Abstract
AMD has been linked to memory deficits, with no established neural mechanisms. In order to describe how resting-state, brain network functional connectivity relates to memory and AMD status in older adults without dementia, we collected resting-state brain fMRI and standardized verbal recall tests from 42 older adults with AMD and 41 age-matched controls. We used seed-based whole brain analysis to quantify the strength of functional connectivity between hubs of the default mode network (DMN) and a network of medial temporal regions relevant for memory. Our results indicated neither memory performance nor network connectivity differed by AMD status. However, the AMD participants exhibited stronger relationships than the controls between memory performance and connectivity from the memory network hub (left parahippocampal) to two other regions: the left temporal pole and the right superior/middle frontal gyri. Also, the connectivity between the medial prefrontal cortex and posterior cingulate cortex of DMN correlated more strongly with memory performance in AMD compared to control. We concluded that stronger brain-behavior correlation in AMD may suggest a role for region-specific connectivity in supporting memory in the context of AMD.
Keywords: AMD, DMN, episodic memory, older adults, LPHC, visual impairment
Introduction
Epidemiological studies have found that visually impaired patients exhibit lower cognitive function and cognitive testing scores(Lin et al., 2004; Reyes-Ortiz et al., 2005; Tay et al., 2006; Chen et al., 2017)and may be at higher risk of dementia(Rogers and Langa, 2010). Age-related macular degeneration (AMD) is the most common cause of permanent vision loss in US older adults(CDC, 2017). It affects 30% of older adults over the age of 75(NIH, 2011) and incurs enormous medical cost of up to 575 million dollars annually in the United States for direct AMD treatment(Rein et al., 2006). In addition to causing central vision loss, AMD has been associated with more prevalent and progressive cognitive impairment(Wong et al., 2002; Clemons et al., 2006; Baker et al., 2009; Rozzini et al., 2014; Zhou et al., 2016) and dementia(Klaver et al., 1999; Tsai et al., 2015).The co-occurrence of cognitive and visual impairment in this population is troubling, as older adults with both impairments have twice the odds of disability compared to older adults with visual impairment alone(Whitson et al., 2007).
AMD is associated with cognitive impairment in a wide range of domains, including verbal fluency(Wong et al., 2002; Clemons et al., 2006), memory(Woo et al., 2012; Lindekleiv et al., 2013; Rozzini et al., 2014), and visual-spatial processing(Woo et al., 2012). There could be many explanations for the observed relationship between AMD and poor performance on cognitive tests, and these explanations are not mutually exclusive(Monge and Madden, 2016; Evans, 2017). Some of the association may be explained by testing artifact if cognitive tests are administered in a way that relies on visual ability(Chen et al., 2017). A second possibility is that some confounding factor (e.g., smoking) was simultaneously related to increased risk for AMD and cognitive decline(Greenland et al., 1999; Whitson et al., 2010; Whitson et al., 2012). In epidemiological studies in older populations that have adjusted for potential confounding or mediating factors, except vision, in the relationship between AMD and cognition, the association is reduced but not completely attenuated(Wong et al., 2002; Clemons et al., 2006). Furthermore, in one case-control study, AMD patients were still more susceptible to cognitive impairment than non-AMD controls after adjusting for visual acuity(Woo et al., 2012). This leads to speculations of a third possibility, which is that neurological changes associated with AMD exert a direct or indirect effect on cognition. In theory, AMD-related neurological changes could be the result of either sensory-level factors such as diminished signaling from the damaged macula, or behavioral factors associated with the loss of vision-dependent activities (e.g., reading, driving, and social interaction) important in maintaining cognitive reserve in an aging brain(Prins et al., 2016).
In cognitive tasks, such as memory recall, AMD patients may be at a disadvantage in memory formation and retrieval, due to diminished visual cueing to create and store memories, or due to degraded visual system pathways that would normally play a role in memory retrieval. Although research from our group and others has suggested brain functional reorganization may be associated with AMD, including changes in the primary visual cortex(Baker et al., 2005; Masuda et al., 2008) and the fronto-parietal control networks(Szlyk and Little, 2009; Zhuang et al., 2017), it remains unclear whether such reorganization plays a role in cognitive function in AMD. Understanding the neuronal connectivity related to cognitive function in AMD would be the first step towards intervention for AMD related cognitive impairment.
It is worth noting that despite the literature mentioned above, there have been a few AMD studies where cognitive function is relatively preserved. In a Canadian study, AMD patients were found to have similar cognitive test results compared to controls(Varin et al., 2020). The authors attributed this surprising finding to differences in age and education in the two groups and relatively short duration of visual loss in the AMD group. In an earlier study from Australia, cognitive impairment was also only observed in late but not early AMD (Pham et al., 2006). Considering this disparity and our finding of AMD-related neuronal reorganization, it is possible that recruitment of compensating cortices may mask the cognitive deficit in early AMD. Further investigating this possibility calls for functional brain analysis in AMD.
In populations without AMD or other sensory disorders, brain imaging research on neural underpinnings of cognitive impairment has indicated structural changes associated with cognitive decline(Shankar, 2010; Morrison and Baxter, 2012). Alzheimer’s disease (AD), which is the leading cause of dementia, has been associated with atrophy in medial temporal, posterior cingulate/precuneus, and temporoparietal regions (Hyman et al., 1984; Mitchell et al., 2002). In age-related cognitive decline, cognitive deficits have also been linked to prominent loss of white matter integrity, especially in the frontal and prefrontal cortices(Sowell et al., 2003; Raz, 2005; Barrick et al., 2010; Fjell and Walhovd, 2010; Madden et al., 2012; Madden and Parks, 2017). The brain’s white matter axons and synapses comprise networks that are activated or deactivated simultaneously to support cognitive function(Andrews-Hanna et al., 2007). The disintegration of these functional neural networks has been linked with age-related decline in cognitive function(Bennett and Madden, 2014; Fjell et al., 2017; Madden et al., 2017).
Studying the functional networks of the brain requires a different technique than measuring static anatomy and physiology, and functional magnetic resonance imaging (fMRI) scans using blood oxygen level dependent (BOLD) signal have proved useful for this purpose(Raichle and Gusnard, 2005; Raichle and Mintun, 2006). fMRI is sensitive to the rate of oxygen consumption across the brain—an indicator of brain activity(Raichle and Gusnard, 2005; Raichle and Mintun, 2006).When measured in serial scans, subtle connections and interactions between regions of the brain can be inferred, revealing functional networks(Greicius et al., 2003; Fox et al., 2005; Vincent et al., 2006). Of the functional networks that change with aging, the DMN and its medial-temporal-lobe subsystem have been frequently implicated in memory and cognition in general(Greicius et al., 2003; Tromp et al., 2015).
The DMN is a resting-state network that consists of several hubs across the brain, including the anterior medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC), bilateral lateral parietal cortex, the bilateral hippocampal formation, and the bilateral parahippocampal cortex (PHC)(Greicius et al., 2003; Andrews-Hanna et al., 2007).The DMN has been significantly related to cognition(Greicius et al., 2004; Andrews-Hanna et al., 2007). Its activation has been associated with self-related processes such as autobiographical memory, self-referential and affective decision-making, and thinking about one’s future(Buckner et al., 2008).This activity is significantly downregulated during active cognitive tasks(Buckner et al., 2008), particularly stimulus-driven goal-directed cognitive tasks(Anticevic et al., 2012).The temporal hubs of the DMN—hippocampal cortices and PHC—contribute to a separate network that dynamically interacts with the DMN during memory retrieval, especially without external stimuli(Huijbers et al., 2011; Andrews-Hanna et al., 2014; Ward et al., 2014). This network contains the medial temporal structures often cited in memory formation and retrieval, and has thus been referred to as a medial temporal memory network(Lech and Suchan, 2013). Although far less studied with respect to memory, other hubs of the DMN, such as the frontal and parietal cortices, also appeared supportive for memory, possibly due to the need for attention and concentration during memory tasks(Cabeza and Nyberg, 2000; Kramer et al., 2005). Empirically, lower resting DMN connectivity has also been correlated with worse performance in specific aspects of cognition, including memory, processing speed and executive function, after controlling for age(Andrews-Hanna et al., 2007; Ward et al., 2015). Overall, higher resting-state connectivity of the DMN and the medial temporal memory network have both been positively associated with cognition in older adults(Jeong et al., 2015). On the other hand, a systemic review by Anthony and Lin had suggested that both DMN and medial temporal regions are related to cognitive reserve, but they found few studies that focused on network connectivity as a compensation for cognitive deficits(Anthony and Lin, 2018).
To our knowledge, prior research has not investigated whether hubs and connectivity of the DMN and the medial temporal memory network play a role in supporting cognition or in potentially compensating for AMD-related cognitive deficits. Given previously established associations between these networks, their hubs, and memory, we hypothesized that these networks, as individual hubs or collectively, may play an amplified role in supporting memory in the context of AMD, which may or may not be able to fully compensate for the deficit. To test this hypothesis, we compared brain-behavior relationships (i.e., memory performance relating to resting-state network connectivity) in a group of AMD-affected older adults and age-matched controls with healthy eyes.
Methods
Participants
The study involved a total of 42 participants with vision loss secondary to AMD and 41 controls with normal eyes, matched by age (Table 1). Out of the AMD group, all disease subgroups, including non-neovascular AMD with/without geographic atrophy and neovascular AMD, are represented (Supplementary Table 1 and 2). Participants with known or suspected diagnoses of dementia, based on either chart review or reports from patients/companions, were excluded from the study. We did not exclude individuals with mild cognitive complaints. All participants were community-dwelling, right hand dominant, and fluent speakers of English. AMD patients were recruited from clinical practices at the Duke Eye Center; enrollment criteria for AMD patients required them to have impaired vision in at least one eye, defined as visual acuity of worse than 0.25 according to Logarithm of the Minimal Angle of Resolution (LogMAR) scales (approximately 20/35 Snellen acuity, as 0 in logMAR corresponds to 20/20 in Snellen), and no other significant eye disease besides AMD. Controls were recruited from patients’ friends and family, and from a registry maintained in the Duke Aging Center which included older adults who have expressed a willingness to be contacted about opportunities to participate in research. Individuals who were interested in participating in this protocol and had no known history of AMD or other eye disorders underwent a dilated eye exam by an ophthalmologist to ensure eligibility, which required no significant eye conditions and visual acuity of LogMAR 0.2 or better in both eyes. All participants provided informed consent and were compensated for their time. All experimental procedures were approved by the Duke University Medical Center Institutional Review Board.
Table 1.
Demographics, Visual Acuity and Measures of Memory of Participants
| AMD | Control | P Group Difference (2-sided) | |
|---|---|---|---|
| N | 42 | 41 | |
| Age in years, mean (SD*) | 75.3 (8.9) | 74.5 (7.2) | 0.65 |
| Gender, Proportion female (SD) | 0.67 (0.48) | 0.54 (0.50) | 0.23 |
| Years of Education, mean (SD) | 14.9 (2.7) | 16.5 (3.0) | 0.01 |
| Visual acuity of the better eye/logMAR (SD) | 0.41 (0.06) | 0.04 (0.38) | <0.01 |
| Episodic Memory Factor (SD) | 0.07 (0.95) | −0.07 (0.99) | 0.53 |
| Wechsler Immediate Recall (SD) | 23.8 (6.4) | 25.1 (7.1) | 0.34 |
| Wechsler Delayed Recall (SD) | 19.5 (7.3) | 21.0 (8.1) | 0.36 |
| BTACT Immediate Recall (SD) | 5.8 (2.6) | 5.8 (1.8) | 0.99 |
| BTACT Delayed Recall (SD) | 3.4 (2.8) | 3.2 (2.4) | 0.78 |
SD: standard deviation
BTACT: Brief Test of Adult Cognition for Telephone
Neurocognitive Assessment
All participants were administered a standardized battery of cognitive testing that was designed for use in visually impaired populations such that no items relied on visual cues or visually mediated activities (e.g., no drawing, reading, recognizing pictures or symbols). Tests related to episodic memory included the Logical Memory I and II subtests from the Wechsler Memory Scale, Third Edition (WMS-III) (Corporation, 1997), and Verbal Memory from the Brief Test of Adult Cognition by Telephone (BTACT)(Tun and Lachman, 2006). The Logical Memory test required the participants to recall details of a paragraph read aloud, both immediately (Logical Memory I), and after a delay period (Logical Memory II). The Verbal Memory task required immediate and delayed recall of 15 unrelated items that were read aloud. Factor analysis was performed on the four scores mentioned above and a single unrotated verbal episodic memory factor with eigenvalue >1 was generated. The factor loading for Logical Memory I, Logical Memory II, Verbal Memory-immediate, and Verbal Memory-delayed were 0.85, 0.87, 0.63, and 0.68, respectively. Logical memory tests and item recall lists have been used previously to characterize memory performance in cohorts of patients with macular disease or visual impairment(Whitson et al., 2010; Whitson et al., 2012).
fMRI analysis
Imaging Acquisition and Processing
Participants were scanned in a 3.0 Tesla GE MR 750 whole-body 60 cm bore human scanner equipped with 40 mT/m gradients and a 150 T/m/s slew rate, with an eight-channel head coil that was used for radio frequency (RF) reception (General Electric, Milwaukee Wisconsin, USA). Sagittal T-1 weighted localizer images were acquired and used to define a volume for data collection and high order shimming. A semi-automated high-order shimming program was used to ensure global field homogeneity. High-resolution structural images were acquired using a 3Dfast spoiled gradient echo(fSPGR) pulse sequence (repetition time [TR] = 8.156 ms; echo time [TE] = 3.18 ms; TI = 450 ms; field of view [FOV] = 25.6 cm2; flip angle =12°; voxel size = 1 × 1 × 1 mm; 166 contiguous slices, sensitivity encoding [SENSE]factor = 2).
Two runs of functional images were acquired during a resting state, in which participants were instructed to close their eyes (to avoid visual sensory activation), and to not let their minds fixate on anything in particular using a gradient echo sequence (TR = 2 s, TE = 27 ms, flip angle = 77°, FOV = 24 cm2, SENSE factor = 1, voxel size = 3.75 ×3.75 ×4 mm, 34 contiguous oblique axial slices, parallel to the anterior-posterior commissure [AC-PC] line, interleaved acquisition). Verbal communication between runs confirmed that participants were awake. In each resting-state run, four initial RF excitations were performed to achieve steady state equilibrium and were subsequently discarded. Each resting state run consisted of the acquisition of a time series of 180 brain volumes (images), which lasted for 6 minutes.
The resting-state data were preprocessed using an in-house developed pipeline (https://wiki.biac.duke.edu/biac:analysis:resting_pipeline) based on tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL version 4.0, www.fmrib.ox.ac.uk/fsl). The first four images were removed for each resting state run to achieve steady state equilibrium. All remaining functional images were aligned with respect to the first image within each run to correct for head movements during data acquisition. A bandpass filter was applied to filter the functional data in the time dimension so that frequencies were retained between 0.001 Hz and 0.08 Hz. The aligned and filtered images were normalized to the MNI space using a 12 degree of freedom affine transformation implemented in FSL’s Linear Image Registration Tool (FLIRT). We further removed constant offsets and linear drift over each run (Fair et al., 2007), and regressed out the six rigid body head motion parameters, the signal averaged over the white matter and the signal averaged over the cerebrospinal fluid regions to reduce non-neuronal influence to BOLD corrections(Van Dijk et al., 2010). The normalized images were smoothed using an isotropic Gaussian kernel of 5 mm FWHM.
Functional Correlation Analysis
On the resting-state series, the regions of interest (ROI)used to identify the DMN and medial temporal memory network were derived from established anatomical locations(Andrews-Hanna et al., 2007). Based on the previous descriptions of these networks, we based our identification of the DMN on the mPFC region and the medial temporal memory network on the PHC regions. We defined the medial temporal memory network based on PHC, rather than hippocampus, to limit overlap with other memory networks activated by external stimuli and in concordance with findings that the PHC is the primary hub of this network that links with DMN through PCC(Ward et al., 2014). Time series (activity) of an ROI were calculated by averaging the activity of each voxel within the 8mm-radius ROI. Seed-based whole brain correlation analyses were then performed by correlating activity from each voxel of the brain to that of the given ROI of the DMN or memory network, to produce a functional connectivity map for an individual.
For each ROI, we analyzed the spatial maps for all participants using a multiple regression method in a group random-effects model in SPM12 (Statistical Parametric Mapping, Wellcome Institute of Cognitive Neurology, London, UK. www.fil.ion.ucl.ac.uk), under MATLAB (MathWorks Inc., Natick, MA, USA). For network-behavioral correlations, the design matrix consisted of four modulators (regressors): age, years of education, the memory factor scores of all AMD patients, and the memory factor scores of all controls. In the third modulator, the memory score of each AMD patient was mean-corrected within the AMD group, while the memory score of each control was coded as 0. In the fourth modulator, the memory score of each control was mean-corrected within the control group, while the memory score of each AMD patient was coded as 0. For both whole brain analysis and network-behavior correlations, we included age and years of education as two extraneous variables so that any potential group difference between AMD patients and controls would not be confounded with individual differences in these extraneous variables.
Significant clusters were identified at two stages. First, connectivity strength was thresholded at p < 0.005, uncorrected, at the voxel level. Second, multiple comparisons were performed to filter out significant clusters only when they survived p < 0.05, cluster level corrected. SPM coordinates of significant cluster peaks are reported in MNI space. Neural connectivity between ROI and each voxel within each significant cluster were extracted in SPM and the mean β value for that cluster is presented as a measure of the effect size. Regions were identified by using the AAL atlas(Tzourio-Mazoyer et al., 2002) and Brodmann templates as implemented in MRIcron (University of South Carolina, McCausland Center for Brain Imaging, Columbia, SC, USA. http://www.MRicro.com/MRicron).
Results
The demographics of all participants by AMD status are presented in Table 1. The groups were well matched for age, gender and race, but the years of education differed significantly, with controls better educated than AMD participants. There was no significant difference in the episodic memory factor between AMD and control participants (t=0.64, p=0.26), and this was not affected by the subgroup status of AMD (supplementary table 1 and 2).
Resting-state Networks
Seed-based whole brain analysis of all the participants’ data (i.e., healthy and AMD participants combined) defined our medial temporal memory network using the LPHC seed region (Table 2, Figure 1a). The network appeared to include bilateral medial and dorsal temporal cortices and part of the insula. Analysis using RPHC demonstrated a very similar network; we elected to focus on the network identified by the LPHC because we were specifically interested in verbal memory, which may be a more left-lateralized ability. The same analysis was carried out in AMD and control groups separately. No significant differences in resting connectivity of the memory network were demonstrated in AMD compared to control participants, or among subgroups of AMD participants.
Table 2.
Areas of significant connectivity for the memory and default mode network (DMN)in all participants*, controlling for age and years of education.
| Cluster regions | Brodmann Areas | size (voxels) | max Z | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| memory network | ||||||
| Seed region: LPHC | ||||||
| L/R Fusiform, STG, MTG, ITG, TP, PHC, HF, Amygdala, Thalamus, MCC, PCC, Calcarine, Cerabellum, and L MOG, MTG, AG | 17,18,19,20,21,30,37,38,39 | 39891 | Inf | −26 | −38 | −16 |
| R MTG, MOG, AG | 37,39 | 1551 | 6.12 | 48 | −66 | 14 |
| L MFG, SFG** | 8,9 | 884 | 4.79 | −22 | 26 | 38 |
| DMN | ||||||
| Seed region: mPFC | ||||||
| L/R ACC, MCC, PCC, Precuneus, SFG, MFG, SMFG, Calcarine, AG*** | 9,10,11,23,24,26,30,32,39,40,48 | 60540 | Inf | 0 | 40 | 20 |
| Cerebellum, Vermis | 595 | 6.12 | −2 | −42 | −28 | |
Threshold: p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected
LPHC: left parahippocampal cortex, L: left, R: right, STG: superior temporal gyrus, MTG: middle temporal gyrus, ITG: inferior temporal gyrus, HF: hippocampal formation, MCC: middle cingulate cortex, PCC: posterior cingulate cortex, MOG: middle occipital gyrus, AG: angular gyrus, SFG: superior frontal gyrus
mPFC: medial pre-frontal cortex, ACC: anterior cingulate cortex, MFG: middle frontal gyrus, SMFG: superior medial frontal gyrus
Brain regions in italics correlated to MNI coordinates for peak voxel, corresponding to highlighted regions in Figure 1 A and B.
Figure 1.
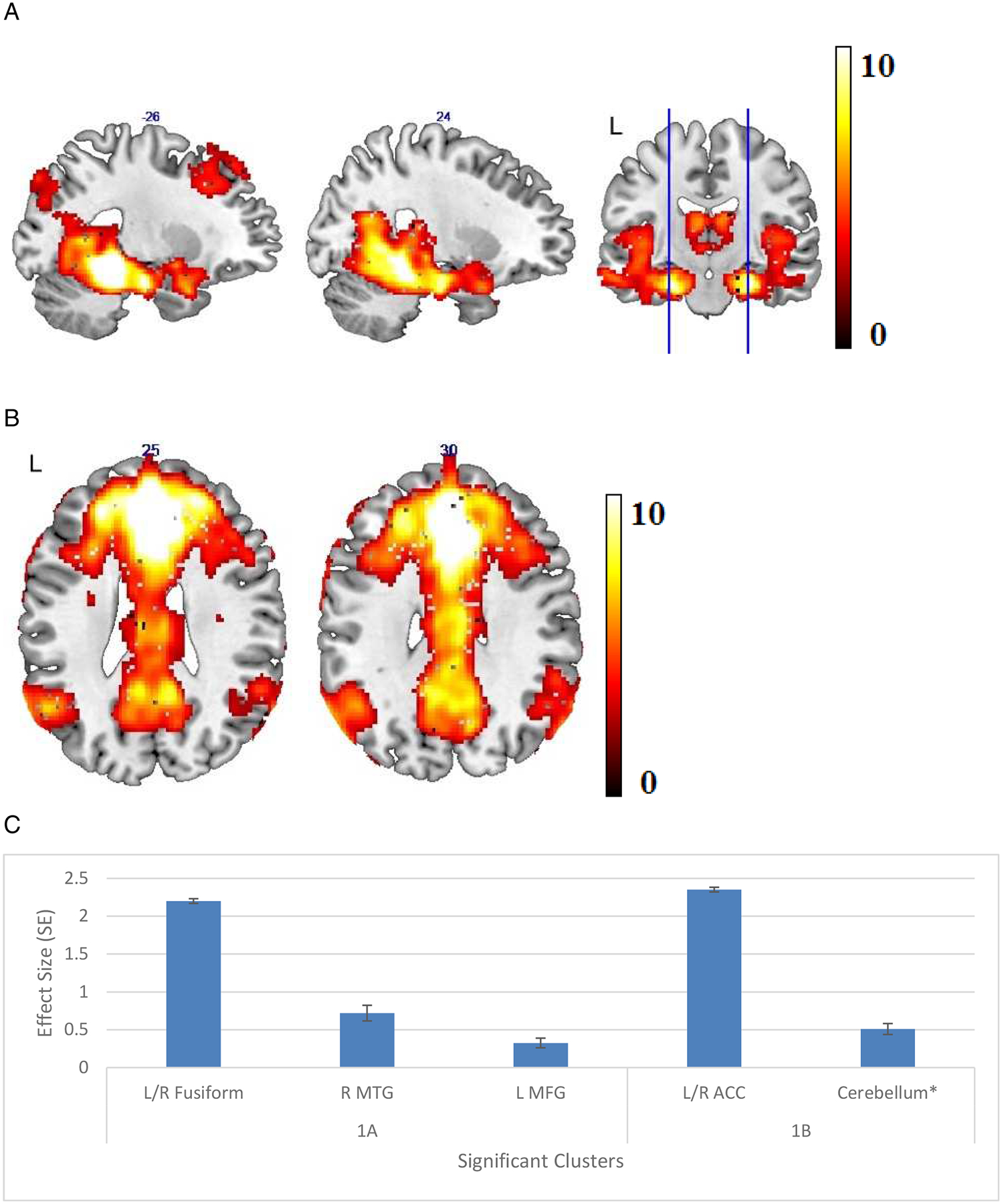
(A) Areas of significant connectivity of the memory network for all participants in a whole brain correlation analysis with the left parahippocampal cortex (LPHC) as a seed region. (B) Areas of significant connectivity of the default mode network (DMN) for all participants in a whole brain correlation analysis with the medial prefrontal cortex (mPFC) as a seed region. (C) Effect size (average β value) by clusters with significant connectivity with seed regions with standard error (SE) plotted. Cluster regions are detailed in Table 2, labeled by the brain region containing peak voxel for the cluster.
* L: left, R: right, MTG: middle temporal gyrus, MFG: middle frontal gyrus, ACC: anterior cingulate cortex
The DMN was demonstrated using mPFC as the seed region in Table 2, Figure 1b, consisting of the bilateral PCC, anterior cingulate cortices, and precuneus. While comparing the whole-brain analysis results of all participants, AMD and control groups, no significant differences in resting DMN connectivity were identified in AMD compared to control participants, or among subgroups of AMD participants. For both networks, the effect sizes and standard errors of significant clusters can be found in Figure 1c.
Resting Network Connectivity Correlating with Memory
For both networks, a stronger brain-behavior relationship was observed in AMD compared to control participants, controlling for age and education. For the memory network, the connections LPHC—L inferior and medial temporal gyri, temporal pole (ITG, MTG and TP), and LPHC—R inferior/middle/superior frontal gyri (IFG, MFG and SFG) were more positively correlated with episodic memory performance in AMD compared to control participants (Figure 2a). The same significant brain-behavior relationship was demonstrated in AMD participants alone (Figure 2b), but not in controls. Of note, no significant brain-behavior relationship was detected using RPHC seed region.
Figure 2.
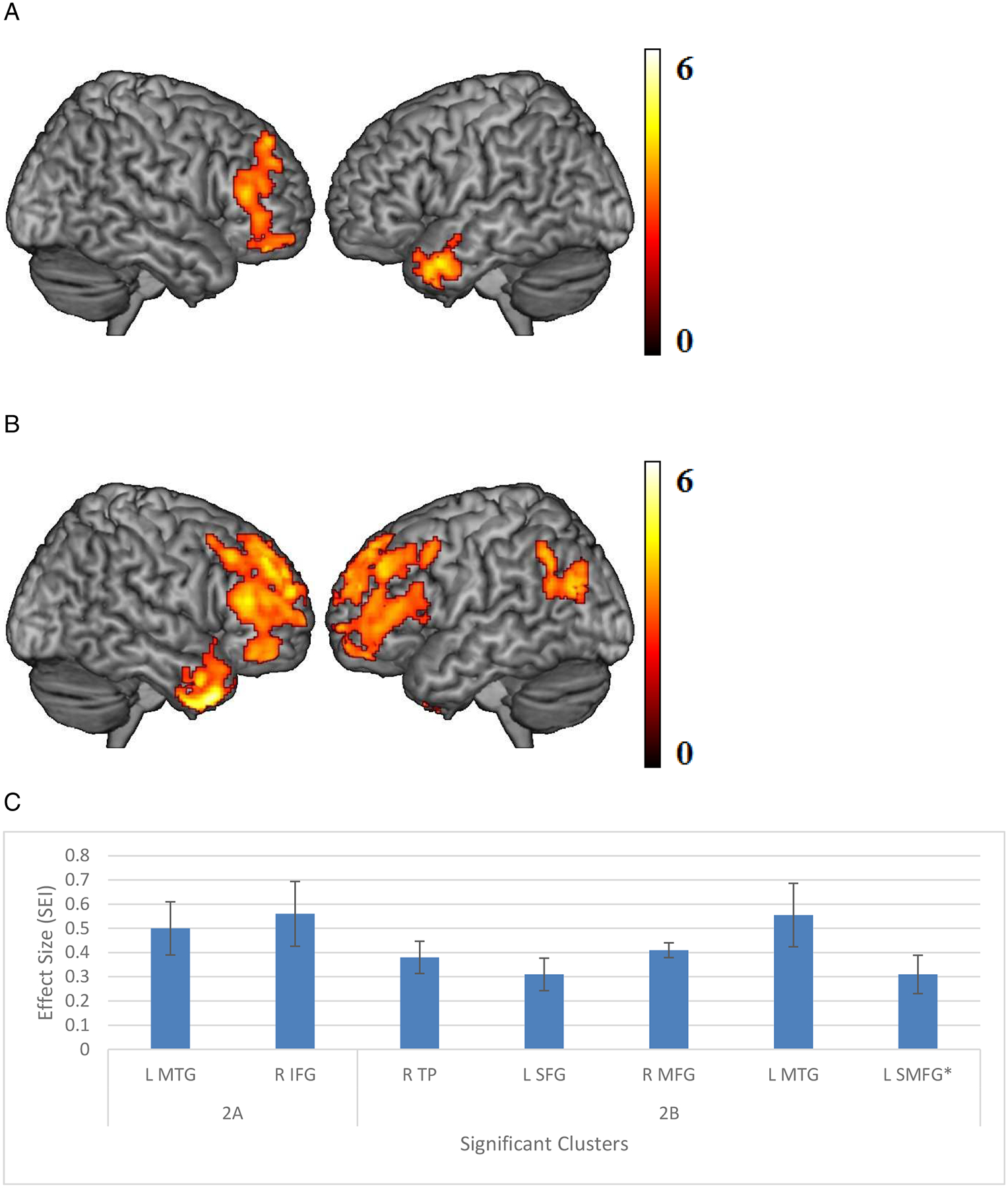
(A) Areas of significant connectivity of the memory network that correlates positively with episodic memory, using left parahippocampal cortex (LPHC) seed region in age-related macular degeneration (AMD) compared to controls. (B) Areas of significant connectivity of the memory network that correlates positively with episodic memory, using LPHC seed region in AMD participants only. (C) Effect size (average β value) by clusters with significant connectivity with seed regions with standard error (SE) plotted. Cluster regions are detailed in Table 3, labeled by the brain region containing peak voxel for the cluster.
(Threshold: p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected)
* L: left, R: right. MTG: middle temporal gyrus, IFG: inferior frontal gyrus, TP: temporal pole, SFG: superior frontal gyrus, MFG: middle frontal gyrus, SMFG: superior medial frontal gyrus
Within the DMN, the mPFC—PCC connectivity was more strongly correlated with episodic memory performance in AMD compared to control participants (Figure 3a). This significant correlation was also observed in AMD participants alone (Figure 3b) but not in controls. No regions outside the DMN showed increased connectivity with the DMN hubs that correlated with behavior. The effect sizes and standard errors of significant clusters can be found in Figure 2c and Figure 3c, respectively.
Figure 3.
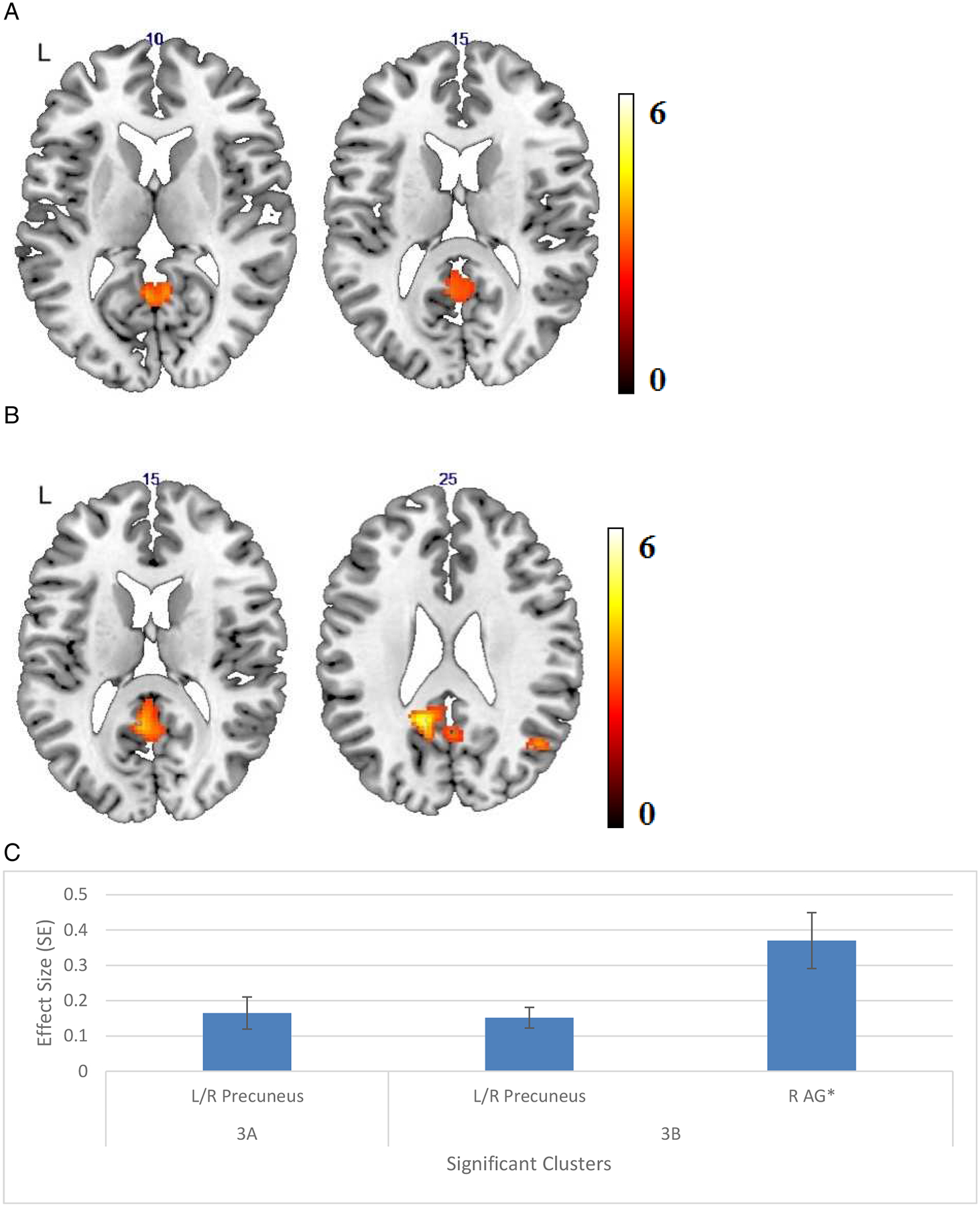
(A) Areas of significant connectivity of the default mode network (DMN) that correlated positively with episodic memory, using medial prefrontal cortex (mPFC) seed region in age-related macular degeneration (AMD) compared to controls. (B)Areas of significant connectivity of the DMN that correlated positively with episodic memory, using mPFC seed region in AMD participants only. (C) Effect size (average β value) by clusters with significant connectivity with seed regions with standard error (SE) plotted. Cluster regions are detailed in Table 3, labeled by the brain region containing peak voxel for the cluster.
(Threshold: p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected)
* L: left, R: right, AG: angular gyrus
Discussion
To our knowledge, this is the first attempt to investigate how resting-state functional network connectivity relates to AMD-associated cognitive function. Our analyses pointed to significant AMD-related differences in how memory performance is linked to connectivity of hubs in a medial temporal memory network and DMN. On average, AMD and control participants demonstrated similar verbal episodic memory scores and connectivity of the two resting networks. When considering episodic memory, AMD participants demonstrated a stronger positive correlation between memory scores and functional connectivity than non-AMD controls in the LPHC—LTP, LPHC—RSFG/MFG, and mPFC—PCC. These results provided an interesting comparison to brain-behavioral connectivity studies in both healthy and cognitively impaired populations and allowed us to speculate on possible mechanisms that may relate to memory performance in AMD.
The PHC—frontal and mPFC—PCC connectivity has been previously studied with respect to memory in other populations(Andrews-Hanna et al., 2007; Barredo et al., 2015).The PHC—frontal linkage was previously found to be significant in functional imaging studies of episodic memory related to memory encoding(Tromp et al., 2015).The resting mPFC—PCC connectivity, two major hubs of the DMN, has been positively associated with memory performance in normal aging(Andrews-Hanna et al., 2007; Ward et al., 2015) and was more tightly linked with better memory performance in individuals with traumatic brain injury compared to controls(Sharp et al., 2011). Other reports have suggested disconnection of the DMN, especially the mPFC—PCC, is often significant in early and late AD(Gili et al., 2011; Brier et al., 2012; Hafkemeijer et al., 2012; Griffanti et al., 2015), leading to suggestions of using DMN connectivity as a screening tool for the progression of the disease(Mohan et al., 2016). Our results also showed that both mPFC—PCC and LPHC—R IFG/MFG/SFG connections, not within the memory network, correlated more positively with verbal episodic memory for AMD participants compared to controls. Thus, although the observational nature of the study and its cross-sectional design do not permit us to make definitive conclusions about the mechanism that accounts for the observed differences, our findings could be consistent with the theory of useful compensation, where flexibility of the brain seems to allow additional neuro-behavioral connections to develop to offset cognitive deficits that arise with normal aging, AD, or other insults such as AMD (Sharp et al., 2011; Tromp et al., 2015). Interestingly, a study of post-traumatic stress disorder patients and controls showed that decreased PHC-PCC connectivity associated with impaired memory performance, with subsequent normalization alongside recovery of memory during follow-up(De Simoni et al., 2016). This and the useful compensation theory suggest that the neuro-behavioral relationship we observed may be dynamic and makes it a worthy objective for longitudinal study. Furthermore, the relationship observed in our study is specific for LPHC, corresponding to an activation pattern related to verbal memory(Smagula et al., 2018). Given that activation of the prefrontal cortex and the medial temporal structures were significant in a review for episodic memory encoding and retrieval(Cabeza and Nyberg, 2000), while mPFC and PCC were both activated for memory retrieval tasks(Cabeza and Nyberg, 2000), the connectivity we identified might be involved in memory consolidation between encoding and retrieval tasks, requiring further studies to test the hypothesis.
One inconsistency with this theory is the fact that we did not observe significantly worse memory performance in AMD patients compared to controls, or among subgroups of AMD participants in this study. Previous studies have reported that AMD patients under-performed on memory tasks, compared to normal-sighted peers, even after adjusting for age and education(Woo et al., 2012). The fact that we did not observe a group difference may reflect either a selection bias (with AMD participants who are willing to undergo brain MRI and extensive neuropsychological assessment being above-average performers or people with exceptionally high cognitive reserve), a delay in cognitive decline due to useful compensation, or a lack of statistical power to detect a true difference.
Another possibility that cannot be fully addressed in the current study is the role that comorbid early AD may have played in each group. Although we excluded people with known or suspected dementia diagnoses, it is possible that some participants had early AD, which can be difficult to distinguish from mild cognitive impairment or early dementia. In the current study, we lacked full clinical assessments and AD biomarker data, which would be helpful in further classifying dementia or AD diagnosis. Especially considering that AMD and AD may share some pathological features(Klaver et al., 1999) and that our findings suggest, similarly to AD, that memory performance in AMD was especially dependent on DMN connectivity, future research should probe the role of comorbid AD in the brain-behavior relationships we have described in AMD.
The LPHC— L ITG/TP/MTG connection has been less well studied with respect to episodic memory, but our observation here could be similarly explained by the theory of brain flexibility(Sharp et al., 2011; Tromp et al., 2015). Previously, activity in the temporal lobe was shown to increase significantly with increasing retrieval demand on the memory network(Barredo et al., 2015), a high-stress situation comparable to stress from cognitive challenges of AMD. Our study highlighted the LPHC—L ITG/TP/MTG, an unusual linkage that may connect the medial temporal memory network to other memory-related temporal regions to support memory facing AMD-related cognitive stressor. Further studies to observe the cohort longitudinally will be required to determine if causality exists between LPHC— L ITG/TP/MTG connection and AMD-associated cognitive changes.
Several limitations of the current study should be highlighted. First, our study is cross-sectional and observational, so we are unable to observe possible development of AMD-related cognitive impairment and fMRI changes, and we are unable to infer the causality of the described relationships. We are also unable to dissociate the effect of AMD status and AMD-related vision loss from the group comparisons, as most of our AMD participants suffered substantial vision impairment. Third, we used resting-state fMRI data to identify two functionally connected networks that we presume to be relevant to memory function, based on descriptions in the literature of similar networks that were linked to memory. These networks could not be proven to be engaged during active memory tasks. Fourth, the memory tests choices were limited by our attempt to avoid visually administered tests, and these tests do not have established sensitivity in the AMD population, despite elsewhere employed(Clemons et al., 2006; Whitson et al., 2010; Whitson et al., 2012; Lindekleiv et al., 2013). However, this also raises the possibility that significant cognitive deficits in AMD would be detected in aspects of memory that rely heavily on vision. Last but not least, the group comparisons we performed failed to demonstrate a cognitive deficit. As a result, we are unable to distinguish between two potential explanations: 1) that memory is intact and unaffected in our sample of AMD patients, or 2) that we identified a successful compensation mechanism in the cohort. The fact that we observed neural differences, but not cognitive differences, between the groups provides weak evidence in favor of compensation. Additional insights may be gained from observing the cohort longitudinally to assess memory trend associated with network connectivity, which may differentiate effective vs ineffective compensation. Our results also call for meta-analysis that compares AMD and controls in cognitive aspects, as published reports have been inconsistent.
In the context of epidemiology studies showing lower cognitive function in people with AMD(Klaver et al., 1999; Wong et al., 2002; Clemons et al., 2006; Woo et al., 2012; Rozzini et al., 2014; Chen et al., 2017), our findings support the hypothesis that AMD patients recruited other cognitive resources (such as connections between memory-supportive networks and outside-network reserves, or a well-connected DMN) to maintain memory performance. Specifically, resting LPHC—L temporal lobe, LPHC—R frontal lobe, and mPFC—PCC connectivity may be supportive of memory, especially verbal episodic memory performance, in the context of conditions such as AMD-related vision loss that threaten memory performance, and this compensation may be masking the early cognitive deficit that would otherwise be observed. Future research is needed to elucidate the mechanistic and clinical importance of the brain-behavior patterns described here in the AMD population.
Supplementary Material
Table 3.
Areas of significant connectivity for the memory and default mode network (DMN) correlating positively with episodic memory function in age-related macular degeneration (AMD) compared to control participants and in AMD participants alone*, controlling for age and years of education.
| Cluster regions | Brodmann Area | size (voxels) | max Z | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Memory Network | ||||||
| Seed region: LPHC | ||||||
| AMD - Control | ||||||
| L MTG, TP, ITG | 21,38 | 360 | 4.22 | −54 | 8 | −28 |
| R IFG, MFG, SFG** | 45,9,46 | 850 | 4 | 50 | 34 | 14 |
| AMD | ||||||
| R TP, MTG, ITG | 20,21,38 | 668 | 5.17 | 40 | 14 | −40 |
| L SFG, MFG, IFG | 45,48 | 1338 | 4.36 | −34 | 66 | 0 |
| R MFG, SFG, SMFG | 9,10,45,46 | 1819 | 4.28 | 28 | 46 | 38 |
| L MTG, AG, I PL | 39,40 | 473 | 4.06 | −54 | −70 | 24 |
| L SMFG*** | 8,9,10 | 398 | 3.87 | −8 | 46 | 48 |
| DMN | ||||||
| Seed region: mPFC | ||||||
| AMD - Control | ||||||
| L/R Precuneus, L PCC, MCC, R Calcarine**** | 30,23,17 | 397 | 3.65 | −12 | −52 | 22 |
| AMD | ||||||
| L/R Precuneus, PCC, calcarine | 30,23,26 | 870 | 4.84 | −14 | −50 | 26 |
| R AG, MTG, SPL, MPL, SOG, MOG***** | 39,19,7 | 489 | 4.49 | 54 | −62 | 40 |
Threshold: p < 0.005, voxel-level uncorrected, and p < 0.05, cluster-level corrected
LPHC: left parahippocampal cortex, MTG: middle temporal gyrus, TP: temporal pole, ITG: inferior temporal gyrus, IFG: inferior frontal gyrus, MFG: middle frontal gyrus, SFG: superior frontal gyrus
SMFG: superior medial frontal gyrus, AG: angular gyrus, IPL: inferior parietal lobule
PCC: posterior cingulate cortex, MCC: middle cingulate cortex
SPL: superior parietal lobule, MPL: middle parietal lobule, MOG: middle occipital gyrus, SOG: superior occipital gyrus
Acknowledgement
The authors gratefully acknowledge the contributions of study staff members including Alice Ventura, Abigail Maciejewski, Xuan Duong Fernandez, and Kala Allen. This work was supported by the National Institute of Health R01AG043438 (HEW), K23EY026988 (EL), R01 AG039684 (DJM PI), and R56 AG052576 (DJM PI). The following grants further contributed to the efforts and ideas presented here: P30AG028716, U13AG054139, UL1TR002553.
Funding
This work was supported by the National Institute of Health R01AG043438 (HEW), K23EY026988 (EL), R01 AG039684 (DJM PI), and R56 AG052576 (DJM PI). The following grants further contributed to the efforts and ideas presented here: P30AG028716, U13AG054139, UL1TR002553.
Abbreviations:
- AMD
age-related macular degeneration
- DMN
default mode network
- mPFC
anterior medial prefrontal cortex
- PCC
posterior cingulate cortex
- PHC
parahippocampal cortex
- TP
Temporal pole
- SFG/MFG
Superior/middle frontal gyrus
- fMRI
functional Magnetic Resonance Imaging
- BOLD
blood oxygen level dependent
- WMS-III
Wechsler Memory Scale, Third Edition
- BTACT
Brief Test of Adult Cognition by Telephone
- ROI
regions of interest
- AD
Alzheimer’s disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest.
References
- Andrews-Hanna JR, Smallwood J, and Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316, 29–52. doi: 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. (2007). Disruption of large-scale brain systems in advanced aging. Neuron 56(5), 924–935. doi: 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony M, and Lin F (2018). A Systematic Review for Functional Neuroimaging Studies of Cognitive Reserve Across the Cognitive Aging Spectrum. Arch Clin Neuropsychol 33(8), 937–948. doi: 10.1093/arclin/acx125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, and Krystal JH (2012). The role of default network deactivation in cognition and disease. Trends Cogn Sci 16(12), 584–592. doi: 10.1016/j.tics.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CI, Peli E, Knouf N, and Kanwisher NG (2005). Reorganization of visual processing in macular degeneration. Journal of Neuroscience 25(3), 614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ML, Wang JJ, Rogers S, Klein R, Kuller LH, Larsen EK, et al. (2009). Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Arch Ophthalmol 127(5), 667–673. doi: 10.1001/archophthalmol.2009.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J, Oztekin I, and Badre D (2015). Ventral fronto-temporal pathway supporting cognitive control of episodic memory retrieval. Cereb Cortex 25(4), 1004–1019. doi: 10.1093/cercor/bht291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, and Markus HS (2010). White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage 51(2), 565–577. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, and Madden DJ (2014). Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience 276, 187–205. doi: 10.1016/j.neuroscience.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. (2012). Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci 32(26), 8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, and Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Cabeza R, and Nyberg L (2000). Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12(1), 1–47. [DOI] [PubMed] [Google Scholar]
- CDC (2017). Common Eye Disorders [Online]. Atlanta, GA: Centers for Disease Control and Prevention; Available: https://www.cdc.gov/visionhealth/basics/ced/index.html [Accessed Aug 21 2019]. [Google Scholar]
- Chen SP, Bhattacharya J, and Pershing S (2017). Association of vision loss With cognition in older adults. JAMA Ophthalmol 135(9), 963–970. doi: 10.1001/jamaophthalmol.2017.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons TE, Rankin MW, McBee WL, and Age-Related Eye Disease Study Research, G. (2006). Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Arch Ophthalmol 124(4), 537–543. doi: 10.1001/archopht.124.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corporation TR (1997). WAIS-III WMS-III Technical Manual.(pp. San Antonio: Harcourt Brace & Company. [Google Scholar]
- De Simoni S, Grover PJ, Jenkins PO, Honeyfield L, Quest RA, Ross E, et al. (2016). Disconnection between the default mode network and medial temporal lobes in post-traumatic amnesia. Brain 139(Pt 12), 3137–3150. doi: 10.1093/brain/aww241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J (2017). Complex relationships between vision and cognition in older people. JAMA Ophthalmol 135(9), 971–972. doi: 10.1001/jamaophthalmol.2017.2843 [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, et al. (2007). A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage 35(1), 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Sneve MH, Grydeland H, Storsve AB, and Walhovd KB (2017). The disconnected brain and executive function decline in aging. Cereb Cortex 27(3), 2303–2317. doi: 10.1093/cercor/bhw082 [DOI] [PubMed] [Google Scholar]
- Fjell AM, and Walhovd KB (2010). Structural brain changes in aging: courses, causes and cognitive consequences. Rev in the Neurosci 21, 187–221. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, and Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, et al. (2011). Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. J Neurol Neurosurg Psychiatry 82(1), 58–66. doi: 10.1136/jnnp.2009.199935 [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, and Robins JM (1999). Causal diagrams for epidemiologic research. Epidemiology 10(1), 37–48. [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, and Menon V (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100(1), 253–258. doi: 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, and Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101(13), 4637–4642. doi: 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Dipasquale O, Lagana MM, Nemni R, Clerici M, Smith SM, et al. (2015). Effective artifact removal in resting state fMRI data improves detection of DMN functional connectivity alteration in Alzheimer’s disease. Front Hum Neurosci 9, 449. doi: 10.3389/fnhum.2015.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, and Rombouts SA (2012). Imaging the default mode network in aging and dementia. Biochim Biophys Acta 1822(3), 431–441. doi: 10.1016/j.bbadis.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, and Daselaar SM (2011). The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One 6(4), e17463. doi: 10.1371/journal.pone.0017463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, and Barnes CL (1984). Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225(4667), 1168–1170. [DOI] [PubMed] [Google Scholar]
- Jeong W, Chung CK, and Kim JS (2015). Episodic memory in aspects of large-scale brain networks. Front Hum Neurosci 9, 454. doi: 10.3389/fnhum.2015.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, and de Jong PT (1999). Is age-related maculopathy associated with Alzheimer’s Disease? The Rotterdam Study. Am J Epidemiol 150(9), 963–968. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, Schuff N, Hollnagel C, Weiner MW, et al. (2005). Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology 19(6), 799–805. doi: 10.1037/0894-4105.19.6.7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech RK, and Suchan B (2013). The medial temporal lobe: memory and beyond. Behav Brain Res 254, 45–49. doi: 10.1016/j.bbr.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Lin MY, Gutierrez PR, Stone KL, Yaffe K, Ensrud KE, Fink HA, et al. (2004). Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 52(12), 1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x [DOI] [PubMed] [Google Scholar]
- Lindekleiv H, Erke MG, Bertelsen G, Peto T, Arntzen KA, Schirmer H, et al. (2013). Cognitive function, drusen, and age-related macular degeneration: a cross-sectional study. Eye (Lond) 27(11), 1281–1287. doi: 10.1038/eye.2013.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, and Song AW (2012). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta 1822(3), 386–400. doi: 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, and Parks EL (2017). Age differences in structural connectivity: Diffusion tensor imaging and white matter hyperintensities Cognitive neuroscience of aging: Linking cognitive and cerebral aging (2nd.) (pp. 71–103) New York: Oxford. [Google Scholar]
- Madden DJ, Parks EL, Tallman CW, Boylan MA, Hoagey DA, Cocjin SB, et al. (2017). Sources of disconnection in neurocognitive aging: cerebral white-matter integrity, resting-state functional connectivity, and white-matter hyperintensity volume. Neurobiol Aging 54, 199–213. doi: 10.1016/j.neurobiolaging.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Dumoulin SO, Nakadomari S, and Wandell BA (2008). V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cerebral Cortex 18, 2483–2493. doi: 10.1093/cercor/bhm256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, et al. (2002). Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol 51(2), 182–189. [DOI] [PubMed] [Google Scholar]
- Mohan A, Roberto AJ, Mohan A, Lorenzo A, Jones K, Carney MJ, et al. (2016). The significance of the Default Mode Network (DMN) in neurological and neuropsychiatric disorders: a review. Yale J Biol Med 89(1), 49–57. [PMC free article] [PubMed] [Google Scholar]
- Monge ZA, and Madden DJ (2016). Linking cognitive and visual perceptual decline in healthy aging: The information degradation hypothesis. Neurosci Biobehav Rev 69, 166–173. doi: 10.1016/j.neubiorev.2016.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, and Baxter MG (2012). The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13(4), 240–250. doi: 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (2011). Facts about Age Related Macular Degeneration [Online]. National Eye Institute; Available: http://www.nei.nih.gov/health/maculardegen/armd_facts.asp [Accessed Nov 20 2011]. [Google Scholar]
- Pham TQ, Kifley A, Mitchell P, and Wang JJ (2006). Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology 52(6), 353–358. doi: 10.1159/000094984 [DOI] [PubMed] [Google Scholar]
- Prins D, Hanekamp S, and Cornelissen FW (2016). Structural brain MRI studies in eye diseases: are they clinically relevant? A review of current findings. Acta Ophthalmol 94(2), 113–121. doi: 10.1111/aos.12825 [DOI] [PubMed] [Google Scholar]
- Raichle ME, and Gusnard DA (2005). Intrinsic brain activity sets the stage for expression of motivated behavior. Journal of Comparative Neurology 493(1), 167–176. [DOI] [PubMed] [Google Scholar]
- Raichle ME, and Mintun MA (2006). Brain work and brain imaging. Annu. Rev. Neurosci 29, 449–476. [DOI] [PubMed] [Google Scholar]
- Raz N (2005). The aging brain observed in vivo: Differential changes and their modifiers, in Cognitive neuroscience of aging: Linking cognitive and cerebral aging, eds. Cabeza R, Nyberg L & Park D. (Oxford: Oxford University Press; ), 19–57. [Google Scholar]
- Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. (2006). The economic burden of major adult visual disorders in the United States. Arch Ophthalmol 124(12), 1754–1760. doi: 10.1001/archopht.124.12.1754 [DOI] [PubMed] [Google Scholar]
- Reyes-Ortiz CA, Kuo YF, DiNuzzo AR, Ray LA, Raji MA, and Markides KS (2005). Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc 53(4), 681–686. doi: 10.1111/j.1532-5415.2005.53219.x [DOI] [PubMed] [Google Scholar]
- Rogers MA, and Langa KM (2010). Untreated poor vision: a contributing factor to late-life dementia. Am J Epidemiol 171(6), 728–735. doi: 10.1093/aje/kwp453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L, Riva M, Ghilardi N, Facchinetti P, Forbice E, Semeraro F, et al. (2014). Cognitive dysfunction and age-related macular degeneration. Am J Alzheimers Dis Other Demen 29(3), 256–262. doi: 10.1177/1533317513517032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SK (2010). Biology of aging brain. Indian J Pathol Microbiol 53(4), 595–604. doi: 10.4103/0377-4929.71995 [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. (2011). Default mode network functional and structural connectivity after traumatic brain injury. Brain 134(Pt 8), 2233–2247. doi: 10.1093/brain/awr175 [DOI] [PubMed] [Google Scholar]
- Smagula SF, Karim HT, Rangarajan A, Santos FP, Wood SC, Santini T, et al. (2018). Association of hippocampal substructure resting-state functional connectivity with memory performance in older adults. Am J Geriatr Psychiatry. doi: 10.1016/j.jagp.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, and Toga AW (2003). Mapping cortical change across the human life span. Nature Neuroscience 6, 309–315. [DOI] [PubMed] [Google Scholar]
- Szlyk JP, and Little DM (2009). An FMRI study of word-level recognition and processing in patients with age-related macular degeneration. Investigative ophthalmology & visual science 50(9), 4487–4495. [DOI] [PubMed] [Google Scholar]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, and Mitchell P (2006). Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology 52(6), 386–394. doi: 10.1159/000095129 [DOI] [PubMed] [Google Scholar]
- Tromp D, Dufour A, Lithfous S, Pebayle T, and Despres O (2015). Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res Rev 24(Pt B), 232–262. doi: 10.1016/j.arr.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Tsai DC, Chen SJ, Huang CC, Yuan MK, and Leu HB (2015). Age-related macular degeneration and risk of degenerative dementia among the elderly in taiwan: A population-based cohort study. Ophthalmology 122(11), 2327–2335 e2322. doi: 10.1016/j.ophtha.2015.07.033 [DOI] [PubMed] [Google Scholar]
- Tun PA, and Lachman ME (2006). Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing 35(6), 629–632. doi: 10.1093/ageing/afl095 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, and Buckner RL (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103(1), 297–321. doi: 10.1016/j.neuroimage.2006.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin M, Kergoat MJ, Belleville S, Li G, Rousseau J, Roy-Gagnon MH, et al. (2020). Age-Related Eye Disease and Cognitive Function: The Search for Mediators. Ophthalmology 127(5), 660–666. doi: 10.1016/j.ophtha.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. (2006). Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 96(6), 3517–3531. doi: 10.1152/jn.00048.2006 [DOI] [PubMed] [Google Scholar]
- Ward AM, Mormino EC, Huijbers W, Schultz AP, Hedden T, and Sperling RA (2015). Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiol Aging 36(1), 265–272. doi: 10.1016/j.neurobiolaging.2014.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, and Sperling RA (2014). The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 35(3), 1061–1073. doi: 10.1002/hbm.22234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson HE, Ansah D, Whitaker D, Potter G, Cousins SW, MacDonald H, et al. (2010). Prevalence and patterns of comorbid cognitive impairment in low vision rehabilitation for macular disease. Arch Gerontol Geriatr 50(2), 209–212. doi: 10.1016/j.archger.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, and Cohen HJ (2007). The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc 55(6), 885–891. doi: 10.1111/j.1532-5415.2007.01093.x [DOI] [PubMed] [Google Scholar]
- Whitson HE, Whitaker D, Sanders LL, Potter GG, Cousins SW, Ansah D, et al. (2012). Memory deficit associated with worse functional trajectories in older adults in low-vision rehabilitation for macular disease. J Am Geriatr Soc 60(11), 2087–2092. doi: 10.1111/j.1532-5415.2012.04194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein R, Nieto FJ, Moraes SA, Mosley TH, Couper DJ, et al. (2002). Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. Am J Ophthalmol 134(6), 828–835. [DOI] [PubMed] [Google Scholar]
- Woo SJ, Park KH, Ahn J, Choe JY, Jeong H, Han JW, et al. (2012). Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology 119(10), 2094–2101. doi: 10.1016/j.ophtha.2012.04.026 [DOI] [PubMed] [Google Scholar]
- Zhou LX, Sun CL, Wei LJ, Gu ZM, Lv L, and Dang Y (2016). Lower cognitive function in patients with age-related macular degeneration: a meta-analysis. Clin Interv Aging 11, 215–223. doi: 10.2147/CIA.S102213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Madden DJ, Duong-Fernandez X, Chen N. k., Cousins SW, Potter GG, et al. (2017). Language processing in age-related macular degeneration associated with unique functional connectivity signatures in the right hemisphere. Manuscript Submitted for Publication. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


