Abstract
Neuropsychiatric symptoms are common in Parkinson’s disease (PD). We investigated the relationship between neuropsychiatric symptoms and current and future diagnosis of PD dementia (PDD). Individuals with PD who had a study partner were enrolled (n=696). Study partners were administered the Neuropsychiatric Inventory or Neuropsychiatric Inventory Questionnaire at baseline. Participants were assigned a cognitive diagnosis at baseline and follow up visits. Hallucinations were significantly associated with a diagnosis of PDD cross-sectionally (p<0.001) and with shortened time to dementia longitudinally among initially nondemented participants (n=444; p=0.005). Screening for hallucinations may be useful for assessing risk of dementia in participants with PD.
Keywords: Behavioral Symptoms, Cognition, Dementia, Hallucinations, Parkinson Disease
INTRODUCTION
Parkinson’s disease dementia (PDD) strongly contributes to reduced quality of life, caregiver distress, higher rate of institutionalization, and mortality [1, 2]. Reported point prevalence of PDD ranges from 25-40% [3, 4]; approximately 80% of patients with PD will progress to dementia over the course of their disease [5, 6]. Identifying individuals who are at a higher risk of developing PDD may lessen the disease burden by planning appropriate interventions with regard to medication management and safety issues, as well as permitting patients and caregivers to make arrangements for the future. Consequently, an effort has been made to detect factors that contribute to or predict the development of PDD [2]. Patients with PDD may demonstrate a variety of psychiatric symptoms, such as hallucinations, delusions, depression, and anxiety. Some of these symptoms might precede the development of, and thus serve as a potential marker for, dementia. In our study we aimed to detect the association between neuropsychiatric variables and current and future diagnosis of dementia in patients with PD.
MATERIALS AND METHODS
Subjects
Participants (n = 696) were enrolled in the Pacific Udall Center (University of Washington/Veterans Affairs Puget Sound Health Care System, Oregon Health and Science University/Veterans Affairs Portland Health Care System, or Stanford University) or the Udall Center at the University of Pennsylvania, had a study partner, and met United Kingdom PD Society Brain Bank clinical diagnostic criteria for PD. Two participants were excluded due to an “unknown” cognitive diagnosis at baseline. Participants completing at least one follow up visit were included in the longitudinal analyses (n = 444). Participants were evaluated on average every 1.5 years (range 0.9 – 4.7 years). The institutional review boards at each site provided formal approval for the study procedures. All participants (or legally authorized representative) and study partners provided written informed consent.
Assessments
Study partners completed a baseline Neuropsychiatric Inventory (NPI) [7] or Neuropsychiatric Inventory Questionnaire (NPI-Q) [8], providing yes/no responses to regarding the presence of delusions, hallucinations, agitation, depression, anxiety, elation, apathy, disinhibition, irritability, nighttime behaviors, and eating abnormalities. Participants were administered the Movement Disorder Society revision of the Unified PD Rating Scale (MDS-UPDRS), part III [9], Modified Hoehn and Yahr [10], 15-item Geriatric Depression Scale (GDS-15) [11], Montreal Cognitive Assessment (MoCA) [12], and a full neuropsychological battery (Table S.1.) [13, 14]. Participants were genotyped for the APOE ε4 allele and underwent full sequencing of the GBA coding region (combining mutations and E326K into a single group) as previously described [15, 16]. Motor and cognitive diagnoses were assigned at a clinical consensus conference; cognitive diagnoses were made using published diagnostic criteria for PDD and PD with mild cognitive impairment (PD-MCI) as previously described [13].
Statistical Analysis
Logistic regression models tested whether each NPI/NPI-Q variable predicted baseline cognitive status (nondemented vs. PDD). Next, all NPI/NPI-Q variables were entered into a single logistic regression to determine if any variables were associated with PDD over and above the other variables. Logistic regression analyses were then conducted on the initially nondemented sample to determine whether baseline NPI/NPI-Q variables predicted conversion to PDD. Cox regression analyses were conducted to determine whether NPI/NPI-Q variables at baseline predicted time to PDD. Kaplan-Meier estimates were calculated, and log-rank tests were performed to determine whether there was a significant difference between the curves. To determine whether longitudinal associations were impacted by a higher rate of baseline PD-MCI in the PDD conversion group, analyses were run separately controlling for baseline cognitive diagnosis (no cognitive impairment, PD-MCI). Covariates included age, education, sex, PD motor symptom duration, total levodopa equivalent daily dose [17], MDS-UPDRS part III, GDS-15, and study site. Longitudinal analyses additionally controlled for length of follow up and number of study visits. Secondary analyses were conducted that included genetic status (APOE ε4 or GBA variant) and MoCA (to account for the full range of cognitive performance in the nondemented group). The Bonferroni adjustment was used to control the family wise type I error set a priori at 0.05; since there were 10 NPI variables, a significance level of 0.05/10 = 0.005 was used. All analyses were performed in Stata 15.1.
RESULTS
Baseline associations between cognitive diagnosis and NPI variables
Baseline participant characteristics are provided in the Supplement (Table S.2.). There was a significant association between a diagnosis of PDD and the presence of delusions, hallucinations, anxiety, apathy, and disinhibition (Table 1). When genetic status (APOE or GBA) was included, the results did not significantly change. When the MoCA was included, the relationship between PDD and apathy was no longer statistically significant, suggesting that apathy was more associated with global cognition than with PDD diagnosis per se.
Table 1.
Associations between NPI variables and cognitive diagnosis
| Nondemented N (%) |
PDD N (%) |
OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Delusions | 14 (2.4) | 18 (16.7) | 7.3 | 3.0 – 17.7 | <0.001† |
| Hallucinations | 43 (7.3) | 40 (37.4) | 7.5 | 4.0 – 14.1 | <0.001† |
| Agitation | 75 (12.8) | 30 (27.8) | 1.9 | 1.1 - 3.5 | 0.029 |
| Depression | 203 (34.6) | 52 (48.2) | 1.5 | 0.9 – 2.4 | 0.142 |
| Anxiety | 94 (16.0) | 33 (30.6) | 2.9 | 1.7 – 5.0 | <0.001† |
| Elation | 11 (1.9) | 5 (4.6) | 3.1 | 0.9 – 10.3 | 0.069 |
| Apathy | 103 (17.6) | 48 (44.4) | 2.2 | 1.3 – 3.6 | 0.004† |
| Disinhibition | 46 (7.9) | 20 (18.5) | 2.8 | 1.4 – 5.5 | 0.002† |
| Irritability | 104 (17.8) | 32 (29.6) | 1.9 | 1.1 – 3.4 | 0.022 |
| Aberrant Motor Behavior | 39 (6.7) | 17 (15.7) | 1.8 | 0.9 – 3.8 | 0.108 |
Significant after correcting for multiple comparisons (p<0.005)
Abbreviations: CI, confidence interval; NPI, Neuropsychiatric Inventory; OR, odds ratio; PDD, Parkinson’s disease dementia
When all NPI variables were entered into a single logistic regression, only hallucinations were associated with a PDD diagnosis over and above all other variables (odds ratio=5.4 95% confidence interval (CI) 2.7 – 10.8, p<0.001). This did not change when MoCA, GBA status, or APOE ε4 genotype were entered.
Associations between conversion to PDD and NPI variables
Of the participants who were non-demented at baseline, 444 (76%) completed at least one follow up visit. Mean length of follow up was 4.5 (SD=2.6) years; PDD converters did not significantly differ from nonconverters in length of follow up. Baseline characteristics for PDD converters and non-converters are provided in the Supplement (Table S.3.).
Comparison of baseline NPI/NPI-Q variables between converters and nonconverters yielded no significant differences. However, time to convert to dementia was significantly shorter for those participants whose study partners reported hallucinations at baseline (hazard ratio [HR] = 2.4 95% CI 1.3 – 4.3, p=0.005; Figure 1). The log rank test indicated the difference in Kaplan-Meier survival curves was statistically significant (χ2 = 11.84, p=0.0006). When baseline cognitive status was included as a covariate, the HR was 2.2, 95% CI 1.2 – 4.0, p = 0.013. These results did not change significantly when genetic variables were entered. When the analyses were performed only on those participants with a MoCA available (n=298), the results were nonsignificant whether or not the MoCA was included, suggesting that smaller sample size may have precluded detection of statistically significant differences.
Figure 1.
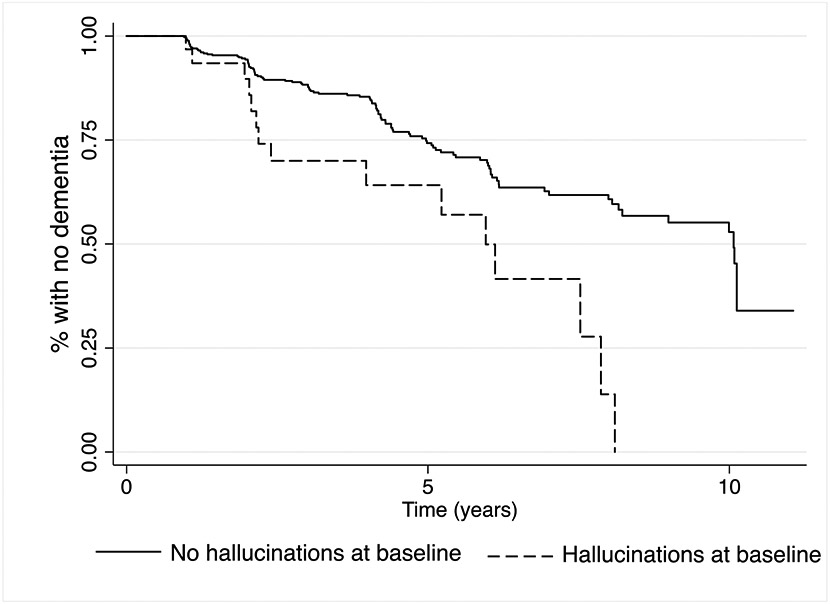
Kaplan-Meier survival estimates of time to dementia for initially non-demented participants with and without study partner reported hallucinations at baseline
DISCUSSION
This study examined the relationship between study partner reported psychiatric symptoms and dementia in participants with PD. Baseline analyses demonstrated that, while PDD was associated with many neuropsychiatric features, the presence of hallucinations had the strongest association over and above other psychiatric variables. Hallucinations were also the only symptom associated with a shortened time to PDD in initially non-demented participants. Although past studies showed strong cross-sectional associations between hallucinations and dementia of PD [1, 18-24] as well as associations with global cognitive decline in PD [25], ours is the first to explicitly demonstrate decreased time to dementia diagnosis in initially non-demented PD individuals with hallucinations.
It is well-recognized that individuals with PDD may experience pronounced hallucinations as part of the disease process amongst other neuropsychiatric problems [26-28]. Our analyses corroborated this finding by showing that although multiple neuropsychiatric variables were associated with a baseline diagnosis of PDD (delusions, hallucinations, anxiety, apathy, and disinhibition), only hallucinations were associated with PDD when correcting for other variables. The current literature suggests that the underlying process responsible for development of hallucinations is interconnected with a mechanism of cognitive deterioration in PD patients, albeit the pathophysiology is not yet fully understood [18, 22-24, 27].
Our research demonstrates that time to convert to dementia was significantly shorter for the non-demented participants whose study partners reported hallucinations at baseline. Thus, the presence of hallucinations in PD may foreshadow more insidious disease progression. Management of dementia and its accompanying symptoms necessitates multidisciplinary and multilayered approaches which take more time, effort, and resources [29]. Therefore, it is important to anticipate and recognize these symptoms early, for example with screening tools such as NPI, in order set up a care plan that is best fitted for the affected patient.
Unfortunately based on the NPI/NPI-Q, we were unable to differentiate between types of hallucinations, which might be important, as there are studies that link certain cognitive domain deficits with visual hallucinations specifically as opposed to other forms of hallucinations [19, 22]. Additionally, the NPI/NPI-Q are completed by the study partner rather than patient, leading to possible inability to detect earlier onset and subtler forms of hallucinations. Finally, MoCA scores were not available for a substantial proportion of participants, making it difficult to distinguish whether hallucinations were a primary factor influencing the rate of decline to PDD or developed in conjunction with worsening cognitive impairment. However, when baseline cognitive diagnosis was controlled for in the larger sample, faster time to dementia was still seen among those with hallucinations at baseline.
Our research demonstrates a significant association between multiple neuropsychiatric symptoms and PDD, and hallucinations in non-demented participants predicted a shorter time to PDD. Our study substantiates the link between psychiatric symptoms, especially hallucinations, and dementia in PD. Psychiatric screening batteries such as the NPI/NPI-Q may provide useful information to clinicians by alerting them to non-motor symptoms that may herald impending dementia. Additional work to determine factors that underlie the association between hallucinations and cognitive decline are important future steps.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Neurological Disorders and Stroke (P50 NS062684) and Department of Veterans Affairs (101 CX001702). Data contributed by the current project Center On Alpha-Synuclein Strains in Alzheimer’s Disease & Related Dementias at the Perelman School of Medicine at the University of Pennsylvania (U19 AG062418, Trojanowski JQ – PI; Weintraub D; Chen-Plotkin A; Dahodwala N; Morley JF; Duda JE) and former Morris K. Udall Center at the Perelman School of Medicine at the University of Pennsylvania (P50 NS053488, Trojanowski JQ – PI). Dr. Gryc and Dr. Roberts were supported by the Veterans Affairs Advanced Fellowship Program in Parkinson's Disease. We sincerely thank all of the individuals who participated in this study.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to declare.
REFERENCES
- [1].Anang JBM, Gagnon J-F, Bertrand J-A, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB (2014) Predictors of dementia in Parkinson disease. Neurology 83, 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Levy G, Tang M-X, Louis ED, Côté LJ, Alfaro B, Mejia H, Stern Y, Marder K (2002) The association of incident dementia with mortality in PD. Neurology 59, 1708–1713. [DOI] [PubMed] [Google Scholar]
- [3].Aarsland D, Zaccai J, Brayne C (2005) A systematic review of prevalence studies of dementia in Parkinson's disease. Movement Disorders 20, 1255–1263. [DOI] [PubMed] [Google Scholar]
- [4].Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders 22, 1689–1707. [DOI] [PubMed] [Google Scholar]
- [5].Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P (2003) Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Archives of Neurology 60, 387–392. [DOI] [PubMed] [Google Scholar]
- [6].Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 23, 837–844. [DOI] [PubMed] [Google Scholar]
- [7].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [8].Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST (2000) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12, 233–239. [DOI] [PubMed] [Google Scholar]
- [9].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- [10].Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, Movement Disorder Society Task Force on Rating Scales for Parkinson's D (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19, 1020–1028. [DOI] [PubMed] [Google Scholar]
- [11].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [12].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [13].Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu SC, Edwards KL, Montine TJ, Leverenz JB (2013) Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis 3, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Watson GS, Cholerton BA, Gross RG, Weintraub D, Zabetian CP, Trojanowski JQ, Montine TJ, Siderowf A, Leverenz JB (2013) Neuropsychologic assessment in collaborative Parkinson's disease research: a proposal from the National Institute of Neurological Disorders and Stroke Morris K. Udall Centers of Excellence for Parkinson's Disease Research at the University of Pennsylvania and the University of Washington. Alzheimers Dement 9, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, Ritz B, Rausch R, Factor SA, Wood-Siverio C, Quinn JF, Chung KA, Peterson-Hiller AL, Goldman JG, Stebbins GT, Bernard B, Espay AJ, Revilla FJ, Devoto J, Rosenthal LS, Dawson TM, Albert MS, Tsuang D, Huston H, Yearout D, Hu SC, Cholerton BA, Montine TJ, Edwards KL, Zabetian CP (2016) GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov Disord 31, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Hurtig HI, Van Deerlin VM, Ritz B, Rausch R, Rhodes SL, Factor SA, Wood-Siverio C, Quinn JF, Chung KA, Peterson AL, Espay AJ, Revilla FJ, Devoto J, Hu SC, Cholerton BA, Wan JY, Montine TJ, Edwards KL, Zabetian CP (2014) APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol 71, 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25, 2649–2653. [DOI] [PubMed] [Google Scholar]
- [18].Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel-Larsen T, Lolk A, Kragh-Sørensen P (2004) The Rate of Cognitive Decline in Parkinson Disease. Archives of Neurology 61, 1906–1911. [DOI] [PubMed] [Google Scholar]
- [19].Creese B, Albertyn CP, Dworkin S, Thomas RS, Wan YM, Ballard C (2018) Executive function but not episodic memory decline associated with visual hallucinations in Parkinson's disease. Journal of Neuropsychology. [DOI] [PubMed] [Google Scholar]
- [20].Forsaa EB, Larsen JP, Wentzel-Larsen T, Goetz CG, Stebbins GT, Aarsland D, Alves G (2010) A 12-Year Population-Based Study of Psychosis in Parkinson Disease. Archives of Neurology 67, 996–1001. [DOI] [PubMed] [Google Scholar]
- [21].Kandiah N, Narasimhalu K, Lau P-N, Seah S-H, Au WL, Tan LCS (2009) Cognitive decline in early Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society 24, 605–608. [DOI] [PubMed] [Google Scholar]
- [22].Ramirez-Ruiz B, Junque C, Marti M-J, Valldeoriola F, Tolosa E (2007) Cognitive changes in Parkinson's disease patients with visual hallucinations. Dementia and Geriatric Cognitive Disorders 23, 281–288. [DOI] [PubMed] [Google Scholar]
- [23].Santangelo G, Trojano L, Vitale C, Ianniciello M, Amboni M, Grossi D, Barone P (2007) A neuropsychological longitudinal study in Parkinson's patients with and without hallucinations. Movement Disorders 22, 2418–2425. [DOI] [PubMed] [Google Scholar]
- [24].Zhu K, van Hilten JJ, Marinus J (2014) Predictors of dementia in Parkinson's disease; findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism & Related Disorders 20, 980–985. [DOI] [PubMed] [Google Scholar]
- [25].Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC, Parkinson Study Group DI (2009) Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 73, 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Emre M (2003) Dementia associated with Parkinson's disease. The Lancet Neurology 2, 229–237. [DOI] [PubMed] [Google Scholar]
- [27].Fénelon G (2008) Psychosis in Parkinson's disease: Phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS spectrums 13, 18–25. [DOI] [PubMed] [Google Scholar]
- [28].Hanagasi HA, Tufekcioglu Z, Emre M (2017) Dementia in Parkinson's disease. Journal of the Neurological Sciences 374, 26–31. [DOI] [PubMed] [Google Scholar]
- [29].Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM (2013) Monetary costs of dementia in the United States. N Engl J Med 368, 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


