Abstract
BACKGROUND:
Since the discovery of the bladder microbiome (urobiome), interest has grown in learning whether urobiome characteristics have a role in clinical phenotyping and/or provide opportunities for novel therapeutic approaches for women with common forms of urinary incontinence (UI).
OBJECTIVES:
To test our hypothesis that the bladder urobiome differs between continent women and women affected by UI by assessing associations between UI status and the cultured urobiome.
STUDY DESIGN:
With IRB oversight, transurethral catheterized urine specimens were collected from 309 adult women, who were categorized into three groups using response to the validated Pelvic Floor Distress Inventory (PFDI): Continent Controls (N=150) and 2 Urinary Incontinence (UI cohorts) - Stress Urinary Incontinence (SUI) (N=50) and Urgency Urinary Incontinence (UUI) (N=109). Symptom severity was assessed with the Urinary Distress Inventory (UDI) subscale score of the PFDI. Microbes were assessed by the Expanded Quantitative Urine Culture protocol, which detects the most common bladder microbes (bacteria and yeast). Microbes were identified to the species level by MALDI-TOF mass spectrometry. Alpha diversity indices were calculated for culture-positive samples and compared across the three groups. The association between UDI scores versus alpha diversity indices and species abundance were estimated.
RESULTS:
Participants had a mean age of 53 years (range 22-90); most were Caucasian (65%). Women with UI were slightly older (Control=47, SUI=54, UUI=61). By design, UDI symptom scores differed (Control = 8.43 (10.1), SUI = 97.95 (55.36), UUI = 93.71 (49.12), p<0.001). While most participants (216, 70%) had Expanded Quantitative Urine Culture-detected bacteria, the UI cohorts had a higher detection frequency than did the Control cohort (Control=57%, SUI 86%, UUI 81%, p<0.001). The most frequently detected species were as follows: Controls, Lactobacillus iners (12.7%), Streptococcus anginosus (12.7%), L. crispatus (10.7%), and L. gasseri (10%); SUI, S. anginosus (26%), L. iners (18%), Staphylococcus epidermidis (18%), and L. jensenii (16%); UUI, S. anginosus (30.3%), L. gasseri (22%), Aerococcus urinae (18.3%), and Gardnerella vaginalis (17.4%). However, only Actinotignum (formerly Actinobaculum) schaalii, A. urinae, A. sanguinicola, and Corynebacterium lipophile group were found at significantly higher mean abundances in at least one of the UI cohorts when compared to the Control cohort (Wilcoxon p<0.02), and no individual genus differed significantly between the two UI cohorts. Both UI cohorts had increased alpha diversity relative to continent controls with indices of species richness, but not evenness, strongly associated with UI.
CONCLUSIONS:
In adult women, the composition of the culturable bladder urobiome is associated with UI, regardless of common incontinence subtype. Detection of more unique living microbes was associated with worse incontinence severity. Culturable species richness is significantly greater in the UI cohorts than continent controls.
Keywords: Urinary Incontinence, Stress Urinary Incontinence, Urgency Urinary Incontinence, Continence, Urobiome, Human Microbiome, Biodiversity, Enhanced Urine Culture
CONDENSATION:
The composition of the adult female bladder urobiome is associated with urinary incontinence and more unique microbes are associated with worse urinary incontinence symptoms.
INTRODUCTION
Symptoms, urodynamic findings and presumed etiologic mechanisms are used to subgroup women with urinary incontinence (UI) into categories, including the two most common forms of UI, stress urinary incontinence (SUI) and urgency urinary incontinence (UUI). These subgroups are used to initiate clinical treatment. In an effort to further refine clinical algorithm and treatment efficacy, multiple experts have expressed the need for additional phenotyping of affected patients. Since the discovery and confirmation of the human bladder urobiome (an umbrella term used to describe the microbiota and/or their genomes) 1–4, interest has grown in learning in whether bladder urobiome characteristics have a role in clinical phenotyping and/or provide opportunities for novel therapeutic approaches 5–16 However, investigations of a single UI subgroup have focused predominantly on UUI and thus comparisons with SUI have been lacking. Two complementary techniques have been used to characterize the human urobiome, enhanced culture and DNA sequencing 7 In this study, we tested our hypothesis that the urobiome differs between continent adult women and adult women affected by UI. To assess associations between UI status and urobiome composition, we used an enhanced culture method called Expanded Quantitative Urine Culture (EQUC) coupled to MALDI-TOF mass spectrometry. The former quantification of the most common microbes (bacteria and yeast), while the latter identifies those microbes to the species level 3,17
MATERIALS AND METHODS
Study Design and Patient Population
Following Institutional Review Board (IRB) approval, we enrolled 309 women seeking care at Loyola University Medical Center between December 2013 and December 2015 using identical study protocols, procedures and equipment. On the day of enrollment, each participant was without clinical evidence of urinary tract infection (UTI) (i.e., standard urine culture-negative and absence of clinical UTI diagnosis or treatment).
Using responses to the Pelvic Floor Disease Inventory (PFDI) questionnaire18, we grouped these patients into continent controls (N=150), SUI (N=50), and UUI (N=109). Participants were categorized as continent controls when their PFDI responses were negative on all UI questions and they subjectively reported no UI. Participants were categorized into the SUI cohort when they responded yes to questions 20 (Do you usually experience urine leakage related to coughing, sneezing or laughing?), 21 (Do you usually experience urine leakage related to physical exercise such as walking, running, aerobics or tennis?) or 22 (Do you usually experience urine leakage related to lifting or bending over?) and reported a bother score of ≥2. Participants were categorized into the UUI cohort when they responded yes to questions 18 (Do you usually experience a strong feeling of urgency to empty your bladder?) and 19 (Do you usually experience urine leakage associated with a feeling of urgency, that is, a strong sensation of needing to go to the bathroom?) and reported a bother score. Patients that answered yes to one or both questions from each set were considered to have mixed symptoms and were excluded. We also excluded females <18 years old, those with systemic antibiotic use in prior 4 weeks, those with current pregnancy, current therapeutic catheterization (indwelling or intermittent) or insufficient English skills to complete study measures. One of the PFDI subscales, the Urogenital Distress Inventory (UDI), measures severity of urinary symptoms, with a range of 0-100, with higher scores indicating worsening symptom severity. Participants gave verbal and written research consent for use of left-over urine (after clinically-indicated testing); they also provided permission for abstraction of their demographic and clinical information from the electronic medical record. Characteristics of all women in the control cohort have been previously reported 3,5–7,19; 59 women in the UUI cohort have also been previously reported 3,5–7.
Sample Collection
In participants who were undergoing urogynecologic surgery, we collected the urine sample via a urinary catheter placed consistent with surgical protocols after induction of surgical anesthesia and prior to surgical antibiotic administration. For ambulatory participants, we collected the sample via urethral catheter consistent with clinical protocols during their clinical care visit. A portion of each urine sample was placed in a BD Vacutainer® Plus C&S Preservative Tube for culturing.
Urine Culture Protocols
The standard urine culture protocol, as performed by the Loyola University Medical Center Clinical Microbiology Laboratory, used 1 μL of urine, spread quantitatively onto 5% sheep blood (BAP) and MacConkey agars (BD BBL™ Prepared Plated Media, Cockeysville, MD) and incubated aerobically at 35°C for 24 hours. The detection limit was 1000 colony forming units per milliliter (CFU/ml). Each sample also was tested with the EQUC protocol 3, in which 100 μL of urine sample was spread quantitatively onto BAP, Chocolate, and Colistin Naladixic Acid (CNA) agars (BD BBL™ Prepared Plated Media) and incubated in 5% CO2 at 35°C for 48 hours, onto BAP incubated aerobically at 35°C for 48 hours, and onto CDC Anaerobic 5% sheep blood (Anaerobic BAP) agar (BD BBL™ Prepared Plated Media) incubated anaerobically at 35°C for 48 hours. The detection limit was 10 CFU/ml; when EQUC was unable to detect bacteria, the culture-negative sample was interpreted as below the detection threshold. Each distinct colony morphology was sub-cultured at 48 hours to obtain pure culture for microbial identification. Microbial identification was determined using a Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometer (MALDI-TOF MS, Bruker Daltonics, Billerica, MA).
Alpha Diversity Measures
The bacterial compositions of urine samples were analyzed using multiple measures of alpha diversity, including measures of richness, evenness, abundance and combinations thereof. Richness was calculated using the Chao1 index and the Abundance-Based Coverage Estimator (ACE). Both estimate the number of unique microbial species present; samples with larger values are richer. Evenness, the distribution of microbial species within the sample, was calculated with Pielou’s Index, which ranks samples from 0 to 1, with 1 being completely even. A sample with a Pielou index score close to 1 contains all species present in nearly equal abundance, while a smaller index score indicates that certain species are more abundant than others. The Shannon and Simpson (1-D) indices describe the diversity of a community, taking into account evenness, richness and/or abundance. The Shannon index combines interactions between richness and evenness, while the Simpson (1-D) index combines interactions between richness and abundance. Thus, larger Shannon diversity values indicate more diverse communities with greater richness and/or evenness. Larger Simpson (1-D) diversity values indicate more diverse communities with greater richness and/or abundance.
Statistical Analyses
The alpha diversity indices described above were calculated for all EQUC-positive samples using the vegan package in R 20. The pair-wise Wilcoxon Rank Sum test then was used to compare alpha diversity indices between the three groups (non-UI controls, SUI, UUI). Three methods (Pearson, Kendall and Spearman) were used to estimate correlations between UDI scores and alpha diversity indices, as well as UDI scores and the abundance of each bacterial species (log10 transformed). The average log10 transformed species abundance, Pearson’s product moment correlation coefficient, Kendall’s tau and Spearman’s rho were reported along with their p-values. The Wilcoxon Rank Sum test was applied to the comparisons of log10 transformed species abundance between the three groups (controls, SUI, and UUI). The Benjamini-Hochberg procedure was used for multiple testing correction for all analyses.
RESULTS
Demographics
Table 1 displays the demographic characteristics of the three cohorts (Continent Controls, SUI, and UUI). The mean ages (yrs) differed (Control=47, SUI=54, UUI=61, p<0.001) and a larger proportion of the Control cohort reported being sexually active (p<0.001). Minor demographic differences were also noted in race/ethnicity, UUI medication, hormonal treatment, and some comorbidities. Other than age (p=0.001), the UUI and SUI cohorts were similar (Supplemental Table 1). By design, the Control group had a lower UDI score (mean [SD]) than the UI participants (Control = 8.43 (10.1), SUI = 97.95 (55.36) and UUI = 93.71 (49.12), p<0.001) (Table 1).
Table 1.
Comparison between Controls, SUI, and UUI cohorts for various demographic and clinical variables
| Patient and Clinical Variables | Total Cohort (N = 309) | Continent Controls (N = 150) | SUI (N = 150) | UUI (N=109) | p-value |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 53 (15) | 47 (14) | 54 (14) | 61 (13) | <0.001a |
|
Race/Ethnicity -White -Hispanic -Black -Asian -Other |
200 (65%) 34 (11%) 61 (20%) 6 (2%) 8 (3%) |
88 (59%) 14 (9%) 41 (27%) 5 (3%) 2 (2%) |
33 (66%) 8 (16%) 3 (6%) 1 (2%) 5 (10%) |
79 (72%) 12 (11%) 17 (16%) 0 (0%) 1 (1%) |
0.04b |
| BMI (kg/m2), mean (SD) | 30.2 (7.8) | 29.2 (7.1) | 30.2 (6.9) | 31.7 (8.8) | 0.42a |
| Sexually Active (N=199) | 123 (62%) | 70 (71%) (N=99) | 27 (54%) (N=50) | 26 (52%) (N=50) | <0.001 |
| Current Anticholinergic Treatment | 10 (3%) | 0 (0%) | 2 (4%) | 8 (7%) | 0.02 |
| Use of Hormone Replacement Therapy | 27 (9%) | 8 (5%) | 4 (8%) | 15 (14%) | 0.06 |
| Diabetes | 28 (9%) | 6 (4%) | 9 (18%) | 13 (12%) | 0.003 |
| Heart Disease | 18 (6%) | 1 (1%) | 3 (6%) | 14 (13%) | <0.001 |
| Hypertension | 96 (31%) | 30 (20%) | 18 (36%) | 48 (44%) | <0.001 |
|
Urine Dipstick: (N=308) White Blood Cells (WBC) Nitrites Red Blood Cells (RBC) |
2 (1%) 0 (0%) 25 (8%) |
(N=149) 0 (0%) 0 (0%) 2 (1%) |
0 (0%) 0 (0%) 6 (12%) |
2 (2%) 0 (0%) 17 (16%) |
0.64 1 <0.001 |
| Urinary Distress Inventory (UDI) Score, mean (SD) | 39.99 (50.86) | 8.43 (10.1) | 97.95 (55.36) | 93.71 (49.12) | <0.001a |
| EQUC-Positive Specimen | 216 (70%) | 85 (57%) | 43 (86%) | 88 (81%) | <0.001 |
Chi-square test used unless otherwise indicated.
SD = standard deviation.
– Analysis of Variance test
– Fisher’s exact test
Bold – significant p values
Cohorts Differ by Microbial Abundance and Diversity
Bacteria were cultured in 216 (70%) urine samples; they were detected more frequently in the UI cohorts than in the Control cohort (Control=57%, SUI 86%, UUI 81%, p<0.001) (Table 1). Although mean age differed between the Control and UI cohorts, there was no correlation between culture results (in terms of CFU/mL) and age for any of the cohorts (Control, p=0.39; SUI, p=019; UUI, p=0.97).
The most frequently detected species were as follows: Controls, Lactobacillus iners (12.7%), Streptococcus anginosus (12.7%), L. crispatus (10.7%), and L. gasseri (10%); SUI, S. anginosus (26%), L. iners (18%), Staphylococcus epidermidis (18%), and L. jensenii (16%); UUI, S. anginosus (30.3%), L. gasseri (22%), Aerococcus urinae (18.3%), and Gardnerella vaginalis (17.4%). However, as shown in Figure 1, only Actinotignum (formerly Actinobaculum) schaalii, A. urinae, A. sanguinicola, and Corynebacterium lipophile group were found at significantly higher mean abundances in at least one of the UI cohorts when compared to the Control cohort (Wilcoxon p<0.02), and no individual genus differed significantly between the two UI cohorts. Figure 2 shows that the bacterial communities in the Control cohort were significantly less rich than those in each of the two UI cohorts; in contrast, evenness was similar. The Chao1 and ACE indices (which calculate alpha diversity measures of richness) were significantly lower in the Control cohort compared to either UI cohort (Figure 2A & B). The Shannon index (which assesses richness and evenness) and the Simpson index (which assesses richness and abundance) were lower in the Control cohort (Figure 2C & D); however, the Pielou index of evenness was not statistically different (Figure 2E).
Figure 1. Bacteria with different abundances in the three cohorts.
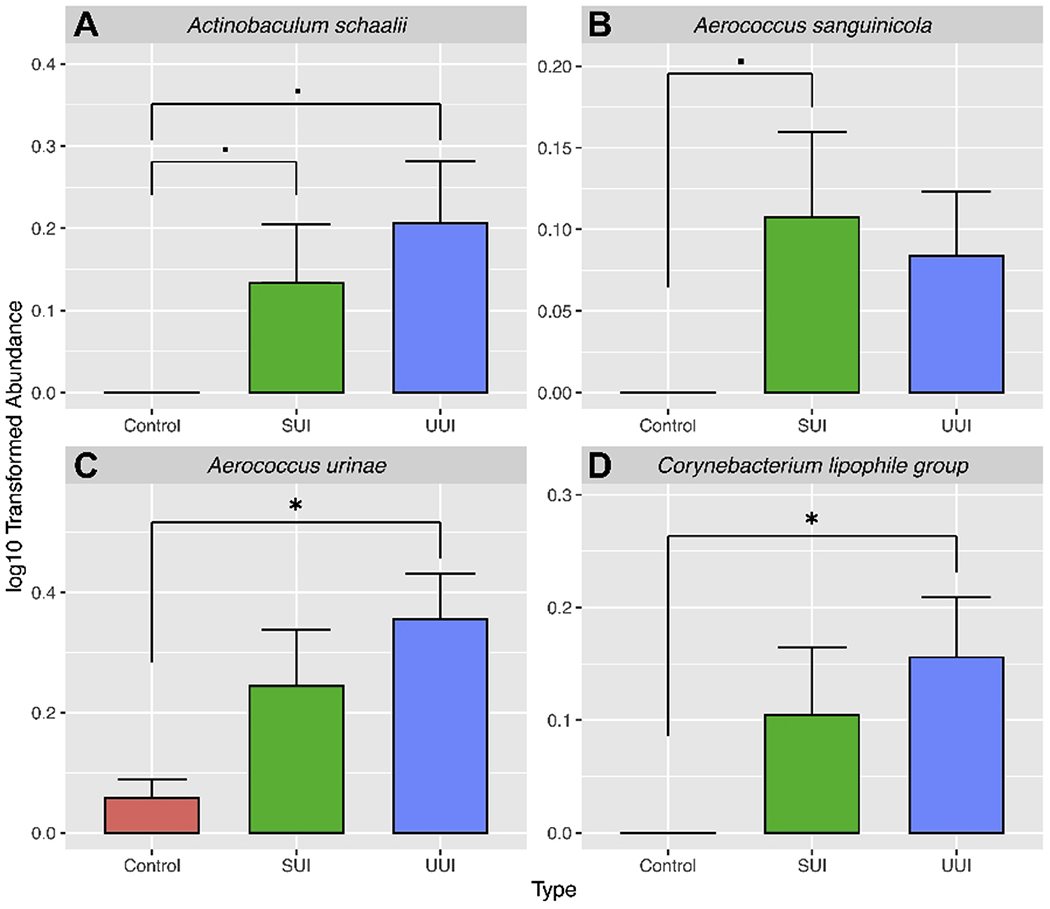
*, p <0.05; ·,p <0.1.
Figure 2. Alpha diversity values.
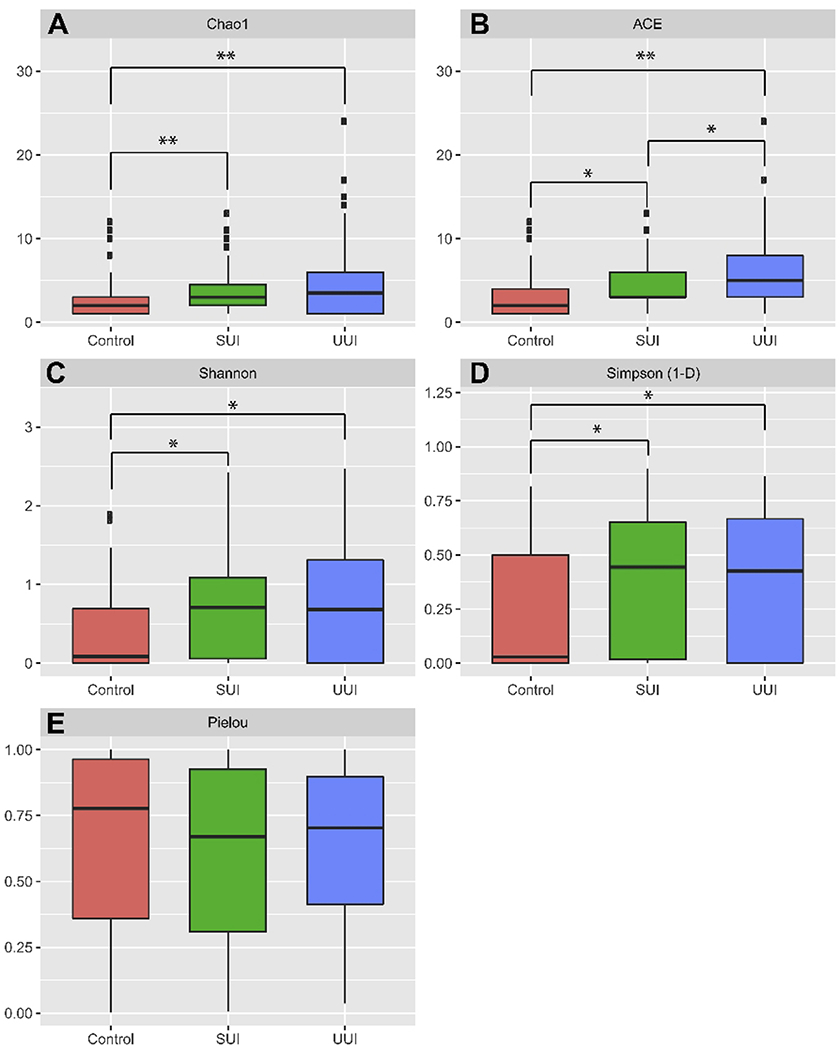
Boxplots depict the distribution of values for Control (red), SUI (green), and UUI (blue) cohorts calculated from EQUC data. A, Chao1; B, ACE; C, Shannon; D, Simpson (1-D); E, Pielou. Statistical significant comparisons after multiple test correction between group are denoted as *, < 0.05; **,< 0.001.
UDI Score Relates to Microbial Abundance and Diversity
UDI scores were significantly lower in the Control cohort, regardless of EQUC findings (Figure 3A & B). There was no strong correlation between UDI scores and the abundance of specific taxa. However, we detected a weak negative correlation between Lactobacillus iners and UDI score for all the EQUC-positive samples (Pearson, r = − 0.293, P = 0.056; Kendall, tau = −0.189, P = 0.087; Spearman, rho = −0.266, P = 0.085).
Figure 3. UDI score distribution among cohorts.
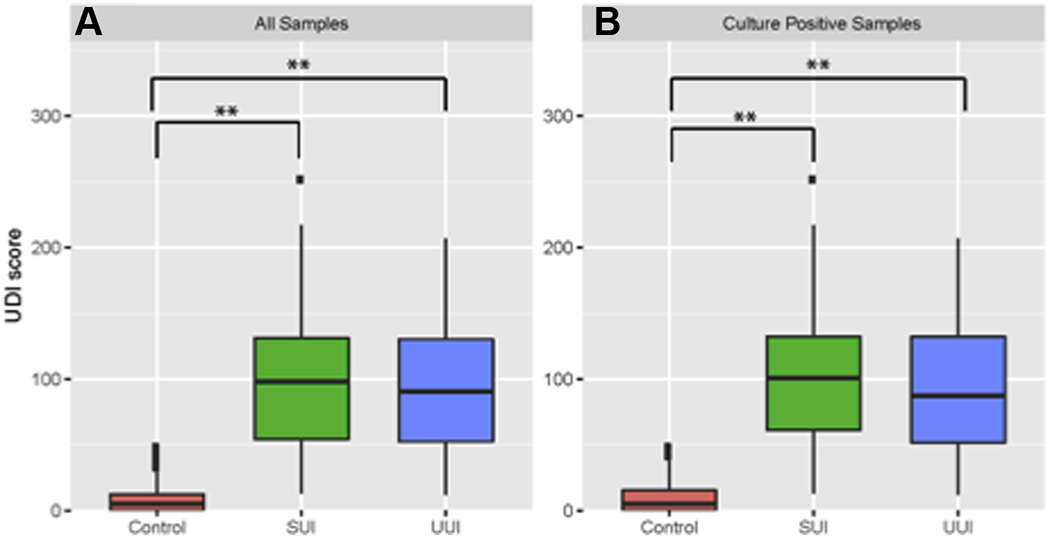
Boxplots depict the distribution of Urinary Distress Inventory (UDI) scores for Control (red), SUI (green), and UUI (blue) cohorts calculated from all patient samples. A, All Samples; B, Culture-positive only. Statistical significant comparisons after multiple test correction between group are denoted as *,< 0.05; **, < 0.001.
In contrast, the Chao1, ACE, and Shannon’s Indices were significantly correlated to UDI scores of the overall participant population, and Simpson’s Index approached significance for this trend; however, there was no correlation with Pielou values (Figure 4, Table 2). Thus, UDI scores correlated with richness, but not evenness.
Figure 4. Correlation between alpha diversity and UDI Scores.
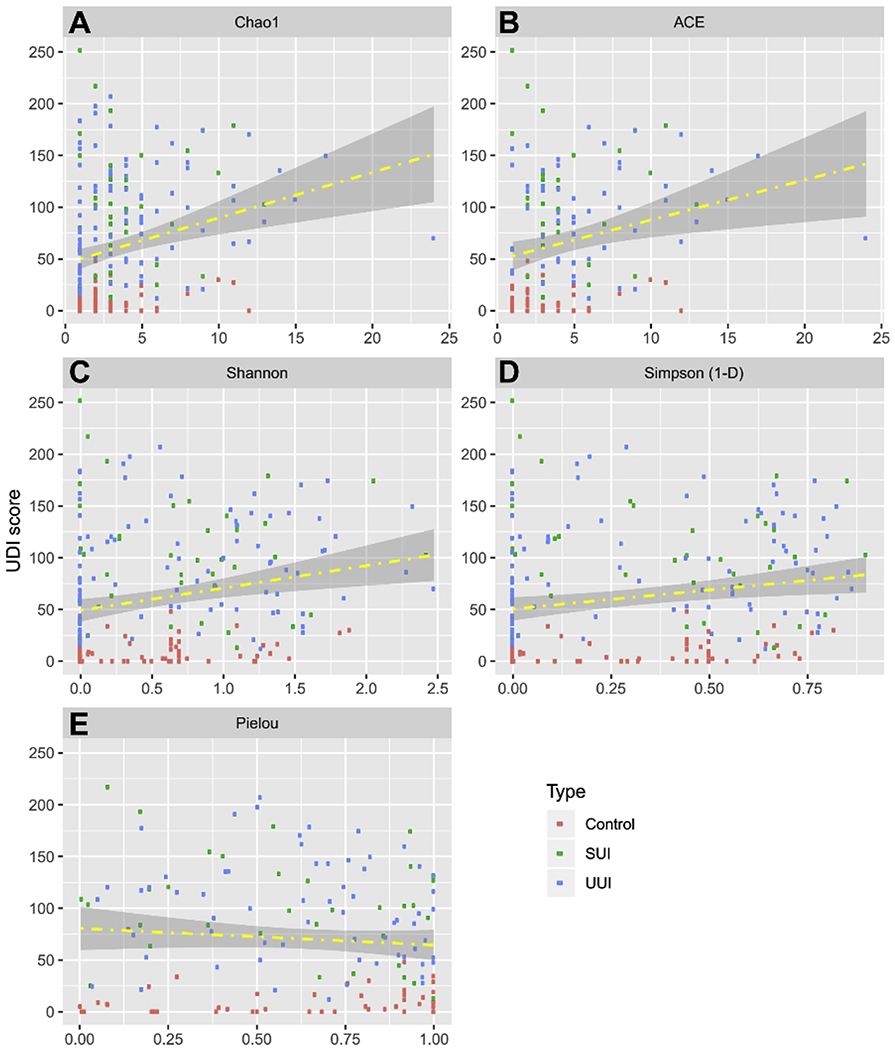
Scatter plots depict the distribution of alpha diversity scores calculated from EQUC data on the x-axis. The distribution of UDI Scores is on the y-axis. Fitted regression lines are in yellow. The area between the confidence bands is shaded in grey. Each data point is colored according to cohort, where red is Control, green is SUI, and blue is UUI. A, Chao1; B, ACE; C, Shannon; D, Simpson; E, Pielou. The regression line in the figure serve as a visual aid to indicate the direction of correlation between UDI score and the alpha diversity as quantitatively measured by Pearson, Kandell and Spearman correlation in Table 2.
Table 2.
Association estimates between alpha diversity indices and UDI score.
| Indices | Average | Pearson | Kendall | Spearman | |||
|---|---|---|---|---|---|---|---|
| cor | P-value | tau | P-value | rho | P-value | ||
| Chao1 | 3.671 | 0.255 | 0.000 | 0.217 | 0.000 | 0.303 | 0.000 |
| ACE | 4.724 | 0.249 | 0.004 | 0.201 | 0.001 | 0.283 | 0.001 |
| Shannon | 0.866 | 0.139 | 0.093 | 0.125 | 0.026 | 0.173 | 0.036 |
| Simpson | 0.454 | 0.064 | 0.442 | 0.098 | 0.082 | 0.140 | 0.089 |
| Pielou | 0.628 | −0.088 | 0.289 | −0.071 | 0.205 | −0.093 | 0.261 |
COMMENT
Principal Findings
There are differences in the cultured bladder urobiome of adult women with the major subtypes of UI compared to continent controls. These differences, which relate to detection and richness, may be useful for UI phenotyping. Women with UI were more likely to have detectable microbes than controls and detection of more unique living microbes was associated with worse incontinence symptom severity (i.e., richness indices were positively correlated with UDI scores). Furthermore, the two cohorts of incontinent women were more similar to each other than to continent controls and each UI cohort differed significantly from the control cohort. Specifically, we found statistically significant trends in terms of species richness by UI subgroup and by UDI score. These findings raise the possibility that dysbiotic microbial populations may play a role in UI etiologies. Indeed, two of the species found at significantly elevated levels (A. urinae and A. schaalii) were previously shown to be significantly elevated in women with UI 13 These findings also raise the possibility that the two most common subtypes of UI are associated with bladder urobiome richness, and these changes are based on the presence of UI itself; however, the differences in urobiome richness were not indicative of a particular UI subtype. Although our findings cannot provide evidence of causation, this association suggests that the urobiome changes with UI, either through some etiologic pathway or as a consequence of the incontinent condition.
Clinical Implications
While the cultured bladder urobiome characteristics in this study do not appear to be useful for distinguishing current clinical subtypes of UI, these findings may provide other phenotyping opportunities that would allow for a more nuanced categorization of women within UI overall (e.g.,within current subtypes or in newly identified subtypes) that relate to clinical outcomes of interest. For example, although the optimal characteristics of the urobiome are poorly understood, it is unlikely that extremes of diversity are a preferred or advantageous biological state. Minimal diversity may be related to the absence of beneficial microbes. Additionally, the clinical significance of a EQUC-negative status is unclear. A higher proportion of control women were EQUC-negative, suggesting that this status is not associated with symptoms. However, the lack of detectable microbes has been associated with elevated risk of post-operative UTI 6,16 In the other extreme, increased diversity may reflect a loss of urobiome regulatory mechanisms that do not allow for a healthy microbial community. As urobiome research progresses, urobiome characteristics may be a therapeutic target for UI treatment as a primary or adjunctive UI therapy.
The conventional UI subtypes of UUI and SUI have been propelled by clinical algorithms of care. While it is well recognized that many adult women with UI have elements of both SUI and UUI, these are generally considered to be two separate conditions, with different etiologies and treatment approaches. Typically, SUI responds well to surgical intervention. Recently, Sung and co-workers reported improvements in UUI symptoms in women with both UUI and SUI who underwent mid-urethral sling surgery 21. Although current clinical evidence does not support first-line surgical therapy for UUI, these findings may provide some insight into the mechanisms associated with UUI symptom resolution in that subset of women.13 Future research to assess longitudinal peri-operative urobiome characteristics may advance our understanding of this observation.
The conventional first-line therapies for UUI reflect the evidence for heterogeneous etiologies; treatments include behavioral/lifestyle approaches, pelvic floor muscle education and oral medications (in one of two drug classes). The clinical efficacy of these interventions is more modest than patients or their providers would like 22 Previous studies suggest that the etiologic heterogeneity of UI may be associated with the variability in clinical response. For example, in a prior study of women using solifenacin for UUI, bladder urobiome characteristics were associated with clinical treatment response; participants with elevated microbial richness did not respond well or at all to the pharmacologic intervention, whereas patients whose urobiome was more similar to unaffected women had good clinical responses 5
Research Implications
This study complements prior work by Thomas-White et al in which they evaluated 197 urine samples from women planning surgery for uncomplicated stress urinary incontinence. Many of those women, who were evaluated by a different validated instrument (MESA – the Medical, Epidemiological and Social Aspects of Aging) also had UUI symptoms; women with mixed symptoms were not excluded in that analysis. Comparisons of these two populations may stimulate future hypothesis-drives work to clarify relevant relationships between the urobiome and common forms of urinary incontinence in adult women.
It is possible, and biologically plausible, that the reported urobiome changes are a consequence of UI; further research is warranted into the microbial effects of the “wet environment” (i.e., wet pads), as the growth conditions for microbes in this environment may vary significantly. Every clinician has been asked questions along the lines of “are these wet pads making things worse?” It is quite plausible that the wet pads alter the perineal microbial environment, which may in turn alter the nearby niches, including the lower urinary tract.
Strengths and Limitations
This study has several major strengths. First, we used validated measures to prospectively characterize three cohorts of clinically relevant women. Second, we used catheterized urine specimens, which most closely reflect the bladder urobiome itself, without contribution of microbial flora from the genital tract. Third, we used EQUC 3,17, which (1) provides robust evidence that the microbes of interest are alive; (2) permits quantification of those microbes (in contrast to 16S rRNA gene sequencing, which provides relative abundance at most, due to primer bias and variable numbers of 16S rRNA genes per species); (3) when coupled to MALDI-TOF mass spectrometry, allows us to identify microbes to the species level while 16S rRNA gene sequences are typically too short for reliable species-level classification; and (4) represents a relatively inexpensive opportunity for pre-treatment phenotyping, as opposed to more expensive sequence-based approaches.
There are also limitations to these findings. Most importantly, this is a convenience sample and the control cohort in this study was younger and more sexually active, two factors that plausibly relate to urobiome status. Whereas age was not correlated to culture result in terms of CFU/mL, it could be possible that the species differences we observed are attributable to age alone; thus, future studies that include larger, population-based cohorts should match more closely for age. In addition, several medical conditions were more common in the UI cohorts. It is also plausible that the urobiome of the UI cohorts may have been altered during UI evaluations, such as cystoscopy or urodynamic testing. As human microbiome knowledge expands, the need to adequately address these factors (e.g., antibiotic use, urine infection history, and vaginal estrogen status) is becoming clear. Although there is no evidence that the site of sample collection differs between operating room and ambulatory clinic settings, this has not been investigated. In this study, we used standardized protocols to characterize participants with regard to continence status and followed a standard protocol for specimen collection regardless of collection site. Given the wide scope of known and unknown factors that can potentially modulate the urobiome, future studies will need to balance the number of clinical variables that are controlled with the feasibility and practical aspects of participant recruitment. Clinical researchers may benefit from alternative recruitment strategies that take advantage of advancing analytic approaches for large bioinformatic datasets. Finally, non-bacterial microbes, such as viruses, may be of interest for future studies. Since EQUC preferentially grows bacteria and only a small subset of fungal species, further studies may be needed to test hypothesis regarding non-bacterial microbes.
Conclusion
In conclusion, these findings offer strong evidence that adult women with UI have bladder urobiomes that differ from unaffected continent control women. Once replicated, these findings should prompt investigators to address whether the detected changes are an etiologic factor for UI or a consequence of the condition itself.
Supplementary Material
AJOG at a Glance:
-
Why was the study conducted?
To determine whether bladder urobiome characteristics have a role in clinical phenotyping common forms of urinary incontinence.
-
What are the key findings?
Bladder urobiome composition is associated with urinary incontinence regardless of urinary incontinence subtype.
Women with urinary incontinence were more likely to have detectable microbes than continent controls.
Detection of more unique living microbes was associated with worse incontinence severity.
-
What does this study add to what is already known?
Women with urinary incontinence have urobiomes that differ from unaffected continent control women. Once replicated with appropriate controls for clinical variables of interest, these findings should prompt investigators to address whether these detected changes are etiologic factors for or consequences of urinary incontinence.
ACKNOWLEDGMENTS:
We thank Mary Tulke RN for her assistance with participant recruitment and sample collection, and Cynthia Brincat MD who greatly assisted with recruitment efforts of the study population.
FUNDING: This work was supported by R01 DK104718, R56 DK104718, R21 DK097435 and P20 DK108268 and a grant from the Falk Foundation (LU#202567). The funders did not play a part in the design or conduct of the study.
DISCLOSURE STATEMENT: Dr. Thomas-White discloses membership on the advisory board of LiveUTIFree. Dr. Mueller discloses research support from NIH, Astellas and Boston Scientific, Dr. Wolfe discloses research support from NIH, Astellas and Kimberly Clark, Dr. Brubaker discloses research funding from NIH and editorial stipends from Female Pelvic Medicine and Reconstructive Surgery, UpToDate and JAMA. The remaining authors (Price, Lin, Gao, Hilt, Dong) report no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PAPER PRESENTATIONS: Some of the data was previously presented:
Travis K. Price, Linda Brubaker, Elizabeth R. Mueller, Cynthia Brincat, Alan J. Wolfe. “The Female Urinary Microbiota Differ by Primary Lower Urinary Tract Disorder.” International Continence Society (ICS), Florence, Italy, September 12-15, 2017.
Travis K. Price, Linda Brubaker, Elizabeth R. Mueller, Cynthia Brincat, Alan J. Wolfe. “The Female Urinary Microbiota Differ by Primary Lower Urinary Tract Disorder.” 39th Annual American Urogynecology Society (AUGS) Meeting, Providence, RI, October 3-7, 2017
REFERENCES
- 1.Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol 2012;50:1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khasriya R, Sathiananthamoorthy S, Ismail S, et al. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol 2013;51:2054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014;52:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med 2012;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas-White K, Brady M, Wolfe AJ, Mueller ER. The bladder is not sterile: History and current discoveries on the urinary microbiome. Curr Bladder Dysfunct Rep 2016;11:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce MM, Zilliox MJ, Rosenfeld AB, et al. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol 2015;213:347 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 2014;5:e01283–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karstens L, Asquith M, Davin S, et al. Does the Urinary Microbiome Play a Role in Urgency Urinary Incontinence and Its Severity? Front Cell Infect Microbiol 2016;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komesu YM, Richter HE, Carper B, et al. The urinary microbiome in women with mixed urinary incontinence compared to similarly aged controls. Int Urogynecol J 2018;29:1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller ER, Wolfe AJ, Brubaker L. Female urinary microbiota. Curr Opin Urol 2017;27:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas-White KJ, Kliethermes S, Rickey L, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol 2016;216:55, e1–.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas-White KJ, Gao X, Lin H, et al. Urinary microbes and postoperative urinary tract infection risk in urogynecologic surgical patients. Int Urogynecol J 2018;29:1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fok CS, Gao X, Lin H, et al. Urinary symptoms are associated with certain urinary microbes in urogynecologic surgical patients. Int Urogynecol J 2018;29:1765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Phan MD, Bates LJ, et al. The urinary microbiome in patients with refractory urge incontinence and recurrent urinary tract infection. Int Urogynecol J 2018;29:1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe AJ, Brubaker L. Urobiome updates: advances in urinary microbiome research. Nat Rev Urol 2018;16: 73–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brubaker L, Nager CW, Richter HE, et al. Urinary bacteria in adult women with urgency urinary incontinence. Int Urogynecol J 2014;25:1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price TK, Dune T, Hilt EE, et al. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J Clin Microbiol 2016;54:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol 2001;185:1388–95. [DOI] [PubMed] [Google Scholar]
- 19.Price TK, Hilt EE, Thomas-White K, Mueller ER, Wolfe AJ, Brubaker L. The urobiome of continent adult women: a cross-sectional study. BJOG 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oksanen J, Kindt R, Legendre P, et al. “Community Ecology Package.” R package version 22–1 2010; http://CRAN.R-project.org/package=vega. [Google Scholar]
- 21.Sung VW, Borello-France D, Newman DK, et al. Effect of Behavioral and Pelvic Floor Muscle Therapy Combined With Surgery vs Surgery Alone on Incontinence Symptoms Among Women With Mixed Urinary Incontinence: The ESTEEM Randomized Clinical Trial. JAMA 2019;322:1066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds WS, McPheeters M, Blume J, et al. Comparative Effectiveness of Anticholinergic Therapy for Overactive Bladder in Women: A Systematic Review and Meta-analysis. Obstet Gynecol 2015;125:1423–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


