Abstract
Neocortical Aβ-amyloid deposition, one of the hallmark pathologic features of Alzheimer’s disease (AD), begins decades prior to the presence of clinical symptoms. As clinical trials move to secondary and even primary prevention, understanding the rates of neocortical Aβ-amyloid deposition and the age at which Aβ-amyloid deposition becomes abnormal is crucial for optimising the timing of these trials. As APOE-ε4 carriage is thought to modulate the age of clinical onset, it is also important to understand the impact of APOE-ε4 carriage on the age at which neocortical Aβ-amyloid deposition becomes abnormal. Here, we show that, for 455 participants with over three years of follow-up, abnormal levels of neocortical Aβ-amyloid were reached on average at age 72 (66.5-77.1). The APOE-ε4 carriers reached abnormal levels earlier at age 63 (59.6-70.3), however, non-carriers reached the threshold later at age 78 (76.1-84.4). No differences in rates of deposition were observed between APOE-ε4 carriers and non-carriers after abnormal Aβ-amyloid levels had been reached. These results suggest that primary and secondary prevention trials, looking to recruit at the earliest stages of disease, should target APOE-ε4 carriers between the ages of 60 and 66 and non-carriers between the ages of 76 and 84.
Keywords: Aβ-amyloid, APOE, Alzheimer’s disease, Longitudinal, Biomarkers
1. Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterised pathologically by the extracellular accumulation of Aβ-amyloid and intracellular accumulation of tau in the neocortex (Jack et al., 2018). Neocortical accumulation of Aβ-amyloid is a key part of the cascade of pathological changes leading to the onset of clinical symptoms in AD (Hardy and Selkoe, 2002; Karran et al., 2011) and is a process that initiates decades prior to clinical manifestation of the disease (Jack et al., 2013a; Villemagne et al., 2013). Increased understanding of onset and rates of neocortical Aβ-amyloid deposition would provide improved disease staging criteria particularly for pre-clinical AD. This is increasingly important with clinical trials aimed at preventative treatment.
Carriage of an APOE-ε4 allele is a well-established risk factor for AD (Harold et al., 2009), reported to impact the levels of neocortical Aβ-amyloid (Liu et al., 2013; Reiman et al., 2009; Rowe et al., 2010; Villemagne et al., 2011), however the nature of this impact is unclear. The literature appears to agree that APOE-ε4 carriage is associated with deposition of neocortical Aβ-amyloid at an earlier age (Bilgel et al., 2019; Fleisher et al., 2013; Mishra et al., 2018) as well as an earlier onset of disease (Corder et al., 1995). Some contributions report that APOE-ε4 carriage is associated with an increased rate of neocortical Aβ-amyloid deposition (Bilgel et al., 2019; Jack et al., 2013a; Mishra et al., 2018; Toledo et al., 2019), others only report a difference in those with low neocortical Aβ-amyloid burden (Lim et al., 2017), whilst others report no difference in neocortical Aβ-amyloid accumulation rates between carriers and non-carriers (Corder et al., 1995; Resnick et al., 2015; Saunders, 2000). Accounting for the temporal relationship between neocortical Aβ-amyloid deposition and disease stage/progression may provide a clearer understanding of the impact of APOE-ε4 carriage on neocortical Aβ-amyloid deposition.
In this study we evaluate the age at which abnormal levels of neocortical Aβ-amyloid deposition can be detected and test our hypotheses that carriage of an APOE-ε4 allele would be associated with a) a younger age of onset and b) faster rates of neocortical Aβ-amyloid deposition. For that purpose, natural history modelling in conjunction with survival analyses is employed to jointly consider onset and rates of neocortical Aβ-amyloid accumulation in reference to disease stage and progression.
2. Materials & Methods
2.1. AIBL Cohort
The Australian Imaging, Biomarker and Lifestyle (AIBL) cohort study of ageing combines data from neuroimaging, biomarkers, lifestyle, clinical, and neuropsychological assessments. Two study centres in Melbourne, VIC, and Perth, WA, Australia recruit mild cognitively impaired (MCI) individuals and individuals with Alzheimer’s disease from primary- care physicians or tertiary Memory Disorders Clinics. Cognitively healthy normal controls (NC) were recruited through advertisement or from spouses of participants in the study. Exclusion criteria were a history of non-Alzheimer’s disease dementia, Parkinson’s disease, schizophrenia, bipolar disorder, obstructive sleep apnoea, serious head injury, current depression (Geriatric Depression Score >5 out of 15), cancer in the past two years (with the exception of basal-cell skin carcinoma), symptomatic stroke, uncontrolled diabetes, or current regular alcohol use. Between Nov 3, 2006, and Oct 30, 2008, AIBL recruited 1112 eligible volunteers, who were aged 60 years or older and fluent in English. An enrichment cohort of 86 patients with Alzheimer’s disease (AD), 124 MCI and 389 NC were recruited by AIBL between March 30, 2011, and June 29, 2015. At baseline, the AIBL study participants were an average of 72 years of age, consisted of 58% women, and 36% were APOE-ε4 carriers. The institutional ethics committees of Austin Health, St Vincent’s Health, Hollywood Private Hospital, and Edith Cowan University approved the AIBL study, and all volunteers gave written informed consent before participating.
2.1.1. PET Aβ-Amyloid
AIBL Aβ-Amyloid positron emission tomography (PET) studies consisted of a 30-minute acquisition starting 40 minutes after injection of 370 MBq of 11C-Pittsburgh compound-B (11C-PiB). For semi-quantitative analysis, PET images were spatially normalised with CapAIBL® using an adaptive atlas (Bougeat et al., 2015). The summed and spatially normalised PET images were then scaled to the recommended reference region, cerebellar cortex, to generate a tissue ratio termed SUV ratio (SUVR), and sampled using a pre-set template of narrow cortical volumes of interest. A global measure of the Aβ-amyloid level was computed using the mean SUVR in the frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions. The abnormal threshold for levels of Aβ-amyloid in AIBL participants was set as 1.4 SUVR (Jack et al., 2013b).
2.1.2. Assessment of APOE genotype
APOE genotype was determined through TaqMan® genotyping assays (Life Technologies) for rs7412 (Assay ID: C____904973_10) and rs429358 (Assay ID: C____3084793_20). TaqMan® genotyping assays were performed on a QuantStudio 12K Flex™ Real-Time-PCR systems (Applied Biosystems, Foster City, CA) using the TaqMan® GTXpress™ Master Mix (Life Technologies) methodology as per manufacturer instructions. APOE carrier status was defined by the presence (1 or 2 copies) or absence (0 copies) of the APOE-ε4 allele.
2.2. ADNI Cohort
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD paticipants. Subjects were recruited from 57 sites across the United States and Canada and are followed-up annually. ADNI initially (ADNI 1) recruited 200 NC subjects, 400 MCI subjects and 200 subjects with early AD. In addition, ADNI GO, launched in 2009 included 200 subjects identified as having early mild cognitive impairment (EMCI). In 2011, ADNI 2 [11] recruited 150 NC, 100 EMCI participants, 150 late mild cognitive impairment (LMCI) participants and 150 AD participants. More recently, ADNI 3 was launched (September 2016) to recruit an additional 1,200 volunteers.
2.2.1. PET Aβ-Amyloid
ADNI Aβ-Amyloid PET studies consisted of an acquisition of 4 x 5-minute frames commencing 50-70 minutes after injection of 10 mCi of 18F Florbetapir (FBP). In the same manner as the AIBL images, the ADNI PET images were spatially normalised with CapAIBL® using an adaptive atlas (Bougeat et al., 2015). The summed and spatially normalised PET images were then scaled to a white matter reference region (a composite of the centrum semiovale and corpus callosum)(Chen et al., 2015) to generate a tissue ratio termed SUV ratio (SUVR), and sampled using the same pre-set template of narrow cortical volumes of interest as for the AIBL cohort. A global measure of the Aβ-amyloid level was computed using the mean SUVR in the frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions. The abnormal threshold for levels of Aβ-amyloid in ADNI participants was set as 0.61 SUVR (equivalent to 1.4 SUVR for 11C-PiB and 1.10 for FBP whole cerebellum correction (Clark et al., 2012)).
2.2.2. Assessment of APOE genotype
A 3 mL aliquot of blood was taken in ethylenediaminetetraacetic acid (EDTA)-containing vacutainer tubes from ADNI participants, and genomic DNA was extracted at Cogenics (now Beckman Coulter Genomics) using the QIAamp DNA Blood Maxi Kit (Qiagen, Inc, Valencia, CA) following the manufacturer’s protocol. The two SNPs (rs429358, rs7412) that define the APOE epsilon 2, 3, and 4 alleles were evaluated by polymerase chain reaction amplification, followed by HhaI restriction enzyme digestion, resolution on 4% Metaphor Gel, and visualization by ethidium bromide staining (Potkin et al., 2009; Saykin et al., 2010).
2.3. Statistical Analysis
AIBL (n=209) and ADNI participants (n=246) with at least three years of follow-up evaluations for Aβ-amyloid with 11C-PiB (AIBL) or 18F Florbetapir PET (ADNI), respectively, who were considered to be accumulating Aβ-Amyloid (rate of deposition > 0.0 SUVR/year(Villemagne et al., 2013)), and had been genotyped for APOE were included in this study. The following analyses were produced in parallel for both the AIBL participants and the ADNI participants. Further, all analyses were again replicated for the NC participants (156 AIBL NC and 106 ADNI NC) in a sensitivity analysis. For comparison purposes, in ADNI, EMCI and LMCI participants were both considered as MCI to align with the classifications in AIBL. All analyses were performed in the R environment (R Development Core Team, 2017).
Demographics:
Baseline differences between APOE-ε4 carriers and non-carriers were assessed with one-way t tests for continuous data (age), χ2 testing for categorised data (sex, years of education, disease classification), and Kruskal-Wallis testing for non- normally distributed data (length of follow-up). This was replicated for the individuals excluded from the study as they were not accumulating Aβ-Amyloid (46 AIBL and 14 ADNI participants).
The differences in rates of Aβ-amyloid deposition: Each individual’s rate of deposition (SUVR/year) was estimated using a linear model regressing their neocortical Aβ-amyloid levels (SUVR) against time since baseline evaluation (years). Differences in these rates between APOE-ε4 carriers and non-carriers, as well as between those above or below the neocortical Aβ-amyloid threshold at baseline, were evaluated using one-way t tests, and presented using box and jitter plots. This analysis was also replicated for a combined cohort of those accumulating and not accumulating Aβ-Amyloid in a sensitivity analysis.
Natural history of deposition: Individual’s rates of Aβ-amyloid deposition, calculated above, were combined to estimate the overall natural history of Aβ-amyloid deposition using the 4-step procedure described previously (Budgeon et al., 2017; Villemagne et al., 2013) and stratified by APOE-ε4 carriage. Briefly, the 4-step procedure comprises 1) estimating the mean and slope of each individuals’ Aβ-amyloid using linear models, 2) fitting a polynomial to the estimated means and slopes across all individuals, 3) integrating the reciprocal of the fitted polynomial, 4) inverting the function to obtain the natural history trajectory. Confidence intervals for the natural history curves were created using the bootstrapping procedure described previously (Budgeon et al., 2017). Note: this analysis was replicated with stratification by sex.
Age of Onset: Cox proportional hazards model of survival, corrected for sex, and years of education were utilised to estimate the age at which participants reached abnormal levels of neocortical Aβ-amyloid. This analysis was replicated with APOE-ε4 carriage stratification, to assess the effect of APOE-ε4 carriage on the age at which participants reached abnormal levels of Aβ-amyloid. Survival was defined as the time between birth and having a PET scan indicating abnormal levels of Aβ-Amyloid, withdrawal from the study, or the last completed follow-up examination. The event was classified as having a PET scan indicating abnormal levels of Aβ-amyloid. For some individuals the date at which their amyloid levels would have become abnormal was imputed, further details on the imputation are provided in supplementary material. The median age at which participants reached abnormal levels of Aβ-Amyloid, represented by the age at which 50% of the cohort reached abnormal levels of Aβ-Amyloid, was reported.
2.4. Data Availability
All ADNI and a subset of the AIBL data including images are shared through the LONI Image & Data Archive (http://adni.loni.usc.edu), a secure research data repository. Applications for access to the entirety of the AIBL data can be made via application through the AIBL website (https://aibl.csiro.au/).
3. Results
3.1. Demographics
There were a significantly higher proportion of NC participants in the AIBL APOE-ε4 non-carriers compared to carriers (p=0.001), for the ADNI participants this relationship held as a trend (p=0.057). Within AIBL, there were significantly more Males among the APOE-ε4 carriers compared to non-carriers (p=0.026), a finding not observed in the ADNI participants (p=0.683). The ADNI APOE-ε4 non-carriers were significantly older than carriers (p=0.005), no differences were observed for age between APOE-ε4 carriers and non-carriers in AIBL (p=0.196). No differences were observed between APOE-ε4 carriers and non-carriers, in either AIBL or ADNI, for Years of Education, or length of follow-up (Table 1).
Table 1.
Demographics table for AIBL and ADNI participants stratified by APOE-ε4 carriage
| APOE-ε4 carriage in AIBL | p-value | APOE-ε4 carriage in ADNI | p-value | |||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| Number of Participants [N] | 123 | 86 | 142 | 104 | ||
| Clinical Classification NC/MCI/AD [N] | 102/15/6 | 54/16/16 | 0.001 | 69/73/0 | 37/67/0 | 0.057 |
| Gender: Males [N (%)] | 51 (41.46) | 50 (58.14) | 0.026 | 62 (43.66) | 49 (47.12) | 0.683 |
| Age (years) [mean (sd)] | 72.48 (6.99) | 71.18 (7.23) | 0.196 | 72.82 (6.83) | 70.33 (6.87) | 0.005 |
| Years of Education [N (%)] | ||||||
| <9 | 8 (6.5) | 5 (5.81) | 0.952 | 0 (0) | 1 (0.96) | 0.620 |
| 9-12 | 50 (40.65) | 38 (44.19) | 20 (14.09) | 12 (11.54) | ||
| 13-15 | 24 (19.51) | 17 (19.77) | 29 (20.42) | 20 (19.23) | ||
| >15 | 41 (33.33) | 26 (30.23) | 93 (65.49) | 71 (68.27) | ||
| Years of Follow-up [Mean (sd)] | 6.93 (1.19) | 6.68 (1.35) | 0.149 | 5.00 (1.20) | 5.09 (1.31) | 0.708 |
There were no significant differences between the APOE-ε4 carriers and non-carriers for these demographic measures in the AIBL participants deemed to be non-accumulators (Supplementary Table 1), caution should be applied to these findings due to the small sample size however. Due to small the sample size comparisons could not be drawn for the ADNI non-accumulators (Supplementary Table 1). There appeared to be more Males and shorter follow-up in the non-accumulators compared to the accumulators, again the small sample size of the excluded non-accumulators should be noted.
3.2. Rates of Aβ-amyloid deposition
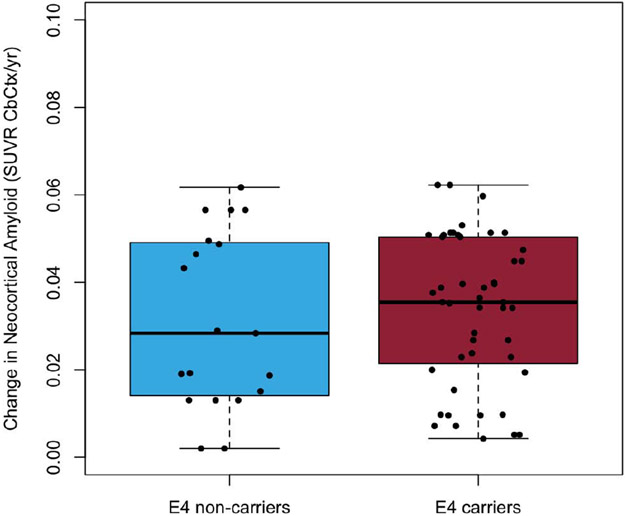
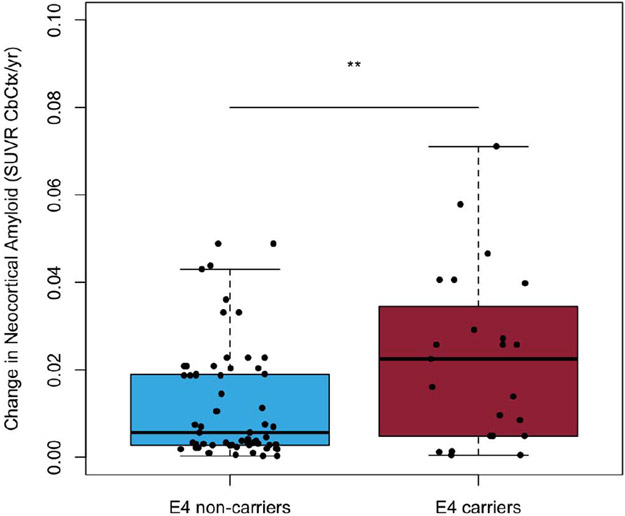
A one-way t-test comparison suggested that APOE-ε4 carriers and non-carriers did not have significantly (p=0.60) different rates of deposition in those above the threshold for Aβ-amyloid at baseline (mean rates of deposition of 0.03±0.02 and 0.03±0.02 SUVR/year, respectively; equivalent to 1.7%/year), (Figure 1A). However, prior to reaching the abnormal threshold AIBL APOE-ε4 carriers appeared to have had significantly (p=0.005) faster rates of Aβ-amyloid deposition (0.02±0.02 SUVR/year; 2.1%/year) in comparison to AIBL APOE-ε4 non-carriers (0.01±0.01 SUVR/year; 1.1%/year), (Figure 1B).
Figure 1A.
Boxplots detailing the rates of Aβ-amyloid deposition for AIBL participants above the abnormal threshold for Aβ-amyloid at baseline (11C-PiB PET SUVR≥1.4) stratified by APOE-ε4 carriage
Figure 1B.
Boxplots detailing the rates of Aβ-amyloid deposition for AIBL participants below the abnormal threshold for Aβ-amyloid at baseline (11C-PiB PET SUVR≥1.4) stratified by APOE-ε4 carriage
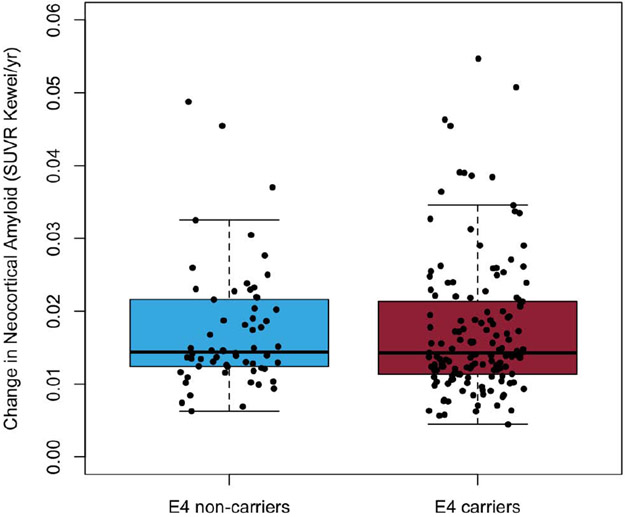
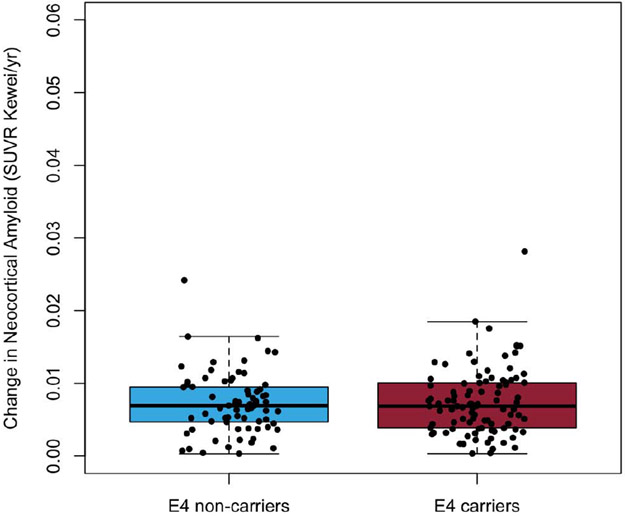
A one-way t-test comparison of individual ADNI participants’ rate of deposition of Aβ-amyloid suggest that APOE-ε4 carriers and non-carriers did not have significantly different rates of deposition either after or prior to reaching the abnormal threshold (p=0.99 and p=0.82, respectively). The mean rates of Aβ-amyloid deposition for ADNI participants beyond the abnormal threshold were 0.02±0.01 SUVR/year (2.2%/year) for both APOE-ε4 carriers and non-carriers, (Figure 1C). Prior to reaching the abnormal threshold, ADNI APOE-ε4 carriers and non-carriers both had rates of Aβ-amyloid deposition of 0.01±0.005 SUVR/year (1.3%/year; Figure 1D).
Figure 1C.
Boxplots detailing the rates of Aβ-amyloid deposition for ADNI participants above the abnormal threshold for Aβ-amyloid at baseline (18F- Florbetapir SUVR≥0.61) stratified by APOE-ε4 carriage
Figure 1D.
Boxplots detailing the rates of Aβ-amyloid deposition for ADNI participants below the abnormal threshold for Aβ-amyloid at baseline (18F- Florbetapir SUVR≥0.61) stratified by APOE-ε4 carriage
In a sensitivity analysis considering only the NC participants, the findings were equivalent with the only statistically significant difference (p=0.001) being found in the AIBL participants below the threshold (Supplementary Figure 1).
Including the non-accumulators to the full data set resulted in no significant differences between APOE-ε4 carriers and non-carriers either above or below the threshold, for AIBL or ADNI participants (Supplementary Figure 2).
3.3. Natural history of neocortical Aβ-amyloid deposition
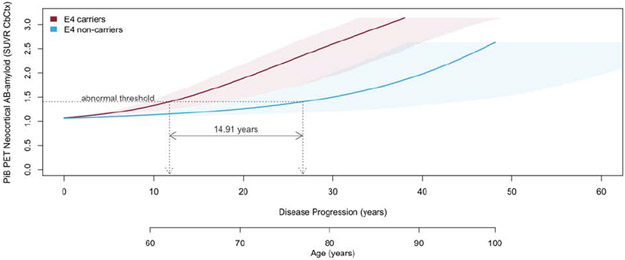
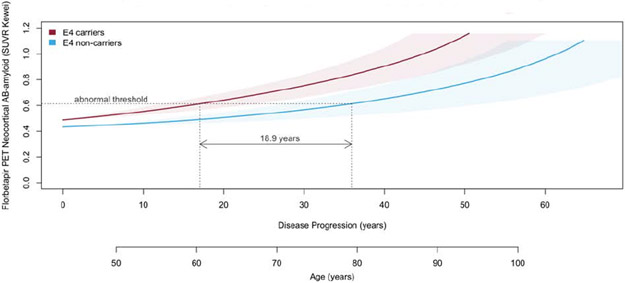
Stratifying the natural history of neocortical Aβ-amyloid deposition by APOE-ε4 carriage indicated that on average AIBL APOE-ε4 carriers reached the abnormal threshold 14.9 (0.3-35.2) years prior to AIBL non-carriers, (Figure 2A). Similarly, on average ADNI APOE-ε4 carriers reached the abnormal threshold 18.9 (CI: 3.5-40.1) years prior to ADNI non-carriers, (Figure 2B). Plots for individuals’ longitudinal data (Step 1 in the method) and slope vs mean plots (Step 2) stratified by APOE-ε4 carriage are provided in Supplementary Figure 3 for AIBL and Supplementary Figure 4 for ADNI. Note: when stratified by sex females appeared to reach the abnormal threshold 2 years prior to males but this was not statistically significant, results not presented.
Figure 2A.
The natural history of deposition of neocortical Aβ-amyloid in AIBL participants stratified by APOE-ε4 carriage. Shaded areas indicate 95% confidence intervals.
Figure 2B.
The natural history of deposition of neocortical Aβ-amyloid in ADNI participants stratified by APOE-ε4 carriage. Shaded areas indicate 95% confidence intervals.
Replicating this in a sensitivity analysis of the NC, indicated that on average NC AIBL APOE-ε4 carriers reached the abnormal threshold 11.1 (−3.9-34.6) years prior to CN AIBL non-carriers, (Supplementary Figure 5). Please note that due to the small numbers of CN in the ADNI cohort, specifically APOE-ε4 carriers (N=37) the models did not converge, and results are not presented.
3.4. Age of onset using survival analysis
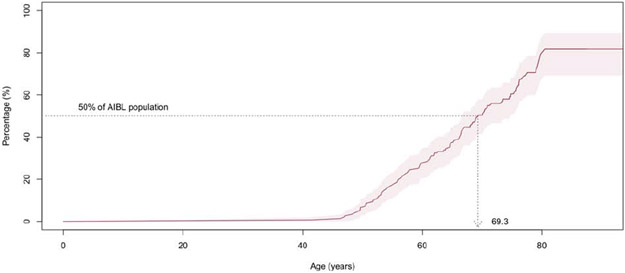
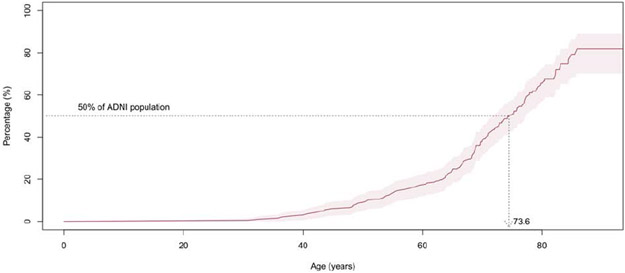
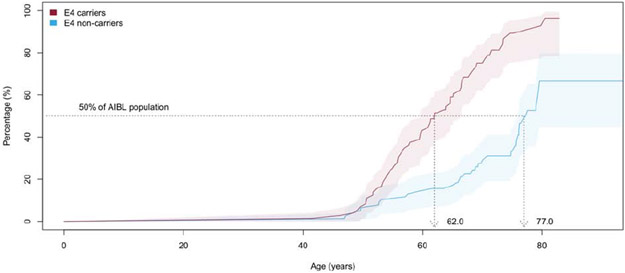
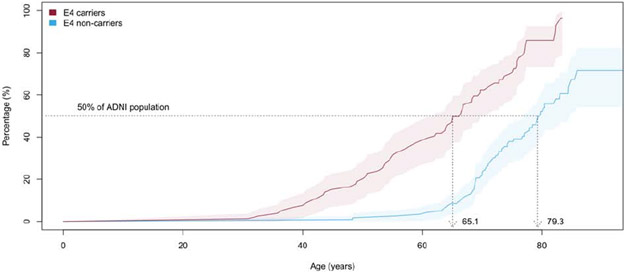
Survival analysis indicated that 50% of the AIBL and ADNI participants reached abnormal levels of Aβ-amyloid by ages of 69.3 (66.5-73.5) and 73.6 (CI: 71.2-77.1), respectively, (Figures 3A and B). Stratifying the participants by APOE-ε4 carriage and replicating the survival analysis indicated 50% of APOE ε4 carriers reached abnormal levels of Aβ-amyloid by ages 62.0 (CI: 59.6-66.5) and 65.1 (CI: 62.0-70.3) in AIBL and ADNI, respectively. In contrast, 50% of the APOE-ε4 non-carriers reached abnormal levels of Aβ-amyloid by ages 77.2 (CI 76.1-NA) and 79.3 (CI 75.9-84.4) in AIBL and ADNI, respectively. These findings suggest that on average APOE-ε4 carriers reached abnormal levels of Aβ-amyloid 15.2 years prior to APOE-ε4 non-carriers in AIBL and 14.2 (2.5-20.5) years in ADNI (Figures 3C and D).
Figure 3A.
Kaplan-Meier plot detailing, by age, the prevalence of AIBL participants with high levels of Aβ-amyloid at baseline (11C-PiB PET SUVR≥1.4). Shaded areas indicate 95% confidence intervals.
Figure 3B.
Kaplan-Meier plot detailing, by age, the prevalence of ADNI participants with high levels of Aβ-amyloid at baseline (18F-Florbetapir SUVR≥0.61). Shaded areas indicate 95% confidence intervals.
Figure 3C.
Kaplan-Meier plot detailing, by age, the prevalence of AIBL participants with high levels of Aβ-amyloid at baseline (11C-PiB PET SUVR≥1.4) stratified by APOE-ε4 carriage. Shaded areas indicate 95% confidence intervals.
Figure 3D.
Kaplan-Meier plot detailing, by age, the prevalence of ADNI participants with high levels of Aβ-amyloid at baseline (18F-Florbetapir SUVR≥0.61) stratified by APOE-ε4 carriage. Shaded areas indicate 95% confidence intervals.
In the CN sub-groups, 50% of CN APOE ε4 carriers reached abnormal levels of Aβ-amyloid by ages 66.2 (CI: 63.6-76.1) and 66.4 (CI: 63.8-NA) in AIBL and ADNI, respectively. In contrast, 50% of the CN APOE-ε4 non-carriers reached abnormal levels of Aβ-amyloid by ages 77.6 (CI 71.6-NA) and 79.3 (CI 76.7-NA) in AIBL and ADNI, respectively (Supplementary Figure 6).
4. Discussion
Survival analyses indicated the average age that AIBL and ADNI participants reached abnormal levels of neocortical Aβ-amyloid was seventy years of age, with confidence intervals (CI) ranging from 66 to 77 years of age. Stratifying the survival analyses by APOE-ε4 carriage suggested that on average APOE-ε4 carriers reached the abnormal threshold in their early sixties, 15 (CI: 6-24) years earlier than non-carriers who reached the threshold late in their seventies. Further, evaluation of the natural history of deposition of neocortical Aβ-amyloid also suggested that APOE-ε4 carriers reached the abnormal threshold of neocortical Aβ-amyloid deposition approximately 15-19 (CI: 4-40) years prior to non-carriers, in line with previous findings (Bilgel et al., 2019; Fleisher et al., 2013; Mishra et al., 2018).
When restricting the analysis to only consider the cognitively normal participants, cognitively normal APOE-ε4 carriers reached the abnormal threshold in their mid-sixties, 12 (CI: 0-24) years earlier than cognitively normal non-carriers who reached the threshold in their midseventies.
It is noted that whilst the age of onset and natural history analyses are not independent, there was exceptional consistency in the findings across the methods as well as across the two cohort studies, despite the use of different Aβ-amyloid tracers. The findings are also consistent with literature looking at the clinical onset of AD which reports APOE-ε4 carriage moves the age of clinical onset earlier by 10-20 years in comparison to non-carriers (Bilgel et al., 2016; Corder et al., 1993; Jack et al., 2014; Jansen et al., 2015).
Based on group comparisons, APOE-ε4 carriers and non-carriers appeared to have similar rates of neocortical Aβ-amyloid deposition, with the only exception being AIBL participants prior to reaching the threshold for neocortical Aβ-amyloid. In this group APOE-ε4 carriers appeared to have significantly faster rates of deposition than non-carriers.
Overall, the findings presented in this paper suggest that the natural history of neocortical Aβ-amyloid deposition in APOE-ε4 carriers starts approximately 15 years earlier but has a similar trajectory to that of APOE-ε4 non-carriers. For the same burden of neocortical Aβ-amyloid, the rate of deposition is similar for both APOE-ε4 carriers and non-carriers (demonstrated by drawing horizontal lines through Figures 2 A and B). These findings fit with the previous literature that APOE-ε4 carriage is not associated with the rate of disease progression, only with earlier onset of disease (Corder et al., 1995; Resnick et al., 2015; Saunders, 2000). Further, they go some way to explaining the conflicting reports that APOE- ε4 carriage is also associated with rate of deposition and/or disease progression (Bilgel et al., 2019; Craft et al., 1998; Hoyt et al., 2005; Jack et al., 2013a; Lim et al., 2017; Mishra et al., 2018; Toledo et al., 2019; Villemagne et al., 2011): if an age matched population was considered (or age corrected modelling used) then the rate of deposition would appear to be higher in APOE-ε4 carriers versus non-carriers. This would be due to APOEεe4 carriers being 15 years further along in disease progression and having higher neocortical Aβ-amyloid burden as well as potentially higher rates of deposition (demonstrated by drawing vertical lines through Figures 2 A and B). Therefore, the difference in rate of deposition between APOE-ε4 carriers and non-carriers previously reported in the literature may be a function of a difference in disease stage opposed to a difference in APOE-ε4 carriage. The temporal relationship between onset and rate is an important consideration and previous evaluations considering these as independent factors or not utilising longitudinal data may have limited their ability to draw valid conclusions.
When stratifying by sex, no significant differences between males and females were observed in the natural history evaluations. As the effect of sex was of a much smaller magnitude at 2 years than that of APOE-ε4 at 15 years, it is possible that this study was not powered to observe a statistically significant difference.
This study has a number of other limitations. Firstly, there were not enough APOE-ε4 homozygotes to enable evaluations on the dose-effect of APOE-ε4 genotype to be undertaken. Secondly, a lack of APOE-ε2 carriers prevented further evaluations to understand the implications of APOE-ε2 carriage and its interplay with APOE-ε4 carriage. Thirdly, given the focus on rates of Aβ-amyloid deposition, only accumulators were included in most of this study which may contrast with other reports and might preclude the generalisability of the findings. Analysis of the small number of non-accumulators available resulted in loss of statistical significance of the difference in rates of change between AIBL APOE-ε4 carriers and non-carriers prior to reaching the threshold, no other differences were found. Fourthly, the analysis is restricted to the longitudinal evaluation of neocortical Aβ-amyloid and it will be necessary to extrapolate this analysis to incorporate peripheral Aβ-amyloid and large longitudinal tau studies once they become available. The participants were volunteers who were not randomly selected from the community, and were generally well educated, thus these findings might only be valid in similar cohorts and this limitation precludes the generalisation of the findings to the general population. Also, in view of the stringent selection criteria in both AIBL and ADNI, which excluded individuals with cerebrovascular disease or other dementias, the effect of other comorbidities on the trajectories might be underestimated. Lastly, longitudinal Aβ-amyloid levels were obtained from 11C PiB PET imaging in AIBL and 18F Florbetapir PET imaging in ADNI and while both underwent the same CapAIBL normalisation, differences in PET scanner and tracer kinetics may contribute a somewhat larger variance in the results.
It has been established that rates of neocortical Aβ-amyloid deposition impact disease progression (Villemagne et al., 2013), earlier onset of Aβ-amyloid deposition may therefore lead to earlier disease onset. Therefore, understanding the age-related, temporal, deposition of neocortical Aβ-amyloid as well as the impact of APOE-ε4 carriage has essential implications for understanding disease mechanisms and informing the timing for therapeutics and diagnostics (Ungar et al., 2014). This is of paramount importance when considering disease staging and/or clinical trial inclusion criteria, for instance clinical trials will potentially need to consider alternative recruitment criteria such as younger age ranges for APOE-ε4 carriers in comparison to non-carriers. The ability to accurately target individuals at appropriate stages of the disease for inclusion in relevant clinical trials could afford such trials a better chance of success in the quest to delay and prevent AD.
Supplementary Material
Highlights:
APOE-ε4 carriers reached abnormal levels of Aβ ~15 years earlier than non-carriers
APOE-ε4 carriers and non-carriers had no differences in Aβ deposition rates beyond the threshold
Primary and secondary prevention trials should target APOE-ε4 carriers aged between 60 and 66
Primary and secondary prevention trials should target APOE-ε4 non-carriers aged between 76 and 84
5. Acknowledgements
We thank the participants who took part in the study and their families.
ADNI Acknowledgement list: Michael W. Weiner, MD Paul Aisen, MD Ronald Petersen, MD, PhD Clifford R. Jack, Jr., MD William Jagust, MD John Q. Trojanowki, MD, PhD Arthur W. Toga, PhD Laurel Beckett, PhD Robert C. Green, MD, MPH Andrew J. Saykin, PsyD John Morris, MD Leslie M. Shaw Zaven Khachaturian, PhD Greg Sorensen, MD Maria Carrillo, PhD Lew Kuller, MD Marc Raichle, MD Steven Paul, MD Peter Davies, MD Howard Fillit, MDFranz Hefti, PhD David Holtzman, MD M. Marcel Mesulam, MD William Potter, MD Peter Snyder, PhD Tom Montine, MD, PhD Gustavo Jimenez, MBS Michael Donohue, PhD Devon Gessert, BS Kelly Harless, BA Jennifer Salazar, MBS Yuliana Cabrera, BS Sarah Walter, MSc Lindsey Hergesheimer, BS Danielle Harvey, PhD Matthew Bernstein, PhD Nick Fox, MD Paul Thompson, PhD Norbert Schuff, PhD Charles DeCArli, MD Bret Borowski, RT Jeff Gunter, PhD Matt Senjem, MS Prashanthi Vemuri, PhD David Jones, MD Kejal Kantarci Chad Ward Robert A. Koeppe, PhD Norm Foster, MD Eric M. Reiman, MD Kewei Chen, PhD Susan Landau, PhD Nigel J. Cairns, PhD, FRCPath Erin Householder, MS John Q. Trojanowki, MD, PhD Virginia Lee, PhD, MBA Magdalena Korecka, PhD Michal Figurski, PhD Arthur W. Toga, PhD Karen Crawford Scott Neu, PhD Andrew J. Saykin, PsyD Tatiana M. Foroud, PhD Steven Potkin, MD UC Li Shen, PhD Kelley Faber, MS, CCRC Sungeun Kim, PhD Kwangsik Nho, PhD.
AIBL Acknowledgement List: Arti Appannah, Mary Barnes, Kevin Barnham, Justin Bedo, Shayne Bellingham, Lynette Bon, Pierrick Bourgeat, Belinda Brown, Rachel Buckley. Samantha Burnham, Ashley Bush, Graeme Chandler, Karren Chen, Roger Clarnette, Steven Collins, Ian Cooke, Tiffany Cowie, Kay Cox, Emily Cuningham, Elizabeth Cyarto, Phuong Anh Vu Dang, David Darby, Patricia Desmond, James Doecke, Vincent Dore, Harriet Downing, Belinda Dridan, Konsta Duesing, Michael Fahey, Maree Farrow, Noel Faux, Michael Fenech, Shane Fernandez, Binosha Fernando, Chris Fowler, Maxime Francois, Jurgen Fripp, Shaun Frost, Samantha Gardener, Simon Gibson, Petra Graham, Veer Gupta, David Hansen, Karra Harrington, Andy Hill, Eugene Hone, Maryam Hor, Malcolm Horne, Brenda Huckstepp, Andrew Jones, Gareth Jones, Adrian Kamer, Yogi Kanagasingam, Lisa Keam, Adam Kowalczyk, Betty Krivdic, Chiou Peng Lam, Fiona Lamb, Nicola Lautenschlager, Simon Laws, Wayne Leifert, Nat Lenzo, Hugo Leroux, Falak Lftikhar, Qiao-Xin Li, Florence Lim, Lucy Lim, Linda Lockett, Kathy Lucas, Mark Mano, Caroline Marczak, Georgia Martins, Paul Maruff, Yumiko Matsumoto, Sabine Bird, Simon McBride, Rachel McKay, Rachel Mulligan, Tabitha Nash, Julie Nigro, Graeme O’Keefe, Kevin Ong, Bernadette Parker, Steve Pedrini, Jeremiah Peiffer, Sveltana Pejoska, Lisa Penny, Keyla Perez, Kelly Pertile, Pramit Phal, Tenielle Porter, Stephanie Rainey-Smith, Parnesh Raniga, Alan Rembach, Carolina Restrepo, Malcolm Riley, Blaine Roberts, Jo Robertson, Mark Rodrigues, Alicia Rooney, Rebecca Rumble, Tim Ryan, Olivier Salvado, Mather Samuel, Ian Saunders, Greg Savage, Brendan Silbert, Hamid Sohrabi, Julie Syrette, Cassandra Szoeke, Kevin Taddei, Tania Taddei, Sherilyn Tan, Michelle Tegg, Philip Thomas, Darshan Trivedi, Brett Trounson, Robyn Veljanovski, Giuseppe Verdile, Victor Villemagne, Irene Volitakis, Cassandra Vockler, Michael Vovos, Freda Vrantsidis, Stacey Walker, Andrew Watt, Mike Weinborn, Bill Wilson, Michael Woodward, Olga Yastrubetskaya, Paul Yates, Ping Zhang
6 Funding
Core funding for the AIBL study was provided by the CSIRO Flagship Collaboration Fund and the Science and Industry Endowment Fund (SIEF) in partnership with the CRC for Mental Health, Edith Cowan University (ECU), Mental Health Research institute (MHRI), Alzheimer’s Australia (AA), National Ageing Research Institute (NARI), Austin Health, Macquarie University, CogState Ltd, Hollywood Private Hospital, and Sir Charles Gairdner Hospital. The study also received funding from the National Health and Medical Research Council (NHMRC), Dementia Collaborative Research Centres program (DCRC), and McCusker Alzheimer’s Research Foundation, and operational infrastructure support from the Government of Victoria. Funding for this study was provided by the NHMRC (Grant Number 1156891).
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Competing Interest
Samantha C. Burnham: reports speaker honoraria from Novartis outside the scope of the submitted work
Simon Laws: personal fees from Alzhyme outside the scope of the submitted work
Charley Budgeon: Declares no conflicts of interest
Vincent Doré: Declares no conflicts of interest
Tenielle Porter: Declares no conflicts of interest
Pierrick Bourgeat: Declares no conflicts of interest
Rachel Buckley: Declares no conflicts of interest
Kevin Murray: Declares no conflicts of interest
Kathryn Ellis: Declares no conflicts of interest
Berwin Turlach: Declares no conflicts of interest
Olivier Salvado: reports a patent PCT/AU2012001536 pending to CSIRO
David Ames: reports receipt of financial assistance to his employer in order to assist with an international drug trail of an anti-Alzheimer’s agent owned by Eli Lilly
Ralph N Martins: Declares no conflicts of interest Dorene Rentz: Declares no conflicts of interest
Colin Masters: reports personal fees from Prana Biotechnology, Eli Lilly, and Actinogen outside the scope of the submitted work
Christopher C. Rowe: reports speaker honoraria from GE Healthcare and Avid Radiopharmaceuticals, consulting fees from Avid Radiopharmaceuticals, AstraZeneca, and Piramal Imaging, and research grants from Avid Radiopharmaceuticals, GE Healthcare, and Piramal Imaging all outside the scope of the submitted work
Victor L. Villemagne: reports speaker honoraria from GE Healthcare, Piramal Imaging, and Avid Radiopharmaceuticals, and consulting fees from Lundbeck, Abbvie, Shanghai Green Valley Co and Hoffmann La Roche, all outside the scope of the submitted work.
Previous Publication
This work has not been published in any other form, nor is under consideration at any other publication.
Ethics Approvals
The institutional ethics committees of Austin Health, St Vincent’s Health, Hollywood Private Hospital, and Edith Cowan University approved the AIBL study, and all volunteers gave written informed consent before participating.
Institutional Review Boards and Research Ethics Boards according to applicable State and Federal requirements for each participating location provided approval for ADNI evaluations, all participants gave informed consent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Samantha C. Burnham, Centre of Excellence for Alzheimer’s Disease Research & Care, School of Medical Sciences, Edith Cowan University, Joondalup, WA, Australia.
Simon M. Laws, Collaborative Genomics Group, Centre of Excellence for Alzheimer’s Disease Research & Care, School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia; School of Biomedical Sciences, Faculty of Health Sciences, Curtin Health Innovation Research Institute, Curtin University, Bentley, WA, Australia; Cooperative Research Centre for Mental Health, http://www.mentalhealthcrc.com.
Charley A. Budgeon, Centre for Applied Statistics, University of Western Australia, Crawley, Western Australia, Australia; eHealth, CSIRO Health and Biosecurity, Floreat, WA, Australia.
Vincent Doré, eHealth, CSIRO Health and Biosecurity, Herston, QLD, Australia; Department of Nuclear Medicine and Centre for PET, Austin Health, Heidelberg, VIC, Australia.
Tenielle Porter, Collaborative Genomics Group, Centre of Excellence for Alzheimer’s Disease Research & Care, School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia; Cooperative Research Centre for Mental Health, http://www.mentalhealthcrc.com.
Pierrick Bourgeat, eHealth, CSIRO Health and Biosecurity, Herston, QLD, Australia.
Rachel F. Buckley, Florey Institute, University of Melbourne, Parkville, VIC, Australia; Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Melbourne School of Psychological Sciences, University of Melbourne, Parkville, VIC, Australia.
Kevin Murray, School of Population and Global Health, University of Western Australia, Crawley, Western Australia, Australia.
Kathryn A. Ellis, Academic Unit for Psychiatry of Old Age, Department of Psychiatry, University of Melbourne, Parkville, VIC, Australia.
Berwin A. Turlach, Centre for Applied Statistics, University of Western Australia, Crawley, Western Australia, Australia.
Olivier Salvado, eHealth, CSIRO Health and Biosecurity, Herston, QLD, Australia; Florey Institute, University of Melbourne, Parkville, VIC, Australia.
David Ames, University of Melbourne Academic Unit for Psychiatry of Old Age, St George’s Hospital, Kew, VIC, Australia; National Ageing Research Institute, Parkville, VIC, Australia.
Ralph N Martins, Centre of Excellence for Alzheimer’s Disease Research & Care, School of Medical Sciences, Edith Cowan University, Joondalup, WA, Australia.
Dorene Rentz, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Colin L. Masters, Florey Institute, The University of Melbourne, Parkville, VIC, Australia.
Christopher C. Rowe, Department of Molecular Imaging & Therapy, Austin Health, Heidelberg, VIC, Australia; Department of Medicine, Austin Health, University of Melbourne, Heidelberg, VIC, Australia.
Victor L. Villemagne, Department of Molecular Imaging & Therapy, Austin Health, Heidelberg, VIC, Australia; Department of Medicine, Austin Health, University of Melbourne, Heidelberg, VIC, Australia
References
- Bilgel M, An Y, Zhou Y, Wong DF, Prince JL, Ferrucci L, Resnick SM, 2016. Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimer's & Dementia 12(4), 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel M, Jedynak BM, Initiative, A.s.D.N., 2019. Predicting time to dementia using a quantitative template of disease progression. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring 11, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougeat P, Villemagne V, Dore V, Brown B, Macaulay L, Martins R, Masters C, Ames D, Ellis K, Rowe C, Salvado O, Fripp J, 2015. Comparison of MR-less PiB SUVR quantification methods. Neurobiology of Aging 36(Supplement 1), 8. [DOI] [PubMed] [Google Scholar]
- Budgeon CA, Murray K, Turlach BA, Baker S, Villemagne VL, Burnham SC, 2017. Constructing longitudinal disease progression curves using sparse, short-term individual data with an application to Alzheimer's disease. Statistics in Medicine. [DOI] [PubMed] [Google Scholar]
- Chen K, Roontiva A, Thiyyagura P, Lee W, Liu X, Ayutyanont N, Protas H, Luo JL, Bauer R, Reschke C, 2015. Improved power for characterizing longitudinal amyloid-b PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med 56(4), 560–566. [DOI] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, 2012. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. The Lancet Neurology 11(8), 669–678. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Rimmler J, Locke P, Conneally P, Schmader K, Tanzi R, 1995. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology 45(7), 1323–1328. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G.a., Roses A, Haines J, Pericak-Vance MA, 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123), 921–923. [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D, 1998. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease Relationship to severity of dementia and apolipoprotein E genotype. Neurology 50(1), 164–168. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Liu X, Ayutyanont N, Roontiva A, Thiyyagura P, Protas H, Joshi AD, Sabbagh M, Sadowsky CH, 2013. Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiology of aging 34(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. science 297(5580), 353–356. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, 2009. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nature genetics 41(10), 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS, 2005. Individual Growth Curve Analysis of APOE ε4-Associated Cognitive Decline in Alzheimer Disease. Archives of neurology 62(3), 454–459. [DOI] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association 14(4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, 2013a. Brain β-amyloid load approaches a plateau. Neurology 80(10), 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, Mielke MM, Jones DT, Senjem ML, Gunter JL, 2013b. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81(20), 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Preboske GM, 2014. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. The Lancet Neurology 13(10), 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, 2015. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama 313(19), 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, Mercken M, De Strooper B, 2011. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nature reviews Drug discovery 10(9), 698. [DOI] [PubMed] [Google Scholar]
- Lim YY, Mormino EC, initiative, A.s.D.N., 2017. APOE genotype and early β-amyloid accumulation in older adults without dementia. Neurology 89(10), 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G, 2013. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology 9(2), 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Blazey TM, Holtzman DM, Cruchaga C, Su Y, Morris JC, Benzinger TL, Gordon BA, 2018. Longitudinal brain imaging in preclinical Alzheimer disease: impact of APOE ε4 genotype. Brain 141(6), 1828–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, Saykin AJ, Orro A, Lupoli S, Salvi E, 2009. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer's disease. PloS one 4(8), e6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, 2017. R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, 2009. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proceedings of the National Academy of Sciences 106(16), 6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Bilgel M, Moghekar A, An Y, Cai Q, Wang M-C, Thambisetty M, Prince JL, Zhou Y, Soldan A, 2015. Changes in Aβ biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiology of aging 36(8), 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O'Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL, 2010. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging 31(8, Sp. Iss. SI), 1275–1283. [DOI] [PubMed] [Google Scholar]
- Saunders AM, 2000. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. Journal of Neuropathology & Experimental Neurology 59(9), 751–758. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, 2010. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimer's & dementia: the journal of the Alzheimer's Association 6(3), 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Habes M, Sotiras A, Bjerke M, Fan Y, Weiner MW, Shaw LM, Davatzikos C, Trojanowski JQ, Initiative, A.s.D.N., 2019. APOE Effect on Amyloid-β PET Spatial Distribution, Deposition Rate, and Cut-Points. Journal of Alzheimer's Disease(Preprint), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar L, Altmann A, Greicius MD, 2014. Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain imaging and behavior 8(2), 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. The Lancet Neurology 12(4), 357–367. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, 2011. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Annals of neurology 69(1), 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All ADNI and a subset of the AIBL data including images are shared through the LONI Image & Data Archive (http://adni.loni.usc.edu), a secure research data repository. Applications for access to the entirety of the AIBL data can be made via application through the AIBL website (https://aibl.csiro.au/).