Abstract
Introduction
Previous work has shown that the vaginal microbiome decreases in Lactobacillus predominance and becomes more diverse following menopause. It has also been shown that estrogen therapy restores Lactobacillus-dominance in the vagina, and that topical estrogen is associated with OAB symptom improvement. We now know that the bladder contains a unique microbiome, and increased bladder microbiome diversity is associated with OAB. However, there is no understanding of how quickly each pelvic floor microbiome responds to estrogen or if those changes are associated with symptom improvement.
Study Design
Analysis of data from post-menopausal participants in two trials (NCT02524769 and NCT02835846) who chose vaginal estrogen as their primary OAB treatment and used 0.5 grams of conjugated estrogen (Premarin Cream, (Pfizer, New York City, NY)) twice weekly for 12 weeks. Baseline and 12-week follow-up data included the OAB-q questionnaire and participants provided catheterized urine, vaginal swabs, perineal swabs, and voided urine. Microbes were detected by an enhanced culture protocol. Linear mixed models were used to estimate microbiome changes over time. Urinary AMP activity was assessed by a bacterial growth inhibition assay and correlated with relative abundance of members of the urobiome.
Results
Twelve weeks of estrogen treatment resulted in decreased microbial diversity within the vagina (Shannon, p=0.047; Richness, p=0.043), but not in the other niches. A significant increase in Lactobacillus was detected in the bladder (p=0.037), but not the vagina (p=0.33), perineum (p=0.56), or voided urine (p=0.28). The change in Lactobacillus levels in the bladder was associated with modest changes in urgency incontinence symptoms (p=0.02). The relative abundance of the genus Corynebacterium correlated positively with urinary AMP activity after estrogen treatment.
Conclusion
Estrogen therapy may change the microbiome of different pelvic floor niches. The vagina begins to decrease in diversity and the bladder experiences a significant increase in Lactobacillus levels; the latter is correlated with a modest improvement in the symptom severity sub-scale of the OAB-q.
Keywords: enhanced urine culture, estrogen, overactive bladder, urgency urinary incontinence, urinary microbiome, urinary urgency, vaginal microbiome, 16S rRNA gene sequencing, antimicrobial peptides
Condensation
Estrogen therapy for overactive bladder resulted in decreased bladder bacterial diversity and increased bladder Lactobacillus, which were associated with modest changes in urgency incontinence symptoms.
INTRODUCTION
Loss of estrogen in post-menopausal women has long been associated with increased pelvic floor symptoms and disorders, such as urinary tract infections and overactive bladder (OAB). The emergence of these symptoms is associated with a change of the vaginal microbiota. Premenopausal women tend to have low microbial diversity and are more likely to be predominated by Lactobacillus than postmenopausal women1,2, and hormone replacement therapy reverses this change3,4.
In post-menopausal women, use of vaginal estrogen improves symptoms of OAB5, a syndrome characterized by urinary urgency, often associated with frequency and nocturia, with or without urgency urinary incontinence in the absence of infection or other pathology6. Although the precise mechanism of symptom relief is not understood, vaginal estrogen increases vaginal blood flow and reduces density of vaginal autonomic and sensory nerves5,7.
The normal loss of estrogen during menopause promotes structural and chemical changes throughout the urogenital tract by reducing urothelial thickness and the abundance of tight junction proteins8,9, which can presumably facilitate pathogen colonization via impaired urothelial barrier function. Low estrogen also reduces the production of several endogenous anti-microbial peptides (AMPs). Elements of both the vaginal and urinary innate immune systems that exhibit direct microbicidal activity9, AMPs help minimize bacterial dysbiosis and facilitate normal epithelial barrier function in the female urogenital tract. Although transvaginal medications likely alter nearby bacterial niches (e.g. the bladder), no study has reported the response of the urinary microbiota to vaginal estrogen.
In the last decade, the female urinary microbiota (urobiome) has been confirmed and associations with clinical conditions of interest have been reported. Relevant to this study, we previously reported increased urobiome diversity in women with OAB10. We further reported that, in women planning oral anti-cholinergic therapy for OAB treatment, pre-treatment urobiome status stratifies patients into treatment response groups; women with less diverse urobiomes are more likely to respond to anti-cholinergic OAB therapy11. These reports provide evidence that the urobiome could factor in lower urinary tract symptoms and that urobiome diversity contributes to lower urinary tract symptoms and lower urinary tract symptom treatment response.
We also have reported that AMP levels and/or activity correlate with urinary tract infection risk in women undergoing urogynecological surgery12. Although urinary AMP activity has been assessed in several urinary pathologies9,12–14, no studies have assessed the role of estrogen in modulating urinary AMP activity as a therapeutic for women with OAB. Given that estrogen promotes epithelial differentiation, which is associated with greater AMP expression and activity, estrogen likely regulates urinary AMPs to optimize urobiome equilibrium9,14,15 and impact the severity of OAB.
Given the proximity of the bladder and vaginal microbiota16, we hypothesize that the urobiome may become less diverse following vaginal estrogen treatment, which may improve OAB symptoms. We further hypothesize that vaginal estrogen treatment in hypoestrogenic women with OAB will be associated with 1) alteration of other urobiome characteristics 2) a correlation between OAB symptoms and reduced urobiome diversity, and 3) altered urinary AMP activity.
MATERIALS AND METHODS
This study features a quasi-experimental design and pools data from participants in two IRB-approved single-armed interventional registered trials with identical study aims and study protocol and statistical analysis plans. The first (NCT02524769, local IRB LU#207152) enrolled 27 participants as a fellowship thesis project, which continued as an investigator-initiated project (NCT02835846, local IRB LU#207777 funded by Kimberly-Clark Corporation) that enrolled 37 additional participants.
Study design and patient recruitment
Women seeking OAB treatment were recruited in the ambulatory urogynecology clinic at Loyola University Medical Center (12/2015–11/2017) after being clinically counseled on evidence-based first-line options for OAB treatment, including behavioral modifications, physical therapy, and vaginal estrogen for treatment of atrophy, as appropriate. Women who chose vaginal estrogen as their primary OAB treatment were invited to participate. Women recruited to the study were also allowed, but not required, to participate in pelvic floor physical therapy as part of their treatment. Eligibility criteria were broad to facilitate subsequent generalization. We included women with OAB symptoms who (1) had higher weighted scores on urge incontinence questions as compared to stress incontinence questions on the Medical, Epidemiological and Social Aspects of Aging (MESA) urinary incontinence questionnaire17; (2) had clinical indication for vaginal estrogen use; (3) were postmenopausal by history, defined as twelve months or greater since last menstrual period or over the age of 55 if they have had a previous hysterectomy; (4) had no current vaginal estrogen therapy and (5) had English skills sufficient to complete questionnaires. Women were excluded for the following: (1) use of systemic hormone replacement therapy (HRT) currently or within the past three months; (2) personal history of estrogen-dependent malignancies (breast, endometrial); (3) contraindication or allergy to local estrogen therapy; (4) insufficient language skills to complete study tasks; (5) urinary tract infection at baseline assessment; (6) use of antibiotics within the past two weeks; (7) pelvic organ prolapse stage III (>1 cm beyond hymen); (8) unwillingness to use vaginal estrogen preparation; or (9) currently on anti-cholinergic medication, desire for anti-cholinergic medication at this visit, or who have received anti-cholinergic medication within the past three months.
Symptom assessment and sample collection
Consented participants were asked to complete the OAB-q, a 33 item self-administered validated questionnaire for OAB-related symptoms and quality of life. It has an 8-item symptom bother scale and a 25-item health related quality of life scale with four domains; coping, concern, sleep, and social interaction. The subscale and total scores are transformed into a 0 – 100 scale. For the symptoms severity subscale, higher values indicate more severe symptoms whereas for the quality of life outcome higher scores indicate improved quality of life18. Demographics and clinical characteristics were abstracted from medical records. Physical examination included pelvic organ prolapse quantification (POP-q)19. Each participant contributed baseline samples in the following order: voided urine, perineal swab, vaginal swab, and catheterized urine. Free of charge, participants were provided 0.625 mg conjugated estrogen/gram of cream (Premarin, Pfizer, New York City, NY) and instructed to use 0.5 grams with an applicator twice weekly for 12 weeks based on the Society for Gynecological Surgeons’ clinical practice guidelines for vaginal estrogen use in postmenopausal women with urogynecological complaints20. Participants kept a simple medication diary for estrogen use during the course of the study. Follow-up occurred 12 weeks after the initial visit. Participants contributed follow-up samples identical to baseline and again completed the OAB-q.
Sample collection and preparation
Voided and catheterized urine specimens were collected in separate sterile blue cap-collection cups (BD #364956) and distributed as follows: a gray-top culture tube was filled for processing within 4 h of collection by the Expanded Quantitative Urine Culture protocol.28 Vaginal and perineal swabs were collected using the BD ESwab (BD#220245); each swab was swirled in 1 ml of bacterial preservative and an aliquot was provided for Expanded Quantitative Urine Culture as described for urine samples.
Expanded Quantitative Urine Culture
Catheterized urine samples were tested by two culture techniques (details in Supplementary Table 1): a standard urine culture method with detection level of 1000 colony forming units (CFU)/mL and the Expanded Quantitative Urine Culture method with detection level of 10 CFU/mL. Due to large biomass, voided urine and swabs were tested by the Expanded Quantitative Urine Culture method with detection levels of 100 or 1000 CFU/mL. The taxonomic identity of all distinct colony morphologies were tested by MALDI-TOF, as described21.
Cluster analysis and diversity measurements
The relative abundance of each taxon was determined for both the baseline and 12-week time points and clustered using the Bray Curtis Dissimilarity index, a measure of beta (between sample) diversity. Bacterial compositions of urine and swab specimens were compared using four measures of alpha diversity. Richness was determined by counting the number of unique species. The distribution of microbial species within samples (evenness) was calculated with Pielou’s Index. Combined interactions were calculated with the Shannon Index (richness and evenness) and Simpson Index (richness and species abundance). All calculations were performed in RStudio (R version 3.5.1)22.
HPLC fractionation of urine and antimicrobial radial diffusion assay
AMP activity is highly dependent upon the degree of peptide hydrophobicity23. High pressure liquid chromatography can be used to isolate and purify AMPs using increasing concentration of acetonitrile to elute peptides based upon their degree of hydrophobicity (Supplementary Figure 1A). Thus, voided urine was subjected to high pressure liquid chromatography fractionation using a C18 column and tested for antimicrobial activity by radial diffusion assay, as described 12,13. The 20 fractions were classified into 4 levels (groups) based on increasing levels of eluted peptide hydrophobicity: Level 1: Fractions 1–2: 10–15% Acetonitrile; Level 2: F3–6: 16–40% Acetonitrile; Level 3: F7–F11: 40–55% Acetonitrile; Level 4: F12–20: 56–90% Acetonitrile. Each fraction was subjected to a radial diffusion assay to assess their capacity to inhibit the growth of a Gram-positive AMP-susceptible mutant (Staphylococcus aureus ΔmprF). The resultant zone of bacterial growth inhibition in mm2 was measured using ImageJ Software and normalized to the total peptide bond concentration measured at UV 214nm (Supplementary Figure 1B).
Statistical methods
Descriptive statistics for baseline demographics and clinical characteristics were calculated; comparisons were tested using Wilcoxon rank sum tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables. Medians and interquartile ranges were presented for OAB-q subscales at each time point, and Wilcoxon signed rank tests assessed statistical significance of change in OAB-q. Each diversity measure was modeled as a separate dependent variable in a linear mixed effects regression, with independent variables including time period, OAB-q symptom severity, physical therapy completed, weeks of estrogen compliance, and body mass index and included random intercepts for subjects. Spearman’s rho was calculated for change in Lactobacillus with change in the OAB-q symptom severity (12-week minus baseline values) for each sample type. Culture data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). For AMP data analyses, Spearman’s Rank Correlation was used to test associations between change in relative bacterial abundance at the genus level and change in area of bacterial growth inhibition per fraction. A Bonferroni correction was used to compensate for multiple testing (type 1 error). All AMP data analyses were performed in RStudio (R version 3.5.1)22.
RESULTS
Baseline samples were collected from 62 participants; 41 participants contributed data at the 12-week follow-up visit. Ten of the 21 women who did not return for follow-up stopped using vaginal estrogen due to concerns about risks or side effects of the medication; two additional participants found the study medication “cumbersome” and discontinued use. Eight participants were not responsive to follow-up calls and one participant was unexpectedly out of the country for family care. Study completers were similar to non-completers in demographics, pelvic floor history, physical exam findings and baseline OAB-q subscales, although prolapse stage differed slightly (Table 1). Among those who completed the study, all were compliant to estrogen treatment for at least 6 weeks, and 24 (58.5%) were compliant for 12 weeks. Physical therapy was also offered to patients; 13 (31.7%) did not do any physical therapy, 8 (19.5%) completed some sessions, and 20 (48.8%) completed all sessions.
Table 1:
Baseline characteristics by study completion
| Completed study n=41 (66.1%) | Did Not Complete Study n=21 (33.9%) | p-value | |
|---|---|---|---|
| Age, median (IQR*) | 68 (62–73) | 69 (64–74) | 0.84 |
| Race/ethnicity, n (%) | |||
| Caucasian | 28 (68.3) | 14 (66.7) | 0.56 |
| African American | 8 (19.5) | 5 (23.8) | |
| Asian | 1 (2.4) | 2 (9.5) | |
| Hispanic | 2 (4.9) | 0 (0.0) | |
| Other | 2 (4.9) | 0 (0.0) | |
| BMI, median (IQR) | 29 (26–35) | 30 (26–36) | 0.46 |
| Vaginal deliveries, median (IQR) | 3 (2–3) | 2 (2–4) | 0.99 |
| Prior hysterectomy, n (%) | 18 (43.9) | 11 (52.4) | 0.53 |
| Stage of prolapse, n (%) | |||
| 0 | 13 (31.7) | 8 (38.1) | 0.008 |
| 1 | 9 (22.0) | 11 (52.4) | |
| 2 | 19 (46.3) | 2 (9.5) | |
| Ovaries removed, n (%) | 11 (28.2) | 5 (25.0) | 0.79 |
| Previous incontinence surgery, n (%) | 4 (9.8) | 2 (9.5) | 0.99 |
| Post Void Residual (mL), median (IQR) | 30 (15–60) | 40 (25–60) | 0.30 |
| Baseline OAB-q** subscales, median (IQR) | |||
| Symptom severity | 48 (43–70) | 55 (40–65) | 0.69 |
| Coping | 65 (43–83) | 55 (20–78) | 0.61 |
| Concern | 57 (37–71) | 46 (17–83) | 0.55 |
| Sleeping | 64 (32–76) | 52 (36–84) | 0.92 |
| Social | 92 (80–100) | 80 (68–100) | 0.29 |
| HRQOL*** | 67 (46–78) | 60 (33–77) | 0.59 |
IQR: interquartile range
OAB-q: Overactive Bladder quantified
HRQOL: Health Related Quality of Life
At the 12-week visit, all OAB-q sub-scale scores improved (Table 2). Most participants (n=30, 73%) reported at least a 10-point improvement in symptom severity. For those who completed the study, the median symptom severity score decreased from 48 (IQR:43–70) to 25 (IQR:8–50). Older women were less likely to report symptom improvement. When we compared women with a symptom severity improvement of > 10 points to those with less than 10 points (minimal clinically difference (MCID)) 24, there were no differences in physical therapy attendance rates (53% with symptom improvement attended all sessions versus 36% without symptom improvement; p=0.34) or compliance with estrogen use (57% with symptom improvement were compliant all weeks versus 64% without symptom improvement; p=0.74).
Table 2:
OAB-q* subscale scores between baseline and follow-up
| Baseline | Follow-up | Change Post - Pre | p-value | |
|---|---|---|---|---|
| OAB-q* subscales, median (IQR**) | ||||
| Symptom severity | 48 (43–70) | 25 (8–50) | −23 (−38– −5) | <0.001 |
| Coping | 65 (43–83) | 88 (75–100) | 18 (3–38) | <0.001 |
| Concern | 57 (37–71) | 86 (57–97) | 23 (6–34) | <0.001 |
| Sleeping | 64 (32–76) | 84 (60–92) | 16 (0–24) | <0.001 |
| Social | 92 (80–100) | 100 (95–100) | 4 (0–16) | 0.006 |
| HRQOL*** | 67 (46–78) | 86 (71–97) | 17 (6–27) | <0.001 |
OAB-q: Overactive Bladder quantified
IQR: interquartile range
HRQOL: Health Related Quality of Life
bold: statistically significant, p<0.05
Linear mixed regression model showed minimal differences in diversity between baseline and the 12-week follow-up. Specifically, catheterized and voided samples showed no change in diversity while some decreases in diversity were seen in the vaginal swabs (Shannon index p=0.047, species richness p=0.043) and perineal swabs (Peilou p=0.034) (Table 3).
Table 3:
Microbiome diversity measurements between baseline and follow-up based on expanded quantitative urine culture results
| Baseline, Adjusted mean (SE) | Follow-up, Adjusted mean (SE) | Change, Adjusted mean (SE) | p-value | |
|---|---|---|---|---|
| Catheterized urine samples | ||||
| Shannon index | 0.83 (0.12) | 0.65 (0.12) | −0.17 (0.16) | 0.30 |
| Simpson index | 0.49 (0.06) | 0.49 (0.06) | 0.00 (0.09) | 0.97 |
| Species richness | 3.94 (0.49) | 3.41 (0.48) | −0.53 (0.66) | 0.43 |
| Pielou’s evenness | 0.65 (0.07) | 0.57 (0.07) | −0.09 (0.10) | 0.39 |
| % Lactobacillus | 10.8 (5.4) | 25.1 (5.3) | 14.4 (6.6) | 0.037 |
| Vaginal swab samples | ||||
| Shannon index | 1.29 (0.08) | 1.04 (0.08) | −0.25 (0.12) | 0.047 |
| Simpson index | 0.61 (0.04) | 0.54 (0.04) | −0.07 (0.05) | 0.19 |
| Species richness | 8.83 (0.52) | 7.36 (0.52) | −1.46 (0.70) | 0.043 |
| Pielou’s evenness | 0.62 (0.03) | 0.56 (0.03) | −0.06 (0.05) | 0.21 |
| % Lactobacillus | 17.7 (5.2) | 24.2 (5.1) | 6.6 (6.6) | 0.33 |
| Perineal swab samples | ||||
| Shannon index | 1.37 (0.09) | 1.46 (0.09) | 0.08 (0.11) | 0.47 |
| Simpson index | 0.65 (0.03) | 0.71 (0.03) | 0.06 (0.04) | 0.15 |
| Species richness | 9.66 (0.58) | 8.30 (0.56) | −1.36 (0.72) | 0.07 |
| Pielou’s evenness | 0.60 (0.04) | 0.71 (0.04) | 0.10 (0.05) | 0.034 |
| % Lactobacillus | 6.7 (3.1) | 9.2 (3.0) | 2.5 (4.2) | 0.56 |
| Voided urine samples | ||||
| Shannon index | 1.10 (0.11) | 0.99 (0.10) | −0.12 (0.15) | 0.45 |
| Simpson index | 0.59 (0.05) | 0.54 (0.05) | −0.05 (0.07) | 0.43 |
| Species richness | 7.56 (0.73) | 7.08 (0.69) | −0.48 (0.78) | 0.55 |
| Pielou’s evenness | 0.54 (0.05) | 0.55 (0.05) | 0.01 (0.07) | 0.87 |
| % Lactobacillus | 13.4 (6.3) | 22.5 (5.5) | 9.2 (8.3) | 0.28 |
Bold: statistically significant, p<0.05
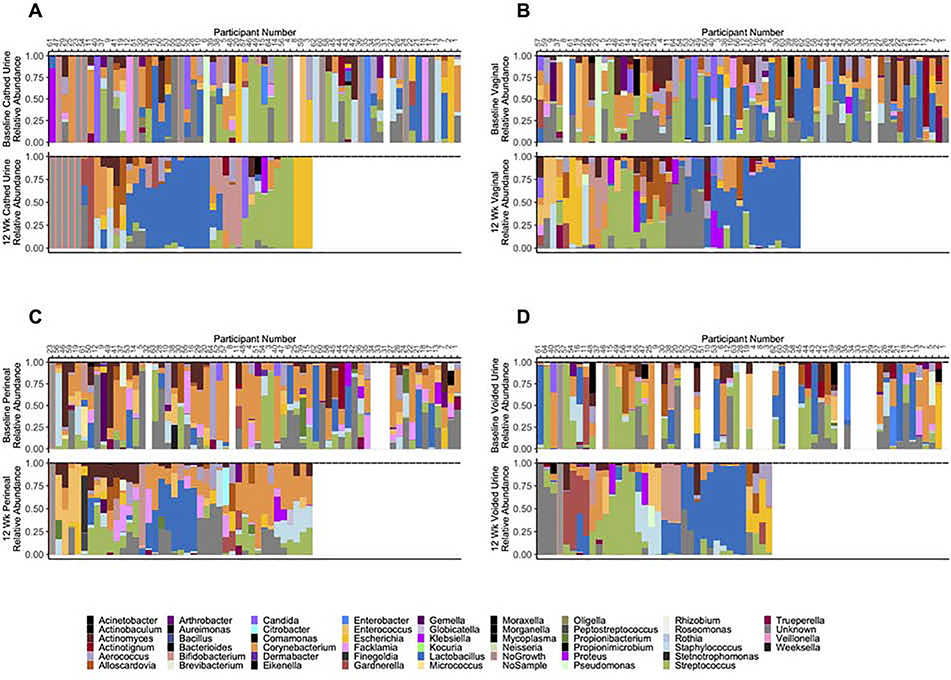
Measuring overall diversity did not provide insight into any increase or decrease in the abundance of specific taxa. For that reason, we also used a separate model to identify if there were significant differences in the abundance of Lactobacillus from baseline to 12 weeks. Catheterized urines had significantly more Lactobacillus by 12-weeks (p=0.037), but no corresponding increase was seen in vaginal (p=0.33), perineal (p=0.56), or voided (p=0.28) samples (Table 3). The observation of more Lactobacillus-predominance was apparent on a participant-by-participant basis for catheterized urine samples (Figure 1A). While the figures do seem to indicate a greater predominance of Lactobacillus by 12-weeks in the vagina (Figure 1B), perineum (Figure 1C), and voided urines (Figure 1D), our model did not show this to be statistically significant (Table 3). Additionally, the change in Lactobacillus levels in catheterized urine from baseline to 12-weeks was associated with an improvement in the symptom severity sub-scale of the OAB-q (p=0.02), but no correlations was seen for Lactobacillus levels in the vagina (p=0.22), perineum (p=0.72), or voided urine (p=0.14) (Table 4).
Figure 1. Microbiota of catheterized urine (A), vaginal swabs (B), perineal swabs (C) and voided urine (D) at baseline and after 12 weeks of estrogen therapy.
Top: baseline; bottom: 12-week. Baseline data arranged according to 12-week microbiota. Figure constructed in R (version 3.5.1) with Vegan (version 2.5–5) 36, Cowplots (version 1.0.0) 37, ggplotify (version 0. 0.5), and ggplot2 (version 3.2.1) 38,39 packages.
Table 4:
Comparison of change in Lactobacillus levels to change in symptom over 12 weeks
| Spearman’s rho | 95% CI | p-value | |
|---|---|---|---|
| Catheterized Urine | −0.42 | −0.67, −0.06 | 0.02 |
| Vaginal swab | −0.21 | −0.49, 0.12 | 0.22 |
| Perineal swab | −0.06 | −0.38, 0.27 | 0.72 |
| Voided urine | −0.32 | −0.64, 0.12 | 0.14 |
Bold: statistically significant, p<0.05
We then assessed the correlation between the bacteria cultured from voided urine and urinary AMP activity. In terms of relative abundance, the genera Corynebacterium and Streptococcus (rho=0.56–0.79) correlated with increased AMP activity, whereas the genus Actinomyces correlated negatively (rho=−0.66) after estrogen treatment. Significance for Corynebacterium and Actinomyces was mostly observed for urine fractions eluted at moderately high or high levels of hydrophobicity; significance for Streptococcus was observed at moderately low hydrophobicity levels (Table 5). Following Bonferroni correction, only Corynebacterium remained highly significant (p=0.005). The results were similar when calculated in terms of CFU/mL (Supplementary Table 2).
Table 5:
Correlation between changes in urinary AMP activity and genus relative abundance
| Genus | AMP fraction/level | p value | S | rho | adjusted p value |
|---|---|---|---|---|---|
| Actinomyces | 11/3 | 0.015 | 603.3 | −0.66 | 0.058 |
| Corynebacterium | 16/4 | 0.001 | 76.2 | 0.79 | 0.005 |
| Corynebacterium | 20/4 | 0.048 | 161.0 | 0.56 | 0.191 |
| Streptococcus | 5/2 | 0.031 | 146.9 | 0.60 | 0.126 |
Fractions were classified into 4 Levels (L1-L4) of increasing levels of hydrophobicity: Level 1: F1−2, 10−15% Acetonitrile; Level 2: F3−6, 16−40% Acetonitrile; Level 3: F7-F11, 40−55% Acetonitrile; Level 4: F12−20, 56−90% Acetonitrile; Bold: statistically significant, p<0.05
DISCUSSION/COMMENT
Principal Findings
Our findings indicate that post-menopausal women seeking treatment for OAB who are treated with vaginal estrogen for twelve weeks do have measurable changes in their pelvic floor microbiota. The vagina and perineum decrease in diversity, and catheterized urine (but not vaginal swabs) increases in Lactobacillus. The increased Lactobacillus in catheterized urine correlates with clinically significant differences in the symptom severity sub-scale of the OAB-q. Finally, AMP activity correlates positively with detection of Corynebacterium.
Results
This study provides longitudinal data to supplement previous cohort comparison studies that provided evidence for depletion of vaginal Lactobacillus species in untreated post-menopausal women1. It also provides evidence that similar changes occur in the bladder and that these changes can be reversed with topical estrogen treatment.
Clinical Implications
It is already known that low dose vaginal estrogen can improve symptoms of OAB in post-menopausal women25–29. This study suggests changes in the urinary microbiome as one possible mechanism for symptom improvement. It is a relatively low risk treatment option that may be added in combination with other evidence-based treatments for OAB.
Research Implications
Previous work has shown that postmenopausal women using topical estrogen cream have a greater amount of vaginal Lactobacillus compared to age-matched controls not using the cream3,30. However, because these studies used populations of women who had been on hormones for years, we do not know how long it takes for Lactobacillus predominance to occur. Shen and colleagues observed an increase in vaginal Lactobacillus predominance following 4 weeks of low dose oral estrogen in a population of postmenopausal women with atrophic vaginitis4. However, no one has looked at the changes in the microbiota over 12 weeks of topical treatment in a population of women with OAB. In this study, we took hypoestrogenic women, sampled them prior to and following 12-weeks of topical estrogen treatment and we observed changes in the microbiota in different pelvic floor niches. Although we did see a decrease in diversity in the vagina, we did not see the expected increase in Lactobacillus. We hypothesize that this is due to the short duration of our study.
Bacterial communities, like other ecosystems, can withstand different amounts of disturbance without changing state. This is called ecological resilience31. It is possible that 12 weeks of hormone are enough of a disturbance to change the state of the bladder ecosystem, but not enough for the vaginal ecosystem. Longer-term studies are needed to determine if longer time points (e.g. 6 months or 1 year) would be sufficient to observe shifts in the vaginal microbiota. The mechanisms of change in the urobiome will require further study to determine whether they are modulated directly via an effect of vaginal estrogen15 or as a secondary phenomenon related to changes in the vulva/vagina that favor Lactobacillus32.
As hypothesized, estrogen treatment increased the urinary AMP activity in several patients, which correlated with higher Corynebacterium abundance. The urinary microenvironment following estrogen treatment may enable the proliferation of Corynebacterium by providing key metabolites that are normally limited in the urine of hypoestrogenic women. Multiple recent reports of Corynebacterium isolation from urine samples, particularly from females, indicate that members of this genus may play an important role in female urinary health33. It is also possible that enhanced AMP activity against other bacteria following estrogen treatment serves as a selective advantage to promote Corynebacterium colonization and/or proliferation in the urinary tract of OAB patients. Estrogen may promote a shift in urinary AMPs to encourage bacterial tolerance in the urinary tract and protect the bladder from other microbes associated with OAB symptoms, as we previously determined that hBD1 was critical in protecting women with pelvic organ prolapse from UTI 12. Further studies are necessary to better define the role that Corynebacterium and AMP activity may play in OAB development and persistence.
Strengths and Limitations
Strengths of this study include its longitudinal aspect, samples from four relevant pelvic floor niches, and the use of each woman as her own control. Another strength was that we supplied estrogen therapy at no cost to the participant. This eliminated issues of insurance coverage and monetary constraints and helped to avoid recruitment bias. The use of the expanded quantitative urine culture protocol allowed for speciation and quantification of the bacteria in each niche, and limited the errors inherent with 16S sequencing (such as PCR and sequencing errors or errors in classification). However, our expanded quantitative urine culture protocol does miss detection of strict anaerobes and non-Candida fungi.
A major weakness of the study is the lack of a control group, which does not allow a causal connection to be made between estrogen treatment and OAB symptom improvement. Patients were also allowed to participate in pelvic floor physical therapy and counseled on behavioral changes, both of which may have been confounders and played a part in symptom improvement, although women with no symptom improvement had the same physical therapy compliance as those who had symptom improvement. Another weakness was the high dropout rate. Approximately one-third of the patients who enrolled in the study discontinued use of the estrogen cream prior to completion of the study. Other studies have also found discontinuation rates as high as 89% in the first year of use of locally applied estrogen creams34. Those who discontinued use may have been less likely to note symptom improvement and the burden of use was greater than perceived benefit. This does have the potential to introduce bias causing overestimation of symptom improvement and amplify the change in microbiomes and AMPs. Finally, we modeled the microbiome data in terms of relative abundance. It should be noted that a relative increase of one taxon does not necessarily represent an absolute increase in that taxon, but could represent decreases of other taxa.
As observed clinically35, some participants were not compliant with vaginal estrogen therapy despite appropriate counseling about the benefits and risks. The patient information sheet that accompanies prescribed vaginal estrogen cream is identical to the sheet provided for all doses and routes of estrogen, essentially equating higher dose oral ingestion with low dose vaginal use and causing patients to avoid low-dose therapy. Changes to this federally mandated information might be warranted to more appropriately advise patients concerning dose, systemic absorption, and route-related risks.
Conclusion
This study builds on the growing knowledge of the urobiome in various health states and provides direct evidence of urobiome changes, comparable with changes in other nearby relevant pelvic microbial niches. These findings can be used to inform future interventional studies, and refine hypotheses for further testing in both clinical and laboratory settings.
Supplementary Material
AJOG at a Glance.
A. Why was this study conducted?
Determine if estrogen treatment of post-menopausal women with overactive bladder decreases urobiome diversity.
B. What are the key findings?
Estrogen therapy increased Lactobacillus levels in bladder, but not vagina.
This increase associated with modest changes in urgency incontinence symptoms.
C. What does this study add to what is already known?
Others analyzed estrogen’s effect on vaginal microbiota of atrophic vaginitis patients, or compared cohorts not on estrogen to those on estrogen for years.
We tracked women with overactive bladder over 12 weeks of estrogen treatment.
We compared microbiota of vaginal swabs, perineal swabs, urine obtained by void (which samples the genitourinary tract) and urine obtained by transurethral catheter (which samples the bladder).
We observed the estrogen effect on microbiota of multiple pelvic floor niches.
Acknowledgements
We would like to acknowledge and thank all the members of the Loyola Urinary Education and Research Collaborative (LUEREC), particularly our research nurse Mary Tulke RN and technician Thomas Halverson.
Source of Funding
This study was funded by Loyola Urogynecology Division Research Fund, an Investigator Initiated grant from the Kimberly Clark Corporation, two NIH awards to Drs. Wolfe and Brubaker (R56DK104718 and R01 DK104718) and the Arthur J Schmitt Dissertation Fellowship. The funders had no input in the design, execution or interpretation of the study.
Disclosure statement
Dr. Thomas-White discloses membership on the advisory board of LiveUTIFree. Dr. Radek discloses research support from Kimberly Clark Corporation. Dr. Brubaker discloses editorial stipends from JAMA, Female Pelvic Medicine and Reconstructive Surgery and UpToDate. Dr. Mueller discloses Astellas Scientific and Medical Affairs (research support), Boston Scientific (Advisory Board), UpToDate (royalties) and Bulter-Snow/Ethicon (Legal review). Dr. Wolfe discloses research support from Astellas Scientific and Medical Affairs and Kimberly Clark Corporation. The other authors (Drs. Taege, Joyce, Brincat, Hilt, Mac-Daniel) report no conflicts of interest.
Footnotes
Clinical Trial ID number and URL
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Canadian journal of microbiology. 2005;51(9):777–781. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Song N, Williams CJ, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep. 2016;6:24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardozo L, Lose G, McClish D, Versi E. A systematic review of the effects of estrogens for symptoms suggestive of overactive bladder. Acta obstetricia et gynecologica Scandinavica. 2004;83(10):892–897. [DOI] [PubMed] [Google Scholar]
- 6.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourology and urodynamics. 2010;29(1):4–20. [DOI] [PubMed] [Google Scholar]
- 7.Griebling TL, Liao Z, Smith PG. Systemic and topical hormone therapies reduce vaginal innervation density in postmenopausal women. Menopause. 2012;19(6):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HY, Chen CJ, Chen WC, Wang SJ, Chen YH. A promising protein responsible for overactive bladder in ovariectomized mice. Taiwan J Obstet Gynecol. 2017;56(2):196–203. [DOI] [PubMed] [Google Scholar]
- 9.Luthje P, Brauner H, Ramos NL, et al. Estrogen supports urothelial defense mechanisms. Science translational medicine. 2013;5(190):190ra180. [DOI] [PubMed] [Google Scholar]
- 10.Pearce MM, Hilt EE, Rosenfeld AB, et al. The Female Urinary Microbiome: a Comparison of Women with and without Urgency Urinary Incontinence. mBio. 2014;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas-White KJ, Hilt EE, Fok C, et al. Incontinence medication response relates to the female urinary microbiota. International urogynecology journal. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nienhouse V, Gao X, Dong Q, et al. Interplay between bladder microbiota and urinary antimicrobial peptides: mechanisms for human urinary tract infection risk and symptom severity. PloS one. 2014;9(12):e114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plichta JK, Holmes CJ, Nienhouse V, et al. Cutaneous Burn Injury Modulates Urinary Antimicrobial Peptide Responses and the Urinary Microbiome. Crit Care Med. 2017;45(6):e543–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichler T, Bender K, Murtha MJ, et al. Ribonuclease 7 Shields the Kidney and Bladder from Invasive Uropathogenic Escherichia coli Infection. J Am Soc Nephrol. 2019;30(8):1385–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luthje P, Hirschberg AL, Brauner A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas. 2014;77(1):32–36. [DOI] [PubMed] [Google Scholar]
- 16.Thomas-White K, Forster SC, Kumar N, et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nature communications. 2018;9(1):1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog AR, Fultz NH. Prevalence and incidence of urinary incontinence in community-dwelling populations. J Am Geriatr Soc. 1990;38(3):273–281. [DOI] [PubMed] [Google Scholar]
- 18.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11(6):563–574. [DOI] [PubMed] [Google Scholar]
- 19.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. American journal of obstetrics and gynecology. 1996;175(1):10–17. [DOI] [PubMed] [Google Scholar]
- 20.Rahn DD, Ward RM, Sanses TV, et al. Vaginal estrogen use in postmenopausal women with pelvic floor disorders: systematic review and practice guidelines. International urogynecology journal. 2014. [DOI] [PubMed] [Google Scholar]
- 21.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. Journal of clinical microbiology. 2014;52(3):871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team R RStudio: Integrated Development for R [computer program]. Boston, MA: URL http://www.rstudio.com/:RStudioInc; 2015. [Google Scholar]
- 23.Giangaspero A, Sandri L, Tossi A. Amphipathic alpha helical antimicrobial peptides. European journal of biochemistry / FEBS. 2001;268(21):5589–5600. [DOI] [PubMed] [Google Scholar]
- 24.Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the overactive bladder questionnaire. The Journal of urology. 2006;176(2):627–632; discussion 632. [DOI] [PubMed] [Google Scholar]
- 25.Benness C, Wise B, Cutner A, Cardozo L. Does low dose vaginal estradiol improve frequency and urgency in postmenopausal women? (Abstract). Proceedings of the American Urogynecology Society, 13thannual meeting; 1992; Cambridge, Massechussetts. [Google Scholar]
- 26.Eriksen PS, Rasmussen H. Low-dose 17 beta-estradiol vaginal tablets in the treatment of atrophic vaginitis: a double-blind placebo controlled study. European journal of obstetrics, gynecology, and reproductive biology. 1992;44(2):137–144. [DOI] [PubMed] [Google Scholar]
- 27.Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR Jr. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause. 2011;18(9):962–966. [DOI] [PubMed] [Google Scholar]
- 28.Tseng LH, Wang AC, Chang YL, Soong YK, Lloyd LK, Ko YJ. Randomized comparison of tolterodine with vaginal estrogen cream versus tolterodine alone for the treatment of postmenopausal women with overactive bladder syndrome. Neurourology and urodynamics. 2009;28(1):47–51. [DOI] [PubMed] [Google Scholar]
- 29.Cardozo LD, Wise BG, Benness CJ. Vaginal oestradiol for the treatment of lower urinary tract symptoms in postmenopausal women--a double-blind placebo-controlled study. J Obstet Gynaecol. 2001;21(4):383–385. [DOI] [PubMed] [Google Scholar]
- 30.Devillard E, Burton JP, Hammond JA, Lam D, Reid G. Novel insight into the vaginal microflora in postmenopausal women under hormone replacement therapy as analyzed by PCR-denaturing gradient gel electrophoresis. European journal of obstetrics, gynecology, and reproductive biology. 2004;117(1):76–81. [DOI] [PubMed] [Google Scholar]
- 31.Gunderson L Ecological Resilience - In Theory and Application. Annual Review of Ecology and Systematics. 2000;31:425–539. [Google Scholar]
- 32.Mirmonsef P, Modur S, Burgad D, et al. Exploratory comparison of vaginal glycogen and Lactobacillus levels in premenopausal and postmenopausal women. Menopause. 2015;22(7):702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salem N, Salem L, Saber S, Ismail G, Bluth MH. Corynebacterium urealyticum: a comprehensive review of an understated organism. Infect Drug Resist. 2015;8:129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portman D, Shulman L, Yeaw J, et al. One-year treatment persistence with local estrogen therapy in postmenopausal women diagnosed as having vaginal atrophy. Menopause. 2015;22(11):1197–1203. [DOI] [PubMed] [Google Scholar]
- 35.Manson JE, Goldstein SR, Kagan R, et al. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. 2014;21(9):911–916. [DOI] [PubMed] [Google Scholar]
- 36.Oksanen J, Blanchet F, Friendly M, et al. vegan: Community Ecology Package. R package version 2.5–5 [computer program]. 2019: https://CRAN.R-project.org/package=vegan.
- 37.Wilke C cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. R package version 1.0.0 [computer program]. 2019: https://CRAN.R-project.org/package=cowplot.
- 38.Yu G ggplotify: Convert Plot to ‘grob’ or ‘ggplot’ Object. R package version 0.0.5. [computer program]. 2020: https://CRAN.R-project.org/package=ggplotify. [Google Scholar]
- 39.Wickham H ggplot2: Elegant Graphics for Data Analysis [computer program]. Springer-Verlag; New York; 2016: https://ggplot2.tidyverse.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.