Abstract
Connexins (Cx) are essential for cardiovascular regulation and maintenance of cardio-renal response involving the natriuretic peptide family. Changes in the expression of connexins promote intercellular communication dysfunction and may induce hypertension, atherosclerosis, and several other vascular diseases. This study analyzed the expression of the genes involved in the renin-angiotensin system (RAS) and the relation of the connexins gene expression with the renovascular hypertension 2K1C in different tissues. The insertion of a silver clip induced renovascular hypertension 2K1C into the left renal artery. Biochemical measurements were made using commercial kits. Gene expression was evaluated in the liver, heart, and kidneys by RT-PCR. The genes investigated were LDLr, Hmgcr, Agt, Ren, Ace, Agtr1a, Anp, Bnp, Npr1, Cx26, Cx32, Cx37, Cx40 and Cx43. All genes involved in the RAS presented increased transcriptional levels in the 2K1C group, except hepatic Agt. The natriuretic peptides (Anp; Bnp) and the receptor genes (Npr1) appeared to increase in the heart, however, Npr1 decreased in the kidneys. In hepatic tissue, hypertension promoted increased expression of Cx32, Cx37, and Cx40 genes however, Cx26 and Cx43 genes were not influenced. Expression was upregulated for Cx37 and Cx43 in cardiac tissue in the 2K1C group, but Cx40 did not demonstrate any difference between groups. The stenotic kidney showed an upregulated expression for Cx37 vs Sham and contralateral kidney, although Cx40 and Cx43 were downregulated. Hypertension did not modify the transcriptional expression of Cx26 and Cx32. Therefore, this study indicated that RAS and cardiac response were regulated transcriptionally by renovascular hypertension 2K1C. Moreover, the results of connexin gene expression demonstrated differential transcriptional regulation in different tissues studied and suggest a relationship between cardiac and renal physiological changes as an adaptive mechanism to the hypertensive state.
Keywords: Genetics, Transcriptomics, Biochemistry, Renovascular hypertension 2K1C, Renin-angiotensin system, Connexins
Genetics; Transcriptomics; Biochemistry; Renovascular hypertension 2K1C; Renin-angiotensin system; Connexins
1. Introduction
Hypertension is a major risk factor for cardiovascular disease and contributes to morbidity and mortality worldwide [1]. Among the different types of high blood pressure, there is renovascular hypertension, which is characterized by increased activation of the renin-angiotensin system (RAS) [2]. Vascular occlusion of the kidneys produces an increase in systemic blood pressure, being one of the pathogenic mechanisms of hypertension [3].
The maintenance of the cardio-renal response involves the natriuretic peptide family (NP), which includes atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and natriuretic peptide type C (CNP). Activation of ANP or BNP induces vasodilation and increases natriuresis and diuresis, counterbalancing the main effects of RAS [4]. In addition, ANP acts as a suppressor of renin secretion in juxtaglomerular cells, and in vivo, ANP and BNP may reduce plasma renin activity [5].
Communication via gap junctions is essential for the regulation of cardiovascular function. Gap junctions are complex structures that promote communication and signaling between adjacent cells and this intercellular communication is mediated by connexons, constituted by the family of connexin (Cx) proteins [6]. The activity of the connexons is modulated by the number, functional status and permeability of gap junctions in the plasma membrane. These events are coordinated by interactions between connexins and other proteins and lipids [7]. Association between specific phospholipids and different isoforms of connexins promotes the regulatory and structural connections of these proteins with lipid membranes [8]. Similarly, the connexin expression can be modulated by diet, which demonstrates an essential relation between them and the genes involved in cholesterol metabolism [9].
Alterations in the expression of connexins lead to dysfunction of intercellular communication, which can induce hypertension, atherosclerosis, and several other vascular diseases [10]. Physiological changes in the expression of connexins are closely associated with changes in vascular tone, with the most common being Cx37, Cx40, Cx43 and Cx45 [11, 12]. In the kidneys of rats and mice, Cx40 is highly expressed in endothelium and renin-secreting cells of the afferent arterioles [13]. Cx37 is highly expressed in the juxtaglomerular apparatus and smooth muscle cells of renal arteries and arterioles and Cx43 expressed in endothelial cells of the renal vasculature [14]. Hepatocytes express Cx32 and, to a lesser extent, Cx26, 90 and 5%, respectively, of all connexins present in the liver of rats and humans [15, 16]. Considering the importance of connexins in the maintenance of tissue homeostasis, we hypothesized that elevated levels of angiotensin II associated with renovascular hypertension could modulate gene expression of connexins. Thus, the aim of this study was to evaluate the effects of hypertension on the expression of genes involved in the RAS and its relation with gene expression of the connexins in different tissues.
2. Materials and methods
The studies were conducted according to experimental standards and biodiversity rights of the National Institute of Health for the Care and Use of Laboratory Animals (NHI Publication 80-23, revised in 2011). The experiments were carried out in accordance with the rules established by the Arouca Law (11794/2008) and were approved by the Animal Use Ethics Committee (CEUA) of the University Center of the Hermínio Ometto Foundation - FHO (protocol no. 036/2014). The work followed with the animal experimentation ethical standards of the Brazilian College of Animal Experimentation (COBEA). The animals were kept in cages with two to three rats per cage, at a room with controlled temperature (22 ± 1 °C) and humidity at 60%, 12 h light/dark cycle, and free access to water and feed.
2.1. Experimental protocol
Renovascular hypertension 2-kidney, 1-clip (model, 2K1C) was induced as previously described by Goldblatt et al. [17]. Male Wistar rats weighing 180–200 g (n = 17) were anesthetized intraperitoneally with ketamine (100 mg/kg) and xylazine (10 mg/kg) and a U-shaped silver clip with an internal diameter of 0.2 mm was placed around the left renal artery (2K1C/hypertensive group, n = 10). The Sham group (normotensive, n = 7) performed the same surgical procedure, but except the insertion of the clip into the renal artery.
Systolic blood pressure (SBP) was evaluated using tail plethysmography, in the awake animals after they were heated in a cabinet at 37 °C for 15 min. The pressure change data were captured by a Power Lab 4/S analog-to-digital converter (AD Instruments Ltd., Castle Hill, Australia) and the results were represented as the average of three consecutive measurements for each animal. Before the first measurement, for 5 days, the animals were adapted to the procedure to minimize stress-induced SBP fluctuations. The animals were considered hypertensive when systolic blood pressure was above 160 mmHg [18]. In this study, 17% of animals were not considered hypertensive. Systolic blood pressure and body mass were evaluated weekly for 4 weeks, and then the animals were euthanized, and the organs removed.
2.2. Biochemical dosages
The blood of the animals was collected by cardiac puncture after a fasting for 10–12 h. The serum was obtained by centrifuging this material for 15 min at 3500 rpm, and then frozen until the time of analysis. The concentration of glucose, total cholesterol, triacylglycerol, creatinine, uric acid, urea, creatine kinase-MB (CK-MB) and creatine kinase- NAC (CK-NAC) was determined using colorimetric kits (Laborclin and Labtest, Brazil). The free fatty acid (FFA) was measured using a commercial colorimetric kit (NEFA, Randox, USA), according to the manufacturer's specifications.
2.3. Analysis of gene expression by RT-PCR
The hepatic, cardiac (left ventricle), and renal (right and left) tissue were removed and immediately frozen in liquid nitrogen and stored at – 80 °C. Total RNA was isolated from approximately 100 mg of tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and digested with amplification grade DNAse I (Invitrogen), according to the manufacturer's instructions. The concentration of RNA was then determined using UV spectrophotometry at wavelengths of 260 and 280 nm. The conversion of total RNA (2 μg/μl) to cDNA was performed using random primers and reverse transcriptase (RT) enzyme (SuperScript II, Invitrogen) in a final volume of 20 μl. The expression of the transcripts of genes involved in cholesterol metabolism - LDLr (LDL receptor) and Hmgcr (3-hydroxy-3-methylglutaryl CoA reductase), Renin-Angiotensin System - Agt (Angiotensinogen), Ren (Renin), Ace (Angiotensin Converting Enzyme I), Agtr1a (Angiotensin II Receptor, type 1a), cardiovascular homeostasis – Anp (Atrial Natriuretic Peptide), Bnp (Brain Natriuretic Peptide), Npr1 (Natriuretic Peptide Receptor 1) and intercellular communication - Cx26, Cx32, Cx37, Cx40, Cx43 were estimated by semiquantitative RT-PCR (Amplitherm Thermal Cyclers PCR) according to Santolim [9] using specific primers described in Table 1. The PCR products were separated by electrophoresis in 1.5% agarose gel and stained with ethidium bromide. The gel was visualized and photographed using Syngene G:Box® photodocumentation system, followed by densitometric quantification of the bands using Scion Image Software (Scion Corp., Frederick, MD, USA). The β-actin gene was used for the housekeeping control. Data are reported as intensity target genes/intensity β-actin (β-act).
Table 1.
Primers sequences used for gene expression analysis.
| Gene | Sense (5´→ 3′) | Antisense (5´→ 3′) |
|---|---|---|
| LDLr | CCAGTGCGGCGTAGGATT | GGGACTCATCGGAGCCAT |
| Hmgcr | CTTGACGCTCTGGTGGAATG | TGGCTGAGCTGCCAAATTGG |
| Agt | CCCTTTCATCTCCTCTACTAC | CTTGCCTCACTCAGCATCTT |
| Ren | GTGGACACTGGCACATCCTA | CTTGGAGAGCCAGTATGCAC |
| Ace | TGCCTCCCAACGAGTTAGAA | GAGAAGTCCACGTACTTTGG |
| Agtr1a | GGAAACAGCTTGGTGGTGAT | ACATAGGTGATTGCCGAAGG |
| Anp | GGATTTCAAGAACCTGCTAG | CAGAGCCCTCAGTTTGCTT |
| Bnp | GAACAATCCACGATGCAGAAG | GTCTTCCTAAAACAACCTCAG |
| Npr1 | GTGCGTGAACGCCTCAACAT | GCATCCGGAGAACTGCAGAT |
| Cx26 | GGTGTGGGGAGATGAGCAAG | GACTTCCCTGAGCAATACCT |
| Cx32 | AATGAGGC GGATGAACTGG | CCTCAAGCCGTAGCATTTTC |
| Cx37 | GATCACAGGTGGTTCTGGAAT | CAGCCTACATCAGTGCCTTC |
| Cx40 | ATCTCCCACATTCGTTACTG | AGGAAGATCCCATAGAGGAG |
| Cx43 | GATTGAAGAGCACGGCAAGG | GTGTAGACCGCGCTCAAG |
| β-act | AGAGGGAAATCGTGCGTGACA | CGATAGTGATGACCTGACCGTCA |
2.4. Statistical analyses
The results were analyzed comparatively between the groups using the Student's t-test or ANOVA (Kidneys) followed by the Bonferroni post-hoc test. Statistical analyses were performed with GraphPad Prism software, version 5.0 (GraphPad Software, Inc., USA). The data were expressed as mean ± standard error of the mean (X ± S.E.M.), considering the results of two independent experiments. The significance level adopted was 5% (P < 0.05).
3. Results and discussion
3.1. Characteristics of the animals after the induction of hypertension
After the experimental period, animals with renal artery stenosis (2K1C) had an increase in body weight, SBP (Figure 1) and heart mass. Likewise, hypertension promoted an increase in the right kidney vs. Sham and compared to the left of the 2K1C group, which indicates right renal hypertrophy and left renal atrophy. These findings indicate hyperactivity of the right kidney relative to the stenotic kidney and cardiac hypertrophy, characteristics of the renovascular hypertension model 2K1C [19]. The others biometric parameters evaluated were not different between the groups (Table 2).
Figure 1.
Body weight (A) and systolic blood pressure (B) of the experimental animals. The values are expressed as mean ± S.E.M and significant differences between groups are indicated by ∗∗∗P < 0.0001 vs Sham.
Table 2.
Biometric and biochemical parameters of renovascular hypertension 2K1C.
| Parameters | Sham (n = 7) | 2K1C (n = 10) | |
|---|---|---|---|
| Liver weight (g) | 9.4 ± 0.25 | 9.7 ± 0.34 | |
| IPF (g/g) | 0.032 ± 0.0007 | 0.032 ± 0.0006 | |
| Heart weight (g) | 0.835 ± 0.012 | 1.163 ± 0.013∗∗∗ | |
| IPC (g/g) | 0.0027 ± 0.0001 | 0.0036 ± 0.0001∗∗ | |
| Kidney weight (g) | Right | 1.097 ± 0.034 | 1.392 ± 0.056∗# |
| Left | 1.106 ± 0.020 | 0.933 ± 0.037∗ | |
| IPR (g/g) | Right | 0.0035 ± 0.0001 | 0.0043 ± 0.0002∗# |
| Left | 0.0035 ± 0.0003 | 0.0028 ± 0.0001∗ | |
| Glycemia (mg/dL) | 78 ± 4.5 | 88 ± 3.3 | |
| Total cholesterol (mg/dL) | 75 ± 9.2 | 80 ± 4.3 | |
| Triglycerides (mg/dL) | 74 ± 7.2 | 58 ± 7.8 | |
| Free Fatty Acids (mmol/L) | 0.3 ± 0.04 | 0.5 ± 0.09 | |
| Creatinine (mg/dL) | 2.2 ± 0.2 | 2.1 ± 0.1 | |
| Urea (mg/dL) | 59.9 ± 4.3 | 70.5 ± 7.8 | |
| Uric acid (mg/dL) | 3.1 ± 0.4 | 3.5 ± 0.6 | |
| CK-MB (U/L) | 32.5 ± 4.9 | 27.1 ± 5.1 | |
| CK-NAC (U/L) | 1707 ± 227.8 | 1691 ± 261.4 | |
Values are expressed as mean ± S.E.M of 2 independent experiments. Number of animals (n); Liver weight index (IPF) (IPF = liver weight, body weight); Heart rate index (CPI); Kidney Weight Index (IPR); Creatine kinase-MB (CK-MB); Creatine kinase- NAC (CK-NAC). ∗P < 0.05, ∗∗P < 0.01 e ∗∗∗P < 0.001 vs. Sham. #P = 0.001 vs. contralateral kidney 2K1C.
Usually, hypertension raises blood levels of LDL-c, total cholesterol, and triglyceride and, in contrast, decreases the amount of HDL-c [20]. However, within 4 weeks of hypertension induction, it was not possible to observe changes in serum cholesterol profile (Table 2). The results found can be justified by the similarity of the gene expression of LDLr and Hmgcr between the groups (Figure 2). The enzyme 3-hydroxy-3-methylglutaryl-CoA reductase participates in the biosynthesis of the cholesterol molecule and acts on homeostasis of blood pressure and hypertension [21]. Serum levels of triglycerides, free fatty acids, glucose, urea, uric acid, CK-MB, CK-NAC and creatinine were similar for the two groups. Increased creatinine levels are associated of risk of cardiovascular [22] disease and reduction in creatinine clearance, as well as increased serum creatinine is found in subjects with mild or moderate renal impairment [23].
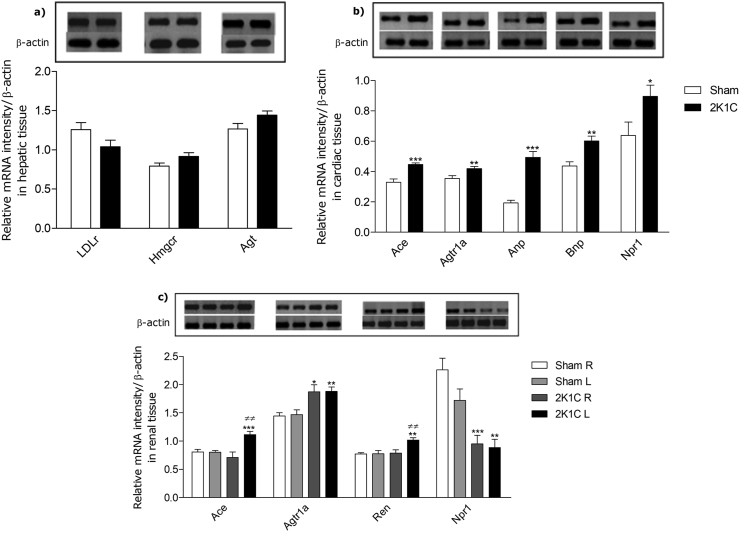
Figure 2.
Expression of target genes of cholesterol metabolism and RAS demonstrated by RT-PCR analysis. Relative densitometry values were calculated after normalization with β-actin gene and the expression changes of the transcripts between the Sham and 2K1C groups were expressed as mean ± S.E.M. The upper panels show the representative bands of the gel in liver (a), heart (b), kidneys (c) (R = right; L = left) (see supplemental data). Significant differences between groups are indicated by ∗P < 0.05, ∗∗P < 0.001, and ∗∗∗P < 0.0001 vs. Sham; ≠≠ P < 0.001 vs. contralateral kidney.
3.2. Transcriptional analysis of the renin-angiotensin system and cardiovascular homeostasis
Renovascular hypertension 2K1C is an experimental model that stimulates the renin-angiotensin system and plays a key role in developing and maintaining high blood pressure [24]. Thus, we performed a comparative analysis of Agt, Ren, Ace, Agtr1a gene expression among normotensive (Sham) and hypertensive (2K1C) animals in different tissues. All RAS genes studied, except hepatic Agt, were upregulated by hypertension (Figure 2).
While several cell types produce AGT, knockout animals for hepatic AGT demonstrate marked reductions in plasma concentrations of AGT and decreased blood pressure [25]. On the other hand, a study provides evidence that AGT also has independent effects of Ang II [26]. However, the Agt genotype has no effect on the 2K1C model, in which renin is stimulated [27]. Thus, these factors may justify that Agt gene expression in the liver is not increased in 2K1C animals.
In the heart, renovascular hypertension increased the expression of Ace, Agtr1a, Anp, Bnp and Npr1 genes in the left ventricle of 2K1C animals when compared to Sham (Figure 2). Studies suggest that ACE inhibition generates cardiac protection, being important given to hypertension patients, but its excess generates left ventricular hypertrophy [28]. Similarly, Agtr1a is required for the development of hypertension and cardiac hypertrophy [29]. Therefore, as expected, renal artery stenosis caused hypertension and cardiac hypertrophy through the overexpression of the Agtr1a gene.
The RAS and NPs present antagonistic mechanisms for the control of arterial hypertension. However, in renovascular hypertension and heart failure, plasma levels of renin, ANP and BNP are also increased [30]. Renovascular hypertension increased gene expression of the NPs and Npr1 in response to renal artery stenosis to attenuate the release of renin (Figure 2). In genetically modified and spontaneously hypertensive rats, the activity of the natriuretic peptide-A receptor (NPR-A) is altered in relation to Npr1 mRNA expression, suggesting abnormal transcriptional control in hypertension [31]. Npr1 is identified as an anti-hypertrophic gene that negatively regulates abnormal cardiac remodeling and cardiac failure [32]. Consequently, increased expression of the Npr1 gene suggests a response to cardiac hypertrophy.
Although the plasma levels of renin, ACE and ANG II were not studied in this study, the gene expression of Ren, Ace, and Agtr1a in the kidneys was upregulated by hypertension (Figure 2), which suggests the transcriptional regulation of this system. The increase of the mRNA of Ren and Ace occurred in the stenotic kidney of the 2K1C animals when compared to the Sham and the contralateral kidney. However, transcripts of the Agtr1a gene increased in both kidneys of the 2K1C vs. Sham animals in an attempt to regulate local blood pressure [33]. Npr1 exhibits negative transcriptional regulation when there are high levels of Agt II in renal cells [34] and increased expression of Agtr1a is followed by decreased expression of Npr1 in 2K1C hypertension.
3.3. Modulation of connexin mRNA expression by renovascular hypertension
The connexins most frequently expressed in hepatocytes are Cx26 and Cx32, while the Cx43 in hepatic non-parenchymal cells [35]. Santolim [9] demonstrated the direct relationship between the increase in Hmgcr gene expression and expression of Cx26 transcripts. In this study, the gene expression of the Cx26 was not affected by hypertension, which may be associated with the transcriptional levels of the Hmgcr in this tissue (Figure 3). Cx32 plays a protective role in hepatic tissue and is downregulated in liver injury, inflammation and oxidative stress [36]. Microvascular changes related to hypertension are a major risk factor that predisposes the liver to disorders [37]. Data from our study demonstrate an increase in expression level of Cx32 in 2K1C animals. This finding suggests a response to the imbalance caused by hypertension, early protecting liver tissue (Figure 3). Generally, gap junctions mediate intercellular communication and support liver homeostasis. Therefore, the upregulation of Cx43 expression is stimulated in pathological conditions, including inflammation and oxidative stress [38]. However, Cx43 gene expression was not modulated by hypertension in the experimental period (Figure 3). Considering that the Cx37 and Cx40 are expressed in vascular cells [16], and that liver damage depends on the degree of microcirculation exposed to the increase in blood pressure [37], the increase of transcripts of these connexins in 2K1C animals is likely to be an adaptive protective response of the liver to allow better cell-to-cell communication.
Figure 3.
Modulation of the gene expression of connexins by hypertension analyzed by RT-PCR in (a) liver, (b) heart and (c) kidneys (R = right; L = left). Relative densitometry values were calculated after normalization with β-actin gene and the expression changes of the transcripts between the Sham and 2K1C groups were expressed as mean ± S.E.M. The top panels show the representative bands of the gel (see supplemental data). Significant differences between groups are indicated by ∗P < 0.05, ∗∗P < 0.001 vs. Sham; #P < 0.05, ≠ P < 0.001 and ≠≠≠ P < 0.0001 vs. contralateral kidney.
Cardiac hypertrophy induced by hypertension increased gene expression of the Cx37 and Cx40 in the heart (Figure 3). In rats, Cx37 and Cx40 are expressed in the endothelium, emphasizing the importance of these channels of intercellular communication in the control of cardiovascular homeostasis coordinating specific signaling processes [12]. The increased gene expression of Cx40 may be due to electrical stimulation generated by hypertension [39]. The increased expression of Cx43 was evidenced in hypertension, hypertrophy, hypercholesterolemia and post-infarction remodeling [40]. Thus, from the increase in Cx43 gene transcripts found in 2K1C animals and reports that connexin 43 protein is also upregulated by hypertension, we can suggest a transcriptional regulatory event for the Cx43 gene in hypertension through organ hypertrophy.
In the kidneys, Cx26 is predominantly present in the proximal tubules, and Cx32 is expressed by the cortical and medullary region of the renal tubules. The Cx32 acts as a tumor suppressor in renal cells and the loss of its function of the Cx26 and Cx32 was not related to changes in blood pressure in humans and rats [41, 42] justifying the maintenance of tissue mRNA levels in this study. However, the increased gene expression of Cx37 in the stenotic kidney of 2K1C animals is due to the increased inflammatory process, since this type of connexin may participate in the inflammatory process during the development of the disease [43]. Gene expression of Cx40 is decreased when compared to their contralateral kidney and Sham group. This decrease may be associated with gene expression Ren increase and renin secretion [44]. Rats with Cx40 deficiency, the renin secretion are increased and there is an increase in the number of renin-secreting cells [45]. In renovascular hypertension 2K1C, there is an increase in mRNA of Cx43 in the non-clipped kidney, which is exposed to increased blood pressure. The increased expression of Cx43 in the non-clipped kidney may be an adaptation of changes occurred in vascular remodeling and consequently associated with the development of hypertension [46]. This may justify decreased gene expression of Cx43 in the left kidney of 2K1C animals, when compared to Sham and contralateral kidney, and increased expression in the right kidney vs. Sham. Data related to the expression of Cx40 and Cx43 corroborate with left renal atrophy.
4. Conclusion
In conclusion, the present study demonstrated that renovascular hypertension 2K1C transcriptionally modulated RAS by increasing the Ren, Ace, and Agtr1a gene expression. It indicated a cardiac response, mediated by the increase of the natriuretic peptides gene expression, in the attempt of normalization of the arterial pressure. LDLr and Hmgcr genes, associated with cholesterol metabolism, were not modulated by 4 weeks of hypertension, as were serum cholesterol and triglyceride levels. Connexins transcripts were differentially regulated in the tissues studied, suggesting a response to hypertension, which might be a possible way to reestablish tissue homeostasis.
Declarations
Author contribution statement
C. de Oliveria: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
A. Enes: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
C. Vicente, J, Grégio and C. Clecêncio: Performed the experiments.
M. do Amaral: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Fundação Hermínio Ometto/FHO.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to Ph.D Cibele M. Prado for her teachings provided in the animal surgery.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Dinh Q.N., Drummond G.R., Sobey C.G. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014;2014:406960. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faselis C., Doumas M., Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int. J. Hypertens. 2011;2011:236239. doi: 10.4061/2011/236239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garovic V.D., Textor S.C. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112:1362–1374. doi: 10.1161/CIRCULATIONAHA.104.492348. [DOI] [PubMed] [Google Scholar]
- 4.Sabrane K., Kruse M.N., Fabritz L. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J. Clin. Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schweda F., Kurtz A. Regulation of renin release by local and systemic factors. Rev. Physiol. Biochem. Pharmacol. 2011;161:1–44. doi: 10.1007/112_2008_1. [DOI] [PubMed] [Google Scholar]
- 6.Maeda S., Tsukihara T. Structure of the gap junction channel and its implications for its biological functions. Cell. Mol. Life Sci. 2011;68:1115–1129. doi: 10.1007/s00018-010-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascio M. Connexins and their environment: effects of lipids composition on ion channels. Biochim. Biophys. Acta. 2005;1711:142–153. doi: 10.1016/j.bbamem.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Locke D., Harris A.L. Connexin channels and phospholipids: association and modulation. BMC Biol. 2009;7:52. doi: 10.1186/1741-7007-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santolim L.V., Amaral M.E.C., Fachi J.L. Vitamin E and caloric restriction promote hepatic homeostasis through expression of connexin 26, N-cad, E-cad and cholesterol metabolism genes. J. Nutr. Biochem. 2017;39:86–92. doi: 10.1016/j.jnutbio.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 10.C Brisset A., Iasakson B.E., Kwak B.R. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 2009;11:267–282. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wit C C., Hoepfl B., Wolfle S.E. Endothelial mediators and communication through vascular gap junctions. Biol. Chem. 2006;387:3–9. doi: 10.1515/BC.2006.002. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa X.F., Duling B.R. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanner F., Sorensen C.M., Holstein-Rathlou N.H. Connexins and the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1143–1155. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Hill C.E. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 2005;68:1171–1185. doi: 10.1111/j.1523-1755.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 15.Bode H.P., Wang L., Cassio D. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36:631–640. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 16.Shiojiri N., Niwa T., Sugiyama Y. Preferential expression of connexin37 and connexin40 in the endothelium of the portal veins during mouse liver development. Cell Tissue Res. 2006;324:547–552. doi: 10.1007/s00441-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 17.Goldblatt H., Lynch J., Hanzel R.F. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischaemia. J. Exp. Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Najafipour H., Vakili A., Shahouzehi B. Investigation of changes in apelin receptor mRNA and protein expression in the myocardium and aorta of rats with two-kidney, one-clip (2K1C) Goldblatt hypertension. J. Physiol. Biochem. 2015;71:165–175. doi: 10.1007/s13105-015-0394-z. [DOI] [PubMed] [Google Scholar]
- 19.Janicki J.S., Brower G.L., Gardner J.D. The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail. Rev. 2004;9:33–42. doi: 10.1023/B:HREV.0000011392.03037.7e. [DOI] [PubMed] [Google Scholar]
- 20.Mora S., Glynn R.J., Ridker P.M. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J., Lecanu L., Han Z. Inhibition of adrenal cortical steroid formation by procaine is mediated by reduction of the cAMP-induced 3-hydroxy-3-methylglutaryl-coenzyme A reductase messenger ribonucleic acid levels. J. Pharmacol. Exp. Therapeut. 2013;307:1148–1157. doi: 10.1124/jpet.103.055178. [DOI] [PubMed] [Google Scholar]
- 22.Wannamethee S.G., Shaper A.G., Perry I.J. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke. 1997;28:557–563. doi: 10.1161/01.str.28.3.557. [DOI] [PubMed] [Google Scholar]
- 23.Weir M.R., Bakris G.L., Weber M.A. Renal outcomes in hypertensive Black patients at high cardiovascular risk. Kidney Int. 2012;81:568–576. doi: 10.1038/ki.2011.417. [DOI] [PubMed] [Google Scholar]
- 24.Navar L.G., Zou L., Von Thun A. Unraveling the Mystery of Goldblatt hypertension. News Physiol. Sci. 1998;13:170–176. doi: 10.1152/physiologyonline.1998.13.4.170. [DOI] [PubMed] [Google Scholar]
- 25.Yiannkouris F., Wang Y., Shoemarker R. Deficiency of angiotensinogen in hepatocytes markedly decreases blood pressure in lean and obese male mice. Hypertension. 2015;66:836–842. doi: 10.1161/HYPERTENSIONAHA.115.06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H., Wu C., Howatt D.A. Angiotensinogen exerts effects independent of angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2016;36:256–265. doi: 10.1161/ATVBAHA.115.306740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Handtrack C., Cordasic N., Klanke B. Effect of the angiotensinogen genotype on experimental hypertension in mice. J. Mol. Med. (Berl). 2007;85:343–350. doi: 10.1007/s00109-007-0166-5. [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P., Iliev I., Costa G.M. Preventing left ventricular hypertrophy by ACE inhibition in hypertensive patients with type 2 diabetes: a prespecified analysis of the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Diabetes Care. 2008;31:1629–1634. doi: 10.2337/dc08-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowley S.D., Gurley S.B., Herrera M.J. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. U.S.A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra S., Cherian D., Verghese P.P. Physiology and clinical significance of natriuretic hormones. Indian J. Endocrinol. Metab. 2013;17:83–90. doi: 10.4103/2230-8210.107869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay J., Hum D.H.F., Sanchez R. TA repeat variation, Npr1 expression, and blood pressure: impact of the Ace locus. Hypertension. 2003;41:16–24. doi: 10.1161/01.hyp.0000042664.75193.1b. [DOI] [PubMed] [Google Scholar]
- 32.Pandey K.N. Biology of natriuretic peptides and their receptors. Peptides. 2005;26:901–932. doi: 10.1016/j.peptides.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Gurley S.B., Riquier-Brison A.D.M., Schnermann J. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metabol. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg R., Pandey K.N. Angiotensin II-mediated negative regulation of Npr1 promoter activity and gene transcription. Hypertension. 2003;41:730–736. doi: 10.1161/01.HYP.0000051890.68573.94. [DOI] [PubMed] [Google Scholar]
- 35.Maes M., Decrock E., Cogliati B. Connexin and pannexin (hemi)channels in the liver. Front. Physiol. 2013;4:405. doi: 10.3389/fphys.2013.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiburcio T.C., Willebrords J., da Silva T.C. Connexin32 deficiency is associated with liver injury, inflammation and oxidative stress in experimental non-alcoholic steatohepatitis. Clin. Exp. Pharmacol. Physiol. 2017;44:197–206. doi: 10.1111/1440-1681.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaffri J.M., Mohamed S., Ahmad I.N. Effects of catechin-rich oil palm leaf extract on normal and hypertensive rats' kidney and liver. Food Chem. 2011;128:433–441. doi: 10.1016/j.foodchem.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 38.Balasubramaniyan V., Dhar D.K., Warner A.E. Importance of Connexin-43 based gap junction in cirrhosis and acute-on-chronic liver failure. J. Hepatol. 2013;58:1194–1200. doi: 10.1016/j.jhep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Leaf D.E., Feig J.E., Vasquez C. Connexin 40 imparts conduction heterogeneity to atrial tissue. Circ. Res. 2008;103:1001–1008. doi: 10.1161/CIRCRESAHA.107.168997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boengler K., Schulz R. Connexin 43 and Mitochondria in cardiovascular Health and disease. Adv. Exp. Med. Biol. 2017;982:227–246. doi: 10.1007/978-3-319-55330-6_12. [DOI] [PubMed] [Google Scholar]
- 41.Kurtz A. Renal connexins and blood pressure. Biochim. Biophys. Acta. 2012;1818:1903–1908. doi: 10.1016/j.bbamem.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Hagiwara H., Sato H., Shirai S. Connexin 32 down-regulates the fibrinolytic factors in metastatic renal cell carcinoma cells. Life Sci. 2006;78:2249–2254. doi: 10.1016/j.lfs.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 43.Toubas J., Beck S., Pageaud A.L. Alteration of connexin expression is an early signal for chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2011;301:F24–32. doi: 10.1152/ajprenal.00255.2010. [DOI] [PubMed] [Google Scholar]
- 44.Wagner C., de Wit C., Kurtz L. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ. Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 45.Machura K., Neubauer B., Muller H. Connexin 40 is dispensable for vascular renin cell recruitment but is indispensable for vascular baroreceptor control of renin secretion. Pflugers Arch. 2015;467:1825–1834. doi: 10.1007/s00424-014-1615-y. [DOI] [PubMed] [Google Scholar]
- 46.Haefliger J.A., Krattinger N., Martin D. Connexin43-dependent mechanism modulates renin secretion and hypertension. J. Clin. Invest. 2006;116:405–413. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.