Abstract
Transcranial Direct Current Stimulation (tDCS) has been used as an alternative treatment for pain reduction in fibromyalgia. In this study, in addition to behavioral measures, we analyzed oscillations in alpha 2 frequency band in the frontal, occipital, and parietal regions, in response to the application of two neuromodulation protocols in fibromyalgia. The study was a randomized, double-blind, placebo-controlled clinical trial with 31 women diagnosed with fibromyalgia. The participants were allocated to three groups with the anodic stimulation applied on the left motor cortex: Group 1, for five consecutive days; Group 2, for 10 consecutive days; and Group 3, sham stimulation for five consecutive days. Statistical analysis showed a reduction in pain intensity after treatment for groups in general [F (1.28) = 8.02; p = 0.008; η2 = 0.223], in addition to a reduction in alpha 2 in the frontal (p = 0.039; d = 0.384) and parietal (p = 0.021; d = 0.520) regions after the treatment on five consecutive days. We conclude that neuromodulation protocols produced similar effects on pain reduction, but differed with respect to the changes in the alpha 2 frequency band in the frontal and parietal regions.
Subject terms: Neuroscience, Diseases, Health care
Introduction
Fibromyalgia (FM) is a clinical condition characterized by the presence of generalized and disabling chronic pain, and may involve symptoms of depression and anxiety1. Its diagnosis is based on exclusively clinical criteria, and there are no complementary tests that contribute to its identification.
A challenge in relation to FM is the therapeutic possibilities, as the drugs available for the treatment of chronic pain have provided only modest relief for these patients2. Thus, the technique of Transcranial Direct Current Stimulation (tDCS) has shown promising results in the treatment of chronic pain in this population3. The application of tDCS has been widely studied in other pain syndromes2,4,5. However, the current evidence is still very limited in relation to the ideal treatment protocol, such as frequency and duration of stimulation3,4, especially in relation to FM.
The application of anodic tDCS over M1 has shown positive results in pain levels2 and that repeated stimulation causes superior results in analgesia in individuals with FM6. The effectiveness of tDCS for five days was found by Fagerlund, Hansen and Aslaksen7, who found that stimulation was able to promote pain relief without providing serious adverse effects when testing the effect of tDCS in participants with FM in a hospital environment. Mendonca et al.8, with a similar protocol, found that the intervention with tDCS provided a reduction in pain and anxiety in individuals with FM. On the other hand, Valle et al.9 used a 10-day consecutive tDCS protocol, and also achieved improvement in pain intensity in FM and long-term clinical benefits with stimulation in M1. Research capable of comparing different protocols would help to clarify the best parameters and duration of treatment to be used for pain in FM, enabling the consolidation of a therapeutic protocol.
In the present study, we compared the two more frequently used protocols for the pain treatment of FM, in which the anodic stimulation is applied to M1 for five and ten consecutive days. An advantage of comparing protocols with different durations is to identify the tDCS protocol that produces satisfactory responses with a smaller number of sessions, consequently, minimizing the occurrence of adverse effects and reducing the total time and costs of the therapeutic intervention.
In addition to pain measures, we analyzed the cortical electrical activity associated to the tDCS stimulation, which had not yet been investigated with these protocols in this population. Studies providing an electrophysiological measure of response to the tDCS treatment may help to provide another data in addition to the behavioral one. In this sense, the electroencephalogram (EEG) stands out as a tool for monitoring response to treatment10,11.
Although there is disagreement in the literature, in general, the analysis of chronic pain in FM through the EEG shows amplitude of the altered alpha wave, being more studied in the frontal, parietal and occipital regions10,12. Alpha is commonly related to the state of relaxation13. Villafaina et al.14 observed that individuals with FM showed a decrease in the alpha 2 power range in the resting condition, suggesting that chronic pain in these patients modulates this frequency range throughout time. For this reason, the alpha 2 frequency band was the subject of our study. Theoretically, the manipulation of the amplitude of the frequency bands could be associated with behavioral changes13. Therefore, the scarcity of studies that offer behavioral and physiological measures of response to treatment with tDCS underscore the importance of this study.
In the present research, we compared two tDCS protocols for pain and their electroencephalographic correlations at rest in women with FM in the frontal, parietal and occipital regions. Our general hypothesis was that different tDCS protocols would lead to a decreased in pain, and differentially modulate the cortical electrical activity in women with fibromyalgia.
Results
The average age of the participants was 44.81 years old (SD = 8.8) and the average level of pain reported was 6.66 (SD = 1.70) in the VAS. The mean time of diagnosis of FM was 6.60 years (SD = 5.38) and there was no difference between this measure in the groups [F (2, 28) = 2.84; p = 0.075]. In addition, 77.40% reported medication use, 25.8% practiced physical activity and 32.30% did psychotherapy. These measures also did not differ between groups [drugs (χ2 (2) = 0.26, p = 0.878); physical activity (χ2 (2) = 1.67, p = 0.404); psychotherapy (χ2 (2) = 0.59, p = 0.747)]. The participants did not report any complaints of adverse effects after the stimulation sessions, nor did they report any neurological complaints or comorbidities, assessed by the CIRS. Also, the groups did not differ from each other before treatment in terms of pain level [F (2,28) = 2.08 p = 0.143], anxiety levels [F (2,28) = 0.57 p = 0.575], depression [F (2, 28) = 0.36 p = 0.699] and cognitive status [F (1, 28) = 2.91, p = 0.071].
There was an effect of the time factor on the pain variable [F (1, 28) = 8.02; p = 0.008; η2 = 0.223], with a reduction in pain levels in general after treatment. However, there were no statistically significant differences for the group factor [F (2, 28) = 0.24; p = 0.792; η2 = 0.017], and no interaction between time and group factors [F (2, 28) = 0.90; p = 0.417; η2 = 0.061].
Regarding the electrophysiological variables, there was an effect of the interaction between time and group on the frontal alpha 2 [F (1 28) = 3.62; p = 0.040; η2 = 0.261] and parietal alpha 2 [F (1, 28) = 4.95; p = 0.014; η2 = 0.261] variables, and the participants who received stimulation for five consecutive days showed a significant reduction in the mean alpha spectral power post-intervention in the frontal (p = 0.039; d = 0.384 ) and parietal (p = 0.021; d = 0.520) regions. For the alpha 2 in the occipital region, there was no interaction effect [F (1, 28) = 2,452; p = 0.104; η2 = 0.149]. Means groups for electrophysiological and pain variables are shown in Table 1.
Table 1.
Comparison between the three groups of participants before and after the tDCS treatment on the outcome measures.
| Variable | 5-days | 10-days | Sham | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | Aafter | Before | After | Groups (5-days vs 10-days vs sham) | Times (before vs after treatment) | Interaction between groups and times | |
| Pain | 7.00 (1.73) | 5.05 (2.49) | 5.72 (1.56) | 5.22 (3.35) | 7.09 (1.61) | 4.82 (3.34) |
p = 0.792 η2 = 0.017 |
p = 0.008 η2 = 0.223 |
p = 0.417 η2 = 0.061 |
| Frontal alpha 2 | 1.07 (3.30) | − 0.24* (3.51) | 1.67 (2.41) | 1.80 (3.07) | 1.95 (2.79) | 2.92 (3.62) |
p = 0.297 η2 = 0.083 |
p = 0.854 η2 = 0.001 |
p = 0.040* η2 = 0.261 |
| Parietal alpha 2 | 3.77 (3.14) | 2.00* (3.63) | 5.31 (2.79) | 5.76 (4.07) | 2.82 (4.74) | 4.19 (5.10) |
p = 0.302 η2 = 0.082 |
p = 0.964 η2 = 0.001 |
p = 0.014* η2 = 0.261 |
| Occipital alpha 2 | 6.15 (2.99) | 4.35 (3.31) | 5.80 (3.57) | 5.99 (4.27) | 6.20 (5.23) | 7.20 (4.86) |
p = 0.680 η2 = 0.027 |
p = 0.716 η2 = 0.005 |
p = 0.104 η2 = 0.149 |
Values are presented as a function of means and standard deviations. For ANOVAs, p and η2 values are presented for the main factors (times and groups) and for the interaction between them. *Post hoc analyzes indicated a decrease in spectral power in frontal and parietal regions after the application of the 5-days tDCS protocol. Level of significance considered was of p < 0.05. SD Standard deviation; η2: Partial eta-squared as a measure of size effect.
Discussion
In the present study, we compared two tDCS protocols for pain and their associated electroencephalographic changes in the frontal, parietal and occipital regions in women with FM. We found no difference between the tDCS protocols applied to M1 for 5 days and 10 days on the reported pain. These results are in line with other studies that found no statistically significant difference between active tDCS and sham on pain levels in FM15, but disagree with the findings of Fagerlund et al.7 and Valle et al.9, who reported pain symptom alleviation after the tDCS protocols applied to M1 for 5 days and 10 days, respectively, when compared to placebo.
It is important to highlight that the optimal parameters of the tDCS administration still need to be defined4. Considering that studies vary in different aspects, information about the optimal parameters cannot be easily obtained from a comparison between these distinct studies16. Previous findings demonstrate limits of the tDCS technique in inducing changes in the cortical excitability17–19. Therefore, it is possible that our results indicate a ceiling effect of the cortical changes, so that it would be necessary to test longer protocols to overcome an eventual plateau of brain responses induced by a 5 to 10-days protocol.
In the present study, we found that tDCS modulated cortical electrical activity, with a decrease in alpha 2 spectral power in the parietal and frontal regions after the treatment with the 5-days stimulation protocol. Similarly, Spitoni et al.20 found changes in alpha activity in the frontal and parietal regions, but not in the occipital region. The increased alpha amplitude is commonly associated with cortical deactivation and inhibition21. Anodic tDCS is commonly associated with increased cortical excitability, so a decline in alpha amplitude is expected after anodic stimulation20 as observed in our study for the 5-days stimulation group. In the other two groups, we observed an increase in alpha 2 activity, but below a statistically significant level. Perhaps, this phenomenon corresponds to the effects of tDCS on inhibitory neurons, which could increase the alpha amplitude after stimulation20. Considering that tDCS is capable of promoting changes in neuronal excitability3, the neuromodulation of the cortical activity may be associated with the decrease in pain.
At the same time, the present results indicate a placebo response to the application of the sham protocol. Other tDCS studies reported similar findings22. Placebo analgesic effects can be brought about by the expectation of symptom improvement17–19. Moreover, a recent meta-analysis of randomized controlled trials showed that the placebo treatment is clinically effective in reducing pain in FM, and stronger in people with greater pain intensity23. The magnitude of the placebo effect in FM may also be influenced by other factors as well as age, gender, disease duration, and expected strength of treatment23. This influence of expectations in the tDCS outcomes has been reported in other studies24,25. Here, another possible explanation for the placebo effect is the parallel design of the study. A recent metanalysis of non-invasive brain stimulation work showed a significant effect of the placebo in parallel designs, but not in crossover studies26. Therefore, further studies may compare tDCS protocols using a parallel design, with the inclusion of control without treatment (waiting list). Likewise, the brain's ability to modify activity in some of its specific structures in response to analgesia due to the placebo effect27,28 may justify the fact that there was analgesia associated with an increase in mean potency alpha2 spectral in the parietal cortex in the sham group.
The results reported here must be considered in light of some limitations. First, the high variability in the diagnosis time, and, consequently, the time living with pain may have influenced the results. To minimize this bias, it was ensured that the groups would be homogeneous in relation to the time of diagnosis. Second, the electric field distribution of the tDCS is influenced by the anatomical distribution of the head tissue. Hence, there is only a limited control on the resulting current distribution in the brain29. Inter-individual variations of the generated fields are likely a key factor that contributes to the observed physiological and behavioral variability, which may explain the differences between groups with regard to the cortical changes. A computational model may provide information on the distribution of tDCS current in the brain as a function of anatomical factors. However, this method is costly and requires the participants to undergo an MRI, previously to the tDCS application30. Nevertheless, future studies may perform the computational modeling of the current in order to control the variability among the individuals with FM.
In conclusion, we found that the tDCS protocols, with the anodic stimulation on M1, for five and 10 consecutive days, as well as the sham protocol, produced similar results in the reduction of pain in women with FM. Nevertheless, the two tDCS protocols modulated alpha 2 cortical electrical activity in the frontal and parietal cortex in different ways. The alpha band is normally associated with a relaxed, passive, and defocused attention state, therefore the modulation of alpha 2 may be related to behavioral changes in women with FM13. Future studies may analyze the effects of longer-term tDCS protocols on pain and brain activity modulation.
Methods
The present study was a longitudinal, randomized, double-blind, placebo-controlled clinical trial, developed for women with fibromyalgia. The project was approved by the Research Ethics Committee of the Health Sciences Center of the Federal University under CAAE: 39796914.5.0000.5188. Written authorization was collected for the participation of each volunteer in the research, through the Informed Consent Form. Participants' autonomy and anonymity was guaranteed, ensuring their privacy regarding confidential data, as regulated by Resolution 466/2012 of the National Health Council. The ethical principles expressed in the Declaration of Helsinki were respected. Our clinical trial was registered on the Clinical Trials platform on 12/28/2017 and is available for public access on the website clinicaltrials.gov through the protocol NCT03384888. It was also registered on the ReBec platform (ensaiosclinicos.gov.br) with protocol RBR-5XBWJK on 06/24/2020. The criteria of the Consolidated standards for test reports—CONSORT were followed.
Sample
The sample was not probabilistic, and comprised 31 volunteers, aged between 27 and 58 years old, who met the following inclusion criteria: (1) having a diagnosis of fibromyalgia, according to the criteria of the American College of Rheumatology; (2) having been diagnosed at least three months ago; (3) be female; (4) be in the age group between 25 and 60 years old; and (5) signing the Informed Consent Form. It was excluded women with a score below 24 on the Mini Mental State Examination (MMSE); metal implants located on the head, cochlear implants and cardiac pacemaker; illiterate; pregnant women; history of seizure; and severe depression, with a score greater than 35 in the Beck Depression Inventory (BDI).
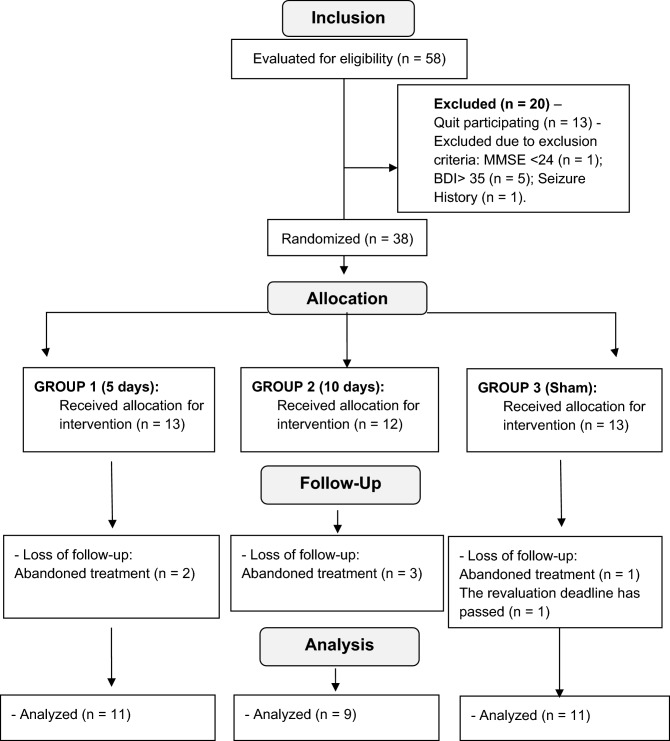
Participants were randomly assigned to three groups: 11 women in Group 1, with anodic stimulation on the left M1 and cathodic stimulation on the right supraorbital region on five consecutive days; nine women in Group 2, with anodic stimulation on the left M1 and cathodic stimulation on the right supraorbital region on 10 consecutive days (excluding the weekend); and 11 women in Group 3 (sham), with simulated type stimulation, following the protocol of Group 1. All volunteers were reevaluated within seven days after the end of care to ensure the measurement of the effects resulting from the application of the current. Prior to the start of consultations, training was conducted with the examiners to minimize random errors and between researchers. The training ended after the standardization of the process was ensured. The flow of participants in the study is described in Fig. 1. The evaluations and neuromodulation sessions were carried out in the Neuroscience Laboratory, individually.
Figure 1.
Flowchart of CONSORT showing the outline of the study groups and the respective sample losses.
Randomization and blinding
The participants were randomly distributed, by one of the researchers, with block exchange at the rate of 1:1:1 using the online randomization program (www.random.org). After randomization, the generated codes were placed in sequential numbered, opaque envelopes and sealed in order to hide the allocation. These envelopes were delivered to the researcher responsible for neurostimulation the day before the start of the sessions. The outcome evaluators and patients were blinded to the type of stimulation applied and the person responsible for neurostimulation blinded to the performance achieved by patients in the evaluations.
Friction and adhesion
As a friction, it was considered the fault in two sessions or a single fault without replacement. In addition to the insertion of medication for continuous use after the initial evaluation. In order to facilitate the participants' adherence to the study, flexible hours for appointments were organized. It was also allowed to miss a day of attendance, being replaced at the end of the sessions, in addition to making periodic calls in order to maintain contact and avoid evasion from the study.
Outcome assessment tools
The instruments used for data collection were: the Sociodemographic and Clinical Questionnaire to characterize the sample; the Cumulative Illness Rating Scale (CIRS)31, for the analysis of existing comorbidities; the Visual Analogue Scale (VAS)32, to check the level of pain at the time of the evaluation; the Mini Mental State Examination (MMSE)33, to assess the participants' cognitive status and serve as an exclusion criterion from the study; the Beck Depression Inventory34, to exclude participants with severe depression; the Beck Anxiety Inventory (BAI)35, to verify that all were homogeneous in terms of anxiety level; and the electroencephalogram, to assess cortical electrical activity. The study phases are shown in Table 2.
Table 2.
Demonstrative schedule of the study phases—CONSORT 2010.
| Time | Recruitment | Allocation | Post-allocation step services | Conclusion | |
|---|---|---|---|---|---|
| − t1 | 0 | t1 1ª week |
t2 2ª week |
t3 | |
| Recruitment | |||||
| Screening selection | x | ||||
| Informed consent | x | ||||
| Other procedures | x | ||||
| Allocation | x | ||||
| Interventions | |||||
| Group 1 intervention | x | ||||
| Group 2 Intervention | x | x | |||
| Group 3 Intervention | x | ||||
| Assessments: | x | ||||
| Revaluations | x | ||||
The clinical trial was conducted between 2018 and 2019.
Evaluation protocol with electroencephalogram
The data collection process with the EEG was made from 32 electrodes placed on the scalp through an adjustable cap, following the EEG International System 10–20, with impedance below 20kΩ36. The amplifier used was the ActiChamp, with a sampling rate of 500 Hz. During the collection, the participants sat comfortably in a chair and were instructed to avoid excessive body and eye movements, in addition to relaxing the mandibular musculature and avoiding muscle contractions in the face region, to decrease the presence of artifacts in the records during the acquisition of the data. Data was collected at rest, 6 min with the participant with eyes open and 6 min with eyes closed37. The time was divided into 2 min separated by small intervals and repeated three times, ending in 12 min for the acquisition of data38.
Neuromodulation protocol
The consultations with the tDCS were performed in an individual session and the electrodes were placed in C3, which corresponds to the region of the primary motor cortex (M1), according to SI 10–20 of the EEG39. The protocol used was 20 min of stimulation per day, the first group was stimulated for five consecutive days, and the second group for two weeks (excluding weekends), totaling 10 sessions. The protocol for sham stimulation was identical to the first group, but the device was turned off 30 s after the start of stimulation, so as not to induce clinical effects. The TCT research equipment was used, with electrodes wrapped in 5 × 7 cm sponges, moistened with saline (NaCl 0.9%). The applied current was 2 mA, the current density being equivalent to 0.05 A / m2. At the end of each session, the participants were asked about the experience of adverse effects, in order to monitor the safety of applying the current.
Analysis of electroencephalographic data
The power spectra of the frequency bands for the electrodes F3, F4, P3, P4, O1 and O2 were analyzed, each representing a cortical area—left and right frontal, left and right parietal, left and right occipital—respectively40, according to the EEG International System 10–20. The analyzes were performed using EEGLAB, a MATLAB toolbox. In the pre-processing, the data filtering was done using the 0.5 Hz high pass and 30 Hz low pass filter. The average of the electrodes was used as a reference, in order to remove possible spatial biases10,36 and, in sequence, the Multiple Artifact Rejection Algorithm—MARA41 for removing artifacts. Only the data corresponding to the participants with their eyes closed were processed37.
Statistical analysis
Statistical analyses were carried out using the Software Statistical Package for the Social Sciences (SPSS) version 22.0 for Windows. First, descriptive analyses were performed, using measures of central tendency and dispersion, to characterize the sample. For inferential analyses, initially the Shapiro–Wilk test was used, which indicated that the data had a normal distribution. The chi-square test was used to compare groups with respect to the practical variables of physical activity, psychotherapy and medication use. The one-way ANOVA test was performed to verify homogeneity between groups before the start of treatment. For pre- and post-treatment evaluation, the ANOVA factorial statistical test with mixed design was used, referring to the three groups (Group 1, Group 2 and Sham) x two times (pre and post treatment). The level of significance considered was p < 0.05. For peer comparison, the Bonferroni-Sidak post hoc test was used. Finally, we used the effect size calculation from the partial eta squared for each variable within each group, with values of 01, 0.06, and > 0.14 reflecting small, medium, and large effects, respectively and Cohen´s d for comparisons between pairs, with values of 0.20, 0.50, and 0.80 reflecting small, medium, and large effects, respectively42.
Acknowledgements
This work was supported in part by funding from the Brazilian National Council for Scientific and Technological Development – CNPQ (nº 307366/2017-0, nº 311910/2017-3), Beatriz Galindo Programme of General Secretariat of Universities at the Ministry of Science, Innovation, and Universities (BGP18/00077) to BF-C; and Grant 008/2019, Paraíba State Research Foundation (FAPESQ).
Author contributions
G.A.M. and N.T. conceived the experiments; G.A.M and B.F-C. analyzed and interpreted the data; N.T., S.M.M.S.A. and E.A.O. designed the work; All authors reviewed the manuscript. All authors have approved the submitted version and have agreed both to be personally accountable for the author's own contributions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Junior MH, Goldenfum MA, Siena CAF. Fibromyalgia: clinical and occupational aspects. Rev. Assoc. Med. Bras. 2012;58:358–365. [PubMed] [Google Scholar]
- 2.DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp. 2011;51:1–11. doi: 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruccu G, et al. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur. J. Neurol. 2016;23:1489–1499. doi: 10.1111/ene.13103. [DOI] [PubMed] [Google Scholar]
- 4.Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology. 2016;55:1507–1517. doi: 10.1093/rheumatology/kew205. [DOI] [PubMed] [Google Scholar]
- 5.Ngernyam, N & Jensen, MP. Transcranial Direct Current Stimulation in Neuropathic Pain. J. Pain Relief.s3, 1–13 (2014). [DOI] [PMC free article] [PubMed]
- 6.Cummiford CM, et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res. Ther. 2016;18:40–51. doi: 10.1186/s13075-016-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015;156:62–71. doi: 10.1016/j.pain.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 8.Mendonca ME, et al. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front. Hum. Neurosci. 2016;10:1–12. doi: 10.3389/fnhum.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valle A, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham- controlled longitudinal clinical trial. J. Pain Manag. 2010;2:353–361. [PMC free article] [PubMed] [Google Scholar]
- 10.González-Roldán AM, Cifre I, Sitges C, Montoya P. Altered dynamic of Eeg oscillations in fibromyalgia patients at rest. Pain Med. 2016;17:1058–1068. doi: 10.1093/pm/pnw023. [DOI] [PubMed] [Google Scholar]
- 11.Jones AKP, Huneke NTM, Lloyd DM, Brown CA, Watson A. Role of functional brain imaging in understanding rheumatic pain. Curr. Rheumatol. Rep. 2012;14:557–567. doi: 10.1007/s11926-012-0287-x. [DOI] [PubMed] [Google Scholar]
- 12.Pinheiro ES, et al. Electroencephalographic patterns in chronic pain: a systematic review of the literature. PLoS ONE. 2016;11:1–26. doi: 10.1371/journal.pone.0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl. Psychophysiol. Biofeedback. 2011;36:193–200. doi: 10.1007/s10484-011-9159-9. [DOI] [PubMed] [Google Scholar]
- 14.Villafaina S, Collado-Mateo D, Fuentes-García JP, Cano-Plasencia R, Gusi N. Impact of fibromyalgia on alpha-2 EEG power spectrum in the resting condition: a descriptive correlational study. BioMed Res. Int. 2019;2019:1–6. doi: 10.1155/2019/7851047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riberto M, Alfieri FM, de Benedetto Pacheco KM, Leite VD, Kaihami HN, Fregni F, Battistella LR. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5:45–50. doi: 10.2174/1874312901105010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012;5:208–213. doi: 10.1016/j.brs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Benwell CSY, Learmonth G, Miniussi C, Harvey M, Thut G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: evidence from biparietal tDCS influence on lateralized attention bias. Cortex. 2015;69:152–165. doi: 10.1016/j.cortex.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Montenegro R, Okano A, Gurgel J, Porto F, Cunha F, Massaferri R, Farinatti P. Motor cortex tDCS does not improve strength performance in healthy subjects. Motriz. 2015;21:185–193. [Google Scholar]
- 19.Furuya S, Klaus M, Nitsche MA, Paulus W, Altenmüller E. Ceiling effects prevent further improvement of transcranial stimulation in skilled musicians. J. Neurosci. 2014;34:13834–13839. doi: 10.1523/JNEUROSCI.1170-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitoni GF, Di Russo F, Cimmino RL, Bozzacchi C, Pizzamiglio L. Modulation of spontaneous alpha brain rhythms using low-intensity transcranial direct-current stimulation. Front. Hum. Neurosci. 2013;7:529–537. doi: 10.3389/fnhum.2013.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Alghamdi F, et al. Effect of transcranial direct current stimulation on the number of smoked cigarettes in tobacco smokers. PLoS ONE. 2019;14:1–14. doi: 10.1371/journal.pone.0212312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Zou K, Abdullah N, Whiteside N, Sarmanova A, Doherty M, Zhang W. The placebo effect and its determinants in fibromyalgia: meta-analysis of randomised controlled trials. Clin. Rheumatol. 2017;36:1623–1630. doi: 10.1007/s10067-017-3595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabipour S, Wu AD, Davidson PSR, Iacoboni M. Expectations may influence the effects of transcranial direct current stimulation. Neuropsychologia. 2018;119:524–534. doi: 10.1016/j.neuropsychologia.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Ray MK, et al. The effect of expectation on transcranial direct current stimulation (tDCS) to suppress food craving and eating in individuals with overweight and obesity. Appetite. 2019;136:1–7. doi: 10.1016/j.appet.2018.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dollfus S, Lecardeur L, Morello R, Etard O. Placebo response in repetitive transcranial magnetic stimulation trials of treatment of Auditory Hallucinations in Schizophrenia: a meta-analysis. Schizophr. Bull. 2016;42:301–308. doi: 10.1093/schbul/sbv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medoff ZM, Colloca L. Placebo analgesia: understanding the mechanisms. Pain Manag. 2015;5:89–96. doi: 10.2217/pmt.15.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. Neural bases of conditioned placebo analgesia. Pain. 2010;151:816–824. doi: 10.1016/j.pain.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Saturnino GB, Madsen KH, Thielscher A. Electric field simulations for transcranial brain stimulation using FEM: an efficient implementation and error analysis. J. Neural Eng. 2019;16:1–15. doi: 10.1088/1741-2552/ab41ba. [DOI] [PubMed] [Google Scholar]
- 30.Thair H, Holloway AL, Newport R, Smith AD. Transcranial direct current stimulation (tDCS): a beginner's guide for design and implementation. Front Neurosci. 2017;11:641–653. doi: 10.3389/fnins.2017.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 32.Huskisson EC. Measurement of pain. The Lancet. 1974;304:1127–1131. doi: 10.1016/S0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 35.Beck AT, Brown G, Epstein N, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 36.Tiemann L, et al. Behavioral and neuronal investigations of hypervigilance in patients with fibromyalgia syndrome. PLoS ONE. 2012;7:1–8. doi: 10.1371/journal.pone.0035068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hargrove JB, et al. Quantitative electroencephalographic abnormalities in fibromyalgia patients. Clin. EEG Neurosci. 2010;41:132–139. doi: 10.1177/155005941004100305. [DOI] [PubMed] [Google Scholar]
- 38.Hassan MA, Fraser M, Conway BA, Allan DB, Vuckovic A. The mechanism of neurofeedback training for treatment of central neuropathic pain in paraplegia: a pilot study. BMC Neurol. 2015;15:200–212. doi: 10.1186/s12883-015-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lefaucheur J-P, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin. Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 40.Vanneste S, Ost J, Van Havenbergh T, De Ridder D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS ONE. 2017;12:1–20. doi: 10.1371/journal.pone.0178516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler I, Haufe S, Tangermann M. Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav. Brain Funct. 2011;7:30–44. doi: 10.1186/1744-9081-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New Jersey: L. Erlbaum Associates; 1988. [Google Scholar]