Abstract
Growth hormone (GH) and its mediator, insulin-like growth factor-1 (IGF-1), have long been recognized as central to human growth physiology. IGF-1 is known to complex with IGF binding proteins as well as with the acid labile subunit (ALS) in order to prolong its half-life in circulation. Factors regulating the bioavailability of IGF-1 (i.e. the balance between free and bound IGF-1) were less well understood. Recently, pregnancy-associated plasma protein-A2 (PAPP-A2) was discovered as a protease which specifically cleaves IGF-binding protein (IGFBP)-3 and -5. PAPP-A2 deficient patients present with characteristic findings including growth failure, elevated total IGF-1 and -2, IGFBPs, and ALS, but decreased percentage of free to total IGF-1. Additionally, patients with PAPP-A2 deficiency have impairments in glucose metabolism and bone mineral density (BMD). Treatment with recombinant human IGF-1 (rhIGF-1) improved height SD scores, growth velocity, body composition, and dysglycemia. Mouse models recapitulate many of the human findings of PAPP-A2 deficiency. This review summarizes the function of PAPP-A2 and its contribution to the GH–IGF axis through an examination of PAPP-A2 deficient patients and mouse models, thereby emphasizing the importance of the regulation of IGF-1 bioavailability in human growth.
Keywords: PAPP-A2, Free IGF-1, IGFBPs, GH–IGF axis, IGF-1 bioavailability, Growth
1. Introduction
The growth hormone (GH) – Insulin-like growth factor (IGF) axis is widely known as one of the critical biological pathways involved in regulating human growth. It has been investigated by many researchers since the late 1940s (Yakar et al., 2018). The GH–IGF axis has traditionally consisted of Somatostatin, GH releasing hormone (GHRH), GH, IGFs, their receptors, and downstream signaling molecules including STAT5B, IGF-binding proteins (IGFBPs), and the acid-labile subunit (ALS). In this manuscript, we will discuss a more recently discovered component to this axis, a regulator of IGF bioavailability, pregnancy-associated plasma protein-A2 (PAPP-A2) (Overgaard et al., 2001), a protease that cleaves IGF binding protein-3 and -5. The secretion of GH from the anterior pituitary is stimulated by GHRH, released by the hypothalamus. Sequentially, GH promotes the production of IGFs, IGFBPs, and ALS from the liver and other tissues. Under normal circumstances, free IGF-1 can circulate with a half-life of up to 10 min in serum. However, the majority of IGF-1 exists in a ternary complex with IGFBP-3 or -5 and the ALS, resulting in an increased half-life of IGF-1 (up to 15 h) (Renes et al., 2019,Yakar et al., 2018). As free IGF-1 is the only form that binds to the IGF receptors, IGF-1 must be liberated from IGFBPs. Therefore, a protease such as PAPP-A2 and/or other proteases are required to cleave IGFBPs, subsequently leading to increased IGF signaling resulting in cell proliferation and growth.
In this review, we discuss PAPP-A2’s contribution to human growth physiology and describe the effects of PAPP-A2 deficiency on serum biochemistry, glucose metabolism, and bone development by exploring the phenotype of human patients bearing PAPPA2 mutations.
2. Physiology and function of PAPP-A2
In 2001, Overgaard et al. (2001) identified and cloned cDNA encoding the PAPP-A2 protein, a protein with 46% homology to the functionally related protein PAPP-A (Overgaard et al., 2001). The PAPPA2 gene, located at chromosome 1q25.2, contains the elongated zinc-binding motif, HEXXHXXGXXH, which works as the catalytic zinc-binding site, similar to PAPP-A (Boldt et al., 2001). PAPP-A2 and PAPP-A were classified as “pappalysins,” new members of the metzincin family of metalloproteinases (Boldt et al., 2001). Given that PAPP-A was known to target select IGF binding proteins for proteolysis, the experiments incubating PAPP-A2 with each of the six IGFBPs revealed that IGFBP-3 and -5 were specific substrates for PAPP-A2 (Overgaard et al., 2001). PAPP-A2 is expressed abundantly in the human placenta, and it’s also detected in other tissues throughout the body, including the kidney, mammary tissue, pituitary, brain, and pancreas. Unlike PAPP-A which cleaves its target IGFBP-4 while anchored to the cell surface, PAPP-A2 does not bind the cell surface but is rather found in circulation (Laursen et al., 2002,Oxvig, 2015). PAPP-A2 protein has been detected at extremely high levels in the circulation of women during pregnancy (Kloverpris et al., 2013,Wang et al., 2009). Therefore, it is thought that PAPP-A works as a local modulator for IGF-1 bioavailability by cleavage of IGFBP-2, -4, and -5 (Argente et al., 2017,Oxvig, 2015). However, PAPP-A2 may play a larger role in increasing serum free IGF-1 concentration systemically via liberating IGF-1 from its ternary complex with IGFBP-3 or -5 and ALS. It is also possible that PAPP-A2 has local tissue effects as well but this requires further exploration.
Stanniocalcin-1 (STC1) and -2 (STC2) are secreted glycoproteins that are widely expressed in human and mouse tissues and are highly conserved throughout evolution from fish to higher vertebrates (Chang et al., 2008,Chang et al., 1998). Kløverpris et al. (2015) and Jepsen et al. (2015) reported that STCs work as protease inhibitors for PAPP-A and PAPP-A2, suggesting that they too may play an important role in regulating the GH–IGF axis (Jepsen et al., 2015,Kloverpris et al., 2015). Interestingly, while STC1 appears to play a role in calcium regulation, transgenic overexpression of human STC2 in mice causes growth failure (Gagliardi et al., 2005). To date, there have been no human cases with a pathogenic mutation in either STC1 or 2.
Recent genomic studies have highlighted the importance of PAPPA, PAPPA2, and STC2 in height biology. In 2010, the Genetic Investigation of Anthropometric Traits Consortium performed a large genome-wide association study (GWAS) examining the role of common genetic variation in determining adult stature (Lango Allen et al., 2010). They identified 180 different loci associated with height and then examined the biological connections between the genes within these loci. This analysis highlighted a number of biological pathways and included the PAPPA, PAPPA2, and STC2 genes. In a follow-up GWAS (Marouli et al., 2017), the same group of investigators identified 83 rare and low frequency coding variants to be associated with height. Of these 83 variants, a missense variant in STC2 was found to have the largest effect on height. The heights of carriers with this rare STC2 missense variant were approximately 2.1 cm taller than non-carriers. These studies highlight that genetic variation around the PAPPA, PAPPA2, and STC2 genes play a role in determining an individual’s height in the general population.
3. PAPP-A2 deficiency in humans
3.1. Descriptions of patients with PAPP-A2 deficiency
In 2016, the first two families bearing rare mutations in PAPPA2 were identified using whole-exome sequencing (Dauber et al., 2016). The two families were of Spanish (Family 1) and Palestinian (Family 2) ancestry and the affected children presented with short stature and markedly elevated IGF-1 levels. In the Spanish family, two of four siblings were found to carry a novel homozygous frameshift mutation in PAPPA2 (c.1927_1928insAT, p.D643fs25*). The affected children presented with heights in the low-normal range (−1.1 and −0.96 SDS) but significantly below their mid-parental target height. Their biochemical profiles at presentation were notable for extreme elevations in IGF-1 and milder elevations of IGFBP-3. PAPP-A2 levels were undetectable. Subtle dysmorphic features consisted of small chins, microcephaly, and long fingers and toes (Dauber et al., 2016). In the second family, three of five siblings carried a novel homozygous missense mutation in PAPPA2 (c.3098C > T, p.A1033V). They presented with more severe postnatal growth retardation and short stature with heights ranging from −2.8 to −3.8 SDS. They had a very similar biochemical profile with extreme elevations in IGF-1 and milder elevations of IGFBP-3 but these patients had detectable levels of PAPP-A2. In vitro studies demonstrated that the missense mutation led to the inability of the mutated PAPP-A2 to cleave IGFBP-3 and IGFBP-5. Similar to the children in the Spanish family, the affected siblings, had small chins, microcephaly, long thin fingers, and delayed dental eruption. In addition, two of the five affected patients were born small for gestational age (SGA). The parents of both families bearing heterozygous mutations in PAPPA2 were of normal height. Follow up studies of the growth profile from the two older siblings in the second family showed the absence of an apparent pubertal growth spurt (Cabrera-Salcedo et al., 2017,Dauber et al., 2016).
3.2. Biochemical consequences of PAPP-A2 deficiency
As noted above, PAPP-A2 protein cleaves IGFBP-3 and -5 thereby freeing IGF-1 from its ternary complex allowing it to bind to its receptor. Therefore, PAPP-A2 deficiency should result in a decrease in free IGF-1 levels leading to a lack of negative feedback at the level of the pituitary ultimately resulting in increased growth hormone levels. This subsequently should cause an increase in all of the growth hormone dependent proteins including total IGF-1 itself as well as IGF-2, IGFBP-3, IGFBP-5, and ALS levels (Figure 1). Further investigations into the affected patients supported this hypothesis. All affected patients presented with elevated serum total IGF-1 and IGFBP-3 levels (Dauber et al., 2016). Bioactive IGF-1 levels measured by a cell-based kinase receptor activation (KIRA) assay (Chen et al., 2003,Sorensen et al., 2015) were reduced in the three prepubertal patients, while those levels in pubertal patients were within normal range. Additionally, free IGF-1 levels measured by enzyme-linked immunosorbent assay (ELISA) showed the same trend as bioactive IGF-1 levels. However, when analyzed as a percentage of bioactive IGF-1 or free IGF-1 compared to the total IGF-1 (i.e. bioactive/total IGF-1 or free/total IGF-1), these percentages were significantly decreased in all patients (Fujimoto et al., 2020,Sorensen et al., 2015). This suggests that the absolute circulating levels of free IGF-1 may be a less important biomarker of PAPP-A2 function, and perhaps of IGF-1 action, than the percentage of free IGF-1. Additionally, extreme elevations in total IGF-I values are a useful clinical clue to suggest the diagnosis of PAPP-A2 deficiency. While mild elevations may be present in heterozygous IGF1R mutations, the degree of elevation seen in the PAPP-A2 deficient patients would only be seen in individuals with homozygous mutations of IGF1R which are extremely rare and lead to severe short stature and microcephaly. As predicted by the hypothesis stated earlier, the patients’ spontaneous GH secretion or peak GH values after stimulation tests were also elevated. Furthermore, other growth hormone dependent factors including serum IGF-2, IGFBP-5, and ALS levels were elevated in the majority of patients. All of the heterozygous unaffected siblings had normal biochemical profiles.
Figure 1. Schematic of the GH-IGF axis highlighting the effects of PAPP-A2 deficiency.
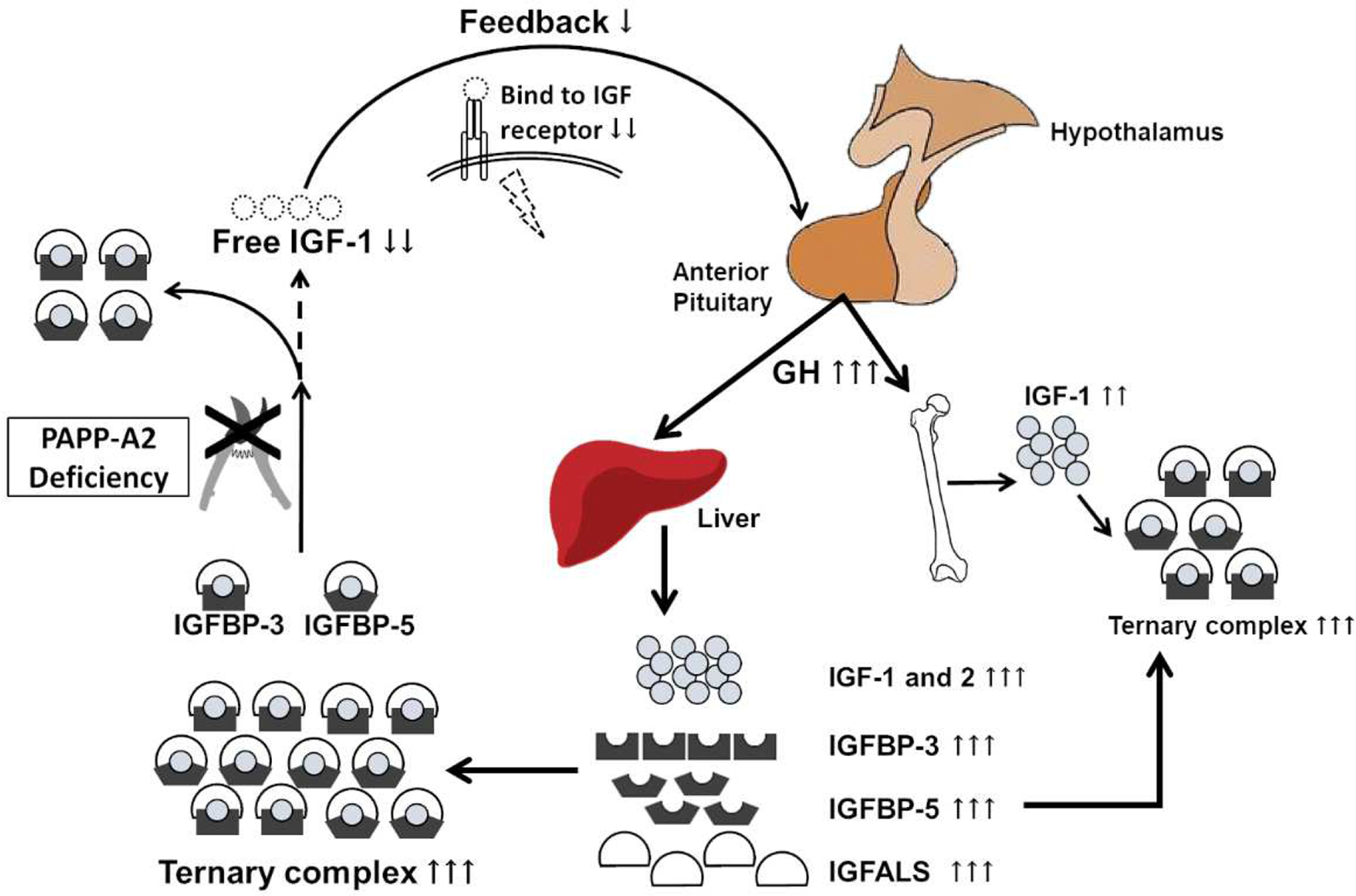
PAPP-A2 deficiency results in insufficient cleavage of the ternary complex containing IGFs, IGFBP-3 or -5, and ALS, resulting in decreased levels of free IGF-1. Reduced free IGF-1 leads to a lack of negative feedback at the level of the pituitary, resulting in increased GH secretion. Ultimately, this leads to increased IGFBPs, ALS, and total IGF-1 without an increase in free IGF-1.
3.3. Glucose metabolism in PAPP-A2 deficiency
PAPP-A2 most likely plays a role in glucose metabolism. Fasting glucose levels in all PAPPA2 deficient patients were normal to high-normal with hyperinsulinemia suggesting a degree of insulin resistance (Cabrera-Salcedo et al., 2017,Dauber et al., 2016,Munoz-Calvo et al., 2016). Oral glucose tolerance tests performed in the second family revealed that two of the patients had elevated blood glucose levels (141 and 152 mg/dL, respectively) at the two-hour time point. The exact mechanism of dysglycemia in our patients is not completely known but may be due to the effects of high growth hormone levels and low free IGF-1 levels caused by PAPP-A2 deficiency. Elevated baseline GH levels induce insulin resistance via effects on hepatic gluconeogenesis, glyconenolysis, and lipolysis (Pal et al., 1992,Vijayakumar et al., 2010). However, the administration of a very low dose of recombinant GH in healthy young adults (19–29yrs) improved insulin sensitivity presumably through the effects of increases in free IGF-1 (Yuen et al., 2004). The administration of recombinant IGF-1 for patients with type 1 and type 2 diabetes mellitus, without PAPP-A2 deficiency, increased serum IGF-1 levels resulting in improved insulin sensitivity (Simpson et al., 2004). This data suggests that free IGF-1 levels may play an important role in modulating insulin sensitivity (Yuen et al., 2007).
3.4. Bone mineral density (BMD) in patients with PAPP-A2 deficiency
All patients had varying degrees of decreased bone mineral density (BMD) with the majority of patients having BMD in the low-normal range (Argente et al., 2019,Cabrera-Salcedo et al., 2017,Hawkins-Carranza et al., 2018). In Family 1, bone microarchitecture strength was measured using the Trabecular Bone Score (TBS). TBS was mildly low in one of the siblings and increased in both siblings after two years of treatment with recombinant human IGF-1 (rhIGF-1). In Family 2, all of the patients had bone density below the mean for the population with one of the brothers having a whole-body BMD z-score below −2 SD. The youngest sibling showed significant gains in BMD after 1 year of treatment with rhIGF-1.
3.5. Treatment of patients with PAPP-A2 deficiency
Currently, there is no approved treatment for PAPP-A2 deficiency. However, two therapeutic approaches have been attempted; rhIGF-1 and plasma transfusion as a means of PAPP-A2 replacement (Andrew et al., 2018,Cabrera-Salcedo et al., 2017,Munoz-Calvo et al., 2016). Four of the five affected patients underwent treatment with subcutaneous rhIGF-1 injection (40–120 μg/kg/dose, twice a day, progressively increased). In Family 2, a detailed pharmacokinetic study was performed. After 120 μg/kg/day of subcutaneous rhIGF-1 injection, the peak free IGF-1 levels were measured and the pharmacokinetic parameters were similar between the patients, their heterozygous relatives and unaffected controls (Cabrera-Salcedo et al., 2017). Three of the patients were successfully treated with rhIGF-1 therapy resulting in a first-year change in height SDS of +0.4 SDS in all three patients and accelerated growth velocity from −1.97 SD to +1.29 SD. Additionally, treatment resulted in resolution of their hyperinsulinemia and improvement in fasting blood glucose levels. Treatment outcomes of the patients are summarized in Table 1. Basal GH levels decreased with rhIGF-1 treatment which may be the cause of the improvement seen in glucose metabolism.
Table 1.
Treatment outcomes
| Case | Sex | Treatment period | rhIGF-1 therapy | Age (y.o.) | Height, cm (SDS) | ΔHtSDS | GV, cm/y (SDS) | Bone age (y.o.) | BMD (SDS) | Body composition change | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1-P1 | F | 0 | 40–80 μg/kg, twice daily | 10.5 | 132 (−1.25) | - | 3.7 (−1.5) | 10.8 | −0.1* | - | |
| 12M | 120 μg/kg, twice daily | 11.5 | 139.6 (−0.86) | +0.4 | 7.6 (+1.6) | 11.3 | −0.4* | Increases in the percentage of LBM (+4.8 %) and decreases in PBFM (-4.5 %) | |||
| After 24M, −0.3* | |||||||||||
| F1-P2 | M | 0 | 40–80 μg/kg, twice daily | 6.1 | 111.5 (−0.74) | - | 5.8 (-1.6) | 4.5 | −0.1* | - | |
| 12M | 120 μg/kg, twice daily | 7.1 | 118.5 (−0.34) | +0.4 | 7.0 (+1.1) | 5.5 | −0.7* | Increases in the percentage of LBM (+5.0 %) and decreases in PBFM (-4.7 %) | |||
| After 24M, −0.2* | |||||||||||
| F2-P1 | F | Untreated | - | 18.6 | 138.4 (−3.81) | - | NA | NA | −0.5† | NA | |
| F2-P2 | M | 0–1 | 60 μg/kg, twice daily | 14.5 | 142.4 (−2.9) | - | 4.3 (−1.6) | 13.5 | −2.5† | - | |
| 12M | 15.5 | 147.2 (−3.0) | −0.1 | 4.8 (+1.5) | 14.5 | −2.2† | Increases in LBM and decreases in PBFM | ||||
| F2-P3 | M | 0 | 60 | 10.4 | 122.0 (−2.9) | - | 3.0 (−2.8) | 9.0 | −0.7† | - | |
| 12M | 120 μg/kg, twice daily | 11.4 | 128.2 (−2.5) | +0.4 | 6.2 (+1.2) | 10.0 | −0.1† | Increases in LBM and decreases in PBFM | |||
| F1-P1 | F | 0 | 957 | 1.4 | ND‡ | 5912 | 3745 (U/L) | 4.8 | 15.1 | ||
| 12M | No significant change | No significant change in bioactive IGF-1 | NA | No significant change | NA | 0.8 | 11 | ||||
| F1-P2 | M | 0 | 882 | 0.3 | ND‡ | 4850 | 3625 (U/L) | 5.3 | 27 | ||
| 12M | No significant change | No significant change in bioactive IGF-1 | NA | No significant change | NA | 0.7 | 8 | ||||
| F2-P1 | F | Untreated | 1060 | 2.0 | 0.2 | 4557 | 2445 (U/L) | NA | Hyperinsulinemia | ||
| F2-P2 | M | 0 | 573.2 | 5.6 | 0.1 | 8997 | 23 | 0.1 | 17.1 | ||
| 12M | 756.2 | 6.1 | 0.1 | 8983 | 17 | 0.1 | Hyperinsulinemia | ||||
| F2-P3 | M | 0 | 546.8 | 0.8 | 0.1 | >10000 | 18 | 0.2 | 7 | ||
| 12M | 709.1 | 1.2 | 0.2 | 6362 | 14 | 0.1 | Improved | ||||
The height-adjusted Z-score was for the lumber spine.
The height-adjusted Z-score was for the whole body less head.
ELISA kit could not detect affected PAPP-A2 caused by the frameshift mutation.
Abbreviations: BA, bone age; BMI, body mass index; HC, head circumference; GV, growth velocity F1, Spanish family; F2, Palestinian family; P1, P2, P3 – Proband 1, 2, 3; LBM, lean body mass; PBFM, percentage of total body fat mass; ND, not detectable; NA, not available
In one of the four treated patients, intracranial hypertension and headache occurred early during rhIGF-1 treatment resulting in discontinuation of therapy (Dauber et al., 2016). No patient experienced any episodes of hypoglycemia.
Prior to initiation of rhIGF-1 treatment in Family 2, an attempt was made to explore the possibility of PAPP-A2 replacement therapy via plasma transfusion. A single transfusion of 20 mL/kg of fresh frozen plasma was given to the eldest affected sibling with PAPP-A2 deficiency (Andrew et al., 2018). The plasma transfusion resulted in a 2.5-fold increase of free IGF-I levels on day 1 post-transfusion but there were no significant changes in total IGF-1, total IGFBP-3 or intact IGFBP-3 levels. Additionally, no detectable changes in PAPP-A2 levels were found suggesting that this transfusion did not substantially increase circulating PAPP-A2 levels. However, the increase in free IGF-1 raises the possibility that recombinant PAPP-A2 could be an effective medication for PAPP-A2 deficiency and growth failure. Additional studies are needed to understand the pharmacokinetics and pharmacodynamics of PAPP-A2.
4. Regulation of GH–IGF axis related proteins by PAPP-A2
4.1. Insights from mouse studies
To date, there have been three mouse models described with PAPP-A2 deficiency and each shows reduced body weight and size with significant decreases in free IGF-1 compared to the wild-type control. Conover et al. (2011) described the first global knock-out (KO) mouse model in which PAPP-A2 protein was absent from all tissue in which it is normally expressed (Conover et al., 2011). Serum from these KO mice was subsequently assayed for free and total IGF-1, and similar to the human patients, PAPP-A2 deficiency led to markedly decreased levels of free IGF-1 despite elevation in total IGF-1 (Dauber et al., 2016). Next, Christians et al. (2015) created a second Pappa2 KO mouse model (Christians et al., 2015). Like the KO created by Conover, Christians saw a significant increase in total IGF-1 in the KO mouse (945 ± 24 ng/mL) compared to littermate wild-type (WT) controls (582 ±28 ng/mL). Finally, a knock-in (KI) mouse model (p.V1034A) was created via CRISPR-Cas9 which recapitulates the human PAPPA2 missense mutation (p.V1033A) (Fujimoto et al., 2019). Similar to the Christians model, homozygous KI mice both show extremely reduced free IGF-1 levels compared to WT littermate controls and markedly higher circulating levels of total IGF-1 in the homozygous KI compared to WT with heterozygotes having an intermediate phenotype.
As seen in the humans, the pattern of reduced body size is noted in every mouse model paper to date. The first KO mouse model showed postnatal growth retardation in male and female mice of 10% and up to 30% compared to WT controls, respectively (Conover et al., 2011). The reduction of body weight and length were also noted in the KI mouse model. Again, males showed ~10% reduction in body length and weight, while females show a 20% reduction in body weight compared to littermate WT controls, both with reduced relative lean body mass (Fujimoto et al., 2019). However, in a reanalysis of the data from Fujimoto et al. (2019), there was no significant sex by genotype interaction either for body length or body weight at 8 and 16 weeks. Similar to the biochemical data, the heterozygous KI and KO mice display as expected, an intermediate phenotype compared to the homozygous KI and WT littermates (Christians et al., 2015,Fujimoto et al., 2019). In addition, bone appears to be affected in all three described mouse models. Embryonic KO mice at E16.5 have smaller skeletons compared to wild-type controls (Conover et al., 2011). In the KI mice, adults have inhibited periosteal bone expansion which results in slender bones (Fujimoto et al., 2019). However, bone mineral density does not appear to be affected in either model. Also reported, Pappa2 KO mice at all ages have shorter bone growth (Christians et al., 2018) and without PAPP-A2 produced locally in bone osteoblasts, normal growth will not occur (Amiri et al., 2015). Finally, in a thorough examination of bone regulation in a Pappa2 deletion mouse model, Christians et al. (2019) showed that PAPP-A2 plays a role in bone structure and mass through a possible regulation of IGFBP-5 in a sex- and age- specific manner (Christians et al., 2019).
In further biochemical studies, Fujimoto et al. (2019) monitored circulating levels of intact IGFBP-3 as a measure of PAPP-A2 activity. As such, serum collected from the KI homozygous mice showed significant increases in intact IGFBP-3 recapitulating the human increases in total IGFBP-3 seen in those with PAPP-A2 deficiency. However, Christians et al. (2015) did report elevations of IGFBP-5 but IGFBP-3 levels showed an approximate 15-fold decrease in KO mice compared to WT controls. As of today, there is no sufficient explanation for these differences in IGFBP-3 levels between models.
To follow up on the human data suggesting impaired glucose tolerance, Fujimoto et al. (2019) performed glucose and insulin tolerance tests (GTT and ITT) in the KI mouse model. In GTT, homozygous KI mice presented significantly elevated glucose levels after glucose injection (glucose values; 422 mg/dL (KI-M), 307 mg/dL (KI-F); 323 mg/dL (WT-M), 234 mg/dL (WT-F)). In ITT, homozygous KI mice did not decrease blood glucose significantly compared to WT after insulin injection (the lowest glucose values; 221 mg/dL (KI-M), 175 mg/dL (KI-F); 125 mg/dL (WT-M), 119 mg/dL (WT-F)). Therefore, both insulin resistance and glucose intolerance were noted in homozygous KI mice. KI male and female mice both show significant decreases in % lean mass and increased levels of % fat mass. These changes in lean muscle and fat mass correspond with insulin resistance in humans and mirror the changes seen in the PAPP-A2 deficient humans (Cabrera-Salcedo et al., 2017). In sum, the totality of the mouse data supports a causal role of PAPP-A2 deficiency in short stature and associated metabolic derangements seen in the human patients.
4.2. Relationship between PAPP-A2 and GH–IGF axis related proteins in the general population
Recently, Fujimoto et al. (2020) and DiPrisco et al. (2019) assayed PAPP-A2 levels throughout childhood or at birth and examined the relationship between PAPP-A2 levels and GH-related proteins (DiPrisco et al., 2019,Fujimoto et al., 2020). PAPP-A2 levels consistently decreased throughout childhood (3–18 years) (Age, 50%ile value [male, female] (ng/mL); 3.5 year-old, 0.345, 0.334; 17.5 year-old, 0.104, 0.132). Mean cord blood PAPP-A2 levels in infants were 2.2 ng/mL. However, in adulthood (20–70 years), PAPP-A2 levels were positively correlated with age (Steinbrecher et al., 2017). In short, these findings suggest that PAPP-A2 levels show a U-shaped curve throughout the lifespan. As expected, PAPP-A2 had a strong inverse correlation with serum intact IGFBP-3 levels, while positively correlated with the percentage of free to total IGF-1 in childhood (Fujimoto et al., 2020). This supports PAPP-A2’s role in regulating IGF-1 levels on a larger population wide scale.
DiPrisco et al. (2019) reported that PAPP-A2 levels in cord blood inversely associated with birth weight and length (DiPrisco et al., 2019). PAPP-A2, even in adjusted linear regression models for gestational age, race, sex, delivery mode, maternal age, and Apgar scores, was inversely associated with birth length Z-score (β = −0.279, P = 0.017). Cord blood total IGF-1 levels in small for gestational age (SGA) infants was significantly lower than in appropriate for gestational age (AGA) infants as previously reported (Randhawa, 2008,Tzschoppe et al., 2015). In contrast, PAPP-A2 levels in the SGA group were significantly higher than in the AGA group. This unexpected finding may be a compensatory response to increase the percentage of free IGF-1 in these children with low IGF-1 and growth impairment.
5. Conclusions
PAPP-A2 has recently been recognized as an important component to the GH–IGF axis. It appears to play a key role in regulating the percentage of free IGF-1 through its actions as a protease of IGFBP-3 and IGFBP-5. PAPP-A2 deficiency results in growth impairment in humans with expected derangements in biochemical parameters including increased levels of total IGF-1, IGFBP-3, ALS, and growth hormone. Mouse models now exist which recapitulate the human phenotype of PAPP-A2 deficiency. Early studies exploring the role of PAPP-A2 in the general population provide supportive evidence for it being an important player in growth physiology.
Highlights:
PAPP-A2, an IGFBP-3 and -5 protease, is a key regulator of free IGF-1.
PAPP-A2 deficiency causes growth failure with high total IGF-1 and IGFBP-3 levels.
PAPP-A2 levels positively correlate with the percentage of free IGF-1 in childhood.
rhIGF-1 treatment may improve PAPP-A2 deficient patients’ growth and metabolism.
Funding
This work was supported by the National Institutes of Health (NIH), National Institute of Child Health and Human Development (NICHD) [grant numbers R01HD093622] to A.D.
Abbreviations
- AGA
appropriate for gestational age
- GH
growth hormone
- GTT
glucose tolerance test
- IGFBP
insulin-like growth factor-binding protein
- ITT
insulin tolerance test
- KI
knock-in
- KO
knock-out
- PAPP-A
Pregnancy-associated plasma protein-A
- PAPP-A2
Pregnancy-associated plasma protein-A2
- SGA
small-for-gestational age
- STC
Stanniocalcin
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
M.F and M.A have nothing to disclose. A.D has a patent pending for the use of recombinant PAPPA2 as a growth promoting agent.
References
- Amiri N and Christians JK, 2015. PAPP-A2 expression by osteoblasts is required for normal postnatal growth in mice, Growth Horm IGF Res. (2015), 25, 274–280. 10.1016/j.ghir.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Andrew M, Liao L, Fujimoto M et al. , 2018. PAPPA2 as a Therapeutic Modulator of IGF-I Bioavailability: in Vivo and in Vitro Evidence, J Endocr Soc (2018), 2, 646–656. 10.1210/js.2018-00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente J, Chowen JA, Perez-Jurado LA et al. , 2017. One level up: abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology, EMBO Mol Med. (2017), 9, 1338–1345. 10.15252/emmm.201707950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente J, Tatton-Brown K, Lehwalder D et al. , 2019. Genetics of Growth Disorders—Which Patients Require Genetic Testing?, Frontiers in Endocrinology. (2019), 10 10.3389/fendo.2019.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt HB, Overgaard MT, Laursen LS et al. , 2001. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin, Biochem J. (2001), 358, 359–367. https://www.ncbi.nlm.nih.gov/pubmed/11513734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Salcedo C, Mizuno T, Tyzinski L et al. , 2017. Pharmacokinetics of IGF-1 in PAPP-A2-Deficient Patients, Growth Response, and Effects on Glucose and Bone Density, J Clin Endocrinol Metab. (2017), 102, 4568–4577. 10.1210/jc.2017-01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AC, Hook J, Lemckert FA et al. , 2008. The murine stanniocalcin 2 gene is a negative regulator of postnatal growth, Endocrinology. (2008), 149, 2403–2410. 10.1210/en.2007-1219. [DOI] [PubMed] [Google Scholar]
- Chang AC, Jeffrey KJ, Tokutake Y et al. , 1998. Human stanniocalcin (STC): genomic structure, chromosomal localization, and the presence of CAG trinucleotide repeats, Genomics. (1998), 47, 393–398. 10.1006/geno.1997.5120. [DOI] [PubMed] [Google Scholar]
- Chen JW, Ledet T, Orskov H et al. , 2003. A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum, Am J Physiol Endocrinol Metab. (2003), 284, E1149–1155. 10.1152/ajpendo.00410.2002. [DOI] [PubMed] [Google Scholar]
- Christians JK, Bath AK and Amiri N, 2015. Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity, Growth Horm IGF Res. (2015), 25, 232–239. 10.1016/j.ghir.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Christians JK, Lennie KI, Huicochea Munoz MF et al. , 2018. PAPP-A2 deficiency does not exacerbate the phenotype of a mouse model of intrauterine growth restriction, Reprod Biol Endocrinol. (2018), 16, 58 10.1186/s12958-018-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Boldt HB, Bale LK et al. , 2011. Pregnancy-associated plasma protein-A2 (PAPP-A2): tissue expression and biological consequences of gene knockout in mice, Endocrinology. (2011), 152, 2837–2844. 10.1210/en.2011-0036. [DOI] [PubMed] [Google Scholar]
- Dauber A, Munoz-Calvo MT, Barrios V et al. , 2016. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability, EMBO Mol Med. (2016), 8, 363–374. 10.15252/emmm.201506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPrisco B, Kumar A, Kalra B et al. , 2019. Placental proteases PAPP-A and PAPP-A2, the binding proteins they cleave (IGFBP-4 and -5), and IGF-I and IGF-II: Levels in umbilical cord blood and associations with birth weight and length, Metabolism. (2019), 100, 153959 10.1016/j.metabol.2019.153959. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Andrew M, Liao L et al. , 2019. Low IGF-I Bioavailability Impairs Growth and Glucose Metabolism in a Mouse Model of Human PAPPA2 p.Ala1033Val Mutation, Endocrinology. (2019), 160, 1363–1376. 10.1210/en.2018-00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Khoury JC, Khoury PR et al. , 2020. Anthropometric and biochemical correlates of PAPP-A2, free IGF-I, and IGFBP-3 in childhood, Eur J Endocrinol. (2020), 182, 363–374. 10.1530/EJE-19-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi AD, Kuo EY, Raulic S et al. , 2005. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs, Am J Physiol Endocrinol Metab. (2005), 288, E92–105. 10.1152/ajpendo.00268.2004. [DOI] [PubMed] [Google Scholar]
- Hawkins-Carranza FG, Munoz-Calvo MT, Martos-Moreno GA et al. , 2018 rhIGF-1 Treatment Increases Bone Mineral Density and Trabecular Bone Structure in Children with PAPP-A2 Deficiency, Horm Res Paediatr. (2018), 89, 200–204. 10.1159/000486336. [DOI] [PubMed] [Google Scholar]
- Jepsen MR, Kloverpris S, Mikkelsen JH et al. , 2015. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis, J Biol Chem. (2015), 290, 3430–3439. 10.1074/jbc.M114.611665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloverpris S, Gaidamauskas E, Rasmussen LC et al. , 2013. A robust immunoassay for pregnancy-associated plasma protein-A2 based on analysis of circulating antigen: establishment of normal ranges in pregnancy, Mol Hum Reprod. (2013), 19, 756–763. 10.1093/molehr/gat047. [DOI] [PubMed] [Google Scholar]
- Kloverpris S, Mikkelsen JH, Pedersen JH et al. , 2015. Stanniocalcin-1 Potently Inhibits the Proteolytic Activity of the Metalloproteinase Pregnancy-associated Plasma Protein-A, J Biol Chem. (2015), 290, 21915–21924. 10.1074/jbc.M115.650143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lango Allen H, Estrada K, Lettre G et al. , 2010 Hundreds of variants clustered in genomic loci and biological pathways affect human height, Nature. (2010), 467, 832–838. 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Weyer K et al. , 2002. Cell surface targeting of pregnancy-associated plasma protein A proteolytic activity. Reversible adhesion is mediated by two neighboring short consensus repeats, J Biol Chem. (2002), 277, 47225–47234. 10.1074/jbc.M209155200. [DOI] [PubMed] [Google Scholar]
- Marouli E, Graff M, Medina-Gomez C et al. , 2017. Rare and low-frequency coding variants alter human adult height, Nature. (2017), 542, 186–190. 10.1038/nature21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Calvo MT, Barrios V, Pozo J et al. , 2016. Treatment With Recombinant Human Insulin-Like Growth Factor-1 Improves Growth in Patients With PAPP-A2 Deficiency, J Clin Endocrinol Metab. (2016), 101, 3879–3883. 10.1210/jc.2016-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard MT, Boldt HB, Laursen LS et al. , 2001. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase, J Biol Chem. (2001), 276, 21849–21853. 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- Oxvig C, 2015. The role of PAPP-A in the IGF system: location, location, location, J Cell Commun Signal. (2015), 9, 177–187. 10.1007/s12079-015-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal BR, Phillips PE, Matthews DR et al. , 1992. Contrasting metabolic effects of continuous and pulsatile growth hormone administration in young adults with type 1 (insulin-dependent) diabetes mellitus, Diabetologia. (1992), 35, 542–549. 10.1007/BF00400482. [DOI] [PubMed] [Google Scholar]
- Randhawa RS, 2008. The insulin-like growth factor system and fetal growth restrictionn, Pediatr Endocrinol Rev. (2008), 6, 235–240. https://www.ncbi.nlm.nih.gov/pubmed/19202510. [PubMed] [Google Scholar]
- Renes JS, van Doorn J and Hokken-Koelega ACS, 2019. Current Insights into the Role of the Growth Hormone-Insulin-Like Growth Factor System in Short Children Born Small for Gestational Age, Horm Res Paediatr. (2019), 92, 15–27. 10.1159/000502739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HL, Jackson NC, Shojaee-Moradie F et al. , 2004. Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes, J Clin Endocrinol Metab. (2004), 89, 425–432. 10.1210/jc.2003-031274. [DOI] [PubMed] [Google Scholar]
- Sorensen JS, Birkebaek NH, Bjerre M et al. , 2015. Residual beta-cell function and the insulin-like growth factor system in Danish children and adolescents with type 1 diabetes, J Clin Endocrinol Metab. (2015), 100, 1053–1061. 10.1210/jc.2014-3521. [DOI] [PubMed] [Google Scholar]
- Steinbrecher A, Janke J, Poy MN et al. , 2017. Pregnancy-Associated Plasma Protein-A2 and Anthropometry, Lifestyle, and Biochemical Factors in a Human Adult Population, Sci Rep (2017), 7, 10455 10.1038/s41598-017-10629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschoppe A, Riedel C, von Kries R et al. , 2015. Differential effects of low birthweight and intrauterine growth restriction on umbilical cord blood insulin-like growth factor concentrations, Clin Endocrinol (Oxf). (2015), 83, 739–745. 10.1111/cen.12844. [DOI] [PubMed] [Google Scholar]
- Vijayakumar A, Novosyadlyy R, Wu Y et al. , 2010. Biological effects of growth hormone on carbohydrate and lipid metabolism, Growth Horm IGF Res. (2010), 20, 1–7. 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qiu Q, Haider M et al. , 2009. Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse, J Endocrinol. (2009), 202, 337–345. 10.1677/JOE-09-0136. [DOI] [PubMed] [Google Scholar]
- Yakar S, Werner H and Rosen CJ, 2018. Insulin-like growth factors: actions on the skeleton, J Mol Endocrinol. (2018), 61, T115–T137. 10.1530/JME-17-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen K, Frystyk J, Umpleby M et al. , 2004. Changes in free rather than total insulin-like growth factor-I enhance insulin sensitivity and suppress endogenous peak growth hormone (GH) release following short-term low-dose GH administration in young healthy adults, J Clin Endocrinol Metab. (2004), 89, 3956–3964. 10.1210/jc.2004-0300. [DOI] [PubMed] [Google Scholar]
- Yuen KC and Dunger DB, 2007. Therapeutic aspects of growth hormone and insulin-like growth factor-I treatment on visceral fat and insulin sensitivity in adults, Diabetes Obes Metab. (2007), 9, 11–22. 10.1111/j.1463-1326.2006.00591.x. [DOI] [PubMed] [Google Scholar]


