Abstract
In the animal production industry, plant-derived antimicrobial phytobiotics are used as an alternative to antibiotics. Here we investigated the role sanguinarine-based phytobiotic in broiler recovery from Necrotic Enteritis (NE) infection. A total of 100 one-day-old broiler chicks (Ross 308) were randomly allocated to four treatments: negative control CTR (no challenge, no phytobiotic supplementation); positive control NE (NE challenged); phytobiotic SG (sanguinarine phytobiotic, 0.12 g/kg); and SG + NE, (sanguinarine phytobiotic, 0.12 g/kg and NE challenge). Sanguinarine-based phytobiotic supplementation caused significant changes between the groups in performance, livability and histological measurements, however, these changes were not significantly different between SG + NE and NE groups. Significant improvement was detected in NE lesion score of the duodenum and ileum of SG + NE birds compared to NE challenged birds at the end of the production cycle at 40 days old, indicating improved post-NE recovery with the addition of phytobiotic. Sanguinarine-based phytobiotic supplementation in NE challenged birds significantly compensated for a NE associated reduction of Firmicutes and an increase in Bacteroidetes. Functional profile of sanguinarine-based phytobiotic supplemented birds microbiota was distinct from CTR functional profile. NE challenge was associated with a significant increase in cecal propionic acid, while sanguinarine-based phytobiotic supplementation resulted in an increase in cecal acetic acid.
Keywords: Microbiology, Food safety, Food microbiology, Nutrition, Infectious disease, Sanguinarine, Phytobiotic, Necrotic enteritis, Broiler, Intestine
Microbiology; Food safety; Food microbiology; Nutrition; Infectious disease; Sanguinarine; Phytobiotic; Necrotic enteritis; Broiler; Intestine.
1. Introduction
Phytobiotics are described as distinctive bioactive plant products with multiple beneficial characteristics. Phytobiotics can be added to the feed as growth promoters to maintain balanced gastrointestinal microbiome, improve immune readiness and performance, reduce oxidative and heat stress [1, 2, 3, 4, 5, 6, 7]. Several phytobiotics have been recognized as safe by the Food and Drug Administration (FDA) of the United States [8]. In the animal production industry, phytobiotics are primarily applied as an alternative to antibiotic growth promoters (AGPs) [6, 9]. Although their mode of action varies considerably based on the chemical structure of the bioactive component, it was suggested that phytobiotics present their benefits by modulating the gut microbiota and reducing the susceptibility toward pathogenic organisms through the changes in membrane permeability to hydrogen ions (H+) [3, 7]. The beneficial properties of a phytobiotic depend on several factors such as plant species, geographical location, harvesting period, manufactural processing, and storage conditions [2, 7, 10].
The use of sanguinarine-like alkaloid supplements in poultry feed has a positive influence on growth performance, anti-inflammatory properties, and antimicrobial activity against enteric pathogens [11, 12, 13, 14, 15]. Karimi et al. [16], investigated the impact of commercial sanguinarine-based phytobiotic on broiler performance, small intestine morphology, and immune function. The results indicated that feed intake and small intestinal morphology were not significantly altered. However, serum antibody titers were notably improved, thereby enhancing the immune state of birds. The supplementation improved both bird's performance and carcass yield. Abudabos et al. [17], evaluated the impact of sanguinarine-based phytobiotic on growth traits and blood biochemical parameters of S. Typhimurium challenged broilers and concluded that commercial sanguinarine-based phytobiotic supplementation is a valid contributor to maintaining growth performance and biochemical profile of Salmonella challenged broilers.
The current investigation aimed to evaluate the capability of sanguinarine-based phytobiotic to benefit in maintaining microbial community and intestinal health in broilers recovering from necrotic enteritis.
2. Materials and methods
2.1. Ethical statement
This study was completed in agreement with the Gloucestershire County Council's (GCC) Animal Welfare Act recognized and officially approved in Royal Decree No. (M/44) by the Saudi Arabian government. The Research Ethics Committee (Deanship of Scientific Research, Vice-Rectorate for Graduate Studies & Scientific Research at King Saud University, Riyadh) approved this study under the authorization number KSU-SE-18-38.
2.2. Study design, dietary treatments and sample collection
One hundred broiler chicks (Ross 308) were acquired as one day old. Chicks of the average initial weight of 201 g were obtained from Alwadi Poultry Farms Ltd, Co., Alkharj, Saudi Arabia and randomly distributed to treatment groups. The chicks were reared in controlled units under the same managerial and hygienic settings using cage system. Chicks were distributed randomly into four dietary treatments and allocated into five replicate cages with five chicks per cage (total of 25/treatment). Treatment groups were a negative control with no challenge, and no supplemented phytobiotic (CTR), NE challenged (NE), sanguinarine-based phytobiotic (0.12 g/kg as recommended by a supplier) supplemented group (SG) and both sanguinarine-based phytobiotic supplemented (0.12 g/kg) and NE challenged group (SG + NE). We used commercial phytobiotic Sangrovit®, which contains a minimum of 1.5% sanguinarine, a quaternary benzo[c]phenanthridine alkaloid (QBA) extracted from plume poppy (Macleaya cordata) [11].
Birds were fed a standard starter (0-21d) and finisher diets (21–40 d) and had constant access to feed and fresh water (Table 1). Induced necrotic enteritis was applied following the infection model previously described by Prescott et al. [18], with minor modifications. Briefly, on day 23, birds from NE and SG + NE groups were orally co-infected with Eimeria sp. using13 times the recommended dose of Coccidia™-D vaccine, along with C. perfringens (type A strain) challenge [19] at the rate of 4 × 108 CFU for three consecutive days; 26, 27, and 28. NE model success was confirmed by the investigation of microscopic and macroscopic lesion characteristic representative of NE in broilers small intestine. From the first day of C. perfingens challenge onwards, post mortem dissections were performed to confirm if the mortality was caused by NE, by assessing representative signs of confluent necrosis and sloughing epithelium of the intestinal tract.
Table 1.
Nutritional components and calculated nutrient analysis of broilers starter and finisher feed.
| Ingredients | Starter | Finisher |
|---|---|---|
| Yellow corn | 50.635 | 65.39 |
| Soybean meal | 42.40 | 20.70 |
| Wheat bran | 0.00 | 0.60 |
| Corn Gluten meal | 0.00 | 0.70 |
| Choline chloride CL 60 | 0.05 | 0.05 |
| Corn oil | 3.60 | 3.00 |
| Dicalcium Phosphate | 1.270 | 1.027 |
| Ground Limestone | 1.080 | 1.04 |
| Salt | 0.300 | 0.30 |
| Phytase xp 10000 TPT | 0.005 | 0.005 |
| Digestive L-methionine | 0.295 | 0.22 |
| Lysine-HCL | 0.080 | 0.36 |
| Threonine | 0.085 | 0.11 |
| Vitamin- Mineral premix1 | 0.200 | 0.200 |
| Total |
100 |
100 |
| Analysis | ||
| ME, kcal/kg | 3000 | 3200 |
| Crude protein, % | 23.0 | 19.5 |
| Non phytate P, % | 0.48 | 0.359 |
| Calcium, % | 0.96 | 0.81 |
| Digestive Lysine, % | 1.28 | 1.03 |
| Sulfur amino acids, % | 0.85 | 0.8 |
| Threonine, % | 0.86 | 0.69 |
Vitamin-mineral premix contains in the following per kg: vitamin A, 12000000 IU; vitamin D3, 5000000 IU; vitamin E, 80000 IU; vitamin K3, 3200 mg; vitamin B1, 3200 mg; vitamin B2, 8600 mg; vitamin B3, 65000 mg; pantothenic acid, 20000 mg; vitamin B6, 4300 mg; biotin 220 mg; antioxidant (BHA + BHT), 50000 mg; B9, 2200 mg; B12, 17 mg; copper, 16000 mg; iodine, 1250 mg; iron, 20000 mg; manganese, 120000 mg; selenium, 300 mg, and zinc, 110000 mg.
Following NE challenge broilers were allowed to reach the end of production time at 40 days before sample collection: cecum contents were collected randomly for microbiota examination and SCFA analysis. Cecum samples were transferred immediately on ice and stored at -80 °C. Ileum tissue was collected from six birds per treatment for histological analysis. Tissue samples were washed in phosphate-buffered saline (PBS) and fixed in 10% buffered formaldehyde.
2.3. Performance measurements
Records for average body weight gain (BWG), feed intake (FI) and feed conversion ratio (FCR) were obtained weekly. Broiler's feed intake was calculated by subtracting the volume of rejected feed from the offered feed. Occurred mortality was recorded, and FCR was adjusted and computed for each treatment group. The Production Efficiency Factor (PEF) was calculated according to the following equation:
2.4. DNA extraction, 16S rRNA amplification and sequencing
Genomic DNA was extracted from chicken cecal samples for microbiota investigation using a protocol defined previously [20, 21] with minor modifications. Extracted DNA was quantified and quality-assured using a Nanodrop spectrophotometer. Amplicons of the V3–V4 hypervariable regions of the 16S rDNA gene were generated in by using primers forward ACTCCTACGGGAGGCAGCAG, and reverse GGACTACHVGGGTWTCTAAT. Primers were designed with barcodes, spacers and Illumina linkers, according to Fadrosh et al., [22]. The sequencing library was prepared following the manufacturer's protocol (Illumina Inc., San Diego, CA, USA). Sequencing of amplicons was performed on the Illumina MiSeq platform using 2 × 300 bp paired-end sequencing.
2.5. Data processing
The sequencing data were analyzed using Quantitative Insights into Microbial Ecology (QIIME v.1.9.1) [23]. Paired-end joining was performed using Fastq-Join algorithm with no mismatches within the overlap region. Only sequences with Phred quality threshold higher than 20 were included in further analysis. The sequences were clustered into operational taxonomic units OTUs at 97% identity using UCLUST algorithm [24]. All taxonomic allocations were completed in QIIME using GreenGenes reference database and QIIME default arguments [25]. OTUs with abundance under 0.01% were removed. The data quality filtering and removal of samples with low sequencing coverage resulted in 52 sequenced cecum samples in total. Hellinger transformed [26] OTU table was used in the analysis. Data visualization and statistical analysis were performed using Calypso (http://cgenome.net/calypso/) [27]. Alpha diversity was assessed with Chao1, Shannon, Richness, Evenness, and Simpson diversity indexes. Untransformed data is presented in figures comparing relative abundance. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis was performed using KEGG Orthologue functional classifications and was performed using default settings on publics data analysis server https://huttenhower.sph.harvard.edu/galaxy/. Significant alterations between treatment groups were detected using ANOVA. Sequencing dataset from this trial is publicly accessible on the MG-RAST server (http://metagenomics.anl.gov/) under accession library number mgl816605.
2.6. Short-chain fatty acid analysis
Extraction of short-chain fatty acids (SCFAs) metabolites was done using an acidified water-extraction method proposed by Zhao, Nyman [28], and the final extracted solution was filtered using a 0.45 μm syringe filter. Standard acids were obtained from Dr. Ehrenstorfer (Augsburg, Germany) and included; acetic acid (C2) (99.9%), propionic acid (C3) (99.9%), butyric acid (C4) (99.5%), i-butyric acid (i-C4) (99.0%), n-valeric acid (C5) (99.3%) and i-valeric acid (i-C5) (99.0%). All standards concentrations were adjusted in methanol to 1000 ug/ml (ppm), and calibration curves were constructed using standard stock solutions that contained all six acids.
The GC-MS system on an Agilent (Palo Alto, CA) 7890A with an Agilent350 °C column (30 m × 250 μm x 0.25 μm) was used to analyze SCFA from cecal samples. GC-MS was operated in total ion chromatogram (TIC) scan mode to measure the retention time of each SCFA and the mixture of SCFAs. Separation of the SCFAs was done following methods described previously [29, 30, 31, 32]. GC/MS was operated in a single ion monitoring (SIM) mode according to the following instrumental parameters: oven temperature program started at 50 °C and held for 1 min, then ramped at 6 °C per min to a temperature of 100 °C, and finally ramped at 25 °C per min until the final temperature of 270 °C was reached and held for 1 min (a total of 18.133 min run program), and 2 min post run of 300 °C. A 2 μL sample volume was injected at heater at 250 °C with helium as the carrier gas in a split-less mode. The pressure was maintained at 11.747 psi at 24.4 mL/min through the entire flow cycle.
2.7. Microscopy and lesion scoring
Ileal tissue samples approximately two-centimetre in length were collected from the mid-section for histological analysis from six individual birds per treatment, samples were fixed for 72 h in 10% buffered formaldehyde, dehydration in graded alcohol, embedded in paraffin and stained by hematoxylin and eosin (H&E) stain described by Samanya and Yamauchi [33]. Photomicrographs were scanned using a Nikon Eclipse Ni–U microscope with a camera (Nikon, Tokyo, Japan).
The histological analysis consisted of villi height, villi width, and calculation of total villi surface area performed by using an IX71 Inverted Olympus Microscope (eyepiece: WH10x; objective lens: 4x), digitalized by Olympus DP72 microscope digital camera (Olympus NV, Aartselaar, Belgium) and analyzed using CellSens digital imaging software tools. Measurements were assessed based on at least ten well-oriented villi/bird. Calculation of the villus surface area was based on the following formula:
Lesion scoring examination of the small intestine was conducted by scoring for lesions at three sites: duodenum, ileum, and jejunum on a scale of 0–3 based on their severity, according to the grading method described by Gholamiandehkordi et al., [34]. A score of zero represented clear intestine with absences of lesions, and the score of three signified diffuse necrosis illustrative of field cases. A sample-blinded histopathology expert performed histological and lesion examination.
2.8. Statistical analysis
Data presented in Tables 2, 3, and 4 were analyzed using general linear models (GLM) of SAS (2009) software, and one-way analysis of variance (ANOVA) was used for randomized complete block design. Means of treatment groups were compared by the least significant difference (LSD) test, and differences were considered significant at P-value < 0.05.
Table 2.
Effect of sanguinarine-based phytobiotic supplementation and NE challenge on cumulative feed intake, body weight gain, feed conversion ratio and production efficiency factor of broilers during different stages of the experiment.
| Treatment group | Performance |
Livability % |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d 0–21 (Pre-Infection) |
d 21–28 (Necrotic Enteritis) |
d 28–35 (Recovery) |
d 0–35 (Overall) |
|||||||||||||||
| FI, g | BWG, g | FCR, g:g | PEF | FI, g | BWG, g | FCR, g:g | PEF | FI, g | BWG, g | FCR, g:g | PEF | FI, g | BWG, g | FCR, g:g | PEF | Final body weight, kg | ||
| CTR | 971.7 | 804.3 | 1.209 | 333.79 | 738.9 | 540.8a | 1.367c | 362.6a | 858.5 | 587.8 | 1.461b | 378.6a | 2569.1 | 1932.9 | 1.329b | 415.8a | 1.934 | 100a |
| NE | 970.9 | 795.3 | 1.221 | 325.9 | 669.6 | 430.9b | 1.556ab | 291.1b | 914.5 | 546.9 | 1.674a | 284.4c | 2555.0 | 1773.0 | 1.441a | 329.7b | 1.800 | 93b |
| SG | 919.4 | 760.6 | 1.207 | 325.9 | 676.3 | 455.2b | 1.483b | 302.1b | 853.2 | 582.2 | 1.473b | 356.0ab | 2448.9 | 1798.0 | 1.361b | 384.4ab | 1.831 | 100.0a |
| SG + NE | 933.5 | 770.0 | 1.212 | 286.2 | 689.9 | 435.4b | 1.586a | 292.9b | 912.0 | 573.8 | 1.596a | 324.9bc | 2535.5 | 1779.2 | 1.426a | 334.0b | 1.809 | 96ab |
| SEM± | 28.23 | 19.68 | 0.014 | 13.33 | 31.23 | 19.24 | 0.029 | 11.28 | 23.7 | 23.99 | 0.032 | 15.95 | 64.97 | 44.49 | 0.014 | 18.42 | 0.047 | 1.369 |
| p-value | 0.52 | 0.46 | 0.91 | 0.16 | 0.57 | 0.016 | 0.002 | 0.007 | 0.19 | 0.68 | 0.0014 | 0.01 | 0.60 | 0.16 | 0.0008 | 0.03 | 0.36 | 0.005 |
CTR: Negative Control; NE: Positive Control, NE challenge; SG: Sanguinarine-Based phytobiotic, unchallenged; SG + NE: Sanguinarine-Based phytobiotic and NE challenge.BWG, bodyweight gain; FI, feed intake; FCR, feed conversion ratio; PEF, production efficiency factor.
abcdMeans in the column with different superscripts differ significantly.
NS, not significant.
Table 3.
Ileum histomorphometric measurements of broilers at (40 d).
| Treatment group | Histomorphometric Measurements |
||
|---|---|---|---|
| L (μm) | W (μm) | SA (μm) | |
| CTR: Negative control | 624.8 | 75.9 | 0.147 |
| NE: Positive control, NE challenge | 566.3 | 75.6 | 0.142 |
| SG: Sanguinarine-based phytobiotic, unchallenged | 652.6 | 69.8 | 0.146 |
| SG + NE: Sanguinarine-based phytobiotic, NE Challenge | 620.7 | 70.5 | 0.143 |
| SEM1 | ±22.72 | ±4.67 | ±0.011 |
| p-Value | 0.059NS | 0.69NS | 0.9877 NS |
L: villus length; W: villi width; SA: surface area.
NS; not significant.
SEM: standard error of the mean.
Table 4.
Intestinal macroscopic NE lesion scores (0–3) of broilers at 40 d.
| Treatment group | Macroscopic NE Lesion Scores (points) |
||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| CTR: Negative control | 0.00c | 0.00b | 0.00b |
| NE: Positive control, NE challenge | 1.86a | 1.21a | 1.07a |
| SG + NE: Sanguinarine-based phytobiotic, NE challenge | 0.71b | 1.00a | 0.29b |
| SEM1 | ±0.163 | ±0.211 | ±0.1029 |
| p-Value | 0.0001∗∗∗ | 0.0005∗∗∗ | 0.0001∗∗∗ |
ab Means values within a column with different superscripts are significantly different ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
NS, not significant.
SEM: standard error of the mean.
3. Results
In the current study, we investigated the beneficial effects of sanguinarine-based phytobiotic supplementation on birds challenged with NE by following the birds up to the end of productive life, to investigate overall benefit for the production and the influence of sanguinarine product on post-NE recovery. While most NE studies focus on the peak of infection, several other studies also followed the birds through the recovery stage [35, 36, 37, 38, 39].
3.1. Growth performance
Growth performance was calculated following different stages of the trial in conjugation with different phases of NE infection, as following: pre-infection (0-21d), NE active infection (21-28d), recovery (28-35d), and cumulative performance (0-35d), in addition to overall livability and final body weight (Table 2). No significant differences were observed in any measured parameters across all treatment groups throughout the pre-infection period. Significant changes were observed in the remaining periods (21-28d), (28-35d) and in overall performance (0-35d), however, there were no differences in any of the performance parameters in any of the growth stages between NE challenged birds, and NE challenged SG supplemented birds indicating that, based on our number of birds pretreatment, there was no performance improvement in NE challenge resulting from SG supplementation. There was a significant difference between the groups in livability (P = 0.005), again, not significantly different between the NE and SG + NE group.
3.2. Microscopic and macroscopic effects of phytobiotic supplementation
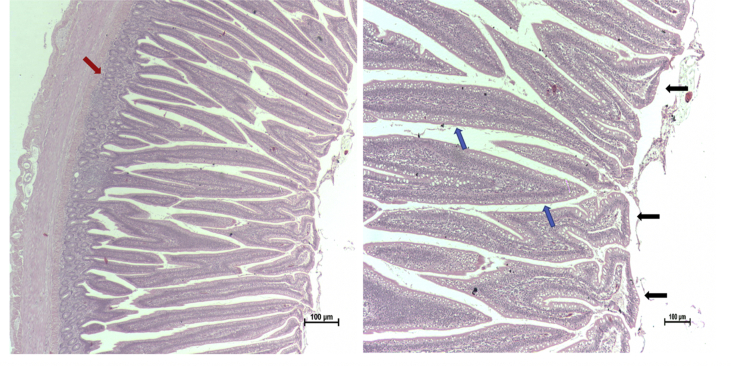
Table 3 represents the impact of sanguinarine-based phytobiotic administration and NE challenge on ileal histological measurements, including villus length, width, and calculation of total surface area in broilers at (40 d). Histological analysis showed no significant differences between any of the treatments in the ileum. Figures 1, 2, and 3 represent the histopathological changes observed in the bird's small intestine. Birds that were NE challenged (NE) illustrated several microscopic presentations of necrotic enteritis (NE) starting with disorganized villi with flattening and fusion, lymphocytes and polymorphonuclear cells, crypt hyperplasia and edema, in addition to macroscopic NE lesion from jejunum region (Figure 2). Broilers under NE challenge and phytobiotic supplementation (SG + NE) showed slightly improved villus structure with broad and thickened villus tips (Figure 3).
Figure 1.
Ileum photomicrograph of negative control CTR (10X magnification), Haematoxylin and Eosin (H&E) stain.
Figure 2.
Microscopic and macroscopic presentations of NE in broilers small intestine (NE), (left): ileum photomicrograph of broilers under NE challenge presenting disorganized villi with fusion and flattening, infiltration of lymphocytes and polymorphonuclear cells, crypt hyperplasia, and severe edema, (right) macroscopic examination of NE lesion in the jejunum region.
Figure 3.
Ileum photomicrograph of broilers under NE challenge and phytobiotic supplementation (SG + NE). It is showing the separation of villus with broad and thickened villus tips in many villi and an increase in absorptive surfaces without exudate into the lumen (black arrows) with hyperplastic villus epithelium containing abundant goblet cells (blue arrows). Crypt hyperplasia is still present but with shorter and normal-sized Glands of Lieberkühn (red arrows). Haematoxylin and Eosin (H&E) stain.
Lesion scoring in broiler small intestine included duodenum, jejunum and ileum regions, at 40 d. Significant differences were detected across all examined sections, where NE challenged (NE) represented the highest scores. Birds exposed to NE challenge and had their diet supplemented with sanguinarine phytobiotic (SG + NE), showed significant improvement in both duodenum and ileum regions compared to challenged un-supplemented group (P = 0.0001), while no significant changes were detected in jejunum between these two groups (Table 4).
3.3. Microbiota composition
Overall, the cecal microbiota structure of broilers in this experiment was represented by five main phyla: dominant Firmicutes followed by Bacteroidetes, Actinobacteria, Tenericutes, and Proteobacteria, respectively. At a genus level, regarding known genera, cecal microbiota profile was dominated by Faecalibacterium followed by Oscillospira, Ruminococcus, Coprococcus, Bacteroides, Lactobacillus, Blautla, Eubacterium, Dorea, Coprobacillus, and Eggerthella.
There were no significant variations in the alpha diversity between treatment groups (Shannon, Richness, Chao1, Evenness, and Simpson). Using Adonis multivariate analysis, only the phylum level showed significant differences between treatments (P = 0.006) while genus and OTU levels were not significantly altered. Phylum level differences were due to NE induced significant drop in Firmicutes and expansion of Bacteroidetes. This major NE driven phylum shift was significantly (Tukey test) corrected with the addition of SG (Figure 4) with NE and SG + NE showing a significant difference (P = 0.0023 and P = 0.0084 for Firmicutes and Bacteroidetes respectively).
Figure 4.
Phylum level alterations in cecal microbiota were due to NE induced shift in Firmicutes and Bacteroides abundance.
The lack of major differences between genera based on multivariate analysis was confirmed with slight differences between treatments (ANOVA) at the genus level, where only 4 genera significantly (P < 0.05) differed between the treatments: unclassified Barnesiellaceae, Dehalobacterium, Bacteroides and Allobaculum. Only unclassified Barnesiellaceae were significantly different (Tukey test) between SG supplemented, and unsupplemented NE challenged birds (P(NE vs SG+NE) = 0.0074), showing sharp increase only under the NE challenge compared to all other groups including SG + NE. Discriminant analysis of principal components (DAPC) plot demonstrates group to group similarities showing that microbiota of the birds in SG + NE group overlaps with SG rather than with NE group (Figure 5A).
Figure 5.
Discriminant analysis of principal components (DAPC) plot demonstrates group to group variations in microbiota community structure (A) and in microbiota functional (B) profiles.
3.4. Functional analysis
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) analysis on KEGG Ortholog inferred functional capabilities of the microbial communities showed minor overall alterations in bacterial function between the groups (multivariate Adonis analysis P = 0.298), with several significantly altered individual functions, however, the differences were strongly significant (P < e−4) only between CTR and NE and CTR and SG, with no significant alterations between NE and SG + NE groups.
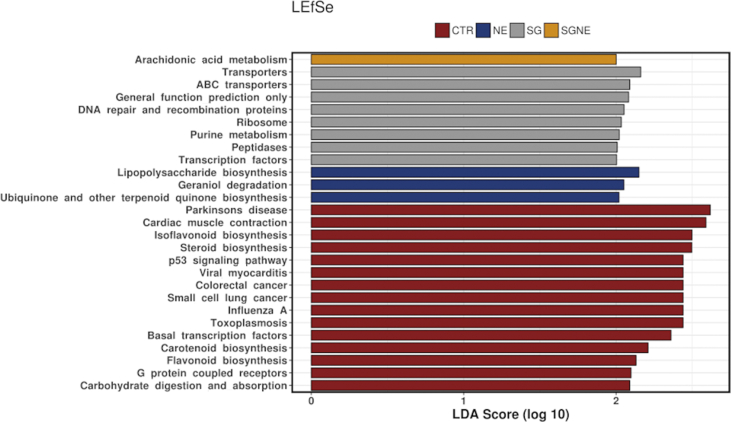
NE challenge altered challenged birds microbial community towards less capacity to energy metabolism, signal transduction, peptidoglycan biosynthesis, bacterial secretion system, vitamin and lipid metabolism (Figure 6). Still, surprisingly NE and SG had an analogous effect on functional changes in microbiota compared to control. To further investigate this incongruity, we again used discriminant analysis of principal components (DAPC) and, unlike in microbiota structure, where SG + NE group overlapped with SG, in functional capability SG + NE group overlapped with NE rather than with SG (Figure 5B). Microbiota DAPC plot (Figure 5A) shows an overlap between the CTR and SG, but in terms of function, CTR and SG are quite distinct from one another (Figure 5B). The biomarker discovery tool, Linear Discriminant Analysis Effect Size (LEfSe) plot in Figure 7 shows functional categories that are strongly enhanced within individual groups.
Figure 6.
PICRUSt analyzed functional capabilities differences between the treatments. Significance is given by ANOVA while Tukey test was showing significant differences only between CTR and NE and CTR and SG.
Figure 7.
The biomarker discovery tool, LEfSe, plot showing functional categories that are enhanced within individual groups on PICRUSt inferred KEGG Orthologue functional categories.
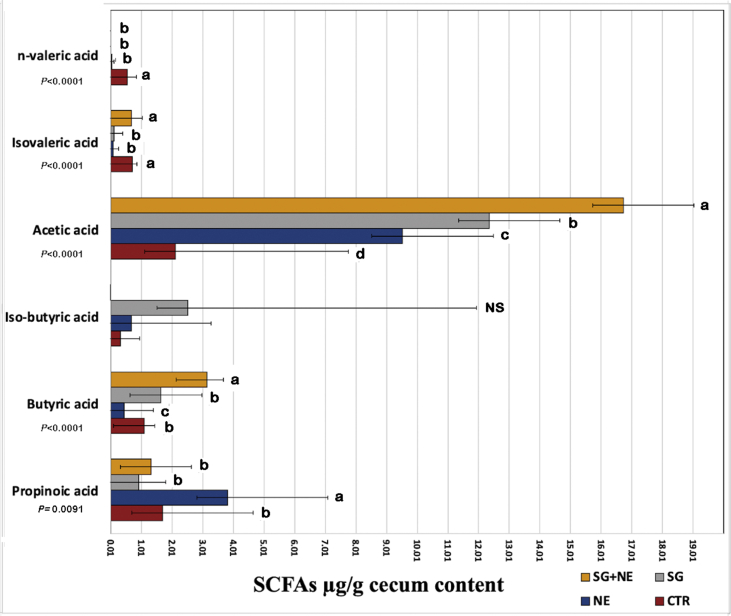
3.5. Short-chain fatty acid production
SCFA production analysis included acetic acid, propionic acid, butyric acid, i-butyric acid, n-valeric acid, and i-valeric acid. Sanguinarine phytobiotic supplemented group (SG + NE) was distinctive by a significant increase in acetic acid (16.74 μg/g cecum content) followed by SG, NE, and CTR, respectively (P < 0.0001). The butyric acid concentration was also the highest in phytobiotic supplemented group (SG + NE). A significant increase in propionic acid concentration was associated with NE challenged group (NE) compared to the rest of the groups (P = 0.0091), and n-valeric acid was significantly elevated in negative control broilers (CTR) (Figure 8).
Figure 8.
Impact of NE challenge and sanguinarine based-phytobiotic administration on six SCFA concentrations in broiler chicken cecum. Error bars represent standard deviation (±SD). abcd Means in the bar with different superscripts differ significantly. NS: not significant.
4. Discussion
Quaternary benzo[c]phenanthridine alkaloids have extended attention because of their low level of toxicity and their multiple beneficial biological activities [40, 41]. QBA's have been extracted from various plant families including Meliaceae, Rutacea, Fumariaceae, Papaveraceae, and Caprifoliaceae [42]. QBA's can be described as phytoallexines due to their antimicrobial, anti-fungal, antitumor activities [42, 43, 44, 45, 46, 47]. Detailed chemical structure of QBA's is available in Stiborová et al. [47]. Sanguiritrin (a mixture of sanguinarine, chelerythrine, and other minor QBA's) have been used as an antimicrobial in myopathy cases. An additional QBA example is fagaronine that exhibits antileukemic properties and has been viewed as a potential novel antitumor drug [43]. In the current trial, the supplementation of sanguinarine-based phytobiotic did not affect performance parameters in the post-NE induced infection period. Others reported an increase in body weight, enhanced performance, and carcass yield [11, 16]. Recently El-Sheikh et al. [48], reported an improvement in the clinical signs of NE in birds under C. perfringens and sanguinarine phytobiotic supplementation, including intestinal lesion scores. Authors attributed this to the presence of essential oils in phytobiotics that are linked to enhanced nutrient digestibility due to the improved activities of amylase and trypsin [49, 50]. These observations are in agreement with our results, as we observed a significant reduction in intestinal lesion score in phytobiotic treated birds in both duodenum, and ileum. At the same time, NE challenged birds represented with the highest scores (P = 0.0001). NE challenge and phytobiotic supplementation did not introduce significant changes in cecal microbiota composition nor in the diversity measures in our dataset. This is opposite to the previous report indicated that NE in chickens could be linked to modifications in both alpha and beta diversity [51], however, the two studies were done using very different NE challenge model.
In the GIT tract, sanguinarine like alkaloids are converted initially to non-toxic dihydrosanguinarine; both compounds are discharged through feces and urine [41]. Previous studies on broiler chickens have demonstrated that sanguinarine phytobiotic can modify gut microbiota and significantly increase the abundance of lactic acid bacteria [50]. However, we did not observe any significant changes in lactic acid related genera as the ceca of the birds in our trial were dominated by Faecalibacterium.
One of the key health determinates of the host is the capability for the absorption of nutrients is the condition of the intestinal mucosa and intestinal morphology which is immensely influenced by bird feed and microbiota [52]. Dietary supplementation of quaternary benzophenanthridinze and protopine alkaloids combination has been related to increasing of villi height, villi width, villus/crypt ratio, and surface area of jejunum in broilers [53]. Conversely, Karimi et al. [16], and Zhang et al. [54], reported that dietary supplementation with sanguinarine had no effect on intestinal histology which is more in line with our data. Comparing ileum photomicrograph of broilers under NE challenge and phytobiotic supplementation represented a slight improvement in villus structure.
Previous studies described benefits of SCFAs in birds include improving the colonic musculature, intestinal blood flow, enterocyte growth, stimulating fluid uptake, regulating intestinal mucin composition, improving intestinal immune functions, and controlling bacterial pathogenesis [55, 56, 57, 58]. Sanguinarine phytobiotic administration was correlated with a significant increase in acetic acid concentrations (P < .0001). Butyric acid has been described in several investigations as beneficial to bird's energy, performance and intestinal development [59, 60]. In addition, acetic acid serves as a carbon and energy source for intestinal bacteria via the activation of glyoxylate pathway enzymes [61]. The data presented in Figure 8 is showing that SG and NE, in combination, have a unique SCFA profile different from their individual treatments. Intense SG associated boost in acetic acid can be related to beneficial effects on the lesion score. Increased levels of naturally produced acetic acid have been associated with improvement of colitis symptoms [62], but externally administered high concentrations are often used as a model for inducing intestinal injury [63].
Another interesting finding is a similar effect of NE challenge and SG supplementation on reduced functional capability of microbiota towards lipid and vitamin metabolism and energy metabolism. The PICRUSt analysis cannot distinguish in which direction this would change actual concentrations as it does not differentiate anabolic from catabolic alterations. Similarly, peptidoglycan biosynthesis and toxin secretion microbiota capability may be inhibited by both NE and SG; in NE via competitive exploits of C. perfringens and in SG via exhibiting previously reported beneficial effects via boosting of probiotic species. The metagenomic analysis could provide an answer if this is an area worth further exploration.
Declarations
Author contribution statement
Mashael R. Aljumaah: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Manal M. Alkhulaifi: Conceived and designed the experiments; Wrote the paper.
Riyadh S Aljumaah: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Alaeldein M. Abudabos: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abdulaziz Alabdullatif, Gamal M. Suliman: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Mu’ath Q. Al-Ghadi: Conceived and designed the experiments; Performed the experiments.
Dragana Stanley: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Deanship of Scientific Research, King Saud University for funding this research through Research Group Project No. RGP-1441-273.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86(14_suppl):E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- 2.Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 2010;158(1):1–14. [Google Scholar]
- 3.Abou-Bakr S. Effect of some plant extracts on fungal and aflatoxin production. Int. J. Acad. Res. 2011;3(4) [Google Scholar]
- 4.Dhama K., Tiwari R., Ru K., Chakraborty S.M.G., Karthik K. 2014. Growth Promoters and Novel Feed Additives Improving Poultry Production and Health, Bioactive Principles and Beneficial Applications: the Trends and Advances: A Review; pp. 129–159. [Google Scholar]
- 5.Gopi M., Karthik K., Manjunathachar H.V., Tamilmahan P., Kesavan M., Dashprakash M. Essential oils as a feed additive in poultry nutrition. Adv. Anim. Vet. Sci. 2014;2(1):1–7. [Google Scholar]
- 6.Karangiya V.K., Savsani H.H., Patil S.S., Garg D.D., Murthy K.S., Ribadiya N.K. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World. 2016;9(3):245–250. doi: 10.14202/vetworld.2016.245-250. PubMed PMID: 27057106; PubMed Central PMCID: PMCPMC4823283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav A.S., Kolluri G., Gopi M., Karthik K., Singh Y. Exploring alternatives to antibiotics as health promoting agents in poultry-a review. J. Exp. Biol. 2016;4 [Google Scholar]
- 8.Chambers J.R., Gong J.S. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 2011;44(10):3149–3159. PubMed PMID: WOS:000298617800003. [Google Scholar]
- 9.Khaksar V., Van Krimpen M., Hashemipour H., Pilevar M. Effects of thyme essential oil on performance, some blood parameters and ileal microflora of Japanese quail. J. Poultry Sci. 2012;49(2):106–110. [Google Scholar]
- 10.Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187(2):182–188. doi: 10.1016/j.tvjl.2010.03.003. PubMed PMID: 20382054. [DOI] [PubMed] [Google Scholar]
- 11.Vieira S.L., Berres J., Reis R., Oyarzabal O.A., Coneglian J.L.B., Freitas DMd. Studies with sanguinarine like alkaloids as feed additive in broiler diets. Rev. Bras. Ciência Avícola. 2008;10(1):67–71. [Google Scholar]
- 12.Newton S.M., Lau C., Gurcha S.S., Besra G.S., Wright C.W. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 2002;79(1):57–67. doi: 10.1016/s0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 13.Kosina P., Gregorova J., Gruz J., Vacek J., Kolar M., Vogel M. Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia. 2010;81(8):1006–1012. doi: 10.1016/j.fitote.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Dvořák Z., Vrzal R., Maurel P., Ulrichová J. Differential effects of selected natural compounds with anti-inflammatory activity on the glucocorticoid receptor and NF-κB in HeLa cells. Chem. Biol. Interact. 2006;159(2):117–128. doi: 10.1016/j.cbi.2005.10.105. [DOI] [PubMed] [Google Scholar]
- 15.Niu X., Fan T., Li W., Xing W., Huang H. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Eur. J. Pharmacol. 2012;689(1-3):262–269. doi: 10.1016/j.ejphar.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Karimi M., Foroudi F., Abedini M. Effect of Sangrovit on performance and morphology of small intestine and immune response of broilers. Biosci. Biotech. Res. Asia. 2014;11:855–861. [Google Scholar]
- 17.Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ. Sci. Pollut. Res. Int. 2016;23(23):24151–24157. doi: 10.1007/s11356-016-7665-2. Epub 2016/09/21. PubMed PMID: 27646442. [DOI] [PubMed] [Google Scholar]
- 18.Prescott J.F., Smyth J.A., Shojadoost B., Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45(3):317–322. doi: 10.1080/03079457.2016.1141345. Epub 2016/01/28. PubMed PMID: 26813025. [DOI] [PubMed] [Google Scholar]
- 19.Aljumaah M.R., Alkhulaifi M.M., Abudabos A.M., Aljumaah R.S., Alsaleh A.N., Stanley D. Bacillus subtilis PB6 based probiotic supplementation plays a role in the recovery after the necrotic enteritis challenge. PloS One. 2020;15(6) doi: 10.1371/journal.pone.0232781. Epub 2020/06/20. PubMed PMID: 32555739; PubMed Central PMCID: PMCPMC7302482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley D., Denman S.E., Hughes R.J., Geier M.S., Crowley T.M., Chen H. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012;96(5):1361–1369. doi: 10.1007/s00253-011-3847-5. PubMed PMID: 22249719. [DOI] [PubMed] [Google Scholar]
- 21.Yu Z., Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36(5):808–812. doi: 10.2144/04365ST04. Epub 2004/05/22. PubMed PMID: 15152600. [DOI] [PubMed] [Google Scholar]
- 22.Fadrosh D.W., Ma B., Gajer P., Sengamalay N., Ott S., Brotman R.M. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2(1):6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. Epub 2010/04/13. doi: nmeth.f.303 [pii] 10.1038/nmeth.f.303. PubMed PMID: 20383131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 25.DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legendre P., Gallagher E.D. Ecologically meaningful transformations for ordination of species data. Oecologia. 2001;129(2):271–280. doi: 10.1007/s004420100716. [DOI] [PubMed] [Google Scholar]
- 27.Zakrzewski M., Proietti C., Ellis J.J., Hasan S., Brion M.-J., Berger B. Calypso: a user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics. 2016;33(5):782–783. doi: 10.1093/bioinformatics/btw725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao G., Nyman M., Jonsson J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006;20(8):674–682. doi: 10.1002/bmc.580. Epub 2005/10/06. PubMed PMID: 16206138. [DOI] [PubMed] [Google Scholar]
- 29.Harrison C. 2010. Quantification of Short-Chain Fatty Acids in Cecal Material by Gas Chromatography-Mass Spectrometry. [Google Scholar]
- 30.Kloos D.P., Gay E., Lingeman H., Bracher F., Müller C., Mayboroda O.A. Comprehensive gas chromatography–electron ionization mass spectrometric analysis of fatty acids and sterols using sequential one-pot silylation: quantification and isotopologue analysis. Rapid Commun. Mass Spectrom. 2014;28(13):1507–1514. doi: 10.1002/rcm.6923. [DOI] [PubMed] [Google Scholar]
- 31.Hoving L.R., Heijink M., van Harmelen V., van Dijk K.W., Giera M. Clinical Metabolomics. Springer; 2018. GC-MS analysis of short-chain fatty acids in feces, cecum content, and blood samples; pp. 247–256. [DOI] [PubMed] [Google Scholar]
- 32.He L., Prodhan M.A.I., Yuan F., Yin X., Lorkiewicz P.K., Wei X. Simultaneous quantification of straight-chain and branched-chain short chain fatty acids by gas chromatography mass spectrometry. J. Chromatogr. B. 2018;1092:359–367. doi: 10.1016/j.jchromb.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samanya M., Yamauchi K.E. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2002;133(1):95–104. doi: 10.1016/s1095-6433(02)00121-6. Epub 2002/08/06. PubMed PMID: 12160875. [DOI] [PubMed] [Google Scholar]
- 34.Gholamiandehkordi A.R., Timbermont L., Lanckriet A., Van Den Broeck W., Pedersen K., Dewulf J. Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol. 2007;36(5):375–382. doi: 10.1080/03079450701589118. PubMed PMID: 17899461. [DOI] [PubMed] [Google Scholar]
- 35.Gharib-Naseri K., Kheravii S., Keerqin C., Morgan N., Swick R., Choct M. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poultry Sci. 2019;98(12):6422–6432. doi: 10.3382/ps/pez480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue G., Barekatain R., Wu S., Choct M., Swick R. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poultry Sci. 2018;97(4):1334–1341. doi: 10.3382/ps/pex444. [DOI] [PubMed] [Google Scholar]
- 37.Keerqin C., Wu S.-B., Svihus B., Swick R., Morgan N., Choct M. An early feeding regime and a high-density amino acid diet on growth performance of broilers under subclinical necrotic enteritis challenge. Anim. Nutr. 2017;3(1):25–32. doi: 10.1016/j.aninu.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues I., Svihus B., Bedford M., Gous R., Choct M. Intermittent lighting improves resilience of broilers during the peak phase of sub-clinical necrotic enteritis infection. Poultry Sci. 2018;97(2):438–446. doi: 10.3382/ps/pex315. [DOI] [PubMed] [Google Scholar]
- 39.M’Sadeq S.A., Wu S.-B., Swick R.A., Choct M. Dietary acylated starch improves performance and gut health in necrotic enteritis challenged broilers. Poultry Sci. 2015;94(10):2434–2444. doi: 10.3382/ps/pev219. [DOI] [PubMed] [Google Scholar]
- 40.Psotova J., Vecera R., Zdarilova A., Anzenbacherova E., Kosina P., Svobodova A. Safety assessment of sanguiritrin, alkaloid fraction of Macleaya cordata, in rats. Vet. Med. Praha. 2006;51(4):145. [Google Scholar]
- 41.Kosina P., Walterova D., Ulrichová J., Lichnovský V., Stiborová M., Rýdlová H. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem. Toxicol. 2004;42(1):85–91. doi: 10.1016/j.fct.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Šimánek V. The Alkaloids: Chemistry and Pharmacology. Vol. 26. Elsevier; 1985. Benzophenanthridine alkaloids; pp. 185–240. [Google Scholar]
- 43.Walterova D., Ulrichova J., Valka I., Vicar J., Vavreckova C., Taborska E. Benzo [c] phenanthridine alkaloids sanguinarine and chelerythrine: biological activities and dental care applications. Acta Univ. Palacki. Olomuc. Fac. Med. 1995;139:7–16. [PubMed] [Google Scholar]
- 44.Wink M., Schmeller T., Latz-Brüning B. Modes of action of allelochemical alkaloids: interaction with neuroreceptors, DNA, and other molecular targets. J. Chem. Ecol. 1998;24(11):1881–1937. [Google Scholar]
- 45.Verpoorte R. Springer; Alkaloids: 1998. Antimicrobially Active Alkaloids; pp. 397–433. [Google Scholar]
- 46.Faddeeva M., Beliaeva T. Sanguinarine and ellipticine cytotoxic alkaloids isolated from well-known antitumor plants. Intracellular targets of their action. Tsitologiia. 1997;39(2-3):181. [PubMed] [Google Scholar]
- 47.Stiborová M., Šimánek Vm, Frei E., Hobza P., Ulrichová J. DNA adduct formation from quaternary benzo [c] phenanthridine alkaloids sanguinarine and chelerythrine as revealed by the 32P-postlabeling technique. Chem. Biol. Interact. 2002;140(3):231–242. doi: 10.1016/s0009-2797(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 48.El-Sheikh S.M., Khairy M.H., Eleiwa N.Z., Abdalla O.E., El-Monsef A.G.A. Veterinary Medicine In-Between Health & Economy (VMHE)–16-19 October 2018. 2018. Effect OF sanguinarine phytobiotic, sodium butyrate compared to ampicillin ON controlling necrotic enteritis IN broiler chickens; p. 55. (20-Suppl) [Google Scholar]
- 49.McReynolds J., Waneck C., Byrd J., Genovese K., Duke S., Nisbet D. Efficacy of multistrain direct-fed microbial and phytogenetic products in reducing necrotic enteritis in commercial broilers. Poultry Sci. 2009;88(10):2075–2080. doi: 10.3382/ps.2009-00106. [DOI] [PubMed] [Google Scholar]
- 50.Lee K.-W., Kim J.-S., Oh S.-T., Kang C.-W., An B.-K. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J. Poultry Sci. 2015 [Google Scholar]
- 51.Stanley D., Wu S.B., Rodgers N., Swick R.A., Moore R.J. Differential responses of cecal microbiota to fishmeal, Eimeria and Clostridium perfringens in a necrotic enteritis challenge model in chickens. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0104739. Epub 2014/08/29. PubMed PMID: 25167074; PubMed Central PMCID: PMCPMC4148237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sittiya J., Yamauchi K., Takata K. Effect of replacing corn with whole-grain paddy rice and brown rice in broiler diets on growth performance and intestinal morphology. J. Anim. Physiol. Anim. Nutr. 2016;100(2):381–390. doi: 10.1111/jpn.12357. PubMed PMID: 26122821. [DOI] [PubMed] [Google Scholar]
- 53.Reansoi A., Ruangpanit Y., Attamangkune S., editors. Annual Conference Kasetsart, Bankok, Thailand. 2015. Effect of quaternary benzophenantridine and protopine alkaloids on growth response and gut health of broiler under hot climate management. http://www annualconference ku ac th/cd53/02_019_O253 pdf Accessed January. [Google Scholar]
- 54.Zhang R., Wang X.-W., Zhu J.-Y., Liu L.-L., Liu Y.-C., Zhu H. Dietary sanguinarine affected immune response, digestive enzyme activity and intestinal microbiota of Koi carp (cryprinus carpiod) Aquaculture. 2019;502:72–79. [Google Scholar]
- 55.Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. PubMed PMID: 11427691. [DOI] [PubMed] [Google Scholar]
- 56.Sanderson I.R. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J. Nutr. 2004;134(9):2450S–2454S. doi: 10.1093/jn/134.9.2450S. PubMed PMID: 15333741. [DOI] [PubMed] [Google Scholar]
- 57.Hooper L.V., Midtvedt T., Gordon J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. PubMed PMID: 12055347. [DOI] [PubMed] [Google Scholar]
- 58.Sun Y., O’Riordan M.X. Regulation of bacterial pathogenesis by intestinal short-chain Fatty acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. PubMed PMID: 23942149; PubMed Central PMCID: PMCPMC4029053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panda A., Rao S.R., Raju M., Sunder G.S. Effect of butyric acid on performance, gastrointestinal tract health and carcass characteristics in broiler chickens. Asian-Australas. J. Anim. Sci. 2009;22(7):1026–1031. [Google Scholar]
- 60.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabol. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. PubMed PMID: 21531334; PubMed Central PMCID: PMCPMC3099420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark D.P., Cronan J.E. Escherichia coli and Salmonella: Cellular and Molecular Biology. second ed. ASM Press; Washington, DC: 1996. Two-carbon compounds and fatty acids as carbon sources; pp. 343–357. [Google Scholar]
- 62.Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015;6:6734. doi: 10.1038/ncomms7734. PubMed PMID: 25828455. [DOI] [PubMed] [Google Scholar]
- 63.Hou Y., Wang L., Yi D., Ding B., Chen X., Wang Q. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br. J. Nutr. 2014;111(10):1748–1758. doi: 10.1017/S0007114514000038. PubMed PMID: 24506942. [DOI] [PubMed] [Google Scholar]