Abstract
Objective:
Mechanical ventilation of patients with ARDS should balance lung and diaphragm protective principles, which may be difficult to achieve in routine clinical practice. Through a Phase I clinical trial, we sought to determine whether a computerized decision support (CDS) based protocol (Real-time Effort Driven ventilator management (REDvent)) is feasible to implement, results in improved acceptance for lung and diaphragm protective ventilation and improves clinical outcomes over historical controls.
Design:
Interventional non-blinded pilot study
Setting:
Pediatric Intensive Care Unit
Patients:
Mechanically ventilated children with ARDS
Interventions:
A CDS tool was tested which prioritized lung protective management of ΔP (PIP-PEEP), PEEP/FiO2, and ventilator rate. Esophageal manometry was used to maintain patient effort in a physiologic range. Protocol acceptance was reported, and enrolled patients were matched 4:1 with respect to age, initial OI, and percentage of immune compromise to historical control patients for outcome analysis.
Measurements and Main Results:
Thirty-two patients were included. Acceptance of protocol recommendations was over 75%. 128 matched historical controls were used for analysis. Compared to historical controls, patients treated with REDvent received lower ΔP and VT, and higher PEEP when FiO2 was > 0.60. REDvent was associated with 6 more ventilator free days, shorter duration until the first spontaneous breathing trial and 3 fewer days on MV amongst survivors (all p = < 0.05) in comparison to historical controls, while maintaining no difference in the rate of reintubation.
Conclusions:
A CDS based protocol prioritizing lung protective ventilation balanced with reduction of controlled ventilation to maintain physiologic levels of patient effort can be implemented and may be associated with shorter duration of ventilation.
Keywords: Mechanical Ventilation, Computerized Decision Support, Clinical Decision Support, Critical Care, ARDS
Introduction
Mechanical ventilation of adults and children with acute respiratory failure necessitates balancing lung and diaphragm protective ventilation. This includes application of Positive End Expiratory Pressure (PEEP) to prevent atelectrauma, limiting tidal volume and driving pressure to prevent over-distension, and use of permissive hypercapnia. However, when this results in sub-physiologic levels of patient effort, ventilator induced diaphragm dysfunction (VIDD) is common. [1–10] VIDD is associated with longer length of ventilation, higher rates of weaning and extubation failure, and higher post-ICU morbidity and mortality. [8] The goal is to achieve lung protective levels of PEEP, driving pressure, and tidal volume while still promoting physiologic levels of patient effort of breathing.
However, implementing such a strategy at the bedside is challenging. Computerized decision support (CDS) offers advantages in circumstances where complex decisions need to be made to weigh potentially competing risks, depending on the physiologic state of the patient. We have developed such an approach using a CDS tool which promotes implementation of lung protective ventilation protocols for PEEP, Fraction of Inspired Oxygen (FiO2), tidal volume, inspiratory pressure and ventilator rate coupled with a target of maintaining patient effort of breathing in a physiologic range (as measured by pressure-rate product from esophageal manometry) whenever spontaneous breathing is permitted. This decision support tool is called Real-time Effort Driven ventilator management (REDvent).
Before embarking on a randomized controlled trial (RCT) focused on clinical outcomes, we performed a Phase I, intervention only, pilot study in children with pediatric Acute Respiratory Distress Syndrome (PARDS). The primary goal of this pilot study was to evaluate if the protocol could achieve a high rate of adherence. In addition we sought to evaluate if there would be separation in ventilator settings between intervention patients and historical controls, and compare clinical outcomes between intervention and historical control patients to help guide sample size estimates for an RCT.
Materials and Methods
We screened intubated children admitted to the pediatric intensive care unit at Children’s Hospital Los Angeles (CHLA) between June 2015 and October 2017. Screening and patient enrollment did not occur on weekends, unless a study investigator was already present in the ICU. Inclusion criteria were an anticipated length of intubation > 48 hours and Pediatric Acute Lung Injury Consensus Conference (PALICC) defined Pediatric Acute Respiratory Distress Syndrome: Oxygen Saturation index (100 × MAP × FiO2/SpO2) >= 5 or Oxygenation Index (OI= 100 × MAP × FiO2/PaO2) >=4, evidence of pulmonary parenchymal disease on chest imaging, and an ARDS trigger. [11] Patients with left ventricular dysfunction could be included if hypoxemia was not entirely explained by heart failure. Patients were excluded if they had a corrected gestation age < 37 weeks, contraindications to nasoesophageal catheter placement (nasopharyngeal or esophageal abnormalities), had significant lower airway obstruction, contraindications to permissive hypercapnia (elevated intracranial pressure, severe pulmonary hypertension, etc.), or required extracorporeal life support. The protocol was registered with clinicaltrials.gov (NCT02989246), approved by the CHLA Institutional Review Board, and informed consent was obtained from each child’s parent or guardian.
Study Protocol
Details of the study protocol are summarized in Figure 1. After consent was obtained, an age-appropriate esophageal balloon catheter was placed and ventilator settings were adjusted at least every 4 hours by a bedside respiratory therapist during a clinical assessment using a CDS tool which was accessible from a tablet or laptop computer at the patient’s bedside. When performing an assessment with the CDS, physiologic and ventilator data could be entered either manually or pulled from the patient monitor as median values from the previous 15 minutes. Range checks were implemented on all fields to identify typographical errors. Esophageal pressure was displayed on a laptop computer, using custom hardware platforms (New Life Box, Applied Biosignals, Weener, Germany and Bicore II, Carefusion, Yorba Linda, CA) and custom software module (Polybench, Applied Biosignals), as previously described. [12–14] Just-in-time training was given to the bedside nurse, respiratory therapist, and physicians. The intervention protocol was divided into acute and weaning phases. The acute phase was defined as the time of consent until the first spontaneous breathing trial. The weaning phase was from the time of first spontaneous breathing trial until extubation.
Figure 1:
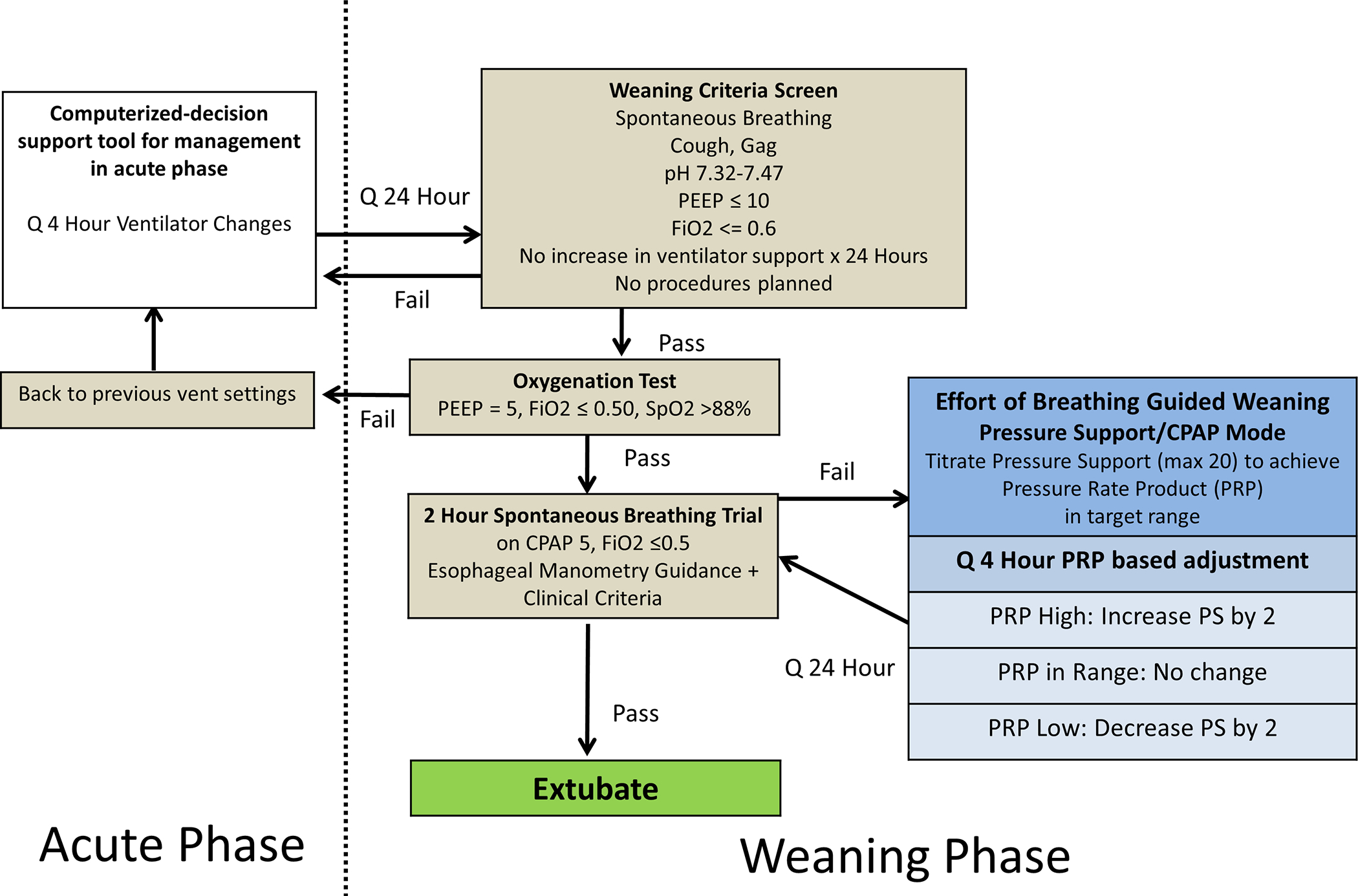
Flow chart for study protocol. Acute and weaning phase are separated by the vertical dotted line. Effort of breathing (EOB) guided weaning while on pressure support (PS) ventilation occurs after failing the first spontaneous breathing trial (SBT).
Acute Phase Intervention:
All patients were managed in Synchronized Intermittent Mandatory Ventilation Pressure Control plus Pressure Support (SIMV-PC/PS), or High Frequency Oscillatory Ventilation (HFOV). The decision to switch between HFOV and SIMV PC/PS was left to the bedside clinician. At the time of each evaluation, the esophageal balloon was inflated to a pre-specified amount (which was determined daily for each patient by research personnel using previously published algorithms [15]), and if the patient was breathing spontaneously, then the pressure-rate product (PRP, peak-to-trough change in esophageal pressure times respiratory rate) was calculated over a median of 10 breaths. PRP is a well-accepted method to measure effort of breathing in children, is easy to calculate using only the esophageal pressure waveform, and is associated with clinical outcomes. [13,14,16–19]
Clinical data including blood gas values, ventilator settings, physiologic monitors (SpO2, End-tidal CO2) were extracted automatically and verified by the bedside respiratory therapist, or entered manually into the CDS tool. The CDS tool then made recommendations to adjust ΔP, ventilator rate, PEEP, FiO2. These recommendations sought to balance lung and diaphragm protective ventilation by managing PEEP/FiO2 using the ARDSNet low-PEEP/FiO2 table, minimizing ΔP and promoting permissive hypercapnia when spontaneous effort was low, and increasing ΔP or rate if effort was very high and outside of a defined range. A PRP of 200–400 was deemed the target physiologic range, based on previous work from our group. [13] Specific details of the rules underlying the CDS tool are provided in the appendix. If the respiratory therapist agreed with the recommendations, they were implemented. If they were unsure or disagreed, they would discuss the recommendations with the most senior critical care physician available who would ultimately decide to accept or reject them. Once daily the patient was assessed for weaning phase criteria (stable ventilator support for 24 hours, spontaneous breathing, PEEP ≤ 10 cmH2O, FiO2 ≤ 60%) and if they met criteria they had an oxygenation test where the PEEP was reduced to 5 cmH2O and FiO2 <= 0.5. If the patient maintained SpO2 > 88% on these settings for at least 30 minutes, the patient moved on to receive a standardized airway occlusion maneuver (negative inspiratory force [NIF]) for 5–10 breaths, measuring the maximal change in airway pressure (aPiMax) to assess respiratory capacity, which has been described as a risk factor for extubation failure. [14] Next we performed a spontaneous breathing trial (SBT) on CPAP of 5 cmH2O (without pressure support) for a goal of 2 hours. If they failed the oxygenation test, they went back to previous ventilator settings, and the acute phase continued for another 24 hours.
During the two hour SBT, patients were continuously monitored at the bedside by research personnel for failure criteria including SpO2 < 90% with FiO2 > 0.5, end tidal PCO2 increase of 10 mmHg from baseline, HR increase of 40 bpm from baseline, Rapid Shallow Breathing Index (RSBI/kg (>= 12)), moderate or severe retractions, or pressure-rate product (PRP) > 500. If any failure criteria were met and sustained over one minute, the bedside clinician was alerted of failure and the clinician determined whether to end the SBT, or extubate the patient. If the patient failed the SBT and was not extubated, he/she was moved to the weaning phase (see below). If no failure criteria were met at the end of the 2 hour SBT, the CDS tool recommended extubation. If the patient was not extubated within 6 hours of passing the SBT, he/she was also moved to the weaning phase.
Weaning Phase
During the weaning phase, the patient was managed in a pressure support/CPAP mode of ventilation. Here the CDS tool continued to make recommendations at least every 4 hours to adjust the level of pressure support to maintain the pressure rate product (PRP) in a physiologic range of 200–400. PEEP could be adjusted between 5–10 cmH2O, as per the clinical team. A daily SBT was performed until extubation or 28 days. All interventions stopped at extubation, death, or 28 days, whichever came first.
Research Questions and Outcomes
Our primary objective was to determine the feasibility of achieving a high level of protocol adherence (target > 75% acceptance) for all recommendations. This 75% target was selected based on previous literature, and was felt to be the minimum necessary adherence to understand the true performance of the protocol. [20–23] Each individual oxygenation, ventilation, and weaning phase recommendation could be accepted or rejected by the bedside provider, and the reason for rejection was collected in the CDS tool. We calculated the percentage of acceptance and stratified this by the acute phase (oxygenation and ventilation recommendations) and the weaning phase recommendations, and reviewed all the reasons for rejection periodically. After the first 20 patients were enrolled, we considered changes to the protocol to improve adherence. A run chart evaluating per patient and cumulative adherence as a function of patients enrolled was used to understand how iterative changes to the protocol improved adherence.
We also monitored ventilation parameters associated with lung protective ventilation. We evaluated ΔP (PIP-PEEP), Tidal Volume, PEEP as a function of FiO2, and CO2 levels over the first 7 days of mechanical ventilation for REDvent patients, compared to matched historical controls. Each REDvent study patient was randomly matched to four historical controls from a previously published dataset which included children with pediatric ARDS admitted to our ICU between 2009 and 2012. Patients were matched based on age category (< 1 year, 1–6 years, 6–12 years, 12–18 years), initial oxygenation index, and whether or not he/she was immunocompromised. Differences between groups were evaluated with a Mann-Whitney U test for continuous data, and a Chi-squared test for categorical data. Data collection for mechanical ventilation parameters for both intervention and historical control patients began at intubation or PICU admission (Time 0 for reference), whichever came later (pre-ICU ventilator settings were not used).
In addition, we compared clinical outcomes between REDvent and historical controls, to determine the potential magnitude of the treatment effect to assist with sample size estimates for a Phase II trial. Outcomes evaluated included ICU mortality, time until the first SBT was attempted (defined as first time Pressure Support was ≤ 10 cmH2O with PEEP ≤ 6 cmH2O), 28-day ventilator free days (death = 0), length of mechanical ventilation among survivors, and re-intubation within 48 hours. Significance was assessed with t-tests, Mann-Whitney U tests, or Chi-squared tests. Kaplan-Meier survival analysis was performed to evaluate the difference in length of ventilation and the time until the first SBT between REDvent and historical control patients.
Results
Forty-four patients were approached for consent for this study, and thirty-two patients agreed to participate in this Phase I intervention pilot study. The most common reason families chose not to participate was related to placement of the esophageal catheter. Median age was 8 years (IQR 2.8, 12.5), and initial Oxygenation Index (OI) was 21.6 (12.7, 29.7). Seven children died, 41% were immunocompromised.
Primary Objective: Protocol Adherence
Cumulatively over the duration of the study, 2179/2853 (76%) protocol recommendations were followed by bedside providers. In the acute phase, 893/1193 (75%) oxygenation recommendations (PEEP and FiO2) and 894/1193(75%) ventilation recommendations (ΔP and ventilator rate) were accepted. During the weaning phase 392/467 (84%) recommendations (pressure support) were accepted.
Based on periodic evaluation of protocol adherence after the first 20 patients, four main adjustments to the protocol were made: (1) migration of the protocol from Microsoft Access to a web-based platform; (2) allowing 3 ranges for effort of breathing based titration (including a middle physiologic target range in which no change was made) instead of 2 ranges which either always increased or decreased support; (3) lowering the upper ventilator rate limit from 35 to 25 for patients who were found to have evidence of air trapping after enrollment; and (4) adding rules for weaning inhaled Nitric Oxide when it was used by the clinical team. [23] Overall adherence and per-patient adherence improved over time and with iterative adjustments (Figure 2). Before migration to the web-based platform overall adherence was 72%, and the three most common reasons for rejecting recommendations were: pulmonary hypertension concerns when reducing oxygenation parameters, 13%; high work of breathing concerns when reducing ventilation parameters, 10%; desaturation concerns when reducing oxygenation or ventilation parameters, 7%. After final adjustments to the protocol and implementation of the inhaled Nitric Oxide rules overall adherence increased to 92%, and the three most common reasons for rejecting recommendations changed to: (1) plans to attempt a new type of therapy, 13%; (2) patient not tolerating a similar adjustment in the past 6 hours, 13%; (3) concerns that PCO2 is too high when adjusting ventilation parameters, 13%. The most common recommendation type was a reduction in ventilator settings (48% of all recommendations), with adherence increasing from 67% to 89% between the two time periods. Maintaining current ventilator settings was a less common recommendation type (36% of all recommendations), and adherence increased from 87% to 99%. The least common recommendation type was increasing ventilator support (16% of all recommendations), and adherence also increased from 50% to 87%.
Figure 2:
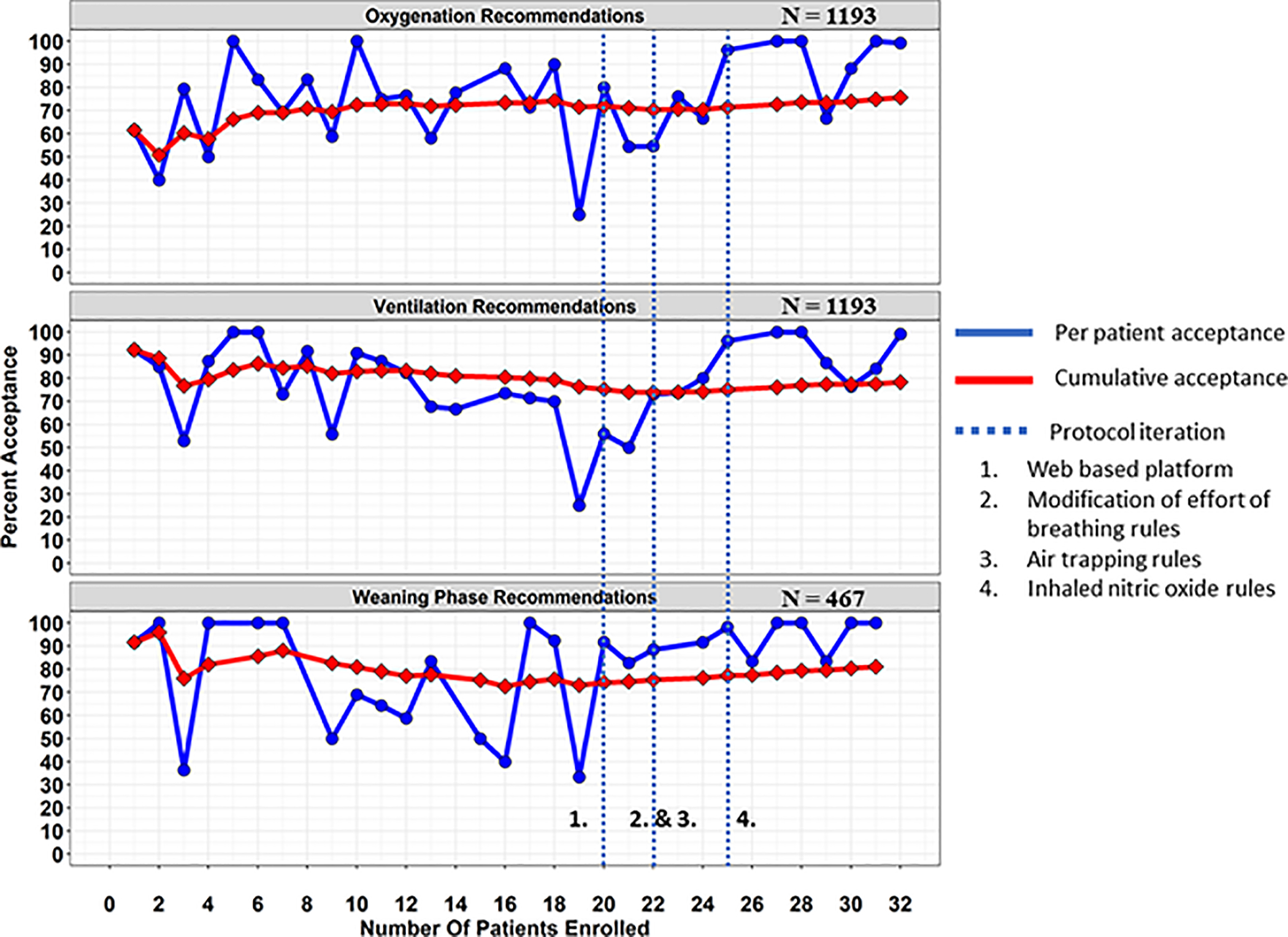
Run chart for REDvent CDS showing the per patient percent acceptance (blue) and cumulative percent acceptance (red) over the course of the pilot study. Iterative adjustments were made based on clinician feed-back throughout and shown as the vertical blue dashed-lines with corresponding numbers: (1) porting the CDS from an Microsoft Access to a web-based version (2) modification of the rules that incorporate effort of breathing (3) adjustments to the upper limit of respiratory rate when air trapping is present (4) incorporating adjustments for inhaled nitric oxide when a clinician decides to use it. Near the end of the study, per patient acceptance was above 90%.
Ventilation Parameters
REDvent patients were similar to matched historical control patients with respect to age, weight, gender, initial oxygenation index, and immune suppressed status (Table 1). The REDvent intervention started a median of 26 [17, 36] hours after Time 0 (intubation or PICU admission, whichever came later). There were no significant differences in PCO2, ΔP, or Peak Inspiratory Pressure on study day 0, although tidal volume was lower (p=0.003) (Figure 3). By study day 1, 25/32 (78%) of REDvent patients were initiated on the protocol. Median ΔP was statistically significantly lower for REDvent patients compared to historical control patients on day 1 of MV (p=0.025), with similar trends from day 1–4 of mechanical ventilation. Tidal volume was significantly lower in REDvent patients compared to historical control patients from days 0–4 of MV (p < 0.05). In addition, when looking at all combinations of PEEP and FiO2 over the entire course of mechanical ventilation, REDvent patients (n=8,290 PEEP/FiO2 combinations) had PEEP escalated, as FiO2 was increased, with more PEEP used in the REDvent group compared to historical controls (n=2007 PEEP/FiO2 combinations) particularly when FiO2 was > 0.6. (Figure 4).
Table 1:
Demographics and outcomes for REDvent patients and matched historical controls. Median (IQR) and P value from Mann-Whitney-U test for continuous data. n (%) and P value reported from Chi-squared test for categorical data.
| Variable | REDvent (N = 32) | Control (N = 128) | P |
|---|---|---|---|
| Age (mo) | 100.7 (33.7,150.4) | 70.3 (13.3,158) | 0.45 |
| Weight (kg) | 34 (9.9,50) | 17.3 (9.3,41) | 0.2 |
| Male | 19 (59.4%) | 67 (52.3%) | 0.6 |
| Initial OI | 21.6 (12.7,29.7) | 18.1 (10.7,27.7) | 0.54 |
| Immunosuppressed | 13 (40.6%) | 48 (37.5%) | 0.9 |
| Mortality | 7 (21.9%) | 37 (28.9%) | 0.56 |
| 28 D VFDs (days) | 20.17 (9.21, 22.54) | 14.33 (0.00, 20.67) | 0.03 |
| Days to first SBT – Survivors (days) | 4.42 (2.92, 7.08) | 6.42 (3.42, 11.33) | < 0.01 * |
| LMV - Survivors (days) |
6.33 (4.33, 9.17) |
9.83 (5.50,15.42) | < 0.01 * |
| Re-intubation | 0 (0%) | 10 (7.8%) | 0.10 |
Significance was assessed with Kaplan-Meier survivor analysis.
Figure 3:
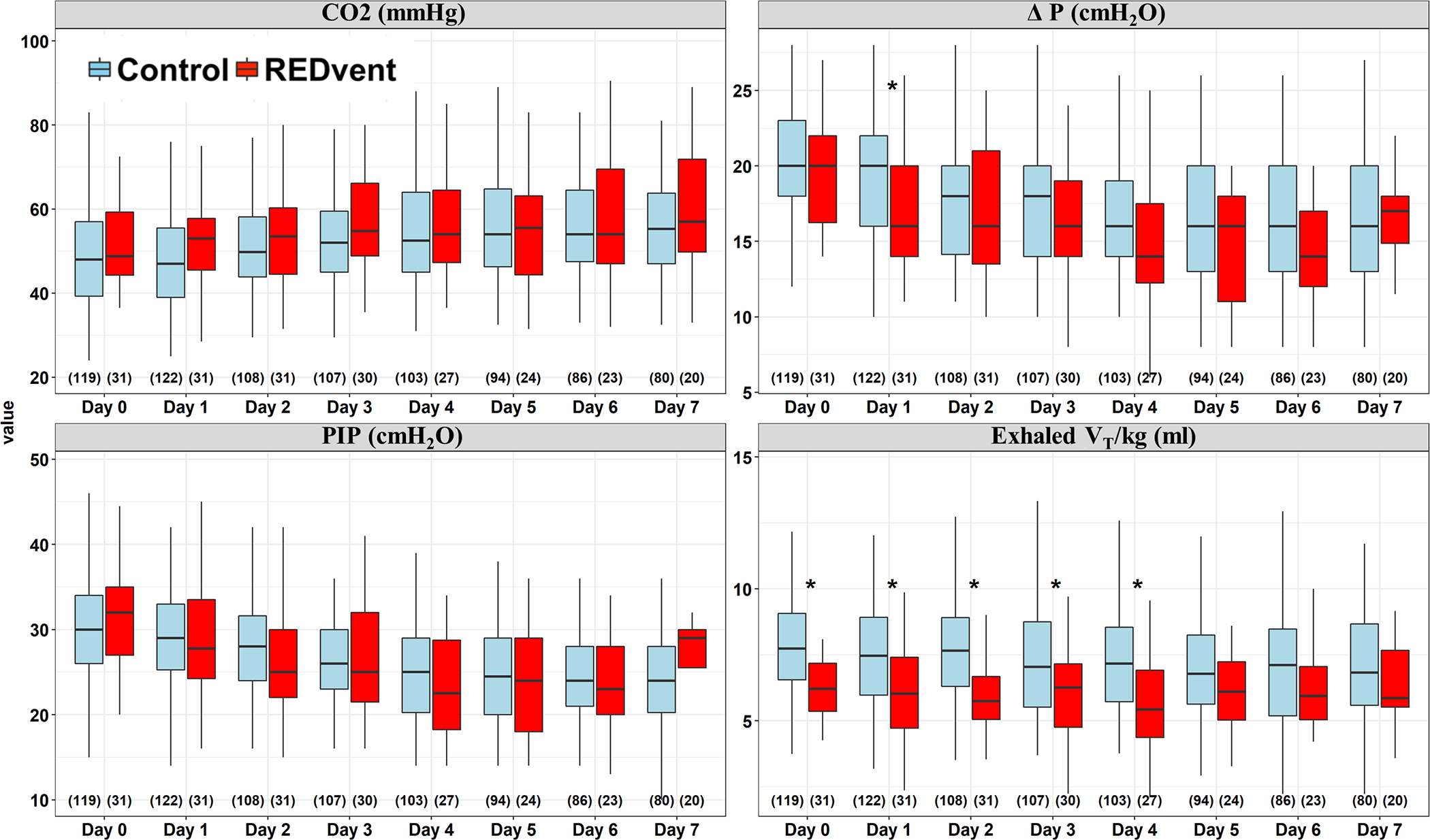
Ventilation parameters for REDvent (red), historical controls (blue), and N( ) as a function of time. For each subject, study day, and parameter, the median value was used. On day 1, ΔP is lower in REDvent with similar trends maintained through study day 4. Exhaled tidal volume per kg is significantly less on day 0 and also maintains significance through study day 4. * Indicates significance for a Mann-Whitney-U test, P < 0.05.
Figure 4:
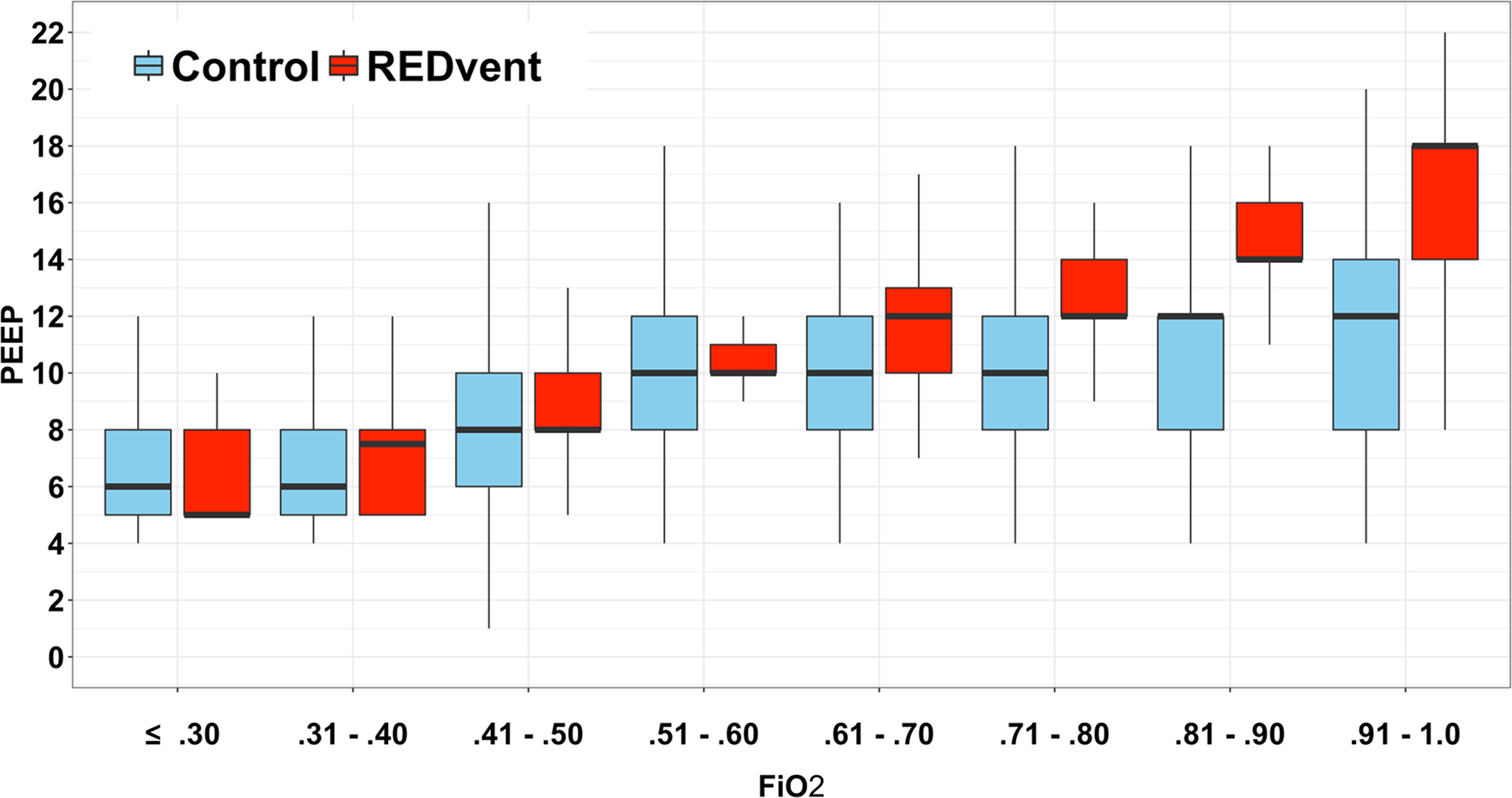
FiO2 as a function of PEEP between REDvent (red) and historical controls (blue) over the entire course of mechanical ventilation. As FiO2 was increased, PEEP increased at a greater rate in the REDvent group particularly when FiO2 was > 0.6.
Clinical Outcomes
Mortality was similar between REDvent (22%) and historical control patients (29%, Table 1) P = 0.26. REDvent was associated with a median of six more ventilator free days (p = 0.03), and approximately three fewer days on MV amongst survivors compared to historical controls, 6.33, [4.33, 9.17] vs. 9.83 [5.50,15.42], (p=0.04). Survival analysis showed shorter time until first SBT and shorter length of mechanical ventilation among survivors (P < 0.01) in the REDvent group compared to historical controls. No patients in the REDvent group were re-intubated within 48 hours, compared to 10 (7.8%) in the matched historical controls (p=0.1). aPiMax was obtained on all REDvent patients at the time of extubation, with a median value of 50 (40, 60).
Discussion
We have demonstrated the feasibility of using a CDS tool to achieve a high level of adherence to a complex ventilator protocol targeting lung and diaphragm protective ventilation. Furthermore, patients managed with this protocol were ventilated with more lung protective levels of tidal volume, inspiratory pressure and PEEP than historical controls. Clinical outcomes such as length of mechanical ventilation and time to a spontaneous breathing trial were also shorter in REDvent patients compared to historical controls. Together, these findings justify using this tool in a larger, randomized clinical trial, which is currently ongoing (NCT03266016). [24]
The physiological principles of lung protective ventilation have been advocated for the last 20 years. A variety of consensus and evidence based recommendations for both adults and children with ARDS specifically focus on limiting tidal volume and driving or plateau pressure, embracing permissive hypercapnia, using PEEP to prevent atelectotrauma, and minimizing FiO2 exposure. [11,25–28] Despite these recommendations, observational studies show that in actual practice, these principles are frequently not implemented. [25,29,30] Tidal volume is often higher than recommended, and is frequently not reduced even when driving pressure is high or lung compliance is poor. In children, PEEP is often not escalated to be in line with even the low-PEEP/FiO2 ARDSnet protocol [30], with current evidence suggesting that a large subset of patients with ARDS are likely to benefit from PEEP levels much higher than suggested by the low-PEEP/FiO2 ARDSnet protocol. [31,32] Importantly, in recent years we have begun to better recognize that lung protective controlled mechanical ventilation can have deleterious effects on the respiratory muscles. Patient effort of breathing during mechanical ventilation, in both adult and pediatric studies, are frequently well below normal, physiologic values. [4,5,13] This leads to ventilator induced diaphragm dysfunction and may prolong weaning from mechanical ventilation, increase extubation failure rates, and have longer term consequences. Routine measurement of airway occlusion pressure during maximal inspiration (aPiMax) was not available in the historical control patients, but we found that REDvent patients had median values of 50, with only one patient having aPiMax < 30 cmH2O. Previous studies we have done in over 400 mechanically ventilated patients in our ICU highlights that the median aPiMax value was 40 [30,50] with 35% of patients having aPiMax <30 cmH2O. This data suggests that the REDvent protocol may have an effect on preventing respiratory muscle weakness.
While it is likely true that an expert clinician can balance the (sometimes competing) risks of lung and diaphragm protective ventilation with careful attention to setting the mechanical ventilator and adjusting it as patient conditions change, this complex process can benefit from automation and diagnostic aids. This is precisely where CDS can play a role in medicine, by reducing the complexity of decision making and balancing competing risks in a systematic and reproducible way. We have implemented this CDS tool in an open loop fashion, as it allows the clinicians to make the final decision about whether or not to adjust the ventilator settings in the suggested manner, and incorporate variables which are not currently a part of the CDS tool (e.g. hemodynamic status, air leak syndrome, etc). However, with continued refinement, repeated external validation with controlled trials and incorporation of additional variables, it may be possible to eventually close the loop.
This study also highlights that development of CDS is an iterative process which requires constant evaluation and education. Adherence to recommendations improved over time because we incorporated user feedback and suggestions, and when necessary modified the rule sets to make them consistent with clinical practice, while still achieving the goals of the protocol. Furthermore, there is a trust that eventually develops with CDS tools, which may not be present upon initial deployment. Hence, over time adherence may continually improve, as practitioners become more comfortable with the recommendations, and potentially relinquishing some control. [20–23]
This study has a number of important limitations. First, this was designed primarily as a pilot phase 1 feasibility trial to test the potential acceptability of the intervention. Comparisons of outcomes and ventilator settings to historical controls were made to understand the potential benefit such a protocol could have. It is unlikely that the actual treatment effect would be this high in a randomized controlled trial, as we cannot control for unmeasured differences between groups and the effect of time. Hence, the conclusions about treatment effect and separation between groups likely represent the best-case scenario. Nonetheless, we believe this helps justify more definitive testing in a randomized controlled trial. Second, we chose to implement a modification of the ARDSNetwork protocols for lung protective ventilation which uses the low PEEP/FiO2 table, and a modified ruleset for ventilator rate and pressure management in a pressure control mode. This protocol is in line with important physiological principles of lung protective ventilation, but it may not be the best protocol for an individual patient as it is focused primarily on gas exchange and not individualized respiratory mechanics (except the effort of breathing titration). Third, we used esophageal manometry and the Pressure-Rate Product to target physiological levels of patient effort. This “physiological range” for PRP was based on previous studies in over 400 children at the time of extubation, targeting the 25–75 percentile of PRP 60 minutes after extubation for the population of children who did well after extubation (N = 375). [13] While there is some individual variation in this range based on pre-existing comorbidities, this represents the best guess at values for patients that have achieved reasonable resolution of respiratory disease. Fourth, patients enrolled in this study (both intervention and historical controls) generally had severe ARDS [11], with a large percentage of immune compromised children. These are the children who generally have the worst outcomes, and longest length of mechanical ventilation. [33] This same magnitude of treatment effect may not occur in children with less severe disease. Finally, the CDS tool has its own limitations. Recommendations are generated only when requested by the user thus preventing its ability to function as a continuous and automated monitoring tool. We rely on a bedside clinician to interact with the CDS and enter physiologic and ventilator information that is either assisted by pulling in monitor data, or manually entered. Manually entered data may contain bias when observing multiple parameters that are constantly changing. While the possibility of entering incorrect information is minimized by adding range checks and alerts to the CDS, data entry errors are still possible and improved error detection is in further development.
Conclusions
A CDS based protocol targeting lung and diaphragm protective ventilation including esophageal manometry can achieve high rates of protocol adherence in children with ARDS. It was associated with more lung protective ventilation and better clinical outcomes than historical control patients. A Phase II randomized controlled clinical trial is ongoing to determine if this intervention improves clinical outcomes such as length of weaning and length of mechanical ventilation.
Supplementary Material
Funding Source:
NIH/NHLBI R01HL124666
Copyright form disclosure: Mr. Hotz’s institution received funding from National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute R01HL124666. Mr. Hotz, and Drs. Newth, and Khemani received support for article research from the NIH. Drs. Newth and Khemani’s institution received funding from the NIH, and they received funding from Hamilton Medical. Dr. Newth received funding from Philips Research North America. Dr. Khemani received funding from Securysin Medical, Massimo, and OrangeMed. The remaining authors have disclosed that they do not have any potential conflicts of interest
Footnotes
This article has electronic supplementary material.
References
- 1.Martin AD, Smith BK, Gabrielli A: Mechanical ventilation, diaphragm weakness and weaning: A rehabilitation perspective. Respir Physiol Neurobiol 2013; 189:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di mussi R, Spadaro S, Mirabella L, et al. : Impact of prolonged assisted ventilation on diaphragmatic efficiency: NAVA versus PSV. Crit Care Med 2016; 20:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinino E, Gartman EJ, Sethi JM, McCool FD: Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014; 69:423–427 [DOI] [PubMed] [Google Scholar]
- 4.Emeriaud G, Larouche A, Ducharme-Crevier L, et al. : Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. Intensive Care Med 2014; 40:1718–1726 [DOI] [PubMed] [Google Scholar]
- 5.Goligher EC, Fan E, Herridge MS, et al. : Evolution of diaphragm thickness during mechanical ventilation: Impact of inspiratory effort. Am J Respir Crit Care Med 2015; 192:1080–1088 [DOI] [PubMed] [Google Scholar]
- 6.Heunks L, Doorduin J, van der Hoeven JG: Monitoring and preventing diaphragm injury. Curr Opin Crit Care 2015; 21:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matamis D, Soilemezi E, Tsagourias M, et al. : Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 2013; 39:801–810 [DOI] [PubMed] [Google Scholar]
- 8.Supinski GS, Callahan LA: Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 2013; 17:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooijman PE, Beishuizen A, Witt CC, et al. : Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med 2015; 191:1126–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf GK, Walsh BK, Green ML, Arnold JH: Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med 2011; 12:e220–e224 [DOI] [PubMed] [Google Scholar]
- 11.Jouvet P, Thomas NJ, Willson DF, et al. : Pediatric Acute Respiratory Distress Syndrome: Consensus Recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khemani RG, Hotz J, Morzov R, et al. : Evaluating Risk Factors for Pediatric Post-extubation Upper Airway Obstruction Using a Physiology-based Tool. Am J Respir Crit Care Med 2016; 193:198–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khemani RG, Hotz J, Morzov R, et al. : Pediatric extubation readiness tests should not use pressure support. Intensive Care Med 2016; 42:1214–1222 [DOI] [PubMed] [Google Scholar]
- 14.Khemani RG, Sekayan T, Hotz J, et al. : Risk factors for pediatric extubation failure: The importance of respiratory muscle strength. Crit Care Med 2017; 45:e798–e805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotz JC, Sodetani CT, Van Steenbergen J, et al. : Measurements obtained from esophageal balloon catheters are affected by the esophageal balloon filling volume in children with ARDS. Respir Care 2018; 63:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diblasi RM, Zignego JC, Tang DM, et al. : Noninvasive respiratory support of juvenile rabbits by high-amplitude bubble continuous positive airway pressure. Pediatr Res 2010; 67:624–629 [DOI] [PubMed] [Google Scholar]
- 17.Ross PA., Hammer J, Khemani R, et al. : Pressure-rate product and phase angle as measures of acute inspiratory upper airway obstruction in rhesus monkeys. Pediatr Pulmonol 2010; 45:639–644 [DOI] [PubMed] [Google Scholar]
- 18.Argent AC, Hatherill M, Newth CJL, Klein M: The effect of epinephrine by nebulization on measures of airway obstruction in patients with acute severe croup. Intensive Care Med 2008; 34:138–147 [DOI] [PubMed] [Google Scholar]
- 19.Argent AC, Newth CJL, Klein M: The mechanics of breathing in children with acute severe croup. Intensive Care Med 2008; 34:324–332 [DOI] [PubMed] [Google Scholar]
- 20.Evans RS, Pestotnik SL, Classen DC, et al. : A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338:232–238 [DOI] [PubMed] [Google Scholar]
- 21.Lobach DF, Hammond WE: Computerized decision support based on a clinical practice guideline improves compliance with care standards. Am J Med 1997; 102:89–98 [DOI] [PubMed] [Google Scholar]
- 22.Morris AH: Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 2000; 132:373–383 [DOI] [PubMed] [Google Scholar]
- 23.Khemani AG, Hotz JC, Sward KA, Newth CJL: The role of computer-based clinical decision support systems to deliver protective mechanical ventilation. Curr Opin Crit Care 2020; 26:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khemani RG, Hotz JC, Klein MJ, et al. : A Phase II randomized controlled trial for lung and diaphragm protective ventilation (Real-time Effort Driven VENTilator management). Contemp Clin Trials 2020; 88:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellani G, Laffey JG, Pham T, et al. : Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 26.Amato MBP, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2014; 372:747–755 [DOI] [PubMed] [Google Scholar]
- 27.Mercat A, Richard J-CM, Vielle B, et al. : Positive End-Expiratory Pressure Setting in Adults With Acute Lung Injury and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA 2008; 299:646–655 [DOI] [PubMed] [Google Scholar]
- 28.Ranieri VM, Rubenfeld GD, Thompson BT, et al. : Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 29.Khemani RG, Sward K, Morris A, et al. : Variability in usual care mechanical ventilation for pediatric acute lung injury: The potential benefit of a lung protective computer protocol. Intensive Care Med 2011; 37:1840–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khemani RG, Parvathaneni K, Yehya N, et al. : Positive end-expiratory pressure lower than the ARDS network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med 2018; 198:77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briel M, Meade M, Mercat: A Higher vs Lower Positive End-Expiratory Pressure in Patients With Acute Lung Injury. JAMA 2010; 303:865–873 [DOI] [PubMed] [Google Scholar]
- 32.Talmor D, Sarge T, Malhotra A, et al. : Mechanical Ventilation Guided by Esophageal Pressure in Acute Lung Injury. N Engl J Med 2008; 359:2095–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khemani RG, Smith L, Lopez-Fernandez YM, et al. : Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 2019; 7:115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


