Abstract
Background
Hyperbilirubinemia occurs in over 80% of newborns, and severe bilirubin toxicity can lead to neurological dysfunction and death. Unbound bilirubin (Bf) levels predict the risk of neurodevelopmental handicap, although total serum bilirubin (TSB) is used to manage care.
Objective
To measure Bf levels in healthy infants, its relationship to TSB, and its response to phototherapy. We hypothesize unexpectedly high Bf levels, poor correlation with TSB and unpredictable response to phototherapy.
Design/Methods
Healthy infants were studied with simultaneous TSB and Bf measurements. The clinical data recorded included ethnicity, gender, birth weight, gestational age, and mode of delivery, Apgar scores, breast/formula feeds, and phototherapy.
Results
One hundred thirty-two infants (3248.9±509.2g, GA 38.7±1.4 weeks), at mean age of the initial sample of 28.5±15.6 hours, had a TSB of 7.9±2.7 mg/dl, and a Bf of 5.2±3.2 nM. The correlation between Bf and TSB was significant but not between Bf and TSB for TSB >12 mg/dl. Bf >11nm were in 22.7% and >17 nM in 3.8% of infants. Post-phototherapy TSB and Bf levels were similar to those before treatment.
Conclusions
The relationship between TSB and Bf in healthy infants is complex, with the inability of one to predict the other’s level in infants with elevated TSB. The mechanism of bilirubin-related neurotoxicity suggests that the management of jaundice in healthy infants requires Bf measurements. Management of jaundice with TSB may result in more infants exposed to phototherapy. However, unexpected elevations of Bf occur in an apparently healthy population.
Keywords: Unbound bilirubin, phototherapy, infant
Introduction
Neonatal jaundice caused by hyperbilirubinemia occurs in more than 80% of newborn infants. (1) Although mild hyperbilirubinemia is physiologic and possibly even neuroprotective (2, 3), severe hyperbilirubinemia can lead to neurologic dysfunction and death. Total serum bilirubin (TSB) is used currently in the clinical management and to quantify the risk of adverse neurodevelopmental outcomes, despite their low sensitivity and specificity for bilirubin-mediated neonatal encephalopathy. (4)
The treatment of neonatal jaundice was altered over 60 years ago by the discovery of the effect of phototherapy on TSB levels by Cremer (5), saving a countless number of infants from the more invasive treatment of hyperbilirubinemia, exchange transfusion. However, important questions remain regarding the efficacy and safety of this intervention. (6) TSB-directed treatment with phototherapy leads to the exposure of over 1000 infants to light treatment to avoid a single exchange transfusion,(7) even though most infants reaching exchange levels do not manifest bilirubin neurotoxicity. (8, 9) In addition, phototherapy is often overused—initiated at lower levels than those recommended by the American Academy of Pediatrics (10) presumably because of concern by clinicians that neurotoxicity can occur despite borderline TSB concentrations. Finally, the possibility that phototherapy-induced bilirubin breakdown products, such as lumirubin, possess neuro-inflammatory effects has recently been raised, albeit more so in preterm rather than term infants. (11) Given these concerns, reducing exposure to phototherapy is an essential clinical objective.
Most bilirubin in the serum compartment is bound to albumin, but a minute fraction of serum unbound bilirubin (Bf) exists, which can cross the blood-brain barrier and expose the brain to higher bilirubin levels and potential neurotoxicity (12–14). Previously, we used a Bf specific probe composed of a mutant fatty acid-binding protein labeled with acrylodan to monitor Bf levels. (15) In this study, we use a new version of this method in which Bf measured with near-infrared fluorescence using 5 μl of undiluted plasma. The goal of this investigation was to examine Bf levels in healthy term and late preterm newborn infants, to correlate it with TSB levels, examine the frequency of unexpected Bf elevations, (14, 16) and investigate the Bf response to phototherapy.
Methods
Patients
Term and late preterm infants admitted into the regular care nursery of RWJMS were eligible for enrollment. Infants with chromosomal or genetic abnormalities were excluded from this observational study. Enrollment was carried out after parental informed consent was obtained. The study was approved by the IRBs of Rutgers and Chesapeake IRB for Fluoresprobe Sciences.
Study Design
Plasma Bf and TSB levels were measured in all enrolled infants after clinicians decided that obtaining a TSB level test was necessary. The number and timing samples in an individual infant were determined by the clinical staff, with the postnatal age at sample measurement indicated below (Table 2). Phototherapy was instituted following the guidelines of the American Academy of Pediatrics. (17)
Table 2.
Total and Unbound Bilirubin Levels in Infants Treated and Untreated with Phototherapy
| Sample-defined Period | n | Age (hours) | TSB (mg/dL) | Bf (nM) | ||||
|---|---|---|---|---|---|---|---|---|
| No Photo | Photo | No Photo | Photo | No Photo | Photo | No Photo | Photo | |
| 1 | 102 | 31 | 29.9±15.1 | 23.5±16.7@ | 7.5±2.5 | 9.1±3.1@** | 4.8±2.7 | 6.7±4.1@** |
| 2 | 62 | 31 | 42.4±16.5 | 35.2±16.7 | 9.1±2.5 | 11.0±2.3** | 5.9±3.1 | 8.2±7.2* |
| 3 | 26 | 29 | 48.1±14.9 | 44.2±17.6 | 10.4±2.6 | 11.2±2.1 | 6.5±6.5 | 8.7±5.1 |
| 4 | 16 | 29 | 56.5±12.8 | 55.3±17.5 | 11.7±2.7 | 11.4±2.3 | 7.4±4.2 | 8.3±3.6 |
| Post-phototherapy (all treated infants) | 10.9±1.9 | 8.3±4.6 | ||||||
Before phototherapy
p<0.05
p<0.01
Blood Sampling
During the first week of life, research plasma samples were collected from residual blood drawn for clinical indications, processed, and frozen. De-identified samples were stored at −70°C and then shipped to Fluoresprobe Sciences for the determination of Bf. A preliminary investigation determined that freezing at this temperature does not alter the Bf analysis.
Bf and TSB measurements
The UBCheck Bf assay is an updated version of our earlier method for measuring Bf. (15) This new version measures equilibrium Bf directly in 5μL of undiluted plasma samples in a disposable cartridge, which is scanned in a reader and yields the Bf concentration in 90 seconds. The sample cartridge contains the Bf sensor, which is a fatty acid-binding protein, labeled with a near Infrared fluorophore that emits at 700 nm plus a second protein labeled with a fluorophore that emits at 820 nm. Upon binding Bf, the fluorescence at 700 nm is quenched, but the one at 820 nm is not changed, and therefore, the degree of quenching of the 700 nm/820 nm ratios is used to calculate the Bf concentration. (18)
The accuracy of the Bf probe measurements was confirmed by comparison with the Arrows UB2 Analyzer, which uses the peroxidase method. (15) In a preliminary investigation of term and near-term infants, UBCheck Bf levels ranged from 2.6 to 11.8 nM while the Arrows Bf levels from 0.9 to 11.9 nM in the same sample. Both methods were well correlated by Deming regression with a correlation coefficient of 0.90 (95%CI: 0.72–1.06). (19) The UBCheck had an average CV of 7% for repeated measurements. The device determines the equilibrium Bf concentration directly in a single analysis and is insensitive to substances that can interfere with the peroxidase measurement. TSB was measured using the diazo method at the RWJMS clinical laboratory. While there are questions that with the Arrows’ peroxidase method Bf in undiluted plasma may be significantly higher than in a diluted sample (20), this usually pertains to levels over 30 nm, and agreement is generally good for lower Bf levels. (19)
Outcome data
Recorded information included postnatal age (in hours) of each sample, age at initiation and discontinuation of phototherapy, TSB, and Bf concentrations, hearing screening results, and age at discharge. The age at which a sample was obtained was used to create timed categories, such that all initial samples constituted the first category, second samples formed the second, etc. The following clinical data were collected including illumination intensity, gender, birth weight, gestational age, blood group incompatibility, hemolysis, postnatal age at the start of phototherapy, TSB and Bf at beginning and end of phototherapy, change in TSB and Bf over time, calculated as the difference in first and last value divided by the intervening hours.
Statistical analysis
Changes in TSB and Bf as a function of demographic and clinical variables were analyzed by repeated-measures analysis and verified by the generalized estimating equation semi-parametric regression method. Multiple regression analysis was performed to evaluate the correlation between TSB and Bf, taking into account confounding variables including birth weight, gestational age, gender, race, treatment interventions, and 5 minute Apgar and the analysis was verified by non-parametric Spearman correlation. The analyses were performed with Statistica (StatSoft); p<0.05 was considered significant. Although controversial, elevated Bf thresholds were designated at levels of 11, 17, and 22 nM, based on prior reports. (13, 14, 16)
Results
The study population consisted of 132 term and late-preterm infants who had a mean birth weight of 3248.9±509.2 g and gestational age of 38.7±1.4 weeks (Table 1). Three hundred thirty-three Bf samples were obtained in this population during the first week of life, with initial sampling at a mean age of 28.5±15.6 hours that yielded an initial TSB of 7.9±2.7 mg/dl and Bf of 5.2±3.2 nM. There were no sex or race differences in the initial and subsequent Bf levels in the population. However, 30 infants (22.7%) demonstrated Bf levels >11 nM, 5 (3.8%) had Bf levels > 17 nM, and 4 (3.0%) had Bf levels > 22 nM. (21) Of the total number of samples, 48 (14.4%), 14 (4.2%), and 4 (1.2%) exceeded these thresholds, respectively.
Table 1.
Demographic Characteristics of Study Infants
| Number of infants enrolled | 132 |
|
| |
| Gestational Age (weeks) | 38.7±1.4 |
|
| |
| Birthweight (g) | 3248.9±509.2 |
|
| |
| Male, n (%) | 72 (54.5) |
|
| |
| Race/Ethnicity, n (%) | |
| Asian | 42 (31.8) |
| Black | 10 (7.6) |
| White | 76 (57.6) |
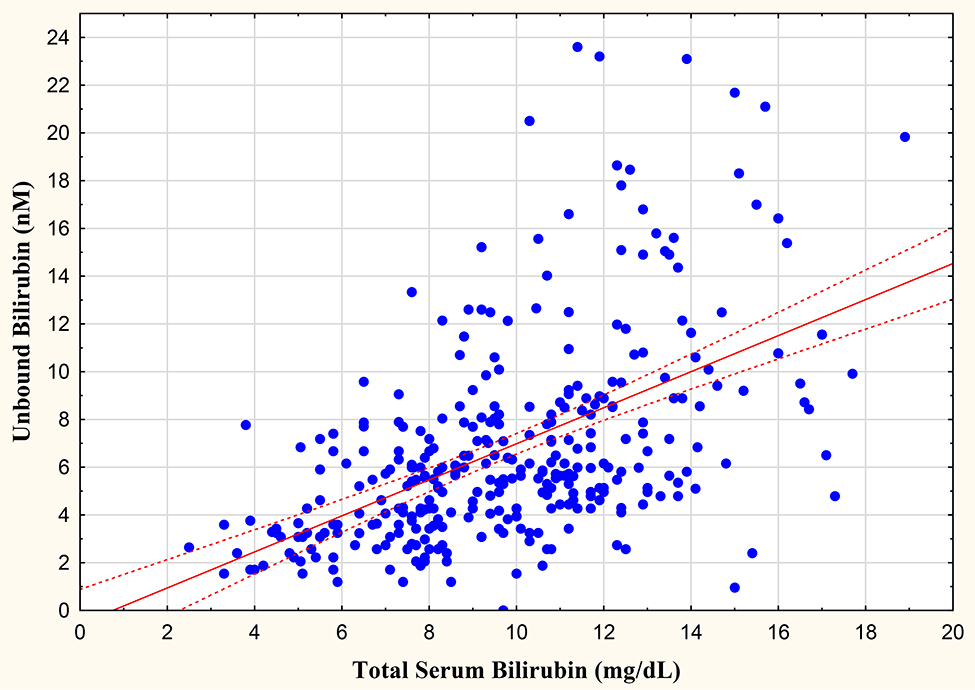
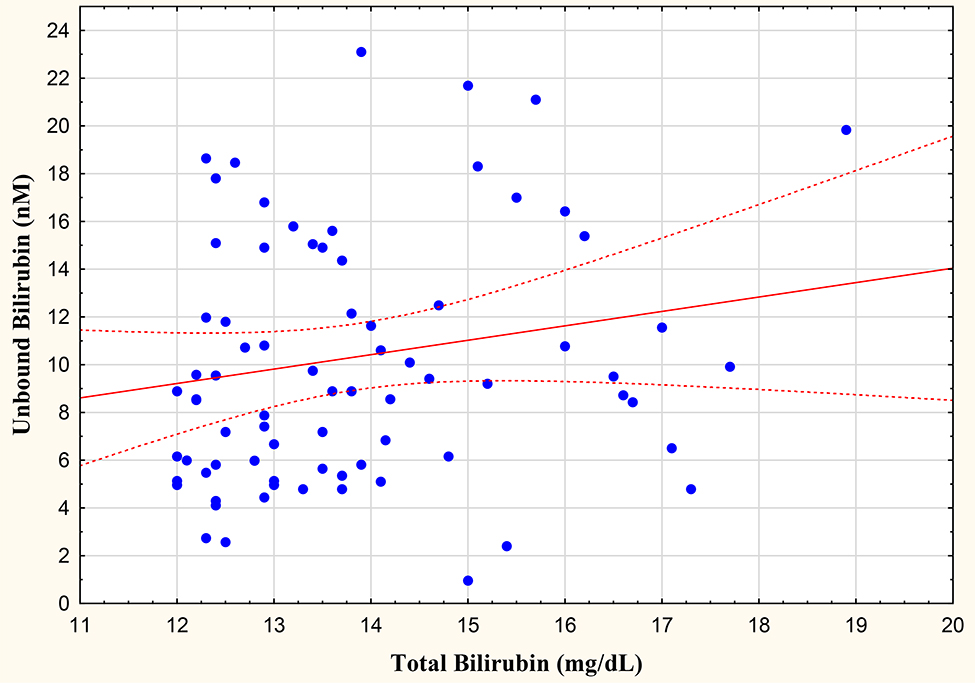
In the total population, the examination of all samples yielded significant correlations between TSB and Bf at r=0.499 (Figure 1). The relationship between TSB and Bf was unaffected by phototherapy, with significant correlations in treated (r=0.426) and untreated (r=0.480) infants. Of importance, assessment of correlations for TSB samples > 12 mg/dl were not significant, with TSB and Bf yielding an r=0.156. Multiple linear regression was performed at each Bf sample with the following selected variables: weight at sample, gestational age, gender, race, and 5 minute Apgar score. As expected, none of the population characteristics significantly impact Bf levels.
Figure 1.
Relationship between total and unbound bilirubin levels.
Thirty-one phototherapy-treated and 102 untreated infants were similar in birth weight (3228.4±523.5 vs. 3330.5±455.2 g), gestational age (38.7±1.4 vs. 38.9±1.4 weeks), sex and race distribution, but the phototherapy group contained five infants suffering from Coomb’s positive ABO incompatibility. In the treated group, phototherapy was initiated at a mean age of 38.9±19.7 hours at a TSB of 11.0+/−2.3 mg/dl and a Bf of 8.2+/−7.2 nM, terminated at 60.5±19.6 hours at a TSB of 11.4+/−2.3 mg/dl and Bf of 8.3+/−3.6 nM of age, lasting for 21.9±13.2 hours. The TSB and Bf levels in the serial samples, listed under sample-defined periods in treated (photo group) and untreated (no photo group) infants are shown in Table 2. Obtained at similar postnatal ages, TSB and Bf levels in infants treated with phototherapy were higher than in the untreated group. In the treated group, post-phototherapy TSB and Bf levels were similar to the results prior to light termination. The percent reduction from maximum levels during phototherapy to the first post-phototherapy value was 4.4% for TSB and 4.6% for Bf. The rates of TSB and Bf increases before treatment were 0.46±0.22 mg/dl/hour and 0.26±0.23 nM/hour, respectively, and correlated significantly at r=0.639. Bf rate of rise correlated with the duration of phototherapy (r=0.564), but the TSB rate of increase did not.
Hearing screening data were available in all infants, with four initial failures and one failure when the examination was repeated. Maximum TSB and Bf among these infants were 11.8 mg/dl and 9.2 nM, respectively. Length of stay analysis was limited to the 83 infants born via the vaginal route, 57 of whom were discharged at ≤ 48 hours of age compared to 26 released after that time. There were no significant differences between the two discharge groups with respect to birth weight, gestational age, TSB maximum (18.9 vs. 18.1 mg/dl), number of infants with TSB ≥ 15 mg/dl (two in each group), Bf maximum (23.6 and 18.6 nM), and number of infants with Bf ≥ 11 nM (two and three). However, 15.8% of earlier discharged infants were treated with phototherapy compared to 53.8% in the latter group.
Discussion
While the neuropathogenesis of bilirubin toxicity in newborn infants is unclear, previous research has identified that bilirubin enters the brain only in the unbound form (Bf) after dissociation from the albumin-bilirubin complex (22, 23) Therefore, bilirubin toxicity depends on Bf levels and not TSB concentrations. (24–26)
In July 2004, the American Academy of Pediatrics published guidelines for the management of neonatal jaundice in term and late-preterm infants calling for the measurement of TSB in all newborns that are jaundiced within 24 hours of birth or appear excessively jaundiced for their age (27). A study of more than 50,000 infants born in 11 Northern California hospitals between 1995 and 1996 indicated that approximately 27% of those newborns received at least one TSB test (28). Assuming the California study is representative of the national average, at least 1 million bilirubin tests are performed on the 4 million infants born in the United States annually (29). This figure is probably a low estimate because it does not consider additional tests for those newborns that require therapeutic intervention or follow-up. Because of the weak correlation between TSB and bilirubin encephalopathy; however, the significance of such measurements for determining the necessity of therapeutic intervention is widely debated (30–32). We also found unnecessary phototherapy exposure and a longer hospital stay in infants managed with TSB, which could potentially increase the risk of the low-frequency complications of this treatment. (7, 33, 34) We recognize that the study was not designed to use Bf as a guide to treatment, thus we could not demonstrate that its use would reduce light exposure and hospital stay.
However, we did demonstrate that Bf levels could not be ascertained from TSB levels; especially in infants, whose TSB exceeded 12 mg/dl. In a prior investigation, we noted similar results in preterm infants, due to the effects of Intralipid infusion on bilirubin binding by albumin in that population. (35) We have no such explanation in the term population, but this earlier observation led to examining and later discarding the role of feeding on this relationship. The rate Bf increase is a function of TSB, controlled by the concentration of albumin and how tightly the albumin binds bilirubin as measured by the association binding constants. (36) The variation in Bf as a function of TSB may have been due to different albumin concentrations and variable bilirubin binding constants. (39, 41), which, unfortunately, were not measured. However, we had no expectations that albumin levels would vary greatly in healthy infants. (37)
Measuring Bf is essential in managing the risk of neurotoxicity at any given TSB level because it provides information on the distribution of the bilirubin pool in the body. Clinical jaundice increases as bilirubin is leaking from the vascular space, which is reflected by Bf levels. (38) Shifting of vascular bilirubin to the extravascular space resulting from sulfa drug administration, for example, can cause kernicterus even at low TSB levels in preterm newborns. (39)
Unfortunately, the levels at which Bf level indicate neurotoxicity have not been clearly defined and remain controversial. Ahlfors used the peroxidase test to determine a Bf exchange transfusion threshold of 1.3 μg/dL (22 nmol/L) per kilogram birth weight with a maximum allowable Bf of 4μg/dL (66 nmol/L). (14) Infants with unbound bilirubin levels near or above 1 μg/dL (17nM) may have subtle bilirubin-induced wave latency and amplitude changes detected by ABR testing, but this level was frequently found in infants with no ABR abnormalities. (13) In late preterm and term infants, Amin noted auditory abnormalities at Bf levels as low 11 nM., (16) but this level would not be considered for intervention even in preterm infants in a study by Nakamura. (40) Our results noted that a proportion of healthy infants manifested unexpectedly elevated levels above ranges that concern some investigators, but we were unable to demonstrate any complications in infants at these levels.
Still, there is substantial evidence that the Bf level is a superior predictor of the risk of bilirubin toxicity. (4, 39, 41) In the past, several methods were developed to measure Bf, but the only one to quantify it directly was the UB Analyzer (Arrows) that was based on the peroxidase assay. (42) This method has several limitations, including the requirement of sample dilution, which resulted in severe underestimates of Bf in the presence of a competitor of bilirubin-albumin binding. (21, 43) In contrast, UBCheck used no dilution and was able to measure Bf accurately in blood in samples as small as 5μL compared to ≥ 20 μL diluted 52-fold for the UB Analyzer. (44)
There are various guidelines for the use of phototherapy, and they all suffer from weak evidence, mainly from 50 and 60-year-old exchange transfusion data and reliance on expert opinion. (6) Approximately 10% of term and late-preterm infants undergo phototherapy treatment in the nursery (1), following the established TSB recommendations by the American Academy of Pediatrics. (17) However, newborns with TSB levels below these thresholds are often treated (45) because clinicians consider subthreshold phototherapy to be safe, and increased exposure may be preferable to more invasive intervention, such as an exchange transfusion. This practice resulted in reducing the number of hospital readmissions for phototherapy but increased the number of infants unnecessarily receiving this intervention and delayed hospital discharge. (46) Our present investigation confirmed these observations, with a large proportion of the infants in the study treated with phototherapy despite safe levels of TSB, which was primarily responsible for the delayed discharge.
Phototherapy produced a weaker Bf response compared to TSB, albeit a significant difference was not achieved in our healthy population of infants. The impact of phototherapy was most evident not in reducing hearing failures, but in extending length of stay.
In conclusion, our investigation demonstrated that the routine use of TSB is ineffective in predicting Bf levels, especially in infants with elevated TSB levels. As noted above, a possible explanation for this observation is that albumin levels may be lower in infants with higher TSB (47). However, in our experience the use of TSB leads to the overuse of phototherapy and further studies on the use of Bf in this context are needed to demonstrate its effectiveness in minimizing light exposure,(9) because overexposure lengthens hospitalization, potentially compromises maternal-infant bonding,(48), and exposes the infant to unexpected long term risks. (34, 49)
There are clear limitations to this observational study including the variability of clinician use of phototherapy, the lack of Bf use to manage treatment, and the absence of neurotoxicity in the healthy population, which would be needed to confirm Bf’s predictive accuracy. However, demonstrating the Bf measurement in small samples by the bedside is easy and feasible and should stimulate future investigations to answer some of the research questions.
Figure 2.
Relationship between unbound and total (> 12 mg/dl) bilirubin levels.
Acknowledgments
Funding source: 1] NIH 2R44HD080412-05A1, 2] Fluoresprobe Sciences
Abbreviations
- TSB
total serum bilirubin
- Bf
unbound bilirubin
- IRB
institutional review board
- RWJMS
Robert Wood Johnson Medical School
Footnotes
Conflict of Interest: All authors have indicated they have no potential conflicts of interest to disclose. No honorarium, grant, or another form of payment was given to anyone to produce the manuscript.
Financial Disclosure: Kleinfeld is the founder of and partner in Fluoresprobe Sciences.
Huber is Director of Research at Fluoresprobe Sciences & Fluoresprobe Sciences pay his salary.
No reprints requested.
References
- 1.Bhutani VK, Stark AR, Lazzeroni LC, Poland R, Gourley GR, Kazmierczak S, et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. The Journal of pediatrics. 2013;162(3):477–82 e1. [DOI] [PubMed] [Google Scholar]
- 2.Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994;349(2):197–200. [DOI] [PubMed] [Google Scholar]
- 3.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–6. [DOI] [PubMed] [Google Scholar]
- 4.Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. The New England journal of medicine. 2013;369(21):2021–30. [DOI] [PubMed] [Google Scholar]
- 5.Cremer RJ, Perryman PW, Richards DH. Influence of light on the hyperbilirubinaemia of infants. Lancet. 1958;1(7030):1094–7. [DOI] [PubMed] [Google Scholar]
- 6.Hansen TWR, Maisels MJ, Ebbesen F, Vreman HJ, Stevenson DK, Wong RJ, et al. Sixty years of phototherapy for neonatal jaundice - from serendipitous observation to standardized treatment and rescue for millions. J Perinatol. 2019. [DOI] [PubMed] [Google Scholar]
- 7.Newman TB, Kuzniewicz MW, Liljestrand P, Wi S, McCulloch C, Escobar GJ. Numbers needed to treat with phototherapy according to American Academy of Pediatrics guidelines. Pediatrics. 2009;123(5):1352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbesen F, Bjerre JV, Vandborg PK. Relation between serum bilirubin levels >/=450 mumol/L and bilirubin encephalopathy; a Danish population-based study. Acta Paediatr. 2012;101(4):384–9. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, Kuzniewicz MW, Wickremasinghe AC, Walsh EM, Wi S, McCulloch CE, et al. Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: a population-based study. JAMA Pediatr. 2015;169(3):239–46. [DOI] [PubMed] [Google Scholar]
- 10.Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. The New England journal of medicine. 2008;358(9):920–8. [DOI] [PubMed] [Google Scholar]
- 11.Jasprova J, Dal Ben M, Hurny D, Hwang S, Zizalova K, Kotek J, et al. Neuro-inflammatory effects of photodegradative products of bilirubin. Sci Rep. 2018;8(1):7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashore WJ, Oh W. Unbound bilirubin and kernicterus in low-birth-weight infants. Pediatrics. 1982;69(4):481–5. [PubMed] [Google Scholar]
- 13.Funato M, Tamai H, Shimada S, Nakamura H. Vigintiphobia, unbound bilirubin, and auditory brainstem responses. Pediatrics. 1994;93(1):50–3. [PubMed] [Google Scholar]
- 14.Ahlfors CE, Wennberg RP, Ostrow JD, Tiribelli C. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clinical chemistry. 2009;55(7):1288–99. [DOI] [PubMed] [Google Scholar]
- 15.Huber AH, Zhu B, Kwan T, Kampf JP, Hegyi T, Kleinfeld AM. Fluorescence sensor for the quantification of unbound bilirubin concentrations. Clin Chem. 2012;58(5):869–76. [DOI] [PubMed] [Google Scholar]
- 16.Amin SB, Saluja S, Saili A, Laroia N, Orlando M, Wang H, et al. Auditory toxicity in late preterm and term neonates with severe jaundice. Developmental medicine and child neurology. 2017;59(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. [DOI] [PubMed] [Google Scholar]
- 18.Kleinfeld A, Huber A, Kameyama H, Sun AT, Hegyi T The effect of conjugated bilirubin on measurements of unbound bilirubin using the UBCheck and Arrows UB analyzer methods. Pediatric Academic Societies Meeting; Baltimiore, MD.2019. [Google Scholar]
- 19.Kleinfeld A, Huber A, Hegyi T Unbound bilirubin measurements by Arrows and UBCheck analyzers. Pediatric Academies Societies Meeting; May 6, 2018; Toronto, CA2018. [Google Scholar]
- 20.Weisiger RA, Ostrow JD, Koehler RK, Webster CC, Mukerjee P, Pascolo L, et al. Affinity of human serum albumin for bilirubin varies with albumin concentration and buffer composition: results of a novel ultrafiltration method. The Journal of biological chemistry. 2001;276(32):29953–60. [DOI] [PubMed] [Google Scholar]
- 21.Ahlfors CE, Wennberg RP, Ostrow JD, Tiribelli C. Unbound (Free) Bilirubin: Improving the Paradigm for Evaluating Neonatal Jaundice. Clin Chem. 2009;55(7):1288–99. [DOI] [PubMed] [Google Scholar]
- 22.Bratlid D How bilirubin gets into the brain. Clinics in perinatology. 1990;17(2):449–65. [PubMed] [Google Scholar]
- 23.Turkel SB. Autopsy findings associated with neonatal hyperbilirubinemia. Clinics in perinatology. 1990;17(2):381–96. [PubMed] [Google Scholar]
- 24.Ahlfors CE. Unbound bilirubin associated with kernicterus: a historical approach. The Journal of pediatrics. 2000;137(4):540–4. [DOI] [PubMed] [Google Scholar]
- 25.Wennberg RP, Ahlfors CE, Bhutani VK, Johnson LH, Shapiro SM. Toward understanding kernicterus: a challenge to improve the management of jaundiced newborns. Pediatrics. 2006;117(2):474–85. [DOI] [PubMed] [Google Scholar]
- 26.Hanko E Unbound bilirubin and risk assessment in the jaundiced newborn: possibilities and limitations. Pediatrics. 2006;117(2):526–7. [DOI] [PubMed] [Google Scholar]
- 27.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–7. [DOI] [PubMed] [Google Scholar]
- 28.Newman TB, Escobar GJ, Gonzales VM, Armstrong MA, Gardner MN, Folck BF. Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics. 1999;104(5 Pt 2):1198–203. [PubMed] [Google Scholar]
- 29.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. Natl Vital Stat Rep. 2003;52(10):1–113. [PubMed] [Google Scholar]
- 30.Newman TB, Maisels MJ. Less aggressive treatment of neonatal jaundice and reports of kernicterus: lessons about practice guidelines. Pediatrics. 2000;105(1 Pt 3):242–5. [PubMed] [Google Scholar]
- 31.Newman TB, Liljestrand P, Jeremy RJ, Ferriero DM, Wu YW, Hudes ES, et al. Outcomes among newborns with total serum bilirubin levels of 25 mg per deciliter or more. The New England journal of medicine. 2006;354(18):1889–900. [DOI] [PubMed] [Google Scholar]
- 32.Ip S, Lau J, Chung M, Kulig J, Sege R, Glicken S, et al. Hyperbilirubinemia and kernicterus: 50 years later. Pediatrics. 2004;114(1):263–4. [DOI] [PubMed] [Google Scholar]
- 33.Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective Cohort Study of Phototherapy and Childhood Cancer in Northern California. Pediatrics. 2016;137(6). [DOI] [PubMed] [Google Scholar]
- 34.Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal Phototherapy and Infantile Cancer. Pediatrics. 2016;137(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegyi T, Kleinfeld A, Huber A, Weinberger B, Memon N, Shih WJ, et al. Effects of Soybean Lipid Infusion on Unbound Free Fatty Acids and Unbound Bilirubin in Preterm Infants. The Journal of pediatrics. 2017;184:45–50 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobsen J Binding of bilirubin to human serum albumin - determination of the dissociation constants. FEBS Lett. 1969;5(2):112–4. [DOI] [PubMed] [Google Scholar]
- 37.van Imhoff DE, Dijk PH, Weykamp CW, Cobbaert CM, Hulzebos CV, Group BAS. Measurements of neonatal bilirubin and albumin concentrations: a need for improvement and quality control. European journal of pediatrics. 2011;170(8):977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid R, Hammaker L. Metabolism and Disposition of C14-Bilirubin in Congenital Nonhemolytic Jaundice. The Journal of clinical investigation. 1963;42:1720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage. The New England journal of medicine. 2014;370(10):979. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura H, Yonetani M, Uetani Y, Funato M, Lee Y. Determination of serum unbound bilirubin for prediction of kernicterus in low birthweight infants. Acta Paediatr Jpn. 1992;34(6):642–7. [DOI] [PubMed] [Google Scholar]
- 41.Ahlfors CE, Parker AE. Bilirubin binding contributes to the increase in total bilirubin concentration in newborns with jaundice. Pediatrics. 2010;126(3):e639–43. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen J, Wennberg RP. Determination of unbound bilirubin in the serum of newborns. Clin Chem. 1974;20(7):783. [PubMed] [Google Scholar]
- 43.Wennberg RP. Measuring free bilirubin: the clinical perspective. Clin Chem. 2012;58(5):811–3. [DOI] [PubMed] [Google Scholar]
- 44.Huber AH, Hegyi T, Kleinfeld AM. Free fatty acids produced from Intralipid and sulfisoxazole displace similar amounts of bilirubin from albumin. Pediatric Academic Societies; May 2, 2014; Vancouver, CA: 2014. [Google Scholar]
- 45.Kuzniewicz MW, Escobar GJ, Newman TB. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics. 2009;124(4):1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickremasinghe AC, Kuzniewicz MW, McCulloch CE, Newman TB. Efficacy of Subthreshold Newborn Phototherapy During the Birth Hospitalization in Preventing Readmission for Phototherapy. JAMA Pediatr. 2018;172(4):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reshad M, Ravichander B, Raghuraman TS A study of cord blood albumin as a predictor of significant neonatal hyperbilirubinemia in term and preterm neonates. Int J Res Med Sci. 2016;4(3):887–90. [Google Scholar]
- 48.Xiong T, Qu Y, Cambier S, Mu D. The side effects of phototherapy for neonatal jaundice: what do we know? What should we do? European journal of pediatrics. 2011;170(10):1247–55. [DOI] [PubMed] [Google Scholar]
- 49.Newman TB, Wu YW, Kuzniewicz MW, Grimes BA, McCulloch CE. Childhood Seizures After Phototherapy. Pediatrics. 2018;142(4). [DOI] [PubMed] [Google Scholar]