Abstract
Introduction
3D printing has widespread applications in orthopaedics including creating biomodels, patient-specific instruments, implants, and developing bioprints. 3DGraphy or printing 3D models enable the surgeon to understand, plan, and simulate different procedures on it. Despite widespread applications in non-healthcare specialties, it has failed to gain traction in healthcare settings. This is perhaps due to perceived capital expenditure cost and the lack of knowledge and skill required to execute the process.
Purpose
This article is written with an aim to provide step-by-step instructions for setting up a cost-efficient 3D printing laboratory in an institution or standalone radiology centre. The article with the help of video modules will explain the key process of segmentation, especially the technique of edge detection and thresholding which are the heart of 3D printing.
Conclusion
This is likely to enable the practising orthopaedician and radiologist to set up a 3D printing unit in their departments or even standalone radiology centres at minimal startup costs. This will enable maximal utilisation of this technology that is likely to bring about a paradigm shift in planning, simulation, and execution of complex surgeries.
Electronic supplementary material
The online version of this article (10.1007/s43465-020-00254-9) contains supplementary material, which is available to authorized users.
Keywords: Thresholding, 3D printing, 3DGraphy, Patient-specific instruments, Segmentation, 3D printers, 3D materials, 3D model, STL, 3D slicer
Introduction
Radiography is a technique to visualize the internal structure of the human body using ionizing or non-ionizing radiation. Similarly, the term 3Dgraphy has been coined by us to describe the technique of producing 3D printed biomodel of human anatomical structures [1]. Such biomodels are invaluable for the clinician for surgical planning and simulation. The information obtained by the tactile understanding of a real-size 3D printed biomodel (tactile stereotaxy) is incomparable and is a useful adjunct for studying complex and rare pathoanatomy [2]. 3DGraphy is a technological evolution which began as an upgrade from the conventional two-dimensional X-rays and CT scan/3D reconstruction images. (Fig. 1).
Fig. 1.
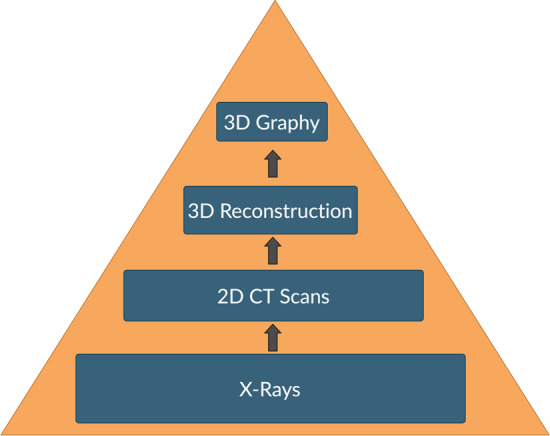
From X-rays to 3D graphy—technological upgrade
3D printing as a specialty started in the mid-1980s for mainly industrial use [3, 4]. It was a complete change from the conventional production process and involved additive manufacturing instead of subtractive manufacturing or mold casting that was prevalent at the time [5–8]. As it ensured rapid production, decreased industrial wastage, and opened up space for newer complex designs—it was heralded as the third industrial revolution.
In the 2000s, the use of 3D printing technology in the medical and dental world started to gain traction, and today it is an inevitable part of surgical planning of complex orthopedic, oncologic, maxillofacial, and dental procedures [3, 4, 8–11]. 3D printing in the orthopedic field, is used to print anatomic models for the purpose of educating peers, preoperative planning, and surgical simulation. Furthermore, it is used to create custom cutting jigs, drill/trajectory guides which go a long way in reducing the technicality of a procedure and the duration of surgery. A hands-on model dramatically increases the understanding of a boney pathology (fracture, deformity, etc.), thereby helping in improving the surgical outcome especially for young surgeons. Various scientific studies have proven the utility of this technology in clinical settings [3, 7, 8, 10]. Many hospitals around the world are investing in an “in-house” 3D printing lab with the help of radiology departments. Invariably, a clinician who cannot keep up with this new technology will be at a disadvantage in the near future. Radiologists, due to their intimate understanding of cross-sectional anatomy, have a key role to play in the process of development of a 3D printed biomodel.
The purpose of this article is to simplify the key technical aspects of the 3D printing process, specifically segmentation, which is an important step in the process. We have used orthopedic anatomical models examples, primarily because bone models are easier to segment. The reader can extrapolate this information, to other applications in orthopedics and also to other specialties as they gain expertise in this field.
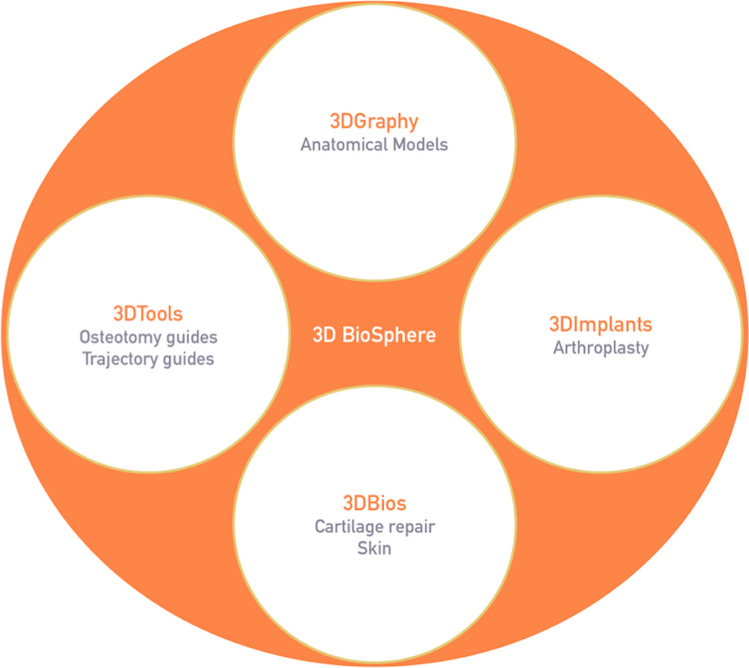
3D BioSphere
The term ‘3D BioSphere’ and subsequent sub-headings have been coined by us to describe the entire spectrum of possibilities that 3D printing covers. This also makes it easier for an orthopedic surgeon to understand the utilities of 3D printing in the field. We have categorized the applications of 3D printing into a 3D BioSphere (Fig. 2):
3DGraphy: anatomical models
3DTools: osteotomy guides, trajectory guides for acetabular screws, and pedicles.
3DImplants: custom fit implants, e.g. in arthroplasty
3DBios: printing living tissue, i.e. cartilage repair, skin
Fig. 2.
3DBioSphere—spectrum of functional capabilities
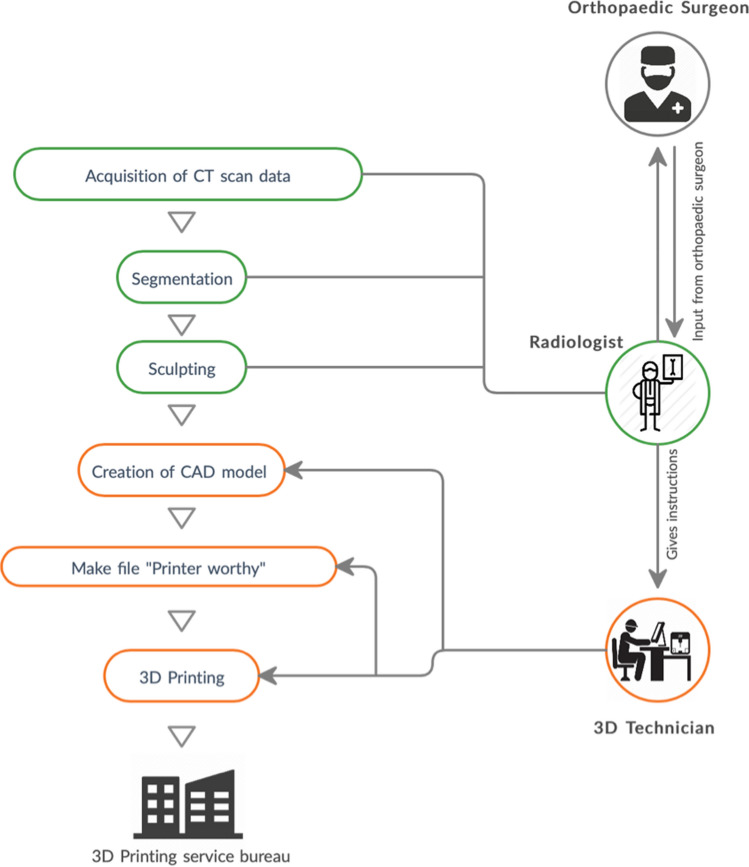
3DGraphy Workflow
3DGraphy involves well-defined sequential steps to achieve the desired biomodel. Each step requires the expertise of a particular member of a strong interdisciplinary team [12]. The key members of this team are radiologists, surgeons, technologists, and engineers [12, 13]. The 3D printing workflow has been depicted in Fig. 3.
Fig. 3.
3DGraphy workflow—process of 3D printing
Acquisition of Images
CT scans are the primary sources of DICOM (Digital Imaging and Communications in Medicine) data for orthopedic applications of 3Dgraphy. However, MRI too can be used for specific indications [14]. Before the acquisition of the images, the radiologist should understand the primary requirements of the surgeon. This will determine the region of interest (ROI; region desired to be printed) and whether intravenous contrast is required. For example, for printing a pelvic tumor, the surgeon may want to visualize the tumor with respect to major vessels. For this purpose, intravenous contrast is necessary as it is almost impossible to segment blood vessels in CT without their opacification.
Furthermore, in the pediatric age group, to reduce radiation exposure, CT scans using the ALARA principle (As low as reasonably achievable) along with noise-reducing algorithms should be utilized [15]. This principle helps in reducing the radiation exposure to the child. Some orthopedic patients may have metal implants. Beam hardening metallic artifacts in CT make the process of segmentation exhaustive and tedious. The radiologists have chosen artifact reducing imaging protocols to reduce these artifacts.
Various CT protocols exist which are utilised in other specialties [16]. However, in case of musculoskeletal radiology, standardized protocols like medical rapid prototyping computed tomography protocol (MRCP) by Bagaria et al. [17] is commonly used as a guideline for image acquisition in 3D printing (Table 1). The slice thickness should be set to 1–1.25 mm with a voxel size of 0.2–0.3 mm. A voxel is a “three-dimensional pixel” used to quantify the resolution of 3D biomodels. Thick slices produce biomodels with a “stair-case” effect which is akin to a pixelated photograph. In contrast, very thin slices (0.6 mm) will unnecessarily add to the segmentation effort. Besides, the computer model or mesh created from very thin slices is invariably very complex and increases the printing time without adding any value to the biomodel. Therefore, a slice thickness of 1–1.25 mm is recommended for boney models. As with volume-rendered 3D images, soft tissue reconstruction kernel is the best for segmentation and 3Dgraphy. The term ‘kernel’ refers to a process used to alter the frequency content of a projected image in a CT scanner. It can be thought of as a ‘filter’ applied to a photographic image to highlight different aspects of the said image. Depending upon the anatomic application, various kernels like standard, soft tissue, bone, lung, chest, etc., have been developed. For the purpose of this paper, we will focus on standard soft tissue and bone kernels only. We recommend using the soft tissue kernel in most cases as it produces smoother CT scan images with reduced noise. Bone kernel, on the other hand sharpens the region of interest and thus looks better virtually; however, comes with the cost of increased noise which is not ideal for 3D printing [18].
Table 1.
MRCP protocol for acquisition of an ideal CT scan image for 3D printing biomodels
| Parameter | Description |
|---|---|
| Field of view | FOV measuring 12 × 12 inches is adequate |
| Region of interest | To be determined by the clinician with guidance from the radiologist |
| mA/kV | Automatic |
| Slice thickness/slice increment | 1–1.25 mm/1–1.5 mm |
| Collimation | 1.25–1.5 mm |
| Kernel/algorithm | Soft tissue (do not use ‘bone’) |
After the acquisition of the DICOM images, the next step would be to import these image sets to 3D Slicer [19] which is an open-source software with all the necessary features to create a good quality 3D model. Though various software like Invesalius (Centro de Tecnologia da Informação Renato Archer, Brazil), Vesalius 3D (PS-Medtech, Netherlands), ITKSnap (https://www.itksnap.org), Seg3D (Scientific computing and Imaging institute, University of Utah), OsiriX (Pixmeo, Switzerland) etc., are available, one of the authors’ preferred software is 3D slicer. Although not American FDA approved, it is inexpensive, relatively intuitive, and created by a team of researchers at Harvard as an open-source interdisciplinary project. Computing requirements include a minimum resolution of 1024 × 768 pixels, over four gigabyte of RAM (random access memory), dedicated graphics hardware, and memory (1 GB). More sophisticated configurations would decrease the processing time and, therefore, more complex printing projects should be undertaken in a computer with higher RAM and computing capability.
Segmentation
The process of volume rendering using DICOM workstations can generate a virtual 3D model which is commonly sought after by the orthopaedic surgeon (3D reconstructed images of CT scan). However, this information cannot be read and interpreted by 3D printers. As opposed to volume rendering, a surface rendered model is required for 3D printing. Segmentation is the initial step required to generate a surface rendered 3D model. It is the process of labeling the region of interest (ROI) (e.g. bone on CT) or voxels on cross-sectional CT images using specific criteria. The criteria for a voxel to be included in the segmentation may be defined as per its Hounsfield units within a specific range (Thresholding) or its connection to a selected point/voxel (Seed point) [20]. Alternatively, the voxels within a specific predefined geometric region can be included to be part of the model. This process may be unfamiliar to the orthopedist as it involves special software. The expertise of a radiologist or a trained orthopedic surgeon is required the most for this step. The process requires a detailed cross-sectional anatomical knowledge of the ROI. Therefore, coordination between a surgeon and radiologist is very important to obtain an accurate model. Although this step over time can be performed by a trained technologist, the accuracy of the segmentation needs to be confirmed by the clinician or the musculoskeletal (MSK) radiologist before the creation of the CAD model (3D printable model).
There are two methods of segmentation; manual and automatic [21]. A set of cross-sectional DICOM images for particular anatomy, for example, pelvic bone, usually has hundreds, if not thousands of slices [22]. Manually segmenting each slice to identity and “paint” the ROI (bone) is impossible. The segmentation software can automate this process. For orthopedic models, as the bone has a high contrast differential compared to surrounding soft tissue, automated techniques such as thresholding work quite well. Frequently, a combination of both methods is used by initiating the segmentation using automated techniques followed by manual corrections.
We will use an example of pelvic bone segmentation on an open-source software called 3D slicer to describe the steps. Appended videos (Video 1 and 2) will help in understanding the steps of this process.
Manual segmentation: in this mode, instead of relying on the ‘artificial intelligence’ of the software (3D Slicer), we manually select the region of interest from every slice of the CT scan. This manual process, however, is very time consuming and cumbersome; hence, we do not recommend using this mode. This method is used for fine-tuning—editing and correcting errors made by automated segmentation (also called sculpting). To understand the sculpting process, you can see the video example 2, timestamp 2:45 onwards.
Automatic segmentation: in automated segmentation, the algorithm automatically delineates the selected region of interest using various tools (e.g. thresholding, safe island, growing seeds) which are described further in this section. For orthopedic models, as the bone has a high contrast differential compared to surrounding soft tissue, automated techniques such as thresholding work quite well.
To understand the process of segmentation better, we have used two examples (thresholding and region growing) of automated segmentation techniques using open-source software called 3D slicer. Alternate open-source segmentation tool includes ITK Snap, Vesalius, and 3D seg apart from numerous paid proprietary software-like MIMICS [23].
Thresholding
This is a technique where we define a range of pixels (by selecting a range of HU) which we want the software to highlight. This demonstration is done using the thresholding module (National Alliance for Medical Image Computing) of 3DSlicer software.
Example 1: Congenital Scoliosis Biomodel
[Video 1] In this example, we have demonstrated the use of thresholding which is one of the most common automated segmentation techniques in most medical specialties. Due to the high contrast differential of bone with respect to surrounding tissues, thresholding is frequently the first step in segmenting bone. Voxels are selected on the bases of a defined range of HUs. For bone, the upper limit is usually the max value of 3000 and the lower limit varies depending upon the region (cortical/cancellous) and quality of bone (osteopenic). This is a quick and reliable method in most situations except for CT scans with metal artifacts. However, doing this will often lead to the inclusion of surrounding structures. This can be mitigated using the ‘Island effect’ tool as depicted in Video 1 (Timestamp 2:05). In case more control is desired in accurately highlighting the region of interest, scissor, and eraser tool can be used. (Timestamp: 2:30).
Region Growing
The region-growing module is based on the principle that voxels of a particular object will have comparable values. Based on this principle, the region-growing module compares the HU of the selected voxel with the neighbouring voxels. If the values are comparable, the software considers them to be a part of the same object and highlights the object till their edges. The edges are detected using the edge detection module, which is based on the same principle. It compares the change in HU of one voxel with the neighbouring voxels. If a drastic change is detected, then it defines it as the edge of the model. For example, in the case of a vertebra, the HU value of the superior endplate maybe 1000, and the HU value of the adjacent disc maybe 50. The software detects this sudden change in HU values and automatically outlines the superior endplate of the vertebra as its border/edge.
Example 2: Acetabulum Model with the Subtraction of the Femoral Head
[Video 2] In this example, we have demonstrated the use of region or seed growing method to segment femoral head and acetabulum separately. If thresholding is used, both femoral head and acetabulum get segmented as one unit, and it is then tedious to separate these two anatomical regions as the hip joint is highly conformal. The seed growing method can segment femoral head separately from the acetabulum. Once completed, the femoral head segmentation can be deleted leaving behind the acetabulum.
To use the region growing tool (National Alliance for Medical Image Computing), the user has to label (yellow color) voxels of the femoral head and then using a different label (green color), mark the voxels of the surrounding acetabulum. When the algorithm is initiated, these “seeds” grow and fill up the respective anatomical regions based on their similarity with adjacent voxels and using the principle of edge detection. If the red-colored segmentation is deleted, then only the acetabulum can be modeled. Final editing can be done by manually painting (labeling) [Video 2, Timestamp—0:50], erasing, or combining regions (sculpting).
Thresholding is used to highlight all the voxels in a predefined range of Hounsfield units while region growing highlights the voxels only adjacent to the ‘selected point’ as described above. Thresholding can be thought of as a local selection while region growing is hyper-local. We recommend using thresholding initially to feature all the bones in the selected region of interest and then applying the region-growing module to highlight the specific bone of interest. (Example: perform thresholding to highlight all the boney elements of the lumbar spine DICOM image and then the region-growing module can be used to highlight a particular vertebra, e.g. L5)
Creating a CAD Model
The collection of segmented voxels now has to be transformed into a 3D model (CAD model). This process is called tessellation and it involves the generation of a surface model represented as a collection of triangles or other polygons. The most common file format for this CAD model is an STL file (surface tessellation language) [4]. The mesh of triangles is the geometrical representation of a 3D surface. The quality of the 3D model is dependent on the number of oriented triangles. However, models with too many triangular facets can lead to unnecessary complexity and require more computing power and printing time. Usually, 300,000 triangles for the femur and 850,000 triangular facets of the full spine are sufficient to create a reasonably accurate model [24].
The unique aspect of the STL file is that these describe only the surface geometry of a three-dimensional object without any representation of texture, color, or any other common CAD model attributes. Recently, an OBJ file has been developed by Wavefront Technologies which contains all such information, i.e. color, texture, etc. 3D slicer supports exporting the configured model in both STL and OBJ format [25].
At this juncture, the STL model or mesh may not be printer-ready [26]. This part of the process can be referred to the engineer who is equipped with the technical knowledge and skills to create a ‘printer-ready’ model. However, we have given a brief description of the common errors that may need to be addressed. If the mesh is “open” and does not enclose a volume, then it cannot be printed. Non-manifold edge is another type of error in which more than two triangles (surfaces) are connected to the same edge. Such errors need to be repaired. Smoothening is another tool to improve the surface of the model. If simulation surgery is planned on the bone biomodel, then the model can have an infill density and surface thickness that closely mimics bone. Support structures are then added, especially if the model has overhanging features. Examples of software that can achieve this are Autodesk Meshmixer, Meshlab, Blender (open source), and Mimics (proprietary). The steps are carried out by the engineer or technologist under the supervision of the radiologist.
Several tools are available in various software to help with mesh manipulation and refinement. They are:
Cleaning: post-segmentation, there may be artifacts present in the form of surface irregularities and unwanted bridges between sections. Mesh cleaning algorithms are present in most software, which create a surface with more uniformly arranged triangles resulting in a better quality model. The unwanted connection between sections, for example, a bridge across a joint or a fracture gap will have to be removed manually using the eraser tool. Again this may not be well understood by the engineers, requiring the constant supervision of a radiologist.
Smoothening: the errors commonly known as ‘stair-casing’ appear following the process of segmentation and can be mitigated by smoothening of the surface of the model with a software. However, caution should be exercised to not “over-smoothen” the model to avoid distorting the surface. Reducing the complexity of the mesh may be desirable when required. If a model is required for geometric analysis, then this step should be used to a bare minimum. In situations where the purpose is a simulation or for anatomic study, a smoother biomodel is desired which can be achieved by increased smoothening.
Printing 3DGraphy Model
The above process will result in a pictorial representation of the desired three-dimensional image. Depending on the surgeons’ experience, it may not be necessary to print the model since the 3D image will provide one with adequate information to carry out the procedure. This can even be done on handheld devices using software like Emb3D (Transform and Lighting Srl). On the other hand, inexperienced or junior surgeons may want a more hands-on feel of the model to understand the complexity of the pathology, wherein 3D printing becomes essential.
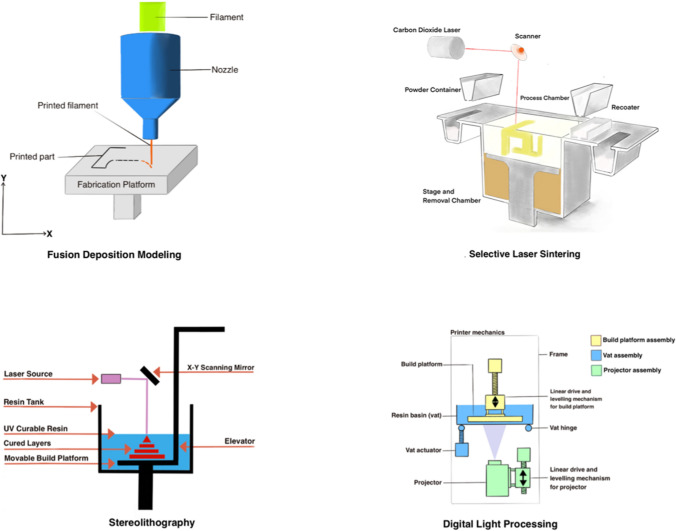
3D printers started to become popular in 1980 and initially, the process of 3D printing was called rapid prototyping [5, 10, 27–31]. STL or virtual reality modeling language (VRML) files are the standard files which are most commonly used to print the 3D model. There are several ways in which STL/VRML (virtual reality modeling language) files can be used to print the 3D model. The main differences between the various printers are the way layers are deposited to create parts and the nature of polymers or materials used to create them. Each of these has its advantages and disadvantages as summarized in Table 2. While choosing the most appropriate printer, the main consideration is speed, the hardware costs, the raw material used, choice and cost of the material, and the color capabilities. Each printer has resolution limits and, therefore, the radiologist needs to know this step as it can drastically affect the quality of the model. For example, certain printers utilize filaments to create the biomodel. If the filament is too thick, it will result in an inaccurate model with poor quality, and intensive work put into the processing of the image will be in vain. The various types of printers are illustrated in Fig. 4. Moreover, we have highlighted the pros/cons and compatibility of each 3D printing material with various 3D printers in Table 3.
Table 2.
Characteristics of 3D printer
| Mechanism of Action | Supports required; Cost |
Accuracy/resolution | Application | Pros | Cons | |
|---|---|---|---|---|---|---|
| SLA | Utilizes light to cause cross linking of chemical monomer and oligomers into polymers |
Yes; Moderately priced |
High/high |
Trajectory guides Cutting jigs |
Smooth finish Fast |
Not user friendly Sensitive to long exposure to UV light Less resemblance to bone |
| SLS | Laser melts and fuses nylon-based powder to the desired object |
No; Highly priced |
High/average |
Anatomy models Surgery guides Drilling simulation |
Smooth finish Closest to bone-like feel |
Brittle Expensive Prone to shrinkage Slow printing process |
| Multi-jet | Similar to SLA, based on photo polymerization |
No; Highly priced |
Both average | Colored anatomy models | Color options available | Expensive |
| FDM | Printer head extrudes heated filament which is laid over each other |
Yes; Low priced |
Moderate/low |
Anatomy models Preoperative templating |
Ease of use Affordable High tensile strength |
Low detailing Materials deform at high temperature |
| DLP | Photo polymerization where light source is a digital light projector screen |
Yes; Moderately priced |
Both average | Cutting guides | Affordable | Object size limited to 10–15 cm |
Fig. 4.
Types of 3D printers
Table 3.
Commonly used 3D printer materials
| Materials | Pros | Cons | Cost | Compatible printer | Autoclavable |
|---|---|---|---|---|---|
| Acrylonitrile butadiene styrene |
Tough, impact resistant Natural color similar to bone |
Unpleasant fumes High melting point |
$20–50/kg | Extrusion printers | Only high temperature ABS |
| Polylactic acid |
Biodegradable Easier to print than ABS |
Rough texture Print degrades over time |
$20–50/kg | FDM | No |
| Polyvinyl alcohol |
Water soluble Good for supports |
Releases toxic vapors | > $100/kg | FDM with multiple extruders | No |
| Nylon |
Medically approved Tough Choice for medical guides |
Requires high temperatures to print | $18–70/kg |
FDM SLS MJF |
Yes |
| Metals | Custom implants can be made | Expensive | $300–600/kg |
DMLS SLS |
Yes |
MJF multi-jet fusion, DMLS direct material laser sintering
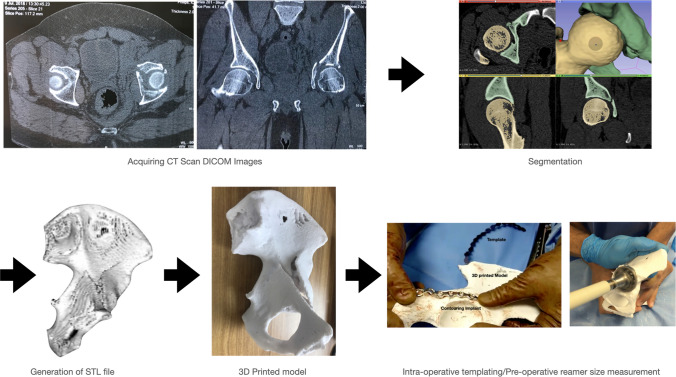
The actual process of 3D printing can be time consuming. The time taken to 3D print a model depends on the resolution, volume, and selected orientation of a model. With regard to orientation, printing within a layer is faster than printing between layers. For example, a femur bone template lying on its side will print faster than the same template in a standing configuration. Printing within a layer results in a geometrically accurate model. Therefore, depending upon the utility of the model, the orientation of the model can be changed. A case example is shown in Fig. 5 to depict how the process begins with procuring a 2D DICOM image and is finally converted into a 3D anatomical model.
Fig. 5.
Clinical workflow of process: from acquiring DICOM images to a complete 3D printed model
Cost–Benefit Ratio of 3DGraphy Technology for MSK Centers: Achieving an Equilibrium
Despite several utilities of 3D printing in orthopedics, ultimately the question is ‘Who’s going to pay for it?’ Insurance companies are yet to completely comprehend the importance of this technology and how it improves clinical decision-making. The onus is on the medical community to justify its need by demonstrating its importance. Once more data are collected justifying the need for 3D printing, a CPT (Current Procedural Terminology) code can be generated specifically for 3D printing by the insurance companies. This will enable more widespread use of this technology.
In 1936, the first modern computer was invented [32]. At the time, computers were bulky and expensive compared to the cost today. Today, most individuals own a computer which has a basic software like Microsoft Word in it; only how to type quality content using the software has to be learned. Similarly, with the advent of technology, 3D printer bureaus are widely available and open-source software like 3D slicer is also present in the market. Thus, essentially the tools are there for one to use; and with the help of this article, the clinician will be able to use these tools to their advantage.
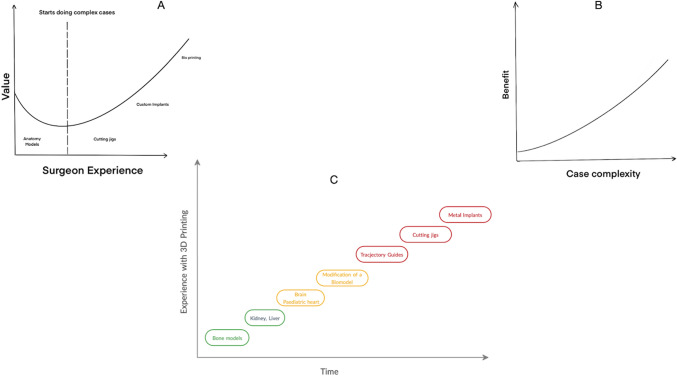
The value of an object is defined as, benefit divided by cost [33]. With advancements in technology, the cost of 3D printing is bound to decrease thereby increasing its value. The benefit is subjective, for a novice surgeon, he/she will benefit more from printing models of simple fractures, but as the surgeon gains more experience, the benefit from printing basic fracture models will reduce. However, once the surgeon starts performing complex surgeries, the benefit of 3D printing bone models will increase (Fig. 6a). Hence, it is safe to say that benefit in 3D printing models is directly proportional to the complexity of cases (Fig. 6b) [34, 35]. Ideally, one should start their 3D printing journey by segmentation of bone models as they are the easiest to segment and then move on to soft tissue which are comparatively harder due to the low differential contrast. This can be followed by printing trajectory guides, cutting jigs, and finally, metal implants which will be the most resource intensive. (Fig. 6c)
Fig. 6.
Value of 3D printing
To create 3D images from DICOM files is not a time-consuming affair and once accustomed can take anywhere between 30 and 60 min for an average user to create a ‘3D printer ready’ file. A surgeon who has familiarized oneself with the process of 3D printing, can segment and generate STL/VRML files without spending any money. The overall time taken from obtaining DICOM images to actual 3D printing a model would take 3–4 working days depending on the type of printer/printing material used. If more complex work like printing 3DTools and 3DImplants is undertaken, then the time taken for the entire process would be naturally more. The cost to procure and maintain a commercial 3D printer is substantial and so we believe for a surgeon just starting with a 3D printing lab, outsourcing may be wiser. With the technical knowledge obtained from this article, any orthopedic surgeon with a basic computer who plans to outsource the final step of “3D printing” can create a 3D biomodel within a short duration. Having said this, sending confidential medical data to a third party should be done with caution. If one still desires to purchase a printer, it should be done keeping in mind the intended service. The cheapest 3D printer costs around INR50,000 or $700, the more sophisticated ones range from INR 5 million to 2 crores ($7000–$260,000).
Conclusion
3D printing looks intimidating in the first instance but once understood proves to be an easy task. It adds tremendous value to the management of the patient. The benefits far outweigh what seems like a laborious process, especially in complex cases. In our opinion, to make the most out of a 3D printing lab in terms of research and patient benefit, the core team should include an orthopedic surgeon and a radiologist. Setting up the 3D printing lab need not be a very cumbersome or expensive proposition. Lowered costs would ensure wide availability of revolutionary technology.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study, informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Darshil Shah, Email: drshahdarshil@gmail.com.
Lokesh Naik, Email: drlokeshortho@gmail.com.
Bhawan Paunipagar, Email: bhawanpaunipagar@gmail.com.
Darshana Rasalkar, Email: drdarshanab@gmail.com.
Kshitij Chaudhary, Email: chaudhary.kc@gmail.com.
Vaibhav Bagaria, Email: bagariavaibhav@gmail.com.
References
- 1.Bagaria V, Chaudhary K. A paradigm shift in surgical planning and simulation using 3Dgraphy: Experience of first 50 surgeries done using 3D-printed biomodels. Injury. 2017;48(11):2501–2508. doi: 10.1016/j.injury.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 2.Green N, Glatt V, Tetsworth K, Wilson LJ, Grant CA. A Practical guide to image processing in the creation of 3D models for orthopedics. Techniques in Orthopaedics. 2016;31(3):153–163. doi: 10.1097/BTO.0000000000000181. [DOI] [Google Scholar]
- 3.Sheikh A, Forster BB. “Holding It in Your Hand”: Musculoskeletal Applications of 3D Printing. Canadian Association of Radiologists journal = Journal l’Association canadienne des radiologistes. 2020;71(2):129–130. doi: 10.1177/0846537119893288. [DOI] [PubMed] [Google Scholar]
- 4.Ghazi AE, Teplitz BA. Role of 3D printing in surgical education for robotic urology procedures. Translational Andrology and Urology. 2020;9(2):931–41.z. doi: 10.21037/tau.2020.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Arsdell GS, Hussein N, Yoo S-J. Three-dimensional printing in congenital cardiac surgery-Now and the future. The Journal of Thoracic and Cardiovascular Surgery. 2020;160(2):515–519. doi: 10.1016/j.jtcvs.2019.12.131. [DOI] [PubMed] [Google Scholar]
- 6.Bagaria V, Kuthe A, Deshpande S. Use of rapid prototyping and three-dimensional reconstruction modeling in management of complex acetabular fracture. Current Orthopaedic Practice. 2009;20(3):337–340. doi: 10.1097/BCO.0b013e3181930263. [DOI] [Google Scholar]
- 7.Bagaria V. Technical note: 3D printing and developing patient optimized rehabilitation tools (port)—a technological leap. International Journal of Neurorehabilitation. 2015;2(3):1–4. doi: 10.4172/2376-0281.1000175. [DOI] [Google Scholar]
- 8.Wang, J., Min, L., Lu, M., Zhang, Y., Wang, Y., Luo, Y., et al. (2020). What are the complications of three-dimensional-printed custom-made integrative hemipelvic endoprostheses in patients with primary malignancies involving the acetabulum, and what is the function of these patients? Clinical Orthopaedics and Related Research. 10.1097/CORR.0000000000001297 [DOI] [PMC free article] [PubMed]
- 9.Fournet, M. E. B., Azaman, F.A., Gunbay, S., Chen, Y. Y., & Devine, D. M. (2019). Polymer-based additive manufacturing. Springer.
- 10.Bagaria V, Bhansali R, Pawar P. 3D printing- creating a blueprint for the future of orthopedics: Current concept review and the road ahead! Journal of Clinical Orthopaedics and Trauma. 2018;9(3):207–212. doi: 10.1016/j.jcot.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye X, Wang L, Li K, Hao Q, Lu J, Chen X, et al. A three-dimensional color-printed system allowing complete modeling of arteriovenous malformations for surgical simulations. Journal of Clinical Neuroscience. 2020;77:134–141. doi: 10.1016/j.jocn.2020.04.123. [DOI] [PubMed] [Google Scholar]
- 12.Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. Journal of Clinical Orthopaedics and Trauma. 2018;9(3):260–268. doi: 10.1016/j.jcot.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Qian C, Shen R, Wu D, Bian L, Qu H, et al. 3D printing technology improves medical interns’ understanding of anatomy of gastrocolic trunk. Journal of Surgical Education. 2020;77(5):1279–1284. doi: 10.1016/j.jsurg.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Parthasarathy J, Krishnamurthy R, Ostendorf A, Shinoka T, Krishnamurthy R. 3D printing with MRI in pediatric applications. Journal of Magnetic Resonance Imaging. 2020;51(6):1641–1658. doi: 10.1002/jmri.26870. [DOI] [PubMed] [Google Scholar]
- 15.de Lima Moreno JJ, Liedke GS, Soler R, da Silveira HED, da Silveira HLD. Imaging factors impacting on accuracy and radiation dose in 3D printing. Journal of Maxillofacial and Oral Surgery. 2018;17(4):582–587. doi: 10.1007/s12663-018-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maria M, et al. A critical review on acquisition and manipulation of CT images of the maxillofacial area for rapid prototyping. Virtual Modeling and Rapid Manufacturing. 2005;6(1):167–173. [Google Scholar]
- 17.Hoque ME, editor. (2011). Advanced applications of rapid prototyping technology in modern engineering InTech Open; pp 1–20.
- 18.Ogden KM, Aslan C, Ordway N, Diallo D, Tillapaugh-Fay G, Soman P. Factors affecting dimensional accuracy of 3-D printed anatomical structures derived from CT data. Journal of Digital Imaging. 2015;28(6):654–663. doi: 10.1007/s10278-015-9803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.3D Slicer. https://www.slicer.org/. Accessed 11 Jul 2020.
- 20.Jacob, S., Pooley, R. A., Thomas M. (2020). Three-dimensional–printed model as a template for chest wall reconstruction. Heart, Lung and Circulation. 10.1016/j.hlc.2020.02.004. [DOI] [PubMed]
- 21.Osti F, Santi G, Neri M, Liverani A, Frizziero L, Stilli S, et al. CT conversion workflow for intraoperative usage of bony models: From DICOM data to 3D printed models. Applied Sciences. 2019;9(4):708. doi: 10.3390/app9040708. [DOI] [Google Scholar]
- 22.Okkalidis N, Marinakis G. Technical Note: Accurate replication of soft and bone tissues with 3D printing. Medical Physics. 2020;47(5):2206–2211. doi: 10.1002/mp.14100. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Belle A, Hargraves RH, Cockrell C, Tang Y, Najarian K. Bone segmentation and 3D visualization of CT images for traumatic pelvic injuries. International Journal of Imaging Systems and Technology. 2014;24(1):29–38. doi: 10.1002/ima.22076. [DOI] [Google Scholar]
- 24.Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK, et al. Medical 3D printing for the radiologist. Radiographics. 2015;35(7):1965–1988. doi: 10.1148/rg.2015140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravorty, D. OBJ File Format—Simply Explained. https://all3dp.com/1/obj-file-format-3d-printing-cad/. 2018. Accessed 11 Jul 2020.
- 26.Friedman T, Michalski M, Goodman TR, Brown JE. 3D printing from diagnostic images: a radiologist’s primer with an emphasis on musculoskeletal imaging-putting the 3D printing of pathology into the hands of every physician. Skeletal Radiology. 2015;45(3):307–321. doi: 10.1007/s00256-015-2282-6. [DOI] [PubMed] [Google Scholar]
- 27.Claflin J, Waits SA. Three dimensionally printed interactive training model for kidney transplantation. Journal of Surgical Education. 2020;77(5):1013–1017. doi: 10.1016/j.jsurg.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Pardo GLA, Conzelmann J, Genske U, Hamm B, Scheel M, Jahnke P. 3D printing of anatomically realistic phantoms with detection tasks to assess the diagnostic performance of CT images. European radiology. 2020;30(8):4557–4563. doi: 10.1007/s00330-020-06808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshmukh TR, Kuthe AM, Chaware SM, Bagaria V, Ingole DS. A novel rapid prototyping and finite element method-based development of the patient-specific temporomandibular joint implant. Computer Methods in Biomechanics and Biomedical Engineering. 2011;15(4):363–370. doi: 10.1080/10255842.2010.538385. [DOI] [PubMed] [Google Scholar]
- 30.Farooqi KM, Saeed O, Zaidi A, Sanz J, Nielsen JC, Hsu DT, et al. 3D printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure. JACC: Heart Failure. 2016;4(4):301–311. doi: 10.1016/j.jchf.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Amin D, Nguyen N, Roser SM, Abramowicz S. 3D printing of face shields during COVID-19 pandemic: A technical note. Journal of Oral and Maxillofacial Surgery. 2020;78(8):1275–1278. doi: 10.1016/j.joms.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahoney MS. The history of computing in the history of technology. IEEE Annals of the History of Computing. 1988;10(2):113–125. doi: 10.1109/MAHC.1988.10011. [DOI] [Google Scholar]
- 33.Tetsworth KD, Mettyas T. Overview of emerging technology in orthopedic surgery: What is the value in 3D modeling and printing? Techniques in Orthopaedics. 2016;31(3):143–152. doi: 10.1097/BTO.0000000000000187. [DOI] [Google Scholar]
- 34.Kang HJ, Kim BS, Kim SM, Kim YM, Kim HN, Park JY, et al. Can preoperative 3D printing change surgeon’s operative plan for distal tibia fracture? BioMed Research International. 2019;2019:1–7. doi: 10.1155/2019/7059413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okoroha KR, Evans TJ, Stephens JP, Makhni EC, Moutzouros V. Three-dimensional printing improves osteochondral allograft placement in complex cases Knee Surgery. Sports Traumatology, Arthroscopy. 2018;26(12):3601–3605. doi: 10.1007/s00167-018-4849-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.