cheng C.Y. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J. Cell. Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- Li M.W., Mruk D.D., Lee W.M., Cheng C.Y. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi A.P., Pisolato R., Vicente C.M., Lazari M.F., Lucas T.F., Porto C.S. Estrogen receptor beta (ERβ) mediates expression of β-catenin and proliferation in prostate cancer cell line PC-3. Mol. Cell. Endocrinol. 2016;430:12–24. doi: 10.1016/j.mce.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Lucas T.F., Nascimento A.R., Pisolato R., Pimenta M.T., Lazari M.F., Porto C.S. Receptors and signaling pathways involved in proliferation and differentiation of Sertoli cells. Spermatogenesis. 2014;4 doi: 10.4161/spmg.28138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T.F., Royer C., Siu E.R., Lazari M.F., Porto C.S. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biol. Reprod. 2010;83:307–317. doi: 10.1095/biolreprod.110.084160. [DOI] [PubMed] [Google Scholar]
- Lucas T.F., Siu E.R., Esteves C.A., Monteiro H.P., Oliveira C.A., Porto C.S., Lazari M.F. 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol. Reprod. 2008;78:101–114. doi: 10.1095/biolreprod.107.063909. [DOI] [PubMed] [Google Scholar]
- Lucas T.F.G., Lazari M.F.M., Porto C.S. Differential role of the estrogen receptors ESR1 and ESR2 on the regulation of proteins involved with proliferation and differentiation of Sertoli cells from 15-day-old rats. Mol. Cell. Endocrinol. 2014;382:84–96. doi: 10.1016/j.mce.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Matzuk M.M., Kumar T.R., Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- Meachem S.J., McLachlan R.I., de Kretser D.M., Robertson D.M., Wreford N.G. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol. Reprod. 1996;54:36–44. doi: 10.1095/biolreprod54.1.36. [DOI] [PubMed] [Google Scholar]
- Meroni S.B., Galardo M.N., Rindone G., Gorga A., Riera M.F., Cigorraga S.B. Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Front. Endocrinol. 2019;10:224. doi: 10.3389/fendo.2019.00224. 1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M.J., Sun J., Carlson K.E., Marriner G.A., Katzenellenbogen B.S., Katzenellenbogen J.Á. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Murray A.W. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- Musnier A., Heitzler D., Boulo T., Tesseraud S., Durand G., Lécureuil C., Guillou H., Poupon A., Reiter E., Crépieux P. Developmental regulation of p70 S6 kinase by a G protein-coupled receptor dynamically modelized in primary cells. Cell. Mol. Life Sci. 2009;66:3487–3503. doi: 10.1007/s00018-009-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni F.D., Hao S.L., Yang W.X. Molecular insights into hormone regulation via signaling pathways in Sertoli cells: with discussion on infertility and testicular tumor. Gene. 2020;753:144812. doi: 10.1016/j.gene.2020.144812. [DOI] [PubMed] [Google Scholar]
- Nicholls P.K., Harrison C.A., Rainczuk K.E., Wayne Vogl A., Stanton P.G. Retinoic acid promotes Sertoli cell differentiation and antagonizes activin-induced proliferation. Mol. Cell. Endocrinol. 2013;377:33–43. doi: 10.1016/j.mce.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Orth J.M., Gunsalus G.L., Lamperti A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- Pisolato R., Lombardi A.P., Vicente C.M., Lucas T.F., Lazari M.F., Porto C.S. Expression and regulation of the estrogen receptors in PC-3 human prostate cancer cells. Steroids. 2016;107:74–86. doi: 10.1016/j.steroids.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Rebourcet D., Darbey A., Monteiro A., Soffientini U., Tsai Y.T., Handel I., Pitetti J.L., Nef S., Smith L.B., O'Shaughnessy P.J. Sertoli cell number defines and predicts germ and Leydig cell population sizes in the adult mouse testis. Endocrinology. 2017;158:2955–2969. doi: 10.1210/en.2017-00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera M.F., Regueira M., Galardo M.N., Pellizzari E.H., Meroni S.B., Cigorraga S.B. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am. J. Physiol. Endocrinol. Metab. 2012;302:E914–E923. doi: 10.1152/ajpendo.00477.2011. [DOI] [PubMed] [Google Scholar]
- Royer C., Lucas T.F., Lazari M.F., Porto C.S. 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol. Reprod. 2012;86:108. doi: 10.1095/biolreprod.111.096891. [DOI] [PubMed] [Google Scholar]
- Rumi M.A., Dhakal P., Kubota K., Chakraborty D., Lei T., Larson M.A., Wolfe M.W., Roby K.F., Vivian J.L., Soares M.J. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155:1991–1999. doi: 10.1210/en.2013-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi M.A.K., Singh P., Roby K.F., Zhao X., Iqbal K., Ratri A., Lei T., Cui W., Borosha S., Dhakal P., Kubota K., Chakraborty D., Vivian J.L., Wolfe M.W., Soares M.J. Defining the role of estrogen receptor beta in the regulation of female fertility. Endocrinology. 2017;158 doi: 10.1210/en.2016-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R.M., McKinnell C., Kivlin C., Fisher J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Simorangkir D.R., de Kretser D.M., Wreford N.G. Increased numbers of Sertoli and germ cells in adult rat testes induced by synergistic action of transient neonatal hypothyroidism and neonatal hemicastration. J. Reprod. Fertil. 1995;104:207–213. doi: 10.1530/jrf.0.1040207. [DOI] [PubMed] [Google Scholar]
- Siu M.K., Wong C.H., Lee W.M., Cheng C.Y. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Skinner M.K., Fritz I.B. Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell functions. Proc. Natl. Acad. Sci. U.S.A. 1985;82:114–118. doi: 10.1073/pnas.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostina N.G., Kipreos E.T. Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol. 2012;22:33–41. doi: 10.1016/j.tcb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer S.R., Coletta C.J., Tedesco R., Nishiguchi G., Carlson K., Sun J., Katzenellenbogen B.S., Katzenellenbogen J.A. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J. Med. Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Tarulli G.A., Stanton P.G., Meachem S.J. Is the adult Sertoli cell terminally differentiated? Biol. Reprod. 2012;87(13):1–11. doi: 10.1095/biolreprod.111.095091. [DOI] [PubMed] [Google Scholar]
- Villalpando I., Lira E., Medina G., Garcia-Garcia E., Echeverria O. Insulin like growth factor 1 is expressed in mouse developing testis and regulates somatic cell proliferation. Exp. Biol. Med. 2008;233:419–426. doi: 10.3181/0708-RM-212. [DOI] [PubMed] [Google Scholar]
- Wong C.H., Cheng C.Y. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: a review of recent data. Dev. Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Xiao X., Mruk D.D., Cheng F.L., Cheng C.Y. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv. Exp. Med. Biol. 2012;763:295–317. doi: 10.1007/978-1-4614-4711-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]