Abstract
Background.
Persons who inject drugs (PWID) have frequent healthcare encounters related to their injection drug use (IDU) but are often not tested for human immunodeficiency virus (HIV). We sought to quantify missed opportunities for HIV testing during an HIV outbreak among PWID.
Methods.
PWID with HIV diagnosed in 5 Cincinnati/Northern Kentucky counties during January 2017–September 2018 who had ≥1 encounter 12 months prior to HIV diagnosis in 1 of 2 Cincinnati/Northern Kentucky area healthcare systems were included in the analysis. HIV testing and encounter data were abstracted from electronic health records. A missed opportunity for HIV testing was defined as an encounter for an IDU-related condition where an HIV test was not performed and had not been performed in the prior 12 months.
Results.
Among 109 PWID with HIV diagnosed who had ≥1 healthcare encounter, 75 (68.8%) had ≥1 IDU-related encounters in the 12 months before HIV diagnosis. These 75 PWID had 169 IDU-related encounters of which 86 (50.9%) were missed opportunities for HIV testing and occurred among 46 (42.2%) PWID. Most IDU-related encounters occurred in the emergency department (118/169; 69.8%). Using multivariable generalized estimating equations, HIV testing was more likely in inpatient compared with emergency department encounters (adjusted relative risk [RR], 2.72; 95% confidence interval [CI], 1.70–4.33) and at the healthcare system receiving funding for emergency department HIV testing (adjusted RR, 1.76; 95% CI, 1.10–2.82).
Conclusions.
PWID have frequent IDU-related encounters in emergency departments. Enhanced HIV screening of PWID in these settings can facilitate earlier diagnosis and improve outbreak response.
Keywords: HIV testing, outbreak response, injection drug use, overdose, skin/soft-tissue infections
Of all human immunodeficiency virus (HIV) diagnoses in the United States during 2012–2017, 4% were related to injection drug use (IDU) and 4% were related to both IDU and male–male sexual contact [1]. Human immunodeficiency virus can be transmitted rapidly in communities with a high prevalence of IDU and injection equipment sharing [2]. This has been demonstrated in recent HIV outbreaks among persons who inject drugs (PWID) in Indiana in 2014–2015 and Massachusetts in 2018 [3–5].
Early HIV detection is important for the rapid initiation of HIV treatment, reducing AIDS-related morbidity and mortality, and decreasing transmission to uninfected partners [6]. In fact, an estimated 38% of all new HIV infections are attributable to the 15% of persons living with HIV who are unaware of their HIV status [7]. The Centers for Disease Control and Prevention (CDC) recommends HIV testing at least once in a lifetime for individuals between the ages of 13 and 64 years and annual testing for groups at increased risk of HIV, including PWID [8]. Unfortunately, the interval of testing among individuals at risk of HIV has declined nationally, and the median time from infection to HIV diagnosis among PWID is estimated to be 2.9 years [9, 10]. In several studies, less than half of PWID reported HIV testing within the past year [11–14] and most had missed opportunities for HIV testing during healthcare encounters prior to diagnosis [15–17].
Persons who inject drugs often suffer complications associated with IDU, leading to frequent encounters with the healthcare system. Despite a decline in opioid overdose deaths since 2017, overdoses from opioids and stimulants are a growing cause of presentations to emergency departments [18, 19]. Injection drug use also increases the incidence of bacterial infections, ranging from skin/soft tissue infections to invasive infections including bacteremia, septic arthritis, osteomyelitis, endocarditis, and epidural abscess [20–22]. To avoid stigma, PWID presenting to healthcare with non–IDU-related problems may not disclose their IDU [23]. Therefore, encounters where IDU is evident are prime opportunities for clinicians to screen patients for HIV and viral hepatitis, offer hepatitis A vaccination, and discuss harm-reduction strategies and substance use disorder treatment.
Diagnosing HIV early and responding rapidly to HIV outbreaks are among the key pillars of the federal initiative on Ending the HIV Epidemic [24]. Increasing HIV testing during an HIV outbreak is crucial to link persons with HIV to treatment and refer persons at risk of infection to HIV-prevention interventions such as pre-exposure prophylaxis [10, 14]. Little is known about HIV testing practices in clinical settings during an HIV outbreak among PWID. This study therefore sought to characterize HIV testing practices during IDU-related encounters and identify missed opportunities for HIV testing among a cohort of PWID with recently diagnosed HIV who were identified as part of an outbreak response.
METHODS
Setting
During January 2017–September 2018, a total of 135 HIV infections were diagnosed among PWID residing in 5 counties in the core Cincinnati metropolitan area, including Northern Kentucky. This represented a significant increase compared with the prior rate of 33 annual HIV diagnoses among PWID in these 5 counties from 2014 to 2016. In October 2018, the Kentucky Department for Public Health, Ohio Department of Health, Northern Kentucky Health Department, and Hamilton County Public Health requested CDC assistance in the investigation and response to this HIV outbreak. As part of this response, a medical record review was conducted to assess the interactions between local healthcare systems and PWID with recently diagnosed HIV.
Medical record review was conducted at 2 large healthcare systems in the Cincinnati metropolitan area. Healthcare system A consisted of 5 community hospitals located in Northern Kentucky with affiliated outpatient clinics. Healthcare system B was a large tertiary care center located in Cincinnati. Both healthcare systems used Epic electronic health record systems and records were accessible across all sites within each healthcare system.
Patient Population
For the overall outbreak response, a case was defined as a person with an HIV diagnosis on or after 1 January 2017, who injected drugs, and who lived at the time of diagnosis in Boone, Campbell, Grant, or Kenton County in Kentucky or Hamilton County in Ohio. Persons who met this case definition and who had at least 1 encounter in either of the 2 healthcare systems were included in the medical record review if their HIV infection had been diagnosed before 30 September 2018.
Data Sources
Persons meeting the case definition were identified and their demographic characteristics were obtained from state health department HIV surveillance datasets. Additional demographic data were derived from enhanced interviews conducted by local health departments during the outbreak response. Name and date of birth were used to identify persons meeting the case definition in the medical records at both healthcare facilities. Encounter notes, laboratory results, and imaging reports were available for medical record abstraction. Medical record data were first abstracted onto a paper form, which was then electronically entered into a de-identified REDCap database. Paper forms with patient-identifying information were then destroyed.
Measurements
Age, gender, race/ethnicity, date and facility of HIV diagnosis, and CD4 count at diagnosis were extracted from state HIV-surveillance datasets. Health department–enhanced interview data and medical record data were combined to determine ever experiencing homelessness/unstable housing or incarceration. The combination of these data sources increased the detection of these events and reduced the number of participants with missing data. Venue of HIV diagnosis was determined from state HIV-surveillance and medical record datasets and categorized as occurring at an outpatient, emergency department, inpatient, or non–healthcare setting. Non–healthcare settings included jails, community testing events, and local health departments.
Healthcare encounter date, level of care, and primary diagnoses were captured for all encounters that occurred 12 months prior to each patient’s HIV diagnosis, including the healthcare encounter of HIV diagnosis, if applicable. Level of care was classified as inpatient, emergency department, or outpatient and was defined as the highest level of care for that encounter. As an example, patients first presenting to the emergency department who were then admitted as an inpatient were classified as inpatients. Human immunodeficiency virus testing during such encounters could have occurred in the emergency department or during the patient’s inpatient admission.
Encounters with primary diagnoses relating to IDU were categorized into 4 categories: substance-induced overdose, skin/soft tissue infections, invasive bacterial infections, or other. Invasive bacterial infections included bacteremia, septic arthritis, osteomyelitis, endocarditis, and epidural abscess. Encounters for opioid withdrawal, medication-assisted treatment, and HIV pre-exposure prophylaxis for IDU were classified as other.
The date of first positive HIV test was extracted from state HIV-surveillance databases. Dates of all negative and the first positive HIV tests were extracted from the electronic health records of both healthcare systems. If the date of first positive HIV test was discordant between the surveillance and medical record datasets, the earlier date was used. The HIV tests performed within 12 months before or at the time of each IDU-related encounter were identified.
A missed opportunity for HIV testing was defined as an IDU-related encounter where an HIV test was not performed and had not been performed in the prior 12 months. Therefore, an encounter was not a missed opportunity for testing if either HIV testing was performed during that visit or at another encounter within the prior 12 months. Since patients may have been aware of their prior HIV testing and communicated this during their IDU-related encounters, healthcare providers were conservatively assumed to be aware of all prior negative HIV tests.
Statistical Analysis
Chi-square tests were done to compare demographics, housing, incarceration history, HIV testing within 12 months of diagnosis, and venue of HIV diagnosis of PWID who had or did not have at least 1 missed opportunity for HIV testing. At the encounter level, chi-square analysis was used to assess whether encounters with HIV testing performed were associated with the healthcare system, level of care, IDU-related diagnosis category, and the presence of HIV testing 12 months prior to the encounter.
To assess the person- and encounter-level determinants of HIV testing during IDU-related encounters, generalized estimating equation (GEE) models were used to account for clustering of encounters within patients. The association of demographic, social, and IDU-related encounter-specific factors on the likelihood of HIV testing during that encounter were estimated with relative risks (RRs) and 95% confidence intervals (CIs). Variables associated with HIV testing during a given encounter with P < .20 in the bivariate GEE analysis were then included in a multivariable GEE model to identify the independent effect of each included factor on HIV testing.
Ethics
This work was reviewed by the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at CDC. All data were collected for the purpose of disease-outbreak investigation and therefore did not require institutional review board review.
RESULTS
Overall, of 135 PWID with HIV infection diagnosed during January 2017–September 2018 in the 5-county area, 109 (80.7%) had medical records in at least 1 of the 2 healthcare systems and were included in the analysis. The majority of PWID with medical records were 20–39 years (83; 76.1%), male (73; 67.0%), and non-Hispanic shite (92; 84.4%) (Table 1). Twelve (11.0%) reported male–male sex in addition to IDU as risk factors for HIV. Most had ever experienced homelessness or unstable housing (77; 70.6%) and incarceration (60; 55.0%). While 70.6% (77/109) of diagnoses occurred in healthcare settings, 80.7% (88/109) did not have prior HIV testing within 12 months of their diagnosis. Of 94 PWID with an initial CD4 value from surveillance data, the median CD4 count per m3 at diagnosis of 424 (interquartile range, 233–611) and 15 (16.0%) had a CD4 count less than 200 at diagnosis.
Table 1.
Sociodemographic and Human Immunodeficiency Virus (HIV) Testing Factors of Persons Who Inject Drugs With HIV Diagnosis by Presence of at Least 1 Missed Opportunity for HIV Testing 12 Months Prior to Diagnosis—Cincinnati/Northern Kentucky, 2017–2018
| Total Patients (N = 109), n (%) | Patients With ≥1 Missed Opportunity (n = 46), n (%) | Patients Without Missed Opportunity (n = 63), n (%) | P | |
|---|---|---|---|---|
| Age at diagnosis | .905 | |||
| 20–29 years | 37 (33.9) | 15 (32.6) | 22 (34.9) | |
| 30–39 years | 46 (42.2) | 21 (45.7) | 25 (39.7) | |
| 40–49 years | 17 (15.6) | 7 (15.2) | 10 (15.9) | |
| ≥50 years | 9 (8.3) | 3 (6.5) | 6 (9.5) | |
| Gender | .691 | |||
| Male | 73 (67.0) | 31 (67.4) | 42 (66.7) | |
| Female | 35 (32.1) | 15 (32.6) | 20 (31.7) | |
| Transgender female | 1 (0.9) | 0 | 1 (1.6) | |
| Race/ethnicity | .302 | |||
| White, non-Hispanic | 92 (84.4) | 41 (89.1) | 51 (81.0) | |
| Black, non-Hispanic | 11 (10.1) | 2 (4.3) | 9 (14.3) | |
| Hispanic/Latino | 3 (2.8) | 2 (4.3) | 1 (1.6) | |
| Other | 3 (2.8) | 1 (2.2) | 2 (3.2) | |
| Housing status | .773 | |||
| Never homeless or unstably housed | 25 (22.9) | 9 (19.6) | 16 (25.4) | |
| Ever homeless or unstably housed | 77 (70.6) | 34 (73.9) | 43 (68.3) | |
| Unknown | 7 (6.4) | 3 (6.5) | 4 (6.4) | |
| Incarceration history | .068 | |||
| Ever incarcerated | 60 (55.0) | 30 (65.2) | 30 (476) | |
| Never incarcerated | 49 (45.0) | 16 (34.8) | 33 (53.4) | |
| Venue of HIV diagnosis | .465 | |||
| Emergency department | 24 (22.0) | 9 (19.6) | 15 (23.8) | |
| Inpatient | 30 (275) | 15 (32.6) | 15 (23.8) | |
| Outpatient | 23 (21.1) | 7 (15.2) | 16 (25.4) | |
| Non-healthcare setting | 32 (29.4) | 15 (32.6) | 17 (270) | |
Person-level Missed Opportunities for Testing Analysis
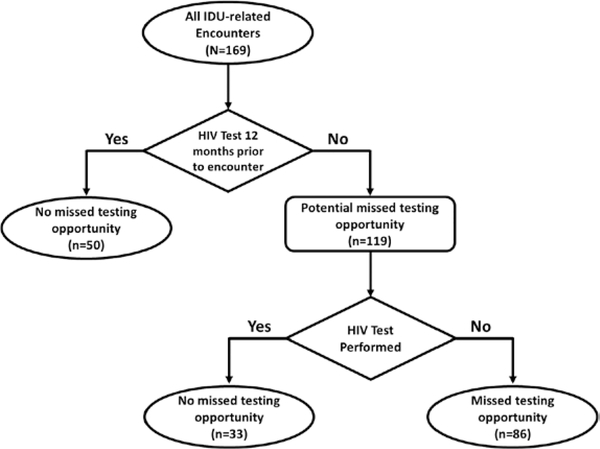
Seventy-five (68.8%) of 109 PWID with medical records had at least 1 IDU-related encounter in the 12-month period before their HIV diagnosis. These 75 PWID had a total of 169 IDU-related encounters in the 12 months before HIV diagnosis, of which 86 (50.9%) were missed opportunities for HIV testing (Figure 1). These 86 missed opportunities for HIV testing occurred among 46 PWID (42.2% of all PWID with medical records). Persons who inject drugs with or without missed opportunities for HIV testing did not differ by demographics, housing status, prior incarceration, or venue of HIV diagnosis (Table 1).
Figure 1.
Flow diagram of defining missed opportunities for HIV testing using a 12-month interval—Cincinnati/Northern Kentucky, 2017–2018. Abbreviations: HIV, human immunodeficiency virus; IDU, injection drug use.
Encounter-level Testing Analysis
Among the 169 IDU-related encounters, healthcare system A accounted for 81 (47.9%) encounters and healthcare system B for 88 (52.1%) encounters (Table 2). Most of the IDU-related encounters occurred in the emergency department (118; 69.8%) compared with inpatient (38; 22.5%) and outpatient (13; 7.7%) settings. Overdose was the most common IDU-related condition (75; 44.4%) followed by skin/soft tissue infections (48; 28.4%) and invasive bacterial infections (20; 11.8%).
Table 2.
Location, Level of Care, Diagnosis, and Prior Human Immunodeficiency Virus (HIV) Testing of Injection Drug Use-Related Encounters by Whether an HIV Test Was Performed—Cincinnati/Northern Kentucky, 2017–2018
| Total Encounters (N = 169) | Encounters With an HIV Test (n = 45) | Encounters Without an HIV Test (n = 124) | P | |
|---|---|---|---|---|
| Healthcare system, n (%) | .052 | |||
| Healthcare system A | 81 | 16 (19.8) | 65 (80.3) | |
| Healthcare system B | 88 | 29 (33.0) | 59 (671) | |
| Level of care, n (%) | <.001 | |||
| Emergency | 118 | 21 (178) | 97 (82.2) | |
| Outpatient | 13 | 2 (15.4) | 11 (84.6) | |
| Inpatient | 38 | 22 (57.9) | 16 (42.1) | |
| IDU-related diagnosis, n (%) | .004 | |||
| Overdose | 75 | 11 (14.7) | 64 (85.3) | |
| Skin/soft tissue infection | 48 | 16 (33.3) | 32 (66.7) | |
| Invasive bacterial infection | 20 | 12 (60.0) | 8 (40.0) | |
| Other | 26 | 6 (23.1) | 20 (76.9) | |
| Prior HIV test within 12 months of encounter, n (%) | .617 | |||
| Yes | 50 | 12 (24.0) | 38 (76.0) | |
| No | 119 | 33 (27.7) | 86 (72.3) |
Percentages are row percentages.
Abbreviation: IDU, injection drug use.
Of the 169 IDU-related encounters, 45 (26.6%) encounters had an HIV test conducted during that encounter. Human immunodeficiency virus testing was more likely to occur during encounters involving inpatient admission (22/38; 48.9%) than during emergency department (21/118; 17.8%) (P < .001) or outpatient (2/13; 15.4%) encounters (P = .003). Further, skin/soft tissue infections (16/48; 33.3%) and invasive bacterial infections (12/20; 60.0%) were associated with an increased likelihood of HIV testing compared with overdose encounters (11/75; 14.7%) (P = .015 and P < .001, respectively). Neither the healthcare system nor the presence of HIV testing within 12 months prior to the encounter was significantly associated (P < .05) with the likelihood of an HIV test being performed at a given encounter.
Regression Analysis
Bivariate GEE regression of IDU-related encounters demonstrated that HIV testing was less likely to occur for patients with a history of incarceration compared with those without incarceration (RR, .58; 95% CI, .34–.99) (Table 3). Conversely, HIV testing was more likely to occur during IDU-related encounters at healthcare system B compared with healthcare system A (RR, 1.66; 95% CI, 1.00–2.76). Human immunodeficiency virus testing occurred more often during inpatient encounters compared with emergency department encounters (RR, 3.23; 95% CI, 2.07–5.04). Compared with overdose encounters, HIV testing occurred more during encounters for skin/soft tissue infections (RR, 2.25; 95% CI, 1.11–4.56) and deep bacterial infections (RR, 4.08; 95% CI, 1.76–9.46). In multivariable analysis, only healthcare system B (adjusted RR, 1.76; 95% CI, 1.10–2.82) and inpatient (adjusted RR, 2.72; 95% CI, 1.70–4.33) encounters were associated with HIV testing occurring during that encounter (Table 3).
Table 3.
Generalized Estimating Equation Regression of the Likelihood a Human Immunodeficiency Virus Test Would Be Performed During an Injection Drug Use-Related Encounter—Cincinnati/Northern Kentucky, 2017–2018
| Bivariate RR for HIV Testing |
Multivariable RR for HIV Testing |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| Age at diagnosis | .242 | |||||
| 20–29 years | Ref | Ref | Ref | |||
| 30–39 years | 2.05 | 1.01,4.17 | .047 | |||
| 40–49 years | 1.53 | .58, 4.04 | .396 | |||
| ≥50 years | 1.79 | .85, 3.78 | .126 | |||
| Female gender | 1.29 | .78, 2.14 | .326 | |||
| Race/ethnicity | .761 | |||||
| White (non-Hispanic) | Ref | Ref | Ref | |||
| Black (non-Hispanic) | 1.40 | .64, 3.07 | .405 | |||
| Hispanic/Latino | 0.63 | .15, 2.60 | .526 | |||
| Other | 0.96 | .15, 5.99 | .961 | |||
| Housing status | .293 | |||||
| Stably housed | Ref | Ref | Ref | |||
| Ever homeless or unstably housed | 0.58 | .30, 1.13 | .110 | |||
| Unknown | 1.02 | .30, 3.50 | .969 | |||
| Ever incarcerated | 0.58 | .34, .99 | .046 | .65 | .39, 1.07 | .093 |
| Healthcare system | .049 | .019 | ||||
| Healthcare system A | Ref | Ref | Ref | Ref | Ref | Ref |
| Healthcare system B | 1.66 | 1.00, 2.76 | 1.76 | 1.10, 2.82 | ||
| Level of care | <.001 | .007 | ||||
| Emergency | Ref | Ref | Ref | Ref | Ref | Ref |
| Inpatient | 3.23 | 2.07 5.04 | <.001 | 2.72 | 1.70, 4.33 | <.001 |
| Outpatient | 0.88 | .14, 5.48 | .894 | .73 | .13, 4.03 | .721 |
| IDU-related diagnosis | .012 | .340 | ||||
| Overdose | Ref | Ref | Ref | Ref | Ref | Ref |
| Skin/soft tissue infection | 2.25 | 1.11,4.56 | .024 | 1.84 | .95, 3.58 | .071 |
| Deep bacterial infection | 4.08 | 1.76, 9.46 | .001 | 1.41 | .53, 3.71 | .489 |
| Other | 1.71 | .59, 4.98 | .324 | 1.33 | .50, 3.54 | .573 |
| Prior HIV test within 12 months of encounter | 0.85 | .49, 1.48 | .572 | |||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; Ref, referent; RR, relative risk.
DISCUSSION
In the setting of an HIV outbreak associated with IDU, this study found that most PWID had at least 1 IDU-related encounter with the healthcare system in the year prior to their HIV diagnosis. Despite these frequent healthcare encounters, half of these IDU-related encounters were missed opportunities for HIV testing according to CDC’s recommendation for annual testing of individuals at increased risk of HIV [8]. The analysis was intentionally limited to IDU-related encounters in order to ensure that the healthcare provider would have been aware of the patient’s IDU and eliminate bias from the nondisclosure of IDU. However, patients can be tested for HIV during any clinical encounter. Therefore, our estimation of healthcare encounters where HIV testing was indicated but not done is likely an underestimation.
These findings highlight potential approaches to improve HIV testing in PWID populations. Persons who inject drugs had several presentations to emergency departments for IDU-related conditions, but HIV testing was infrequent. Given this, emergency departments may be optimal settings to implement targeted HIV testing for PWID. Indeed, state funding supports a program at healthcare system B where counselors review emergency department medical records and offer patients HIV testing 24 hours per day. This program may explain the increased likelihood of receiving HIV testing at healthcare system B. Universal opt-out HIV screening in emergency departments has been found to be superior to risk-based protocols since universal screening can be easier to implement, interfere less in workflow, and reduce stigma [25–29]. Future research characterizing the impact of enhanced universal HIV screening in emergency departments in increasing HIV diagnosis during an outbreak is needed.
This study also found that HIV testing was more likely to occur during inpatient encounters, which could be explained by the increased length of stay, higher acuity, and greater number of providers patients interact with during inpatient encounters. Developing tools beyond the emergency room and throughout the healthcare system may help identify PWID who would benefit from HIV testing. The electronic health record can be leveraged to identify individuals with indications for HIV testing throughout a healthcare system and assist in the implementation opt-out testing programs [30, 31]. Automated algorithm-driven protocols may outperform nurse-driven protocols [32].
Finally, policies that support HIV testing may also increase testing rates. For instance, Kentucky had a statute that allowed only the provider that ordered the test to report its result to the patient. This likely resulted in low testing rates in emergency departments and was recently revised to allow for delegation of reporting to other parties [33]. Integrating routine HIV screening in healthcare settings as part of an HIV outbreak response could improve the rate of testing, facilitate earlier diagnosis and linkage to care, and reduce HIV transmission. However, further research is needed to identify the optimal parameters and methods of HIV screening by healthcare systems in outbreak settings.
There are limitations to consider when interpreting these results. This study was designed to assess healthcare visits of PWID with HIV recently diagnosed during this outbreak. Since medical records of PWID without recent HIV diagnoses were not abstracted, these results may not be generalizable to all PWID. A further limitation was that the health records from 2 healthcare systems in the Cincinnati metropolitan area did not encompass all the healthcare interactions of the patient sample. Not capturing prior negative HIV testing at other local healthcare facilities may overestimate the numbers of encounters that were missed opportunities for HIV testing. Last, the type of physician ordering an HIV test was not captured, so it was not possible to distinguish whether an HIV test was ordered by an emergency or inpatient provider for inpatient encounters. The dates of HIV testing during inpatient encounters suggests that most tests were ordered by inpatient providers, but it is also possible that emergency department providers may have been more likely to order a test if they knew the admitted patient would receive the results during their inpatient encounter. It was also not possible to assess whether an HIV test was offered but refused or whether an HIV test was ordered as a part of an opt-out screening strategy.
Early diagnosis of HIV and rapid response to HIV clusters are foundational pillars of the federal Ending the HIV Epidemic initiative [24]. Testing is crucial in the identification of persons living with HIV and initiation of HIV treatment as well as the referral of individuals at risk of HIV to HIV-prevention interventions such as pre-exposure prophylaxis. Increasing HIV testing at the population level has been shown to reduce the percentage of late-stage HIV diagnoses [34]. Implementation of routine testing can increase the frequency of HIV testing, and improvements in early HIV diagnosis have been documented with routine testing in jails and prisons, medication-assisted treatment programs, and syringe service programs [34–38]. While there were many missed opportunities for HIV testing during IDU-related encounters, the finding that the healthcare system receiving funds to implement targeted emergency department HIV testing had higher rates of testing indicates that healthcare systems can develop programs to increase targeted HIV testing. Overall, increasing HIV testing through universal screening in emergency departments may be an effective response strategy during HIV outbreaks among PWID and can help achieve the goal of Ending the HIV Epidemic by 2030.
Acknowledgments
Financial support. This work was supported by the Centers for Disease Control and Prevention, which supported the epidemiologic field investigation and analysis of the data collected in the field.
Footnotes
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2017; 29. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 2.Van Handel MM, Rose CE, Hallisey EJ, et al. County-level vulnerability assessment for rapid dissemination of HIV or HCV infections among persons who inject drugs, United States. J Acquir Immune Defic Syndr 2016; 73:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters PJ, Pontones P, Hoover KW, et al. HIV infection linked to injection use of oxymorphone in Indiana, 2014–2015. N Engl J Med 2016; 375:229–39. [DOI] [PubMed] [Google Scholar]
- 4.Conrad C, Bradley HM, Broz D, et al. ; Centers for Disease Control and Prevention (CDC). Community outbreak of HIV infection linked to injection drug use of oxymorphone—Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:443–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Cranston K, Alpren C, John B, et al. Notes from the field: HIV diagnoses among persons who inject drugs—northeastern Massachusetts, 2015–2018. MMWR Morb Mortal Wkly Rep 2019; 68: 253–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA 2019; 321:2326–36. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Purcell DW, Sansom SL, Hayes D, Hall HI. Vital signs: HIV transmission along the continuum of care—United States, 2016. MMWR Morb Mortal Wkly Rep 2019; 68:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 9.An Q, Song R, Finlayson TJ, Wejnert C, Paz-Bailey G; NHBS Study Group. Estimated HIV inter-test interval among people at high risk for HIV infection in the U.S. Am J Prev Med 2017; 53:355–62. [DOI] [PubMed] [Google Scholar]
- 10.Dailey AF, Hoots BE, Hall HI, et al. Vital signs: human immunodeficiency virus testing and diagnosis delays—United States. MMWR Morb Mortal Wkly Rep 2017; 66:1300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burt RD, Tinsley J, Glick SN. A decline in HIV testing among persons who inject drugs in the Seattle area, 2004–2015. J Acquir Immune Defic Syndr 2017; 75(Suppl 3): S346–51. [DOI] [PubMed] [Google Scholar]
- 12.Cooley LA, Wejnert C, Spiller MW, Broz D, Paz-Bailey G. Low HIV testing among persons who inject drugs—National HIV Behavioral Surveillance, 20 U.S. cities, 2012. Drug Alcohol Dependence 2016; 165:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnett JC, Broz D, Spiller MW, Wejnert C, Paz-Bailey G. HIV infection and HIV-associated behaviors among persons who inject drugs—20 cities, United States, 2015. MMWR Morb Mortal Wkly Rep 2018; 67:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wejnert C, Prejean J, Hoots B, Hall HI, McCray E, Mermin J; NHBS Study Group. Prevalence of missed opportunities for HIV testing among persons unaware of their infection. JAMA 2018; 319:2555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downing A, Garcia-Diaz JB. Missed opportunities for HIV diagnosis. J Int Assoc Provid AIDS Care 2017; 16:14–7. [DOI] [PubMed] [Google Scholar]
- 16.Liggett A, Futterman D, Umanski GI, Selwyn PA. Missing the mark: ongoing missed opportunities for HIV diagnosis at an urban medical center despite universal screening recommendations. Fam Pract 2016; 33:644–8. [DOI] [PubMed] [Google Scholar]
- 17.Traynor SM, Rosen-Metsch L, Feaster DJ. Missed opportunities for HIV testing among STD clinic patients. J Community Health 2018; 43:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladden RM, O’Donnell J, Mattson CL, Seth P. Changes in opioid-involved overdose deaths by opioid type and presence of benzodiazepines, cocaine, and methamphetamine—25 States, July-December 2017 to January-June 2018. MMWR Morb Mortal Wkly Rep 2019; 68:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital signs: trends in emergency department visits for suspected opioid overdoses—United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep 2018; 67:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlman D, Håkansson A, Kral AH, Wenger L, Ball EL, Novak SP. Behavioral characteristics and injection practices associated with skin and soft tissue infections among people who inject drugs: a community-based observational study. Subst Abus 2017; 38:105–12. [DOI] [PubMed] [Google Scholar]
- 21.Islam S, Piggott DA, Moriggia A, et al. Reducing injection intensity is associated with decreased risk for invasive bacterial infection among high-frequency injection drug users. Harm Reduct J 2019; 16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant staphylococcus aureus infections among persons who inject drugs—six sites, 2005–2016. MMWR Morb Mortal Wkly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend 2019; 198:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 25.Lyons MS, Lindsell CJ, Ruffner AH, et al. Randomized comparison of universal and targeted HIV screening in the emergency department. J Acquir Immune Defic Syndr 2013; 64:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunn A, Towey C, Chan PA, et al. Routine HIV screening in an urban community health center: results from a geographically focused implementation science program. Public Health Rep 2016; 131(Suppl 1):30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haukoos JS, Hopkins E, Conroy AA, et al. ; Denver Emergency Department HIV Opt-Out Study Group. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA 2010; 304:284–92. [DOI] [PubMed] [Google Scholar]
- 28.Hoenigl M, Mathur K, Blumenthal J, et al. Universal HIV and birth cohort HCV screening in San Diego emergency departments. Sci Rep 2019; 9:14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White DAE, Giordano TP, Pasalar S, et al. Acute HIV discovered during routine HIV screening With HIV antigen-antibody combination tests in 9 US emergency departments. Ann Emerg Med 2018; 72:29–40.e2. [DOI] [PubMed] [Google Scholar]
- 30.Felsen UR, Cunningham CO, Heo M, Futterman DC, Weiss JM, Zingman BS. Expanded HIV testing strategy leveraging the electronic medical record uncovers undiagnosed infection among hospitalized patients. J Acquir Immune Defic Syndr 2017; 75:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Mauntel-Medici C, Heinert S, Baghikar S. Harnessing the power of the electronic medical record to facilitate an opt-out HIV screening program in an urban academic emergency department. J Public Health Manag Pract 2017; 23:264–8. [DOI] [PubMed] [Google Scholar]
- 32.White DAE, Todorovic T, Petti ML, Ellis KH, Anderson ES. A comparative effectiveness study of two nontargeted HIV and hepatitis C virus screening algorithms in an urban emergency department. Ann Emerg Med 2018; 72:438–48. [DOI] [PubMed] [Google Scholar]
- 33.General consent to testing for HIV—Emergency procedures—Disclosures of test results—Voluntary testing programs in each county. KRS 214.181 (Kentucky Revised Statutes). KY State Law; 214181. Kentucky, 2019. [Google Scholar]
- 34.Krueger A, Van Handel M, Dietz PM, Williams WO, Patel D, Johnson AS. HIV testing, access to HIV-related services, and late-stage HIV diagnoses across US States, 2013–2016. Am J Public Health 2019; 109:1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaulding AC, Anderson EJ, Khan MA, Taborda-Vidarte CA, Phillips JA. HIV and HCV in U.S. prisons and jails: the correctional facility as a bellwether over time for the community’s infections. AIDS Rev 2017; 19:134–47. [PubMed] [Google Scholar]
- 36.DeBeck K, Cheng T, Montaner JS, et al. HIV and the criminalisation of drug use among people who inject drugs: a systematic review. Lancet HIV 2017; 4:e357–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner LI, Marks G, Strathdee SA, et al. Faster entry into HIV care among HIV-infected drug users who had been in drug-use treatment programs. Drug Alcohol Depend 2016; 165:15–21. [DOI] [PubMed] [Google Scholar]
- 38.Lambdin BH, Kral AH, Comfort M, Lopez AM, Lorvick J. Associations of criminal justice and substance use treatment involvement with HIV/HCV testing and the HIV treatment cascade among people who use drugs in Oakland, California. Addict Sci Clin Pract 2017; 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]