Abstract
Over the past two decades, research has revealed that genetic factors shape the propensity for aggressive, antisocial, and violent behavior. The best-documented gene implicated in aggression is MAOA (Monoamine oxidase A), which encodes the key enzyme for the degradation of serotonin and catecholamines. Congenital MAOA deficiency, as well as low-activity MAOA variants, has been associated with a higher risk for antisocial behavior (ASB) and violence, particularly in males with a history of child maltreatment. Indeed, the interplay between low MAOA genetic variants and early-life adversity is the best-documented gene × environment (G × E) interaction in the pathophysiology of aggression and ASB. Additional evidence indicates that low MAOA activity in the brain is strongly associated with a higher propensity for aggression; furthermore, MAOA inhibition may be one of the primary mechanisms whereby prenatal smoke exposure increases the risk of ASB. Complementary to these lines of evidence, mouse models of Maoa deficiency and G × E interactions exhibit striking similarities with clinical phenotypes, proving to be valuable tools to investigate the neurobiological mechanisms underlying antisocial and aggressive behavior. Here, we provide a comprehensive overview of the current state of the knowledge on the involvement of MAOA in aggression, as defined by preclinical and clinical evidence. In particular, we show how the convergence of human and animal research is proving helpful to our understanding of how MAOA influences antisocial and violent behavior and how it may assist in the development of preventative and therapeutic strategies for aggressive manifestations.
Keywords: Monoamine oxidase A, aggression, violence, antisocial personality disorder, psychopathy, animal models
1. Introduction
Aggression is a multifaceted set of behaviors directed at inflicting harm on another organism for offensive or defensive purposes. In most animals, the key evolutionary goal of this function is to improve the probability of self-preservation and reproduction (Huber and Brennan, 2011). By the same token, human aggression is also regarded as an adaptive response, aimed at enhancing social status (particularly in the context of hierarchical organizations), increasing access to sexual mates and resources, and enabling defensive reactions against attackers (Buss and Shackelford, 1997; Archer, 2009). The spectrum of acceptable aggressive manifestations in our species, however, is limited by social and cultural norms. Considering the complex gamut of motivations underlying aggression, classifications aimed at distinguishing different subtypes can be particularly useful to frame biological and evolutionary variants of this behavioral response and operationalize distinct psychological profiles of aggressive individuals.
The heuristic that has gained the most traction in the classification of aggression dissects it into two dissociable forms: reactive and proactive (Dodge and Coie, 1987; Anderson and Bushman, 2002; Wrangham, 2018). Reactive aggression refers to the manifestation of impulsive, volatile hostility occurring in retaliation to provocation or perceived threat. Conversely, proactive aggression involves instrumental, premeditated aggression, typically aimed at obtaining personal gains. Although these types of aggression diverge by developmental origins and neurobiological mechanisms (Vitaro et al., 2006), they are often tightly correlated in the same individuals (Polman et al., 2007). While both constructs are physiological expressions of different adaptive mechanisms, they are regarded as pathological whenever they are disproportionate to the eliciting stimulus and deviate from social norms and cultural practices. Accordingly, pathological reactive and proactive aggression are often conducive to (or at least associated with) embroilment in illicit and criminal activities, and negatively impact the interpersonal functioning of both perpetrators and victims. There has been a recent proliferation of neuroimaging studies – mainly functional magnetic resonance imaging (fMRI) investigations – exploring the putative neural underpinnings of reactive and proactive aggression (for a synoptic view, see Table 1).
Table 1.
Main neuroimaging studies on reactive and proactive aggression.
| Study | Population | Paradigm/Imaging Modality | Findings | |
|---|---|---|---|---|
| Reactive aggression | Coccaro et al. (2007) | 10 individuals with Intermittent explosive disorder | fMRI socio-emotional processing task | ↑ amygdala reactivity and ↓ orbitofrontal reactivity to angry faces |
| Bubenzer-Busch et al. (2016) | 18 boys with ADHD (10 with comorbid CU) | Modified fMRI Point Subtraction Aggression Game | ↓ activation of ventral ACC and temporoparietal junction during aggressive phase | |
| da Cunha-Bang et al. (2017) | 18 violent offenders (14 with ASPD) | Modified fMRI Point Subtraction Aggression Game | ↑ amygdala and striatum reactivity to provocation | |
| Farah et al. (2018) | 156 males with varying levels of psychopathology | sMRI | right amygdala volumes positively correlated with reactive aggression | |
| Proactive aggression | Lozier et al. (2014) | 30 youth with conduct problems endorsing high and low CU traits | fMRI implicit face-emotion processing task | Right amygdala responses to fearful expressions mediated relationship between CU traits and proactive aggression |
| Craig et al. (2019) | 140 youth with CD or ODD | proton magnetic resonance spectroscopy | proactive aggression was inversely correlated with striatal glutamate concentration |
ACC = anterior cingulate cortex; ADHD = attention deficit hyperactivity disorder; CD = conduct disorder; CU = callous-unemotional; fMRI = functional magnetic resonance imaging; ODD = oppositional defiant disorder; sMRI = structural magnetic resonance imaging
Reactive aggression is generally associated with a hypoactive prefrontal cortex (PFC) and hyperactive limbic regions, including the amygdala and striatum, in response to negative social stimuli, such as provocation, social exclusion, and angry facial expressions (Sterzer et al., 2005; Coccaro et al., 2007; Marsh et al., 2011; Motzkin et al., 2011; Fanning et al., 2017; Coccaro et al., 2018a). Although much less neuroimaging research has targeted the neural correlates of proactive aggression, this subtype has been associated with increased grey matter density in the dorsomedial PFC, reduced grey matter density in the posterior cingulate cortex, and increased functional connectivity between the left precuneus and the PFC (Zhu et al., 2019). Although these results point to very distinct neurobiological signatures of these two constructs, reactive and proactive elements often coexist even within the same aggressive manifestation. Thus, several authors have proposed more refined taxonomies that encompass complementary dimensions, such as the modality, quality, and immediacy of the aggressive act (Anderson and Carnagey, 2004; Bushman and Huesmann, 2010; Krahé, 2013).
Over the past few years, further advances in our understanding of aggression have arisen from the Research Domain Criteria (RDoC) initiative (Cuthbert and Insel, 2013). This research framework focuses on transdiagnostic approaches, as well as biological knowledge of risk factors, to gain insight on different dimensions of psychopathology. Originally, aggression was proposed to be one of the three core constructs in the Negative Valence System (NVS) domain of the RDoC matrix; however, the NVS Workgroup viewed reactive aggression as highly heterogeneous and proposed its further classification into two distinct categories:
Frustrative non-reward, elicited by the inability to obtain a reward despite sustained efforts. This type of reactive aggression is currently defined as one of the five main constructs of the NVS domain.
Defensive aggression, aimed at terminating a real or perceived threat; this type of reactive aggression is partially encompassed by the Response to Acute Threat Construct of the NVS domain.
Even this further classification, however, may prove inadequate to capture the phenomenological complexity of reactive aggression. For example, it has been proposed that aggression propensity may reflect a dysfunctional interplay between the NVS and Cognitive System RDoC domains, indicating a deterioration of inhibitory cognitive control during exposure to threatening stimuli (Verona and Bresin, 2015).
The NVS Workgroup also recommended that NVS-related aggression constructs should be disentangled from offensive/proactive aggression, described as instrumental for the acquisition of resources, social status, and dominant roles, and thus more suitable for inclusion in the Social Processes Domain of the RDoC. Recent work has advocated for the demarcation of appetitive aggression as a new subtype of proactive aggression based on predatory and reward-based mechanisms (Elbert et al., 2010). Appetitive aggression is driven by the pleasure of attacking and hunting someone down, or even by the smell of blood (Weierstall and Elbert, 2011) and is very common among combatants and gang members (Hecker et al., 2012; Weierstall et al., 2013). In particular, some authors have pointed to the possibility that the inherent pleasure associated with appetitive aggression may lead to a form of behavioral addiction (Golden and Shaham, 2018). This argument appears to support the inclusion of this type of proactive aggression in alternative RDoC rubrics, such as the Reward Responsiveness Construct of the Positive Valence System Domain. At the time of this writing (April 2020), however, no specific classification exists for proactive aggression in the RDoC.
An alternative classification for aggression concerns the distinction between physiological and pathological manifestations. Whereas physiological aggression is adaptive, its pathological counterpart is manifested in a fashion either inappropriate or disproportionate to socio-cultural norms and typically entails acts of violence and delinquency. The first systematic attempt to include these behaviors in psychiatric nosography classification came with the first version of the Diagnostic and Statistical Manual of Mental Disorders (DSM). In it, the term sociopathic personality disorder was coined to designate a psychopathological condition characterized by aberrant social behavior and lack of conformity with acceptable standards of conduct (APA, 1952). This nomenclature was, in turn, based on the construct of sociopathy that had been popularized by George Everett Partridge (Partridge, 1930). From the DSM-III onward, however, this category was renamed as antisocial personality disorder (ASPD), and defined as a chronic, pervasive pattern of disregard for (and violation of) the rights of others, as well as manifestation of aggression and violence and a high propensity to engage in criminal activities (APA, 1980). While ASPD is framed as a disorder of adulthood, it is typically preceded by analogous manifestations in adolescence and childhood, the overarching diagnosis for which is conduct disorder. Approximately 2–7% of the adult population meets diagnostic criteria for lifetime ASPD (Kessler et al., 1994; Swanson et al., 1994; Grant et al., 2004; Compton et al., 2005; Coid et al., 2009), and the disorder is 3–7 times more common in males than females (Robins, 1987; Grant et al., 2004; Hamdi and Iacono, 2014). It is worth noting that approximately half of all ASPD patients possess a record of criminal offending, and 85% have a history of violent behavior toward others (Robins and Regier, 1991; Samuels et al., 2004). ASPD is particularly associated with high rates of violence against children, intimate partners, and strangers (Coid et al., 2006).
One taxonomic classification that is relevant to the developmental trajectory of ASPD posits that there are two main subgroups of antisocial behavior (ASB): life-course-persistent (LCP) and adolescent-limited (AL) (Moffitt, 1993). The LCP group comprises individuals whose onset of ASB occurs in childhood and persists into adulthood. The LCP designation would, therefore, capture many individuals with ASPD and some with psychopathy who follow this developmental trajectory (Skilling et al., 2002). By contrast, the AL group includes individuals whose onset of ASB occurs in adolescence and desists before adulthood. By definition, this group would exclude individuals with ASPD and likely psychopathy. AL ASB has been likened to an amplified expression of normal adolescent behavior that stems from social factors, whereas developmental expression of LCP is influenced by genetic predisposition and neuropsychological risk factors, which are aggravated by adverse childhood familial environments (Mofftt, 1993; Moffitt, 2003). In agreement with the strong biological influence postulated for LCP, recent studies have shown that, in contrast with non-antisocial and AL subjects, LCP individuals show a marked thinning and reduction of the frontotemporal cortices (Carlisi et al., 2020).
The current version of the DSM (DSM-5) specifies that ASPD features impairments in personality, self-functioning, and interpersonal functioning, with specific symptoms of antagonism and disinhibition (APA, 2013). Although ASB is not limited to aggressive manifestations, both reactive and proactive aggression are common symptoms of ASPD (Nouvion et al., 2007; Lobbestael et al., 2013). Of these two constructs, reactive aggression is more commonly observed in ASPD, often motivated by hostile attribution bias (Lobbestael et al., 2013) and associated with paranoid personality traits (Lobbestael et al., 2015) and trait neuroticism (Miller and Lynam, 2001). However, it should be noted that manifestations of exclusively reactive pathological aggression are currently classified as intermittent-explosive disorder (IED). This entity, which typically presents with regular, uncontrollable bouts of rage and anger, has been traditionally regarded as mutually exclusive with ASPD; however, recent surveys have shown that 21.9% IED patients exhibit ASPD comorbidity (Coccaro et al., 2018b), potentially indicating broad neurobiological commonalities.
The definitions of sociopathy and ASPD are sometimes mistakenly used interchangeably with the term psychopathy, which refers to a condition characterized by behavioral deviancy, lack of empathy, and callous-unemotional traits (Hare, 2003). While ASB is one of the domains of psychopathy, this construct is also characterized by profound deficits in interpersonal, affective, and lifestyle dimensions (Neumann et al., 2005). Only a subset of ASPD patients fulfill diagnostic criteria for psychopathy (Meyer, 1994; Ogloff, 2006); accordingly, the prevalence rates of ASPD and psychopathy among male incarcerated subjects are estimated at 50% and 15%, respectively (Hare, 1998; Singleton, 1998; Hare et al., 2000; Fazel and Danesh, 2002). It is worth noting that psychopathic traits in ASPD are associated with proactive aggression (Kolla et al., 2013), suggesting that this behavior may serve as an index of ASPD/psychopathy comorbidity.
The Psychopathy Checklist-Revised (PCL-R) (Hare, 2003) is a common tool used to conceptualize the construct of psychopathy. Although the PCL-R total score is thought to be a satisfactory index of overall psychopathy, PCL-R items demonstrate a replicable two-factor structure (Harpur et al., 1988). Factor one (F1) encompasses interpersonal and affective traits of psychopathy, while Factor 2 (F2) indexes impulsive and antisocial behaviors characteristic of the disorder. It has been demonstrated that the application of the two PCL-R factors in tandem as opposed to PCL-R total scores can better predict the use of reactive aggression mediated by negative emotionality. These associations occur, because the unique variance in F1 is weakly negatively related to measures of anger-hostility, whereas the unique variance in F2 strongly predicts these measures (Hicks and Patrick, 2006). Such findings illustrate the presence of mutual suppression, which occurs when two correlated predictors exhibit opposing relations with a specific criterion, such that the inclusion of both predictors concurrently in a regression model increases the correlation of each with the criterion (Blonigen et al., 2010). Although mutual suppression is a relatively uncommon phenomenon in psychopathology research (Loney et al., 2003), the covariance between Factor 1 and Factor 2 scores may cause associations with impulsivity to be overlooked due to suppressor effects (Snowden and Gray, 2011). Finally, in a community sample of adults who participated in a fMRI experiment (Hyde et al., 2014), negative emotionality and amygdala reactivity did not exhibit zero-order relationships with unidimensional measures of psychopathy or ASPD on their own but only after adjusting for the overlapping variance in APD and psychopathy. That is, after adjustment, higher psychopathy scores were related to lower negative emotionality and lower amygdala activity. In comparison, greater ASPD scores were associated with higher negative emotionality and greater amygdala reactivity. Taken together, these results suggest that reactive aggression encompassed by negative emotionality may present divergent neural signatures and operate differently in ASPD and psychopathy.
This background shows that, although classifications of aggression based on phenomenological and clinical parameters have broad empirical support, they fall short of the necessary degree of detail to elucidate the complexity of its neurobiological basis. An interesting alternative approach may be afforded by the analysis of the genes implicated in aggression. Ample research has shown that aggression has a robust genetic underpinning (Craig and Halton, 2009; Anholt and Mackay, 2012; Veroude et al., 2016; Waltes et al., 2016) and that aggressive ASB is highly heritable (h2= 0.60) (Eley et al., 2003). While research implicates many genes in aggression (Zhang-James et al., 2019), the best-characterized of them is MAOA, which encodes the protein monoamine oxidase A (MAOA). This enzyme catalyzes the degradation of several monoamine neurotransmitters implicated in the regulation of aggression, including serotonin (5-hydroxytryptamine; 5-HT) and the catecholamines norepinephrine and dopamine (Bortolato et al., 2008). Strikingly, in a recent cross-species analysis of eight aggression gene lists (encompassing 1767 genes) from adult and children genome-wide association studies (GWASs), transcriptome-wide studies of rodent models, as well as sets from Online Mendelian Inheritance in Man (OMIM) and knockout (KO) mice revealed that MAOA ranked highest by number of occurrences and weighted ranks for aggression (Zhang-James et al., 2019). Furthermore, brain-imaging studies validated that MAOA activity in most brain regions is negatively correlated with the multidimensional personality questionnaire (MPQ) score of trait aggression (Alia-Klein et al., 2008). These data highlight the unique importance of MAOA in the neurobiology of aggression in humans and animals. The following sections summarize the available evidence of the role of MAOA in these interrelated constructs and underscore how the convergence of findings from neuroimaging studies and animal models is helping elucidate the neurobiological mechanisms of these conditions.
2. Molecular characteristics and brain localization of MAOA
2.1. Structure and function of the enzyme MAOA
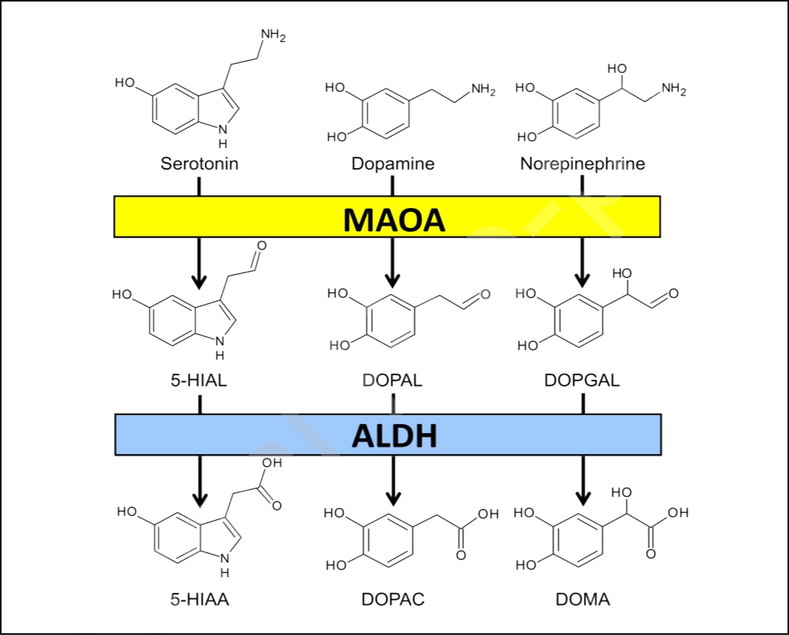
The enzyme MAOA (EC:1.4.3.4) is bound to the outer mitochondrial membrane via a transmembrane segment of 22 amino acids (aa) in its C-terminus (Bach et al., 1988; Hsu et al., 1988; Son et al.,2008) and features a flavin adenine dinucleotide (FAD) covalently bound to a cysteine residue by an 8α-(S cysteinyl)-riboflavin linkage (Igaue et al., 1967; Kearney et al., 1971; Oreland, 1971; Walker et al., 1971). As mentioned above, MAOA catalyzes the oxidative deamination of several monoamine neurotransmitters - including 5-HT and the catecholamines norepinephrine and dopamine (Bortolato et al., 2008) – into aldehydes (Fig. 1), with the synthesis of ammonia and hydrogen peroxide as by-products.
Figure 1.
Role of MAOA and ALDH in the degradation of serotonin, dopamine, and norepinephrine. 5-HIAL, 5-hydroxy indole aldehyde; DOPAL; 3,4-dihydroxy phenyl acetaldehyde; DOPGAL; 3,4-dihydroxy phenyl glycol aldehyde; 5-HIAA; 5-hydroxy indole acetic acid; DOPAC; 3,4-dihydroxy phenylacetic acid; DOMA; 3,4-dihydroxymandelic acid. For further details, see text.
MAOA shares a high degree of similarity with its isoenzyme Monoamine oxidase B (MAOB). While both enzymes have similar length (human MAOA: 527 aa; human MAOB: 520 aa) and weight (~60 KDa), they have a rather different profile of substrate affinity. MAOA has a much higher affinity for 5-HT and norepinephrine, while MAOB is particularly selective for trace amine substrates such as β-phenylethylamine (PEA). In humans, the two isoenzymes have similar affinities for dopamine and tyramine (Bortolato et al., 2008). MAOB, but not MAOA, has also been shown to participate in the catabolism of histamine by catalyzing the oxidative degradation of its metabolite N-methylhistamine (Maintz and Novak, 2007). Finally, MAOB has also been recently implicated in the catabolism of the diamine putrescine, resulting in the synthesis of γ-aminobutyric acid (GABA) (Yoon et al., 2014).
Even with these differences, studies in KO mice have shown some overlap between the catalytic functions of the two isoenzymes; indeed, the deletion of both Maoa and Maob leads to a much greater accumulation of 5-HT and other substrates than the functional loss of each isoenzyme alone (Chen et al., 2004). It should be noted, however, that the catalytic activity of MAOA and MAOB in rodents may not entirely reflect the mechanisms observed in primates. For example, the metabolism of dopamine is primarily served by MAOA in rodents under basal conditions (Hovevey-Sion et al., 1989; Paterson et al., 1991; Fornai et al., 1999); conversely, the contribution of MAOB to dopamine degradation is much more significant in primates, at least in the cortex (Paterson et al., 1995). Conversely, the catabolic action on 5-HT and norepinephrine are equivalent between rodents and humans.
Crystallographic analyses have been instrumental in helping to decode the structure of human MAOA (De Colibus et al., 2005; Son et al., 2008) and MAOB (Binda et al., 2003). These studies show that the substrate specificity of MAOA is primarily contributed by a flexible loop between aa 108 and 118, which controls substrate access and is stabilized by the anchoring of the enzyme in the mitochondrial membrane (Son et al., 2008).
Although the details of the catalytic process of MAOA remain partially unclear (Gaweska and Fitzpatrick, 2011), several proposed mechanisms posit that the oxidation of monoamines may occur in three fundamental steps: i) the oxidation of amine into a corresponding imine, which is accompanied by the reduction of FAD into FADH2; ii) a hydrolytic step, in which the imine is converted into an aldehyde with the formation of ammonia; and iii) an oxidative reaction, in which FADH2 is re-oxidized to FAD with the formation of hydrogen peroxide (Gaweska and Fitzpatrick, 2011). The order of these steps within the catalytic cycle can vary depending on the specific binding of the substrate to the oxidized and reduced form of the enzyme (Ramsay et al., 2011). In the central nervous system, the aldehyde product of the MAOA reaction is typically converted into the corresponding carboxylic acid by an NAD+- dependent aldehyde dehydrogenase (ALDH) (Fig. 1). For instance, MAOA degrades 5-HT into 5-hydroxy-3-indole acetaldehyde (5-HIAL), which is then rapidly converted into 5-hydroxy-3-indoleacetic acid (5-HIAA). Conversely, the other two main neurotransmitters metabolized by MAOA, norepinephrine, and dopamine, are oxidized into 3,4-dihydroxy phenylacetaldehyde (DOPAL) and 3,4-dihydroxy phenyl glycolaldehyde (DOPGAL), respectively. These aldehydes are then further converted into 3,4-dihydroxymandelic acid (DOMA) and 3,4-dihydroxyphenylacetic acid (DOPAC) by ALDH (Fig. 1). In contrast with this mechanism, small quotas of the aldehyde are further reduced to the corresponding alcohols or glycols by aldehyde reductase or alcohol dehydrogenase. This mechanism transforms 5-HIAL, DOPAL, and DOPGAL into 5-hydroxy indole ethanol (5-HIET), 3,4-dihydroxy phenyl ethanol (DOPET), and 3,4-dihydroxy phenylethylene glycol (DOPEG), respectively. Plasma levels of DOPEG are particularly regarded as sensitive indices of MAOA activity (Sunderland et al., 1985).
2.2. Genetic and epigenetic regulation of MAOA
In humans, the MAOA gene is located on the X chromosome (Xp11.23) in a position adjacent to MAOB (Ozelius et al., 1988; Lan et al., 1989). The two genes share similar exon-intron organization, with 15 exons and ~70% sequence identity (Grimsby et al., 1991); furthermore, in both genes, the FAD-binding site is located on exon 12, which is highly conserved, with 93.9% identity (Grimsby et al., 1991). Based on this high homology, the two MAO paralogues are regarded as the result of a tandem duplication of the same ancestor gene (Grimsby et al., 1991). Indeed, only one MAO has been documented in protochordates (Yamamoto and Vernier, 2011) and teleost fish (Chen et al., 1994; Anichtchik et al., 2006), with functional properties overlapping with both mammal MAOA and MAOB (Arslan and Edmondson, 2010). On the other hand, the presence of two MAO enzymes has been acknowledged in anuran amphibians (Nicotra and Senatori, 1988), suggesting that the duplication of the MAO progenitor gene occurred during the transition from fish to amphibians. Notably, available genome data from the > 200 animal species listed in the Gene NCBI database indicate that the MAOA gene is X-linked in eutherian mammals, but not in other subdivisions of tetrapods (including reptiles, birds, monotremes, and marsupials).
The activity of MAOA is directly controlled by a critical region of its promoter, located between −71 and −40 bp, whose deletion reduces activity by 64–83% in different human cell lines (Gupta et al., 2015). Several transcription factors bind to this region, including Sp1 (specificity protein 1) (Zhu et al., 1994), GATA2, and TBP (Gupta et al., 2015). These factors act synergistically to enhance MAOA expression. Notably, MAOA protein levels were found to be reduced by antibody-based inactivation of these three factors or markedly increased by their ectopic expression (Gupta et al., 2015). Sp1 function is also potentiated by the male-specific factor SRY (sex regulatory gene on chromosome Y) (Wu et al., 2009), and its binding site interacts with androgens (Ou et al., 2006), pointing to a sex-dimorphic regulation of MAOA expression. The action of Sp1 on MAOA transcription is also tightly controlled by a repressor molecule, R1 (RAM2/CDCA7L/JPO2), which competes with Sp1 for the same binding site (Chen et al., 2005).
Another key mechanism of regulation of MAOA is afforded by epigenetic mechanisms. Brain MAOA activity in humans is strongly associated with the methylation status of the CpG island (CGI)_1060 within the MAOA promoter, which includes 14 CpG sites (Shumay et al., 2012). These studies are in line with in vitro findings, which documented a negative correlation between MAOA promoter methylation and MAOA expression (Checknita et al., 2015; Schiele et al., 2018). Methylation mechanisms are also responsible for the regulation of a newly discovered MAOA-associated long non-coding RNA (MAALIN), which suppresses MAOA expression in the brain (Labonté et al., 2020).
2.3. Neuroanatomical localization of MAOA
The brain-regional distribution of MAOA has been analyzed in humans and animals using multiple methodological approaches. The available data on brain MAOA density in vivo have been obtained through neuroimaging techniques, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) using radio-ligated MAOA specific inhibitors. The three main PET radiotracers available for MAOA are the irreversible inhibitor [11C]clorgyline (MacGregor et al., 1985; Fowler et al., 2007), and the two reversible blockers [11C]harmine (Bergström et al., 1997; Ginovart et al., 2006), and [11C]befloxatone (Dollé et al., 2003; Bottlaender et al., 2010). The two last radiotracers are uniquely suited to quantify brain MAOA levels, because they are reversible, highly selective for the MAOA isoenzyme, and bind with high affinity to the substrate cavity of MAOA (Son et al., 2008). However, of the two reversible radioligands, only [11C]harmine has been used in human studies to date. SPECT analyses of brain MAOA have ony been performed in rodents, and they used [125I]iodoclorgyline (Ohmomo et al., 1991; Hirata et al., 1995). In these neuroimaging studies, MAOA binding is used as a proxy for its expression, since the density and metabolic activity of MAOA are highly correlated (Saura et al., 1992). Studies on post-mortem brain samples showed that MAOA protein levels (measured by immunoblotting) are strongly correlated with PET data using both radio-ligated clorgyline (R=0.82) and harmine (R=0.86) (Tong et al., 2013). These studies revealed that, in adults, there is a high concentration of MAOA in the cerebral cortex, particularly in the medial frontal and cingulate regions; among the subcortical areas, high concentrations are found in the hippocampal uncus, medial pulvinar of the thalamus and hypothalamus; and very low levels are present in the striatum and globus pallidus. The lowest expression was found in the cerebellar cortex and white matter (Tong et al., 2013).
Most available public repositories (including the Allen Brain Atlas and the Human Protein Atlas) indicate that the highest expression of MAOA transcript is in the brainstem monoaminergic nuclei, including the substantia nigra and ventral tegmental area (VTA; dopaminergic), locus coeruleus (norepinephrinergic) and raphe nuclei (serotonergic), as well as in the nuclei of cranial nerves. The expression of MAOA is also highly abundant in the hypothalamus, followed by the amygdala, habenula, and nucleus accumbens. MAOA distribution is relatively high throughout the cortex (with particularly high levels in the PFC and anterior cingulate cortex) and moderate in the hippocampus. Lower levels are encountered in the thalamus, spinal cord, pituitary gland, and cerebellum. These data are in essential agreement with the neuroanatomical distribution of MAOA in rodents, as shown by in situ hybridization and radioligand binding (Saura et al., 1992; Jahng et al., 1997).
3. Polymorphisms of human MAOA gene
3.1. Tandem repeats
The MAOA gene exhibits several minisatellite polymorphisms, which have been associated with a broad array of behavioral effects. The best-known MAOA tandem repeat polymorphism is a 30bp functional upstream variable-number tandem repeat (uVNTR) in the promoter region, at position −1142 to −1262 relative to the ATG translation initiation codon of the gene (Genbank sequence: M89636; Zhu et al., 1992). Several alleles have been documented, containing 2, 3, 3.5, 4, 5, or 6 copies of a 30-bp sequence (Sabol et al., 1998; Huang et al., 2004). A rare 1-repeat (1R) variant has also been recently described in the Iraqi population (Al-Tayie and Ali, 2018). The 30 bp-sequence (ACCGGCACCG GCACCAGTAC CCGCACCAGT) features five repetitions of the 6-nucleotide motif ACCVGY (Sabol et al., 1998; Huang et al., 2004). Each of these sequences is consistently followed by a motif of 15 bp (ACCGGCACCG GCACC) corresponding to the first half of the repeat; this sequence was not initially included in the first nomenclature of the alleles (Sabol et al., 1998). Some authors have advocated for the use of a more rigorous classification factoring for this adjustment; based on this alternative convention, for example, the 4-repeat (4R) variant has been sometimes named 4.5R, and so on (Jorm et al., 2000; Das et al., 2006; Im et al., 2019). The two most frequent alleles harbor 3 (3R) and four repeats (4R). The frequency of the 3R variant is estimated to be 51–59% in African Americans and 33–37% in Caucasians, based on the most extensive studies on MAOA (Sabol et al., 1998; Beaver et al., 2013; Haberstick et al., 2014). Conversely, the 4R allele is found in 36–43% of African Americans and 60–65% of Caucasians. Frequency data on other ethnicities, such as Asians, non-white Hispanics, and Pacific Islanders, are still unclear, as most estimates of these populations have been based on small cohorts to date. For example, several studies have shown that the frequency of MAOA 3R and 4R variants in Han Chinese in Taiwan (conducted on analyses with up to ~200 control subjects for each study) have shown a predominance of the 3R variant (54–62%) over the 4R allele (37–44%) (Lu et al., 2002; Huang et al., 2007). However, other reports from Taiwan and the People’s Republic of China have not confirmed this predominance (Lin et al., 2008; Wang et al., 2018), suggesting that more extensive studies are needed to verify the distribution of MAOA genotypes in this ethnic group. All other variants are infrequent in the general population; for example, the 2-repeat (2R) variant has been documented in ~5% of African Americans (and in 0.1% of Caucasians) (Beaver et al., 2013; Haberstick et al., 2014). Conversely, the 3.5-repeat (3.5R) allele is found in ~1.5% Caucasians, and 0.01% African Americans (Haberstick et al., 2014).
In vitro data have consistently shown that the 2R and 3R variants (MAOA-L) are associated with lower transcriptional efficiency (Sabol et al., 1998; Deckert et al., 1999; Denney et al., 1999; Jonsson et al., 2000). Conversely, 3.5R and 4R variants are associated with higher transcriptional activity (MAOA-H). Accordingly, MAOA-H alleles have shown a relationship with higher CSF concentrations of the 5-HT and catecholamine metabolites 5-HIAA and homovanillic acid (HVA) (Williams et al., 2003; Zalsman et al., 2005), respectively. The transcriptional efficiency of the other variants is less clear. For example, the 5-repeat (5R) variant has been inconsistently associated with either high (Deckert et al., 1999; Beach et al., 2010) or low activity (Sabol et al., 1998). No activity data are currently available for the 1R variant, which has only been documented in the Iraqi population to date (Al-Tayie and Ali, 2018).
In contrast with in vitro data, post-mortem and brain-imaging studies have shown that MAOA activity is not significantly higher in the brain of adult MAOA-H carriers (Balciuniene et al., 2002; Cirulli and Goldstein, 2007; Fowler et al., 2007). Indeed, no relationship was found between uVNTR genotypes and the levels of MAOA activity in the brain, as measured using [11C]clorgyline (Fowler et al., 2007). While this discrepancy may appear puzzling, the lack of correspondence between activity and genotype in adults likely reflects the influence of other functional MAOA polymorphisms (see below) and the life-long impact of other environmental factors (including stress exposure, aging, and smoking) on the gene transcription and enzyme activity. In support of this hypothesis, one epigenetic study reported that methylation of CpG sites in the core MAOA promoter (obtained from a peripheral blood sample) predicted brain MAOA levels (Shumay et al., 2012). From this perspective, the correspondence between genotype and enzyme activity may likely be much tighter during prenatal and early postnatal developmental stages. Future studies will be needed to verify whether MAOA genotypes can predict MAOA activity in utero and during childhood, the life stages where genetic variants most arguably impact brain circuitry development and behavioral ontogeny the most.
In addition to the uVNTR polymorphism, a second, distal VNTR (dVNTR) has been identified at approximately 500 bp upstream of the uVNTR. This polymorphism is comprised of two types of 10-bp decamer motifs (decamer A: CCCCTCCCCG and decamer B: CTCCTCCCCG, respectively) (Philibert et al., 2011). Five different dVNTR variants have been described, featuring 8, 9, 10, 11, and 12 repeats (Philibert et al., 2011). The 9R and 10R alleles are the most frequent, with the latter showing lower transcriptional efficiency. Because of a significant linkage disequilibrium between dVNTR and uVNTR loci, some haplotype combinations are more common. For example, the 4R uVNTR allele is almost always co-segregated with the 9R dVNTR variant (Manca et al., 2018), while the 3R uVNTR has been found in association with 9,10, or 11R uVNTR alleles (for a full table of the observed dVNTR/uVNTR frequencies, see Manca et al., 2018). Strikingly, the dVNTR alleles may exert an even greater role than the uVNTR on MAOA expression; experiments in vitro have revealed that MAOA mRNA levels are significantly reduced by dVNTR, but not uVNTR, deletion (Manca et al., 2018). Future studies should ascertain whether the lack of alignment between MAOA activity data and uVNTR alleles may be accounted for by the differential influence of dVNTR alleles.
A third VNTR has been described in intron 1 of the MAOA gene, featuring 6, 7, 8, 9, or 10 copies of a 23-bp repeat ((GAACTGTGTT TATATATATA TAT). The two most frequent alleles contain either eight (63.1%) or seven copies (33.5%) (Hinds et al., 1992). Finally, a long dinucleotide (CA)n repeat microsatellite has been described in intron 2 (Black et al., 1991). The functional significance of these polymorphisms, however, remains unknown. Considering the widespread diffusion of VNTRs in the human genome (Näslund et al., 2005), it is likely that MAOA activity may also be controlled by as of yet uncovered tandem-repeat polymorphisms. Determining how MAOA expression is regulated by the interaction of these polymorphisms remains a key research objective to gain a firmer understanding of the transcriptional regulation of MAOA.
3.2. Single nucleotide polymorphisms (SNPs)
Around 20 SNPs of the MAOA gene have been identified and analyzed for functional properties. To date, the dbSNP repository data on MAOA report 14,922 unique polymorphisms (not accounting for alternative nomenclatures of the same polymorphism). Approximately 92.7% of polymorphisms are intronic, while 7.3% are located within coding sequences (Table 2). Of the 37 SNPs that have been targeted by published studies (Table 3), very few have been associated with a functional role in the regulation of MAOA expression. The two main functional SNPs characterized to date, rs6323 and rs1137070, were initially identified as two synonymous polymorphisms that could be teased out based on the activity of the FnuHI and EcoRV restriction endonucleases, respectively (Hotamisligil and Breakefield, 1991). This approach showed that the guanine (G) allele of rs6323 encodes an enzyme with higher activity than the thymine (T) variant; similarly, the thymine (T) variant of rs1137070 is associated with higher activity than that of the cytosine (C) allele (Hotamisligil and Breakefield, 1991). It should be noted, however, that these results are at odds with the findings of a study on gout patients, which documented a marginal trend for greater MAO activity in specimens from C-allele carriers as compared with T-harboring counterparts (Tu et al., 2010).
Table 2.
Number of unique single nucleotide polymorphisms (SNPs) in the MAOA gene, divided by type of mutation. Data collected from the dbSNP repository.
| Polymorphism type | # recorded polymorphisms | % intronic polymorphisms | % coding sequence polymorphisms |
|---|---|---|---|
| Single nucleotide variant (SNV) | 13837 | 12863 | 974 |
| Multinucleotide variant (MNV) | 3 | 2 | 1 |
| Deletion | 214 | 195 | 19 |
| Insertion | 60 | 52 | 8 |
| Deletion/insertion | 808 | 725 | 83 |
| TOTAL | 14922 | 13837 | 1085 |
Table 3.
Best-studied SNPs of MAOA. Data are collected from the dbSNP and SNPedia databases.
| SNP | Alleles and relative frequency | Functional consequence |
|---|---|---|
| rs6323 | G > T | synonymous variant |
| rs1137070 | T > C | synonymous variant |
| rs1800466 | A > G | missense variant |
| rs72554632 | C > T | Stop variant (Brunner syndrome) |
| rs909525 | C > T | intron variant |
| rs979605 | A > G | intron variant |
| rs979606 | C > T | intron variant |
| rs1465107 | A > G | intron variant |
| rs1465108 | A > G | intron variant |
| rs1799835 | T > G | missense variant |
| rs1800659 | G > A,T | intron variant |
| rs1803986 | G > T | missense variant |
| rs2072743 | T > C | intron variant |
| rs2072744 | T > C | intron variant |
| rs2235185 | A > C,G,T | intron variant |
| rs2235186 | A > G | intron variant |
| rs2238968 | C > A | intron variant |
| rs2239448 | T > C | intron variant |
| rs2283724 | G > A | intron variant |
| rs2283725 | A > G,T | intron variant |
| rs3027396 | C > T | intron variant |
| rs3027399 | G > C | intron variant |
| rs3027400 | T > G | intron variant |
| rs3027405 | A > T | intron variant |
| rs3027407 | A > G | 3’ UTR variant |
| rs3788862 | A > G | intron variant |
| rs3788863 | T > A | intron variant |
| rs3810709 | C > T | intron variant |
| rs5905702 | T > G | intron variant |
| rs5905809 | G > C,T | intron variant |
| rs5905859 | C > A,T | intron variant |
| rs5906883 | A > C | intron variant |
| rs5906597 | A > C,G | intron variant |
| rs5953210 | G > A | upstream transcript variant |
| rs6609257 | G > A | downstream variant |
| rs6610845 | C > T | intron variant |
| rs12843268 | A > G | intron variant |
4. The role of MAOA in aggression and ASB: human genetic evidence
4.1. Brunner syndrome
The first finding that documented the involvement of MAOA in antisocial and aggressive behavior came with the description of Brunner syndrome, an X-linked recessive syndrome characterized by a nonsense mutation of the MAOA gene (rs72554632). All affected males in a Dutch pedigree demonstrated disruptive, violent outbursts that manifested in attempted murder, rape, and arson (Brunner et al., 1993a; Brunner et al., 1993b). These behavioral derangements were accompanied by a dramatic reduction in 5-HT and catecholamine urine metabolites. Recent studies, however, have shown that Brunner syndrome features a cohort of symptoms that is much more complex than what was initially described. For example, the few cases of infants with this diagnosis exhibit developmental cognitive disabilities and behavioral changes associated with the autism spectrum (Piton et al., 2014; Bortolato et al., 2018). Recent studies have begun investigating the neurobiological mechanisms underlying Brunner syndrome, using neurons derived from proband pluripotent stem cells. These investigations have revealed a marked increase in N-methyl-D-aspartate (NMDA) glutamate subunits NR2A and NR2B, strikingly akin to the changes identified in Maoa KO mice (see below) (Shi et al., 2019).
4.2. Direct effects of MAOA polymorphisms on aggression, violence, psychopathy, and ASB
Several studies have documented that, in male adolescents and adults, the MAOA-L alleles of the uVNTR polymorphism are inherently associated with a greater propensity for ASB, psychopathy, and, particularly, criminal violence (Buckholtz and Meyer-Lindenberg, 2008; Guo et al., 2008; Williams et al., 2009; Beaver et al., 2010; Beaver, 2013; Beaver et al., 2014; Ficks and Waldman, 2014; Stetler et al., 2014; Tiihonen et al., 2015). Several of these studies have focused on the rare 2R variant, which is strongly associated with a high proclivity to engage in violent delinquency (Guo et al., 2008). These results were confirmed in a larger analysis of 2574 subjects from the National Longitudinal Study of Adolescent Health (NLSAH) (Beaver et al., 2013). Notably, the increased risk for violent behavior in 2R carriers may reflect the extremely low levels of enzyme activity associated with this variant (estimated to be about half of 3R) (Guo et al., 2008). Together with the evidence on the highly violent behavior in Brunner syndrome patients, these results suggest that the proclivity for violence in adulthood may inversely reflect the levels of MAOA activity in early developmental stages. Further studies, possibly through measurements of plasma DOPEG and MHPG in youth, are warranted to validate this possibility.
Aside from the specific relevance of 2R alleles in aggression and ASB, a few studies have focused on the relevance of MAOA-L variants in violent crime. In an initial study, Beaver and colleagues examined data from a subset of the NLSAH (1155 females and 1041 males). They concluded that, in males, these alleles conferred an increased risk for joining a gang and, once becoming a member, using weapons during a fight. The idea that MAOA-L may specifically predispose for violent crime was borne out by subsequent investigations in prisoners. In a study on 89 male convicts in a large Kansas correctional facility, violent crime was found to be highly associated with the MAOA-L genotype, irrespective of any other psychological variable. This association reached full significance in Caucasian subjects, while it was only marginally significant in African Americans (p = 0.08) (Stetler et al., 2014). The association between MAOA-L variants and violent crime was later replicated in a GWAS conducted in 749 Finnish violent offenders and the general Finnish population (Tiihonen et al., 2015). The offender sample was composed of non-violent offenders (n = 215), violent offenders implicated in at least one violent crime (n = 538), and extremely violent offenders who had committed ten or more violent crimes (n = 84). Offenders who had committed only sexual crimes and psychotic individuals were excluded from the analysis. Results revealed that the MAOA-L genotype was related to violent offending in the criminal cohort (odds ratio [OR)] of 1.71. Intriguingly, the association between MAOA-L and violence was most robust for the extremely violent offenders (OR = 2.66). No relationship was detected between MAOA alleles and non-violent offenders, implying that the observed effect was specific for violent offending. Substance abuse or a diagnosis of ASPD did not alter the findings. Risks were the same for males and females and were not modified by a history of childhood abuse. Although the MAOA tandem repeat was related to violence in this study, none of the other MAOA SNPs proved predictive.
Other studies have found a main effect of MAOA-L alleles on variables related to psychopathy, violence, and ASB. One of the first studies to investigate the relationship between MAOA-L and antisocial outcomes found that individuals with ASPD and alcohol use disorders (AUDs) had an increased frequency of the MAOA-L genetic variant in the ASPD+AUD group compared to ASPD without AUD and healthy controls (Samochowiec et al., 1999). These results suggest that the MAOA-L allele may have been related to the presence of an AUD versus ASPD. However, subsequent studies reported that the MAOA-L variant was more common in males with ASPD+AUD than in males with AUD and a depressive-anxious personality disorder, further suggesting that ASPD was driving the association with MAOA-L (Schmidt et al., 2000). Furthermore, although some studies have shown a positive association between MAOA-L variants and alcoholism (Guindalini et al., 2005; Contini et al., 2006), this link has not been confirmed by subsequent research (Lu et al., 2002; Koller et al., 2003; Mokrovic et al., 2008; Samochoviec et al., 2015). In a study on 237 males, the severity of antisocial and psychopathic traits was found to be higher in MAOA-L carriers (Sadeh et al., 2013). Similarly, in a larger study on 4278 Finnish individuals, MAOA-L homozygous female carriers exhibited slightly higher levels of psychopathy than their MAOA-H counterparts (Hollerbach et al., 2018). One investigation genotyped 453 individuals for the MAOA variant, excluded persons with self-reported childhood maltreatment, and analyzed the relationship between MAOA genetic variants and ASPD traits in the remaining sample (Reti et al., 2011). Among Caucasians with no history of childhood abuse, ASPD trait scores were higher in MAOA-L versus MAOA-H carriers. Subjects with MAOA-L variants also exhibited increased anger/hostility, lower agreeableness, and scored higher on measures indicative of psychopathy. Important to note, however, is that not all investigations have reported a main effect of MAOA uVNTR variants on ASPD or psychopathic outcomes. One of the most extensive studies to date (n = 4,316) failed to detect any main effects of MAOA uVNTR variants on ASBs (Haberstick et al., 2014). Finally, some investigations implicate MAOA-H variants in aggression. For example, the 4R MAOA genotype has been linked to violent recidivism in psychopathic offenders (Tikkanen et al., 2011).
It is worth mentioning that, while this evidence powerfully underscores that MAOA-L variants are a key predisposing factor for aggressive and ASB in adolescents and adults, they do not appear to confer an inherent vulnerability for disruptive and aggressive behavior in boys. Strikingly, the available evidence suggests that these outcomes have a stronger association with the MAOA-H rather than the MAOA-L genotype. For example, in an initial study on 50 boys (mean age: 9 years), high aggressive behavior (based on parent and teacher reports) was associated with a greater frequency of 4R alleles (Beichtman et al., 2004). Consistent with this finding, MAOA-H variants were associated with a slight – yet significant – increase in global mental health problems and ASB in 7-year-old boys (Kim-Cohen et al., 2006). A plausible explanation for this discrepancy may lie in the higher association of MAOA-H alleles and (ADHD) (Kim-Cohen et al., 2006), which is, in turn, a key comorbidity with disruptive behavior in childhood (August et al., 1996).
Very few investigations have addressed the role of other MAOA polymorphisms in the control of aggression and related functions. In a study of suicidal individuals, the SNPs rs6323, rs909525, and rs2064070 were associated with outward-expressed anger in male suicidal patients (Antypa et al., 2013). The polymorphism rs1465108 was associated with greater negative urgency, resulting in heightened aggression (Chester et al., 2015). The polymorphism rs6609257, located 6.6 kb downstream of the MAOA gene, impacts the link between working memory and aggressive behavior and is associated with altered brain activity in a network of frontal, parietal and occipital cortex (Ziermans et al., 2012). Finally, the polymorphism rs2235186 has been linked to variations in anger control, aggression, and empathy (Yan et al., 2012; Atramentova and Luchko, 2016).
4.3. Interactive effects of MAOA polymorphisms and environmental factors on aggression and ASB
Behavioral phenotypes are increasingly identified as arising from the complex interplay between gene expression and environmental influences. Perhaps the most studied G × E relationship associated with aggression and ASB occurs between the MAOA-L allele and childhood maltreatment. In 2002, Avshalom Caspi, Terrie Moffitt, and colleagues were the first to study this G × E interaction in 442 males from the Dunedin Multidisciplinary Health and Development Study, a population cohort of 1037 subjects born in New Zealand between April 1972 and March 1973 (Poulton et al., 2015). Some of these individuals had very well-characterized histories of environmental adversity, which had been assessed longitudinally with periodic assessments from the age of 3 years, and antisocial outcomes / criminal histories, obtained via police records and interviews with the subjects as well as familiar individuals. The main result of the study was that male carriers of the MAOA-L genotype who had been maltreated were found to display an increased risk of engaging in ASB than their maltreated peers who possessed the MAOA-H variant (Caspi et al., 2002). Over the past 18 years, this seminal finding has been substantially replicated by many independent groups (Foley et al., 2004; Huang et al., 2004; Widom and Brustowicz, 2006; Weder et al., 2009; Beach et al., 2010; Derringer et al., 2010; Edwards et al., 2010; Åslund et al., 2011; Fergusson et al., 2011; Cicchetti et al., 2012; Fergusson et al., 2012; Choe et al., 2014; Gorodetzky et al., 2014; Zhang et al., 2016). Nevertheless, three independent meta-analyses to this date (Kim-Cohen et al., 2006; Taylor and Kim-Cohen, 2007; Byrd and Manuck, 2014) have validated the existence of this G × E interaction and detected an effect size of approximately 0.18. For a comprehensive presentation on the literature on these studies, the interested reader is referred to Byrd and Manuck, 2014, Ficks and Waldman, 2014, and Nilsson et al., 2018.
One of the first confirmations came from a study with 514 boys (aged between 8 and 17 years) in the Virginia Twin Study for Adolescent Behavioral Development; in this cohort, the interaction between MAOA-L alleles and childhood adversity (defined as the exposure to interparental violence, parental neglect, and inconsistent discipline) was found to significantly increase the risk for conduct disorder (Foley et al., 2004). In a subsequent study involving 766 adults (663 psychiatric outpatients with a mood disorder and 103 non-affected controls), this G × E interaction was found to predict aggression in both sexes, as well as impulsivity in men only (Huang et al., 2004). These results are partially at odds with the findings of another study on a combined population of 235 adult psychiatric patients and healthy volunteers, in which the interaction between MAOA-L alleles and the exposure to early-life traumatic events was found to increase the risk of physical violence only in men, but not in women (Frazzetto et al., 2007).
To further investigate the potential mechanisms of this G × E interaction, several studies have focused on experimental paradigms aimed at testing the reactivity of MAOA-L carriers to specific environmental stressors in a laboratory setting. This research has confirmed that MAOA-L male carriers show higher levels of experimental aggression (as tested by standard paradigms, such as the hot sauce task, the Taylor aggression paradigm, and the point-subtraction aggression paradigm) in response to provocation and social exclusion (McDermott et al., 2009; Gallardo-Pujol et al., 2013; Kuepper et al., 2013). While these data suggest that the aggression associated with this G × E interaction may be primarily reactive, it is worth noting that other studies appear to indicate that this biosocial interplay may also be relevant to aspects of proactive aggression in ASB. For example, a few studies have shown that the interaction of MAOA-L alleles and early life stress increases the risk for fraudulent behavior (Beaver and Holtfreter, 2009). More generally, emerging criminological evidence shows that, while MAOA-L has a direct association with serious criminal activity (as highlighted in the previous section), its interaction with a history of abuse enhances the risk for delinquent behavior and less serious crime (Armstrong et al., 2014). Similarly, Roettger and colleagues (2016) showed that while the 2R uVNTR allele is associated with criminal violence in males, these effects are buffered by a close relationship between the proband and father. An interaction between the MAOA-L genotype and peer delinquency has also been reported to increase the risk of ASB in males (Cooke et al., 2018).
Several studies have also examined the temporal trajectory of the G × E interaction from childhood onwards. Enoch and colleagues (2010) documented that, in a cohort of 6129 4- to 7-year-old children, boys and girls harboring MAOA-L variants with a history of stress exposure during their infancy exhibited greater hyperactivity and behavioral disinhibition. Furthermore, a longitudinal study with 1265 males confirmed that the interplay between MAOA-L haplotype and a history of childhood adversity led to conduct problems and hostility in puberty (Fergusson et al., 2011). Another longitudinal analysis confirmed that a history of childhood maltreatment and the MAOA-L genetic variant predicted lifetime symptoms of ASPD and revealed that childhood adversity predicted adult depressive symptoms in carriers of MAOA-H alleles (Beach et al., 2010), suggesting that different MAOA variants may be critical to defining the long-term psychopathological sequelae of early-life stress. Some insight into the psychological mechanisms by which this G × E interaction may occur has been provided by Galan and coworkers (2017), who reported that MAOA interacts with punitive parenting during childhood to increase the risk of ASB, plausibly through problems in social information processing. Specifically, boys with MAOA-L alleles experiencing punitive discipline at age 1.5 years showed an increase in aggressive responses to perceived threat at age ten years. This observation provides support for the concept that MAOA-L genotypes may increase hostile attribution bias in early developmental stages, thereby impairing social information processing and enhancing the risk of aggressive responses.
In addition to the research on the interaction between MAOA uVNTR alleles and child maltreatment, a few studies have recently focused on the hypothesis that these genetic variants may also interact with prenatal and perinatal stress. These analyses have shown that infants carrying MAOA-L alleles and exposed to prenatal stress exhibited greater negative emotionality (Hill et al., 2013). Interestingly, a recent study conducted on 95 mother-child dyads verified that exposure to Superstorm Sandy during pregnancy was associated with a reduction in placental MAOA activity (Pehme et al., 2018), suggesting that prenatal exposure to adversity may synergize with the effects of MAOA genotype to cause a more profound deficit in enzyme activity (leading to greater neurochemical imbalances). However, another recent study identified that, in males, MAOA-H, rather than MAOA-L variants might interact with early-life stress to predispose to disruptive behavior at five years of age (Massey et al., 2017).
Although these confirmations strongly support the existence of a G × E interaction between MAOA-L and early adversity, several studies have failed to confirm it (Huizinga et al., 2006; Prichard et al., 2008; Kieling et al., 2013; Lavigne et al., 2013; Haberstick et al., 2014; Kiive et al., 2014; Smeijers et al., 2020). These discrepancies may partially reflect the influence of several confounding factors:
Race
Several investigations have denoted that the effects of this G × E interaction may be race-specific. In a study of 600 subjects with a documented history of abuse and neglect before 12 years of age and who were followed up into adulthood, the interaction between MAOA-L variants and child maltreatment was confirmed to increase the risk for violent and ASB only in Caucasians, but not in subjects of other ethnic groups (Widom and Brustowicz, 2006). Similar racial differences were found by Reti and colleagues (2011). These disparities may be due to both genetic and environmental factors. As noted above, the frequency of MAOA-L and MAOA-H variants is significantly different across different ethnic groups. Furthermore, racial differences have been shown for other biological variables that may play a role in aggression, including testosterone and other sex hormones (Richard et al., 2014). Racial differences are also observed in the risk of child maltreatment, mostly due to disparities in socioeconomic status (SES) (Lefebvre et al., 2017; Kim and Drake, 2018). Indeed, the fourth National Incidence Study revealed that the risk of abuse and neglect was approximately 5.8 times for children raised in families with low SES (Sedlak et al., 2010). Finally, studying racial differences in antisocial conduct may be complicated by the criteria used to operationalize violence, given racial inequalities in the criminal justice systems of the United States and other ethnically diverse countries (Rehavi and Starr, 2014; da Silva and Oliveira Lima, 2016).
Sex
Research on the uVNTR polymorphism and ASB has predominantly targeted male populations for several reasons. First, as mentioned above, male sex is a key risk factor for external aggression, ASPD, psychopathy, and violence. Second, at a molecular level, there are differences in MAOA allele pairings that make comparisons of genotypes between sexes more difficult. For instance, females are either homozygous or heterozygous for the MAOA uVNTR allele; conversely, males are hemizygous at the same genetic locus. Furthermore, X-inactivation may occur in females, although some research indicates that the MAOA gene may escape X-inactivation (Benjamin et al., 2000; Carrel and Willard, 2005). Even with these limitations, a few studies have been conducted on how the interaction of MAOA uVNTR variants and environmental factors leads to the development of ASB in females. Some data suggest that MAOA-L variants are associated with conduct-disordered behavior in women. For example, one investigation of Native American females found that the MAOA-L genotype was more common among individuals with ASB and AUD (Ducci et al., 2008). Furthermore, MAOA-L homozygous women who had a history of childhood sexual abuse were more likely to display ASPD and AUD symptoms. This interaction was only reported in women who had experienced childhood sexual abuse. In contrast to these findings, other studies have shown that, in MAOA-H female carriers, early adversity is significantly associated with conduct problems and ASB (Sjöberg et al., 2007; Prom-Wormley et al., 2009; Åslund et al., 2011; McGrath et al., 2012; Ruisch et al., 2019) as well as externalizing behaviors (Kinnally et al., 2009; Beach et al., 2010; Åslund et al., 2011; McGrath et al., 2012; Verhoeven et al., 2012). In their meta-analysis, Byrd and Manuck (2014) showed that, although no clear effect for a G × E interaction was found in females, homozygous MAOA-H female carriers exhibited stronger effects of child maltreatment, pointing to a possible sex-dimorphism of these interplays. The sex dimorphism of MAOA is in keeping with rich evidence on its gene × sex interactions (for a full discussion of this topic, please see Godar and Bortolato, 2014). Multiple mechanisms appear to participate in this interplay, including a different role of testosterone in the transcription of the MAOA gene. In line with this idea, it has been reported that higher CSF testosterone levels are linked to increased unspecified aggression only in males with the MAOA-L variant (Sjöberg et al., 2008). Furthermore, it should be mentioned that the administration of testosterone increases risk-taking responses in MAOA-L carriers (Wagels et al., 2017). These data suggest that the influence of the MAOA-L genotype may vary depending on androgen levels.
Epistatic interactions with other genes and epigenetic factors
Another key element to account for different results across multiple studies is the possibility that epistatic interactions with other genes may be critical to sharpen the long-term impact of child maltreatment on ASB risk. For example, the interaction between MAOA-H, 5-HTTLPR-SS, and a history of sexual abuse has been found to predispose Han Chinese to a greater propensity for aggression (Zhang et al., 2017). Adolescent males with the MAOA-L genotype who were homozygous for the 10R allele of the dopamine transporter 1 (DAT1) gene and who had experienced poor maternal parenting evinced less self-control and engaged in more criminal behavior (Watts and McNulty, 2016).
Other epistatic interactions have been reported in a large cohort of Swedish high-school students, including four-way interactions encompassing the brain-derived neurotrophic factor Val66Met genotype (Nilsson et al., 2014). Another key factor that may modify the interaction between stress and MAOA polymorphic variants is the epigenetic impact of other environmental variables on the MAOA promoter. Hypermethylation of the MAOA promoter has been reported among incarcerated offenders with ASPD (Checknita et al., 2015). These findings suggest that epigenetic modifications of the MAOA promoter region may fortify the association of MAOA-L genotypes and aggression. Indeed, in a small outpatient sample of adolescents misusing substances, maltreated males with the 3R MAOA genetic variant and high exonic methylation had the highest level of aggressive behavior, controlling for substance misuse, tobacco use, and psychotropic medication use (Checknita et al., 2020). At the same time, however, hypermethylation of MAOA may reduce the protective effect of MAOA-H alleles against the impact of early-life trauma.
While most research has linked the MAOA-L variant to the development of ASB with or without a history of childhood maltreatment, some investigations (typically based on smaller samples) have also shown a relationship between the MAOA-H alleles and antisocial and aggressive behavior. These studies have pointed to an association of the MAOA-H genetic variant with aggression among boys and adult men (Manuck et al., 2000; Brownlie et al., 2004). Additionally, an investigation of 174 male, Finnish violent offenders with an AUD (36% had a diagnosis of ASPD) reported that the MAOA-H genetic variant was related to violent recidivism within eight years of follow-up among individuals with a history of childhood maltreatment. No such relationship was discerned for offenders with the MAOA-L genetic variant (Tikkanen et al., 2010). Lastly, in a sample of 119 adolescent women, the MAOA-H genotype was related to increased risk for criminal behavior among those who reported active psychosocial stressors (Sjöberg et al., 2007). The divergence in findings between the effects of MAOA-L and MAOA-H uVNTR alleles could reflect different mechanisms underlying antisocial and aggressive behavior. For example, MRI research has shown that the MAOA-L genotype is associated with hyperactivity of the amygdala and hypoactivity of PFC regulatory regions, which may increase vulnerability for reactive aggression and hypervigilance to perceived threats (Meyer-Lindenberg et al., 2006). On the other hand, the MAOA-H genotype has been linked to psychopathic traits such as callousness and insensitivity, which may moderate the effects of childhood maltreatment (Pickles et al., 2013; Silva et al., 2014). Some studies (Kolla et al., 2014), but not all (Kolla et al., 2018), have also shown a connection between the MAOA-H genetic variant and proactive aggression. This association may be mediated by the level of psychopathic traits present (Kolla et al., 2018), as participants from different studies varied in the severity of psychopathic traits. These theoretical models remain speculative yet propose that different types of aggression in ASPD may be regulated by other MAOA uVNTR variants, with the MAOA-L allele being more common in ASPD with reactive aggression.
4.4. MAOA inhibition as a mechanism for ASB associated with prenatal smoking exposure
In addition to early-life maltreatment, another critical environmental factor for the predisposition to ASB, delinquency, and recidivism, independently of potential confounders and cultural factors, is prenatal exposure to tobacco (Wakschlag et al., 1997; Brennan et al., 1999; Fergusson, 1999; Weissman et al., 1999; Rasanen et al., 1999; Gibson and Tibbetts, 2000; Wakschlag and Keenan, 2001; Wakschlag et al., 2002; Cornelius and Day, 2009).
The odds of developing ASB are 1.5–4 times greater in individuals exposed to tobacco (Wakschlag et al., 2002). Several components of tobacco smoke, including 2,3,6-trimethyl-1,4-naphthoquinone and adducts of 1,2,3,4-tetrahydroisoquinoline, have been shown to inhibit MAOs (Castagnoli et al., 2002; Sari and Khalil, 2015). The β-carboline alkaloids harman and nonharman also inhibit MAOA, even though they may account for no more than 10% of the total effects of tobacco smoking on this enzyme (Truman et al., 2017). PET studies have confirmed that MAOA activity in the brain is reduced in tobacco smokers (Fowler et al., 1996), supporting the inference that fetal brain MAOA inhibition may be a mechanism whereby prenatal tobacco exposure confers a higher propensity for conduct disorder (Baler et al., 2008). Notably, Wakschlag et al. (2010) reported that, in males, MAOA-L variants interact with prenatal tobacco exposure to increase the risk of antisocial symptoms, as measured by a face-processing task.
5. The role of MAOA in aggression: human neuroimaging studies
Only a few investigations have investigated the role of MAOA in aggression and ASB. Here we present the current evidence obtained via PET and RI studies on this issue.
5.1. PET studies on MAOA activity and aggression
PET is a non-invasive neuroimaging technique that provides a window into the neurochemistry of the living human brain, including individuals with ASPD (Kolla and Houle, 2019).
Initial PET studies of MAOA focused on the relationship between trait anger and aggression in healthy participants (Alia-Klein et al., 2008; Soliman et al., 2011). These investigations converged in their findings of an inverse association between trait anger and aggression and lower MAOA binding. That is, individuals higher on personality measures of aggression and angry/hostility had lower MAOA binding in cortical and subcortical structures. Remarkably, Alia-Klein and colleagues (2008) reported that brain MAOA levels explained more than 30% of the variability in trait aggression. Consistent with the animal literature and healthy participant PET studies cited above, we similarly found that MAOA density measured using [11C]harmine PET was lower in the orbitofrontal cortex (OFC) and ventral striatum (VS) of ASPD with high psychopathic traits and aggression compared with the control group (Kolla et al., 2015). Additionally, MAOA binding in the VS was negatively correlated with measures of impulsivity. Not only do these results emphasize the importance of the MAOA KO as critical to understanding the pathophysiology and ASPD and aggression, but they also suggest that MAOA may be a feasible target for therapeutics or preventative strategies.
Other PET studies have analyzed the impact of MAOA genotypes on neurotransmitter functions. In a study with the D2/D3 ligand ([18F]DMFP), MAOA-L carriers, contrary to predictions, showed lower dopamine release and decreases in aggression after watching a violent movie, potentially indicating a role of MAOA uVNTR genotypes in the regulation of dopamine release in response to violent stimuli (Schlüter et al., 2016).
5.2. Structural MRI (sMRI)
One of the first studies of an antisocial population (Romero-Rebollar et al., 2015) used voxel-based morphometry (VBM) to examine the association of MAOA gene-environment interactions with regional changes in brain structure. The experimental group (n = 25) did not undergo diagnostic interviews but were selected based on high psychopathic traits and other measures of aggression. Twenty-eight males scoring low on all instruments comprised the comparison group. All participants were genotyped for the uVNTR polymorphism. Although no differences in grey matter volumes were detected between high and low psychopathic groups or by differing MAOA uVNTR genotypes, a G × E interaction emerged, such that MAOA-L carriers with greater aggression demonstrated grey matter reductions in the right superior temporal pole. The authors proposed that their results were consistent with other neuroimaging studies reporting abnormal connections between this region and other brain areas commonly implicated in ASPD, such as the amygdala or OFC. Other VBM studies have similarly reported a reduction of temporal pole grey matter volume in ASPD (Kolla et al., 2013).
A subsequent investigation (Kolla et al., 2017) analyzed the relationship between the amygdala surface area and OFC thickness with MAOA uVNTR alleles in a well-characterized sample of ASPD males and healthy controls. Compared with healthy MAOA-L carriers, ASPD subjects who possessed the MAOA-L genotype displayed decreased surface area of the right basolateral nucleus of the amygdala (BLA) and increased surface area of the right anterior cortical amygdaloid nucleus. Interestingly, these structural changes were also associated with enhanced psychopathic traits. Since 5-HT is a neuromodulator of amygdala BLA function (Cheng et al., 1998) and is also a substrate of MAOA, it was surmised that genetic influences altering serotonergic neurotransmission during development could affect the structure of the BLA, which is also required for intact fear conditioning (Campeau and Davis, 1995) – a process that is often impaired in psychopathy.
5.3. Functional MAOA Neuroimaging Studies of Aggression and ASPD
As mentioned above, MAOA-L carriers have been shown to exhibit abnormalities in cortico-amygdaloid connectivity (Meyer-Lindenberg et al., 2006). Male carriers of these alleles display an increased volume of the orbitofrontal cortex (OFC), as well as diminished PFC activity during inhibitory control tasks and heightened limbic responsiveness to emotional arousal (Meyer-Lindenberg et al., 2006; Buckholtz et al., 2008; Nymberg et al., 2013). These individuals also exhibit a marked enhancement of PFC activation in response to social provocation or rejection, or negative facial affect (Eisenberger et al., 2007; Lee and Ham, 2008; Denson et al., 2014), supporting the idea that MAOA in the PFC exerts a key role in evaluating the emotional salience of socio-affective cues and enacting adaptive responses. The disruption of this function may increase the sensitivity to early-life adversities and promote a negative socio-cognitive bias (Buckholtz et al., 2008; Dorfman et al., 2014). As mentioned in the introduction, alterations in the connectivity between the PFC and amygdala are a hallmark feature of reactive aggression, IED, and ASPD (Ongur and Price, 2000; Best et al., 2002; Ghashghaei and Barbas, 2002; Machado and Bachevalier, 2006; Coccaro et al., 2007; New et al., 2009; Coccaro et al., 2011; Rosell and Siever, 2015; De Cunha-Bang et al., 2017).
The effects of MAOA genotypes on the connectivity between PFC and amygdala may be age-dependent, possibly in relation to the degree of functional maturation of the cortex across different developmental stages. Indeed, the activation of the left amygdala in MAOA-L carriers in response to rejection words was observed in young adults (aged 23–28 years). In comparison, adolescents (14–16 years) with the same variants had a lower response to the same type of stimuli when compared with MAOA-H counterparts (Sebastian et al., 2010).
An early investigation (Williams et al., 2009) examined event-related potentials (ERPs) in relation to MAOA uVNTR variants among a community sample of 210 males and females. Subjects also completed the Five-Factor Inventory Antisocial Index (FFI-AI), a scale derived from the Revised NEO Personality Inventory (Costa and McCrae, 1992) that assesses the level of antisocial traits. A main effect of genotype on FFI-AI results was observed with MAOA-L carriers achieving higher scores. The investigation also included a facial emotion perceptual task, where the processing of anger in male MAOA-L carriers showed activation in medial frontal, parietal, and superior temporo-occipital regions; in MAOA-L females, effects were limited to the superior temporo-occipital area. Results suggested that the MAOA-L genotype may confer vulnerability for ASB through alteration of neural signals relevant to threat-related emotional cues. Again, caution is warranted in extrapolating these results to clinical populations.
Recent research has highlighted differences that neural networks supporting trait aggression may depend on MAOA gene alleles. For example, healthy males with the MAOA-L genotype showed stronger functional connections between the ventromedial PFC and trait aggression in regions of the default mode network, such as the right angular gyrus, posterior cingulate cortex, and dorsomedial PFC and lower correlations in bilateral supramarginal gyrus. Disengagement of the ventromedial PFC from the default mode network in MAOA-L genotype males may, therefore, be associated with lower trait aggression (Klasen et al., 2018).
The association (Kolla et al., 2018) of resting-state functional connectivity (FC) with MAOA uVNTR genotypes was investigated in a sample of ASPD males and healthy controls. ASPD participants in possession of the MAOA-L genotype endorsed greater proactive aggression than MAOA-H carriers with ASPD. However, proactive aggression among ASPD MAOA-L subjects was positively correlated with ventral striatum FC to the angular gyrus and negatively correlated with FC to the precuneus. These data provide support for the premise that neural endophenotypes underlying aggressive behavior may differ between MAOA-L and MAOA-H genotypes.
A handful of fMRI investigations have analyzed the association between MAOA uVNTR genotypes, neural activation patterns, and trait or behavioral aggression. Results have not been consistent, perhaps because studies have used different fMRI paradigms or scanning sequences. For example, one study of 125 healthy subjects from a high-risk cohort reported an association between amygdala activation and reactive aggression in response to an emotional task among MAOA-H females but not males of either MAOA genotype. In a different investigation, the combined amygdala and thalamic response to a visual linguistic anger task explained most of the variance in anger reactivity among healthy individuals with the MAOA-L genotype (Alia-Klein et al., 2009). Finally, in a sample of ASPD violent offenders, inferior ventral striatum-precuneus activity, measured during resting state, was negatively associated with trait proactive aggression among males with the MAOA-L variant (Kolla et al., 2018). Future fMRI-genetic studies of MAOA uVNTR variants could benefit from the study of additional populations with pathological aggression that employ well-validated paradigms eliciting aggressive behavior.
The effects of the interaction of MAOA genotype and early-life adversity have been analyzed in one study on 125 healthy adults from a high-risk community sample (Holz et al., 2016). The results of this study showed that, during an emotional face-matching task, the degree of activation of the amygdala and hippocampus increased in relation to levels of childhood stress in male MAOA-L carriers and decreased in MAOA-H counterparts. These effects were shown to be sex-dimorphic, with MAOA-H female carriers showing an increased response in those regions as a function of their history of childhood stress (Holz et al., 2016). The same authors found that sex x genotype x stress interaction also influenced response inhibition in a go/no-go task (Holz et al., 2016). While the neurobiological mechanism of this difference between males and females remains unknown, a recent study has shown that, in men, testosterone administration can synergize with MAOA-L genotype to increase the propensity for risk-taking behavior and responsiveness to social provocation, by lowering the activation of the insula (Wagels et al., 2017). The same interaction was found to increase the involvement of brain regions subserving responsivity toward social provocation (such as the cuneus) (Wagels et al., 2019). These findings point to the possibility that androgen hormones may moderate the role of MAOA uVNTR genotypes on corticolimbic connectivity.
6. The role of Maoa in aggression and ASB: animal evidence
While MRI neuroimaging studies are paving the way to understand the neuroanatomic alterations related to the functioning of gene variants, animal models remain a crucial tool to gain mechanistic insight into the neurochemical processes associated with behavioral changes and genetic mutations. Studies in mammalian models are particularly meaningful, given the high homology of human MAOA with those of other placental mammals (Table 4).
Table 4.
Degrees of MAOA sequence identity across humans (H. sapiens sapiens), chimpanzees (P. troglodytes), dogs (C. lupus familiaris), cattle (B. Taurus), mouse (M. musculus) and rat (R. Norvegicus). Data are obtained from the gene NCBI database.
| Human | |||||
| 99.17% | Chimpanzee | ||||
| 89.75% | 89.47% | Dog | |||
| 88.05% | 87.81% | 89.75% | Cattle | ||
| 88.12% | 88.48% | 87.74% | 85.82% | Mouse | |
| 88.00% | 88.30% | 86.83% | 86.07% | 95.98% | Rat |
6.1. Neurobehavioral effects of MAOA in non-human primates
Several studies have been conducted to understand the presence of MAOA polymorphisms in primates. All apes have been found to display a VNTR polymorphism similar to the uVNTR in humans. In chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), a single 30 bp-repeat sequence, identical to that in humans, was discovered (Wendland et al., 2006); however, most individuals display one repeat (Wendland et al., 2006), and an extremely rare, no-repeat allele with no repeats was found in 0.01% of chimpanzees (Inoue-Murayama et al., 2006). In gorillas (Gorilla gorilla), the VNTR features 2, 3, 4, or 5 copies of a shorter repeat, featuring the first 18 bp of the human and chimpanzee copies (ACCGGCACCG GCACCAGT) (Inoue-Murayama et al., 2006). Of these alleles, the 2R variant was by far the most common (78.4% of allelic frequency). Orangutans (Pongo pygmaeus) also exhibited a VNTR of a 12 bp repeat (Inoue-Murayama et al., 2006) with 1.5, 3 or 4 repeat variants; however, because of slight intraspecies variations across repeats and the high degree of repetition within each series, no consensus has been reached as to the exact size of the repeat and the number of copies in the VNTR polymorphism (see Wendland et al., 2006). Finally, similar VNTR polymorphisms have been documented in all the species from the four known genera of gibbons (Hylobates, Nomascus, Symphalangus, and Hoolock) (Choi et al., 2014).
The transfection of VNTR constructs of apes in a human neuroblastoma cell line showed that, unlike the significant difference between human 3R and 4R variants, neither chimpanzee nor orangutan alleles showed any substantial divergence in luciferase activity. The 2R allele of the gorilla VNTR was associated with a slight numeric increase (approximately 12.5%) in comparison with 3R and 5R variants (Inoue-Murayama et al., 2006). Although these data suggest that these polymorphisms are unlikely to be functional, current evidence on the potential contributions of these variants to aggression in other apes remains scant.
Most of the information on MAOA VNTRs in non-human primates and their functional implications comes from a VNTR in Rhesus monkeys (Macaca mulatta), featuring an orthologous polymorphism (rhMAOA-LPR) in the upstream regulatory region of the MAOA gene in macaques, at 1.1 kb from the initiation site of the MAOA gene (Newman et al., 2005). The rhMAOA-LPR features 4, 5, 6, or 7 copies of an 18-bp repeat highly similar to that observed in gorillas (ACCGGCACTG GCACVACT) (Newman et al., 2005; Wendland et al., 2006; Jones et al., 2020). Available data suggest that the four alleles have different frequencies, with a possible predominance of the 7R variant (4R: 0–1%; 5R: 15–35%; 6R: 25–43%; 7R: 40–41%) (Wendland et al., 2006; Jones et al., 2020). Based on in vitro studies, the 5 and 6 R alleles were found to transcribe at a higher rate than 7R variants (Newman et al., 2005). The same 18-bp repeat motif has been identified in the Japanese macaque (Macaca fuscata), the gelada (Theropithecus gelada), and several species of baboons (Papio gen.). However, the distribution of variants diverges greatly within each species; for example, gelada only showed a 6R variant (Wendland et al., 2006), while M. fuscata displays 6R and 7R alleles (Jones et al., 2020). Different species of baboons show 8,9, or 10 copies of the repeat, with varying distributions of frequency depending on their origin (Kalbitzer et al., 2016). The high intraspecies variability of MAOA promoter in M. mulatta has been speculated to reflect the exceptionally wide geographical distribution of this species as well as their high adaptive capability (Wendland et al., 2006). Capitalizing on the knowledge of polymorphic MAOA variants in rhesus macaques, a few studies were performed to verify whether these alleles may interact with early-life environment to modify the predisposition to aggression. These investigations found that male macaques with low-activity rhMAOA-LPR alleles reared by mothers in small social groups had higher aggression scores (Newman et al., 2005; Karere et al., 2009).
Very little information is currently available about MAOA tandem repeats in other species than primates. Eo and coworkers (2016) identified a 90-bp VNTR in the upstream region of the MAOA gene of dogs. All tested breeds, however, showed two copies of this sequence, suggesting that this motif does not affect enzyme expression or function or explain potential differences in aggressiveness.
6.2. Neurobehavioral effects of Maoa deficiency and hypomorphism in mice
While studies in primate models have stressed the importance of early-life environment in shaping the role of MAOA in the ontogeny of aggression, they have not led to significant mechanistic advances on the neurochemical processes mediating the interplay between MAOA-L and early-life stress. Studies in rodents, on the other hand, have been crucial to understanding the mechanisms of action by which variations in MAOA contribute to aggression (Bortolato and Shih, 2011; Godar et al., 2016). The first studies on the neurobehavioral impact of Maoa genetic deficiency came from the serendipitous generation of a line of Maoa KO mice following the integration of an interferon beta cassette in exons 2 and 3 of the Maoa gene of C3H/HeJ mice (Cases et al., 1995). The resulting frameshift mutation resulted in an early truncation of the corresponding protein and the ablation of the native Maoa gene. Strikingly, these mutants exhibited several behavioral characteristics highly reminiscent of the phenotypes of Brunner syndrome (Brunner et al., 1993). Specifically, adult male mice carrying the mutation exhibited high levels of inter-male aggression, which was accompanied by significant elevations of 5-HT and norepinephrine. Of note, the elevation in 5-HT brain levels was found to be particularly pronounced during the first weeks of life (with an overall 10-fold increase in the mutants compared with wild-type littermates); conversely, the greatest increase in norepinephrine levels was more modest and was primarily observed after the third week onward. The increase in whole-brain dopamine was even smaller and was limited to late adolescence and young adulthood. These results suggest that the catabolism of different neurotransmitters by Maoa varies across different developmental stages (as well as brain regions). Similar behavioral and neurochemical phenotypes were observed in a second mouse line harboring a spontaneous point mutation in exon 8 of the gene in a different genetic background (129S6) (Scott et al., 2008). Detailed studies on the behavioral repertoire of Maoa KO mice showed that aggression in these mutants is accompanied by severe deficits in sociability and environmental exploration (Bortolato et al., 2011; Godar et al., 2011; Bortolato et al., 2013). Maoa KO mice were found to display exaggerated defensive responses towards foreign counterparts as well as innocuous objects; conversely, these mutants showed a reduced reaction to several stressors, including physical restraint, cold temperature, and predator cues (Popova et al., 2006; Godar et al., 2011). Notably, the increased aggression in Maoa KO mice is not limited to reactive constructs, as it also encompasses a lower latency in predatory attacks against a cricket (Vishniyetskaya et al., 2007), possibly indicating the link of this genetic alteration with both defensive and proactive aggression.
Adding to the complexity of these behavioral aberrations, the social deficits in Maoa KO mice were also found to be accompanied by a full spectrum of autism-related alterations, including communication deficits and perseverative responses, as well as poor reversal learning and reduced acoustic and tactile sensitivity (Bortolato et al., 2013). In line with these discoveries, several recent reports have documented that children affected by Brunner syndrome show autistic-like abnormalities (Piton et al., 2014; Palmer et al., 2016; Bortolato et al., 2018). Furthermore, autistic patients with MAOA-L variants have thicker cortex as well as greater severity of several symptoms, including arousal regulation deficits, aggressiveness, and communication deficits (Davis et al., 2008; Cohen et al., 2011).
Although the MGI repository lists 229 SNPs in mice, no polymorphism (with the exclusion of nonsense mutations) has yet been documented to impact MAOA activity in this species. To gain insight into the phenotypical outcomes of polymorphic variants associated with low activity, a new line of hypomorphic mice (MaoaNeo) was generated by the insertion of a neomycin resistance cassette into intron 8 of the Maoa gene (Bortolato et al., 2011). This mutation results in a significant reduction in Maoa transcript and enzyme activity in the brain. While these mice share the same socio-communicative deficits as their KO counterparts (Bortolato et al., 2011; Godar et al., 2019), they exhibit only a very modest increase in aggression propensity. These studies appear to indicate that the primary deficit associated with MAOA deficiency is the impairment of social and environmental information processing during early developmental stages. In turn, these alterations may enhance the propensity for the emergence of ASPD and other developmental disabilities. Indeed, poor social information processing is regarded as critical for the ontogeny of aggressive behavior (Schwartz et al., 1997).
In line with human evidence, several studies have shown that both Maoa KO and MAOANeo male mice display functional PFC deficits (Bortolato et al., 2011), as well as alterations in corticogenesis. Both mutant lines are characterized by cytoarchitectural alterations in the columnar organization of the cortex. These anomalies are best observed in the barrel fields of layer IV of the somatosensory cortex (Bortolato et al., 2011; Cases et al., 1995). These structures are spatially well-demarcated, as they correspond to the sensory map of the mystacial vibrissae in the rodent snout (Erzurumlu and Jhaveri, 1990). Cortical columns are posited to integrate input over time and space and are, therefore, essential for integrating environmental information (Hawkins et al., 2017). Thus, it is likely that alterations of columnar organization in the PFC may underlie the information deficit processing in these mutants. It is worth noting that the pyramidal cells in MAOANeo male mice show an exaggerated development of dendritic arbor, which may signify excessive connectivity in this region. In line with this interpretation, a recent multimodal neuroimaging study conducted on more than 200 healthy subjects showed that MAOA-L carriers exhibit cortical hyperconnectivity in relation to a selective impairment of implicit emotion processing (Harneit et al., 2019). The dysfunctional connectivity of the PFC is also signaled by the evidence that Maoa KO mice display deficits of the NMDA glutamate receptors, which serve as primary targets for the integration of different signals at the neuronal level, in the PFC (Bortolato et al., 2012). Such alterations included reduced glycosylation of NR1 subunits in the PFC, which leads to lower levels of release of NMDA from the endoplasmic reticulum and membrane expression (Bortolato et al., 2012; Lichnerova et al., 2015). The relevance of the PFC to the manifestation of aggression in Maoa KO mice is also indicated by evidence indicating that this behavioral abnormality is rescued by the genetic reinstatement of human MAOA in the forebrain areas (Chen et al., 2007).
6.3. Interactive Effects of MAOA genotypes and environmental factors
To study the mechanisms of G × E interactions, a mouse model has been recently developed (Godar et al., 2019), based on subjecting MAOANeo male pups (as well as their wild type littermates) to a regimen of maternal separation and daily intraperitoneal injections - mimicking neglect and physical abuse, respectively - for the first three weeks of postnatal life. The most effective way to deliver this regimen of early stress was to subject mice to these stressors following a pseudorandom schedule (at different times of the day and with variable durations), which increased the unpredictability of each event. This manipulation resulted in the emergence of aggressive responses in adolescent and adult MAOANeo mice; conversely, wild-type littermates exhibited a strikingly different phenotype, including some behavioral alterations more directly akin to depression-like responses, rather than aggression. This result is reminiscent of similar human findings (Beach et al., 2010). The increase in aggressive behavior in MAOANeo mice subjected to early stress is not accompanied by other specific anxiety-like responses but is associated with a low reactivity to environmental threat and stress. These characteristics resemble the low emotional arousal documented in some antisocial individuals (Raine, 2002). According to the low arousal theory, antisocial individuals engage in aggressive and socially unsanctioned behavior to compensate for their low emotional reactivity to stimuli (Raine et al., 1997).
We further qualified that the temporal window to produce the effect was primarily limited to the first week of postnatal life. Indeed, stress during the first week of postnatal life produced significant increases in aggression, while beginning the stressful schedule from the second week did not. This concept highlights the importance of the first week of postnatal life in mice to produce aggressive and antisocial-related behaviors. These data are aligned with previous observations in rodents, which showed that perinatal pharmacological inhibition of MAOA produced impulsive and aggressive responses (Whitaker-Azmitia et al., 1994; Mejia et al., 2002), which are accompanied by long-term perturbations of serotonergic, but not dopaminergic signaling in the cortex and raphe nuclei (Burke et al., 2018). It should be noted that, in mice, the brain expression of the Maoa gene in serotonergic neurons is high during the first four days and progressively declines to reach a minimal level by the third week of postnatal life (Vitalis et al., 2002). Conversely, the activity of MAOA in dopaminergic neurons is very low at birth and progressively increases to reach stability around the 10th day of life. These data further support the relevance of the serotonergic system in the early stages of G × E interaction between MAOA-L and child maltreatment.
Before the onset of aggressive reactions, MAOANeo mice exposed to early stress were also found to exhibit several phenotypic predictors of aggression documented in humans, including low body weight, hyperactivity, communication deficits, and, particularly, low resting heart rate (Raine et al., 1990; Portnoy et al., 2014; Latvala et al., 2015). This last response likely corresponds to low arousal in humans. The analysis of the mechanism whereby the MAOA-L genotype interacts with early-life stress pointed to the selective up-regulation of 5-HT2A receptors in the PFC. Accordingly, the use of antagonists for this receptor during the first postnatal week fully rescued the trajectory to aggression in this model. This evidence aligns with previous studies showing that 5-HT2A receptor antagonism suppresses aggression in Maoa KO mice (Shih et al., 1999) as well as other models of murine aggression (Sakaue et al., 2002) and points to these receptors as critical modulators of the behavioral outcomes of aggression. In line with this evidence, the density of 5-HT2A receptors has also been reported to be increased in the OFC of aggressive personality-disordered patients (Rosell et al., 2010). At the same time, it should be noted that this up-regulation may be specific to certain compartments of the PFC, since other studies have found a reduction in 5-HT2A receptor binding potential in the dorsolateral PFC of violent, aggressive individuals (Meyer et al., 2008). To explain the role of PFC 5-HT2A receptors in the pathophysiology of ASPD and psychopathy, it is important to note that these molecules exert a profound influence on threat reactivity in the amygdala. In particular, Fisher and colleagues (2015) reported that the density of 5-HT2A receptors in the medial PFC was inversely correlated with the reactivity of the right amygdala and that it explained 25–37% of the variability in the response of this region to threat. Ongoing studies in the Bortolato lab demonstrate that MAOANeo mice exposed to early stress exhibit a progressive, age-dependent decline in the responsiveness of glutamatergic receptors in the PFC, suggesting that the early activation of 5-HT2A receptors may lead to an escalating deficit of this region (Fig. 2).
Figure 2.
Hypothesized mechanism of interaction between MAOA-L and child maltreatment in ASB.
On the other hand, the Kolla lab recently reported that glutamine+glutamate (Glx) metabolites measured using magnetic response spectroscopy were greater in the PFC of ASPD with high psychopathic traits compared with individuals with bipolar disorder and healthy controls and that they were positively correlated with a measure of aggression (Smaragdi et al., 2019).
Since a reduced density of NMDA receptors is a typical signature of aging (Segovia et al., 2001), increased Glx could be a candidate biomarker of adult ASPD or psychopathy. Although the mechanisms whereby early-life activation of 5-HT2A receptors may lead to PFC deficits remain elusive, several studies have evidenced that the stimulation of these receptors enhances glutamatergic transmission in the PFC (Marek and Aghajanian, 1998; Mocci et al., 2014), which may lead to altered connectivity due to excitotoxicity or morphological aberrances of dendritic arbors.
Recent evidence has particularly shown that activation of presynaptic 5-HT2A heteroreceptors in glutamatergic thalamocortical terminals enhances glutamate release to the PFC (Barre et al., 2016). Overall, these data suggest that overactivation of 5-HT2A receptors in the PFC during early postnatal life may reduce the threshold for glutamatergic excitotoxicity, which is already low during the first two weeks after birth (McDonald et al., 1992). Future studies should assess whether early-life activation of 5-HT2A receptors in Maoa-deficient mice is conducive to neuronal death in the PFC.
Peripubertal stress, a known risk factor for aggression (Márquez et al., 2013), may also alter the binding of Sirt1 (Imai et al., 2000), a NAD-dependent deacetylase, to the MAOA promoter. In male but not female rats exposed to peripubertal stress, MAOA expression and enzyme activity was reduced in the hypothalamus and increased in the PFC. Subsequent investigation revealed hypomethylation of the Maoa promoter and hypermethylation of the hypothalamus. However, binding of Sirt1 showed an opposite pattern with an increase in the Maoa promoter and a decrease in the hypothalamus. Neither MAOA expression nor alteration of the epigenetic status of MAOA was present in female rats exposed to peripubertal stress, suggesting a pattern of sexual dimorphism in the molecular changes associated with early stressors, MAOA and aggression (Konar et al., 2019).
Another mechanism that may contribute to the pathogenesis of aggression in MAOAdeficient subjects is the elevation in brain dopamine content. Although dopamine levels are only mildly increased in the central nervous system of Maoa KO mice, recent studies have shown that the direct optogenetic stimulation of dopaminergic neurons in the mesocorticolimbic system leads to a marked enhancement of aggressive behaviors (Yu et al., 2014). Given that cortical dopamine is predominantly metabolized by catechol-O-methyltransferase (COMT), low MAOA activity may lead to selective changes in dopamine signaling in the nucleus accumbens. Accordingly, studies in rodents have shown that the accumbal concentrations of this neurotransmitter are increased before, during, and after an aggressive encounter (de Almeida et al., 2005). Clinical evidence has also implicated hyperactive dopaminergic limbic transmission in the genesis of reactive aggression (Comai et al., 2012). Furthermore, given the importance of accumbal dopamine signaling in habit formation, it is likely that this substrate may play a role in the formation of aggressive habits and the perpetuation of ASBs. It is important to note that 5-HT2A receptors in the PFC have been shown to regulate the release of dopamine in the mesolimbic system (Bortolozzi et al., 2005). Thus, activation of these receptors may facilitate the release of dopamine from the VTA to the nucleus accumbens, which in turn sustains aggressive responses through activation of D1 receptors (Frau et al., 2019). As impulsive traits in humans similarly predict nucleus accumbens dopamine release following pharmacological challenge (Buckholtz et al., 2010), novel neuropsychopharmacological interventions that inhibit or attenuate striatal dopaminergic signaling could emerge as potential therapeutics for aggression, ASPD, and psychopathy.
The surge in testosterone during adolescence likely plays a key role in shaping aggression by affecting the homeostatic control of dopamine neurotransmission. Ample evidence has shown that, by acting on androgen receptors, this hormone increases the synthesis of both MAOA as well as tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis (Goldstein et al., 1992; Purves-Tyson et al., 2014). While the upregulation of these two enzymes may maintain a balance in dopamine turnover, it is possible that in MAOA-L subjects, the effects of testosterone could have a greater effect on TH, leading to increased production of dopamine in the mesolimbic pathway. This situation may be particularly problematic in individuals with constitutionally low emotional arousal and deficient PFC functioning.
It is worth noting, however, that, while dopaminergic mechanisms may play a critical role in the ontogeny of aggression of Maoa-deficient mice, the translation of these data to humans is definitely complicated, given that, as mentioned in Section 2 of this article, dopamine catabolism in primates is primarily served by MAOB, rather than MAOA. At the same time, although no clinical condition of isolated MAOB deficiency has been described in humans, low MAOB platelet activity has been associated with novelty-seeking traits (Ruchkin et al., 2005), but not aggression. It is worth noting that the deletion of the Maob gene in mice is associated with a very similar behavioral profile, but no changes in dopamine brain-regional content (Grimsby et al., 1997; Bortolato et al., 2009).
These potential interspecies discrepancies notwithstanding, the findings in Maoa-deficient mice collectively point to the possibility that the ontogeny of ASPD and psychopathy may be reflective of multiple, age-specific neurochemical mechanisms. According to this multi-hit hypothesis, the interaction of MAOA-L variants and early life stress may be mediated by overactive 5-HT2A receptors in the PFC, leading to functional impairments of the amygdala and a reduction in emotional arousal. Throughout adolescence, these problems may be compounded by alterations in the mesolimbic dopaminergic system contributed by testosterone, ultimately leading to the overt expression of aggressive behaviors.
7. Concluding statements and future directions
The findings presented in the previous sections illustrate how the study of MAOA uVNTR polymorphism in relation to ASPD, psychopathy, aggression, and antisocial outcomes among small samples provided the initial landscape for investigation of MAOA uVNTR genetic variants in larger and well-characterized samples of antisocial outcomes. The field was further enhanced with the discovery that MAOA-L genetic variants in concert with adverse environmental outcomes increased risk for aggression and antisocial symptoms. Still, the incredible heterogeneity of the results illustrates that diverse and nuanced factors contribute to this variance. For example, differences in the definition, duration, and type of early adverse experiences may help explain incongruent findings in similar populations (Kolla and Vinette, 2017).
A growing line of research suggests that MAOA uVNTR polymorphism is associated with structural and functional brain changes in ASPD. Imaging genetics holds promise for the elucidation of neural endophenotypes underscoring violence and aggression in ASPD and psychopathy. The preclinical and clinical evidence presented in the previous sections collectively shows that a reduction of in vitro MAOA activity combined with stress during early life increases susceptibility to ASPD. Findings in animal models of G × E interactions highlight a key role of 5-HT2A receptors in the mechanism underpinning this biosocial interplay. In line with this evidence, four polymorphisms of the HT2RA gene (rs6311, rs6313, rs6314, and rs7322347) have been shown to affect aggression and ASB (Bjork et al., 2002; Assal et al., 2004; Nomura et al., 2006; Burt and Mikolajewski, 2008; Banlaki et al., 2015). These findings highlight the possibility that the role of MAOA on ASPD or psychopathy may be moderated by HT2RA polymorphisms. Future studies are warranted to evaluate the role of epistatic MAOA × HT2RA interactions in ASPD and psychopathy. These and other epistatic interactions may be critical to understanding the relationship between MAOA and both reactive and proactive aggression.
Another intriguing corollary of this discovery is that 5-HT2A receptor antagonists may be a viable treatment strategy for premorbid and prodromal manifestations of ASB, which may be highly valuable in reducing the susceptibility for ASPD, psychopathy, and violent behavior. The development of novel, more specific animal models mimicking the role of MAOA in ASPD and psychopathy may be critical to test the efficacy of selective 5-HT2A receptor antagonists as a potential novel therapeutic and preventative tool. From this perspective, it is worth noting that pimavanserin, a novel 5-HT2A receptor inverse agonist, has been approved in 2014 for clinical use as a novel antipsychotic therapy for Parkinson’s disease patients (Hunter et al., 2015).
The availability of novel animal models and advanced neuroimaging technologies affords new translational research tools to elucidate the role of MAOA and its interaction with environmental factors in the ontogeny of aggression and violence. The integration of these strategies with traditional research approaches may prove critical to addressing the following mechanistic problems on the neurobiology of ASPD and psychopathy:
Mechanisms of sex dimorphism in G × E interactions
As mentioned above, evidence on the interaction between MAOA genotypes and early environmental factors in female carriers remains inconclusive, due to the insufficient power of studies that have compared MAOA-LL and MAOA-HH homozygous carriers. Furthermore, given the influence of ovarian hormones on serotonergic transmission and 5-HT2A receptor expression (Biegon et al., 1983; Sumner and Fink, 1995; Cyr et al., 1998; Rubinow et al., 1998), it is conceivable that these alterations may be partially responsible for the association between estradiol and aggression in women (Stanton and Schultheiss, 2007; Vermeersch et al., 2008) as well as for the fluctuations of aggressive responses throughout the menstrual cycle (Ritter, 2003). Animal models may be a useful tool to study the impact of early life stress in Maoa-deficient female mice across different phases of their estrous cycle, and dissect the mechanisms underlying such differences.
Molecular mediators of stress in G × E interactions
The mediator that links stress to interaction with the MAOA genotype, ultimately leading to 5-HT2A receptor activation and aggression, remains unknown. A promising candidate may be corticotropin-releasing factor (CRF), which has been shown to mediate many of the long-term psychopathological sequelae of early life stress (Nemeroff, 2004; Gondre-Lewis et al., 2016; Forster et al., 2018) and up-regulate the expression of 5-HT2A receptors in the PFC (Magalhaes et al., 2010). In line with this possibility, recent evidence from humans reveals that variance in corticotropin-releasing factor receptor 1 (CRF1) genetic polymorphic alleles is associated with violence and aggression in young Han Chinese males (Liu et al., 2019). Synthesis of suitable PET radioligands for CRF1 (Lodge et al., 2014) may allow researchers to acquire in vivo human data of potential CRF1 receptor anomalies in ASPD, psychopathy, and aggression.
Neurodevelopmental mechanisms of G × E interactions
By definition, the antisocial outcomes associated with MAOA variants should be classified as a typical example of LCP ASB. Indeed, one of the key differences between LCP and AL is the strong biological and genetic signature associated with the former subtype (Moffitt, 1993). Even with this persistence, very little is known about the age-specific neurobiological alterations associated with the trajectory of LCP ASB. In particular, the transience of AL antisocial conduct may reflect the maturation of the PFC in the second decade of life, providing a better inhibitory control over the propensity to engage in aggressive and delinquent behavior (Arain et al., 2013); however, these mechanisms appear to have very little to no impact on LCP ASB. Mouse models of G × E interactions of early-life stress and low-activity Maoa genotypes afford an interesting opportunity to examine how the biological impairments caused by this biosocial interplay override the behavioral changes associated with cortical maturation and ensure the persistence of aggressive tendencies through adulthood.
Role of G × E interactions in the pathophysiology of comorbid problems in ASPD and psychopathy
Ample evidence has shown that MAOA uVNTR variants exert sex-dimorphic main effects on the development of AUDs, as well as interactive effects with early-life adversity on the expression of AUDs (Samochoviec et al., 1999; Schmidt et al., 2000; Vanyukov et al., 2004; Guindalini et al., 2005; Contini et al., 2006; Ducci et al., 2008; Nilsson et al., 2011). Furthermore, MAOA-L variants are associated with a younger age of onset of alcohol dependence (Vanyukov et al., 1995; Vanyukov et al., 2004) and antisocial alcoholism (Samochoviec et al., 1999) in males. A history of maltreatment predisposes male MAOA-L carriers or female MAOA-H carriers to a greater risk of alcohol use (Nilsson et al., 2011). In alignment with these findings, we found that MAOA-L males and MAOA-H females with a history of child maltreatment have a higher risk for tobacco use (Fite et al., 2018) and polysubstance use (Fite et al., 2020). Accordingly, the examination of MAOA variants in comorbid conditions known to exacerbate the symptomatology of ASPD and psychopathy may be fruitful avenues to pursue as novel therapeutics that can mitigate the effects of ASPD and psychopathy. From this perspective, the examination of the effects of drugs of abuse on mouse models of G × E interactions is likely to offer a unique platform to examine the reciprocal links and influences between AUD and ASPD.
Verification of the differential susceptibility hypothesis for G × E interactions
The interaction between MAOA alleles and child maltreatment has been traditionally explained through the diathesis-stress framework, which posits that genetic factors confer vulnerability to stress, and the synergism of the two factors leads to a super-additive negative effect (Zuckerman, 1999). Alternatively, the differential susceptibility hypothesis suggests that some genetic factors can predispose to either positive or negative sequelae, depending on the specific environmental interactors (Belsky et al., 2009; Ellis et al., 2011). In the particular case of the uVNTR polymorphism, MAOA-L variants should not only confer greater vulnerability to stress, but also higher responsiveness to a positive environmental influence. Several studies have provided preliminary support for this theoretical framework (Oreland et al., 2007; Simons et al., 2012; Nilsson et al., 2018). For example, in a stratified sample of Swedish youth, the MAOA-L genotype was found to increase or decrease the risk of criminality in boys as a function of high or low psychosocial stress in their environment (Oreland et al., 2007). The same authors further showed that MAOA-L carriers with a history of positive relationships with their parents showed a lower risk of delinquency (Nilsson et al., 2014). Nevertheless, evidence on the protective potential of MAOA-L genotypes for boys raised in a nurturing environment remains inconclusive and awaits further experimental confirmation. From this perspective, the employment of animal models may offer an attractive platform to help confirm the predictions of the differential susceptibility hypothesis. If this framework applies to animal models, it would be expected that exposure of dams and litters to environmental enrichment (such as nesting material and shelters) during pregnancy and lactation may lead to more favorable long-term outcomes in MAOANeo mice. These studies, however, may be complicated by potential experimental caveats and interpretational issues, as previous research has documented that, in several mouse strains, enrichment is often associated with increased levels of intermale aggression (Van Loo et al., 2002; Bayne, 2018). Given the abnormal reactivity of Maoa-deficient mice to contextual stimuli (Godar et al., 2011), it is particularly important to verify what types of environmental enrichment may reduce stress in these mutants, by monitoring neuroendocrine responses.
Role of MAOA variants in reactive and proactive aggression
One of the major unresolved issues about the role of MAOA in aggression concerns its association with reactive or proactive aggression. In general, most evidence appears to suggest that MAOA may have a predominant role in impulsive and reactive aggression. Nevertheless, some evidence indicates a high frequency of MAOA-L genotypes among individuals that engage in proactive aggression. We hypothesize that, while the interaction of low-activity MAOA variants with early-life stress may lead to an increase in impulsive and defensive aggression, the consistent enactment of violence (facilitated by certain settings) from adolescence onwards may gradually increase the likelihood that these individuals also engage in proactive and appetitive aggression. A similar type of progression has, indeed, been described among combatants in war-torn regions. At the same time, these individuals typically experience a strong aversion for the extreme violence of their first killing, they gradually become desensitized towards this sense of repulsion and experience more motivation and pleasure from inflicting harm on their victims (Elbert et al., 2010). Given that engagement in fights increases dopamine in the nucleus accumbens just like any drug of abuse, it is possible that, in a subset of MAOA-deficient individuals, the activation of reward circuits may facilitate the transition from impulsive aggression to a veritable “aggression addiction,” as defined by Golden and Shaham (2018). Similar to addicts, violent offenders engage in appetitive aggression despite their awareness of long-term untoward consequences and have a very high rate of recidivism (relapse). This phenomenology has been replicated in a subset of aggressive mice, which engage in operant behavior to attack subordinate males and establish conditioned preference for fighting-associated contexts (Fish et al., 2002; Golden et al., 2016).
Based on this background, it is tempting to speculate that, exactly like in the escalation from abuse to dependence, engagement in violence in some individuals with low MAOA activity may also shift from initial impulsive episodes to a long-standing habit. Incidentally, the fact that low MAOA activity reduces the catabolism of dopamine, thereby prolonging its synaptic effects on the striatum, may offer a mechanistic support in favor of a facilitated transition to habit formation. Although a first study has failed to find an association between MAOA and appetitive aggression in a community of low-income South-Africans (Hemmings et al., 2018), these results should be confirmed by further studies, particularly since this study did not account for the impact of early-life trauma in aggressive manifestations. Studying the progression of aggressive behaviors in MAOANeo mice subjected to early stress may offer a unique opportunity to study how the trajectory of aggression in these animals may be accompanied by progressive alterations in dopamine release as well as plastic changes associated with the reward system.
The title of this article pays homage to John Steinbeck’s novella Of Mice and Men, which depicts a cruel world where violence begets violence by a seemingly inexorable law of nature. Just as the story of the two protagonists casts light on the diverse motivations promoting this endless cycle of human violence, so too can mouse models of G × E interactions in ASB offer a unique window into the mechanisms underlying aggression. For more than two decades, MAOA has secured its place as one of the most intensely scrutinized genes in psychiatric research and is arguably the most important in the study of human aggression. We fully expect that the synergism of genetic, neuroimaging, and animal research on this gene and its gene product will point to new horizons for understanding the widespread - yet still largely elusive - problem of violence and aggression.
Supplementary Material
HIGHLIGHTS.
This review article discusses the role of monoamine oxidase (MAOA) in aggression
Findings of genetic and neuroimaging studies are discussed
The evidence from animal models of MAOA deficiency is also presented
A potential mechanism to explain the involvement of MAOA in aggression is framed
Future directions and experimental challenges are highlighted
Acknowledgments
Financial Disclosures: The present manuscript was supported by grants from the National Institute of Mental Health (NIH R01 MH104603 to M.B.), as well as funding from the ALSAM Foundation (to M.B.). Grants from the Canadian Institutes of Health Research to NJK also supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang GJ, Volkow ND, Fowler JS, 2008. Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 28, 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Tomasi D, Woicik PA, Moeller SJ, Williams B, Craig IW, Telang F, Biegon A, Wang GJ, Fowler JS, Volkow ND, 2009. Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion. 9, 385–396. 10.1037/a0015904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tayie SR, Ali AA, 2018. Allelic Diversity of uVNTR polymorphism in Monoamine Oxidase A (MAOA) gene in Iraqi Population. J. Pharmaceutical Sci. Res. 10, 3099–3102. [Google Scholar]
- American Psychiatric Association., 1952. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publ., Washington, DC. [Google Scholar]
- American Psychiatric Association., 1980. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publ, Washington,DC. [Google Scholar]
- American Psychiatric Association., 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM, Fifth edit. ed. American Psychiatric Association, Arlington VA. [Google Scholar]
- Anderson CA, Bushman BJ, 2002. Human Aggression. Annu. Rev. Psychol. 53, 27–51. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Carnagey NL, 2004. Violent evil and the general aggression model In Miller AG (Ed.), The social psychology of good and evil (pp. 168–192). New York, NY: Guilford Press. [Google Scholar]
- Anholt RR, Mackay TF, 2012. Genetics of aggression. Annu Rev Genet 46, 145–164. 10.1146/annurev-genet-110711-155514 [DOI] [PubMed] [Google Scholar]
- Anichtchik O, Sallinen V, Peitsaro N, Panula P, 2006. Distinct structure and activity of monoamine oxidase in the brain of zebrafish (Danio rerio). J Comp Neurol. 498, 593–610. [DOI] [PubMed] [Google Scholar]
- Antypa N, Giegling I, Calati R, Schneider B, Hartmann AM, Friedl M, Konte B, Lia L, De Ronchi D, Serr etti A, Rujescu D, 2013. MAOA and MAOB polymorphisms and anger-related traits in suicidal participants and controls. Eur Arch Psychiatry Clin Neurosci. 263, 393–403. 10.1007/s00406-012-0378-8 [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S, 2013. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 9, 449–61. 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J, 2009. The nature of human aggression. Int J Law Psychiatry. 32(4):202–208. [DOI] [PubMed] [Google Scholar]
- Armstrong TA, Boutwell BB, Flores S, Symonds M, Keller S, Gangitano DA, 2014. Monoamine oxidase A genotype, childhood adversity, and criminal behavior in an incarcerated sample. Psychiatr Genet 24, 164–171. 10.1097/YPG.0000000000000033 [DOI] [PubMed] [Google Scholar]
- Arslan BK, Edmondson DE, 2010. Expression of zebrafish (Danio rerio) monoamine oxidase (MAO) in Pichia pastoris: purification and comparison with human MAO A and MAO B. Protein Expr Purif. 70, 290–297. 10.1016/j.pep.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW, 2011. Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behav Genet 41, 262–272. 10.1007/s10519-010-9356-y [DOI] [PubMed] [Google Scholar]
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL, 2004. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol 61, 1249–1253. 10.1001/archneur.61.8.1249 [DOI] [PubMed] [Google Scholar]
- Atramentova LA, Luchko EN, 2016. Aggression and empathy as genetic differentiation factors of urban population. Genetika. 52, 705–712. [PubMed] [Google Scholar]
- August GJ, Realmuto GM, MacDonald AW 3rd, Nugent SM, Crosby R, 1996. Prevalence of ADHD and comorbid disorders among elementary school children screened for disruptive behavior. J Abnorm Child Psychol. 24, 571–595. [DOI] [PubMed] [Google Scholar]
- Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, Seeburg PH, Shih JC, 1988. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 85, 4934–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin E, 2002. Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet 110, 1–7. 10.1007/s00439-001-0652-8 [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND, Fowler JS, Benveniste H, 2008. Is fetal brain monoamine oxidase inhibition the missing link between maternal smoking and conduct disorders? J Psychiatry Neurosci 33, 187–195. [PMC free article] [PubMed] [Google Scholar]
- Banlaki Z, Elek Z, Nanasi T, Szekely A, Nemoda Z, Sasvari-Szekely M, Ronai Z, 2015. Polymorphism in the serotonin receptor 2a (HTR2A) gene as possible predisposal factor for aggressive traits. PLoS One 10, e0117792 10.1371/journal.pone.0117792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre A, Berthoux C, De Bundel D, Valjent E, Bockaert J, Marin P, Bécamel C, 2016. Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A. 113, e1382–1391. 10.1073/pnas.1525586113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne K, 2018. Environmental enrichment and mouse models: current perspectives. Animal Model Exp Med. 1, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Gunter TD, Packer H, Wernett P, Philibert RA, 2010. Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. J Fam Psychol 24, 12–20. 10.1037/a0018074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver KM, 2013. The familial concentration and transmission of crime. Crim Justice Behav 40, 139–155. [Google Scholar]
- Beaver KM, Barnes JC, Boutwell BB, 2014. The 2-repeat allele of the MAOA gene confers an increased risk for shooting and stabbing behaviors. Psychiatr Q 85, 257–265. 10.1007/s11126-013-9287-x [DOI] [PubMed] [Google Scholar]
- Beaver KM, DeLisi M, Vaughn MG, Barnes JC, 2010. Monoamine oxidase A genotype is associated with gang membership and weapon use. Compr Psychiatry 51, 130–134. 10.1016/j.comppsych.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Beaver KM, Holtfreter K, 2009. Biosocial influences on fraudulent behaviors. J Genet Psychol 170, 101–114. 10.3200/GNTP.170.2.101-114 [DOI] [PubMed] [Google Scholar]
- Beaver KM, Wright JP, Boutwell BB, Barnes JC, DeLisi M, Vaughn MG, 2013. Exploring the association between the 2-repeat allele of the MAOA gene promoter polymorphism and psychopathic personality traits, arrests, incarceration, and lifetime ASB. Pers. Individ. Dif. 54, 164–168. [Google Scholar]
- Beitchman JH, Mik HM, Ehtesham S, Douglas L, Kennedy JL, 2004. MAOA and persistent, pervasive childhood aggression. Mol Psychiatry. 9, 546–547. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R, 2009. Vulnerability genes or plasticity genes? Mol Psychiatry. 14, 746–754. 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Van Bakel I, Craig IW, 2000. A novel expression based approach for assessing the inactivation status of human X-linked genes. Eur J Hum Genet 8, 103–108. 10.1038/sj.ejhg.5200427 [DOI] [PubMed] [Google Scholar]
- Bergström M, Westerberg G, Långström B, 1997. 11C-harmine as a tracer for monoamine oxidase A (MAO-A): in vitro and in vivo studies. Nucl Med Biol. 24, 287–293. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF, 2002. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci U S A 99, 8448–8453. 10.1073/pnas.112604099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Reches A, Snyder L, McEwen BS, 1983. Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones. Life Sci. 32, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Binda C, Li M, Hubalek F, Restelli N, Edmondson DE, Mattevi A, 2003. Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc Natl Acad Sci U S A. 100, 9750–9755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Moeller FG, Dougherty DM, Swann AC, Machado MA, Hanis CL, 2002. Serotonin 2a receptor T102C polymorphism and impaired impulse control. Am J Med Genet 114, 336–339. 10.1002/ajmg.10206 [DOI] [PubMed] [Google Scholar]
- Black GC, Chen ZY, Craig IW, Powell JF, 1991. Dinucleotide repeat polymorphism at the MAOA locus. Nucleic Acids Res 19, 689 10.1093/nar/19.3.689-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen DM, Patrick CJ, Douglas KS, Poythress NG, Skeem JL, Lilienfeld SO, Edens JF, Krueger RF, 2010. Multimethod assessment of psychopathy in relation to factors of internalizing and externalizing from the Personality Assessment Inventory: the impact of method variance and suppressor effects. Psychol Assess. 22, 96–107. 10.1037/a0017240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, Farrell MR, Scott AL, Wellman CL, Shih JC, 2011. Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice. Neuropsychopharmacology 36, 2674–2688. 10.1038/npp.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC, 2008. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev 60, 1527–1533. 10.1016/j.addr.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Floris G, Shih JC, 2018. From aggression to autism: new perspectives on the behavioral sequelae of monoamine oxidase deficiency. J Neural Transm 125, 1589–1599. 10.1007/s00702-018-1888-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RC, Shih JC, 2013. Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol 16, 869–888. 10.1017/S1461145712000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M., Godar SC., Davarian S., Chen K., Shih JC., 2009. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology. 34, 2746–2757. 10.1038/npp.2009.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Melis M, Soggiu A, Roncada P, Casu A, Flore G, Chen K, Frau R, Urbani A, Castelli MP, Devoto P, Shih JC, 2012. NMDARs mediate the role of monoamine oxidase A in pathological aggression. J Neurosci 32, 8574–8582. 10.1523/JNEUROSCI.0225-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Shih JC, 2011. Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence. Int Rev Neurobiol. 2011;100:13–42. 10.1016/B978-0-12-386467-3.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Díaz-Mataix L, Scorza MC, Celada P, Artigas F, 2005. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 95, 1597–1607. [DOI] [PubMed] [Google Scholar]
- Bottlaender M, Valette H, Delforge J, Saba W, Guenther I, Curet O, George P, Dollé F, Grégoire MC, 2010. In vivo quantification of monoamine oxidase A in baboon brain: a PET study using [(11)C]befloxatone and the multi-injection approach. J Cereb Blood Flow Metab. 30, 792–800. 10.1038/jcbfm.2009.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mednick SA, 1999. Maternal smoking during pregnancy and adult male criminal outcomes. Arch Gen Psychiatry 56, 215–219. 10.1001/archpsyc.56.3.215 [DOI] [PubMed] [Google Scholar]
- Brownlie EB, Beitchman JH, Escobar M, Young A, Atkinson L, Johnson C, Wilson B, Douglas L, 2004. Early language impairment and young adult delinquent and aggressive behavior. J Abnorm Child Psychol 32, 453–467. 10.1023/b:jacp.0000030297.91759.74 [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA, 1993a. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262, 578–580. 10.1126/science.8211186 [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, van Zandvoort P, Abeling NG, van Gennip AH, Wolters EC, Kuiper MA, Ropers HH, van Oost BA, 1993b. X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am J Hum Genet 52, 1032–1039. [PMC free article] [PubMed] [Google Scholar]
- Bubenzer-Busch S, Herpertz-Dahlmann B, Kuzmanovic B, Gaber TJ, Helmbold K, Ullisch MG, Baurmann D, Eickhoff SB, Fink GR, Zepf FD, 2016. Neural correlates of reactive aggression in children with attention-deficit/hyperactivity disorder and comorbid disruptive behaviour disorders. Acta Psychiatr Scand. 133, 310–323. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A, 2008. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry 13, 313–324. 10.1038/sj.mp.4002020 [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A, 2008. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci 31, 120–129. 10.1016/j.tins.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, Ansari MS,Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH, 2010. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 13, 419–421. 10.1038/nn.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MW, Fillion M, Mejia J, Ervin FR, Palmour RM, 2018. Perinatal MAO Inhibition Produces Long-Lasting Impairment of Serotonin Function in Offspring. Brain Sci. 8 pii: E106 10.3390/brainsci8060106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Mikolajewski AJ, 2008. Preliminary evidence that specific candidate genes are associated with adolescent-onset ASB. Aggress Behav 34, 437–445. 10.1002/ab.20251 [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Huesmann L. R., 2010. Aggression In Fiske ST, Gilbert DT, and Lindzey G (Eds.), Handbook of Social Psychology (5th ed., Vol.2, pp.833–863) Hoboken, NJ: Wiley and Sons. [Google Scholar]
- Buss DM, Shackelford TK, 1997. Human aggression in evolutionary psychological perspective. Clin Psychol Rev 17, 605–619. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Manuck SB, 2014. MAOA, childhood maltreatment, and ASB: meta-analysis of a gene-environment interaction. Biol Psychiatry 75, 9–17. 10.1016/j.biopsych.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M, 1995. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 15, 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisi CO, Moffitt TE, Knodt AR, Harrington H, Ireland D, Melzer TR, Poulton R, Ramrakha S, Caspi A, Hariri AR, Viding E, 2020. Associations between life-course-persistent antisocial behaviour and brain structure in a population-representative longitudinal birth cohort. Lancet Psychiatry. 7, 245–253. 10.1016/S2215-0366(20)30002-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L, Willard HF, 2005. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404. 10.1038/nature03479 [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC, 1995. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 268, 1763–1766. 10.1126/science.7792602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R, 2002. Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854. 10.1126/science.1072290 [DOI] [PubMed] [Google Scholar]
- Castagnoli N Jr., Castagnoli K, Magnin G, Kuttab S, Shang J, 2002. Studies on the oxidation of 1,4-disubstituted-1,2,3,6-tetrahydropyridines. Drug Metab Rev 34, 533–547. 10.1081/DMR-120005655 [DOI] [PubMed] [Google Scholar]
- Checknita D, Bendre M, Ekström TJ, Comasco E, Tiihonen J, Hodgins S, Nilsson KW, 2020. Monoamine oxidase A genotype and methylation moderate the association of maltreatment and aggressive behaviour. Behav Brain Res. 382, 112476 10.1016/j.bbr.2020.112476 [DOI] [PubMed] [Google Scholar]
- Checknita D, Maussion G, Labonté B, Comai S, Tremblay RE, Vitaro F, Turecki N, Bertazzo A, Gobbi G, Côté G, Turecki G, 2015. Monoamine oxidase A gene promoter methylation and transcriptional downregulation in an offender population with antisocial personality disorder. Br J Psychiatry. 206, 216–22. [DOI] [PubMed] [Google Scholar]
- Chen K, Cases O, Rebrin I, Wu W, Gallaher TK, Seif I, Shih JC, 2007. Forebrain-specific expression of monoamine oxidase A reduces neurotransmitter levels, restores the brain structure, and rescues aggressive behavior in monoamine oxidase A-deficient mice. J Biol Chem 282, 115–123. 10.1074/jbc.M609830200, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC, 2004. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biol Chem. 279, 39645–39652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Ou XM, Chen G, Choi SH, Shih JC, 2005. R1, a novel repressor of the human monoamine oxidase A. J Biol Chem. 280, 11552–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Wu HF, Grimsby J, Shih JC, 1994. Cloning of a novel monoamine oxidase cDNA from trout liver. Mol Pharmacol. 46, 1226–1233. [PubMed] [Google Scholar]
- Cheng LL, Wang SJ, Gean PW, 1998. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci 10, 2163–2172. 10.1046/j.14609568.1998.00229.x [DOI] [PubMed] [Google Scholar]
- Chester DS, DeWall CN, Derefinko KJ, Estus S, Peters JR, Lynam DR, Jiang Y, 2015. Monoamine oxidase A (MAOA) genotype predicts greater aggression through impulsive reactivity to negative affect. Behav Brain Res. 283, 97–101. 10.1016/j.bbr.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe DE, Shaw DS, Hyde LW, Forbes EE, 2014. Interactions Between Monoamine Oxidase A and Punitive Discipline in African American and Caucasian Men’s ASB. Clin Psychol Sci. 2, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Jung YD, Ayarpadikannan S, Koga A, Imai H, Hirai H, Roos C, Kim HS, 2014. Novel variable number of tandem repeats of gibbon MAOA gene and its evolutionary significance. Genome. 57, 427–432. 10.1139/gen-2014-0065 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Thibodeau EL, 2012. The effects of child maltreatment on early signs of ASB: genetic moderation by tryptophan hydroxylase, serotonin transporter, and monoamine oxidase A genes. Dev Psychopathol. 24, 907–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Goldstein DB, 2007. In vitro assays fail to predict in vivo effects of regulatory polymorphisms. Hum Mol Genet. 16, 1931–1939. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Cremers H, Fanning J, Nosal E, Lee R, Keedy S, Jacobson KC, 2018b. Reduced frontal grey matter, life history of aggression, and underlying genetic influence. Psychiatry Res Neuroimaging 271, 126–134. 10.1016/j.pscychresns.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL, 2007. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry 62, 168–178. 10.1016/j.biopsych.2006.08.024 [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Shima CK, Lee RJ, 2018a. Comorbidity of personality disorder with intermittent explosive disorder. J Psychiatr Res. 106, 15–21. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Sripada CS, Yanowitch RN, Phan KL, 2011. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry 69, 1153–1159. 10.1016/j.biopsych.2011.02.032 [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Lewis ME, Chudley A, Forster-Gibson C, Gonzalez M, Jenkins EC, Brown WT, Holden JJ, 2011. Autism severity is associated with child and maternal MAOA genotypes. Clin Genet 79, 355–362. 10.1111/j.1399-0004.2010.01471.x [DOI] [PubMed] [Google Scholar]
- Coid J, Yang M, Roberts A, Ullrich S, Moran P, Bebbington P, Brugha T, Jenkins R, Farrell M, Lewis G, Singleton N, 2006. Violence and psychiatric morbidity in the national household population of Britain: public health implications. Br J Psychiatry 189, 12–19. 10.1192/bjp.189.1.12 [DOI] [PubMed] [Google Scholar]
- Coid J, Yang M, Ullrich S, Roberts A, Hare RD, 2009. Prevalence and correlates of psychopathic traits in the household population of Great Britain. Int J Law Psychiatry 32, 65–73. 10.1016/j.ijlp.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Comai S, Tau M, Gobbi G, 2012. The psychopharmacology of aggressive behavior: a translational approach: part 1: neurobiology. J Clin Psychopharmacol 32, 83–94. 10.1097/JCP.0b013e31823f8770 [DOI] [PubMed] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF, 2005. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry 66, 677–685. [DOI] [PubMed] [Google Scholar]
- Contini V, Marques FZ, Garcia CE, Hutz MH, Bau CH, 2006. MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet 141B, 305–308. 10.1002/ajmg.b.30290 [DOI] [PubMed] [Google Scholar]
- Cooke EM, Armstrong T, Boisvert D, Wells J, Lewis RH, Hughes-Stamm S,Gangitano D, 2018. The relationship between the MAOA-uVNTR polymorphism, delinquent peer affiliation, and ASB with a consideration of sex differences. Psychiatr Q. 89, 841–853. 10.1007/s11126-018-9582-7 [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Day NL, 2009. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol 22, 121–125. 10.1097/WCO.0b013e328326f6dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR, 1992. Normal personality assessment in clinical practice: the NEO Personality Inventory. Psychol. Assess. 4, 5–13. [Google Scholar]
- Craig IW, Halton KE, 2009. Genetics of human aggressive behaviour. Hum Genet. 126, 101–13. 10.1007/s00439-009-0695-9 [DOI] [PubMed] [Google Scholar]
- Craig MC, Mulder LM, Zwiers MP, Sethi A, Hoekstra PJ, Dietrich A, Baumeister S, Aggensteiner PM, Banaschewski T, Brandeis D, Werhahn JE, Walitza S, Castro-Fornieles J, Arango C, Schulze UME, Glennon JC, Franke B, Santosh PJ, Mastroianni M, van Asten JJA, Buitelaar JK, Lythgoe DJ, Naaijen J, 2019. Distinct associations between fronto-striatal glutamate concentrations and callous-unemotional traits and proactive aggression in disruptive behavior. Cortex. 121, 135–146. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Bosse R, Di Paolo T, 1998. Gonadal hormones modulate 5-HT2A receptors: Emphasis on the rat frontal cortex. Neuroscience. 83, 829–836. [DOI] [PubMed] [Google Scholar]
- da Cunha-Bang S, Hjordt LV, Perfalk E, Beliveau V, Bock C, Lehel S, Thomsen C, Sestoft D, Svarer C, Knudsen GM, 2017. Serotonin 1B Receptor Binding Is Associated With Trait Anger and Level of Psychopathy in Violent Offenders. Biol Psychiatry. 82(4), 267–274. [DOI] [PubMed] [Google Scholar]
- da Silva RF, Oliveira Lima ME, 2016. Crime and Punishment: the Impact of Skin Color and Socioeconomic Status of Defendants and Victims in Jury Trials in Brazil. Span J Psychol. 19, E77. [DOI] [PubMed] [Google Scholar]
- Das M, Bhowmik AD, Sinha S, Chattopadhyay A, Chaudhuri K, Singh M, Mukhopadhyay K, 2006. MAOA promoter polymorphism and attention deficit hyperactivity disorder (ADHD) in indian children. Am J Med Genet B Neuropsychiatr Genet. 141B, 637–642. [DOI] [PubMed] [Google Scholar]
- Davis LK, Hazlett HC, Librant AL, Nopoulos P, Sheffield VC, Piven J, Wassink TH, 2008. Cortical enlargement in autism is associated with a functional uVNTR in the monoamine oxidase A gene. Am J Med Genet B Neuropsychiatr Genet 147B, 1145–1151. 10.1002/ajmg.b.30738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA, 2005. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 526, 51–64. 10.1016/j.ejphar.2005.10.004 [DOI] [PubMed] [Google Scholar]
- De Colibus L, Li M, Binda C, Lustig A, Edmondson DE, Mattevi A, 2005. Threedimensional structure of human monoamine oxidase A (MAO A): relation to the structures of rat MAO A and human MAO B. Proc Natl Acad Sci U S A. 102, 1268412689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP, 1999. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 8, 621–624. 10.1093/hmg/8.4.621 [DOI] [PubMed] [Google Scholar]
- Denney RM, Koch H, Craig IW, 1999. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet 105, 542–551. 10.1007/s004399900183 [DOI] [PubMed] [Google Scholar]
- Denson TF, Dobson-Stone C, Ronay R, von Hippel W, Schira MM, 2014. A functional polymorphism of the MAOA gene is associated with neural responses to induced anger control. J Cogn Neurosci. 26, 1418–1427. 10.1162/jocn_a_00592 [DOI] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Irons DE, Iacono WG, 2010. Harsh discipline, childhood sexual assault, and MAOA genotype: an investigation of main and interactive effects on diverse clinical externalizing outcomes. Behav Genet 40, 639–648. 10.1007/s10519-010-9358-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Coie JD, 1987. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol 53, 1146–58. [DOI] [PubMed] [Google Scholar]
- Dolle F, Valette H, Bramoulle Y, Guenther I, Fuseau C, Coulon C, Lartizien C, Jegham S, George P, Curet O, Pinquier JL, Bottlaender M, 2003. Synthesis and in vivo imaging properties of [11C]befloxatone: a novel highly potent positron emission tomography ligand for mono-amine oxidase-A. Bioorg Med Chem Lett. 13, 1771–1775. [DOI] [PubMed] [Google Scholar]
- Dorfman HM, Meyer-Lindenberg A, Buckholtz JW, 2014. Neurobiological mechanisms for impulsive-aggression: the role of MAOA. Curr Top Behav Neurosci 17, 297–313. 10.1007/7854_2013_272 [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D, 2008. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry 13, 334–347. 10.1038/sj.mp.4002034 [DOI] [PubMed] [Google Scholar]
- Edwards AC, Dodge KA, Latendresse SJ, Lansford JE, Bates JE, Pettit GS, Budde JP, Goate AM, Dick DM, 2010. MAOA-uVNTR and early physical discipline interact to influence delinquent behavior. J Child Psychol Psychiatry 51, 679–687. 10.1111/j.1469-7610.2009.02196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD, 2007. Understanding genetic risk for aggression: clues from the brain’s response to social exclusion. Biol Psychiatry 61, 1100–1108. 10.1016/j.biopsych.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Elbert T, Weierstall R, Schauer M, 2010. Fascination violence: on mind and brain of man hunters. Eur Arch Psychiatry Clin Neurosci. 260 Suppl 2, S100–5. 10.1007/s00406-010-0144-8 [DOI] [PubMed] [Google Scholar]
- Eley TC, Lichtenstein P, Moffitt TE, 2003. A longitudinal behavioral genetic analysis of the etiology of aggressive and nonaggressive ASB. Dev Psychopathol. 15, 383–402. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH, 2011. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Dev Psychopathol. 2011 Feb;23(1):7–28. 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D, 2010. Early life stress, MAOA, and gene–environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 9, 65–74. 10.1111/j.1601-183X.2009.00535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo J, Lee HE, Nam GH, Kwon YJ, Choi Y, Choi BH, Huh JW,. Kim M, Lee SE, Seo B, Kim HS, 2016. Association of DNA methylation and monoamine oxidase A gene expression in the brains of different dog breeds. Gene. 580, 177–182. 10.1016/j.gene.2016.01.022 [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S, 1990. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Res Dev Brain Res 56, 229–234. 10.1016/0165-3806(90)90087-f [DOI] [PubMed] [Google Scholar]
- Fanning JR, Keedy S, Berman ME, Lee R, Coccaro EF, 2017. Neural Correlates of Aggressive Behavior in Real Time: a Review of fMRI Studies of Laboratory Reactive Aggression. Curr Behav Neurosci Rep. 4, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah T, Ling S, Raine A, Yang Y, Schug R, 2018. Alexithymia and reactive aggression: The role of the amygdala. Psychiatry Res Neuroimaging. 281, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Danesh J, 2002. Serious mental disorder in 23000 prisoners: a systematic review of 62 surveys. Lancet 359, 545–550. 10.1016/S0140-6736(02)07740-1 [DOI] [PubMed] [Google Scholar]
- Fergusson DM, 1999. Prenatal smoking and ASB. Arch Gen Psychiatry. 56(3):223–224. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ, Miller AL, Kennedy MA, 2011. MAOA, abuse exposure and antisocial behaviour: 30-year longitudinal study. Br J Psychiatry 198, 457–463. 10.1192/bjp.bp.110.086991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ, Miller A, Kennedy MA, 2012. Moderating role of the MAOA genotype in antisocial behaviour. Br J Psychiatry. 200, 116–123. 10.1192/bjp.bp.111.093328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficks CA, Waldman ID, 2014. Candidate genes for aggression and ASB: a meta-analysis of association studies of the 5HTTLPR and MAOA-uVNTR. Behav Genet. 44, 427–444. 10.1007/s10519-014-9661-y [DOI] [PubMed] [Google Scholar]
- Fish EW, De Bold JF, Miczek KA, 2002. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology (Berl). 163, 459–466. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Haahr ME, Jensen CG, Frokjaer VG, Siebner HR, Knudsen GM, 2015. Fluctuations in [11C]SB207145 PET binding associated with change in threat-related amygdala reactivity in humans. Neuropsychopharmacology. 40, 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite PJ, Brown S, Hossain W, Manzardo A, Butler MG, Bortolato M, 2019. Tobacco and cannabis use in college students are predicted by sex-dimorphic interactions between MAOA genotype and child abuse. CNS Neurosci Ther. 25, 101–111. 10.1111/cns.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fite PJ, Brown S, Hossain WA, Manzardo A, Butler MG, Bortolato M, 2020. Sex-dimorphic interactions of MAOA genotype and child maltreatment predispose college students to polysubstance use. Front Genetics, 10:1314 10.3389/fgene.2019.01314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B, 2004. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry 61, 738–744. 10.1001/archpsyc.61.7.738 [DOI] [PubMed] [Google Scholar]
- Fornai F, Chen K, Giorgi FS, Gesi M, Alessandri MG, Shih JC, 1999. Striatal dopamine metabolism in monoamine oxidase B-deficient mice: a brain dialysis study. J Neurochem. 73, 2434–2440. [DOI] [PubMed] [Google Scholar]
- Forster GL, Anderson EM, Scholl JL, Lukkes JL, Watt MJ, 2018. Negative consequences of early-life adversity on substance use as mediated by corticotropin-releasing factor modulation of serotonin activity. Neurobiol Stress. 9, 29–39. 10.1016/j.ynstr.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Alia-Klein N, Kriplani A, Logan J, Williams B, Zhu W, Craig IW, Telang F, Goldstein R, Volkow ND, Vaska P, Wang GJ, 2007. Evidence that brain MAO A activity does not correspond to MAO A genotype in healthy male subjects. Biol Psychiatry. 62, 355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP, 1996. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A 93, 14065–14069. 10.1073/pnas.93.24.14065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Fanni S, Serra V, Simola N, Godar SC, Traccis F, Devoto P, Bortolato M, Melis M, 2019. Dysfunctional mesocortical dopamine circuit at pre-adolescence is associated to aggressive behavior in MAO-A hypomorphic mice exposed to early life stress. Neuropharmacology. 159, 107517 10.1016/j.neuropharm.2019.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A, 2007. Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLoS One 2, e486 10.1371/journal.pone.0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan CA, Choe DE, Forbes EE, Shaw DS, 2017. The interaction between monoamine oxidase A and punitive discipline in the development of ASB: Mediation by maladaptive social information processing. Dev Psychopathol 29, 1235–1252. 10.1017/S0954579416001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Pujol D, Andres-Pueyo A, Maydeu-Olivares A, 2013. MAOA genotype, social exclusion and aggression: an experimental test of a gene-environment interaction. Genes Brain Behav 12, 140–145. 10.1111/j.1601-183X.2012.00868.x [DOI] [PubMed] [Google Scholar]
- Gaweska H, Fitzpatrick PF, 2011. Structures and Mechanism of the Monoamine Oxidase Family. Biomol Concepts. 2, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H, 2002. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115, 1261–1279. 10.1016/s0306-4522(02)00446-3 [DOI] [PubMed] [Google Scholar]
- Gibson CL, Tibbetts SG, 2000. A biosocial interaction in predicting early onset of offending. Psychol Rep 86, 509–518. 10.2466/pr0.2000.86.2.509 [DOI] [PubMed] [Google Scholar]
- Ginovart N, Meyer JH, Boovariwala A, Hussey D, Rabiner EA, Houle S, Wilson AA, 2006. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab. 26, 330–344. [DOI] [PubMed] [Google Scholar]
- Godar SC, Bortolato M, 2014. Gene-sex interactions in schizophrenia: focus on dopamine neurotransmission. Front Behav Neurosci. 8, 71 10.3389/fnbeh.2014.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Bortolato M, Frau R, Dousti M, Chen K, Shih JC, 2011. Maladaptive defensive behaviours in monoamine oxidase A-deficient mice. Int J Neuropsychopharmacol 14, 1195–1207. 10.1017/S1461145710001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Fite PJ, McFarlin KM, Bortolato M, 2016. The role of monoamine oxidase A in aggression: Current translational developments and future challenges. Prog Neuropsychopharmacol Biol Psychiatry 69, 90–100. 10.1016/j.pnpbp.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Scheggi S, Devoto P, Moench KM, Strathman HJ, Jones CM, Frau R, Melis M, Gambarana C, Wilkinson B, DeMontis MG, Fowler SC, Coba MP, Wellman CL, Shih JC, Bortolato M, 2019. Gene-environment interactions in ASB are mediated by early-life 5-HT2A receptor activation. Neuropharmacology 10.1016/j.neuropharm.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Shaham Y, 2018. Aggression Addiction and Relapse: A New Frontier in Psychiatry. Neuropsychopharmacology 43, 224–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, Russo SJ, Shaham Y, 2017. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav. 16:44–55. 10.1111/gbb.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein ME, Tank AW, Fossom LH, Hamill RW, 1992. Molecular aspects of the regulation of tyrosine hydroxylase by testosterone. Brain Res Mol Brain Res 14, 79–86. 10.1016/0169-328x(92)90013-2 [DOI] [PubMed] [Google Scholar]
- Gondré-Lewis MC, Warnock KT, Wang H, June HL Jr, Bell K,A, Rabe H, Tiruveedhula VV, Cook J, Lüddens H, Aurelian L, June HL Sr., 2016. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress. 19, 235–47. 10.3109/10253890.2016.1160280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky E, Bevilacqua L, Carli V, Sarchiapone M, Roy A, Goldman D, Enoch MA, 2014. The interactive effect of MAOA-LPR genotype and childhood physical neglect on aggressive behaviors in Italian male prisoners. Genes Brain Behav. 13, 543–549. 10.1111/gbb.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K, S.F.S., 2004. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61(8), 807–816. [DOI] [PubMed] [Google Scholar]
- Grimsby J, Chen K, Wang LJ, Lan NC, Shih JC, 1991. Human monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc Natl Acad Sci U S A 88, 3637–3641. 10.1073/pnas.88.9.3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, Karoum F, Gal J, Shih JC, 1997. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet. 17, 206–210. [DOI] [PubMed] [Google Scholar]
- Guindalini C, Scivoletto S, Ferreira RG, Nishimura A, Zilberman ML, Peluso MM, Zatz M, 2005. Association of MAO A polymorphism and alcoholism in Brazilian females. Psychiatr Genet. 15, 141–144. [DOI] [PubMed] [Google Scholar]
- Guo G, Ou XM, Roettger M, Shih JC, 2008. The uVNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity. Eur J Hum Genet 16, 626–634. 10.1038/sj.ejhg.5201999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Khan AA, Sasi BK, Mahapatra NR, 2015. Molecular mechanism of monoamine oxidase A gene regulation under inflammation and ischemia-like conditions: key roles of the transcription factors GATA2, Sp1 and TBP. J Neurochem. 134, 21–38. 10.1111/jnc.13099 [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Lessem JM, Hewitt JK, Smolen A, Hopfer CJ, Halpern CT, Killeya-Jones LA, Boardman JD, Tabor J, Siegler IC, Williams RB, Mullan Harris K, 2014. MAOA genotype, childhood maltreatment, and their interaction in the etiology of adult ASBs. Biol Psychiatry 75, 25–30. 10.1016/j.biopsych.2013.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdi NR, Iacono WG, 2014. Lifetime prevalence and co-morbidity of externalizing disorders and depression in prospective assessment. Psychol Med 44, 315–324. 10.1017/S0033291713000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD, 1998. Psychopaths and Their Nature: Implications for the Mental Health and Criminal Justice Systems in Psychopathy: Antisocial, Criminal, and Violent Behavior. Guilford Press, New York, NY. [Google Scholar]
- Hare RD, 2003. Manual for the Revised Psychopathy Checklist (2nd ed.). Multi-Health Systems, Toronto, ON, Canada. [Google Scholar]
- Hare RD, Clark D, Grann M, Thornton D, 2000. Psychopathy and the predictive validity of the PCL-R: an international perspective. Behav Sci Law 18, 623–645. [DOI] [PubMed] [Google Scholar]
- Harneit A, Braun U, Geiger LS, Zang Z, Hakobjan M, van Donkelaar MMJ, Schweiger JI, Schwarz K, Gan G, Erk S, Heinz A, Romanczuk-Seiferth N, Witt S, Rietschel M, Walter H, Franke B, Meyer-Lindenberg A, Tost H, 2019. MAOA-VNTR genotype affects structural and functional connectivity in distributed brain networks. Hum Brain Mapp. 40, 5202–5212. 10.1002/hbm.24766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hakstian AR, Hare RD, 1988. Factor structure of the Psychopathy Checklist. J Consult Clin Psychol. 56, 741–747. [DOI] [PubMed] [Google Scholar]
- Hawkins J, Ahmad S, Cui Y, 2017. A Theory of How Columns in the Neocortex Enable Learning the Structure of the World. Front Neural Circuits 11, 81 10.3389/fncir.2017.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker T, Hermenau K, Maedl A, Elbert T, Schauer M, 2012. Appetitive aggression in former combatants--derived from the ongoing conflict in DR Congo. Int J Law Psychiatry. 35, 244–9 10.1016/j.ijlp.2012.02.016 [DOI] [PubMed] [Google Scholar]
- Hemmings SMJ, Xulu K, Sommer J, Hinsberger M, Malan-Muller S, Tromp G, Elbert T, Weierstall R, Seedat S, 2018. Appetitive and reactive aggression are differentially associated with the STin2 genetic variant in the serotonin transporter gene. Sci Rep. 8, 6714 10.1038/s41598-018-25066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Patrick CJ, 2006. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. J Abnorm Psychol. 115, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Breen G, Quinn J, Tibu F, Sharp H, Pickles A, 2013. Evidence for interplay between genes and maternal stress in utero: monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5 weeks. Genes Brain Behav 12, 388–396. 10.1111/gbb.12033 [DOI] [PubMed] [Google Scholar]
- Hinds HL, Hendriks RW, Craig IW, Chen ZY, 1992. Characterization of a highly polymorphic region near the first exon of the human MAOA gene containing a GT dinucleotide and a novel uVNTR motif. Genomics 13, 896–897. 10.1016/0888-7543(92)90181-q [DOI] [PubMed] [Google Scholar]
- Hirata M, Magata Y, Ohmomo Y, Saji H, Murakami K, Takagaki T, Yamamura N, Tanaka C, Konishi J, Yokoyama A, 1995. Evaluation of radioiodinated iodoclorgyline as a SPECT radiopharmaceutical for MAO-A in the brain. Nucl Med Biol. 22, 175–180. [DOI] [PubMed] [Google Scholar]
- Hollerbach P, Johansson A, Ventus D, Jern P, Neumann CS, Westberg L, Santtila P, Habermeyer E, Mokros A, 2018. Main and interaction effects of childhood trauma and the MAOA uVNTR polymorphism on psychopathy. Psychoneuroendocrinology. 95, 106–112. [DOI] [PubMed] [Google Scholar]
- Holz N, Boecker R, Buchmann AF, Blomeyer D, Baumeister S, Hohmann S, Jennen-Steinmetz C, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer-Lindenberg A, Schmidt MH, Esser G, Banaschewski T, Brandeis D, Laucht M, 2016. Evidence for a Sex-Dependent MAOA× Childhood Stress Interaction in the Neural Circuitry of Aggression. Cereb Cortex. 26, 904–14. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Breakefield XO, 1991. Human monoamine oxidase A gene determines levels of enzyme activity. Am J Hum Genet 49, 383–392. [PMC free article] [PubMed] [Google Scholar]
- Hovevey-Sion D, Kopin IJ, Stull RW, Goldstein DS, 1989. Effects of monoamine oxidase inhibitors on levels of catechols and homovanillic acid in striatum and plasma. Neuropharmacology. 28, 791–797. [DOI] [PubMed] [Google Scholar]
- Hsu YP, Weyler W, Chen S, Sims KB, Rinehart WB, Utterback MC, Powell JF, Breakefield XO, 1988. Structural features of human monoamine oxidase A elucidated from cDNA and peptide sequences. J Neurochem 51, 1321–1324. [DOI] [PubMed] [Google Scholar]
- Huang SY, Lin WW, Wan FJ, Chang AJ, Ko HC, Wang TJ, Wu PL, Lu RB, 2007. Monoamine oxidase-A polymorphisms might modify the association between the dopamine D2 receptor gene and alcohol dependence. J Psychiatry Neurosci 32, 185–192. [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ, 2004. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology 29, 1498–1505. 10.1038/sj.npp.1300455 [DOI] [PubMed] [Google Scholar]
- Huber R, Brennan PA, 2011. Aggression. Adv Genet 75, 1–6. 10.1016/B978-0-12-380858-5.00016-2 [DOI] [PubMed] [Google Scholar]
- Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, Grotpeter J, Hewitt JK, 2006. Childhood maltreatment, subsequent ASB, and the role of monoamine oxidase A genotype. Biol Psychiatry. 60, 677–83. [DOI] [PubMed] [Google Scholar]
- Hunter NS, Anderson KC, Cox A, 2015. Pimavanserin. Drugs Today (Barc) 51, 645–652. 10.1358/dot.2015.51.11.2404001 [DOI] [PubMed] [Google Scholar]
- Hyde LW, Byrd AL, Votruba-Drzal E, Hariri AR, Manuck SB, 2014. Amygdala reactivity and negative emotionality: divergent correlates of antisocial personality and psychopathy traits in a community sample. J Abnorm Psychol. 123, 214–224. 10.1037/a0035467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaue I, Gomes B, Yasunobu KT, 1967. Beef mitochondrial monoamine oxidase, a flavin dinucleotide enzyme. Biochem Biophys Res Commun. 29, 562–70. [DOI] [PubMed] [Google Scholar]
- Im S, Jeong J, Jin G, Yeom J, Jekal J, Lee SI, Cho JA, Lee S, Lee Y, Kim DH, Bae M, Heo J, Moon C, Lee CH, 2019. MAOA variants differ in oscillatory EEG & ECG activities in response to aggression-inducing stimuli. Sci Rep. 9, 2680 10.1038/s41598-019-39103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L, 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 403, 795–800. [DOI] [PubMed] [Google Scholar]
- Inoue-Murayama M, Mishima N, Hayasaka I, Ito S, Murayama Y, 2006. Divergence of ape and human monoamine oxidase A gene promoters: comparative analysis of polymorphisms, tandem repeat structures and transcriptional activities on reporter gene expression. Neurosci Lett. 405, 207–11. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Wessel TC, Chen K, Shih JC, Joh TH, 1997. Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse. 25, 30–36. [DOI] [PubMed] [Google Scholar]
- Jones DN, Ruiz CA, Raghanti MA, Tosi AJ, Tanaka H, Goto Y, 2020. Monoamine oxidase polymorphisms in rhesus and Japanese macaques (Macaca mulatta and M. fuscata). J Chem Neuroanat. 103, 101726 10.1016/j.jchemneu.2019.101726 [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Norton N, Gustavsson JP, Oreland L, Owen MJ, Sedvall GC, 2000. A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J Psychiatr Res 34, 239–244. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, Tan X, Easteal S, 2000. Association of a functional polymorphism of the monoamine oxidase A gene promoter with personality and psychiatric symptoms. Psychiatr Genet. 10, 87–90. [DOI] [PubMed] [Google Scholar]
- Kalbitzer U, Roos C, Kopp GH,. Butynski TM, Knauf S, Zinner D, Fischer J, 2016. Insights into the genetic foundation of aggression in Papio and the evolution of two length-polymorphisms in the promoter regions of serotonin-related genes (5-HTTLPR and MAOALPR) in Papionini. BMC Evol Biol. 16, 121 10.1186/s12862-016-0693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP, 2009. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry 65, 770–777. 10.1016/j.biopsych.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney EB, Salach JI, Walker WH, Seng RL, Kenney W, Zeszotek E, Singer TP, 1971. The covalently-bound flavin of hepatic monoamine oxidase. 1. Isolation and sequence of a flavin peptide and evidence for binding at the 8alpha position. Eur J Biochem. 24, 321–327. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS, 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51, 8–19. 10.1001/archpsyc.1994.03950010008002 [DOI] [PubMed] [Google Scholar]
- Kieling C, Hutz MH, Genro JP, Polanczyk GV, Anselmi L, Camey S, Hallal PC, Barros FC, Victora CG, Menezes AM, Rohde LA, 2013. Gene-environment interaction in externalizing problems among adolescents: evidence from the Pelotas 1993 Birth Cohort Study. J Child Psychol Psychiatry. 54, 298–304. 10.1111/jcpp.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiive E, Laas K, Akkermann K, Comasco E, Oreland L, Veidebaum T, Harro J, 2014. Mitigating aggressiveness through education? The monoamine oxidase A genotype and mental health in general population. Acta Neuropsychiatr. 26, 19–28. 10.1017/neu.2013.34 [DOI] [PubMed] [Google Scholar]
- Kim H, Drake B, 2018. Child maltreatment risk as a function of poverty and race/ethnicity in the USA. Int J Epidemiol. 47, 780–787. 10.1093/ije/dyx280 [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE, 2006. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry 11, 903–913. 10.1038/sj.mp.4001851 [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Huang YY, Haverly R, Burke AK, Galfalvy H, Brent DP, Oquendo MA, Mann JJ, 2009. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatr Genet 19, 126–133. 10.1097/YPG.0b013e32832a50a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasen M, Wolf D, Eisner PD, Habel U, Repple J, Vernaleken I, Schlüter T, Eggermann T, Zerres K, Zepf FD, Mathiak K, 2018. Neural networks underlying trait aggression depend on MAOA gene alleles. Brain Struct Funct. 223, 873–881. 10.1007/s00429-017-1528-6 [DOI] [PubMed] [Google Scholar]
- Kolla NJ, Attard S, Craig G, Blackwood N, Hodgins S, 2014. Monoamine oxidase A alleles in violent offenders with antisocial personality disorder: high activity associated with proactive aggression. Crim Behav Ment Heal. 24, 368–372. 10.1002/cbm.1917 [DOI] [PubMed] [Google Scholar]
- Kolla NJ, Dunlop K, Meyer JH, Downar J, 2018. Corticostriatal Connectivity in Antisocial Personality Disorder by MAO-A Genotype and Its Relationship to Aggressive Behavior. Int J Neuropsychopharmacol 10.1093/ijnp/pyy035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Houle S, 2019. Single-Photon Emission Computed Tomography and Positron Emission Tomography Studies of Antisocial Personality Disorder and Aggression: a Targeted Review. Curr Psychiatry Rep 21, 24 10.1007/s11920-019-1011-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Malcolm C, Attard S, Arenovich T, Blackwood N, Hodgins S, 2013. Childhood maltreatment and aggressive behaviour in violent offenders with psychopathy. Can J Psychiatry 58, 487–494. 10.1177/070674371305800808 [DOI] [PubMed] [Google Scholar]
- Kolla NJ, Matthews B, Wilson AA, Houle S, Bagby RM, Links P, Simpson AI, Hussain A, Meyer JH, 2015. Lower Monoamine Oxidase-A Total Distribution Volume in Impulsive and Violent Male Offenders with Antisocial Personality Disorder and High Psychopathic Traits: An [(11)C] Harmine Positron Emission Tomography Study. Neuropsychopharmacology 40, 2596–2603. 10.1038/npp.2015.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Patel R, Meyer JH, Chakravarty MM, 2017. Association of monoamine oxidase-A genetic variants and amygdala morphology in violent offenders with antisocial personality disorder and high psychopathic traits. Sci Rep 7, 9607 10.1038/s41598-017-08351-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla NJ, Vinette SA, 2017. Monoamine Oxidase A in Antisocial Personality Disorder and Borderline Personality Disorder. Curr Behav Neurosci Rep 4, 41–48. 10.1007/s40473-017-0102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller G, Bondy B, Preuss UW, Bottlender M, Soyka M, 2003. No association between a polymorphism in the promoter region of the MAOA gene with antisocial personality traits in alcoholics. Alcohol Alcohol 38, 31–34. 10.1093/alcalc/agg003 [DOI] [PubMed] [Google Scholar]
- Konar A, Rastogi M, Bhambri A, 2019. Brain region specific methylation and Sirt1 binding changes in MAOA promoter is associated with sexual dimorphism in early life stress induced aggressive behavior. Neurochem Int. 129, 104510 10.1016/j.neuint.2019.104510 [DOI] [PubMed] [Google Scholar]
- Krahé B, 2013. The social psychology of aggression, 2nd ed. New York, NY: Psychology Press. [Google Scholar]
- Kuepper Y, Grant P, Wielpuetz C, Hennig J, 2013. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behav Brain Res 247, 73–78. 10.1016/j.bbr.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Labonté B, Abdallah K, Maussion G, Yerko V, Yang J, Bittar T, Quessy F, Golden SA, Navarro L, Checknita D, Gigek C, Lopez JP, Neve RL, Russo SJ, Tremblay RE, Côté G, Meaney MJ, Mechawar N, Nestler EJ, Turecki G, 2020. Regulation of impulsive and aggressive behaviours by a novel lncRNA. Mol Psychiatry. In press. 10.1038/s41380-019-0637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Lai E, Grimsby J, Sparkes RS, Mohandas T, Shih JC, 1989. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics 4, 552–559. [DOI] [PubMed] [Google Scholar]
- Latvala A, Kuja-Halkola R, Almqvist C, Larsson H, Lichtenstein P, 2015. A Longitudinal Study of Resting Heart Rate and Violent Criminality in More Than 700000 Men. JAMA Psychiatry 72, 971–978. 10.1001/jamapsychiatry.2015.1165 [DOI] [PubMed] [Google Scholar]
- Lavigne JV, Herzing LB, Cook EH, Lebailly SA, Gouze KR, Hopkins J, Bryant FB, 2013. Gene × environment effects of serotonin transporter, dopamine receptor D4, and monoamine oxidase A genes with contextual and parenting risk factors on symptoms of oppositional defiant disorder, anxiety, and depression in a community sample of 4-year-old children. Dev Psychopathol. 25, 555–75. [DOI] [PubMed] [Google Scholar]
- Lee BT, Ham BJ, 2008. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. Neuroreport. 19, 515–519. 10.1097/WNR.0b013e3282f94294 [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Fallon B, Van Wert M, Filippelli J, 2017. Examining the Relationship between Economic Hardship and Child Maltreatment Using Data from the Ontario Incidence Study of Reported Child Abuse and Neglect-2013 (OIS-2013). Behav Sci (Basel). 7, pii: E6 10.3390/bs7010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichnerova K, Kaniakova M, Park SP, Skrenkova K, Wang YX, Petralia RS, Suh YH, Horak M, 2015. Two N-glycosylation Sites in the GluN1 Subunit Are Essential for Releasing N-methyl-d-aspartate (NMDA) Receptors from the Endoplasmic Reticulum. J Biol Chem 290, 18379–18390. 10.1074/jbc.M115.656546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YM, Davamani F, Yang WC, Lai TJ, Sun HS, 2008. Association analysis of monoamine oxidase A gene and bipolar affective disorder in Han Chinese. Behav Brain Funct 4, 21 10.1186/1744-9081-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Qiao Y, Shao Y, Yu SY, Zhang C, Zhang R, Wang DX, Zhao M, Xie B, 2019. Association of Corticotropin-Releasing Hormone Receptor-1 Gene Polymorphisms and Personality Traits with Violent Aggression in Male Adolescents. J Mol Neurosci. in press. 10.1007/s12031-019-01396-8 [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Cima M, Arntz A, 2013. The relationship between adult reactive and proactive aggression, hostile interpretation bias, and antisocial personality disorder. J Pers Disord 27, 53–66. 10.1521/pedi.2013.27.1.53 [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Cima M, Lemmens A, 2015. The relationship between personality disorder traits and reactive versus proactive motivation for aggression. Psychiatry Res 229, 155–160. 10.1016/j.psychres.2015.07.052 [DOI] [PubMed] [Google Scholar]
- Lodge NJ, Li YW, Chin FT, Dischino DD, Zoghbi SS, Deskus JA, Mattson RJ, Imaizumi M, Pieschl R, Molski TF, Fujita M, Dulac H, Zaczek R, Bronson JJ, Macor JE, Innis RB, Pike VW, 2014. Synthesis and evaluation of candidate PET radioligands for corticotropin-releasing factor type-1 receptors. Nucl Med Biol. 41(6), 524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney BR, Frick PJ, Clements CB, Ellis ML, Kerlin K, 2003. Callous-unemotional traits, impulsivity, and emotional processing in adolescents with antisocial behavior problems. J Clin Child Adolesc Psychol. 32, 66–80. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA, 2014. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 71, 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu RB, Lee JF, Ko HC, Lin WW, Chen K, Shih JC, 2002. No association of the MAOA gene with alcoholism among Han Chinese males in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry 26, 457–461. 10.1016/s0278-5846(01)00288-3 [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Halldin C, Fowler JS, Wolf AP, Arnett CD, Langström B, Alexoff D, 1985. Selective, irreversible in vivo binding of [11C]clorgyline and [11C]-L-deprenyl in mice: potential for measurement of functional monoamine oxidase activity in brain using positron emission tomography. Biochem Pharmacol. 34, 3207–3210. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J, 2006. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav Neurosci 120, 761–786. 10.1037/0735-7044.120.4.761 [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, Ferguson SS, 2010. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci.13, 622–9. 10.1038/nn.2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maintz L, Novak N, 2007. Histamine and histamine intolerance. Am J Clin Nutr. 85, 1185–1196. [DOI] [PubMed] [Google Scholar]
- Manca M, Pessoa V, Lopez AI, Harrison PT, Miyajima F, Sharp H, Pickles A, Hill J, Murgatroyd C, Bubb VJ, Quinn JP, 2018. The Regulation of Monoamine Oxidase A Gene Expression by Distinct Variable Number Tandem Repeats. J Mol Neurosci. 64, 459–470. 10.1007/s12031-018-1044-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF, 2000. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res 95, 9–23. 10.1016/s0165-1781(00)00162-1 [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK, 1998. The electrophysiology of prefrontal serotonin systems: therapeutic implications for mood and psychosis. Biol Psychiatry. 44, 1118–27. [DOI] [PubMed] [Google Scholar]
- Márquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, Magistretti PJ, Trono D, Sandi C, 2013. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry. 3, e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz ITN, Schechter JC, Yu HH, Pine DS, Blair RJR, 2011. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Res 194, 279–286. 10.1016/j.pscychresns.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Hatcher AE, Clark CAC, Burns JL, Pine DS, Skol AD, Mroczek DK, Espy KA, Goldman D, Cook E, Wakschlag LS, 2017. Does MAOA increase susceptibility to prenatal stress in young children? Neurotoxicol Teratol. 61(Supplement C), 82–91. 10.1016/j.ntt.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott R, Tingley D, Cowden J, Frazzetto G, Johnson DD, 2009. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proc Natl Acad Sci U S A 106, 2118–2123. 10.1073/pnas.0808376106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Trescher WH, Johnston MV, 1992. Susceptibility of brain to AMPA induced excitotoxicity transiently peaks during early postnatal development. Brain Res. 583(1–2), 54–70. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Mustanski B, Metzger A, Pine DS, Kistner-Griffin E, Cook E, Wakschlag LS, 2012. A latent modeling approach to genotype-phenotype relationships: maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Arch Womens Ment Heal. 15, 269–282. 10.1007/s00737-012-0286-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia JM, Ervin FR, Baker GB, Palmour RM, 2002. Monoamine oxidase inhibition during brain development induces pathological aggressive behavior in mice. Biol Psychiatry. 52, 811–821. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Rusjan P, Clark M, Houle S, Woodside S, Arrowood J, Martin K, Colleton M, 2008. Serotonin2A receptor binding potential in people with aggressive and violent behaviour. J Psychiatry Neurosci 33, 499–508. [PMC free article] [PubMed] [Google Scholar]
- Meyer R, Deitsch S, and Wolverton D, 1994. Antisocial personality disorder In Ramachandran V(Ed.), Encyclopedia of Human Behavior. San Diego: Academic Press. [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR, 2006. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A 103, 6269–6274. 10.1073/pnas.0511311103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Lynam D, 2001. Structural models of personality and their relation to ASB: A meta-analytic review. Criminology. 39:765–798. [Google Scholar]
- Mocci G, Jiménez-Sánchez L, Adell A, Cortés R, Artigas F, 2014. Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology. 79, 49–58. 10.1016/j.neuropharm.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Moffitt TE, 1993. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 100, 674–701. [PubMed] [Google Scholar]
- Moffitt TE, 2003. Life-course-persistent and adolescent-limited ASB: A 10-year research review and a research agenda In: Lahey BB, Moffitt TE, Caspi A (eds) Causes of conduct disorder and juvenile delinquency. The Guilford Press, New York, pp 49–75 [Google Scholar]
- Mokrovic G, Matosic A, Hranilovic D, Stefulj J, Novokmet M, Oreskovic D, Balija M, Marusic S, Cicin-Sain L, 2008. Alcohol dependence and polymorphisms of serotonin-related genes: association studies. Coll Antropol 32 Suppl 1, 127–131. [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M, 2011. Reduced prefrontal connectivity in psychopathy. J Neurosci 31, 17348–17357. 10.1523/JNEUROSCI.4215-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näslund K, Saetre P, von Salomé J, Bergström TF, Jareborg N, Jazin E, 2005. Genome-wide prediction of human VNTRs. Genomics. 85, 24–35. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, 2004. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 38, 14–20. [PubMed] [Google Scholar]
- Neumann CS, Vitacco MJ, Hare RD, Wupperman P, 2005. Reconstruing the “Reconstruction” of Psychopathy: A Comment on Cooke, Michie, Hart, and Clark. J Pers Disord 19, 624–640. 10.1521/pedi.2005.19.6.624 [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, Lazarus S, Trisdorfer R, Goldstein KE, Goodman M, Koenigsberg HW, Flory JD, Siever LJ, Buchsbaum MS, 2009. Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biol Psychiatry 66, 1107–1114. 10.1016/j.biopsych.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP, 2005. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry 57, 167–172. 10.1016/j.biopsych.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Nicotra A, Senatori O, 1988. Changes in monoamine oxidase activity by mitochondria isolated from late embryos of Bufo bufo. Comp Biochem Physiol C 89, 5–9. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Åslund C, Comasco E, Oreland L, 2018. Gene-environment interaction of monoamine oxidase A in relation to antisocial behaviour: current and future directions. J Neural Transm (Vienna). 125, 1601–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Åslund C, Nordquist N, Leppert J, Oreland L, 2011. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. 16, 347–55. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Hodgins S, Oreland L, Åslund C,2014. Genotypes do not confer risk for delinquency but rather alter susceptibility to positive and negative environmental factors: gene-environment interactions of BDNF Val66Met, 5-HTTLPR, and MAOA-uVNTR. Int J Neuropsychopharmacol. 18, pii: pyu107 10.1093/ijnp/pyu107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Kusumi I, Kaneko M, Masui T, Daiguji M, Ueno T, Koyama T, Nomura Y, 2006. Involvement of a polymorphism in the 5-HT2A receptor gene in impulsive behavior. Psychopharmacol. 187, 30–35. 10.1007/s00213-006-0398-z [DOI] [PubMed] [Google Scholar]
- Nouvion SO, Cherek DR, Lane SD, Tcheremissine OV, Lieving LM, 2007. Human proactive aggression: association with personality disorders and psychopathy. Aggress Behav 33, 552–562. 10.1002/ab.20220 [DOI] [PubMed] [Google Scholar]
- Nymberg C, Jia T, Lubbe S, Ruggeri B, Desrivieres S, Barker G, Buchel C, Fauth-Buehler M, Cattrell A, Conrod P, Flor H, Gallinat J, Garavan H, Heinz A, Ittermann B, Lawrence C, Mann K, Nees F, Salatino-Oliveira A, Paillere Martinot ML, Paus T, Rietschel M, Robbins T, Smolka M, Banaschewski T, Rubia K, Loth E, Schumann G, Consortium I, 2013. Neural mechanisms of attention-deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biol Psychiatry 74, 607–614. 10.1016/j.biopsych.2013.03.027 [DOI] [PubMed] [Google Scholar]
- Ogloff JR, 2006. Psychopathy/antisocial personality disorder conundrum. Aust N Z J Psychiatry 40, 519–528. 10.1080/j.1440-1614.2006.01834.x [DOI] [PubMed] [Google Scholar]
- Ohmomo Y, Hirata M, Murakami K, Magata Y, Tanaka C, Yokoyama A, 1991. Synthesis of [125I]iodoclorgyline, a selective monoamine oxidase A inhibitor, and its biodistribution in mice. Chem Pharm Bull (Tokyo) 39, 3343–3345. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL, 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10, 206–219. 10.1093/cercor/10.3.206 [DOI] [PubMed] [Google Scholar]
- Oreland L, 1971. Purification and properties of pig liver mitochondrial monoamine oxidase. Arch Biochem Biophys. 146, 410–21. [DOI] [PubMed] [Google Scholar]
- Oreland L, Nilsson K, Damberg M, Hallman J, 2007. Monoamine oxidases: activities, genotypes and the shaping of behaviour. J Neural Transm (Vienna). 114, 817–822. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC, 2006. Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J Biol Chem. 281, 21512–21525. [DOI] [PubMed] [Google Scholar]
- Ozelius L, Hsu YP, Bruns G, Powell JF, Chen S, Weyler W, Utterback M, Zucker D, Haines J, Trofatter JA, et al. , 1988. Human monoamine oxidase gene (MAOA): chromosome position (Xp21-p11) and DNA polymorphism. Genomics 3, 53–58. 10.1016/0888-7543(88)90159-0 [DOI] [PubMed] [Google Scholar]
- Palmer EE, Leffler M, Rogers C, Shaw M, Carroll R, Earl J, Cheung NW, Champion B, Hu H, Haas SA, Kalscheuer VM, Gecz J, Field M, 2016. New insights into Brunner syndrome and potential for targeted therapy. Clin Genet 89, 120–127. 10.1111/cge.12589 [DOI] [PubMed] [Google Scholar]
- Partridge GE, 1930. Current conceptions of psychopathic personality. Am. J. Psychiatry 10, 53–99. [Google Scholar]
- Paterson IA, Davis BA, Durden DA, Juorio AV, Yu PH, Ivy G, Milgram W, Mendonca A, Wu P, Boulton AA, 1995. Inhibition of MAO-B by (−)-deprenyl alters dopamine metabolism in the macaque (Macaca facicularis) brain. Neurochem Res. 20, 1503–1510. [DOI] [PubMed] [Google Scholar]
- Paterson IA, Juorio AV, Berry MD, Zhu MY, 1991. Inhibition of monoamine oxidase-B by (−)-deprenyl potentiates neuronal responses to dopamine agonists but does not inhibit dopamine catabolism in the rat striatum. J Pharmacol Exp Ther. 258, 1019–1026. [PubMed] [Google Scholar]
- Pehme PM, Zhang W, Finik J, Pritchett A, Buthmann J, Dana K, Hao K, Nomura Y, 2018. Placental MAOA expression mediates prenatal stress effects on temperament in 12-month-olds. Infant Child Dev. 27, e2094 10.1002/icd.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Wernett P, Plume J, Packer H, Brody GH, Beach SR, 2011. Gene environment interactions with a novel variable Monoamine Oxidase A transcriptional enhancer are associated with antisocial personality disorder. Biol Psychol. 87, 366–71 10.1016/j.biopsycho.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Hill J, Breen G, Quinn J, Abbott K, Jones H, Sharp H, 2013. Evidence for interplay between genes and parenting on infant temperament in the first year of life: monoamine oxidase A polymorphism moderates effects of maternal sensitivity on infant anger proneness. J Child Psychol Psychiatry 54, 1308–1317. 10.1111/jcpp.12081 [DOI] [PubMed] [Google Scholar]
- Piton A, Poquet H, Redin C, Masurel A, Lauer J, Muller J, Thevenon J, Herenger Y, Chancenotte S, Bonnet M, Pinoit JM, Huet F, Thauvin-Robinet C, Jaeger AS, Le Gras S, Jost B, Gerard B, Peoc’h K, Launay JM, Faivre L, Mandel JL, 2014. 20 ans apres: a second mutation in MAOA identified by targeted high-throughput sequencing in a family with altered behavior and cognition. Eur J Hum Genet 22, 776–783. 10.1038/ejhg.2013.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman H, Orobio de Castro B, Koops W, van Boxtel HW, Merk WW, 2007. A Meta-Analysis of the Distinction between Reactive and Proactive Aggression in Children and Adolescents. J Abnorm Child Psychol.35, 522–535. 10.1007/s10802-007-9109-4 [DOI] [PubMed] [Google Scholar]
- Popova NK, Maslova LN, Morosova EA, Bulygina VV, Seif I, 2006. MAO A knockout attenuates adrenocortical response to various kinds of stress. Psychoneuroendocrinology 31, 179–186. 10.1016/j.psyneuen.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Portnoy J, Raine A, Chen FR, Pardini D, Loeber R, Jennings JR, 2014. HEART RATE AND ASB: THE MEDIATING ROLE OF IMPULSIVE SENSATION SEEKING. Criminology 52, 292–311. 10.1111/1745-9125.12038 [DOI] [Google Scholar]
- Poulton R, Moffitt TE, Silva PA, 2015. The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 50, 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard Z, Mackinnon A, Jorm AF, Easteal S, 2008. No evidence for interaction between MAOA and childhood adversity for ASB. Am J Med Genet B Neuropsychiatr Genet. 147B, 228–332. [DOI] [PubMed] [Google Scholar]
- Prom-Wormley EC, Eaves LJ, Foley DL, Gardner CO, Archer KJ, Wormley BK, Maes HH, Riley BP, Silberg JL, 2009. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychol Med 39, 579–590. 10.1017/S0033291708004170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves-Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS, 2014. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One 9, e91151 10.1371/journal.pone.0091151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, 2002. Annotation: the role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. J Child Psychol Psychiatry 43, 417–434. [DOI] [PubMed] [Google Scholar]
- Raine A, O’Brien M, Smiley N, Scerbo A, Chan CJ, 1990. Reduced lateralization in verbal dichotic listening in adolescent psychopaths. J Abnorm Psychol 99, 272–277. 10.1037//0021-843x.99.3.272 [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA, 1997. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: evidence from the Mauritius Child Health Project. J Am Acad Child Adolesc Psychiatry 36, 1457–1464. 10.1097/00004583-199710000-00029 [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Olivieri A, Holt A, 2011. An improved approach to steady-state analysis of monoamine oxidases. J Neural Transm (Vienna). 118, 1003–1019. 10.1007/s00702-011-0657-y [DOI] [PubMed] [Google Scholar]
- Rasanen P, Hakko H, Isohanni M, Hodgins S, Jarvelin MR, Tiihonen J, 1999. Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in the Northern Finland 1966 Birth Cohort. Am J Psychiatry 156, 857–862. 10.1176/ajp.156.6.857 [DOI] [PubMed] [Google Scholar]
- Rehavi MM, Starr SB, 2014. Racial Disparity in Federal Criminal Sentences. J. Polit. Econ. 122, 1320–1354. 10.1086/677255 [DOI] [Google Scholar]
- Reti IM, Xu JZ, Yanofski J, McKibben J, Uhart M, Cheng YJ, Zandi P, Bienvenu OJ, Samuels J, Willour V, Kasch-Semenza L, Costa P, Bandeen-Roche K, Eaton WW, Nestadt G, 2011. Monoamine oxidase A regulates antisocial personality in whites with no history of physical abuse. Compr Psychiatry 52, 188–194. 10.1016/j.comppsych.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A, Rohrmann S, Zhang L, Eichholzer M, Basaria S, Selvin E, Dobs AS, Kanarek N, Menke A, Nelson WG, Platz EA, 2014. Racial variation in sex steroid hormone concentration in black and white men: a meta-analysis. Andrology. 2014 May;2(3):428–35. 10.1111/j.20472927.2014.00206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter D, 2003. Effects of menstrual cycle phase on reporting levels of aggression using the buss and perry aggression questionnaire. Aggress. Behav. 29, 531–538. 10.1002/ab.10054 [DOI] [Google Scholar]
- Robins LN, 1987. The epidemiology of antisocial personality disorder In: Psychiatry, Michels RO, Cavenar JO (Eds), Vol 3 Lippincott, Philadelphia [Google Scholar]
- Robins LN, Regier DA, 1991. Psychiatric disorders in America : the epidemiologic catchment area study. Free Press ; Collier Macmillan Canada ; Maxwell Macmillan International, New York; Toronto; New York; Oxford. [Google Scholar]
- Roettger ME, Boardman JD, Harris KM, Guo G, 2016. The association between the MAOA 2R genotype and delinquency over time among men: the interactive role of parental closeness and parental incarceration. Crim Justice Behav. 43, 1076–1094. 10.1177/0093854816629184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Rebollar C, Ostrosky-Shejet F, Camarena-Medellín B, Bobes-León MA, Díaz-Galván KX, Pérez-López ML, 2015. Brain morphometric correlates of MAOA-uVNTR polymorphism in violent behavior. Rev. Médica Del Hosp. Gen. México 78, 13–20. [Google Scholar]
- Rosell DR, Siever LJ, 2015. The neurobiology of aggression and violence. CNS Spectr 20, 254–279. 10.1017/S109285291500019X [DOI] [PubMed] [Google Scholar]
- Rosell DR, Thompson JL, Slifstein M, Xu X, Frankle WG, New AS, Goodman M, Weinstein SR, Laruelle M, Abi-Dargham A, Siever LJ, 2010. Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol Psychiatry 67, 1154–1162. 10.1016/j.biopsych.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA, 1998. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 44, 839–850. [DOI] [PubMed] [Google Scholar]
- Ruchkin VV, Koposov RA, af Klinteberg B, Oreland L, Grigorenko EL, 2005. Platelet MAO-B, personality, and psychopathology. J Abnorm Psychol. 114, 477–482. [DOI] [PubMed] [Google Scholar]
- Ruisch IH, Dietrich A, Glennon JC, Buitelaar JK, Hoekstra PJ, 2019. Interplay between genome-wide implicated genetic variants and environmental factors related to childhood ASB in the UK ALSPAC cohort. Eur Arch Psychiatry Clin Neurosci. 269, 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D, 1998. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103, 273–279. 10.1007/s004390050816 [DOI] [PubMed] [Google Scholar]
- Sadeh N, Javdani S, Verona E, 2013. Analysis of monoaminergic genes, childhood abuse, and dimensions of psychopathy. J Abnorm Psychol. 122, 167–79. 10.1037/a0029866 [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Sowa C, Sakamoto Y, Nishihara B, Koyama Y, Baba A, Matsuda T, 2002. Modulation by 5-hT2A receptors of aggressive behavior in isolated mice. Jpn J Pharmacol 89, 89–92. 10.1254/jjp.89.89 [DOI] [PubMed] [Google Scholar]
- Samochowiec A, Chęć M, Kopaczewska E, Samochowiec J, Lesch O, Grochans E, Jasiewicz A, Bienkowski P, Łukasz K, Grzywacz A, 2015. Monoamine oxidase a promoter variable number of tandem repeats (MAOA-uVNTR) in alcoholics according to Lesch typology. Int J Environ Res Public Health. 12, 33173326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samochowiec J, Lesch KP, Rottmann M, Smolka M, Syagailo YV, Okladnova O, Rommelspacher H, Winterer G, Schmidt LG, Sander T, 1999. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Res 86, 67–72. 10.1016/s0165-1781(99)00020-7 [DOI] [PubMed] [Google Scholar]
- Samuels J, Bienvenu OJ, Cullen B, Costa PT Jr, Eaton WW, Nestadt G, 2004. Personality dimensions and criminal arrest. Compr Psychiatry 45, 275–280. [DOI] [PubMed] [Google Scholar]
- Sari Y, Khalil A, 2015. Monoamine Oxidase Inhibitors Extracted from Tobacco Smoke as Neuroprotective Factors for Potential Treatment of Parkinson’s Disease. CNS Neurol Disord Drug Targets 14, 777–785. [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG, 1992. Quantitative enzyme radioautography with 3H-Ro 41–1049 and 3H-Ro 19–6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 12, 1977–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele MA, Ziegler C, Kollert L, Katzorke A, Schartner C, Busch Y, Gromer D, Reif A, Pauli P, Deckert J, Herrmann MJ, Domschke K, 2018. Plasticity of Functional MAOA Gene Methylation in Acrophobia. Int J Neuropsychopharmacol. 21, 822–827. 10.1093/ijnp/pyy050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter T, Winz O, Henkel K, Eggermann T, Mohammadkhani-Shali S, Dietrich C, Heinzel A, Decker M, Cumming P, Zerres K, Piel M, Mottaghy FM, Vernaleken I, 2016. MAOA-VNTR polymorphism modulates context-dependent dopamine release and aggressive behavior in males. Neuroimage. 125, 378–385. 10.1016/j.neuroimage.2015.10.031 [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Sander T, Kuhn S, Smolka M, Rommelspacher H, Samochowiec J, Lesch KP, 2000. Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious-depressive alcoholics. J Neural Transm 107, 681–689. 10.1007/s007020070069 [DOI] [PubMed] [Google Scholar]
- Schwartz D, Dodge KA, Pettit GS, Bates JE, 1997. The early socialization of aggressive victims of bullying. Child Dev 68, 665–675. [PubMed] [Google Scholar]
- Scott AL, Bortolato M, Chen K, Shih JC, 2008. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport 19, 739–743. 10.1097/WNR.0b013e3282fd6e88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, Roiser JP, Tan GC, Viding E, Wood NW, Blakemore SJ, 2010. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain Behav. 2010. 9, 628–637. 10.1111/j.1601-183X.2010.00596.x [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, Li S, 2010. Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress, Executive Summary. Department of Health and Human Services, Administration for Children and Families, Washington, DC: US [Google Scholar]
- Segovia G, Porras A, Del Arco A, Mora F, 2001. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev 122, 1–29. 10.1016/s0047-6374(00)00225-6 [DOI] [PubMed] [Google Scholar]
- Shi Y, van Rhijn JR, Bormann M, Mossink B, Frega M, Hakobjan M, KittelSchneider S, Schubert D, Brunner H, Franke B, Kasri NN, 2019. Brunner Syndrome associated MAOA dysfunction in human induced dopaminergic neurons results in dysregulated NMDAR expression and increased network activity. bioRxiv 741108. 10.1101/741108 [DOI] [Google Scholar]
- Shih JC, Chen K, Ridd MJ, 1999. Role of MAO A and B in neurotransmitter metabolism and behavior. Pol J Pharmacol 51, 25–29. [PubMed] [Google Scholar]
- Shumay E, Logan J, Volkow ND, Fowler JS, 2012. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics 7, 1151–1160. 10.4161/epi.21976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva TC, Grana JL, Gonzalez-Cieza L, 2014. Self-reported physical and emotional abuse among youth offenders and their association with internalizing and externalizing psychopathology: a preliminary study. Int J Offender Ther Comp Criminol 58, 590–606. 10.1177/0306624X12474975 [DOI] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Stewart EA, Brody GH, Beach SR, Philibert RA, Gibbons FX, 2012. Social adversity, genetic variation, street code, and aggression: A genetically informed model of violent behavior. Youth Violence Juv Justice. 10, 3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton N, Meltzer M, Gatward R, Coid J, & Deasy D, 1998. Psychiatric Morbidity among Prisoners in England and Wales. London: The Stationary Office. [Google Scholar]
- Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell’osso L, Virkkunen M, Goldman D, 2008. A non-additive interaction of a functional MAO-A uVNTR and testosterone predicts ASB. Neuropsychopharmacology 33, 425–430. 10.1038/sj.npp.1301417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindstrom L, Oreland L, 2007. Adolescent girls and criminal activity: role of MAOA-LPR genotype and psychosocial factors. Am J Med Genet B Neuropsychiatr Genet 144B, 159–164. 10.1002/ajmg.b.30360 [DOI] [PubMed] [Google Scholar]
- Skilling TA, Harris GT, Rice ME, Quinsey VL, 2002. Identifying persistently antisocial offenders using the Hare Psychopathy Checklist and DSM antisocial personality disorder criteria. Psychol Assess. 14, 27–38. [PubMed] [Google Scholar]
- Smaragdi A, Chavez S, Lobaugh NJ, Meyer JH, Kolla NJ, 2019. Differential Alevels of prefrontal cortex glutamate+glutamine in adults with antisocial personality disorder and bipolar disorder: A proton magnetic resonance spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry 93, 250–255. 10.1016/j.pnpbp.2019.04.002 [DOI] [PubMed] [Google Scholar]
- Smeijers D, Bulten E, Franke B, Buitelaar J, Verkes RJ, 2020. Associations of multiple trauma types and MAOA with severe aggressive behavior and MAOA effects on training outcome. Eur Neuropsychopharmacol. 30, 66–74. 10.1016/j.euroneuro.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Gray NS, 2011. Impulsivity and psychopathy: associations between the Barrett impulsivity scale and the psychopathy checklist revised. Psychiatry Res. 187, 414–417. 10.1016/j.psychres.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Soliman A, Bagby RM, Wilson AA, Miler L, Clark M, Rusjan P, Sacher J, Houle S,Meyer JH, 2011. Relationship of monoamine oxidase A binding to adaptive and maladaptive personality traits. Psychol Med. 41, 1051–1060. 10.1017/S0033291710001601 [DOI] [PubMed] [Google Scholar]
- Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T, 2008. Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A 105, 5739–5744. 10.1073/pnas.0710626105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Schultheiss OC, 2007. Basal and dynamic relationships between implicit power motivation and estradiol in women. Horm. Behav. 52, 571–580. 10.1016/j.yhbeh.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F, 2005. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 57, 7–15. 10.1016/j.biopsych.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Stetler DA, Davis C, Leavitt K, Schriger I, Benson K, Bhakta S, Wang LC, Oben C, Watters M, Haghnegahdar T, Bortolato M, 2014. Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. J Psychiatr Res 58, 69–75. 10.1016/j.jpsychires.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE, Fink G, 1995. Estrogen increases the density of 5-hydroxytryptamine(2A) receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol. 54, 15–20. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Mueller EA, Cohen RM, Jimerson DC, Pickar D, Murphy DL, 1985. Tyramine pressor sensitivity changes during deprenyl treatment. Psychopharmacol. 86, 432–437. 10.1007/bf00427904 [DOI] [PubMed] [Google Scholar]
- Swanson MC, Bland RC, Newman SC, 1994. Epidemiology of psychiatric disorders in Edmonton. Antisocial personality disorders. Acta Psychiatr Scand Suppl 376, 63–70. [PubMed] [Google Scholar]
- Taylor A, Kim-Cohen J, 2007. Meta-analysis of gene-environment interactions in developmental psychopathology. Dev Psychopathol 19, 1029–1037. 10.1017/S095457940700051X [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Rautiainen MR, Ollila HM, Repo-Tiihonen E, Virkkunen M, Palotie A, Pietilainen O, Kristiansson K, Joukamaa M, Lauerma H, Saarela J, Tyni S, Vartiainen H, Paananen J, Goldman D, Paunio T, 2015. Genetic background of extreme violent behavior. Mol Psychiatry 20, 786–792. 10.1038/mp.2014.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen R, Ducci F, Goldman D, Holi M, Lindberg N, Tiihonen J, Virkkunen M, 2010. MAOA alters the effects of heavy drinking and childhood physical abuse on risk for severe impulsive acts of violence among alcoholic violent offenders. Alcohol Clin Exp Res 34, 853–860. 10.1111/j.1530-0277.2010.01157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen R, Auvinen-Lintunen L, Ducci F, Sjöberg RL, Goldman D, Tiihonen J, Ojansuu I, Virkkunen M, 2010. Psychopathy, PCL-R, and MAOA genotype as predictors of violent reconvictions. Psychiatry Res. 2011 Feb 28;185(3):382–6. 10.1016/j.psychres.2010.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, Houle S, Kish SJ, 2013. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab. 33, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman P, Grounds P, Brennan KA, 2017. Monoamine oxidase inhibitory activity in tobacco particulate matter: Are harman and norharman the only physiologically relevant inhibitors? Neurotoxicology 59, 22–26. 10.1016/j.neuro.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Tu HP, Ko AM, Wang SJ, Lee CH, Lea RA, Chiang SL, Chiang HC, Wang TN, Huang MC, Ou TT, Lin GT, Ko YC, 2010. Monoamine oxidase A gene polymorphisms and enzyme activity associated with risk of gout in Taiwan aborigines. Hum Genet. 127, 223–229. 10.1007/s00439-009-0765-z [DOI] [PubMed] [Google Scholar]
- Van Loo PLP, Kruitwagen CLJJ, Koolhaas JM, Van de Weerd HA, Van Zutphen LFM, Baumans V, 2002. Influence of cage enrichment on aggressive behaviour and physiological parameters in male mice. J Appl Anim Behav Sci 76, 65–81. [Google Scholar]
- Vanyukov MM, Maher BS, Devlin B, Tarter RE, Kirillova GP, Yu LM, Ferrell RE, 2004. Haplotypes of the monoamine oxidase genes and the risk for substance use disorders. Am J Med Genet B Neuropsychiatr Genet. 125B(1), 120–125. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Yu LM, Tarter RE, Deka R, 1995. Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAOA gene with early onset alcoholism/substance abuse. Am J Med Genet. 60, 122–126. [DOI] [PubMed] [Google Scholar]
- Verhoeven FE, Booij L, Kruijt AW, Cerit H, Antypa N, Does W, 2012. The effects of MAOA genotype, childhood trauma, and sex on trait and state-dependent aggression. Brain Behav 2, 806–813. 10.1002/brb3.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeersch H, T’Sjoen G, Kaufman JM, Vincke J, 2008. Estradiol, testosterone, differential association and aggressive and non-aggressive risk-taking in adolescent girls. Psychoneuroendocrinology 33, 897–908. 10.1016/j.psyneuen.2008.03.016 [DOI] [PubMed] [Google Scholar]
- Verona E, Bresin K, 2015. Aggression proneness: Transdiagnostic processes involving negative valence and cognitive systems. Int J Psychophysiol 98, 321–329. 10.1016/j.ijpsycho.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Veroude K, Zhang-James Y, Fernàndez-Castillo N, Bakker MJ, Cormand B, Faraone SV, 2016. Genetics of aggressive behavior: An overview. Am J Med Genet B Neuropsychiatr Genet. 171B(1), 3–43. 10.1002/ajmg.b.32364 [DOI] [PubMed] [Google Scholar]
- Vishnivetskaya GB, Skrinskaya JA, Seif I, Popova NK, 2007. Effect of MAO A deficiency on different kinds of aggression and social investigation in mice. Aggress Behav. 33, 1–6. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Fouquet C, Alvarez C, Seif I, Price D, Gaspar P, Cases O, 2002. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. J Comp Neurol. 442, 331–347. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Brendgen M, Barker ED, 2006. Subtypes of aggressive behavior: a developmental perspective. Int. J. Behav. Dev. 30, 12–19. [Google Scholar]
- Wagels L, Votinov M, Kellermann T, Konzok J, Jung S, Montag C, Schneider F, Eisert A, Beyer C, Habel U, 2019. Exogenous testosterone and the monoamine-oxidase A polymorphism influence anger, aggression and neural responses to provocation in males. Neuropharmacology. 156, 107491 10.1016/j.neuropharm.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Wagels L, Votinov M, Radke S, Clemens B, Montag C, Jung S, Habel U, 2017. Blunted insula activation reflects increased risk and reward seeking as an interaction of testosterone administration and the MAOA polymorphism. Hum Brain Mapp 38, 4574–4593. 10.1002/hbm.23685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Keenan K, 2001. Clinical significance and correlates of disruptive behavior in environmentally at-risk preschoolers. J Clin Child Psychol 30, 262–275. 10.1207/S15374424JCCP3002_13 [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, Dukic V, Blair RJ, Leventhal BL, Cox NJ, Burns JL, Kasza KE, Wright RJ, Cook EH Jr., 2010. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth ASB. Mol Psychiatry. 2010. September;15(9):928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL, 1997. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry 54, 670–676. 10.1001/archpsyc.1997.01830190098010 [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E Jr., Benowitz NL, Leventhal BL, 2002. Maternal smoking during pregnancy and severe ASB in offspring: a review. Am J Public Heal. 92, 966–974. 10.2105/ajph.92.6.966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Kearney EB, Seng R, Singer TP, 1971. Sequence and structure of a cysteinyl flavin peptide from monoamine oxidase. Biochem Biophys Res Commun. 44, 287–292. [DOI] [PubMed] [Google Scholar]
- Waltes R, Chiocchetti AG, Freitag CM, 2016. The neurobiological basis of human aggression: A review on genetic and epigenetic mechanisms. Am J Med Genet B Neuropsychiatr Genet. 171, 650–75. 10.1002/ajmg.b.32388 [DOI] [PubMed] [Google Scholar]
- Wang CH, Ning QF, Liu C, Lv TT, Cong EZ, Gu JY, Zhang YL, Nie HY, Zhang XL, Li Y, Zhang XY, Su LY, 2018. Associations of serotonin transporter gene promoter polymorphisms and monoamine oxidase A gene polymorphisms with oppositional defiant disorder in a Chinese Han population. Behav Brain Funct 14, 15 10.1186/s12993-018-0147-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SJ, McNulty TL, 2016. Genes, Parenting, Self-Control, and Criminal Behavior. Int J Offender Ther Comp Criminol. 60, 469–91. 10.1177/0306624X14553813 [DOI] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J, 2009. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol Psychiatry 65, 417–424. 10.1016/j.biopsych.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierstall R, Elbert T, 2011. The Appetitive Aggression Scale-development of an instrument for the assessment of human’s attraction to violence. Eur J Psychotraumatol. 2 10.3402/ejpt.v2i0.8430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weierstall R, Hinsberger M, Kaminer D, Holtzhausen L, Madikane S, Elbert T (2013). Appetitive aggression and adaptation to a violent environment among youth offenders., Peace Confl. 19, 138. [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB, 1999. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry 38, 892–899. 10.1097/00004583-199907000-00020 [DOI] [PubMed] [Google Scholar]
- Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ, 2006. Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet. 36, 163–172. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Zhang X, Clarke C, 1994. Effects of gestational exposure to monoamine oxidase inhibitors in rats: preliminary behavioral and neurochemical studies. Neuropsychopharmacology. 11, 125–132. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM, 2006. MAOA and the “cycle of violence:” childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 60, 684–689. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, Palmer DM, Paul RH, Song L, Costa PT, Schofield PR, Gordon E, 2009. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on n antisocial index. Neuropsychopharmacology 34, 1797–1809. 10.1038/npp.2009.1 [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC, 2003. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology 28, 533–541. 10.1038/sj.npp.1300054 [DOI] [PubMed] [Google Scholar]
- Wrangham RW, 2018. Two types of aggression in human evolution. Proc Natl Acad Sci U S A 115, 245–253. 10.1073/pnas.1713611115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JB, Chen K, Li Y, Lau YF, Shih JC, 2009. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J. 23, 4029–4038. 10.1096/fj.09-139097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Vernier P, 2011. The evolution of dopamine systems in chordates. Front Neuroanat. 5, 21 10.3389/fnana.2011.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XJ, Zhan XH, Hou JL, 2012. Study on the correlation between single nucleotide polymorphism of monoamine oxidase A gene and anger regulation. Zhongguo Zhong Xi Yi Jie He Za Zhi. 32, 1354–1357. [PubMed] [Google Scholar]
- Yoon BE, Woo J, Chun YE, Chun H, Jo S, Bae JY, An H, Min JO, Oh SJ, Han KS, Kim HY, Kim T, Kim YS, Bae YC, Lee CJ, 2014. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 592, 4951–4968. 10.1113/jphysiol.2014.278754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Gingrich JA, Ansorge MS, 2014. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry 19, 688–698. 10.1038/mp.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Harkavy-Friedman JM, Oquendo MA, Ellis SP, Mann JJ, 2005. Relationship of MAO-A promoter (u-uVNTR) and COMT (V158M) gene polymorphisms to CSF monoamine metabolites levels in a psychiatric sample of caucasians: A preliminary report. Am J Med Genet B Neuropsychiatr Genet 132B, 100–103. 10.1002/ajmg.b.30094 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ming Q, Wang X, Yao S, 2016. The interactive effect of the MAOA-uVNTR genotype and childhood abuse on aggressive behaviors in Chinese male adolescents. Psychiatr Genet. 26, 117–123. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ming QS, Yi JY, Wang X, Chai QL, Yao SQ, 2017. Gene-GeneEnvironment Interactions of Serotonin Transporter, Monoamine Oxidase A and Childhood Maltreatment Predict Aggressive Behavior in Chinese Adolescents. Front Behav Neurosci. 11, 17 10.3389/fnbeh.2017.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang-James Y, Fernàndez-Castillo N, Hess JL, Malki K, Glatt SJ, Cormand B, Faraone SV, 2019. An integrated analysis of genes and functional pathways for aggression in human and rodent models. Mol Psychiatry. 24, 1655–1667. 10.1038/s41380-018-0068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Chen K, Shih JC, 1994. Bidirectional promoter of human monoamine oxidase A (MAO A) controlled by transcription factor Sp1. J Neurosci. 14, 73937403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Grimsby J, Chen K, Shih JC, 1992. Promoter organization and activity of human monoamine oxidase (MAO) A and B genes. J Neurosci. 12, 4437–4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhou X, Xia LX, 2019. Brain structures and functional connectivity associated with individual differences in trait proactive aggression. Sci Rep 9, 7731 10.1038/s41598-019-44115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans T, Dumontheil I, Roggeman C, Peyrard-Janvid M, Matsson H, Kere J,Klingberg T, 2012. Working memory brain activity and capacity link MAOA polymorphism to aggressive behavior during development. Transl Psychiatry. 2:e85 10.1038/tp.2012.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, 1999. Diathesis-stress models In Zuckerman M, Vulnerability to psychopathology: A biosocial model (pp. 3–23). American Psychological Association; 10.1037/10316-001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.