Abstract
Glutamate (Glu) is a key molecule in cellular metabolism, the most abundant excitatory neurotransmitter in the brain, and the principal neurotransmitter of cortical efferents. Glutamate dysfunction, on the other hand, is common in neurodegenerative disorders, and likely contributes to age-related declines in behavioral and cognitive functioning. Nonetheless, the extant literature measuring age-related changes in brain glutamate in vivo has yet to be comprehensively and quantitatively summarized. This meta-analysis examines proton spectroscopy (1HMRS) measures of Glu-related brain metabolites across 589 healthy young and older adults. Glu (Cohen’s d = −0.82) and Glu+glutamine (Cohen’s d = −0.51) concentrations were significantly lower in older compared to younger adults, whereas the concentration of glutamine (d = 0.43) was significantly higher in older individuals. Notably, 1HMRS methodological choices impacted effect sizes for age-related Glu differences. Glu metabolite change appears to be a robust marker of aging-related neurological change, however additional studies are needed to elucidate age-related trajectories of glutamatergic alterations and their relationship to cognitive phenotypes.
Keywords: aging, glutamate, spectroscopy (1HMRS), meta-analysis, MRI
1.0. Introduction
Over the next several decades the number of Americans aged 65 and older is projected to double from 46 to 98 million (Mather et al., 2015). This increase is a direct result of meaningful advances in geriatric medicine and healthcare. Despite these advances, age-related cognitive decline remains one of the most dreaded, largely unmodifiable, and poorly understood features of aging. The age-related improvements in cognition (e.g. in memory and executive function) seen from childhood through adolescence (Zelazo et al., 2004) are mirrored by systematic, age-related declines in cognitive functions in the final decades of life (Dempster, 1992; Mayr, 2001). This decline in complex cognitive functioning arises due to a loss of integrity (e.g. a loss of synaptic connectivity) of the neural systems underlying cognition (Craik and Grady, 2002). Among the biological mechanisms proposed to contribute to such integrity loss, alterations in brain glutamate (Glu) are considered critical, given Glu’s ubiquity and involvement in cellular energetics and high-order cognitive functions (Coyle, 2006; Curtis and Johnston, 1974; Erecińska et al., 1990; McEntee and Crook, 1993; Robbins and Murphy, 2006).
Glutamate is the most abundant endogenous excitatory amino acid in the brain (Cotman and Monaghan, 1988) and the primary neurotransmitter used by pyramidal cells in the cerebral cortex (Cotman and Monaghan, 1988). As such, it is not surprising that Glu has been implicated in age-related declines in functioning. Animal studies, for example, have consistently demonstrated aging-related reductions in brain Glu (McEntee and Crook, 1993), as well as a loss of NMDA-type glutamate receptors (Wenk et al., 1991). Studies of brain neurochemistry in humans have relied predominantly on post-mortem tissue (Hynd et al., 2003), yet these studies unfortunately have a number of limitations (e.g. post-mortem interval, time in storage, tissue pH) making quantification challenging. Critically, in recent years the use of high-field, non-invasive neuroimaging techniques has enabled the in vivo study of Glu neurometabolites in the human brain (Soares and Law, 2009), including in aging (Cleeland et al., 2019).
The examination of in vivo Glu can be accomplished using invasive electrodes (Huberfeld et al., 2011), positron emission tomography (PET), and proton magnetic resonance spectroscopy (1HMRS). Unlike electrode measurements that measure mostly extracellular glutamate and PET that measures glutamate receptor availability, 1HMRS measures total glutamate present in both intra- and extra-cellular compartments. 1HMRS allows for the measurement of many, albeit not all, brain metabolites within specific regions of the brain (e.g. (Barker et al., 2001)). Nonetheless, the quantification of Glu by 1HMRS presents a particular challenge, due predominantly to the overlap of Glu and glutamine (Gln) resonances. Consequently, the 1HMRS readouts from these neurochemicals are commonly combined and referred to as “Glx” (Glu + Gln). Still, 1HMRS has been the primary method used for in vivo Glu/Gln/Glx imaging in aging to date. In fact, a recent review reported that brain Glu in healthy older adults appears to be consistently lower when compared to younger individuals, but the size of this effect was not quantified, and potential moderating variables were not investigated statistically (Cleeland et al., 2019). To our knowledge there has not been a systematic, quantitative analysis of the extant Glu 1HMRS data in aging, as has been done with other neurometabolites (see (Haga et al., 2009)).
There have been 13 independent 1HMRS studies (two at 1.5 Tesla (Chang et al., 1996; Raininko and Mattsson, 2010), eight at 3 Tesla (Ding et al., 2016; Hädel et al., 2013; Mooney et al., 2017; Sailasuta et al., 2008; Schubert et al., 2004; Simmonite et al., 2019; Suri et al., 2017; Zahr et al., 2008), two at 4 Tesla (Boumezbeur et al., 2010; Kaiser et al., 2005) and one at 7 Tesla (Marjańska et al., 2017)) that provide age-related measures of Glu-related metabolites in healthy older and younger adults. What is clear from qualitative review of these studies is that Glu-related metabolite concentrations may indeed differ in older and younger adults. The magnitude and pattern of these differences, however, remain unquantified. As noted in a recent review, there is significant heterogeneity across studies in terms of (a) which metabolite(s) (Glu, Gln, and/or Glx) were measured, (b) which brain region(s) Glu-related metabolites were measured in, and (c) what 1HMRS protocol was employed. Critically, these factors may affect the age-related effect sizes reported (Cleeland et al., 2019), and, by extension, our understanding of the neurobiology of aging. Consequently, a systematic analysis of all available data that incorporates diverse moderating variables is needed to better elucidate the nature of age-related changes in brain Glu.
In the current work, we conduct a comprehensive meta-analysis of existing studies examining Glu-related metabolites in healthy aging. This approach allows for results to be consolidated across studies, providing a more powerful estimate of true population differences. We furthermore seek to identify the impact of various potential moderators on previously reported results, including demographic (e.g. sex), methodological (e.g. 1HMRS technique), and neurological (e.g. brain region) moderators. The goals of these moderator analyses are to reveal regional and tissue type-specific changes in Glu-related metabolites in aging, to gain insight into methodological sensitivity, and to provide future guidance for the field. We hypothesized that brain Glu would be lower in healthy older adults when compared to younger adults and anticipated that heterogeneity across studies would be explained by specific methodological choices. Overall, we propose that a refined appreciation of glutamate availability in the human brain throughout aging—and its relationship to molecular, cellular, and cognitive changes—will lead to improvements in our understanding of cognitive aging and offer new targets for intervention in age-related cognitive decline.
2.0. Materials and methods
The extraction of relevant articles and article data and reporting of meta-analytic aims, methods, results, and biases followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) reporting standards (Supplemental Figure 1) (Stroup et al., 2000). The search strategy included an initial broad search in PubMed, MEDLINE, PsychINFO, and Google Scholar databases, followed by a manual review of articles utilizing references from identified original articles and reviews. We used the following search criteria: “aging OR age”, AND “glutamate” OR “GLU” OR “Glx” OR “Glutamine” or “GLN”, AND “spectroscopy” OR “1HMRS”, AND “brain”. The search was limited to articles published prior to September 30, 2019 that were written in English and that enrolled human participants.
Studies included in the meta-analysis met the following criteria: 1) inclusion of 1HMRS measures of Glu, Gln, or Glx; 2) samples comprised of unrelated, healthy young and old adults with no history of major medical, neurological or psychiatric conditions; 3) a minimum age difference of 25 years between ‘young’ and ‘old’ participants as determined by group averages or the age-range provided in the primary study; and 4) data or statistical information that enabled the calculation of effect sizes. Studies reporting results from either independent samples (i.e. younger vs. older) or correlations with age were included. Two authors (MW, DRR) initially reviewed each potential publication for inclusion based on the aforementioned criteria. Consensus of the first (DRR) and senior (PJM) authors was needed to exclude an article. Three authors (VJS, DRR, PJM) were involved in extracting relevant data from publications and quality checking the extracted data for accuracy. Variables extracted for meta-analytic analysis, when provided, included 1HMRS concentration values (absolute or ratio) and standard deviations, age-related correlations with Glu, Gln or Glx, metabolite measured (Glu/Gln/Glx), 1HMRS sequence, MRI field strength, echo time (TE), 1HMRS voxel type, brain region of interest (ROI) studied, old/young sample age, and sex. We identified thirteen (n = 13) independent publications for inclusion that reported age-related measures of Glu/Gln/Glx 1HMRS, with a combined total of 44 effects (k) (Table 1). Four (n = 4) additional articles that were originally considered were ultimately excluded due to: 1) lack of quantitative Glu metabolite values provided with respect to age (Mader et al., 2002; Tisell et al., 2013; Tunc-Skarka et al., 2014) or 2) sample overlap between manuscripts (Zahr et al., 2013). Data included in the final analysis is available in the Supplemental Material and on our GitHub repository: https://github.com/droalf/Aging-Glutamate-MetaAnalysis.git.
Table 1:
Summary table of1HMRS studies reporting Glu, Gln or Glx effects in healthy older and younger adults.
|
Table 1 Study Year |
Age Young# | Age Old# | Sample Age Range* | Sample Size (Total/Young/Old) | ROI | Sex (%F) | MRS Sequence | Field Strength | Voxel Type | Glu Type | Echo Time (TE)msec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boumezbeur 2010 | 26 | 76 | - | 14/7/7 | Midline Occipito-Parietal Lobe | 33 | LASER-POCE | 4.0 T | Single | Glu | 42 |
| Chang 1996 | - | - | 19–78 | 33/-/- | Mid-Frontal Gray Matter; Right-Frontal White Matter | PRESS | 1.5 T | Single | Glx | 30 | |
| Ding 2016 | 25 | 65 | 20–70 | 30/17/13 | Bilateral Frontal and Parietal lobes | 57 | EPSI | 3.0 T | Multi | Glx | 17.6 |
| Hädel 2013 | - | - | 20–56 | 118/-/- | Left Hippocampus; Anterior Cingulate Cortex | 50 | PRESS | 3.0 T | Single | Glu, Gln | 80 |
| Kaiser 2005 | 26 | 54 | 24–68 | 24/11/13 | Corona Radiata; Mesial Motor Cortex | 54 | STEAM | 4.0 T | Single | Glu, Gln | 15 |
| Marjańska 2017 | 21 | 78 | 19–88 | 33/17/16 | Posterior Cingulate Cortex; Occipital Cortex | 52 | STEAM | 7.0 T | Multi | Glu, Gln | 8 |
| Mooney 2017 | 24.6 | 70.3 | 20–83 | 31/15/16 | Primary Motor Cortex | 35 | MEGA-PRESS | 3.0 T | Single | Glx | 68 |
| Raininko 2010 | 32 | 62 | 13–72 | 34/15/19 | Supraventricular White Matter | 44 | PRESS | 1.5 T | Single | Glx | 22 |
| Sailasuta 2008 | - | - | 21–71 | 22/16/6 | Frontal White Matter; Parietal Gray Matter; Basal Ganglia | 40 | PRESS | 3.0 T | Single | Glu | 82^ |
| Schubert 2004 | - | - | 20–60 | 40/-/- | Anterior Cingulate Cortex; Hippocampus | 50 | PRESS | 3.0 T | Single | Glu, Gln | 80^ |
| Simmonite 2019 | 20.7 | 76.5 | 18–87 | 39/20/19 | Occipital Cortex | 56 | PRESS | 3.0 T | Single | Glx | 35 |
| Suri 2017 | 23.9 | 68.9 | 20–85 | 147/30/117 | Posterior Cingulate Cortex | 26 | SPECIAL | 3.0 T | Single | Glu, Gln | 8.5 |
| Zahr 2008 | 25.5 | 77.7 | 19–84 | 24/12/12 | Striatum;Cerebellum; Pons | 50 | CT-PRESS | 3.0 T | Single | Glu, Glx | 139^ |
ROI = region of interest; %F = percent female; Glu = glutamate; Gln = glutamine; Glx = glutamate+glutamine;
average age reported for cohort studies;
minimum and maximum age included in the study;
indicates average TE.
2.1. Statistical Analyses
Meta-analysis was completed across all studies to determine age-related effect sizes, followed by meta-regressions and analyses of publication bias. Analyses were carried out using Comprehensive Meta-Analysis version 2.21.064 (Biostat, 2005), using standard random-effects models. Glu-related metabolite level (Glu, Gln, Glx) differences between older and young participants were standardized using Cohen’s d (effect size; quantified as the difference between the two raw mean scores divided by the pooled standard deviation (SD)). When means and SDs were not available, Cohen’s d was calculated from reported univariate F-tests, t-statistics, r-values, or p-values. Confidence intervals (CI) for each effect are reported. In order to control for differences in sample size during effect size calculation, studies were weighted according to their inverse variance estimates. Prior convention has classified effect sizes as small (d = ±0.2), medium (d = ±0.5), or large (d ≥ ±0.8) based on these methods (Cohen, 1988). Negative effect size values indicate lower brain metabolite level in older relative to younger adults. Homogeneity of effect size across studies was assessed using the Cochran Q-statistic (Hedges and Olkin, 1985).
2.1.1. Moderator Variables and Meta-regression
Meta-regression was used to investigate potential moderating variables that vary across studies (e.g., sex, MRI field strength, etc.) in order to better understand heterogeneity between studies. The following moderator variables were evaluated in follow-up analyses with meta-regression: (a) metabolite (Glu, Gln, Glx), (b) MRI field strength, (c) 1HMRS protocol (d) 1HMRS acquisition voxel type (single vs. multislice), (e) whether Glu values were reported as an absolute concentration or a ratio, (f) brain region, (g) TE, and (h) sex. Additional demographic and clinical characteristics of interest, including race, years of education, and cognitive functioning, were insufficiently reported across studies to be included in formal analyses.
2.1.2. Analysis of Small Study Bias
It is possible that studies with large effects are overrepresented in the extant literature. The specific concern is that studies with relatively large effects are more likely to be published than studies with small effects, even when addressing the same question, especially when results align with theory. This may lead to a small study bias in the literature that can possibly influence the results of a meta-analysis. Small study bias was therefore assessed using several convergent methods: (1) visual inspection of a funnel plot: an asymmetric scatterplot of effect size by study precision may indicate bias; (2) adjusted rank-correlation (e.g. Spearman) test according to the methods of Begg and Mazumdar (Begg and Mazumdar, 1994), Egger et al. (Egger et al., 1997), and Duval and Tweedy (Duval and Tweedie, 2000): a high correlation between study effect sizes and sampling variances is suggestive of bias; and (3) a fail-safe file drawer analysis: a probability-based metric used to determine the number of null studies needed to invalidate the reported effect (Rosenthal, 1979). Finally, to address the potential of certain studies being outliers, a trim-and-fill method was used to adjust average effect size to account for publication bias where appropriate (Duval and Tweedie, 2000).
3.0. Results
3.1. Overall Meta-analysis
Across all studies, the average age for older participants was 69.24 years (range 54–78) and the average age for younger participants was 22.71 years (range 19–26). Analysis of study-specific effect sizes for differences between older and younger participants across all three metabolites (Glu/Gln/Glx) revealed an overall medium effect size (d = −0.43, 95% CI = −0.69 < δ < −0.18, k = 44) that was significantly heterogeneous (QB[43] = 252.42, p < 0.001). Given that the variability in study-specific effect sizes between healthy older and younger groups differed more than would be expected from sampling error alone, analyses of potential moderator variables were undertaken.
3.2. Moderator Analyses
3.2.1. Metabolite Type.
Follow-up moderator analysis of metabolite type revealed significant heterogeneity across metabolites (QB[2] = 23.50, p < 0.001). Effect sizes for Glu (d = −0.82, CI = −1.17 < δ < −.47, k = 17) and Glx (d = −0.51, CI = −0.81 < δ < −0.21, k = 18) were homogenous (QB[1] = 1.78, p = 0.18) in magnitude and direction (Young > Old, Figure 1). The effect size for Gln (d = 0.43, CI = 0.82 < δ < 0.03, k = 9), on the other hand, was significantly different from both Glu (QB[1] = 22.09, p < 0.001) and Glx (QB[1] = 13.84, p < 0.001) and opposite in direction (Young < Old, Figure 1). Given the differences in magnitude and direction of metabolite effect sizes and the relatively small number of Gln effects (k = 9), additional moderator analyses are limited to Glu and Glx.
Figure 1. Individual study and average effect sizes displayed by 1HMRS metabolite type.
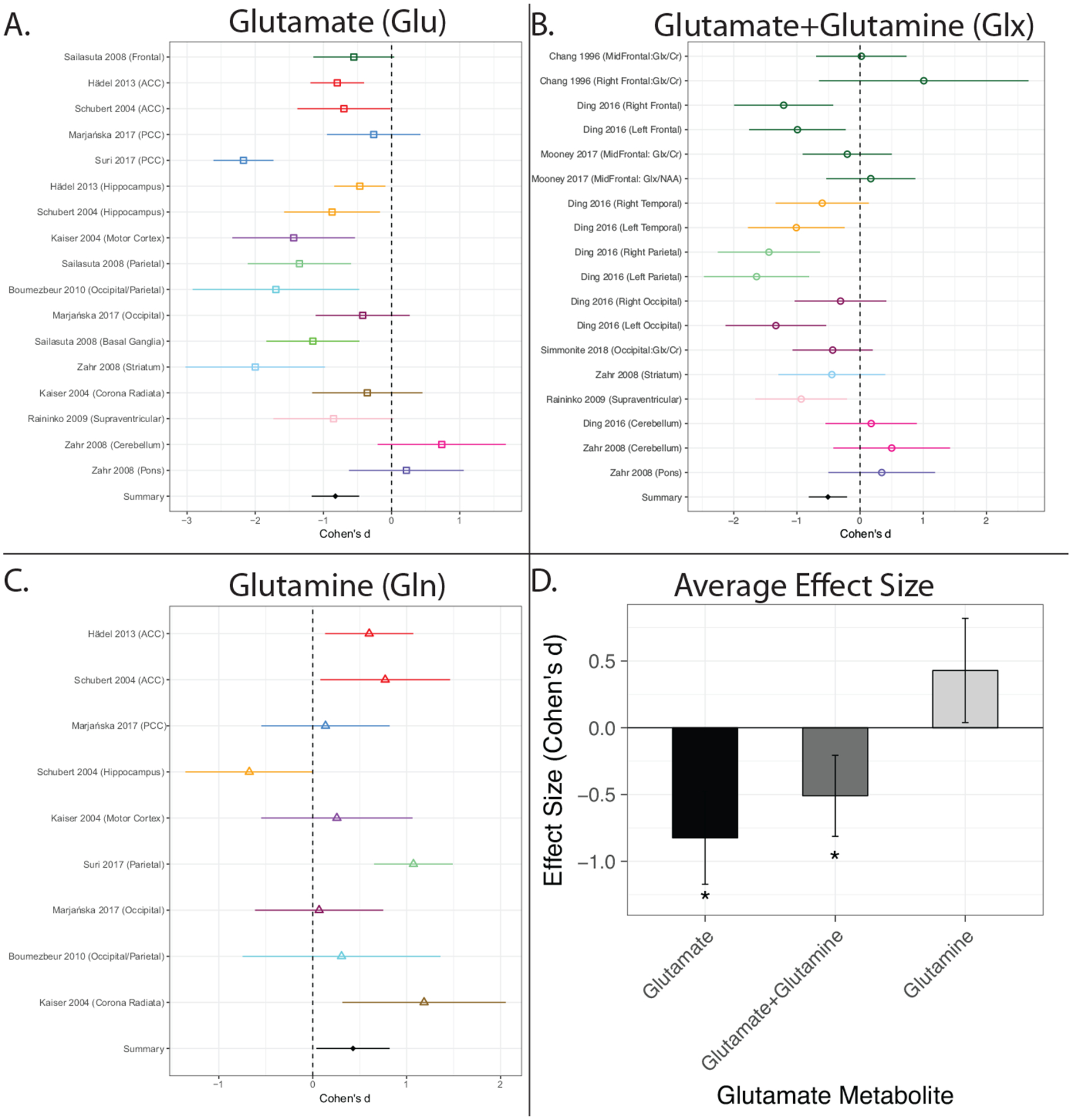
A-C: Effects sizes are shown separately for glutamate (A), glutamate+glutamine (B), and glutamine (C). Effects are shown as mean Cohen’s d (effect size) with corresponding 95% confidence intervals. (D). The meta-analytic derived average effect size ((+/ 95% CI) for each of the three metabolite types. Effects shown in A-C are ordered and colored by brain region. In all panels, negative effect sizes indicate lower metabolite levels in older compared to younger adults. *indicates significantly different than glutamine. ACC=Anterior Cingulate Cortex; PCC=Posterior Cingulate Cortex.
Recalculating the overall meta-analytic effect based only on studies of Glu and Glx resulted in a moderate-to-large negative effect size (d = −0.66, CI = −0.90 < δ < −0.43, k = 35) indicating that Glu (k = 17) and Glx (k = 18) levels are lower in older as compared to younger adults. However, effects remained significantly heterogeneous (QB[34] = 127.11, p < 0.001) between studies, as can been seen in panels A and B of Figure 1.
3.2.2. MRI Field Strength.
MRI field strength can affect the ability to quantify Glu and Glx due to spectral overlap effects; quantification at higher fields is expected to be more precise. Across all studies, reported 1HMRS effects for Glu and Glx were most frequent at 3T (k = 26), followed by 1.5T (k = 4), 4T (k = 3), and 7T (k = 2). Effect sizes did not significantly differ by MRI field strength (Figure 2A) (QB[3] = 3.15, p = 0.36). Average effect sizes were nominally largest at 4.0T (d = −1.09, 95% CI = −1.93 < δ < −0.25), followed by 3.0T (d = −0.68, 95% CI = −0.96 < δ < −0.40), then 1.5T (d = −0.37, 95% CI = −1.07 < δ < 0.32) and 7T (d = −0.34, 95% CI = −0.82 < δ < 0.14). It should be noted, however, that there has only been one 7T study, which contributed both 7T effects.
Figure 2. Meta-analytic average effect sizes by methodological moderator variable.
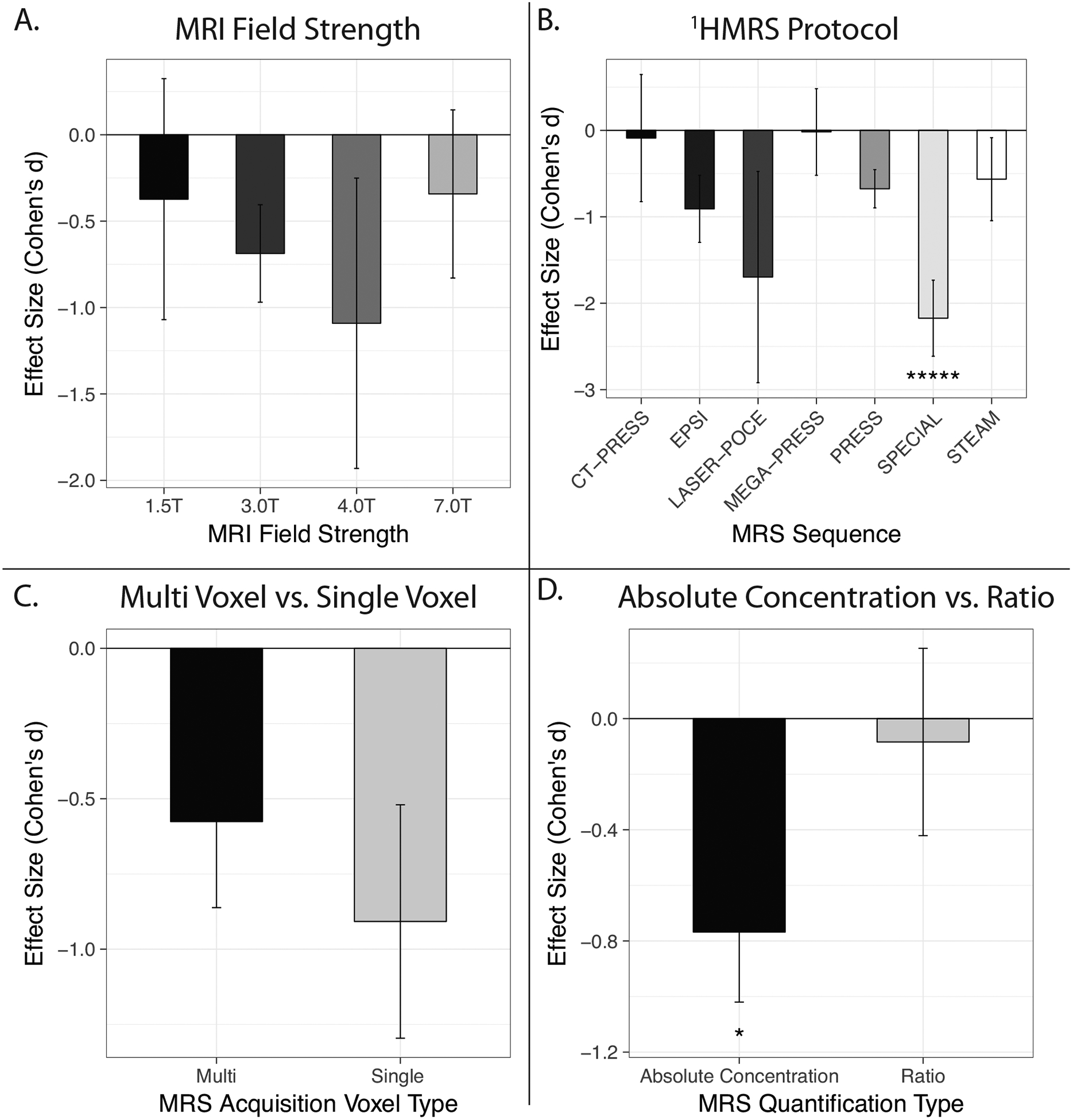
Average effect sizes (+/ 95% CI) shown for each variable were calculated from all available Glu and Glx effects. Effect sizes did not significantly differ by MRI Field Strength (A) or Voxel Type (C). Effect sizes for 1HMRS Protocol (B) and Quantification Method (D) were significantly heterogeneous.
3.2.3. 1HMRS Protocol.
1HMRS protocols vary in their sensitivity and specificity for brain glutamate metabolites. Unsurprisingly, there was significant variability in the 1HMRS protocols employed across included studies. PRESS (k = 12), CT-PRESS (k = 6), and STEAM (k = 4) were the most commonly used protocols, followed by MEGA-PRESS (k = 2), SPECIAL (k = 1), and LASER-POCE (k = 1). One EPSI study reported nine effects (k = 9). Analysis revealed significant heterogeneity in effect sizes across 1HMRS sequence types (QB[6] = 54.91, p < 0.001). Significant heterogeneity remained after excluding studies only contributing one effect. Studies implementing SPECIAL (d = −2.17, 95% CI = −2.61 < δ < −1.73), LASER-POCE (d = −1.70, 95% CI = −2.92 < δ < −0.48), and EPSI (d = −0.91, 95% CI = −1.30 < δ < −0.52) sequences showed the largest effects, on average; however, for each protocol type, one study contributed all effects. The smallest average effect sizes were found for CT-PRESS (d = −0.09, 95% CI = −0.82 < δ < 0.64) protocols and for MEGA-PRESS protocols (d = −0.02, 95% CI = −0.52 < δ < 0.48). CT-PRESS and MEGA-PRESS summary effects were smaller than effects for PRESS (d = −0.68, 95% CI = −0.90 < δ < −45) and STEAM (d = −0.57, 95% CI = −1.05 < δ < −0.09) sequences (Figure 2B). Pairwise comparisons across all 1HMRS protocols are shown in Supplemental Table 1.
3.2.4. Voxel Type.
Single voxel spectroscopy (SVS; k = 26), wherein data is collected from one large (~30cm3) voxel in the brain, is the most commonly acquired type of 1HMRS data. Across all studies included in this meta-analysis, only one study (k = 9) acquired multivoxel data covering the whole brain. Effect sizes appeared homogenous across voxel type (single v. multivoxel) (QB[1] = 1.82, p = 0.17), i.e., while the effect sizes for multivoxel data (d = −0.90 95% CI = −1.29 < δ < −0.52) were larger than for SVS data (d = −0.57 95% CI = −0.86 < δ < −0.28), this difference was not statistically significant (Figure 2C).
3.2.5. Concentration vs. Ratio.
1HMRS studies can report absolute concentration values or ratios (e.g. Glu/Creatine). Moderator analysis revealed significant heterogeneity among study-specific effect sizes for reported Glu differences between older and younger individuals (QB[1] = 10.16, p = 0.001) depending on the measurement approach used. Effect sizes for absolute concentration values were significantly larger (d = −0.77 95% CI = −1.02 < δ < −0.52) than for ratios (d = −0.08 95% CI = −0.42< δ < 0.25; Figure 2D).
3.2.6. Brain Tissue Type.
The majority of studies reported metabolite effects derived from mixed tissue (k = 18), followed by gray matter (GM; k = 13), then white matter (WM; k = 4). Moderator analysis indicated a non-significant trend in effect sizes dependent on tissue type (QB[2] = 5.80, p = 0.055; Figure 3A). Effect sizes in GM were nominally larger (d = −1.00 95% CI = −1.30 < δ < −0.70) than effects in regions with a mixed tissue (d = −0.44 95% CI = −0.80 < δ < −0.08) or WM (d = −0.60 95% CI = −1.13 < δ < −0.07) composition.
Figure 3. Meta-analytic average effect sizes by neurological moderator variable.

Average effect sizes (+/ 95% CI) shown for each variable were calculated from all available Glu and Glx effects. Effect sizes did not differ by (A) Brain Tissue Type but did differ by (B) Brain Region of Interest (*indicates significantly different from Cerebellum. ^indicates significantly different from Brain Stem). Meta-regression of age-related glutamate effect size on (C) echo time (TE) and (D) sex were significant.
3.2.7. Brain Region.
Metabolite levels were measured across a wide range of cortical and subcortical ROIs. Effects were grouped by brain region to facilitate meaningful comparisons across studies. Regions included: frontal lobe (k = 9), parietal lobe (k = 5), temporal lobe (k = 2), occipital lobe (k = 4), subcortical areas (k = 6), cerebellum (k = 3), brain stem (k = 2), and mixed cortical (k = 4). The meta-regression revealed significant heterogeneity among study effect sizes across these brain regions (QB[7] = 33.22, p < 0.001; Figure 3B). Meta-analytic average effect sizes for frontal (d = −0.51, 95% CI = −0.91 < δ < −0.12), parietal (d = −1.39, 95% CI = −2.10 < δ < −0.68), temporal (d = −0.80, 95% CI = −1.33 < δ < −0.27), occipital (d = −0.59, 95% CI = −1.02 < δ < −0.17), subcortical (d = −0.84, 95% CI = −1.19 < δ < −0.50), and mixed cortical (d = −0.85, 95% CI = −1.31 < δ < −0.39) regions were lower in older as compared to younger adults (Young > Old). Effects within the parietal lobe were significantly larger than those within the frontal lobe (QB[1] = 4.49, p = 0.03). Effects in all other cortical regions were roughly equivalent. Effects within the cerebellum (d = 0.42, 95% CI = −0.07 < δ < 0.90) were in the opposite direction (Young < Old) and significantly differed from all other ROIs except the Brainstem (d = 0.28, CI = −0.31 < δ < 0.87). Pairwise comparisons across brain regions are shown in Supplemental Table 2. In addition, effect sizes are colored by ROI in Figures 1A–C to allow for comparison across studies within specific brain regions.
3.2.8. Echo Time.
Glutamate metabolites are best detected in the human brain using short TEs (Wijtenburg and Knight-Scott, 2011). TEs in the included studies ranged from 8ms to 139ms. Several studies used multiple TEs during acquisition, as such the effective TE was used for analysis. Analysis of TE indicated a significant age-related difference in glutamate metabolite (Z = 4.41, p < .001) in the expected direction—studies using shorter TEs resulted in larger age-related glutamate effect sizes (Figure 3C).
3.2.9. Sex.
Analysis of sex composition of the samples revealed a significant age-related sex difference in glutamate metabolite effect sizes (Z = 4.02, p < .001). Samples with a greater percentage of females exhibited smaller age effects (Figure 3D).
3.3. Sensitivity Analysis: Comparing Univariate and Multivariate Approaches
Several studies included in this meta-analysis reported 1HMRS effects from more than one brain region. Thus, it is possible that considering each effect size as an independent effect could bias the meta-analytic outcome. As such, we performed sensitivity analyses using mixed-effects multivariate models that account for dependency amongst effects derived from the same study (Cheung, 2019; Scott et al., 2015). We defined a two-level mixed-effects model, where Level 1 is represented by multiple effect sizes within studies, and Level 2 is represented by the different studies. This model examines the variability of effects sizes between studies (random factor) and the association between various explanatory variables (fixed factors) and effect sizes. To apply this model to meta-analytic data, we used the standardized mean effect sizes (Cohen’s d) and determined the sampling variance of each effect size. The model considers the Level 1 effect-size variances as fixed/known (as calculated). The fixed- and random-effects parameters and their variances and covariance are estimated via adaptive quadrature, a robust and flexible numeric integration approach that allows for heteroscedastic Level 1 variances (Rabe-Hesketh et al., 2004, 2005). Models were fit using the program ‘gllamm’ of Stata Version 13 (Grilli and Rampichini, 2006; Rabe-Hesketh et al., 2004; StataCorp, 2013).
The summary effects derived using multivariate analyses aligned with our univariate approach (See Supplemental Results). Most effect sizes and comparisons between moderators were similar. The only notable difference was that the effect sizes across brain tissue type (GM, WM, Mixed), which were considered homogenous in the univariate approach (p = 0.055), were significantly different using the multivariate approach (p<0.001). The direction of the individual comparisons was the same (GM>Mixed>WM). Thus, it appears that the univariate approach employed in this meta-analysis is robust to the inclusion of multiple effects from the same study and offers conservative effect size estimates.
3.4. Publication Bias
Investigations into potential small study publication biases for studies examining Glu and Glx revealed a symmetric funnel plot (Supplemental Figure 2) and non-significant Begg (p = 0.36) and Egger (p = 0.13) tests. To further examine biases, we estimated the number of potential outlier studies using the Duval and Tweedie (Duval and Tweedie, 2000) “trim and fill” method. This procedure indicated that one study could be considered an outlier and could be trimmed from analysis. This process generated an estimated effect size of d = −0.72 that was slightly larger than the original estimate. Lastly, calculation of a fail-safe N revealed that 1,037 “null” studies would need to be found and incorporated into the analysis to negate the observed effect. As such, these methods support the notion that the current meta-analytic data accurately represent the extant literature concerning glutamate across the lifespan.
4.0. Discussion
The present findings are in alignment with our hypothesis that aging results in significant alterations in glutamatergic neurometabolite concentrations. The meta-analytically derived summary effect size for Glu and Glx differences between younger and older individuals was substantial (d = −0.66), and most, though not all, studies reported at least a trend towards lower brain Glu/Glx in older adults. Of note, metabolite (Glu/Glx/Gln) studied, 1HMRS protocol, measuring ratio versus absolute concentration values, brain region, echo time, and sex were moderating factors that significantly influenced reported age-related effect sizes. In contrast, effect sizes across studies were robust to differences in MRI field strength, brain tissue type (GM/WM/mixed), and voxel type selection (single versus multi).
Overall, the present findings indicate that age-related changes in Glu neurometabolites result in nearly global lower levels of Glu and Glx. While effect sizes for Glu and Glx did not significantly differ, it is important to note that the effect size for Glu was, on average, 60% larger than that for Glx. Smaller effects for Glx are likely the result of the mixing of Glu and Gln due to spectral overlap in 1HMRS studies, particularly at lower MRI field strengths (Ramadan et al., 2013). Alterations in glutamate levels have vast implications for the aging brain. At the cellular level, age-related changes in the brain include reductions in neuron size, neuron density, dendritic arborization, and axonal length (Drachman, 2006), all of which may be related to, or account for, alterations in brain Glu. Finally, age-related reductions in Glu metabolites likely affect the balance of neural transmission, ultimately affecting cognitive functioning (e.g. learning and memory).
Interestingly, meta-analysis of Gln effects uncovered an opposite pattern: older adults consistently showed higher levels of Gln than younger individuals. The dissociation between Glu/Glx and Gln changes during aging provides insight into putative underlying biological mechanisms, given that Glu is primarily located within neurons whereas Gln localizes to glia cells. Glia—most notably astrocytes—undergo significant aging-associated changes that may influence synthesis or conversion of glutamine (Palmer and Ousman, 2018). Nonetheless, given the limited number of effects (k = 9) reported for Gln in aging in vivo, we were unable to perform a comprehensive meta-analysis of Gln. As such, additional studies are needed to elucidate the role of brain Gln in the aging process, and to assess cell-type related mechanisms. Future studies probing age-related changes should consider the magnitude and direction of expected changes in Glu, Glx and Gln when interpreting findings.
Methodological heterogeneity across studies accounted for a considerable amount of variation in Glu/Glx effect sizes. Quite importantly, 1HMRS acquisition protocol (i.e. sequence) and TE accounted for significant heterogeneity. Interestingly, studies utilizing more advanced MRS techniques, including SPECIAL, LASER-POCE, and EPSI sequences, reported more robust effects compared to those that used the more commonly employed PRESS or STEAM sequences. These advanced techniques attempt to combine the benefits of multiple ‘traditional’ MRS approaches (e.g. combining the benefits of STEAM-like TEs and full signal intensity of PRESS) in order to better optimize measurement of brain metabolites like Glu. It must be noted, however, that studies using these more advanced techniques were limited in number, thus these results should be considered preliminary. As expected, studies using shorter TEs reported larger effect sizes (Wijtenburg and Knight-Scott, 2011).
There are a multitude of factors that must be considered when choosing what 1HMRS acquisition scheme to employ, including (but not limited to) scanner and sequence availability, hardware design, B1 homogeneity, shimming capabilities, and the availability of short echo time sequences. Still, sequence parameters will affect the quantification of Glu metabolites in vivo (Ramadan et al., 2013), and thus must be considered in future efforts that aim to study Glu in aging. For example, PRESS may be selected over LASER as the large number of RF pulses used in LASER results in higher RF power requirement and longer TEs, neither of which are optimal for measuring brain Glu at 3T (Zhu and Barker, 2011). PRESS and STEAM sequences have been compared in detail and the most significant difference is that PRESS has twice the signal as STEAM (Zhu and Barker, 2011). STEAM sequences are often preferred when short TEs are required (e.g. at 7T), however, modified PRESS sequences can achieve short TEs of ~20ms, thus achieving TE close to that of STEAM while retaining more signal. Whereas the PRESS sequence does include chemical shift artifacts for Glu and NAA, these artifacts can be minimized by setting water acquisition spectrum excitation and refocusing pulses in resonance with the water peak at 4.7ppm. Nonetheless, overall, the ‘traditional’ sequences (e.g. PRESS, STEAM) still provided evidence of robust and significant age-related change in Glu metabolites.
More recently, studies have compared PRESS and MEGA-PRESS sequences for measuring glutamate metabolites (Glu, Glx, Gln). MEGA-PRESS studies are implemented to measure J-coupled metabolites, such as GABA, but are not traditionally use to report Glu, Glx, or Gln. Still, two recent studies (Maddock et al., 2018; van Veenendaal et al., 2018) directly compared PRESS and MEGA-PRESS for the measurement of Glu, Glx and Gln. Van Veenendaal et al. (2018) found only a small positive correlation between glutamate measures when using PRESS and MEGA-PRESS (r = 0.30), while Maddock et al. (2018) found the correlation between glutamate measured using these two types of sequences to be much larger (r = 0.88); notably both studies indicated that correlations were stronger for Glu as compared to Glx. There were significant methodological differences between these two studies (Maddock et al., 2018; van Veenendaal et al., 2018), including acquisition parameters (TR/TE), that could explain some of the discrepant results. Overall, while these studies suggest that MEGA-PRESS protocols provide comparable in vivo results as PRESS sequences for Glu and Glx, caution should be taken when directly comparing the results given the limited associations. Notably, we report larger age-related effect sizes for PRESS (d = −0.68) as compared to MEGA-PRESS (d = −0.02).
Other advanced techniques have not necessarily been directly compared to PRESS or STEAM in older samples. Notably, the use of whole brain EPSI techniques have emerged in recent years and comparability data in older samples is lacking. This technique offers unprecedented neurometabolic information as it generates 1HMRS spectra across the brain via one (~20 minute) acquisition, yielding metabolite concentrations comparable to those obtained from conventional SVS (Maghsudi et al., 2019). While standardization across the field is a challenge given the physical limitations at any given research site, we believe that the present data provide evidence in favor of more advanced approaches using shorter TEs when investigating age-related changes in brain Glu.
The majority of effects included in this meta-analysis reported absolute concentrations of metabolites; the remainder reported ratios (e.g. Glu/Creatine). Those utilizing absolute concentration produced significantly larger age-related effects than those that analyzed ratios. Although many clinical studies do indeed utilize metabolite ratios when comparing values within affected and unaffected brain tissue, techniques that quantify absolute concentrations are readily available and can be successfully applied, particularly at high field strength. Absolute concentrations may be particularly relevant to use in aging, as simultaneous decreases in Glu and creatine could occur—in this case, ratios would obscure neurobiologically-relevant decreases in Glu. Furthermore, relative quantification may introduce substantial errors, lead to erroneous metabolite values, and introduce misinterpretation of spectral data (Jansen et al., 2006). Hence, it is not surprising that most of the extant aging research has used absolute concentration values, or that these absolute effects were larger.
While the meta-analytically derived effect size was only nominally larger within gray matter than within white matter and mixed tissue, brain region analyzed varied by study and significantly affected the age-related difference in Glu metabolites observed. Regional effects were largest within the parietal lobe, followed by the subcortex and temporal, occipital, and frontal lobes. Notably, only effects within the cerebellum indicated significantly larger amounts of Glu/Glx with age, but this should be interpreted with caution as there were only two reported effects from this ROI. The regional pattern of aging-associated Glu/Glx changes observed in this meta-analysis align to some degree with reported patterns of aging-associated brain volume change (Mueller et al., 2007; Resnick et al., 2000) and aging- and disease-related change in the neuronal marker N-acetyl aspartate (NAA, a putative marker of neuronal integrity) (Angelie et al., 2001; Schuff et al., 2002). Alterations in brain Glu thus appear to overlap anatomically with decreases in brain volume. It is of interest for future studies to delineate whether age-related changes in brain glutamate precede, or are a product of, changes in regional brain volume. Longitudinal studies are particularly apposite for resolving this question convincingly. Of note, ROI selection itself was very heterogeneous across the included studies, which makes synthesis of these data challenging. Consequently, it is difficult to make conclusions about the degree of spatial specificity of glutamatergic deficits in aging (though currently, effects seem largely diffuse), or to discern interrelationships among Glu metabolite levels across diverse brain regions.
Higher MRI field strength is known to increase the spectral resolution of 1HMRS, especially for glutamate and glutamine (Moser et al., 2012). Greater MRI field strength was associated with larger effect sizes at lower field strengths (1.5>3.0T>4.0T), but results from 7T did not follow this pattern. 1HMRS studies at 3T were by far the most numerous, and these did evince nominally larger age-related effects than 1.5T studies. Effect sizes for 4T studies were additionally larger than for 3T studies, but effect sizes reported for the one study at 7T were the smallest. This relatively small (n = 33) sole 7T study measured Glu and Gln from the posterior cingulate cortex and the occipital cortex. Effect sizes for Glu were larger than for Gln, and the Glu effect size within the occipital lobe was similar to those observed in other studies at lower field strength. Clearly, it is inappropriate to make definitive conclusions about findings at 7T based upon a few effects from one study. Other studies have, however, reported improved estimates of Glu metabolites at 7T using 1HMRS methods (Mekle et al., 2009), thus data collection at higher field strengths appears promising for improving our understanding of age-related Glu metabolite change. Still, 3T MRIs are the most widely available, and are capable of identifying robust age-related effects in Glu metabolites. It will be interesting to compare results from the present analysis to those obtained by other novel imaging methods than can specifically measure brain glutamate, such as Glutamate Chemical Exchanges Saturation Transfer (GluCEST), which can only be employed at 7T (Cai et al., 2012; Davis et al., 2015; Nanga et al., 2018; Roalf et al., 2017), but offers highly sensitive measures of brain Glu independent of Gln.
Finally, sex composition of the study moderated the overall effect, with a larger percentage of females in the study sample resulting in a smaller age-related glutamate effect size. The majority of effects (k = 31, average Cohen’s d = −0.40) were from studies with well-balanced samples (50–57% female) and only a handful (k = 9; average Cohen’s d = −0.62) were from studies (k = 9) that include a greater number of males than females. This meta-analytic result may indicate that there are sex differences in the aging processes that affect brain Glu, further suggesting that sex should be considered a relevant biological factor in future studies. Continued examination of sex effects will help to clarify the impact of sex on brain aging as well as reconcile inconsistencies between studies reporting sex differences in Glu metabolites in young adults (O’Gorman et al., 2011) and across the lifespan (Hädel et al., 2013; Marjańska et al., 2017; Sailasuta et al., 2008; Zahr et al., 2013), versus those finding no sex differences (Ding et al., 2016; Kaiser et al., 2005; Raininko and Mattsson, 2010). Yet, the predominance of studies reviewed here did not directly measure sex effects (Boumezbeur et al., 2010; Chang et al., 2009; Kumar et al., 2018; Mooney et al., 2017; Schubert et al., 2004; Suri et al., 2017; Zahr et al., 2008).
4.1. Limitations and Future Directions
The present meta-analysis should be considered in context with its limitations. First, this field is still relatively small, and this meta-analysis includes only 13 studies reporting effects of Glu-related metabolites in healthy aging. Larger studies of glutamate in aging populations are needed.
As noted above there is little consistency in the acquisition of 1HMRS data, and, unsurprisingly, some methodological choices affect the measurement of brain Glu. Variability across the methodological space may have made the present synthesis across studies less precise. Findings under certain methodological conditions (e.g. 7T MRI) should be considered tentative and future studies are warranted.
Moderator and meta-regression analyses were performed only on those studies where relevant data was reported. Many studies did not report other data that may be relevant to the Glu changes observed in the meta-analysis, including medication status, cognitive function, or general clinical symptoms. Amazingly, only three studies measured the relationship between brain Glu and aging-related cognitive changes (Simmonite et al., 2019; Zahr et al., 2008; Zahr et al., 2013). All three studies suggest that lower Glu is associated with poorer cognitive performance in older adults, but again these samples are small. As such, future studies should explore age-related Glu change in relation to clinical symptoms and cognitive performance. We echo previous calls (Haga et al., 2009) for large-scale studies of middle aged and older adults to provide more robust data on age-related Glu changes. This would allow a more nuanced view of brain glutamate changes in healthy aging, enable better understanding of how non-linear age-related trajectories of structural brain change (Vinke et al., 2018) are associated with glutamate, and offer more insight into disease-related changes. It is our contention that researchers should continue to investigate Glu metabolite change in aging and neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease, while using consistent and comparable methodologies and reporting sufficient phenotypic data. This will allow for study outcomes to be better synthesized in the future.
In conclusion, the present meta-analytic review is the first to quantitatively synthesize data on glutamatergic metabolite change in the aging brain. The results of this meta-analysis demonstrate clear alterations in glutamate metabolite levels (Glu/Glx) in older individuals. Future studies examining glutamate-related metabolite concentrations in conditions such as mild cognitive impairment are warranted; this has been an area of limited investigation despite initial work suggesting that reduced glutamate may be a biomarker of aging-related cognitive decline (Zeydan et al., 2017). Investigations linking age-related glutamate change to clinical and cognitive changes, and studies of the underlying genetic and neurobiological mechanisms contributing to this age-related decline, will improve our understanding of the aging brain.
Supplementary Material
Meta-analysis demonstrates reductions in glutamate metabolite levels in older adults.
Methodological heterogeneity across study accounts for sizable variation of effect size.
Brain glutamate may serve as a biomarker for delineating typical and atypical aging
Funding Sources:
This work was supported by the National Institute of Mental Health grants R01MH119185 to DRR & PJM and R01MH120174 to DRR. Support for Dr. J. Cobb Scott was from VA Office of Research & Development I01RX002699 and the Life Span Brain Institute (LiBI)—a collaboration between the University of Pennsylvania School of Medicine and Children’s Hospital of Philadelphia. This work was also supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (DRR), and NBIB under Grant No. P41 EB015893 (RR).
The funding sources were not directly involved in study design, collection, data analysis or interpretation, nor manuscript writing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures/Conflicts of Interest:
David R. Roalf, PhD reports no potential conflicts of interest or financial disclosures related to this work.
Valerie J. Sydnor, BA reports no potential conflicts of interest or financial disclosures related to this work.
Madison Woods reports no potential conflicts of interest or financial disclosures related to this work.
David Wolk, MD reports no potential conflicts of interest or financial disclosures related to this work.
J. Cobb Scott, PhD reports no potential conflicts of interest or financial disclosures related to this work.
Ravinder Reddy reports no potential conflicts of interest or financial disclosures related to this work.
Paul J. Moberg reports no potential conflicts of interest or financial disclosures related to this work.
References
- Angelie E, Bonmartin A, Boudraa A, Gonnaud P-M, Mallet J-J, Sappey-Marinier D, 2001. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. American Journal of Neuroradiology 22(1), 119–127. [PMC free article] [PubMed] [Google Scholar]
- Barker PB, Hearshen DO, Boska MD, 2001. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn. Reson. Med 45(5), 765–769. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M, 1994. Operating characteristics of a rank correlation test for publication bias. Biometrics, 1088–1101. [PubMed] [Google Scholar]
- Boumezbeur F, Mason GF, De Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF, 2010. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab 30(1), 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R, 2012. Magnetic resonance imaging of glutamate. Nat. Med 18(2), 302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ, 1996. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci 58(22), 2049–2056. [DOI] [PubMed] [Google Scholar]
- Chang L, Jiang CS, Ernst T, 2009. Effects of age and sex on brain glutamate and other metabolites. Magn. Reson. Imaging 27(1), 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MW-L, 2019. A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychol. Rev, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland C, Pipingas A, Scholey A, White D, 2019. Neurochemical changes in the aging brain: A systematic review. Neurosci. Biobehav. Rev [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences, 2nd ed Erlbaum, Hillsdale, New Jersey. [Google Scholar]
- Cotman CW, Monaghan DT, 1988. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu. Rev. Neurosci 11(1), 61–80. [DOI] [PubMed] [Google Scholar]
- Coyle JT, 2006. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell. Mol. Neurobiol 26(4), 363–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Grady CL, 2002. Aging, memory, and frontal lobe functioning
- Curtis DR, Johnston GA, 1974. Amino acid transmitters in the mammalian central nervous system, Ergebnisse der Physiologie Reviews of Physiology, Volume 69 Springer, pp. 97–188. [DOI] [PubMed] [Google Scholar]
- Davis KA, Nanga RPR, Das S, Chen SH, Hadar PN, Pollard JR, Lucas TH, Shinohara RT, Litt B, Hariharan H, 2015. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci. Transl. Med 7(309), 309ra161–309ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster FN, 1992. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Dev. Rev 12(1), 45–75. [Google Scholar]
- Ding X-Q, Maudsley AA, Sabati M, Sheriff S, Schmitz B, Schütze M, Bronzlik P, Kahl KG, Lanfermann H, 2016. Physiological neuronal decline in healthy aging human brain—An in vivo study with MRI and short echo-time whole-brain 1H MR spectroscopic imaging. Neuroimage 137, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA, 2006. Aging of the brain, entropy, and Alzheimer disease. Neurology 67(8), 1340–1352. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R, 2000. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2), 455–463. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M, Zaleska MM, Nelson D, Nissim I, Yudkoff M, 1990. Neuronal glutamine utilization: glutamine/glutamate homeostasis in synaptosomes. J. Neurochem 54(6), 2057–2069. [DOI] [PubMed] [Google Scholar]
- Grilli L, Rampichini C, 2006. A review of random effects modelling using gllamm in Stata Department of Statistics, University of Florence. [Google Scholar]
- Hädel S, Wirth C, Rapp M, Gallinat J, Schubert F, 2013. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J. Magn. Reson. Imaging 38(6), 1480–1487. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM, 2009. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol. Aging 30(3), 353–363. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I, 1985. Statistical methods for meta-analysis Academic press. [Google Scholar]
- Huberfeld G, de La Prida LM, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R, 2011. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat. Neurosci 14(5), 627. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Lewohl JM, Scott HL, Dodd PR, 2003. Biochemical and molecular studies using human autopsy brain tissue. J. Neurochem 85(3), 543–562. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME, 2006. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology 240(2), 318–332. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW, 2005. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol. Aging 26(5), 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Liddle EB, Fernandes CC, Palaniyappan L, Hall EL, Robson SE, Simmonite M, Fiesal J, Katshu MZ, Qureshi A, 2018. Glutathione and glutamate in schizophrenia: a 7 T MRS study. Mol. Psychiatry, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Caton MD, Ragland JD, 2018. Estimating glutamate and Glx from GABA-optimized MEGA-PRESS: Off-resonance but not difference spectra values correspond to PRESS values. Psychiatry Research: Neuroimaging 279, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader I, Seeger U, Karitzky J, Erb M, Schick F, Klose U, 2002. Proton magnetic resonance spectroscopy with metabolite nulling reveals regional differences of macromolecules in normal human brain. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 16(5), 538–546. [DOI] [PubMed] [Google Scholar]
- Maghsudi H, Schmitz B, Maudsley AA, Sheriff S, Bronzlik P, Schütze M, Lanfermann H, Ding X, 2019. Regional metabolite concentrations in aging human brain: Comparison of short-TE whole brain MR spectroscopic imaging and single voxel spectroscopy at 3T. Clin. Neuroradiol, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjańska M, McCarten JR, Hodges J, Hemmy LS, Grant A, Deelchand DK, Terpstra M, 2017. Region-specific aging of the human brain as evidenced by neurochemical profiles measured noninvasively in the posterior cingulate cortex and the occipital lobe using 1H magnetic resonance spectroscopy at 7 T. Neuroscience 354, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Jacobson L, Pollard K, 2015. Population Bulletin: Aging in the United States, Population Reference Bureau; Washington, D.C. [Google Scholar]
- Mayr U, 2001. Age differences in the selection of mental sets: the role of inhibition, stimulus ambiguity, and response-set overlap. Psychol. Aging 16(1), 96. [DOI] [PubMed] [Google Scholar]
- McEntee WJ, Crook TH, 1993. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl.) 111(4), 391–401. [DOI] [PubMed] [Google Scholar]
- Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R, 2009. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 61(6), 1279–1285. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Cirillo J, Byblow WD, 2017. GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J. Neurophysiol 118(1), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Stahlberg F, Ladd ME, Trattnig S, 2012. 7-T MR--from research to clinical applications? NMR Biomed 25(5), 695–716. [DOI] [PubMed] [Google Scholar]
- Mueller S, Stables L, Du A, Schuff N, Truran D, Cashdollar N, Weiner M, 2007. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4 T. Neurobiol. Aging 28(5), 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanga RPR, DeBrosse C, Kumar D, Roalf D, McGeehan B, D’Aquilla K, Borthakur A, Hariharan H, Reddy D, Elliott M, 2018. Reproducibility of 2D GluCEST in healthy human volunteers at 7 T. Magn. Reson. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E, 2011. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J. Magn. Reson. Imaging 33(5), 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AL, Ousman SS, 2018. Astrocytes and aging. Front. Aging Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A, Pickles A, 2004. GLLAMM manual
- Rabe-Hesketh S, Skrondal A, Pickles A, 2005. Maximum likelihood estimation of limited and discrete dependent variable models with nested random effects. Journal of Econometrics 128(2), 301–323. [Google Scholar]
- Raininko R, Mattsson P, 2010. Metabolite concentrations in supraventricular white matter from teenage to early old age: a short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta Radiol 51(3), 309–315. [DOI] [PubMed] [Google Scholar]
- Ramadan S, Lin A, Stanwell P, 2013. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed 26(12), 1630–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB, 2000. One-year age changes in MRI brain volumes in older adults. Cereb. Cortex 10(5), 464–472. [DOI] [PubMed] [Google Scholar]
- Roalf D, Nanga R, Rupert P, Hariharan H, Quarmley M, Calkins M, Dress E, Prabhakaran K, Elliott M, Moberg P, 2017. Glutamate imaging (GluCEST) reveals lower brain GluCEST contrast in patients on the psychosis spectrum. Mol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER, 2006. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol. Sci 27(3), 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, 1979. The file drawer problem and tolerance for null results. Psychol. Bull 86(3), 638. [Google Scholar]
- Sailasuta N, Ernst T, Chang L, 2008. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn. Reson. Imaging 26(5), 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, Rinneberg H, 2004. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 21(4), 1762–1771. [DOI] [PubMed] [Google Scholar]
- Schuff N, Capizzano A, Du A, Amend D, O’neill J, Norman D, Kramer J, Jagust W, Miller B, Wolkowitz O, 2002. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology 58(6), 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, Schweinsburg BC, 2015. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull 141(1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite M, Carp J, Foerster BR, Ossher L, Petrou M, Weissman DH, Polk TA, 2019. Age-related declines in occipital GABA are associated with reduced fluid processing ability. Acad. Radiol 26(8), 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D, Law M, 2009. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin. Radiol 64(1), 12–21. [DOI] [PubMed] [Google Scholar]
- StataCorp L, 2013. Stata Statistical Software: Release 13 College Station Texas. [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15), 2008–2012. [DOI] [PubMed] [Google Scholar]
- Suri S, Emir U, Stagg CJ, Near J, Mekle R, Schubert F, Zsoldos E, Mahmood A, Singh-Manoux A, Kivimäki M, 2017. Effect of age and the APOE gene on metabolite concentrations in the posterior cingulate cortex. Neuroimage 152, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisell A, Leinhard OD, Warntjes JBM, Lundberg P, 2013. Procedure for quantitative 1H magnetic resonance spectroscopy and tissue characterization of human brain tissue based on the use of quantitative magnetic resonance imaging. Magn. Reson. Med 70(4), 905–915. [DOI] [PubMed] [Google Scholar]
- Tunc-Skarka N, Meier S, Demirakca T, Sack M, Weber-Fahr W, Brusniak W, Wolf I, Matthäus F, Schulze TG, Diener C, 2014. Effects of normal aging and SCN1A risk-gene expression on brain metabolites: evidence for an association between SCN1A and myo-inositol. NMR Biomed 27(2), 228–234. [DOI] [PubMed] [Google Scholar]
- van Veenendaal TM, Backes WH, van Bussel FC, Edden RA, Puts NA, Aldenkamp AP, Jansen JF, 2018. Glutamate quantification by PRESS or MEGA-PRESS: Validation, repeatability, and concordance. Magn. Reson. Imaging 48, 107–114. [DOI] [PubMed] [Google Scholar]
- Vinke EJ, De Groot M, Venkatraghavan V, Klein S, Niessen WJ, Ikram MA, Vernooij MW, 2018. Trajectories of imaging markers in brain aging: the Rotterdam Study. Neurobiol. Aging 71, 32–40. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, Cork LC, 1991. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol. Aging 12(2), 93–98. [DOI] [PubMed] [Google Scholar]
- Wijtenburg SA, Knight-Scott J, 2011. Very short echo time improves the precision of glutamate detection at 3T in 1H magnetic resonance spectroscopy. J. Magn. Reson. Imaging 34(3), 645–652. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV, 2008. Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cereb. Cortex 18(10), 2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Chanraud S, Gu M, Sullivan EV, Pfefferbaum A, 2013. In vivo glutamate measured with magnetic resonance spectroscopy: behavioral correlates in aging. Neurobiol. Aging 34(4), 1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Craik FI, Booth L, 2004. Executive function across the life span. Acta Psychol. (Amst.) 115(2–3), 167–183. [DOI] [PubMed] [Google Scholar]
- Zeydan B, Deelchand DK, Tosakulwong N, Lesnick TG, Kantarci OH, Machulda MM, Knopman DS, Lowe VJ, Jack CR Jr, Petersen RC, 2017. Decreased glutamate levels in patients with amnestic mild cognitive impairment: an sLASER proton MR spectroscopy and PiB-PET study. J. Neuroimaging 27(6), 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Barker PB, 2011. MR spectroscopy and spectroscopic imaging of the brain, Magnetic resonance neuroimaging Springer, pp. 203–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


