Abstract
Whole breast radiotherapy (WBRT) after breast conserving surgery is the standard treatment to prevent recurrence and metastasis of early stage breast cancer. This study aims to compare seven WBRT techniques including conventional tangential, field-in-field (FIF), hybrid intensity-modulated radiotherapy (IMRT), IMRT, standard volumetric modulated arc therapy (STD-VMAT), non-coplanar VMAT (NC-VMAT) and multiple arc VMAT (MA-VMAT). Fifteen patients who were previously diagnosed with left-sided early stage breast cancer and treated in our clinic were selected for this study. WBRT plans were created for these patients and were evaluated based on target coverage and normal tissue toxicities. All techniques produced clinically acceptable WBRT plans. STD-VMAT delivered the lowest mean dose (1.1 ± 0.3 Gy) and the lowest maximum dose (7.3 ± 4.9 Gy) to contralateral breast, and the second lowest LAR (4.1 ± 1.4%) of secondary contralateral breast cancer. MA-VMAT delivered the lowest mean dose to lungs (4.9 ± 0.9 Gy) and heart (5.5 ± 1.2 Gy), exhibited the lowest LAR (1.7 ± 0.3%) of secondary lung cancer, NTCP (1.2 ± 0.2%) of pneumonitis, RCE (10.3 ± 2.7%), and LAR (3.9 ± 1.3%) of secondary contralateral breast cancer. NC-VMAT plans provided the most conformal target coverage, the lowest maximum lung dose (46.2 ± 4.1 Gy) and heart dose (41.1 ± 5.4 Gy), and the second lowest LAR (1.8 ± 0.4%) of secondary lung cancer and RCE (10.5 ± 2.8%). MA-VMAT and NC-VMAT could be the preferred techniques for early stage breast cancer patients after breast conserving surgery.
Keywords: Lumpectomy, whole breast radiotherapy, conventional tangential, field-in-field, intensity-modulated radiotherapy, volumetric modulated arc therapy
Introduction
Breast cancer has the highest incidence rate among women in the US other than skin cancer (www.cancer.org). Women who were diagnosed with early-stage breast cancer and had lumpectomy usually underwent whole breast radiation therapy (WBRT) after surgery, which could lower recurrence and metastasis rates and make lumpectomy as effective as mastectomy.1
The current standard of care (SOC) for WBRT in the US is using parallel-opposed tangential photon fields to treat the whole ipsilateral breast and chest wall, plus additional photon and electron fields to treat supraclavicular, axillary and internal mammary nodes when necessary.2 However, significant dose inhomogeneity can occur within the irradiated volume and can cause poor cosmetic outcomes, especially for women with large breasts.3–5 Field-in-field (FIF) technique is used sometimes to improve dose homogeneity throughout the target volume.3, 6 Intensity-modulated radiation therapy (IMRT) has been used for WBRT and can improve dose conformity and homogeneity, reduce high dose to heart and lung at the expense of increasing overall low doses,7 and has been shown to decrease acute skin toxicity.8 Hybrid IMRT (combination of open tangential and IMRT beams) has been shown to have a good balance of plan complexity and dose coverage/OAR sparing.9–11 Volumetric modulated arc therapy (VMAT) can achieve similar target coverage as IMRT, spare more normal tissues and can significant reduce treatment time.12 Multiple arc VMAT (MA-VMAT) showed good feasibility and OAR sparing for WBRT.13 Non-coplanar VMAT (NC-VMAT) has been shown to improve OAR dosimetry for post-mastectomy breast cancer14 and partial breast cancer,15–18 but has not been evaluated for WBRT.
The purpose of this study was to compare target coverage and risks of developing of radiogenic side effects for a sample of WBRT patients using various modalities, including SOC, FIF, hybrid IMRT, IMRT, VMAT, MA-VMAT and NC-VMAT. There have been multiple treatment planning studies of WBRT,19–28 but most of them did not include hybrid IMRT in the comparison although hybrid IMRT had been recommended as the optimal technique for WBRT10; with the advance of inverse planning techniques, the differences in treatment plan outcomes should be evaluated among different VMAT techniques while none of the previous studies did so; in addition, the radiobiological metrics like normal tissue complication probability (NTCP) of pneumonitis, lifetime attributable risk (LAR) of second cancers, and risk of coronary events (RCE) should be evaluated and compared among various WBRT modalities because it has been shown inclusion of non-dosimetric factors can provide a more robust comparison of different radiotherapy techniques,29 while most of the previous studies only performed dosimetric comparisons.
Methods and Materials
Patient selection
Fifteen early stage left-sided breast cancer patients presenting for WBRT without nodal involvement after breast conserving surgery were included in this study. Computed tomography (CT) scans were obtained when patients were immobilized on a breast wing board with the left arm elevated above the head and free-breathing, and all CT data were anonymized30 for this study. The target definitions were based on RTOG breast cancer Atlas and were approved by a radiation oncologist: clinical target volume (CTV) was defined as the ipsilateral breast with 5 mm skin extraction; planning target volume (PTV) was defined as CTV plus 7 mm expansion; PTV-Eval was based on PTV and defined to be limited anteriorly to exclude the part outside the patient and the first 5 mm of tissue under the skin, and posteriorly no deeper to the anterior surface of the ribs, and PTV-Eval was used in planning and for dose analysis. The contours of organs at risk (OARs) for each patient were approved by the same radiation oncologist and included lungs, whole heart and contralateral breast.
Treatment planning
The prescription dose for all patients was 50 Gy in 25 fractions. The following criteria were required for each treatment plan to be clinically acceptable: the volume of the PTV receiving at least 95% of the prescribed dose is greater than or equal to 95%; the volume of left lung receiving at least 20 Gy is less than 20%31; the volume of heart receiving at least 22.5 Gy is less than 20%.32 Maximum and mean doses for heart, lung and contralateral breast were constrained. All plans were generated in a commercial treatment planning system (TPS) (Pinnacle3 v9.8, Philips Medical Systems, Fitchburg, WI, USA).
SOC plans included two opposed tangential beams of 6, 10 or 15 MV energy depending on patient’s anatomy, and the tangential beam angles were determined by the fiducial markers placed on the skin and were usually around anterior midsternum and ipsilateral lower axilla. Collimator was rotated to shield the heart and lung, and dynamic wedges were used to minimize hotspots within PTV-Eval. FIF, hybrid IMRT and IMRT plans utilized the same beam energies as SOC plans. FIF plans used the same beam angles as SOC plans, and two to three subfields per beam were manually added using multi-leaf collimators (MLCs) to eliminate hotspots after the open field plan was created.6 Hybrid IMRT plans included a pair of open tangent fields and a pair of dynamic IMRT tangent fields, and 80% of prescription dose was delivered by open tangent beams and 20% of the prescription dose was delivered by IMRT beams.11 IMRT plans were generated using the direct machine parameter optimization (DMPO) algorithm, and included seven coplanar beam equidistantly distributed in a sector of 180° that avoided direct exposure to the contralateral breast. All STD-VMAT, NC-VMAT and MA-VMAT plans used 6 MV beam energy and were generated using the SmartArc optimization algorithm. STD-VMAT plans utilized two coplanar partial arcs: the first arc was planned to be delivered counterclockwise (CCW) with starting and stopping gantry angles same as tangential fields, and the second arc was planned to be delivered clockwise (CW) over the same range of gantry angle. NC-VMAT plans utilized two partial arcs: the first arc was planned to be delivered CCW with starting and stopping gantry angles same as tangent fields and with 20° couch angle, and the second arc was planned to be delivered CW over the same range of gantry angle and with 340° couch angle. The collimator was rotated to align with the long axis of PTV in both arcs.33 MA-VMAT plans consisted of six partial arcs (ARC01 to ARC06), each with 50°gantry rotations.13 ARC01 to ARC03 were delivered CW and ARC04 to ARC06 were delivered CCW. The starting angle of ARC01 and stopping angle of ARC03 were the same as SOC technique. The collimator was always rotated to align with the long axis of PTV in each arc.
Plan comparison metrics
Dosimetric parameters were evaluated for target, lungs, heart and contralateral breast. Dose homogeneity index (DHI)34 and conformity index (CI)35 were evaluated for PTV-Eval. Risks of radiogenic side effects were assessed: LAR was computed for secondary lung cancer and contralateral breast cancer using BEIR VII model36 and organ equivalent dose (OED)37. NTCP for pneumonitis was evaluated using Lyman-Kutcher-Burman (LKB) model38–40. Dose-response model in Darby et al.41 was used to evaluate RCE for each patient and Reynolds risk model42 was used to calculate the baseline risk assuming medium risk type. More details of dose-risk models can be found in our previous study.14
The post hoc Tukey test was used to determine the statistical significance of the differences between two WBRT techniques. All statistical analyses were conducted with R software (version 3.2.3) and any difference was considered significant when p < 0.05.
Results
The axial dose distributions and DVHs for a typical WBRT patient are shown in Figs. 1 and 2. Table 1 lists PTV and OARs evaluation metrics for various WBRT techniques. The results of statistical tests are shown in Table 2 where the grey color indicates statistically significance (p values <0.05), e.g., SOC has significantly higher V107% for PTV, Dmean, Dmax, V20 and NTCP for lung, V22.5 and V30 for heart, Dmean and V5 for contralateral breast compared to IMRT, STD-VMAT, NC-VMAT and MA-VMAT plans; SOC has significantly higher Dmax and V107% for PTV compared to FIF and hybrid IMRT plans; STD-VMAT has significantly higher V5 for lung and heart compared to other six WBRT techniques; no statistically significance shown in any comparison between FIF and hybrid IMRT.
Fig. 1.
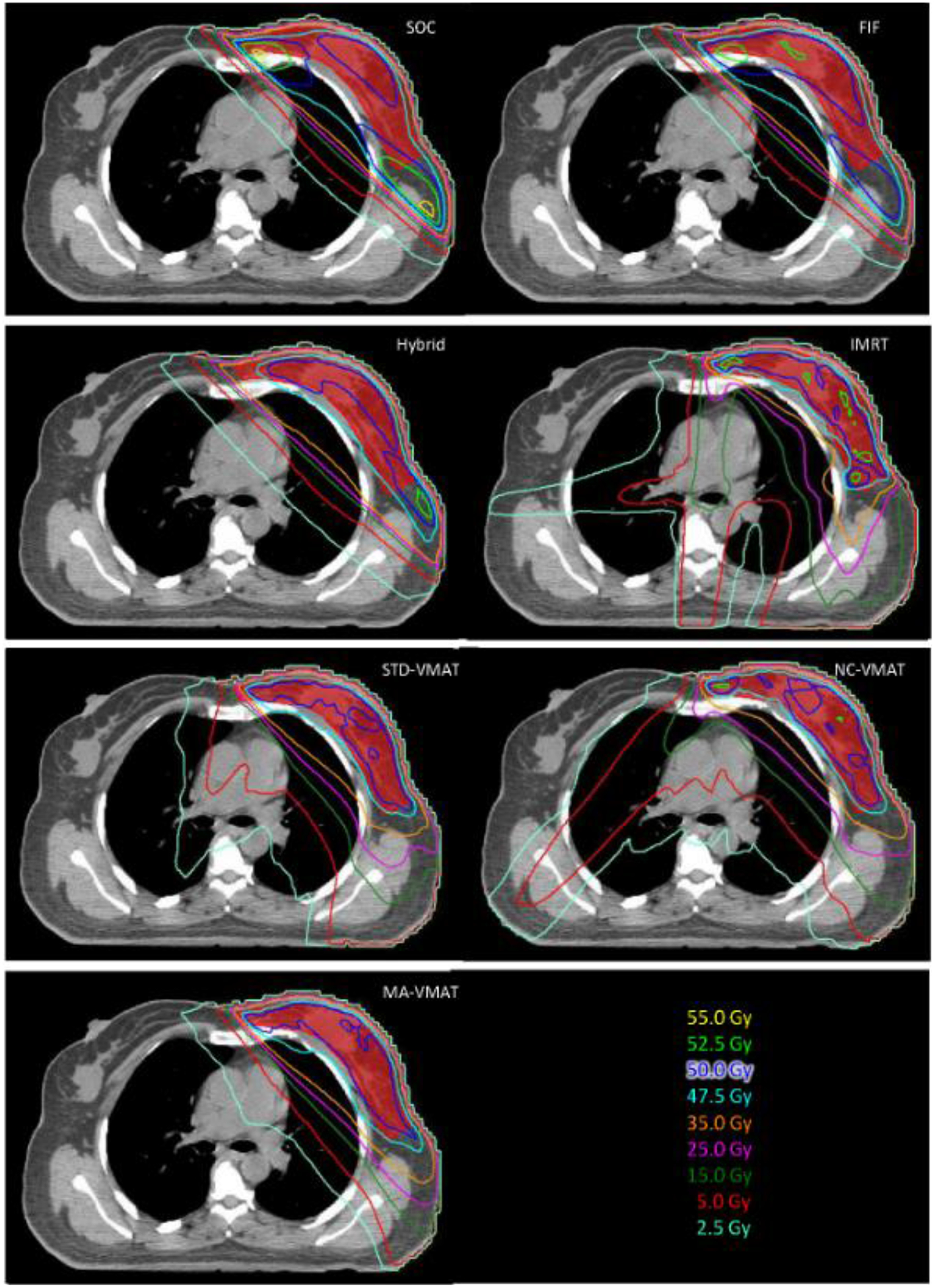
Axial view of isodose distribution for SOC, FIF, Hybrid IMRT, IMRT, STD-VMAT, NC-VMAT and MA-VMAT plans for a typical WBRT patient. The red color wash represents the PTV-Eval.
Fig. 2.

DVHs for SOC, FIF, Hybrid IMRT, IMRT, STD-VMAT, NC-VMAT and MA-VMAT plans for a typical WBRT patient.
Table 1.
MU, PTV and OAR evaluation metrics (mean ± standard deviation). DHI values have more significant figures to show the difference among different techniques. SOC: standard of care; FIF: field in field; Hybrid: hybrid intensity-modulated radiation therapy; IMRT: intensity-modulated radiation therapy; STD-VMAT: standard volumetric modulated arc therapy; NC-VMAT: non-coplanar VMAT; MA-VMAT: multiple arc VMAT; MU: monitor unit; PTV: planning target volume; DHI: Dose homogeneity index; CL breast: contralateral breast. LAR: lifetime attributable risk; RCE: risk of coronary events; NTCP: normal tissue complication probability.
| SOC | FIF | Hybrid | IMRT | STD-VMAT | NC-VMAT | MA-VMAT | |
|---|---|---|---|---|---|---|---|
| Average total MU | 7343±962 | 5647±227 | 8673±1506 | 19300±2471 | 10263±1049 | 10397±1549 | 11523±1167 |
| PTV | |||||||
| Dmean (Gy) | 50.7±0.7 | 50.6±0.5 | 50.4±0.6 | 50.1±0.3 | 50.0 ±0.4 | 49.9±0.4 | 50.0±0.4 |
| Dmax (Gy) | 55.5±1.7 | 53.8±0.9 | 53.8±0.9 | 56.7±0.9 | 55.0±1.4 | 54.7±1.0 | 55.2±1.3 |
| V107% (%) | 0.1±0.1 | 0.0±0.1 | 0.0±0.1 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| CI | 0.5±0.1 | 0.5±0.1 | 0.6±0.1 | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 |
| DHI | 0.150±0.033 | 0.132±0.021 | 0.126±0.024 | 0.142±0.029 | 0.157±0.056 | 0.158±0.044 | 0.164±0.046 |
| Total lung | |||||||
| Dmean (Gy) | 6.7±1.0 | 6.4±0.8 | 6.1±1.0 | 5.9±0.9 | 6.0±0.9 | 5.4±1.1 | 4.9±0.9 |
| Dmax (Gy) | 53.3±1.7 | 51.4±1.4 | 50.3±1.5 | 48.3±1.5 | 47.9±2.3 | 46.2±4.1 | 50.8±2.8 |
| V5 | 18.3±2.2 | 18.6±2.3 | 17.7±2.0 | 26.4±5.0 | 27.0±7.1 | 24.8±8.2 | 19.6±4.2 |
| V10 | 14.8±2.0 | 15.0±2.2 | 14.4±2.0 | 15.1±3.1 | 14.3±2.7 | 12.8±3.6 | 12.5±3.3 |
| V20 | 12.7±2.0 | 12.6±2.1 | 12.2±1.9 | 7.9±2.6 | 7.8±1.6 | 6.6±1.9 | 7.3±1.6 |
| NTCP (%) | 1.8±0.4 | 1.7±0.4 | 1.6±0.3 | 1.5±0.3 | 1.5±0.3 | 1.3±0.3 | 1.2±0.2 |
| LAR (%) | 2.2±0.4 | 2.2±0.4 | 2.0±0.3 | 2.0±0.3 | 2.0±0.4 | 1.8±0.4 | 1.7±0.3 |
| Heart | |||||||
| Dmean (Gy) | 9.6±3.7 | 8.1±3.7 | 8.1±2.8 | 7.4±1.3 | 7.8± 1.5 | 5.8±1.0 | 5.5±1.2 |
| Dmax (Gy) | 51.7±2.2 | 50.1±1.7 | 49.3±1.4 | 43.7±6.2 | 44.5±3.3 | 41.0±5.4 | 45.0±4.1 |
| V5 | 25.3±10.1 | 25.7±9.4 | 23.9±8.7 | 48.3±14.0 | 53.2±8.5 | 30.5±9.0 | 22.1±9.0 |
| V10 | 19.8±8.8 | 19.0±8.0 | 18.4±7.7 | 20.1±4.9 | 18.7±5.7 | 11.7±3.5 | 9.7±2.7 |
| V22.5 | 16.6±8.0 | 14.9±6.9 | 14.7±6.6 | 4.7±3.2 | 6.2±2.7 | 4.3±1.9 | 5.0±1.8 |
| V30 | 15.1±7.5 | 13.3±6.4 | 12.7±5.9 | 2.7±2.9 | 3.9±2.2 | 2.0±1.4 | 3.2±1.9 |
| RCE (%) | 12.4±3.5 | 11.5±3.1 | 11.6±3.1 | 11.4±3.3 | 11.6±3.2 | 10.5±2.8 | 10.3±2.7 |
| CL breast | |||||||
| Dmean (Gy) | 2.8±2.5 | 1.9±1.8 | 1.6±1.2 | 1.4±0.7 | 1.1±0.3 | 1.2±0.7 | 1.2±0.4 |
| Dmax (Gy) | 48.3±7.2 | 45.6±6.8 | 42.8±6.7 | 17.0±4.3 | 7.3±4.9 | 10.3±8.5 | 12.0±8.3 |
| V5 | 4.2±2.6 | 3.8±3.0 | 4.0±3.0 | 0.2±0.3 | 0.3±0.7 | 0.2±0.5 | 0.1±0.1 |
| LAR (%) | 5.8±4.9 | 5.7±2.7 | 5.3±4.7 | 4.8±2.4 | 4.1±1.4 | 4.1±2.2 | 3.9± 1.3 |
Table 2.
Statistic comparison of seven WBRT techniques using post hoc Tukey test. Grey color indicates statistically significant (p values <0.05)
| Variable | SOC vs FIF | SOC vs Hybrid | SOC vs IMRT | SOC vs STD-VMAT | SOC vs NC-VMAT | SOC vs MA-VMAT | FIF vs Hybrid | FIF vs IMRT | FIF vs STD-VMAT | FIF vs NC-VMAT | FIF vs MA-VMAT | Hybrid vs IMRT | Hybrid vs STD-VMAT | Hybrid vs NC-VMAT | Hybrid vs MA-VMAT | IMRT vs STD-VMAT | IMRT vs NC-VMAT | IMRT vs MA-VMAT | STD-VMAT vs NC-VMAT | STD-VMAT vs MA-VMAT | NC-VMAT vs MA-VMAT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV | |||||||||||||||||||||
| Dmean | |||||||||||||||||||||
| Dmax | |||||||||||||||||||||
| V107% (%) | |||||||||||||||||||||
| CI | |||||||||||||||||||||
| DHI | |||||||||||||||||||||
| Lung | |||||||||||||||||||||
| Dmean | |||||||||||||||||||||
| Dmax | |||||||||||||||||||||
| V5 | |||||||||||||||||||||
| V10 | |||||||||||||||||||||
| V20 | |||||||||||||||||||||
| NTCP | |||||||||||||||||||||
| LAR (%) | |||||||||||||||||||||
| Heart | |||||||||||||||||||||
| Dmean | |||||||||||||||||||||
| Dmax | |||||||||||||||||||||
| V5 | |||||||||||||||||||||
| V10 | |||||||||||||||||||||
| V22.5 | |||||||||||||||||||||
| V30 | |||||||||||||||||||||
| RCE (%) | |||||||||||||||||||||
| CL breast | |||||||||||||||||||||
| Dmean | |||||||||||||||||||||
| Dmax | |||||||||||||||||||||
| V5 | |||||||||||||||||||||
| LAR (%) |
By combining the results from Table 1 and Table 2, we found that all seven WBRT techniques analyzed in this study meet clinical requirement of PTV coverage; SOC plans introduce significantly larger hot spots in PTV by showing the highest V107%, and deliver relatively higher dose to OARs than inverse planning techniques (IMRT, STD-VMAT, NC-VMAT and MA-VMAT); FIF and hybrid IMRT plans exhibit better PTV coverage than SOC, deliver relatively lower dose to OARs than SOC, and both show relatively better dose homogeneity in PTV than the other five techniques; STD-VMAT plans provide the lowest Dmean and Dmax for contralateral breast, and the second lowest LAR of secondary cancer in contralateral breast, but significantly increase the low dose cloud like V5 for lung and heart; MA-VMAT plans show the lowest Dmean, V10, NTCP and LAR for lung, the lowest V5 and LAR for contralateral breast, and the lowest Dmean, V5, V10, and RCE for heart; NC-VMAT plans provide the most conformal target coverage, the lowest Dmax, V20 and the second lowest NTCP and LAR for lung, the lowest Dmax, V22.5, V30 and the second lowest RCE for heart compared with other techniques.
Discussion
We evaluated seven WBRT techniques for treating left-sided lumpectomy breast cancer patients. All seven techniques provide clinical acceptable dose coverage to the target volume. For the two forward planning techniques, FIF plans not only show better PTV coverage but also reduce OAR dose than SOC. Five inverse planning techniques show lower OAR doses than the two forward planning techniques. STD-VMAT plans spare contralateral breast well at the cost of larger low dose cloud for lung and heart. MA-VMAT plans show the most optimal OARs doses and minimum risk of developing late side effects among all techniques. NC-VMAT plans provide the most conformal PTV coverage and excellent sparing of lung and heart.
There are plenty of WBRT planning studies in the literature. Considering it is difficult to compare studies with different target definitions, we compared our study with previous work that had the same PTV delineation based on RTOG as ours (Table 3): Descovich et al.43 concluded that hybrid IMRT can reduce the hot spot within PTV compared to FIF for left-sided breast cancer patients and can provide better coverage, which is consistent with our results; Jin et al.19 reported that tangential IMRT has the best balance of target coverage and normal tissue sparing compared with conventional tangential beams, FIF, multi-beam IMRT and VMAT for small breast size, while our study shows IMRT provides inferior target coverage or OAR sparing than VMAT especially the advanced VMAT techniques, which is mainly because the mean PTV volume in our study (910.2 ± 439.8 cc) is much larger than theirs (360.8 ± 149.1 cc). These results show that tangential IMRT may not be the optimal technique for all WBRT patients, and its application should be assessed based on patient’s anatomy; Schubert et al.22 and Haciislamoglu et al.25 both concluded that Tomotherapy (TOMO) may reduce high doses to heart and lung at the cost of increased low dose cloud which may lead to an increased probability of radiogenic side effects, and Han et al.21 concluded that TOMO is recommended for WBRT compared to SOC, FIF, IMRT and VMAT since it exhibits lowest total LAR for OARs. Our study didn’t evaluate TOMO, but shows NC-VMAT and MA-VMAT have comparable or better sparing of OARs than TOMO, e.g. NC-VMAT and MA-VMAT deliver mean lung dose of 5.4 ± 1.1 Gy and 4.9 ± 0.9 Gy, respectively, and mean contralateral breast dose of 1.2 ± 0.7 Gy and 1.2 ± 0.4 Gy, respectively, while Haciislamoglu et al.25 reported mean lung dose of 9.6 ± 2.0 Gy and mean contralateral breast dose of 3.1 ± 0.4 Gy for TOMO. This suggests that NC-VMAT and MA-VMAT could be used as good alternatives when TOMO System is not available; Zhang et al.27 evaluated different IMRT techniques and recommended FIF-DMPO-IMRT because it can reduce doses to lungs and heart and decrease treatment time. Their FIF-DMPO-IMRT consists of 70~80% FIF and 20%~30% IMRT, which is similar to our hybrid IMRT technique that has 80% open tangent beams and 20% IMRT, but they required 95% volume of PTV to receive 100% of prescription dose which makes their dosimetric results not comparable to ours; Viren et al.26 concluded that both tangential VMAT (tVMAT) with two dual arcs of 50°−60° and continuous VMAT (cVMAT) with a dual arc of 240° have improved DHI within PTV and better sparing of heart and ipsilateral lung tissues compared to FIF and tangential IMRT, and cVMAT provided the best target coverage at the cost of significantly increased dose to contralateral breast. In our study, STD-VMAT (dual arc of approximately 180°) also has improved DHI within PTV and better sparing of heart and lung compare to FIF, and STD-VMAT has much better sparing of contralateral breast (mean dose 1.1 Gy) than their cVMAT (mean dose 2.6 Gy) and is similar to their tVMAT (mean dose 1.2 Gy), which suggests that smaller arcs of VMAT can lower the dose to contralateral breast.
Table 3.
Comparison with previous WBRT planning studies that have the same PTV definition as ours.
| Reference | Num. of patients | Breast site | Techniques compared | Key findings |
|---|---|---|---|---|
| Descovich et al. (2010) | 15 | Left | FIF, hybrid IMRT | Hybrid IMRT is preferred since it can reduce hot spot, provide better coverage and require less planning time. |
| Schubert et al. (2011) | 10 | Left | SOC, FIF, tangential IMRT (2 Fields), TOMO, topotherapy | TOMO, topotherapy and tangential IMRT can reduce high dose to target and normal tissue; TOMO results in increased low doses to normal tissue. |
| Jin et al. (2013) | 20 | Left | SOC, FIF, tangential IMRT (2 Fields), IMRT (7 Fields), VMAT (starting and ending angles were same as tangential beam angles) | Tangential IMRT is recommended since it has improved DHI and reduced dose to heart and lung. |
| Viren et al. (2015) | 10 | Left | FIF, tangential IMRT (2 Fields), tangential VMAT (two dual arcs of 50°–60°), continuous VMAT (dual arc of 240°) | Both VMAT techniques show improved DHI and better sparing of heart and ipsilateral lung. Continuous VMAT provides best dose coverage at the cost of significantly increased dose to contralateral breast. |
| Haciislamoglu et al. (2015) | 15 | Left | SOC, FIF, 9-field IMRT, TOMO, VMAT (Starting and ending angles of the arcs were 10° posterior to tangential fields) | TOMO shown reduced high and mean doses to heart and lung at the cost of increased low dose cloud. |
| Han et al. (2016) | 10 | Left and right | SOC, FIF, IMRT (10 to 12 fields), VMAT (3–4 partial arcs spanned from 305° to 152° for the left, 60° to 214° for the right), TOMO | TOMO is recommended since it provides the lowest LAR for all surrounding OARs. |
| Zhang et al. (2018) | 50 | Left | 5-field IMRT, 6-field IMRT, FIF-DMPO-IMRT | FIF-DMPO-IMRT is recommended due to reduced heart and lung doses and treatment time. |
| Our study | 15 | Left | SOC, FIF, hybrid, IMRT, VMAT (starting and ending angle were same as tangential beam angles), MA-VMAT, NC-VMAT | MA-VMAT and NC-VMAT are recommended due to reduced doses and risks for heart, lung and contralateral breast. |
Jeulink et al.10 illustrated that hybrid IMRT is most optimal WBRT technique compared with full IMRT, STD-VMAT and MA-VMAT for the best reduction of mean and low OARs doses, while it is not the most optimal choice in our study. This is possibly because only two tangential IMRT fields are used for hybrid IMRT in our study whereas four tangential IMRT fields were used in theirs. Moreover, left anterior descending coronary artery (LAD) was contoured as an OAR for plan optimization in their study, which may further limit dose to the heart but may significantly increase workload for physicians and dosimetrists. Additionally, the PTV delineation is different since the boost PTV was included in their analysis. These results suggest that hybrid IMRT may not be the best choice for all WBRT patients and its application should be determined based on all clinical factors.
Among all WBRT techniques in our study, MA-VMAT and NC-VMAT have shown superior OARs sparing. Tsai et al.13 reported mean heart dose of 7.6 ± 1.4 Gy and lung dose of 5.6 ± 0.4 Gy for MA-VMAT, which were slightly higher than our mean heart dose (5.5 ± 1.2 Gy) and lung dose (4.9 ± 0.9 Gy). This can be explained by the fact that a slightly higher prescription dose (50.4 Gy delivered in 28 fractions) was used in their study, and different dose constrains were used, e.g. our mean heart dose limit is 7 Gy while their mean heart dose limit was 9 Gy. When multiple arcs were used and collimator angle was adjusted for each arc, treatment plans could be further optimized since more degrees of freedom were provided for MA-VMAT. Smyth et al.44 summarized the recent advancement in non-coplanar radiotherapy and listed different techniques including static couch NC-VMAT, coronal VMAT, trajectory VMAT and dynamic wave arc etc. Our study utilizes static couch NC-VMAT, according to definitions in Smyth et al.44, for WBRT for the first time and shows it can provide excellent sparing of lung and heart compared to other WBRT techniques, which demonstrates that OARs can be spared more by adjusting the couch angle to minimize direct irradiation.
There is a lack of clinical outcome data of radiogenic late effects for advanced WBRT techniques, but our calculated RCE and LARs for SOC WBRT show good agreement with clinical data for breast cancer patients who went through SOC WBRT: Taylor et al.45 reported the annual risk of developing radiogenic lung cancer and contralateral breast cancer was 0.2% and 0.36%, respectively, and our calculated annual risk is 0.22% and 0.38% for SOC; Hooning et al.46 reported the annual cardiac toxicity was 1.19% whereas our estimation is 1.23% for SOC. Based on these good agreements, we expect our estimated radiogenic risks values for advanced WBRT techniques to be reasonable. Prospective clinical studies can validate our calculations and further illustrate the benefit of advanced WBRT techniques.
Cardiac toxicity is a major concern for breast cancer patients who received radiotherapy, especially for left-sided breast cancer patients.41 Our study shows comparable mean heart dose for FIF, IMRT and VMAT as those reported by Viren et al.26. However, our study shows higher mean heart dose for conventional SOC, FIF, IMRT and VMAT than those in Jin et al.19, which is possibly because their extra dose constrains on coronary artery has further limited heart dose, and the larger breast size in our study could inevitably lead to larger fields that will induce higher heart dose in order to provide enough PTV coverage. Furthermore, all patients were treated in supine position in our study, while literature47, 48 has shown that breast irradiation in prone position may result in lower risk of cardiac toxicity and improve dose homogeneity within PTV compared to standard irradiation in supine position. Further studies are needed to evaluate advanced WBRT techniques in prone treatment position.
In order to further reduce heart irradiation, deep inspiration breath hold (DIBH) has been implemented for WBRT and studies have shown that DIBH can minimize irradiation of heart without compromise target coverage for most left-sided breast cancer patient49–55. However, not all patients could benefit from it, e.g. Dell’Oro et al.56 recently reported that DIBH may not be recommended for some patients due to little dosimetric benefit. Several studies54, 56, 57 reported criteria of selecting breast cancer patients for DIBH, including patient’s age, ability to hold breath for a specific duration of time, total lung volume, in-field heart volume, sternal excursion etc. In our study, the fifteen patients were not selected for DIBH in the clinic mainly due to the limited dosimetric benefit for them. The benefit of various WBRT techniques for DIBH patients will be investigated by our group in the near future.
One limitation of our study is that we did not compare the WBRT techniques in terms of planning or treatment time, quality assurance (QA) workload, and patient’s comfort. Among the seven WBRT techniques analyzed in this study, the delivery efficiency may be a concern for non-coplanar VMAT plans,44, 58 and the rotation of gantry and couch between beams can take considerable amount of time. The delivery time for non-coplanar plans can be shorten by implementing the automated machine transitions between beams, e.g., Liang et al.18 illustrated that coronal VMAT which had dynamic patient couch rotation can be delivered in 4.5 minutes for a 3.85 Gy fraction for accelerated partial breast irradiation. Moreover, IMRT, STD-VMAT, NC-VMAT and MA-VMAT require more MUs than SOC, FIF and hybrid IMRT, as shown in Table 1. The increased number of MUs will inevitably result in longer treatment time and increased leakage radiation which may increase patient’s discomfort and risk of developing radiogenic side effects. The planning time for the seven WBRT techniques was comparable. Contouring for one patient took 60 minutes on average, and treatment planning for a patient using one technique took another 45 minutes on average, which is comparable to the literature,7 except for MA-VMAT which needed slightly longer planning time (about 60 minutes) due to increased small fields that required longer optimization time to achieve the objectives. Hybrid IMRT, IMRT, STD-VMAT, NC-VMAT and MA-VMAT all require QA of dosimetric output for every treatment plan,59 and NC-VMAT requires extra specific QA procedures on couth rotation which may further increase the workload of patient’s specific QA.60
Conclusions
Seven WBRT techniques were evaluated in this study. Among them, NC-VMAT was evaluated for WBRT for the first time and showed excellent sparing of lung and heart, STD-VMAT could reduce dose to the contralateral breast, and MA-VMAT showed the best OAR sparing. MA-VMAT and NC-VMAT might be the appropriate WBRT techniques for early stage breast cancer patients after breast conserving surgery.
Acknowledgement
This work was partially supported by National Institutes of Health (NIH) through a National Cancer Institute (NCI) grant K22CA204464 and Louisiana State University (LSU) Economic Development Assistantship Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest.
References
- 1.Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, Halberg F, Hoffman K, Horst K, Moran J, Patton C, Perlmutter J, Warren L, Whelan T, Wright JL and Jagsi R, “Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline,” Pract Radiat Oncol 8, 145–152 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Halperin EC, Perez CA and Brady LW, Perez and Brady’s Principles and Practice of Radiation Oncology, 5th Edition (LWW, Philadelphia, PA, 2008). [Google Scholar]
- 3.Cox JD and Ang KK, Radiation Oncology: rationale, technique, results. 9th ed (MOSBY ELSEVIER, Philadelphia, PA, 2010). [Google Scholar]
- 4.Moody AM, Mayles WP, Bliss JM, A’Hern RP, Owen JR, Regan J, Broad B and Yarnold JR, “The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity,” Radiother Oncol 33, 106–112 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith C, Haviland J, Tsang Y, Sydenham M and Yarnold J, “Large breast size as a risk factor for late adverse effects of breast radiotherapy: is residual dose inhomogeneity, despite 3D treatment planning and delivery, the main explanation?,” Radiother Oncol 100, 236–240 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Lo YC, Yasuda G, Fitzgerald TJ and Urie MM, “Intensity modulation for breast treatment using static multi-leaf collimators,” Int J Radiat Oncol Biol Phys 46, 187–194 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Beckham WA, Popescu CC, Patenaude VV, Wai ES and Olivotto IA, “Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer?,” Int J Radiat Oncol Biol Phys 69, 918–924 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L and Nicolaou N, “Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer,” Am J Clin Oncol 29, 66–70 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Farace P, Zucca S, Solla I, Fadda G, Durzu S, Porru S, Meleddu G, Deidda MA, Possanzini M, Orru S and Lay G, “Planning hybrid intensity modulated radiation therapy for whole-breast irradiation,” Int J Radiat Oncol Biol Phys 84, e115–122 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Jeulink M, Dahele M, Meijnen P, Slotman BJ and Verbakel WF, “Is there a preferred IMRT technique for left-breast irradiation?,” J Appl Clin Med Phys 16, 5266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo CS, Urie MM and Fitzgerald TJ, “Hybrid IMRT plans--concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time,” Int J Radiat Oncol Biol Phys 61, 922–932 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, Wai ES and Otto K, “Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes,” Int J Radiat Oncol Biol Phys 76, 287–295 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Tsai PF, Lin SM, Lee SH, Yeh CY, Huang YT, Lee CC and Hong JH, “The feasibility study of using multiple partial volumetric-modulated arcs therapy in early stage left-sided breast cancer patients,” J Appl Clin Med Phys 13, 3806 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y, Bourgeois D, Guo B and Zhang R, “Postmastectomy radiotherapy for left-sided breast cancer patients: Comparison of advanced techniques,” Med Dosim 45, 34–40 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaitelman SF, Kim LH, Yan D, Martinez AA, Vicini FA and Grills IS, “Continuous arc rotation of the couch therapy for the delivery of accelerated partial breast irradiation: a treatment planning analysis,” Int J Radiat Oncol Biol Phys 80, 771–778 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Popescu CC, Beckham WA, Patenaude VV, Olivotto IA and Vlachaki MT, “Simultaneous couch and gantry dynamic arc rotation (CG-Darc) in the treatment of breast cancer with accelerated partial breast irradiation (APBI): a feasibility study,” J Appl Clin Med Phys 14, 4035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahimian B, Yu V, Horst K, Xing L and Hristov D, “Trajectory modulated prone breast irradiation: a LINAC-based technique combining intensity modulated delivery and motion of the couch,” Radiother Oncol 109, 475–481 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Liang J, Atwood T, von Eyben R, Fahimian B, Chin E, Horst K, Otto K and Hristov D, “Trajectory Modulated Arc Therapy: A Fully Dynamic Delivery With Synchronized Couch and Gantry Motion Significantly Improves Dosimetric Indices Correlated With Poor Cosmesis in Accelerated Partial Breast Irradiation,” Int J Radiat Oncol Biol Phys 92, 1148–1156 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Jin GH, Chen LX, Deng XW, Liu XW, Huang Y and Huang XB, “A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: conventional tangential field, filed-in-filed, tangential-IMRT, multi-beam IMRT and VMAT,” Radiat Oncol 8, 89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, He M, Cheng G, Han D, Wu N, Shi D, Zhao Z and Jin J, “A comparative dosimetric study of left sided breast cancer after breast-conserving surgery treated with VMAT and IMRT,” Radiat Oncol 10, 231 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han EY, Paudel N, Sung J, Yoon M, Chung WK and Kim DW, “Estimation of the risk of secondary malignancy arising from whole-breast irradiation: comparison of five radiotherapy modalities, including TomoHDA,” Oncotarget 7, 22960–22969 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert LK, Gondi V, Sengbusch E, Westerly DC, Soisson ET, Paliwal BR, Mackie TR, Mehta MP, Patel RR, Tome WA and Cannon GM, “Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy,” Radiother Oncol 100, 241–246 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Xie X, Ouyang S, Wang H, Yang W, Jin H, Hu B and Shen L, “Dosimetric comparison of left-sided whole breast irradiation with 3D-CRT, IP-IMRT and hybrid IMRT,” Oncol Rep 31, 2195–2205 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Shiau AC, Hsieh CH, Tien HJ, Yeh HP, Lin CT, Shueng PW and Wu LJ, “Left-sided whole breast irradiation with hybrid-IMRT and helical tomotherapy dosimetric comparison,” Biomed Res Int 2014, 741326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haciislamoglu E, Colak F, Canyilmaz E, Dirican B, Gurdalli S, Yilmaz AH, Yoney A and Bahat Z, “Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric arc therapy,” Phys Med 31, 360–367 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Viren T, Heikkila J, Myllyoja K, Koskela K, Lahtinen T and Seppala J, “Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy,” Radiat Oncol 10, 79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang HW, Hu B, Xie C and Wang YL, “Dosimetric comparison of three intensity-modulated radiation therapies for left breast cancer after breast-conserving surgery,” J Appl Clin Med Phys 19, 79–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Wang J, Hu Z, Tian Y, Ma P, Li S, Dai J and Wang S, “Locoregional irradiation including internal mammary nodal region for left-sided breast cancer after breast conserving surgery: Dosimetric evaluation of 4 techniques,” Med Dosim (2018). [DOI] [PubMed] [Google Scholar]
- 29.Allen Li X, Alber M, Deasy JO, Jackson A, Ken Jee KW, Marks LB, Martel MK, Mayo C, Moiseenko V, Nahum AE, Niemierko A, Semenenko VA and Yorke ED, “The use and QA of biologically related models for treatment planning: short report of the TG-166 of the therapy physics committee of the AAPM,” Med Phys 39, 1386–1409 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Newhauser WD, Jones T, Swerdloff S, Newhauser W, Cilia M, Carver R, Halloran A and Zhang R, “Anonymization of DICOM electronic medical records for radiation therapy,” Computers in Biology and Medicine 53, 134–140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, Martel MK and Jackson A, “Radiation dose-volume effects in the lung,” Int J Radiat Oncol Biol Phys 76, S70–76 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardenbergh PH, Munley MT, Bentel GC, Kedem R, Borges-Neto S, Hollis D, Prosnitz LR and Marks LB, “Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results,” Int J Radiat Oncol Biol Phys 49, 1023–1028 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Nicolini G, Fogliata A and Cozzi L, “Critical appraisal of a non-coplanar technique for radiotherapy of breast minimising lung involvement,” Radiother Oncol 76, 319–325 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Wu Q, Mohan R, Morris M, Lauve A and Schmidt-Ullrich R, “Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results,” Int J Radiat Oncol Biol Phys 56, 573–585 (2003). [DOI] [PubMed] [Google Scholar]
- 35.van’t Riet A, Mak AC, Moerland MA, Elders LH and van der Zee W, “A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate,” Int J Radiat Oncol Biol Phys 37, 731–736 (1997). [DOI] [PubMed] [Google Scholar]
- 36.National Research Council Health Risks from Exposure to Low Levels of Ionizing Radation: BEIR VII - Phase 2. Washington, DC: The National Academies Press, 2006. [PubMed] [Google Scholar]
- 37.Schneider U, Sumila M and Robotka J, “Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy,” Theor Biol Med Model 8, 27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyman JT, “Complication probability as assessed from dose-volume histograms,” Radiat Res Suppl 8, S13–19 (1985). [PubMed] [Google Scholar]
- 39.Kutcher GJ and Burman C, “Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method,” Int J Radiat Oncol Biol Phys 16, 1623–1630 (1989). [DOI] [PubMed] [Google Scholar]
- 40.Seppenwoolde Y, Lebesque JV, de Jaeger K, Belderbos JS, Boersma LJ, Schilstra C, Henning GT, Hayman JA, Martel MK and Ten Haken RK, “Comparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probability,” Int J Radiat Oncol Biol Phys 55, 724–735 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C and Hall P, “Risk of ischemic heart disease in women after radiotherapy for breast cancer,” N Engl J Med 368, 987–998 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Buring JE, Rifai N and Cook NR, “Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score,” JAMA 297, 611–619 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Descovich M, Fowble B, Bevan A, Schechter N, Park C and Xia P, “Comparison between hybrid direct aperture optimized intensity-modulated radiotherapy and forward planning intensity-modulated radiotherapy for whole breast irradiation,” Int J Radiat Oncol Biol Phys 76, 91–99 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Smyth G, Evans PM, Bamber JC and Bedford JL, “Recent developments in non-coplanar radiotherapy,” Br J Radiol 92, 20180908 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J, Dodwell D, Ewertz M, Gray R, Jagsi R, Pierce L, Pritchard KI, Swain S, Wang Z, Wang Y, Whelan T, Peto R and McGale P, “Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials,” J Clin Oncol 35, 1641–1649 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW and van Leeuwen FE, “Long-term risk of cardiovascular disease in 10-year survivors of breast cancer,” J Natl Cancer Inst 99, 365–375 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Merchant TE and McCormick B, “Prone position breast irradiation,” Int J Radiat Oncol Biol Phys 30, 197–203 (1994). [DOI] [PubMed] [Google Scholar]
- 48.Stegman LD, Beal KP, Hunt MA, Fornier MN and McCormick B, “Long-term clinical outcomes of whole-breast irradiation delivered in the prone position,” Int J Radiat Oncol Biol Phys 68, 73–81 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Bruzzaniti V, Abate A, Pinnaro P, D’Andrea M, Infusino E, Landoni V, Soriani A, Giordano C, Ferraro A and Strigari L, “Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast cancer,” J Exp Clin Cancer Res 32, 88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeung R, Conroy L, Long K, Walrath D, Li H, Smith W, Hudson A and Phan T, “Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation,” Radiat Oncol 10, 200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyth LM, Knight KA, Aarons YK and Wasiak J, “The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: a systematic review,” J Med Radiat Sci 62, 66–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vikstrom J, Hjelstuen MH, Mjaaland I and Dybvik KI, “Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage,” Acta Oncol 50, 42–50 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Osman SO, Hol S, Poortmans PM and Essers M, “Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation,” Radiother Oncol 112, 17–22 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Latty D, Stuart KE, Wang W and Ahern V, “Review of deep inspiration breath-hold techniques for the treatment of breast cancer,” J Med Radiat Sci 62, 74–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA and Wong JW, “Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation,” Int J Radiat Oncol Biol Phys 55, 392–406 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Dell’Oro M, Giles E, Sharkey A, Borg M, Connell C and Bezak E, “A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique,” Cancers (Basel) 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim T, Reardon K, Trifiletti DM, Geesey C, Sukovich K, Crandley E, Read PW and Wijesooriya K, “How dose sparing of cardiac structures correlates with in-field heart volume and sternal displacement,” J Appl Clin Med Phys 17, 60–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu VY, Landers A, Woods K, Nguyen D, Cao M, Du D, Chin RK, Sheng K and Kaprealian TB, “A Prospective 4pi Radiation Therapy Clinical Study in Recurrent High-Grade Glioma Patients,” Int J Radiat Oncol Biol Phys 101, 144–151 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Ezzell GA, Galvin JM, Low D, Palta JR, Rosen I, Sharpe MB, Xia P, Xiao Y, Xing L and Yu CX, “Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee,” Med Phys 30, 2089–2115 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Yu VY, Fahimian BP, Xing L and Hristov DH, “Quality control procedures for dynamic treatment delivery techniques involving couch motion,” Med Phys 41, 081712 (2014). [DOI] [PubMed] [Google Scholar]


