Abstract
BACKGROUND:
Children born extremely preterm whose placenta had histologic evidence of chorioamnionitis have early brain dysfunction, but little is known about neurological development at 10 years of age.
OBJECTIVE:
We investigated the association between histologic chorioamnionitis and neurodevelopmental impairment at 10 years among children born <28 weeks gestation (extremely preterm).
METHODS:
The multicenter Extremely Low Gestational Age Newborn (ELGAN) Study enrolled extremely preterm newborns from 2002–2004 at 14 U.S. hospitals. Chorioamnionitis was defined by histologic stage (early, moderate, and advanced) and grade (mild/moderate and severe) of chorionic plate and umbilical cord inflammation. The children were evaluated for cerebral palsy at 2 years, and autism spectrum disorder, cognitive impairment (intelligence quotient >2 standard deviations below the mean), and epilepsy at age 10 years by blinded evaluators using validated measures. Multivariable logistic regression with generalized estimating equations was used.
RESULTS:
Among 805 placenta, 43% (347/805) had histologic chorioamnionitis by moderate or advanced maternal stage, 36% (286/805) by severe maternal grade, 18% (132/737) by moderate or advanced fetal stage, and 1% (10/737) by severe fetal grade. The frequencies of impairments were 11% (88/767) for cerebral palsy, 7% (56/773) for autism spectrum disorder, 15% (120/788) for cognitive impairment, and 7% (52/763) for epilepsy. Adjusted for maternal age, body mass index, race, insurance status, maternal education, tobacco use, infant sex, and multiple gestations, the adjusted odds (AOR) for the association between histologic chorioamnionitis and cerebral palsy years was increased with advanced maternal stage (AOR 2.5, 95% CI 1.6 to 3.9), severe maternal grade (AOR 2.0, 95% CI 1.2 to 3.4), moderate fetal stage (AOR 2.20, 95% CI 2.1 to 2.2), and mild or moderate fetal grade (AOR 1.5, 95% CI 1.0 to 2.2). Similarly, the AOR for the association between histologic chorioamnionitis and epilepsy was increased with advanced maternal stage (AOR 1.5, 95% CI 1.3 to 1.6) and severe fetal grade (AOR 5.9, CI 1.9 to 17.8). In addition, the AOR for the association between histologic chorioamnionitis and autism spectrum disorder was increased with mild or moderate fetal grade (AOR 1.7, 95% CI 1.0 to 2.9). Histologic chorioamnionitis was not associated with cognitive impairment. These findings held after adjustment for gestational age at delivery. In contrast to histological chorioamnionitis, a clinical diagnosis of chorioamnionitis was not associated with neurodevelopmental impairment.
CONCLUSION:
Histologic chorioamnionitis may be associated with some forms of neurodevelopmental impairment at 10 years of life among infants born <28 weeks gestation.
Keywords: Chorioamnionitis, pregnancy, infection outcomes, neurocognition, neurodevelopment, acute placenta inflammatory lesion, neurologic impairment, extremely preterm infant
INTRODUCTION
Histologic chorioamnionitis is a frequent obstetric complication, occurring in 3 to 5% of term placentas and 90% of placentas delivered at 21-to-24 weeks gestation.1 Infants born extremely preterm whose placenta has characteristics of chorioamnionitis are at an increased risk of adverse neurological outcomes documented during the first two postnatal years.2–7 Fortunately, what is seen at age 2 years does not always indicate what will be seen at an older age.8, 9 Furthermore, chorioamnionitis appears to be a treatable disorder when antibiotics are promptly initiated at diagnosis.10–12 Consequently, there is a need to investigate the association between chorioamnionitis and neurological outcomes at school age in these most vulnerable children.
Histologic chorioamnionitis is a biomarker of perinatal inflammation, and placental histopathology is the gold-standard to confirm its diagnosis.14 Chorioamnionitis leads to a fetal immune response syndrome,15 and DNA shed from the placenta can serve as a potential agent initiating the inflammatory response.16
Prior studies assessing the impact of chorioamnionitis on neurodevelopment have been limited by the lack of rigorous prospective ascertainment of neurological outcomes and chorioamnionitis and limited postnatal follow-up of 24 months. Having a greater understanding of the contribution of histologically-confirmed chorioamnionitis to this risk is needed.14, 17
In the current multi-center prospective study, we investigated the association between histologic chorioamnionitis and neurodevelopmental impairment at 10 years of age among children born <28 weeks gestation (i.e., extremely preterm). We refer to chorioamnionitis as any histologic evidence of the chorionic plate OR umbilical cord inflammation. We hypothesized that histologic chorioamnionitis defined using standardized criteria by histopathology would be associated with an increased risk of long-term neurodevelopmental impairment.
METHODS
Study setting:
The Extremely Low Gestational Age Newborns (ELGAN) Study is a multi-center, prospective observational study of the risk of structural and functional neurologic disorders in extremely preterm infants.18, 19 Between 2002–2004, women who delivered <28 weeks gestation at 14 sites in 11 cities in 5 states (Connecticut, Illinois, Massachusetts, Michigan, North Carolina) were asked to enroll in the study. Of 1,200 ELGAN Study survivors, 1,102 (92%) were evaluated at 2 years, and 966 surviving children were recruited at 10 years by mail and then by phone. The families of 889/966 (92%) of these children returned for the follow-up visit at age 10 and 874 children were assessed. This analysis included those with complete exposure and outcome data (n=805 with maternal stage and grade and n=737 with fetal stage and grade). Enrollment and study procedures for this follow-up study were approved by the Institutional Review Boards at all participating institutions.
Participants:
This 10-year evaluation assessed cognition, motor function, and behavior. While the child was tested, the parent or caregiver completed questionnaires regarding the child’s medical and neurological status and behavior. Evaluations lasted 3 to 4 hours and were administered by child psychologists who had undergone training and verification of competency for administering neurocognitive tests. Additionally, those evaluators who assessed for autism spectrum disorder received training in the administration and scoring of the Autism Diagnostic Interview-Revised (ADI-R) and Autism Diagnostic Observation Schedule-2 (ADOS-2) and established acceptable interrater reliability with the study autism expert.
Study exposure:
The primary exposure was a diagnosis of histologically-confirmed chorioamnionitis at the chorionic plate (i.e., maternal inflammatory response),1, 20 and histological evidence of fetal systemic inflammatory response based on umbilical cord inflammation or funisitis (i.e., fetal inflammatory response), which is consistent with the histological classification used in prior studies from this cohort.21, 22 A diagnosis of histologic chorioamnionitis was based on the presence of acute inflammation in either or both the fetal membranes and umbilical cord.23, 24 The exposure was quantified per the Society for Pediatric Pathology reporting guidelines based on maternal and fetal inflammatory response stage (none, 1=early, 2=moderate, and 3=advanced) and grade (none, 1=mild/moderate, and 2=severe).25 The fetal inflammatory response was assessed among the subset of placentas with histologic evidence of maternal inflammatory response. We secondarily assessed any severe inflammation defined as maternal or fetal stage 2 or 3 and/or grade 2. Of note, unlike the stage of inflammation, the grading scale for fetal inflammatory response in the cord (i.e., funisitis) at original pathologic assessment did not conform to the above schema.21 In that original grading system, high-grade was only applied when sections showed panvasculitis extending deep into Wharton’s jelly with or without necrosis. Since less severe lesions are now included in the category of grade 2 fetal inflammatory response, the grade of fetal response based on the original pathologic read was underestimated in a subset of cases in the current analysis.
Representative sections were taken from the umbilical cord (2 sections), a membrane roll, and from the paracentral zone of the placental disc (2 full thickness sections).26 After training on a teaching set of slides to minimize observer variability, study pathologists assessed for histologic characteristics using a standardized form.22, 27 Slides were read by the study pathologists at the time of enrollment.
Based on data suggesting that histologic chorioamnionitis but not clinical chorioamnionitis is a risk factor for cerebral palsy,5 we conducted secondary analyses defining clinical chorioamnionitis based on 1) documentation by the obstetrical provider on the discharge summary and 2) a white blood cell count >20,000 cells/mm3 and/or maternal intrapartum fever >101.4°F, albeit these thresholds are not necessarily consistent with contemporary criteria to define clinical chorioamnionitis but rather reflect threshold values used in original data collection.
Study outcomes:
We assessed the following four measures of neurodevelopmental impairment: 1) cerebral palsy, 2) autism spectrum disorder, 3) cognitive impairment (intellectual quotient [IQ] > 2 standard deviations below the mean), and 4) epilepsy. Cerebral palsy was diagnosed at 2 years adjusted age; the other three outcomes were assessed at 10 years of age. In the case of cerebral palsy, because of brain plasticity, some children lose their motor deficits by the age of 10. However, brain lesions may persist and are associated with cerebral dysfunction, including persistent cognitive effects and seizures.28, 29 Hence, in the current study, the cerebral palsy outcome was assessed at 2 years rather than at 10 years. All outcomes were assessed by blinded evaluators using validated measures.
To diagnose cerebral palsy, neurological examiners used a standardized manual and data collection instrument, including viewing an instructional compact disk to minimize examiner variability.30 Secondarily, we analyzed the association between chorioamnionitis and subtypes of cerebral palsy, namely hemiparesis, diparesis, and quadripareis, because the association between inflammation and cerebral palsy may vary across subtypes.31
To diagnose autism spectrum disorder, all children were initially screened by parent report with the Social Communication Questionnaire (SCQ),32 and those identified to be at risk were then assessed with the Autism Diagnostic Interview-Revised (ADI-R), an in-depth parent interview.33 Children meeting ADI-R modified criteria for autism spectrum disorder34 were then administered the Autism Diagnostic Observation Schedule-2 (ADOS-2).35 All children meeting standardized research criteria for autism spectrum disorder on both the ADI-R and ADOS-2 were classified as having autism spectrum disorder.
To diagnose cognitive impairment, IQ was assessed with the school-age Differential Ability Scale-II (DAS-II) Verbal and Nonverbal Reasoning scales.36, 37 Because both verbal and non-verbal scores were strongly correlated in this sample, the mean value was used as an estimate of overall IQ.38 An IQ score more than 2 standard deviations below the normal mean (i.e., <70) was defined as intellectual disability or cognitive impairment.
Finally, to diagnose epilepsy, identification of seizures involved a two-step process in which parents were asked to complete a validated seizure screen, and for those who screened positive, a structured interview by a pediatric epilepsy specialist was conducted.39, 40 A second epilepsy specialist independently reviewed and rated all events, and a third specialist served as a tie-breaker in the 3% of discordant evaluations. We defined epilepsy as having two or more unprovoked seizures.40
Statistical analyses:
We evaluated the association between the grade and stage of histologic chorioamnionitis and the four assessed measures of neurodevelopmental impairment, cerebral palsy, autism spectrum disorder, cognitive impairment, and epilepsy. The analysis for each individual outcome was restricted to those children for whom we had both exposure AND outcome data: cerebral palsy (N=767 for maternal stage/grade and 702 for fetal stage/grade), autism spectrum disorder (N=773 for maternal stage/grade and 706 for fetal stage/grade), cognitive impairment (N=788 for maternal stage/grade and 720 for fetal stage/grade), and epilepsy (N=763 for maternal stage/grade and 702 for fetal stage/grade). Multivariable logistic regression with generalized estimating equations (GEE) was used, adjusted for the clustering of twin infants. We calculated adjusted odds ratios (AORs) with 95% confidence intervals (95% CI). We a priori used a directed acyclic graph (DAG) to identify covariates to include in multivariable models.41 Models adjusted for maternal age, pre-pregnancy body mass index, self-reported race, insurance status, maternal education, tobacco use in pregnancy, and infant sex. In the primary analysis, we did not adjust for gestational age at delivery because it was considered to be a mediator on the causal pathway between chorioamnionitis and study outcomes rather than a confounder.42, 43 Multiple imputation was used to assess the effect of missing data.44 For each variable with missing data, we generated imputed values for the same study population and the same covariates as the previously described models assuming that variables were missing at random conditional on those covariates. Data imputation was performed for the following covariates: body mass index (3.4%), race (0.1%), education (3.4%), tobacco use (2.2%), and infant sex (1.3%).
In secondary analyses, we defined chorioamnionitis as 1) any severe inflammation by maternal and/or fetal stage 2 or 3 and/or grade 2; and 2) a clinical diagnosis per the discharge summary and then by white blood cell count and/or intrapartum fever. In sensitivity analyses, we re-performed the primary analysis 1) adjusting for gestational age at birth because while gestational age lies on the causal pathway between chorioamnionitis and outcomes, it also provides important information about the risk of adverse prenatal outcomes;45, 46 2) restricting the analysis to singletons after excluding multiple gestations; 3) adjusting for receipt of intrapartum antibiotics; and 4) excluding children with a diagnosis of cerebral palsy at 2 years but without gross motor function deficits at 10 years (n=18) because the outcome of cerebral palsy was defined at 2 years, as explained above. All statistical analyses were performed using STATA (STATACORP, version MP 15.1, College Station, TX).
RESULTS
Of 1,200 ELGAN Study survivors, 1,102 (92%) were evaluated at 2 years. Of these children, 87% (966/1,102) were recruited at 10 years by mail and then by phone. As previously described,47 those who did not participate at 10 years were more likely to have indicators of social disadvantage, including lower maternal education and receipt of public health insurance, but did not differ by gestational age at birth. The families of 92% (889/966) of eligible children returned for the 10-year assessment and 874 children were assessed (Figure 1), 95% (833/874) of whom had placenta data available. After excluding those participants with inadequate placental staging and grading data (n=28), the final study sample consisted of 805 participants with maternal stage and grade and 737 with fetal stage and grade. Participants in the final cohort were similar to the 874 assessed at 10 years by socio-demographic and clinical characteristics listed in Table 1.
Figure 1.
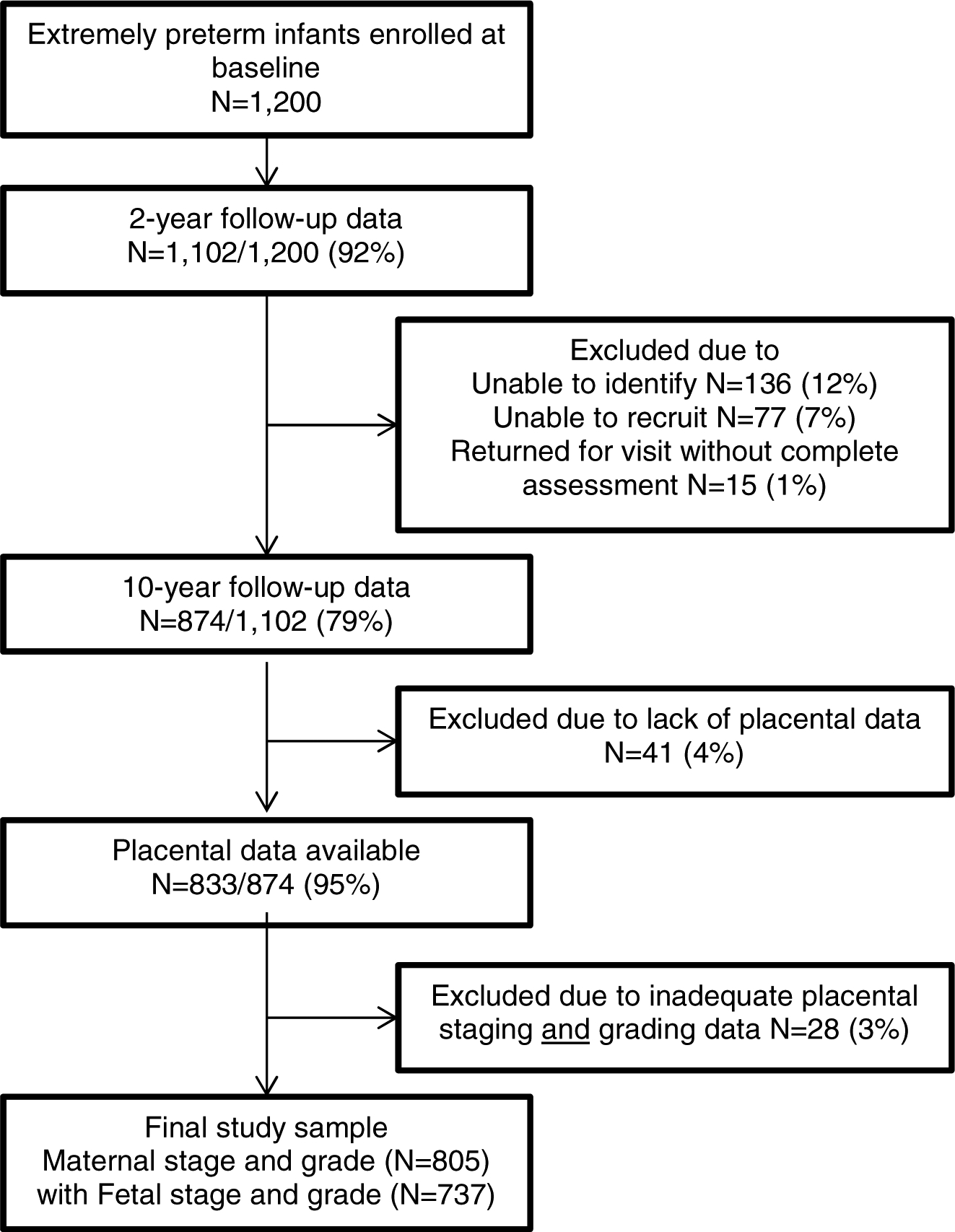
Flowchart of study participants
Table 1.
Socio-demographic and clinical characteristics of assessed women and their infants in the ELGAN cohort (N=805)
| n | % | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age | ||
| Race | ||
| Latina ethnicity | 75/802 | 9.3 |
| Pre-pregnancy body mass index, kg/m2 | ||
| Multifetal gestation | 282 / 805 | 35.0 |
| Education, years | ||
| Single marital status | 322 / 804 | 40.0 |
| Public insurance | 287 / 805 | 35.6 |
| Tobacco use during pregnancy | 109 / 787 | 13.8 |
| Mode of delivery, cesarean | 539 / 805 | 66.9 |
| Duration of labor, mean (SD), hours | 87.8 (201.97) | -- |
| Duration of rupture of membranes, hours | ||
| Newborn characteristics | ||
| Infant sex, female | 383 / 794 | 48.2 |
| 5 minute Apgar score <7 | 222 / 803 | 27.6 |
| Gestational age at birth, weeks | ||
| Birthweight, mean (SD), grams | 833.1 (196.61) | -- |
| NICU length of stay, mean (SD), days | 78.4 (47.24) | -- |
| Chorioamnionitis characteristics | ||
| Chorioamnionitis diagnosis on delivery discharge | 153 / 805 | 19.0 |
| Chorioamnionitis diagnosis as maternal fever >101.4F and/or WBC >20,000 cells/mm3 | 190 / 776 | 24.4 |
| Intrapartum antibiotic receipt | 522 / 805 | 64.8 |
| Maternal fever >101.4F | 44 / 778 | 5.6 |
| Maternal WBC >20,000 cells/mm3 | 162 / 795 | 20.3 |
Missing: race (n=1), ethnicity (n=3), body mass index (n=28), education (n=25), tobacco (n=18), sex (n=11), Apgar score (n=2), fever (n=27)
Abbreviations: WBC (white blood cell count), SD (standard deviation)
The mean maternal age at the time of delivery was 29.2 years (standard deviation, SD: 6.7) (Table 1). Most women (63%) were Caucasian and 9% identified as Latina ethnicity. The mean pre-pregnancy body mass index was 25.5 mg/k2 (SD: 6.9). Over a third (35%) were enrolled in public insurance during pregnancy, 35% had multifetal gestations, and 66% delivered by cesarean section. The mean gestational age at birth was 25.6 weeks (SD: 1.2), and the mean birthweight was 833 grams (SD: 196.6). Almost a half (48%) of infants were female. Over two-thirds of women (64%) received intrapartum antibiotics.
A total of 32% (91/805) of placentas were classified as moderate or advanced maternal stage and 36% (286/805) as severe maternal grade, and 18% (132/737) as moderate or advanced fetal stage and 1% (10/737) as severe fetal grade (Figure 2). Nearly two-thirds or 61% (212/344) of placentas with histologic evidence of chorioamnionitis at the maternal plate (i.e., maternal inflammatory response) had evidence of umbilical cord inflammation (i.e., fetal inflammatory response). The frequency of clinical chorioamnionitis as diagnosed by the obstetrician was 19% (153/805) and by white blood cell count >20,000 cells/mm3 and/or intrapartum fever >101.4°F was 24% (190/776). Overall, 86% (132/153) placentas with evidence of histologic chorioamnionitis by maternal stage/grade and 58% (83/141) by fetal stage/grade had a diagnosis of clinical chorioamnionitis.”
Figure 2.

Frequency of histologic chorioamnionitis by maternal and fetal stage and grade
Cerebral palsy occurred in 11% (88/767), autism spectrum disorder in 7% (56/773), cognitive impairment in 15% (120/788), and epilepsy in 6% (52/763) in the study sample. The frequency of cerebral palsy increased from 9% for none (i.e., no chorioamnionitis by maternal stage) to 19% for advanced maternal stage (p=0.02), but the other three outcomes did not vary by maternal stage (Figure 3). Similarly, the frequency of cerebral palsy increased from 9% for none (i.e., no chorioamnionitis by maternal grade) to 15% for severe maternal grade (p<0.01), and epilepsy increased from 6% for none (i.e., no chorioamnionitis by fetal grade) to 30% for severe fetal grade (p<0.01). The frequency of study outcomes did not vary by fetal stage.
Figure 3.

Frequency of neurodevelopmental outcomes by histopathologic chorioamnionitis
In multivariable analysis, histologic chorioamnionitis was associated with a higher likelihood of cerebral palsy, autism spectrum disorder, and epilepsy at 10 years of life (Table 2). The adjusted odds (AOR) for the association between histologic chorioamnionitis and cerebral palsy was increased with advanced maternal stage (AOR 2.5, 95% CI 1.6 to 3.9), severe maternal grade (AOR 2.0, 95% CI 1.2 to 3.4), moderate fetal stage (AOR 2.2, 95% CI 2.1 to 2.3), and mild or moderate fetal grade (AOR 1.5, 95% CI 1.0 to 2.2). Similarly, the AOR for the association between histologic chorioamnionitis and epilepsy was increased with advanced maternal stage (AOR 1.5, 95% CI 1.3 to 1.6) and severe fetal grade (AOR 5.9, CI 1.9 to 17.8), but not severe maternal grade (AOR: 0.8, 95% CI: 0.7 to 1.0). In addition, the AOR for the association between histologic chorioamnionitis and autism spectrum disorder was increased with mild or moderate fetal grade (AOR 1.7, 95% CI 1.0 to 2.9). Histologic chorioamnionitis was not associated with higher odds of cognitive impairment.
Table 2.
Multivariable analysis of association between histologic chorioamnionitis by maternal and fetal stage and grade and neurodevelopmental outcomes1,2,3,6
| Cerebral Palsy | Autism spectrum disorder | Cognitive impairment | Epilepsy | |
|---|---|---|---|---|
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Primary analysis | ||||
| Maternal stage | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 (early) | 0.58 (0.36 to 0.95)5 | 0.51 (0.19 to 1.34) | 0.44 (0.40 to 1.84) | 0.48 (0.17 to 1.33) |
| 2 (moderate) | 1.37 (0.59 to 3.18) | 1.08 (0.72 to 1.62) | 1.02 (0.53 to 1.95) | 1.15 (0.60 to 2.22) |
| 3 (advanced) | 2.48 (1.59 to 3.85) | 1.65 (0.92 to 2.94) | 1.07 (0.75 to 1.55) | 1.47 (1.33 to 1.63) |
| Maternal grade | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 (mild/moderate) | 0.78 (0.58 to 1.05) | 0.71 (0.31 to 1.64) | 0.80 (0.20 to 3.03) | 0.73 (0.39 to 1.36) |
| 2 (severe) | 2.01 (1.20 to 3.35) | 1.30 (0.69 to 2.48) | 0.97 (0.58 to 1.59) | 0.81 (0.69 to 0.95) |
| Fetal stage | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 (early) | 1.16 (0.87 to 1.53) | 1.85 (0.48 to 7.17) | 1.66 (0.84 to 3.32) | 1.01 (0.57 to 1.76) |
| 2 (moderate) | 2.20 (2.13 to 2.27) | 1.82 (0.75 to 4.43) | 1.02 (0.66 to 1.58) | 0.52 (0.22 to 1.19) |
| 3 (advanced) | 1.33 (0.70 to 2.58) | 1.58 (0.63 to 4.05) | 0.98 (0.40 to 2.40) | 1.08 (0.35 to 3.38) |
| Fetal grade | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 (mild/moderate) | 1.50 (1.04 to 2.18) | 1.69 (1.01 to 2.88) | 0.97 (0.58 to 1.61) | 0.62 (0.37 to 1.03) |
| 2 (severe) | 1.03 (0.31 to 3.45) | --4 | 1.29 (0.60 to 2.82) | 5.87 (1.93 to 17.81) |
We used generalized estimating equations clustered on twin gestations.
Data imputation was performed for the following covariates: body mass index, race, maternal education, tobacco use in pregnancy, and infant sex.
Adjusted models included: maternal age, body mass index, race, insurance status, maternal education, tobacco use in pregnancy, and infant sex.
Not adequate exposure/outcome pairs for model to converge.
Bolded results are statistically significant findings (p<0.05).
Final N for each model: 1) Cerebral palsy maternal (N=767) and fetal (N=702); 2) Autism spectrum disorder maternal (N=773) and fetal (N=706); 3) Cognitive impairment maternal (N=788) and fetal (N=720); and 4) Epilepsy maternal (N=763) and fetal (N=702).
Abbreviations: adjusted odds ratio (AOR) and 95% confidence interval (95% CI).
Secondarily, histologic chorioamnionitis defined as any severe inflammation (i.e., moderate/advanced maternal/fetal stage and/or severe grade) was associated with cerebral palsy (AOR: 1.6, 95% CI: 1.1 to 2.2), but not the other measures of neurodevelopmental impairment (data not shown). The frequency of any severe inflammation did not vary by the phenotype of cerebral palsy, including hemiparesis (50%), diparesis (62%), and quadripareis (40%) versus none (40%) (overall p=0.08). In contrast, the four measures of neurodevelopmental impairment were not associated with clinical chorioamnionitis (either as diagnosed by the obstetrician, or according to elevated white blood cell count and/or maternal fever).
In sensitivity analyses, the primary analyses held when adjusted for gestational age at birth, restriction to only singleton births, adjusted for receipt of intrapartum antibiotic therapy, and for the outcome of cerebral palsy, excluding children with a diagnosis of cerebral palsy at 2 years but without gross motor function deficits at 10 years (data not shown).
COMMENT
Principal findings
In this prospective multi-center cohort of infants born <28 weeks gestation, we found that 10-year old children whose placenta had histologic characteristics of chorioamnionitis were at increased risk of cerebral palsy, autism spectrum disorder, and epilepsy, and that the risk of these measures of neurodevelopmental impairment did not vary appreciably by severity and location of chorioamnionitis.
Clinical implications
We offer several reasons for why we found some associations that might not have been expected based on earlier reports.5, 45, 46, 48, 49 First, prior studies focused on short-term neurodevelopment at 18 to 24 months rather than at 10 years as the current study, and the assessment of cognitive impairment at 2 years (i.e., Bayley Scales) differed considerably from the assessment at 10 years (i.e., DAS).8, 50
Second, chorioamnionitis is a heterogeneous clinical entity that has been variably defined. In the current study, we evaluated the extent and severity of chorioamnionitis. Histologic chorioamnionitis can be inaccurately defined when mild and severe disease are combined into a single histologic diagnosis. Doing so eliminates the opportunity to identify associations evident only with severe disease. Additionally, heterogeneity also applies to combining maternal and fetal disease as one entity. In the current study, our finding associations with maternal disease only when of advanced stage and severe grade contrasts with our finding associations with less advanced fetal stage and less than severe fetal grade. The lack of association of neurodevelopment outcomes with apparent severity of grade and stage of histologic chorioamnionitis may indicate that grade and stage are dependent primarily on location of tissue sampling of the placenta, rather than actual severity of inflammation or inflammatory response.
Finally, it is biologically plausible that intrauterine inflammation (i.e., chorioamnionitis) in the placenta and umbilical cord can produce brain damage in the neonate.51 Infection induced maternal immune activation leads to a cytokine-mediated fetal inflammatory response and immune dysregulation in the developing fetal brain.52 Chorioamnionitis is generally considered to result from a polymicrobial infection.24 Prior studies, including from the current ELGAN cohort, have suggested that the presence of microorganisms in placental parenchyma is associated with brain damage in extremely preterm infants.53
Research implications
As advised by the Perinatal Section of the Society for Pediatric Pathology,20, 25 we assessed histologic chorioamnionitis by current criteria for stage and grade of maternal and fetal inflammatory response. The fetal involvement is likely a more important predictor of neonatal outcomes than isolated maternal or intrauterine inflammation,45, 54 though in the current analysis, the risk did not necessarily vary by site and location. Future studies should classify histologic chorioamnionitis by site and location, as well as by molecular diagnostics, and be designed in ways that will assess to what extent early detection and treatment of chorioamnionitis can affect infant neurodevelopment.55
We also employed rigorous standardized outcome ascertainments in contrast to prior studies. In the ELGAN Study cohort, almost two-thirds of children who had a subnormal IQ (< 70) at age 2 years as assessed with the Bayley Scales were no longer considered to have such a low IQ when assessed with the outcome at 10 years we used for this report.8 This might also explain why our findings are discrepant with reports of assessments at age 2 years or younger.
In the primary analysis, we did not adjust for gestational age at birth because we viewed gestational age as a plausible intermediate variable on the causal pathway between chorioamnionitis, death, and neurodevelopmental outcomes.43 Because adjusting for gestational age may convey information about unmeasured covariates,56 we carried out additional analyses that adjusted for gestational age at delivery. The results differed minimally from the primary analysis. The implication is that gestational age is not a confounder.
Strengths and limitations
Important strengths of the current study include a prospective design, a heterogeneous multicenter cohort, standardized assessments of neurodevelopment at 10 years of age by providers who were not aware of patient’s chorioamnionitis status, rigorous standardized definitions of neurodevelopmental impairment, a contemporary, gold standard definition of chorioamnionitis based on histologic diagnosis, and minimal loss to follow-up at 10 years. Among this study’s limitations are a lack of non-histologic biomarkers (e.g., cytokines or microorganisms in placental parenchyma) and histologic evaluations performed by multiple pathologists. While early efforts to minimize inter-observer reliability were probably effective,21 we did not assess inter-observer reliability after those early efforts. Because this study was limited to extremely preterm infants who were likely at the highest risk of neurodevelopmental impairment regardless of intrapartum infection, these results may not be generalizable to infants born later in the preterm period or term. Selection bias is possible as those who did not participate at 10 years were more likely to have indicators of social disadvantage, including lower maternal education and receipt of public health insurance. However, 95% of participants who followed-up at 10 years had placenta data available. It is possible that chorioamnionitis may not increase the risk of neurodevelopmental impairment among infants born at a later gestational age. The current study included both singleton and multiple gestations, and while we adjusted for twins and conducted sensitivity analyses restricted to singletons, it is possible that the relationship between chorioamnionitis and neurodevelopment may vary between these groups. Finally, clinical practice at the time of delivery has evolved since recruitment in this cohort, and it is possible that current maternal and neonatal interventions in the peripartum period may further impact these findings.
Conclusions
Histologic chorioamnionitis is associated with neurodevelopmental impairment, specifically cerebral palsy, autism spectrum disorders, and epilepsy, at 10 years of age among infants born extremely preterm. Further research is needed to understand whether prevention and treatment of intrapartum infection might improve neurological outcomes of infants born extremely preterm. Our results suggest that intrapartum inflammation can result in lasting neurodevelopmental impairment nearly a decade later in a high-risk population of extremely preterm infants.
CONDENSATION.
Implications and Contributions
A. Why was this study conducted?
Chorioamnionitis might be an indicator of increased risk of long-term neurodevelopmental impairment at 10 years of life among extremely preterm infants.
B. What are the key findings?
Among children born extremely preterm <28 weeks gestation, histologic chorioamnionitis is associated with an increased risk of neurodevelopmental impairments, including cerebral palsy, autism spectrum disorder, and epilepsy at 10 years of life.
C. What does this study add to what is already known?
Prior studies have focused on the association between chorioamnionitis and infant neurodevelopment at up to 2 years of life. This study indicates that the impact of chorioamnionitis on early neonatal brain development may persist through middle childhood. These results highlight the importance of efforts to prevent intrapartum infection, which could improve developmental outcomes of extremely preterm infants.
ACKNOWLEDGEMENTS
ELGAN Study collaborators: Project Lead for ELGAN-2: Julie V. Rollins, MA
Site Principal Investigators: Baystate Medical Center, Springfield, MA: Bhahvesh Shah, MD; Rachana Singh, MD, MS; Boston Children’s Hospital, Boston, MA: Linda Van Marter, MD, MPH and Camilla Martin, MD, MPH; Janice Ware, PhD; Tufts Medical Center, Boston, MA: Cynthia Cole, MD; Ellen Perrin, MD; University of Massachusetts Medical School, Worcester, MA: Frank Bednarek, MD; Jean Frazier, MD; Yale University School of Medicine, New Haven, CT: Richard Ehrenkranz, MD (deceased); Jennifer Benjamin, MD; Wake Forest University, Winston-Salem, NC: T. Michael O’Shea, MD, MPH; University of North Carolina, Chapel Hill, NC: Carl Bose, MD; Diane Warner, MD, MPH; East Carolina University, Greenville, NC: Steve Engelke, MD; Helen DeVos Children’s Hospital, Grand Rapids, MI: Mariel Poortenga, MD; Steve Pastyrnak, PhD; Sparrow Hospital, East Lansing, MI: Padu Karna, MD; Nigel Paneth, MD, MPH; Madeleine Lenski, MPH; University of Chicago Medical Center, Chicago, IL: Michael Schreiber, MD; Scott Hunter, PhD; Michael Msall, MC; William Beaumont Hospital, Royal Oak, MI: Danny Batton, MD; Judith Klarr, MD
Site Study Coordinators: Baystate Medical Center, Springfield, MA: Karen Christianson, RN; Deborah Klein, BSM, RN; Boston Children’s Hospital, Boston MA: Maureen Pimental, BA; Collen Hallisey, BA; Taryn Coster, BA; Tufts Medical Center, Boston, MA: Ellen Nylen, RN; Emily Neger, MA; Kathryn Mattern, BA; University of Massachusetts Medical School, Worcester, MA: Lauren Venuti, BA; Beth Powers, RN; Ann Foley, EdM; Yale University School of Medicine, New Haven, CT: Joanne Williams, RN; Elaine Romano, APRN; Wake Forest University, Winston-Salem, NC: Debbie Hiatt, BSN (deceased); Nancy Peters, RN; Patricia Brown, RN; Emily Ansusinha, BA; University of North Carolina, Chapel Hill, NC: Gennie Bose, RN; Janice Wereszczak, MSN; Janice Bernhardt, MS, RN; East Carolina University, Greenville, NC: Joan Adams (deceased); Donna Wilson, BA, BSW; Nancy Darden-Saad, BS, R; Helen DeVos Children’s Hospital, Grand Rapids, MI: Dinah Sutton, RN; Julie Rathbun, BSW, BSN; Sarah Nota, BS; Teri Crumb, BSN, RN, CCRC; Sparrow Hospital, East Lansing, MI: Karen Miras, RN, BSN; Deborah Weiland, MSN; University of Chicago Medical Center, Chicago, IL: Grace Yoon, RN; Rugile Ramoskaite, BA; Suzanne Wiggins, MA; Krissy Washington, MA; Ryan Martin, MA; Barbara Prendergast, BSN, RN; William Beaumont Hospital, Royal Oak, MI: Beth Kring, RN; Jennifer DeRidder, RN
Psychologists: Baystate Medical Center, Springfield, MA: Anne Smith, PhD; Susan McQuiston, PhD; Boston Children’s Hospital: Samantha Butler, PhD; Rachel Wilson, PhD; Kirsten McGhee, PhD; Patricia Lee, PhD; Aimee Asgarian, PhD; Anjali Sadhwani, PhD; Brandi Henson, PsyD Tufts Medical Center, Boston MA: Cecelia Keller, PT, MHA; Jenifer Walkowiak, PhD; Susan Barron, PhD; University of Massachusetts Medical School, Worcester MA: Alice Miller, PT, MS; Brian Dessureau, PhD; Molly Wood, PhD; Jill Damon-Minow, PhD; Yale University School of Medicine, New Haven, CT: Elaine Romano, MSN; Linda Mayes, PhD; Kathy Tsatsanis, PhD; Katarzyna Chawarska, PhD; Sophy Kim, PhD; Susan Dieterich, PhD; Karen Bearrs, PhD; Wake Forest University Baptist Medical Center, Winston-Salem NC: Ellen Waldrep, MA; Jackie Friedman, PhD; Gail Hounshell, PhD; Debbie Allred, PhD; University Health Systems of Eastern Carolina, Greenville, NC: Rebecca Helms, PhD; Lynn Whitley, PhD Gary Stainback, PhD; University of North Carolina at Chapel Hill, NC: Lisa Bostic, OTR/L; Amanda Jacobson, PT; Joni McKeeman, PhD; Echo Meyer, PhD; Helen DeVos Children’s Hospital, Grand Rapids, MI: Steve Pastyrnak, PhD; Sparrow Hospital, Lansing, MI: Joan Price, EdS; Megan Lloyd, MA, EdS; University of Chicago Medical Center, Chicago, IL: Megan Scott, PhD; William Beaumont Hospital, Royal Oak, MI: Kelly Vogt, PhD.
Additional contributors to ELGAN-1: Brigham and Women’s Hospital, Boston, MA: Hidemi Yamamoto, BA; Stanthia Ryan, Medical Student; Damilola Junaid, BS; Hassan Dawood, BS; Noah Beatty, BS; Ngan Luu, Undergraduate student; Vanessa Tang, MD; Rosaria Rita Sassi, MD; Jenna-Malia Pasicznyk, RN.
We are grateful to ELGAN Study participants and their families for their willingness to be engaged in the study for these many years and for the commitment and extra efforts that have made this work possible. We also acknowledge the inspiration, guidance and collaboration of Elizabeth Allred whose contributions to the ELGAN Study cannot be overstated.
FUNDING: This study was supported by The National Institute of Neurological Disorders and Stroke (5U01NS040069–05; 2R01NS040069 – 06A2) and the National Institute of Child Health and Human Development (5R01HD092374–02 and 5P30HD018655–34) and the Office of the National Institutes of Health Director (1UH3OD023348–01). Dr. Frazier has the following disclosures: receiving research support from Takeda Pharmaceuticals, Fulcrum Therapeutics, Janssen Research and Development and Roche. No funds from these entities supported this project, and none of these entities reviewed/commented on this study. All other authors declare no financial or ethical conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors report no conflict of interest. This manuscript was presented at the Society for Maternal-Fetal Medicine 40th Annual Meeting, Dallas, TX, February 2020.
REFERENCES
- 1.Kim C, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American Journal of Obstetrics and Gynecology 2015; 213:S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. International Journal of Developmental Neuroscience 2011; 29:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark E, Mele L, Wapner RJ, Spong CY, Sorokin Y, Peaceman A, Iams JD, Leveno KJ, Harper M, Caritis SN, Miodovnik M, Mercer BM, Thorp JM, Ramin SM, Carpenter M, Rouse DJ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of fetal inflammation and coagulation pathway gene polymorphisms with neurodevelopmental delay at age 2 years. American Journal of Obstetrics and Gynecology 2010; 203:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Muñoz Rodrigo F, Galán Henríquez GM, Ospina CG. Morbidity and mortality among very-low-birth-weight infants born to mothers with clinical chorioamnionitis. Neonatology 2014; 55:381–386. [DOI] [PubMed] [Google Scholar]
- 5.Shi Z, Ma L, Luo K, Bajaj M, Chawla S, Natarajan G, Hagberg H, Tan S. Chorioamnionitis in the Development of Cerebral Palsy: A Meta-analysis and Systematic Review. Pediatrics 2017; 139:e20163781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlapbach L, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, Nelle M, Bucher HU, Latal B; Swiss Neonatal Network and Follow-Up Group. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011; 128:e348–e357. [DOI] [PubMed] [Google Scholar]
- 7.Ylijoki M, Ekholm E, Ekblad M, Lehtonen L. Prenatal Risk Factors for Adverse Developmental Outcome in Preterm Infants-Systematic Review. Frontiers in Psychology 2019; 26:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Shea T, Joseph RM, Allred EN, Taylor HG, Leviton A, Heeren T, Douglass LM, Frazier JA, Jara H, Kuban KCK; ELGAN Study Investigators. Accuracy of the Bayley-II mental development index at 2 years as a predictor of cognitive impairment at school age among children born extremely preterm. Journal of Perinatology 2018; 38:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson K ‘Outgrowing’ a cerebral palsy diagnosis. Dev Med Child Neurol 2020; 62:12. [DOI] [PubMed] [Google Scholar]
- 10.Lyell D, Pullen K, Fuh K, Zamah AM, Caughey AB, Benitz W, El-Sayed YY. Daily compared with 8-hour gentamicin for the treatment of intrapartum chorioamnionitis: a randomized controlled trial. Obstet Gynecol 2010; 115:344–349. [DOI] [PubMed] [Google Scholar]
- 11.Oh K, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, Yoon BH.. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. American Journal of Obstetrics and Gynecology 2019; 221:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon B, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A, Hong JS.. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology 2019; 221:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roescher A, Timmer A, Erwich JJ, Bos AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLos ONE 2014; 9:e89419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins R, Saade G, Polin RA, Grobman WA, Buhimschi IA, Watterberg K, Silver RM, Raju TN; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns With a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet Gynecol 2016; 127:426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallapur S, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Seminars in reproductive medicine 2014; 32:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartley J, Ferguson BJ, Moffett A. The role of shed placental DNA in the systemic inflammatory syndrome of preeclampsia. American Journal of Obstetrics and Gynecology 2015; 213:268–277. [DOI] [PubMed] [Google Scholar]
- 17.Pierrat V,, Marchand-Martin L, Arnaud C, Kaminski M, Resche-Rigon M, Lebeaux C, Bodeau-Livinec F, Morgan AS, Goffinet F, Marret S, Ancel PY, EPIPAGE-2 writing group. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. British Medical Journal 2017; 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Shea T, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A; ELGAN study Investigators. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Human Development 2009; 85:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuban K, Joseph RM, O’Shea TM, Allred EN, Heeren T, Douglass L, Stafstrom CE, Jara H, Frazier JA, Hirtz D, Leviton A; Extremely Low Gestational Age Newborn (ELGAN) Study Investigators. Girls and Boys Born before 28 Weeks Gestation: Risks of Cognitive, Behavioral, and Neurologic Outcomes at Age 10 Years. Journal of Pediatrics 2016; 173:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redline R Classification of placental lesions. American Journal of Obstetrics and Gynecology 2015; 213:S21–S28. [DOI] [PubMed] [Google Scholar]
- 21.Hecht J, Allred EN, Kliman HJ, Zambrano E, Doss BJ, Husain A, Pflueger SM, Chang CH, Livasy CA, Roberts D, Bhan I, Ross DW, Senagore PK, Leviton A; Elgan Study Investigators. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology 2008; 40:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hecht JL, Onderdonk A, Delaney M, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol 2008; 11:15–22. [DOI] [PubMed] [Google Scholar]
- 23.Tita A, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clinics in perinatology 2010; 37:339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson C, Farzin A, Burd I. Current management and long-term outcomes following chorioamnionitis. Obstetrics and Gynecology Clinics of North America 2014; 41:649–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redline R, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, The Society for Pediatric Pathology. Amniotic Infection Syndrome: Nosology and Reproducibility of Placental Reaction Patterns. Pediatric and Developmental Pathology 2003; 6. [DOI] [PubMed] [Google Scholar]
- 26.Driscoll SG, Langston C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on Methods for Placental Examination. Arch Pathol Lab Med 1991; 115:704–8. [PubMed] [Google Scholar]
- 27.Hecht JL, Allred EN, Kliman HJ, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology 2008; 40:372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston N Plasticity in the developing brain: implications for rehabilitation. Developmental Disabilities Research Reviews 2009; 15:94–101. [DOI] [PubMed] [Google Scholar]
- 29.Kuban K, Leviton A. Cerebral palsy. New England Journal of Medicine 1994; 330:188–195. [DOI] [PubMed] [Google Scholar]
- 30.Kuban K, O’Shea M, Allred E, Leviton A, Gilmore H, DuPlessis A, Krishnamoorthy K, Hahn C, Soul J, O’Connor SE, Miller K, Church PT, Keller C, Bream R, Adair R, Miller A, Romano E, Bassan H, Kerkering K, Engelke S, Marshall D, Milowic K, Wereszczak J, Hubbard C, Washburn L, Dillard R, Heller C, Burdo-Hartman W, Fagerman L, Sutton D, Karna P, Olomu N, Caldarelli L, Oca M, Lohr K, Scheiner A. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. Journal of Child Neurology 2015; 20:829–831. [DOI] [PubMed] [Google Scholar]
- 31.Smilga A, Garfinkle J, Ng P, Andersen J, Buckley D, Fehlings D, Kirton A, Wood E, van Rensburg E, Shevell M, Oskoui M. Neonatal Infection in Children With Cerebral Palsy: A Registry-Based Cohort Study. Pediatric Neurology 2018; 80:77–83. [DOI] [PubMed] [Google Scholar]
- 32.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire - Manual.. Los Angeles, CA: Western Psychological Services, 2003. [Google Scholar]
- 33.Rutter M, Le Couteur A, Lord C. “Autism Diagnostic Interview-Revised.”. Los Angeles, CA: Western Psychological Services, 2003. [Google Scholar]
- 34.Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 2006; 45:1094–103. [DOI] [PubMed] [Google Scholar]
- 35.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule™, second edition Torrance, California: Western Psychological Services, 2012. [Google Scholar]
- 36.Elliott CD. Differential Ability Scales. San Antonio, TX: Pearson, 2007. [Google Scholar]
- 37.Joseph R, Fein D. The significance of IQ and differential cognitive abilities for understanding ASD.. Neuropsychol Autism 2011:281–294. [Google Scholar]
- 38.Hirschberger R, Kuban KCK, O’Shea TM, Joseph RM, Heeren T, Douglass LM, Stafstrom CE, Jara H, Frazier JA, Hirtz D, Rollins JV, Paneth N1; ELGAN Study Investigators. Co-occurrence and Severity of Neurodevelopmental Burden (Cognitive Impairment, Cerebral Palsy, Autism Spectrum Disorder, and Epilepsy) at Age Ten Years in Children Born Extremely Preterm. Pediatric Neurology 2018; 79:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglass L, Kuban K, Tarquinio D, Schraga L, Jonas R, Heeren T, DeBassio WA, Stafstrom CE, Heinrick RJ, Ferguson C, Blumberg L, Wong V. A Novel Parent Questionnaire for the Detection of Seizures in Children. Pediatric Neurology 2016; 54:64–69. [DOI] [PubMed] [Google Scholar]
- 40.Douglass L, Heeren TC, Stafstrom CE, DeBassio W, Allred EN, Leviton A, O’Shea TM, Hirtz D, Rollins J, Kuban K. Cumulative Incidence of Seizures and Epilepsy in TenYear-Old Children Born Before 28 Weeks’ Gestation. Pediatric Neurology 2017; 73:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutcheon J, Moskosky S, Ananth CV, Basso O, Briss PA, Ferré CD, Frederiksen BN, Harper S, Hernández-Díaz S, Hirai AH, Kirby RS, Klebanoff MA, Lindberg L, Mumford SL, Nelson HD, Platt RW, Rossen LM, Stuebe AM, Thoma ME, Vladutiu CJ, Ahrens KA. Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Pediatric and Perinatal Epidemiology 2019; 33:O15–O24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T, Li H, Su P, Yu Y, Sun X, Liu Y, Yuan Z, Xue F. Sensitivity analysis for mistakenly adjusting for mediators in estimating total effect in observational studies. BMJ Open 2017; 7:e015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ananth C, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. American Journal of Obstetrics and Gynecology 2017; 217:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.UCLA Institute for Digital Research and Education. Multiple Imputation in STATA, 2019.
- 45.Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, Walsh MC, Das A, Hale EC, Newman NS, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatrics 2014; 168:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bierstone D, Wagenaar N, Gano DL, Guo T, Georgio G, Groenendaal F, de Vries LS, Varghese J, Glass HC, Chung C, Terry J, Rijpert M, Grunau RE, Synnes A, Barkovich AJ, Ferriero DM, Benders M, Chau V, Miller SP. Association of Histologic Chorioamnionitis With Perinatal Brain Injury and Early Childhood Neurodevelopmental Outcomes Among Preterm Neonates. JAMA Pediatrics 2018; 172:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph R, O’Shea TM, Allred EN, Heeren T, Hirtz D, Paneth N, Leviton A, Kuban KC. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Research 2016; 10:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendson L, Russell L, Robertson CM, Liang Y, Chen Y, Abdalla A, Lacaze-Masmonteil T. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. Journal of Pediatrics 2011; 158:397–402. [DOI] [PubMed] [Google Scholar]
- 49.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’shea TM, Paneth N. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatric Research 2010; 67:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vander Haar E, Gyamfi-Bannerman C. Chorioamnionitis and Neurocognitive Development at Age 2 Years. Obstetrics and Gynecology 2016; 127:437–441. [DOI] [PubMed] [Google Scholar]
- 51.Dammann O, Leviton A.. Intermittent or sustained systemic inflammation and the preterm brain. Pediatric Research 2014; 75:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. American Journal of Reproductive Immunology 2012; 67:287–294. [DOI] [PubMed] [Google Scholar]
- 53.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev 2009; 85:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, Van Marter LJ, Pagano M, Hegyi T, Hiatt M, Sanocka U, Shahrivar F, Abiri M, Disalvo D, Doubilet P, Kairam R, Kazam E, Kirpekar M, Rosenfeld D, Schonfeld S, Share J, Collins M, Genest D, Shen-Schwarz S, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatric Research 1999; 46:566–575. [DOI] [PubMed] [Google Scholar]
- 55.Shatrov J, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstetrics and Gynecology 2010; 116:387–392. [DOI] [PubMed] [Google Scholar]
- 56.Leviton A, Blair E, Dammann O, Allred E. The wealth of information conveyed by gestational age. Journal of Pediatrics 2005; 146:123–127. [DOI] [PubMed] [Google Scholar]


