Abstract
Previous studies have reported a beneficial effect from CMV (cytomegalovirus) reactivation after alloHCT on immune reconstitution. We determined the CMV antigenemia level associated with increased CMV Antigen Specific T-cells (CASTs) at day+100 and decreased CMV reactivation after day+100. CMV reactivation and CASTs were measured with CMV antigenemia and CMV-specific major histocompatibility complex multimers. The analysis consisted of 775 CAST measurements obtained before and 30, 100, and 365 days post-alloHCT from 327 consecutive patients treated between 2008–2016. Detectable CASTs correlated with recipient (p<0.0001) and donor (p<0.0001) CMV seropositivity pre-alloHCT. CMV reactivation before day+100 was associated with a higher proportion of patients who achieved ≥3 CASTs/μL by day+100 (61% with versus 39% without reactivation, p<0.001). In alloHCT recipients at high risk for CMV reactivation (R+D±) with a maximum of grade II acute graft-versus-hostdisease (aGvHD), reactivating CMV before day+100 and achieving ≥3 versus <3 CASTs/μL at day+100 was associated with reduced CMV reactivation from day+100 to +365 (27% vs. 62%, p=0.04). This protective effect was observed with low-level but not high-level CMV reactivation (<5 vs. ≥5/50,000 PMNs+pp65, respectively). These findings suggest low-level CMV reactivation may be beneficial and that treatment may be delayed until progression. These findings will need validation in prospective clinical trials using CMV PCR and antigenemia assays.
Introduction
Cytomegalovirus (CMV) reactivation and disease, defined respectively as viral replication in the blood and end organ damage attributed to CMV, are important causes of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (alloHCT). [1] The primary risk factor for CMV reactivation is recipient / donor (R/D) CMV serology. [2, 3] CMV reactivation occurs in approximately 11-32% of CMV seropositive recipient / donor pairs after alloHCT. [3] Primary CMV infection occurs in approximately 4% of CMV seronegative recipient / donor pairs. [3] Other risk factors for CMV reactivation and disease include the use of high dose corticosteroids, T cell depletion, acute and chronic graft-versus-host disease (GvHD), and the use of human leukocyte antigen (HLA) mismatched or unrelated donors. [4]
Long term control of CMV infection is dependent on CMV specific cytotoxic CD8+ T-cells. [5, 6] CMV specific CD4+ T-cells, natural killer cells, and γδ T-cells also contribute to the control of CMV infection. [7, 8] Optimizing the reconstitution of anti-CMV immunity after alloHCT to prevent CMV reactivation and progression to disease is of vital importance.
Patients with CMV reactivation are typically treated early with valganciclovir, ganciclovir, or foscarnet at the time of CMV detection. These patients can experience considerable morbidity due to neutropenia (from valganciclovir and ganciclovir), renal dysfunction (from foscarnet), and the time and financial costs of receiving intravenous therapy and serial monitoring for CMV reactivation (from ganciclovir and foscarnet). [9, 10] Despite early therapy, the cumulative incidence of CMV disease by 52 weeks was 10.5% in a recent cohort study of allogeneic transplant patients who were CMV seropositive or had a seropositive donor. [11] Of the patients with CMV disease, 37% died within the first year after transplant. The introduction of letermovir in 2014 changed the epidemiology of CMV reactivation and disease. In a phase III randomized trial of letermovir prophylaxis versus no prophylaxis, 17.5% versus 41.8% of patients reactivated CMV, and 5% versus 3% developed CMV disease by week 24. [12] Long term clinical outcomes of letermovir prophylaxis with regard to CMV reactivation and disease have yet to be reported. It is not known if letermovir has delayed the onset of CMV reactivation to a later period after transplant.
An early randomized controlled trial comparing prophylaxis versus early treatment of CMV with ganciclovir demonstrated improved immune reconstitution by ex vivo testing by day+90 in the early treatment arm. [13] Other studies have observed decreased AML relapse in patients with early CMV reactivation. [14, 15] A wide range of CMV reactivation levels were used in these studies to initiate early CMV treatment, leading to the question of which threshold is the optimum for initiating early treatment of CMV reactivation. In this study, we measured CMV reactivation with CMV antigenemia and CASTs with a CMV derived peptide associated major histocompatibility complex (MHC) multimer based flow cytometry assay [16] to determine a CMV antigenemia threshold associated with improved CAST reconstitution and decreased incidence of CMV infection from day+100 to day+365.
Methods
This is a retrospective analysis of prospectively gathered clinical data with laboratory correlates. The Roswell Park Institutional Review Board approved this study. Patients (N=315) undergoing alloHCT from 2008-2016 were prospectively monitored for CMV reactivation / disease by CMV antigenemia testing at least once weekly starting on approximately day+21 after alloHCT and continuing until day+100 regardless of recipient / donor CMV serostatus (R±D±). After day+100, monitoring for CMV reactivation / disease occurred as part of routine follow up until 1 year after alloHCT or immunosuppression was completely tapered, whichever occurred later. Reconstitution of anti-CMV immunity was determined by MHC-multimer testing for CASTs before alloHCT and at days +30, +100, and +365. Seven hundred and seventy-five multimer measurements (62% of expected) were performed.
All patients received routine anti-viral prophylaxis with acyclovir 400 mg twice daily starting day+1 after cellular infusion. Acyclovir prophylaxis was continued until 6 months after all immunosuppression was tapered, the CD4 count was >200/μL, the patient was >2 years after alloHCT, and antibody titers indicated a humoral response to vaccination against varicella-zoster virus. Acyclovir was temporarily discontinued if the patient received anti-CMV therapy and restarted once CMV infection resolved.
Disease Definitions
CMV reactivation was determined by the level of CMV antigenemia (the number of pp65 positive polymorphonuclear leukocyte (PMNs) / counted PMNs, PMNs+pp65) as measured by indirect immunofluorescence microscopy (CMV Brite™ Turbo Kit, IQProducts, the Netherlands). CMV reactivation was defined as ≥2/50,000 PMNs+pp65 observed once, 1/50,000 PMNs+pp65 observed twice consecutively, or 1/50,000 PMNs+pp65 observed once followed by pre-emptive CMV therapy. Repeat CMV antigenemia testing occurred within 2-3 days according to the clinician’s discretion. Absence of CMV reactivation was defined as 0 or <1/100,000 PMNs+pp65 observed, or 1/50,000 PMNs+pp65 observed once without subsequent anti-CMV treatment. Unique episodes of CMV reactivation were defined as starting from the initial CMV antigenemia and ending after 2 weeks of negative CMV antigenemia testing. Detection of CMV by quantitative polymerase chain reaction (PCR) testing was not routinely performed at our institution during this study. CMV disease was defined as end organ dysfunction attributed to CMV infection with demonstration of CMV in tissue by histology or culture, or new or changing pulmonary infiltrates with the presence of CMV in bronchial alveolar lavage by culture, direct fluorescent antibody staining, or cytology.
Treatment Regimens
Prior to 2010, anti-CMV therapy was initiated upon detection of any CMV antigenemia. Beginning in 2010, the institutional practice for anti-CMV therapy changed such that lower levels of CMV antigenemia (<5/50,000 PMNs+pp65) could be observed until a repeat level within 2-3 days confirmed persistent or increasing CMV antigenemia; at that time, anti-CMV therapy would begin. The decision to initiate therapy was made by the attending physician based on his/her clinical judgement which considered the presence of GvHD and intensity of immunosuppression.
Induction therapy consisted of ganciclovir 5 mg/kg intravenously every 12 hours for at least 2 weeks. Maintenance therapy, consisting of ganciclovir 5 mg/kg IV daily, was started after 2 weeks of negative CMV antigenemia testing (0/100,000 PMNs+pp65). Maintenance therapy was discontinued after 2 additional weeks of negative CMV antigenemia testing. Intravenous foscarnet every 12 hours was used as second line therapy in case of CMV resistance to ganciclovir (defined by mutational genetic analysis, or a rising antigenemia after 1 week of therapy) or adverse side effects such as neutropenia.
MHC Multimer Testing for CASTs
Fresh blood samples were analyzed by flow cytometry for CASTs with CMV specific MHC multimer reagents (Dextramer, Immudex, Copenhagen, Denmark, listed in Supplemental Table 1). The Dextramer reagents were restricted to HLA-A01, -A02, - A03, -B07, -B08, and -B35. The CMV peptides displayed by each HLA molecule on the Dextramer were derived from cytomegalovirus pp50 (A*01), pp65 (A*02, B*07, B*35), and IE-1 (A*03, B*08) proteins. Of the predominantly European American patients seen at this transplant center, eighty-seven percent had an HLA-A or B allele that matched the available CMV Dextramers.
Methods for CAST detection and quantification were previously described. [16] Briefly, 200 pL of whole blood were incubated at room temperature for 10 minutes with 10 μL of HLA specific Dextramers followed by incubation at room temperature with the recommended amounts of anti-CD3 PerCP (Becton Dickinson; clone SK7) and CD8 FITC (Dako; clone DK25 or BD; clone SK1) antibodies and LIVE/DEAD™ Fixable Violet Reagent (LDV, Life Technologies). RBCs were lysed for 10 minutes followed by leukocyte pelleting, washing, and resuspension in 500 μL 2% formaldehyde in PBS.
Separately, 100 μL of whole blood were incubated at 4°C for 30 minutes with anti-CD3 PerCP, anti-CD8 FITC, and LDV (hereafter termed CD8 count tube). After labeling, RBCs were lysed and the sample processed as above without washing. 100 μL homogenized AccuCount fluorescent particles (Spherotech) were added to the CD8 count tube.
Fixed cell samples were acquired by flow cytometry within 1 h of labeling using either FACSCanto II (Becton Dickinson), FACSCalibur (Becton Dickinson), FC 500 (Beckman Coulter), or CyAn (Dako) flow cytometers. A forward scatter threshold was applied during the acquisition of samples to eliminate electronic noise and small particles from the flow cytometric data. An irregular region was placed on a bivariate histogram of PerCP CD3-A versus FITC CD8-A, and used to circumscribe the CD3+, CD8+ cell population; for which at least 20,000 region-inclusive events were collected at a medium flow rate (60 μl/minute for FACSCanto II;. 35 μl/minute for FACSCalibur;. 30 μl/minute for FC 500).
CD8 count tubes were acquired using a threshold set and applied based on PerCP CD3+ intensity, such that only CD3+ T cells and brightly-fluorescent enumeration beads were collected. In this manner, lysed cellular debris could be excluded without losing fluorescent enumeration beads. Thereafter, a rectangular region was used to capture the fluorescent enumeration bead population on a bivariate histogram of PerCP CD3-A versus SSC-A, for which at least 10,000 region-inclusive events were collected at a medium flow rate.
Data from the CD8 count tube was analyzed separately to quantify the absolute number of CD3+CD8+ T cells per μl of blood sample. The absolute number of CD3+CD8+cells/μL was determined by:
The absolute number of CASTs/μl of blood sample was determined by:
Determination of CASTs/μL Thresholds
CAST thresholds (CASTs/μL) for statistical analysis were initially determined based on operating parameters for the assay. Thus, a threshold of 1 CAST/μL was chosen as the minimum detectable level. Subsequently, 1, 2, 3, 5, 7, and 10 CASTs/μL were chosen as potential cutoffs to indicate functional immunity against CMV for further analysis. All cutoffs at 3 CASTs/μL and greater were associated with protection against later CMV reactivation, thus 3 CASTs/μL was chosen as the lowest number needed to provide functional immunity against CMV.
Statistical Analysis
The Pearson χ2 or Fisher’s exact tests were used for univariate comparisons of categorical variables. The Mann Whitney rank-sum test was used for univariate comparisons of nonparametric ordinal variables. Observation time for aGvHD was calculated from the date of alloHCT (day 0) until the date of aGvHD onset or day+100 post alloHCT and was analyzed as a competing risk with disease progression / relapse or death without GvHD. Similarly, the cumulative incidence of relapse was analyzed as a competing risk with GvHD and death. Overall survival (OS) time was calculated from day 0 to the date of death due to any cause; patients were censored at last follow-up. Progression free survival (PFS) time was calculated from day 0 to the first date of disease progression/relapse or death due to any cause; patients were censored at last follow-up in the absence of disease progression/relapse. Kaplan-Meier survival curves were constructed and tested using the log-rank statistic. The quantitative value of CMV antigen specific T-cells (CASTs) at each time point (pre-alloHCT, day+30, +100, and +365) was defined as the maximum value measured by all the HLA specific Dextramers informative for each patient. All statistical analyses were performed using SAS version 9.4 with a two-sided type I error rate of 0.05.
The demographic characteristics of the 315 alloHCT recipient/donor pairs studied are presented in Table 1. All pairs had at least one HLA allele for which there was a corresponding Dextramer. No patients received ATG or other in vivo or ex vivo T-cell depletion. The flow and disposition of recipient / donor pairs through the analysis is presented in Figure 1.
Table 1.
Patient characteristics (N=315)
| Median (range) | |
|---|---|
| Recipient age | 57 (19-74) |
| Number (%) | |
| Gender | |
| Male | 196 (62) |
| Female | 119 (38) |
| Race | |
| European American | 307 (98) |
| Other | 8 (2) |
| Diagnosis | |
| AML | 131 (42) |
| MDS/MPD | 71 (23) |
| ALL | 38 (12) |
| NHL/HL | 46 (15) |
| Other | 29 (9) |
| Recipient / donor CMV serostatus | |
| Negative / negative pairs | 121 (38) |
| Positive / negative pairs | 89 (28) |
| Positive / positive pairs | 57 (18) |
| Negative / positive pairs | 48 (15) |
| KPS at time of HCT | |
| ≤80 | 262 (83) |
| ≥90 | 53 (17) |
| Donor HLA matching | |
| MRD | 120 (38) |
| MUD | 187 (59) |
| MMUD | 8 (2) |
| Number of HLA alleles for which Dextramers were available | |
| 1 | 111 (35) |
| 2 | 90 (29) |
| 3 | 83 (26) |
| 4 | 22 (7) |
| 5 | 9 (3) |
| Status at HCT | |
| CR | 163 (52) |
| Not in CR | 152 (48) |
| HCT regimen | |
| Reduced intensity conditioning | 253 (80) |
| Myeloablative | 62 (20) |
| Acute GvHD prophylaxis regimen | |
| FK MMF CY | 2 (1) |
| FK MTX | 18 (6) |
| FK MTX MMF | 288 (91) |
| FK, FK MMF, FK SIR | 7 (2) |
Abbreviations: AML – acute myeloid leukemia, ALL – acute lymphoblastic leukemia, CMV – cytomegalovirus, CR – complete remission, FK – tacrolimus, HCT – hematopoietic cell transplantation, HL – Hodgkin lymphoma, HLA – human leukocyte antigen, KPS – Karnofsky performance status, MDS – myelodysplastic syndrome, MMF – mycophenolate mofetil, MMUD – HLA mismatched unrelated donor, MPD – myeloproliferative disease, MRD – HLA matched related donor, MTX – methotrexate, MUD – HLA matched unrelated donor, N – number, NHL – non-Hodgkin lymphoma, SIR - sirolimus
Figure 1.

Flow of recipient/donor pairs through the analysis.
Results
CMV reactivation and disease
Table 2 summarizes the incidence and timing of CMV reactivation for the population studied. The median time until CMV reactivation for these patients did not vary according to R/D CMV serotype. The incidence of CMV reactivation in R−D+ pairs was significantly lower than in R+D− or R+D+ pairs (p < 0.0001, Supplemental Figure 1). None of the R−D− pairs reactivated CMV. CMV disease occurred in 6/315 (2%) recipients. The organs affected by CMV disease were the intestine (N=3), lung (N=2), and bladder (N=1). Median (range) follow up of the alloHCT recipients was 54 (4-120) months.
Table 2.
Patient outcomes
| Incidence of CMV reactivation by recipient / donor CMV serostatus | N / total | % |
|---|---|---|
| All recipient / donor pairs | 74/315 | 23 |
| Negative / negative pairs | 0/121 | 0 |
| Positive / negative pairs | 47/89 | 53 |
| Positive / positive pairs | 24/57 | 42 |
| Negative / positive pairs | 3/48 | 6 |
| Median time to first CMV reactivation by recipient/donor CMV serostatus pairs | N | Day (range) |
| All recipient / donor pairs | 74 | 41 (24-363) |
| Negative / negative pairs | 0 | NA |
| Positive / negative pairs | 47 | 40 (24-363) |
| Positive / positive pairs | 24 | 42 (25-222) |
| Negative / positive pairs | 3 | 40 (33-56) |
| Graft-versus-host disease | N / total | |
| Number of patients with Grade II and above | 156/315 | 50 |
| Number of patients with Grade III and above | 57/315 | 18 |
| Abbreviations: CMV – cytomegalovirus, N - number | ||
Pre-alloHCT CAST measurements are specific for past immunity against CMV.
Pre-transplant CAST measurements (N=315) in alloHCT recipients were significantly correlated with past exposure to CMV, defined by positive anti-CMV IgG serology (Table 3A). The sensitivity and specificity of CAST detection for past anti-CMV immunity were 55% (95% confidence interval (CI) 46-63%, 80/146) and 99% (95%CI 96-100%, 167/169), respectively. Donor samples for CASTs were available in 51 HLA matched sibling donors and the correlation between CASTs and past anti-CMV immunity in healthy normal HLA matched sibling donors was evaluated (Table 3B) to control for the effects of prolonged lymphodepleting chemotherapy. The recipients and donors were relatively age-matched (median (range) recipients: 54 (21-72) vs. donors: 52 (18-69)). The sensitivity and specificity of CAST detection for past CMV immunity was higher in healthy sibling donors at 77% (95%CI 56-91%, 20/26) and 100% (95%CI 86-100%, 25/25).
Table 3.
Correlation between CAST detection and past anti-CMV immunity in recipients before alloHCT and donors
| A. All recipients | |||
|---|---|---|---|
| Pre-alloHCT CASTs |
Anti-CMV IgG serology before alloHCT | p-value | |
| Negative (N=169) N (%) |
Positive (N=146) N (%) |
||
| < 1 cell / μL | 167 (99) | 66 (45) | <0.0001 |
| ≥ 1 cell / μL | 2 (1) | 80 (55) | |
| B. HLA matched sibling donors | |||
| Pre-alloHCT CASTs |
Anti-CMV IgG serology | p-value | |
| Negative (N=25) N (%) |
Positive (N=26) N (%) |
||
| < 1 cell / μL | 25 (100) | 6 (23) | <0.0001 |
| ≥ 1 cell / μL | 0 | 20 (77) | |
Abbreviations: alloHCT – allogeneic hematopoietic cell transplant, CAST – CMV antigen specific T cell, CMV – cytomegalovirus, HLA – human leukocyte antigen, IgG – immunoglobulin G, N – number
Factors affecting CAST reconstitution after alloHCT.
CAST reconstitution varied according to R/D CMV serostatus. CAST levels were higher for R+D+ and R+D− compared to R-D+ pairs at days +100 (P = 0.0002) and +365 (p = NS, Table 4, Supplemental Figure 2). CAST reconstitution varied according to the HLA antigen utilized in the Dextramer with A02N yielding the highest detectable CAST levels (Supplemental Table 1 and Supplemental Figure 3). The median level of CASTs decreased from baseline levels pre-alloHCT, nadired at day+30, increased to above baseline by day+100, and stabilized by day+365. CAST reconstitution patterns were similar between patients with only 1 versus ≥ 2 informative available HLA specific Dextramer reagents (Supplemental Figure 3).
Table 4.
CAST reconstitution varies according to R/D CMV serostatus
| R/D CMV serostatus | N | Pre-alloHCT Median (range) |
Day+30 Median (range) |
Day+100 Median (range) |
Day+365 Median (range) |
|---|---|---|---|---|---|
| R-D+ | 48 | 0 (0-53.24) | 0.52 (0-7.67) | 0.31 (0-103.76) | 1.36 (0-6.4) |
| R+D− | 89 | 1.08 (0-97.11) | 0.01 (0-20.89) | 2.78 (0-580.47) | 2.03 (0-317.72) |
| R+D+ | 57 | 2.05 (0-235.47) | 1.44 (0-196.21) | 22.27 (0-279.7) | 9.6 (0-51.65) |
Abbreviations: alloHCT – allogeneic hematopoietic cell transplant, CAST – CMV antigen specific T cell, CMV – cytomegalovirus, D – donor, HLA – human leukocyte antigen, IgG – immunoglobulin G, N – number, R - recipient
CMV reactivation stimulates subsequent CAST reconstitution.
CMV reactivation before day+100 was significantly associated with a greater proportion of CMV seropositive pairs (R+D+, R+D-, R-D+) that achieved ≥3 CASTs/μL by day+100 (p<0.0001, Table 5A). This analysis included patients with grade III-IV acute GvHD receiving systemic corticosteroids. All levels of antigenemia before day+100 (1-2, 3-5, 6-9, 10-24, ≥25 PMNs+pp65) were associated with increased CASTs at day+100 (p=0.03, Table 5B). Finally, CMV reactivation between day+100 and day+365 was not associated with achieving ≥3 CASTs/μL by day+365. The majority of these patients (71/91 (81%)) developed chronic GvHD between day+100 and +365.
Table 5.
Effect of CMV reactivation on CASTs
| A. CMV reactivation before day+100 correlates with increased CASTs reconstitution at day+100 in R+D+, R+D-, and R-D+ pairs. Second parenthesis denote horizontal percentages. | |||||||
|---|---|---|---|---|---|---|---|
| Day+100 CAST level |
CMV reactivation before day+100 | p-value | |||||
| No (N=65) N (%) |
Yes (N=54) N (%) |
||||||
| <3/uL (N=57) | 42 (66) (75) | 14 (26) (25) | <0.0001 | ||||
| ≥3/uL (N=62) | 22 (34) (35) | 40 (74) (65) | |||||
| B. A range of CMV antigenemia before day+100 is associated with increased CASTs at day+100 in R+D+, R+D-, and R-D+ pairs. | |||||||
| Change in CASTs from before alloHCT to day+100 |
CMV antigenemia (PMNs+pp65) upon CMV reactivation before day+100 |
p-value | |||||
| None | 1-2 | 3-5 | 6-9 | 10-24 | ≥25 | ||
| Same or lower | 43 (70) | 9 (15) | 3 (5) | 2 (3) | 1 (2) | 3 (5) | 0.03 |
| Higher | 21 (37) | 18 (32) | 7 (12) | 5 (9) | 2 (4) | 4 (7) | |
Abbreviations: alloHCT – allogeneic hematopoietic cell transplant, CAST – CMV antigen specific T cell, CMV – cytomegalovirus, D – donor CMV serotype, N – number, PMNs+pp65 – polymorphonuclear cells positive for pp65 antigen, R-recipient CMV serotype.
Exposure of CASTs to early CMV reactivation was associated with reduced incidence of CMV reactivation after day+100
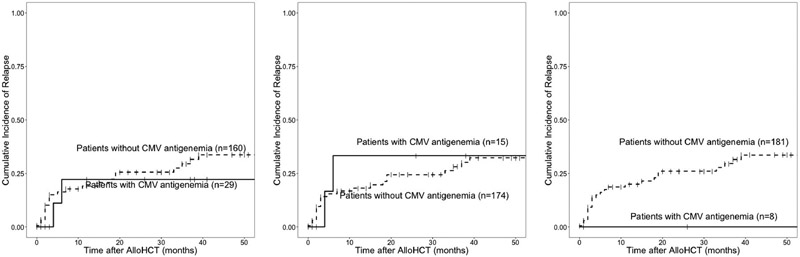
In the 146 patients at high risk to reactivate CMV before day+100 (R+D− and R+D+), 43 reactivated CMV before day+100, had a maximum of grade II acute GvHD before day+100, and could be followed at day+365. (Figure 1A to 1B) For these patients, ≥3 CASTs/μL versus <3 CASTs/μL at day+100 was associated with decreased CMV reactivation between day+100 and day+365 (8/30 (27%) vs. 8/13 (62%) reactivated CMV, respectively, p=0.04, Figure 2, Table 6A). The patients who were not protected by >3/μL CMV exposed CASTs (N=8) were more likely to be female, have R+D− CMV serotype, and have chronic GvHD. Detailed plots of CMV reactivation and CAST reconstitution for these patients are presented in Supplemental Figure 5. This effect of CMV antigenemia exposed CASTs on later CMV reactivation was not significant when patients with Grade III-IV acute GvHD prior to day 100 were included in the analysis (data not shown).
Figure 2.
CAST number at day+100 correlates with subsequent CMV reactivation in R−D+ and R+D− transplant pairs that reactivated CMV before day+100 (p = 0.04).
Table 6.
CMV antigenemia primes CASTs to prevent subsequent CMV reactivation
| A. In R+D± patients with a maximum of grade II acute GvHD, reactivating CMV and achieving ≥3 CASTs/μL by day+100 is associated with decreased CMV reactivation from day+100 to +365. The chronologic pattern of CMV reactivation and CASTs reconstitution for the individual subjects in this table are shown in Supplemental Figure 6. | |||
|---|---|---|---|
| CMV reactivation between day+100 and +365 |
Blood concentration of CASTs by day+100 | p-value | |
| <3/μL (N=13) N (%) |
≥3/uL (N=30) N (%) |
||
| No | 5 (38) | 22 (73) | 0.04 |
| Yes | 8 (62) | 8 (27) | |
| B. In R+D± patients with a maximum of grade II acute GvHD, developing low level CMV antigenemia (<5/50,000 PMNs+pp65) and achieving ≥3 CASTs/μL by day+100 is associated with decreased CMV reactivation from day+100 to +365. | |||
| CMV reactivation between day+100 and +365 |
Blood concentration of CASTs by day+100 | p-value | |
| <3/μL (N=10) N (%) |
≥3/μL (N=18) N (%) |
||
| No | 3 (30) | 13 (76) | 0.04 |
| Yes | 7 (70) | 4 (24) | |
Abbreviations: CAST – CMV antigen specific T cell, CMV – cytomegalovirus, GvHD – graft versus host disease, D – donor CMV serotype, N – number, NS – not significant, PMNs+pp65 – polymorphonuclear cells positive for pp65 antigen, R – recipient CMV serotype.
CMV antigenemia levels before day+100 may modulate the effect of CASTs at day+100 on CMV reactivation after day+100
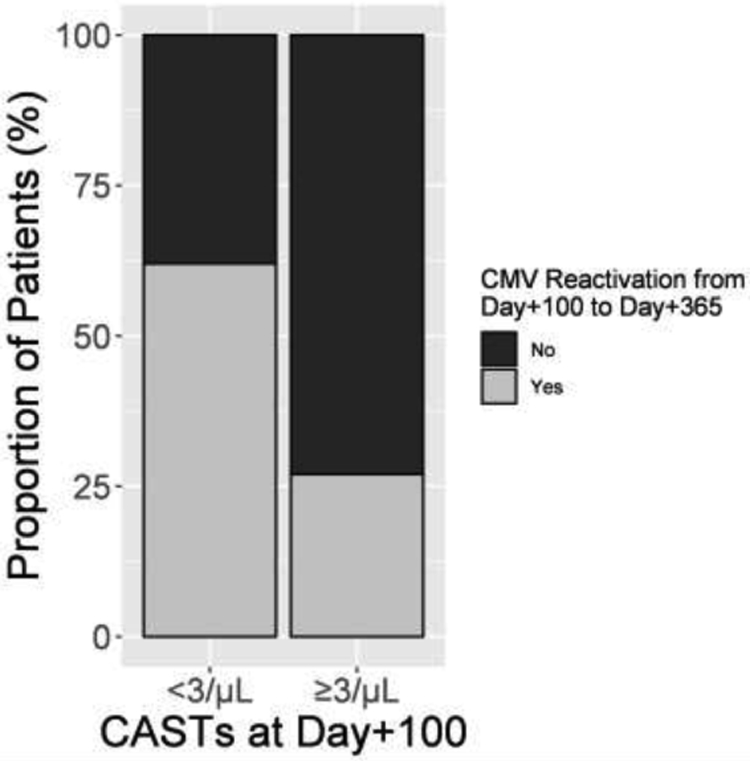
For patients exposed to low-level CMV antigenemia (<5/50,000 PMNs+pp65) before day+100, achieving ≥3 versus <3 CASTs/μL at day+100 was significantly associated with decreased CMV reactivation after day+100 (4/17 (23%) vs. 7/10 (70%) reactivated CMV, respectively, p=0.04, Table 6B). In contrast, for patients exposed to high-level CMV reactivation (≥5/50,000 PMNs+pp65) before day+100, achieving ≥3 versus <3 CASTs/μL by day+100 was not significantly associated with a lower rate of CMV reactivation after day+100 (4/13 (31%) vs. 1/3 (33%) reactivated CMV).
Effect of CASTs and CMV reactivation on relapse.
The cumulative incidence of relapse was not different for patients who achieved a CMV antigenemia of ≥5 or ≥10/50,000 PMNs +pp65. None of the patients who achieved a CMV antigenemia of ≥25/50,000 PMNs +pp65 experienced a relapse, in contrast to 20-22% of patients who never had any positive CMV antigenemia and relapsed by 1 year. (Figure 3). CAST levels at day +100 did not associate with risk of relapse. (Figure 4).
Figure 3.
Sensitivity analysis of CMV antigenemia and disease relapse.
Figure 4.
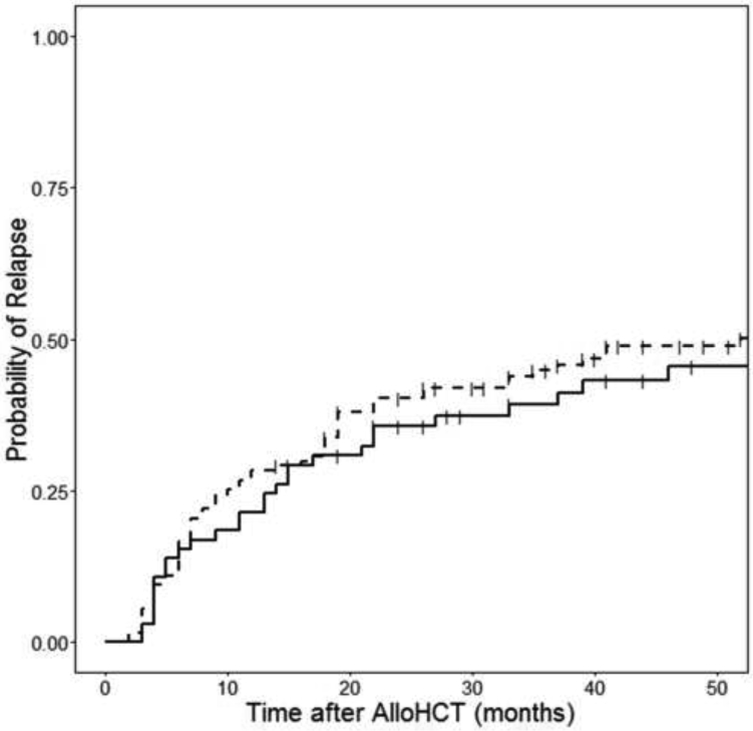
The presence of CASTs at day+100 does not associate with cumulative incidence of relapse.
Discussion
In this study, we demonstrated that any level of CMV antigenemia before day+100 increases the number of CASTs at day+100. We also observed that early low level CMV antigenemia (<5/50,000 PMNs+pp65) was associated with the improved ability of CASTs to protect against subsequent CMV reactivation. These results suggest that treatment of low level CMV infection (<5/50,000 PMNs+pp65 by antigenemia) could be delayed until progression to high level CMV infection without adversely affecting and possibly improving later anti-CMV immunity. Our results are relevant to allogeneic transplant centers using a preemptive CMV treatment strategy as a potential guide for when to initiate therapy.
Randomized trials of CMV prophylaxis versus early treatment with ganciclovir support this notion. [13, 17-19] An early treatment strategy would have allowed low-level CMV viremia to develop while a prophylactic strategy would have more completely suppressed CMV viremia. In one study, ex vivo functional testing of CMV specific CD8+ CASTs demonstrated that CMV reconstitution was equally poor at day+30 in the prophylaxis and pre-emptive therapy arms. [19] Between days +40 to +90, however, CASTs recovered in the preemptive therapy but not the prophylactic arm. These results are consistent with our results reported here showing poor CAST reconstitution at day+30 and an association between early CMV antigenemia and an increase in the number and clinical activity of CASTs.
Recently, a phase III trial of letermovir for CMV prophylaxis demonstrated a significantly reduced incidence of breakthrough CMV reactivation. [12] However, after week 18 and the discontinuation of letermovir, there was an increasing incidence of CMV reactivation. These results suggest that while CMV infection was suppressed, it was likely due to drug effect rather an enduring anti-CMV cytotoxic T cell response.
CMV reactivation after alloHCT has been associated with a salutary effect on cytotoxic T, NK, and γδ T cell reconstitution [18, 20-22] and a an anti-leukemia effect. [14, 15] The mechanism for the anti-leukemic effect of γδ T cells is postulated to be expansion of V62 negative γδ T cells in response to CMV reactivation followed by cross-reaction of the Vγ2 negative γδ T cells with leukemic blasts. [22] The anti-leukemia effect of NK cells may follow a similar mechanism. After alloHCT, CD56dimCD57+NKG2C+ NK cells have also been shown to expand after CMV reactivation. Ex vivo, these NK cells react against the K562 leukemia cell line with increased TNF and IFN-γ cytokine production. [23] CD56dimCD57+NKG2C+ NK cell expansion also trended with decreased disease relapse at 6 months in the population studied. [23]
The findings of this study suggest a potentially beneficial effect from early CMV reactivation. It will be important to evaluate the long-term effects of a prophylactic strategy to control CMV after alloHCT, as suppression of early CMV replication may inhibit later anti-CMV immune reconstitution and potentially impact the late development of CMV reactivation or disease. This later reactivation may ultimately be more clinically significant as surveillance for CMV is generally less frequent beyond the first 3 months after transplant.
An alternative explanation for the association between low level CMV antigenemia before day+100 and improved anti-CMV immunity after day+100 is that low level antigenemia may by the result of better control of CMV reactivation by an inherently more immunocompetent recipient. Patients presenting with high antigenemia levels may have greater immunologic deficits due to immunosuppression, prior chemotherapy, or disease status than patients presenting with low antigenemia and thus be less capable of controlling CMV reactivation. Thus, it may be possible to delay treatment of low level antigenemia until it progresses to high level antigenemia.
Finally, our findings suggest that patients with prior CMV reactivation who have a history of grade III-IV acute GvHD and <3 CASTs/ μL at day+100 are at increased risk for CMV reactivation after day+100 and may require further surveillance for CMV.
Limitations of this study
CMV antigenemia, rather than quantitative CMV PCR, was used to measure the viral load in this study. CMV PCR was not routinely used at our transplant center during the time period of this study. While continuous use of CMV antigenemia allows the comparison of data acquired over time at our center, it limits the applicability of our findings in transplant centers that use CMV PCR. The low level of CMV antigenemia that was found to be associated with improved measures of anti-CMV reconstitution could be converted to an equivalent CMV PCR level. A conversion of 1/100,000 PMNs+pp65 to 1200 IU CMV/mL has been reported. [24] Individual centers using either CMV antigenemia or PCR as a threshold for initiating early CMV treatment would need to make adjustments based upon their own clinical outcomes.
Because CMV antigenemia is less sensitive than quantitative CMV PCR for detecting CMV infection, the viral load associated with reconstitution of CMV immunity in this study may have been overestimated by CMV antigenemia testing. In addition, the incidence of CMV reactivation may have been underestimated. We compensated for a lower sensitivity to CMV reactivation by requiring a ≥2 week period without CMV antigenemia to determine that an episode of CMV reactivation had resolved. This is a shorter period in comparison to other studies such as those from the Center for International Blood and Marrow Transplant Research which require a 60 day CMV-free period. Because the aim of the study was to assess the level of CASTs that conferred protective immunity to prevent first and subsequent reactivation, a shorter period was used to avoid underestimating the control of CMV by disregarding flares of CMV viremia because they were within the 60 day window.
In this study, the number of CASTs increased after CMV reactivation as demonstrated by serial quantitative measurements. The ability of CASTs to increase suggests that they were stimulated to divide after CMV exposure and therefore were not anergic. Although not done in this study, additional immunophenotyping for markers of T-cell activation / exhaustion to determine the in vivo functionality of CASTs might have yielded additional information and is a consideration for future studies.
Alternative methods for identifying CASTs, such as ex vivo stimulation of patient lymphocytes by overlapping CMV protein sequence derived pentadecapeptides followed by flow cytometric detection of intracellular cytokines (such as IFN-γ), can also determine their ex vivo functional potential. [25] However, the ex vivo functional potential may not truly reflect the in vivo function. Furthermore, because peptide binding to HLA is sequence specific, the number of haplotypes peptide stimulation assays can be applied to is limited by the number of different peptides that is added and the cell’s ability to process them into epitopes able to bind individual HLA.
Finally, this was a retrospective analysis of clinical observations and subject to the limitations of the methodology. Our main findings were ultimately drawn from a modest number of patients (N=43), who were at high risk for CMV reactivation and had a maximum of grade II acute GvHD, precluding a definitive conclusion. Because patients who live at a distance from the transplant center and do well are less likely to follow up at the transplant center, the patient population observed may have become enriched for those with ongoing alloHCT complications and thus may not be representative of the total post-transplant population. Another limitation was that the threshold for initiating early CMV therapy was based upon physician judgement rather than a clinical protocol. Consequently, the effect of CMV antigenemia on anti-CMV immune reconstitution may be confounded by patient specific variables such as the presence of grade III-IV acute GvHD or the intensity of immunosuppression. In this study, grade III-IV aGvHD did not affect the development of CASTs but seemed to nullify their protective effect against subsequent CMV reactivation.
Summary
We show that early CMV reactivation induces CASTS and may improve their ability to prevent subsequent CMV infection. These results suggest that a low level CMV infection (<5/50,000 PMNs+pp65 by antigenemia) before day+100 after alloHCT may be beneficial and may not require treatment until progression of CMV reactivation. There are, however, important limitations to this retrospective study which will require confirmation in prospective studies comparing different CMV antigenemia and PCR thresholds for early CMV therapy and the effect on durable anti-CMV immunity.
Supplementary Material
Highlights.
Cytomegalovirus (CMV) reactivation induces CMV antigen specific T-cells which may prevent subsequent CMV reactivation.
Low level CMV antigenemia (<5/50,000 polymorphonuclear cells positive for pp65) associates with protective anti-CMV immunity after day+100 and may be beneficial. Early therapy of low level CMV antigenemia could be delayed until progression to high level CMV antigenemia.
Acknowledgements
Funding. This study was supported by research funding from Immudex and the National Cancer Institute (P30CA016056).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematology/oncology clinics of North America. 2011;25:151–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–82. [DOI] [PubMed] [Google Scholar]
- [3].Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van der Heiden P, Marijt E, Falkenburg F, Jedema I. Control of Cytomegalovirus Viremia after Allogeneic Stem Cell Transplantation: A Review on CMV-Specific T Cell Reconstitution. Biol Blood Marrow Transplant. 2018;24:1776–82. [DOI] [PubMed] [Google Scholar]
- [5].Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–14. [DOI] [PubMed] [Google Scholar]
- [6].Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–41. [DOI] [PubMed] [Google Scholar]
- [7].Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–5. [DOI] [PubMed] [Google Scholar]
- [8].Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rastogi S, Ricci A, Jin Z, Bhatia M, George D, Garvin JH, et al. Clinical and Economic Impact of Cytomegalovirus Infection among Children Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- [10].Huang YT, Su Y, Kim SJ, Nichols P, Burack D, Maloy M, et al. Cytomegalovirus Infection in Allogeneic Hematopoietic Cell Transplantation Managed by the Preemptive Approach: Estimating the Impact on Healthcare Resource Utilization and Outcomes. Biol Blood Marrow Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377:2433–44. [DOI] [PubMed] [Google Scholar]
- [13].Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–71. [PubMed] [Google Scholar]
- [14].Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–12. [DOI] [PubMed] [Google Scholar]
- [15].Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tario JD Jr., Chen GL, Hahn TE, Pan D, Furlage RL, Zhang Y, et al. Dextramer reagents are effective tools for quantifying CMV antigen-specific T cells from peripheral blood samples. Cytometry B Clin Cytom. 2015;88:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118:173–8. [DOI] [PubMed] [Google Scholar]
- [18].Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7. [DOI] [PubMed] [Google Scholar]
- [19].Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–9. [PubMed] [Google Scholar]
- [20].Peggs KS, Verfuerth S, Pizzey A, Khan N, Moss P, Goldstone AH, et al. Reconstitution of T-cell repertoire after autologous stem cell transplantation: influence of CD34 selection and cytomegalovirus infection. Biol Blood Marrow Transplant. 2003;9:198–205. [DOI] [PubMed] [Google Scholar]
- [21].Davis ZB, Cooley SA, Cichocki F, Felices M, Wangen R, Luo X, et al. Adaptive Natural Killer Cell and Killer Cell Immunoglobulin-Like Receptor-Expressing T Cell Responses are Induced by Cytomegalovirus and Are Associated with Protection against Cytomegalovirus Reactivation after Allogeneic Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27:1328–38. [DOI] [PubMed] [Google Scholar]
- [23].Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cardenoso L, Pinsky BA, Lautenschlager I, Aslam S, Cobb B, Vilchez RA, et al. CMV antigenemia and quantitative viral load assessments in hematopoietic stem cell transplant recipients. J Clin Virol. 2013;56:108–12. [DOI] [PubMed] [Google Scholar]
- [25].Kern F, Faulhaber N, Frommel C, Khatamzas E, Prosch S, Schonemann C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.