Abstract
BACKGROUND
Autophagy is highly active in neuroepithelial cells of the developing neuroepithelium, and impaired autophagy leads to neural tube defects (NTDs). We have demonstrated that maternal diabetes induces NTDs, and that impaired autophagy and consequent cellular imbalance, including the endoplasmic reticulum (ER), where critical events occur leading to the induction of diabetic embryopathy. Because the mammalian target of rapamycin (mTOR) pathway suppresses autophagy, we hypothesize that p70S6K1 (70 kDa ribosomal protein S6 kinase 1), a major downstream effector of mTOR, mediates the inhibitory effect of maternal diabetes on autophagy in the developing neuroepithelium.
OBJECTIVE
We investigated whether p70S6K1 mediates the inhibitory effect of maternal diabetes on autophagy during neurulation. We also examined if p70S6K1 deficiency restores autophagy and thus relieves ER stress and inhibits maternal diabetes-induced apoptosis, which leads to reduction in NTD incidence in diabetic embryopathy.
STUDY DESIGN
Female p70S6K1 heterogeneous knockout (p70S6K1+/−) mice were bred with male p70S6K1 heterogeneous knockout (p70S6K1+/−) mice to generate wild type (WT), p70S6K1+/− and p70S6K1 knockout (p70S6K1−/−) embryos. Embryos at embryonic day 8.5 (E8.5) were harvested for the assessment of indices of autophagy, ER stress and apoptosis. NTDs incidence was determined in E10.5 embryos. For in vitro studies, siRNA knockdown of p70S6K1 in C17.2 mouse neural stem cells were used to determine the effect of p70S6K1 deficiency on autophagy impairment and ER stress under high glucose conditions.
RESULTS
Knockout of the Rps6kb1 gene, which encodes for p70S6K1, ameliorated maternal diabetes-induced NTDs and restored autophagosome formation in neuroepithelial cells suppressed by maternal diabetes. Maternal diabetes-suppressed conversion of LC3-I (Microtubule-associated protein 1A/1B-light chain 3) to LC3-II, an index of autophagic activity, in neurulation stage embryos was abrogated in the absence of p70S6K1. p70S6K1 knockdown in neural stem cells also restored autophagosome formation and the conversion of LC3-I to LC3-II. The activation of the major unfolded protein response (UPR), indicated by phosphorylation of IRE1α, PERK and eIF2α, and the increase of the endoplasmic reticulum (ER) stress marker, CHOP, were induced by maternal diabetes in vivo and high glucose in vitro. UPR and ER stress induced by maternal diabetes or high glucose were diminished by Rps6kb1 deletionor p70S6K1 knockdown, respectively. Rps6kb1knockoutblocked maternal diabetes-induced caspase cleavage and neuroepithelial cell apoptosis. The SOD memetic Tempol abolished high glucose-induced p70S6K1 activation.
CONCLUSION
We revealed the critical involvement of p70S6K1 in the pathogenesis of diabetic embryopathy.
Keywords: maternal diabetes, neural tube defects, p70S6K1, autophagy, ER stress, apoptosis, diabetic embryopathy
Introduction
Autophagy (here referred to macroautophagy) is a cellular process by which eukaryotic cells degrade damaged proteins and dysfunctional organelles, and sustain bio-energetic demands under stress conditions1. Autophagy is initiated with the formation of specialized double-membrane autophagosomes, which engulf protein aggregates or dysfunctional organelles and subsequently fuse with lysosomes to enable degradation1–3. Autophagy is important for cellular balance between anabolic and catabolic processes in sustaining cell viability4–7, and thus, autophagy is crucial for cells to adapt to stressors, such as oxidative stress, parasite invasion or chemotherapeutic exposure1, 7–18. Thus, mild oxidative stress induces adaptive responses in stimulating autophagy which can remove oxidative stress-damaged cellular components. However, severe oxidative stress, such as maternal diabetes-induced oxidative stress, suppresses autophagy leading to intracellular imbalance and cell apoptosis19, 20. Autophagy is an effective and highly regulated process in maintaining cellular homeostasis through the removal of unnecessary or defective components8, 21, 22. It is essential for preimplantation development23. Our previous studies have shown that restoring autophagy inhibits maternal diabetes-induced neural tube defects19, 20. Others have shown that autophagy deficiency due to a gene deletion result in neural tube defects (NTDs)24.
Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase, is a negative autophagy regulator25. Inhibiting mTOR activity is a crucial step for autophagy induction under nutrient starvation25. p70S6K1 is a downstream kinase of the mTOR signaling pathway, indicating that p70S6K1 may mediate the inhibitory effect of mTOR on autophagy. However, mTOR suppresses autophagy by directly phosphorylating (or inactivating) the protein of autophagy related gene 1 (ATG1)25. We propose a new function of p70S6K1 in diabetic embryopathy: activation of p70S6K1 suppresses autophagy in neuroepithelial cells leading to cellular organelle stress.
Our previous studies have demonstrated that autophagy in the developing neuroepithelium is suppressed by hyperglycemia of diabetes during pregnancy19, 20, 26, 27. Maternal diabetes disrupts neurulation through oxidative stress leading to neural tube defects (NTDs)28–39, while restoring autophagy ameliorates maternal diabetes-induced NTDs19, 20. However, it is still unclear how maternal diabetes represses autophagy. Maternal diabetes predominantly induces exencephaly, triggers neuroepithelial cell apoptosis and suppresses autophagy in the region between the forebrain and midbrain of the developing embryo31, 40–51. We hypothesize that knockout of the Rps6kb1 gene, encoding for p70S6K1, reverses maternal diabetes-induced autophagy impairment, cellular organelle stress and apoptosis, leading to a reduction of NTD formation.
Cellular organelle stress, including ER stress and mitochondrial dysfunction is manifested in diabetic embryopathy19, 29, 30, 44, 45, 49, 50, 52–55. The ER is morphologically and functionally perturbed in cells of developing organs that are susceptible to maternal diabetes19, 48, 56, 57 Maternal diabetes activates the major unfolded protein response (UPR) sensors, Inositol requiring enzyme 1 alpha (IRE1α) and protein kinase R-like endoplasmic reticulum kinase (PERK), which induce the pro-apoptotic effects of ER stress leading to NTDs48. Thus, activation of the UPR and ER stress mediates the teratogenicity of maternal diabetes48. Autophagy restores intracellular balance by relieving ER stress. Therefore, if p70S6K1 is critically involved in maternal diabetes-induced autophagy impairment, it will impact maternal diabetes-induced UPR and ER stress in the developing embryo.
Our previous studies found maternal diabetes-induced neuroepithelial cell apoptosis41, 50. p70S6K1 deficiency blocks hepatocyte undergoing apoptosis 59, suggesting that p70S6K1 may induce apoptosis. Apoptosis and autophagy are two inter-related cellular processes that regulate cell fate. Some studies show that their regulation is intimately connected and the same regulators can sometimes control both autophagy and apoptosis60, 61. p70S6K1 may be one of these regulators to impact both maternal diabetes-induced autophagy impairment and apoptosis in the developing embryo.
In the present study, we investigated the effect of p70S6K1 deficiency on maternal diabetes-induced autophagy impairment, ER stress and apoptosis in diabetic embryopathy. We found that deleting the Rps6kb1 gene abrogated maternal diabetes-induced autophagy impairment, resolved cellular homeostatic imbalance by preventing UPR activation and ER stress, inhibited maternal diabetes-induced apoptosis, and ultimately reduced NTD formation in the embryos of diabetic pregnant mice.
Materials and Methods
Study design
To investigate whether the oxidative stress-responsive kinase p70S6K1 mediates the inhibitory effect of maternal diabetes on autophagy during neurulation, we used the streptozotocin (STZ)-induced type 1 diabetes mouse model. Diabetes was induced in Wild-type (WT) C57BL/6J mice and heterozygous p70S6K1 knockout (KO) mice in the same background. Indices of autophagy, cellular stress and apoptosis in the developing neuroepithelium were analyzed in WT and KO embryos from nondiabetic and diabetic dams at embryonic day 8.5 (E8.5), a critical time point for mouse neurulation. NTD incidence was morphologically examined at E10.5. For in vitro studies in the C17.2 mouse neural stem cell line, p70S6K1 knockdown by transfection of siRNAs was achieved for the evaluation of autophagy and cellular stress. Cells were cultured under normal (5 mM glucose) and high glucose (25 mM glucose) conditions and transfection of the scramble siRNA (the un-targeting siRNA) served as a control group. For a separate in vitro experiment, cells were treated with the antioxidant Tempol to determine whether oxidative stress is responsible for high glucose-induced p70S6K1 activation (phosphorylation).
Animals and Reagents
WT C57BL/6J mice (median body weight 22 g) were purchased from The Jackson Laboratory (Bar Harbor, ME). p70S6K1 KO mice onC57BL/6J background was previously described62. STZ (Sigma Chemical Company, St. Louis, MO) was dissolved in sterile 0.1mol/L citrate buffer (pH 4.5). The procedures for animal use were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Mouse Models of Diabetic Embryopathy
Our mouse model of diabetic embryopathy has been described previously20, 41, 47 In the diabetic group, female mice were intravenously injected daily with 75 mg/kg STZ over 2 days to induce diabetes before pregnancy was established. Diabetes was defined as a 12-h fasting blood glucose level of greater than or equal to 13.9 mM. Then, these female diabetic mice were mated with nondiabetic male mice. In the nondiabetic group, female mice were treated with vehicle injection. On E8.5, mice were euthanized and conceptuses were dissected out of the uteri for analyses.
Cell Culture and Transfection
C17.2 mouse neural stem cells, originally obtained from the European Collection of Cell Culture (Salisbury, UK), were maintained (more than 3 passages) in DMEM (5 mM glucose) supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. p70S6K1 siRNA and control siRNA were obtained from Invitrogen. Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used according to the manufacturer’s protocol for transfection of siRNA into the cells. After seeding for 12h, the cells were transfected with siRNA and cultured in 1% FBS + DMEM for 8h. Then cells were cultured in 10% FBS + DMEM and cells were harvested after 48h for analyses.
Immunofluorescent staining
Cells were maintained under standard tissue culture conditions at 37 °C, 5% CO2 in a humidified incubator. After 48h in different treatment conditions, cells were washed with DPBS. 1ul of Cyto-ID Green Detection Reagent was added to 1 ml cell culture medium. After Cyto-ID staining, cells were incubated with DAPI (4’,6-diamidino-2-phenylindole) for 10 min for cell nuclei staining. Fluorescence images were taken on a Nikon H600L microscope with the IP Lab imaging system (Scientific Instrument Company, Campbell, CA).
Whole embryos were fixed in 4% paraformaldehyde (PFA) (pH7.4) for 30 min at room temperature and fixed embryos were embedded in optimum cutting temperature medium (OCT) compound (Sakura Finetek, Torrance, CA). Then, 10-μm embryonic frozen sections were obtained and permeabilized with 0.25% Triton-X100 (Sigma, St. Louis, MO) for 10 min. Samples were blocked for 30 min in 10% donkey serum in PBS and incubated with the LC3 antibody (1:200, Cell Signaling Technology, Danvers, MA) overnight at 4°C. After they were washed with PBS, samples were incubated with Donkey anti-Rabbit Secondary Antibody, Alexa Fluor 488 (1:1000, Invitrogen) for 2h, followed by DAPI cell nuclear counterstaining for 10 min and coverslip mounting with aqueous mounting medium. For confocal fluorescent imaging, images were captured by a laser scanning microscope (LSM 510 META, ZEISS, Germany).
Immunoblotting
Immunoblotting was performed as previously described50. To extract protein, samples (embryos or C17.2 cells) were sonicated in lysis buffer (Cell Signaling Technology) containing a protease inhibitor cocktail (Sigma). Equal amounts of protein and the Precision Plus Protein standards (Bio-Rad, Portland, ME) were resolved by SDS-PAGE and transferred onto Immobilon-P membranes (Millipore, Billerica, MA). Membranes were incubated in 5% nonfat milk for 1h and then incubated overnight at 4°C with primary antibodies at their respective dilutions. p-p70S6K1(1:1000, Cell Signaling Technology), p70S6K1(1:1000, Cell Signaling Technology), LC3(1:1000, Cell Signaling Technology), β-actin(1:10,000, Abcam, Cambridge, MA), caspase-3(1:500, Millipore), caspase-8(1:1000, Enzo Life Sciences, Farmingdale, NY), p-S6(1:1000, Cell Signaling Technology), S6(1:1000, Cell Signaling Technology). Membranes were exposed to HRP-conjugated goat anti-rabbit or goat anti-mouse (Jackson Immuno Research Laboratories, West Grove, PA) secondary antibodies. Signals were detected using the Super Signal West Femto Maximum Sensitivity Substrate kit (Thermo Fisher Scientific, Halethorpe, MD). Chemiluminescence emitted from the bands was directly captured using a UVP Bioimage EC3 system.
TUNEL assay
The TUNEL assay was performed using the ApopTag Fluorescein in Situ Apoptosis Detection kit (Chemicon) as previously described48, 63. Briefly, 10-um frozen embryonic sections were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and incubated with TUNEL reaction agents. Three embryos from three different dams (n = 3) each group were used, and two sections per embryo were examined. TUNEL-positive cells in an area (about 200 cells) of the neuroepithelia were counted. The percentage of TUNEL-positive cells was calculated as a fraction of the total cell number, multiplied by 100 and averaged within the sections of one embryo.
Statistical analysis
Data are presented as means ± SEM (standard error). Embryonic samples from each replicate were from a different dam. For overall statistical significance, one-way ANOVA was performed using the Sigma Stat 3.5 software (Systat Software Inc., San Jose, CA). In multiple comparisons, the Tukey-test was used to estimate the significance of the difference between groups. Significant difference between groups in NTDs incidences was analyzed by the Fisher Exact Tests. Statistical significance was accepted at P< 0.05.
Results
p70S6K1 knockdown restores autophagy suppressed by high glucose
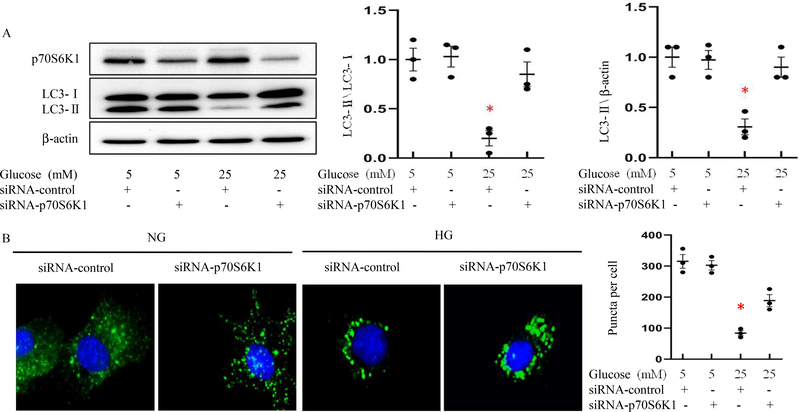
Our previous study demonstrated that high glucose represses autophagy20. To investigate whether removing p70S6K1 restores normal autophagy, even under high glucose conditions, we utilized a specific siRNA to knockdown p70S6K1 expression in C17.2 neural stem cells. Immunoblotting showed thatp70S6K1 siRNA reduced p70S6K1 protein levels (Fig. 1A), indicating the effectiveness of the siRNA-mediated p70S6K1 mRNA silencing. The amount of LC3-II, the lipidated form of LC3-I as an autophagic activity index, was not affected by p70S6K1 siRNA knockdown under normal glucose conditions, but was significantly reduced by high glucose (Fig.1A). High glucose-reduced LC3-II abundance was abrogated by p70S6K1 siRNA knockdown (Fig.1A). Using the Cyto-ID staining method to detect autophagosomes, we observed inhibition of autophagy by high glucose (Fig.1B). Under normal glucose conditions, the number of Cyto-ID staining puncta did not differ between the control group and the p70S6K1 siRNA transfected group (Fig. 1B). However, in the high glucose conditions, the number of Cyto-ID staining puncta was significantly reduced, and p70S6K1 knockdown reversed this puncta reduction (Fig. 1B).
Figure 1. p70S6K1 knockdown reverts high glucose-suppressed autophagy.
A: Quantification of relative protein levels of p70S6K1 and LC3-II (Microtubule-associated protein 1 light chain 3 II, the lipidation form of LC3-I, is an index of autophagy activation) versus LC3-I in normal glucose (NG; 5 mM glucose) and high glucose (HG; 25 mM glucose) culture conditions. B: Representative Cyto-ID staining (green) images and quantification of autophagosomes (green puncta) in C17.2 neural stem cells in normal glucose and high glucose culture conditions. C17.2 cells were transfected with p70S6K1 siRNA. Scramble siRNA (the un-targeting siRNA) was used as a control. In B, cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), bars = 15 μm. Experiments were repeated three times (n=3); * indicates significant difference compared to the other groups (P< 0.05).
Rps6kb1 gene knockout ameliorates maternal diabetes-induced NTDs
To determine whether p70S6K1 plays a role in the induction of NTDs in diabetic pregnancy, we used p70S6K1 knockout mice and examined NTD formation. As shown in Table 1. Under diabetic conditions, 10 out of 31 embryos (32.3%) from Wild-Type (WT) diabetic dams had NTDs. Only 2 of 29 Rps6kb1 gene-deleted embryos (6.9%) exhibited NTDs (Table 1) and this NTD rate was significantly lower than that of WT embryos from diabetic dams (Table 1). Rps6kb1 gene deletion knockout significantly reduced NTD formation in diabetic pregnancy. Under nondiabetic condition, none of Rps6kb1 gene-deleted embryos exhibited NTDs (Table 1), indicating that Rps6kb1 gene deletion knockout did not affect embryonic development. These findings support our hypothesis that p70S6K1 deficiency ameliorates maternal diabetes-induced NTDs.
Table1.
p70S6K1 deficiency ameliorates maternal diabetes-induced neural tube defects.
| Experimental group | Glucose level (mg/dl) | Genotype | embryos | NTD Embryos (NTDs rate%) | |
|---|---|---|---|---|---|
| ND | p70S6K1+/− male × p70S6K1+/− female (11 litters) | 144.8±12.6 | WT | 20 | 0(0.0%) |
| p70S6K1+/− | 39 | 0(0.0%) | |||
| p70S6K1−/− | 15 | 0(0.0%) | |||
| DM | p70S6K1+/− male × p70S6K1+/− female (15 litters) | 381.8±12.5 | WT | 31 | 10(32.3%) |
| p70S6K1+/− | 53 | 10(18.9%) | |||
| p70S6K1−/− | 29 | 2(6.9%) * |
indicates significant difference compared with the other groups in Fisher Exact Tests (P<0.05)
NTDs: neural tube defects; ND: Non- diabetes Mellitus; DM: Diabetes Mellitus; WT: Wild-Type. 11 litters from ND mice and 15 litters from DM mice were used for evaluation of each genotype.
Rps6kb1 gene knockout rescues autophagy suppressed by maternal diabetes
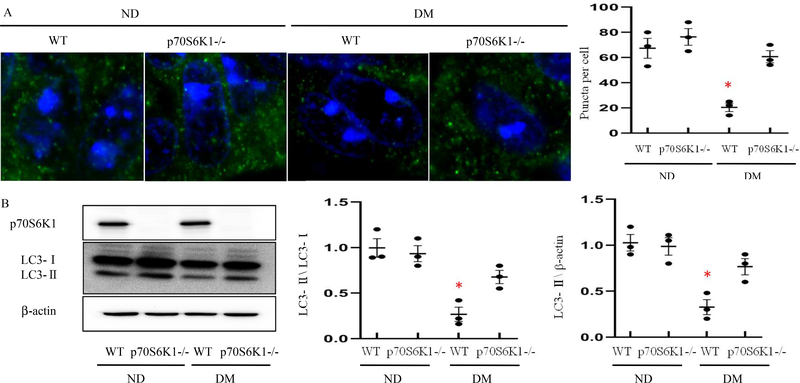
Because impaired autophagy is a critical mechanism by which NTDs occur in offspring of diabetic pregnant dams, we investigated the impact of Rps6kb1 gene knockout on autophagy activity. Rps6kb1 gene knockout prevented maternal diabetes-induced reduction in autophagosome numbers in neuroepithelial cells (Fig. 2A). Immunofluorescent staining showed that the number of autophagic puncta in neuroepithelial cells was not significantly different in WT embryos compared with Rps6kb1 gene deleted embryos under nondiabetic conditions (Fig. 2A). In WT embryos of diabetic dams, the number of autophagic puncta was significantly reduced (Fig. 2A). This reduction of autophagic puncta by diabetes was reversed by Rps6kb1 gene knockout (Fig. 2A). The lipidation of LC3-I into LC3-II in neurulation stage embryos was significantly reduced by maternal diabetes (Fig. 2B), and Rps6kb1 gene knockout abrogated the suppression of LC3-II expression by maternal diabetes (Fig. 2B).
Figure 2. Rps6kb1 gene knockout rescues autophagy suppressed by maternal diabetes.
A: Confocal images of immunostaining for LC3 (Microtubule-associated protein 1 light chain 3) in neuroepithelial cells of E8.5 embryos. LC3 punctate foci which serves as indices of autophagy activation with a diameter ≥ 20 pixels were quantified by Image J. Bars = 5 μm. B: Protein levels of p70S6K1 and LC3 in E8.5 Wild-Type (WT) and Rps6kb1 gene deleted embryos from non-diabetic or diabetic dams. Quantification of relative expression levels of p70S6K1 and LC3-II versus LC3-I were shown in the graphs. Experiments were performed using three embryos from three different dams per group (n=3); * indicates significant difference compared to the other groups (P< 0.05). ND: Nondiabetic; DM: Diabetes Mellitus; WT: Wild-Type.
p70S6K1 deficiency inhibits UPR activation and ER stress
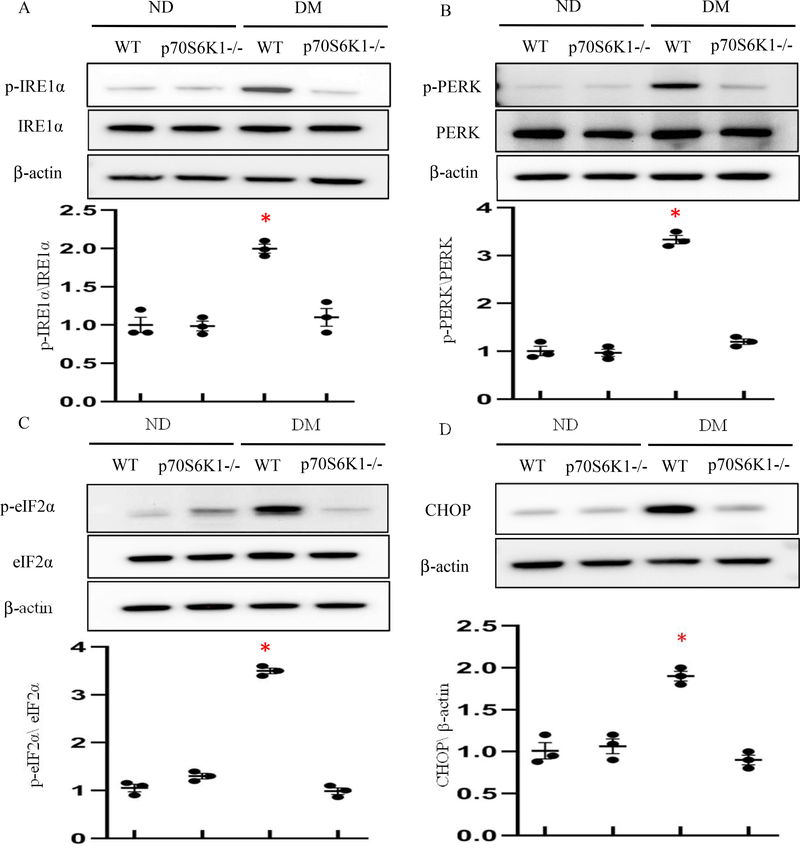
Autophagy can effectively resolve ER stress1. Because Rps6kb1 gene knockout re-activated autophagy in the developing neuroepithelium, we sought to determine whether deleting the Rps6kb1 gene blocks diabetes-induced UPR activation and ER stress. Maternal diabetes triggered the phosphorylation of two major UPR sensors, IRE1α and PERK, and their downstream effectors, CHOP and eIF2α, respectively, in neurulation stage embryos (Fig. 3). Deleting theRps6kbl gene abolished maternal diabetes-induced phosphorylation of IRElα, PERK and eIF2α (Fig. 3), and blocked the increase of CHOP expression (Fig. 3).
Figure 3. Rps6kb1 gene knockout blocks the unfolded protein response (UPR) activation and ER stress.
(A-D) Protein levels of ER stress in E8.5 Wild-Type (WT) and Rps6kb1 gene deleted embryos from nondiabetic or diabetic dams. Quantification of relative expression levels of phosphorylation of IRElα, PERK and eIF2α versus total IRElα, PERK and eIF2α and CHOP were shown in the graphs. Experiments were performed using three embryos from three different dams per group (n=3); * indicates significant difference compared to the other groups (P< 0.05). ND: Nondiabetic; DM: Diabetes Mellitus.
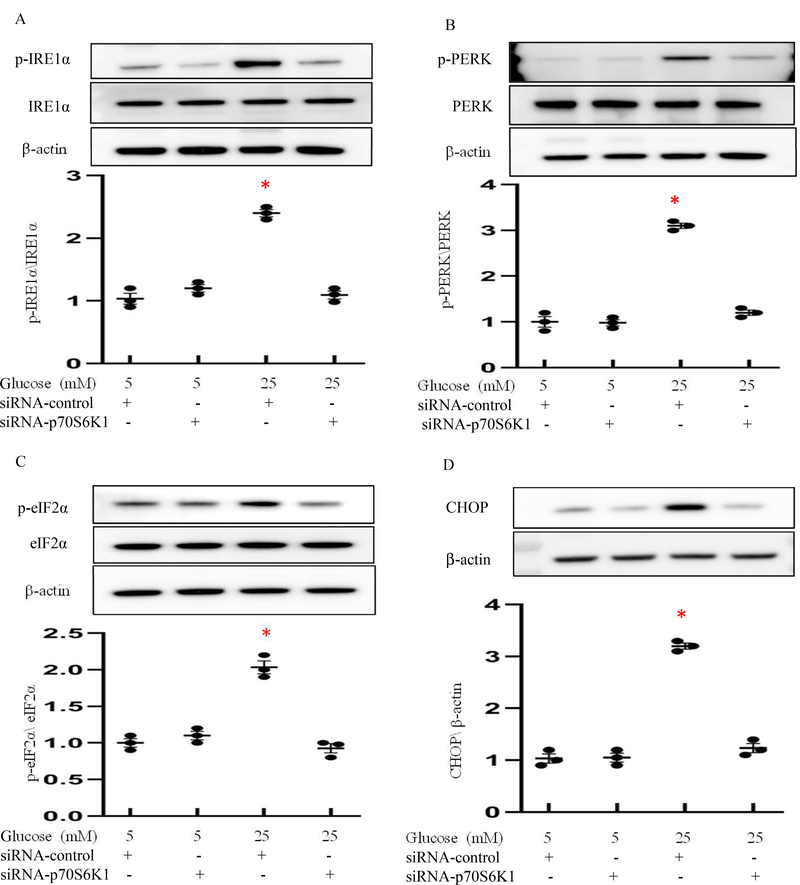
To further determine whether p70S6K1 accounts for UPR and ER stress, we tested the effect of reducing p70S6K1 expression in vitro under high glucose conditions. High glucose triggered the phosphorylation of IRElα, PERK and their downstream effectors, CHOP and eIF2α, while p70S6K1 siRNA knockdown abolished high glucose-induced IRElα, PERK and eIF2α phosphorylation (Fig. 4). Additionally, p70S6K1 siRNA knockdown prevented the increase of CHOP expression (Fig. 4).
Figure 4. p70S6K1 Knockdown inhibits UPR activation and ER stress.
(A-D) Protein levels of ER stress in normal glucose (NG; 5mM glucose) and high glucose (HG; 25mM glucose) culture conditions. Quantification of relative expression levels of phosphorylation of IRElα, PERK and eIF2α versus total IRElα, PERK and eIF2α, and CHOP were shown in the graphs. Experiments were repeated three times (n=3); * indicates significant difference compared to the other groups (P< 0.05).
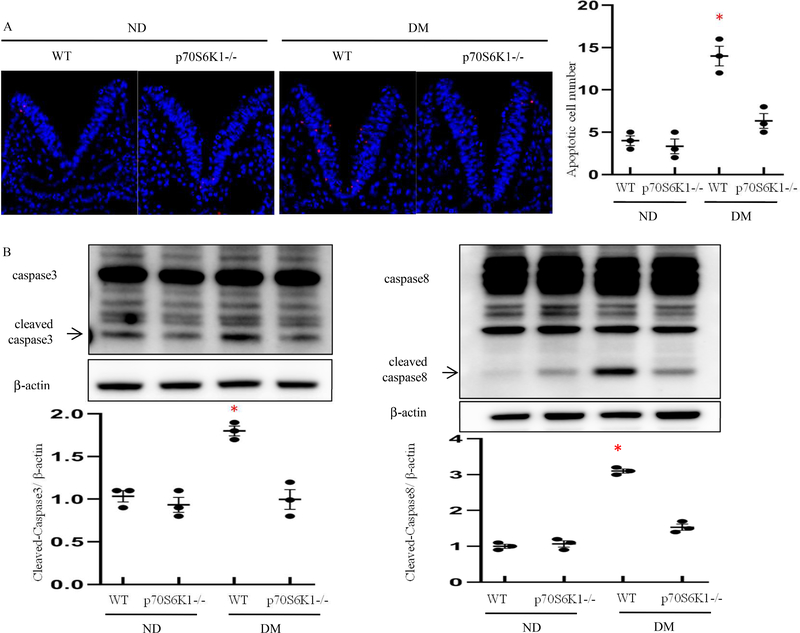
Rps6kb1 gene knockout blocks maternal diabetes-induced apoptosis
Maternal diabetes induces the neuroepithelium apoptosis through caspase-dependent signaling pathway29, 64 We used the TUNEL assay to detect apoptotic cells in the developing neuroepithelium. We found the apoptotic cell number in the neuroepithelia of WT embryos from diabetic dams was significantly higher than that in WT embryos from nondiabetic dams (Fig. 5A). Under diabetic conditions, the apoptotic cell numbers in neuroepithelia of Rps6kb1 gene knockout embryos were significantly lower compared to those in the neuroepithelia of the WT embryos (Fig. 5A). Thus, Rps6kb1 gene knockout prevented maternal diabetes induced-excessive cell apoptosis. To determine whether maternal diabetes induces caspase activation in the neuroepithelium, cleaved caspase 3 and caspase 8 were assessed. Maternal diabetes increased the abundance of both cleaved caspase 3 and caspase 8 in WT embryos, whereas Rps6kb1 gene knockout blocked maternal diabetes-induced caspase cleavage (Fig.5B). These results suggest that Rps6kb1 gene knockout inhibits maternal diabetes-induced apoptosis in the developing neuroepithelium.
Figure 5. Rps6kb1 gene knockout reduces maternal diabetes-induced apoptosis in the developing neuroepithelium.
(A) Representative TUNEL assay images showing apoptotic cells (red dots) in the E8.5 neuroepithelium. Cell nuclei were stained with DAPI (blue), bars = 30 μm. Quantification of TUNEL positive cells per section. Experiments were performed using three embryos from three different dams per group (n=3). (B) Protein levels of cleaved caspase8 and caspase3 in E8.5 embryos. Experiments were repeated three times (n=3); * indicates significant difference compared to the other groups (P< 0.05). ND: Nondiabetic; DM: Diabetes Mellitus; WT: Wild-Type.
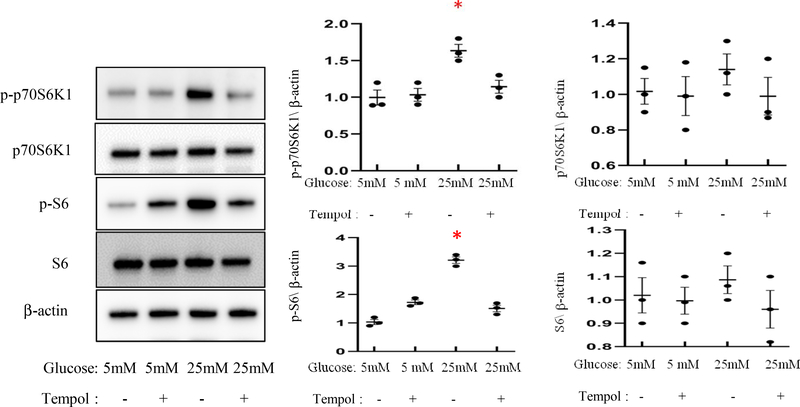
The superoxide dismutase (SOD) memetic Tempol inhibits high glucose-induced p70S6K1 activation
We sought to determine whether mitigating high glucose-induced oxidative stress using the antioxidant enzyme SOD memetic Tempol would block the activation (phosphorylation) of p70S6K1 and its downstream kinase S6. Indeed, we found that levels of phosphorylated- (p-) p70S6K1 and S6 were increased by high glucose, whereas total protein expression of p70S6K1 and S6 was not affected by high glucose (Fig. 6). The ratios of p-p70S6K1/p70S6K1 and p-S6/S6 were significantly increased by high glucose (Fig. 6). Temple treatment blocked high glucose-induced phosphorylation of p70S6K1 and S6 (Fig. 6). These findings demonstrate that oxidative stress is responsible for the activation of p70S6K1 and its major downstream effector S6 induced by high glucose, and further contribute to the oxidative stress hypothesis in the induction of diabetic embryopathy.
Figure 6. Tempol inhibits high glucose-induced p70S6K1 activation.
Protein levels and quantification data of phosphorylation of p70S6K1, S6 and total p70S6K1 and S6 in normal glucose (NG; 5mM glucose) with Tempol and high glucose (HG; 25mM glucose) with Tempol culture conditions. Experiments were repeated three times (n=3); * indicates significant difference compared to the other groups (P< 0.05).
Comment
Autophagy maintains cellular homeostasis and promotes cell survival during embryonic development23. During mouse development, two peaks of massive autophagy occur at the time of fertilization and in the early neonatal period23. At the tissue level, autophagy participates in cell differentiation and tissue patterning. Autophagy in neuroepithelial cells is suppressed by maternal diabetes in pregnancy and may be a primary cause for diabetes-induced NTDs19, 20.
In the present study, we elucidated a mechanism by which maternal diabetes suppresses autophagy in neuroepithelial cells leading to NTD formation. Our evidence in vivo and in vitro supports the hypothesis that p70S6K1 is a negative regulator of autophagy and mediates the inhibitory effect of maternal diabetes on autophagy.
p70S6K1 regulates important biological processes, including protein synthesis, maintenance of cellular energy states, cell growth, proliferation and survival65. It has been demonstrated that p70S6K1 activates anabolic pathways and inhibits catabolic processes65. The role of p70S6K1 in autophagy regulation has been controversial. p70S6K1 negatively regulates autophagy in rat hepatocytes66. Other data have indicated that p70S6K1 is necessary for autophagy in the fat body of Drosophila melanogaster67,68. p70S6K1 is a downstream effector of mTOR signaling, which inhibits autophagy. There is no report in pre-gestational diabetes-induced p70S6K1 activation. However, higher basal phosphorylation of p70S6K1 was observed in gestational diabetes versus control subjects, which is associated with impaired insulin receptor signaling69. In the current study, we observed that p70S6K1 knockdown by specific siRNA increased the abundance of LC3-II and restored autophagosome formation suppressed by high glucose. Results obtained from our in vivo studies showed that removing p70S6K1 restores LC3-II expression and autophagosome formation. Furthermore, deleting the Rps6kb1 gene restored autophagy in the developing neuroepithelium and blocked cellular stress. Not surprisingly, removing p70S6K1 ultimately abrogated maternal diabetes-induced neuroepithelial cell apoptosis and consequent NTD formation.
ER stress and mitochondrial dysfunction are causally involved in diabetic teratogenesis20, 57 Embryos exposed to diabetes exhibit impaired autophagy19, 20. It is well established that ER stress stimulates autophagy70. However, in diabetic embryopathy, ER stress does not enhance autophagy; in fact, autophagy is diminished in neuroepithelia exposed to maternal diabetes20, 26, 27 The signal transduction mechanism underlying enhanced ER stress and diminished autophagy in diabetic embryopathy is distinct to other systems. p70S6K1 activation-induced protein overproduction may override the ER capacity of posttranslational processing resulting in ER stress. ER stress negatively affects mitochondrial function50. Suggesting that p70S6K1 may play a role in the induction of mitochondrial dysfunction. The present study demonstrated that p70S6K1 was required for autophagy impairment in diabetic embryopathy and removing p70S6K1 restored autophagy leading to suppression of cellular stress induced by maternal diabetes. Thus, p70S6K1 is a key autophagy regulator and cellular stress enhancer in the pathogenesis of diabetic embryopathy.
Apoptosis is directly involved in the induction of diabetic embryopathy41, 71. We have consistently shown that maternal diabetes-induced neuroepithelial cell apoptosis is caspase 8-dependent20, 41. It has been reported that ER stress induces caspase 8 activation in other systems72. The Rps6kb1 gene knockout relieves ER stress and reduces maternal diabetes-induced apoptosis.
The C17.2 cell line may not truly reflect the cell biology of the embryonic neuroepithelium. This cell line was derived from neonatal cerebellum, which is developmentally more differentiated than neuroepithelium of the neurulation-stage embryo73. Because using primary neuroepithelial cells is impossible due to the very small size of the neuroepithelium, cell lines are the choices for some experiments that are essentially being done in vitro. Our previous studies have shown that high glucose mimics the in vivo condition of maternal diabetes to trigger cellular stress, suppress autophagy and induce apoptosis in C17.2 cells20,27, 57. Therefore, the C17.2 cell line is suitable for the current study.
In summary, our study reveals a mechanism underlying maternal diabetes-suppressed autophagy in the neuroepithelium leading to NTD formation. We demonstrated that p70S6K1 negatively regulates autophagy. Future studies may aim to reveal the importance of autophagy in other morphogenetic processes that are affected by maternal diabetes.
Clinical Significance
Our studies show that impaired autophagy is critically involved in the pathogenesis of maternal diabetes-induced NTDs. Clinical research has demonstrated that defective autophagy is involved in the pathogenesis of various pregnancy-related diseases. For example, altered autophagy is associated with preterm delivery, intrauterine growth restriction, stillbirth, low birthweight, aberrant placental metabolism and syncytial knot formation74–83. Intrauterine inflammation suppresses autophagy in the fetal brain74. Autophagy is an important contributor to the pathogenesis of preterm birth because altered autophagy is observed in preterm birth caused by fetoscopic laser surgery in twin-twin transfusion syndrome84. Autophagy is inhibited in vaginal epithelial cells infected by Group B streptococcus76. It has been demonstrated that stillbirth and late-term placentas contain larger and arrested autophagosomes than those in 37 to 39-week placentas, indicating inhibition of the autophagic process in these placentas81. Oxidative stress85–88 and pro-apoptotic kinases that negatively regulate autophagy in diabetic pregnancy are critical etiological factors in the pathogenesis of many maternal-fetal complications20, 41, 47, 48. It is still unclear whether autophagy impairment is a significant contributor or just a secondary effect associated with adverse pregnancy outcomes in various maternal conditions. Therefore, further work may determine the potential involvement of autophagy impairment in the induction of specific pregnancy complications.
Research Implications
Autophagy sustains cellular homeostasis and is essential for cell survival during embryonic development23. Thus, determining the cause of autophagy dysregulation is an important area in maternal-fetal complications. Our study sets an example in this research area by demonstrating that autophagy in neuroepithelial cells of the developing embryo is suppressed by maternal diabetes leading to diabetes-induced NTDs19, 20.
p70S6K1 regulates many important biological processes, including protein synthesis, maintenance of cellular energy states, cell growth, proliferation and survival65. There is no report in pre-gestational diabetes-induced p70S6K1 activation. Thus, the present study provides the novel role of p70S6K1 in the etiology of diabetic embryopathy through repressing autophagy.
ER stress and mitochondrial dysfunction are causally involved in diabetic teratogenesis20, 57 Embryos exposed to diabetes exhibit impaired autophagy, ER stress and mitochondrial dysfunction19, 20. The relationship among these three cellular processes in diabetic embryopathy is unclear. It is well established in other system that ER stress stimulates autophagy. However, autophagy is diminished in neuroepithelia exposed to maternal diabetes20, 26, 27. The signal transduction mechanism underlying diabetes-enhanced ER stress and diminished autophagy in diabetic embryopathy is distinct to other system. p70S6K1 activation-induced protein overproduction may override the ER capacity of posttranslational processing leading to ER stress. ER stress negatively affects mitochondrial function50, suggesting that p70S6K1 may play a role in the induction of mitochondrial dysfunction. The present study demonstrated that p70S6K1 was required for autophagy impairment in diabetic embryopathy and removing p70S6K1 restored autophagy leading to suppression of ER stress and restoration of mitochondrial function. Thus, p70S6K1 is a key autophagy regulator and cellular stress enhancer in the pathogenesis of diabetic embryopathy and warrants further study.
Apoptosis is directly involved in the induction of diabetic embryopathy41, 71. Apoptosis and autophagy are two inter-related cellular processes that regulate cell fate. Studies have suggested that their regulation is intimately connected and the same regulators can sometimes control both autophagy and apoptosis60, 61.
We elucidated a mechanism by which deleting the gene encoding p70S6K1 alleviates diabetic embryopathy by resorting autophagy and inhibiting apoptosis, suggesting that autophagy is essential for neuroepithelial cell viability. Future studies may aim to reveal the importance of autophagy in cell survival processes that are affected by maternal diabetes.
Strengths and Weaknesses
Strengthens of our study are 1) the use of the Rps6kb1 gene deletion mouse model in determining the role of p70S6K1 in diabetic embryopathy; 2) the delicate design of the animal studies in revealing the cause of diabetic embryopathy, and 3) the detailed analyses of cellular stress processes including impaired autophagy, ER stress and mitochondrial dysfunction. The findings in these well-designed molecular studies provide the mechanistic basis for autophagy dysregulation-associated pathogenesis.
One of the weaknesses of our study is that there is no immediate clinical impact. Our study is not a human study and animal experiments in the present study may not faithfully reflect the complex human conditions. However, the underlying etiology of maternal diabetes-induced neural tube defects in the fetus is still elusive. Additionally, diabetic embryopathy is a significant health problem affecting both the mother and her newborn. The current study has potential translational value and provides a mechanistic basis for future clinical research.
Conclusions
Our findings show that p70S6k1 mediates the inhibitory effect of maternal diabetes on autophagy in the developing neuroepithelium. p70S6K1 decreases autophagy and induces NTD formation in diabetic embryopathy. Furthermore, p70S6K1 deficiency relieves ER stress and inhibits maternal diabetes-induced apoptosis. Our study reveals a mechanism underlying maternal diabetes-suppressed autophagy in the neuroepithelium leading to NTD formation.
Condensation.
Deficiency of the oxidative stress-responsive kinase p70S6K1 ameliorates diabetic embryopathy via restoring autophagy.
AJOG at a Glance.
Why was this study conducted?
Our previous studies have demonstrated that autophagy in the developing neuroepithelium is suppressed in diabetic embryopathy. Maternal diabetes disrupts neurulation leading to neural tube defects (NTDs). However, it is unclear how maternal diabetes inhibits autophagy leading to NTD formation. Because oxidative stress is critically involved in the induction of diabetic embryopathy, we aim to determine the role of an oxidative stress-responsive kinase, p70S6K1 (70 kDa ribosomal protein S6 kinase 1), in maternal diabetes-suppressed autophagy.
Key Findings
Deleting the Rps6kb1 gene encoding for the oxidative stress-responsive kinase p70S6K1 (70 kDa ribosomal protein S6 kinase 1) reverses maternal diabetes-inhibited autophagy.
Autophagy rescued by the deletion of the Rps6kb1 gene blocks endoplasmic reticulum stress and neuroepithelial cell apoptosis leading to amelioration of diabetic embryopathy.
An antioxidant blocks high glucose-induced p70S6K1 activation.
What does this add to what is known?
The findings reveal the critical involvement of p70S6K1 in the pathogenesis of maternal diabetes-induced NTDs and demonstrate that p70S6K1 is responsible for maternal diabetes-repressed autophagy in neuroepithelial cells of the developing embryo.
Acknowledgments
This work was supported by the NIH grants NIH R01DK083243, R01DK101972, R01DK103024, R01HL131737, R01HL134368, R01HL139060, R01HD100195 and R01 HD1022065. We would like to thank Dr. Sara C. Kozma at the College of Medicine, University of Cincinnati for providing us the p70S6K1 knockout mice. We thank Dr. Julie Rosen, Assistant Professor of Obstetrics, Gynecology & Reproductive Sciences and Executive Director for Medical Research and Scientific Publications at the University of Maryland School of Medicine, for critical reading and editing.
Glossary of Terms
- Diabetic embryopathy
Pregestational maternal diabetes-induced birth defects are collectively termed as diabetic embryopathy
- Neural tube defects (NTDs)
A severe structural birth defects with an open neural tube resulting from the failure of neurulation, a process involving the formation of the primitive brain and spinal cord during embryonic development
- Autophagy
Autophagy (or macroautophagy) is the natural, regulated, and destructive mechanism of the cell that disassembles unnecessary or dysfunctional components. Autophagy allows the orderly degradation and recycling of cellular material. In macroautophagy, targeted cytoplasmic constituents are isolated from the rest of the cell within a double-membrane vesicle known as an autophagosome. The autophagosome eventually fuses with lysosomes, resulting in the degradation and recycling of its contents. Three forms of autophagy are commonly described: macroautophagy, microautophagy, and chaperone-mediated autophagy. In disease, autophagy has been viewed as an adaptive response to stress to promote survival, whereas in other cases it appears to promote cell death and morbidity. In the extreme case of starvation, the breakdown of cellular components promotes cellular survival by maintaining energy levels
- p70S6K1
(Ribosomal protein S6 kinase beta-1) 70 kDa ribosomal protein S6 kinase 1 is encoded by the Rps6kb1 gene in humans. It is a serine/threonine kinase that acts downstream of mTOR, phosphatidylinositol (3,4,5)-trisphosphate and phosphoinositide-dependent kinase-1 in the phosphatidylinositol 3-kinase pathway. p70S6K1phosphorylates the S6 ribosomal protein, which induces protein synthesis
- LC3
LC3 (Microtubule-associated protein 1 light chain 3) is also called Atg8, which is a homologue of Apg8p in yeast. The conversion of cytosolic LC3-I (18 kDa) to autophagosome membrane-bound LC3-II (16 kDa) is a critical event in autophagy
- C17.2
C17.2 is a transformed cell line of NSCs (neural stem cells) isolated from the external germinal layer of neonatal mouse cerebellum. It has potential to differentiate into a variety of cell types, such as neurons, oligodendrocytes, and astrocytes, when transplanted into the appropriate part of the central nervous system in vivo or induced by neural factors in vitro. Therefore, it is an ideal cell model for assessing the in vitro effect of high glucose
- Endoplasmic reticulum (ER) stress
The endoplasmic reticulum (ER) is the cellular organelle that is critical for protein folding and secretion, calcium homeostasis, and lipid biosynthesis. Under various conditions, called ER stress, protein folding in the ER is impaired leading to the accumulation of misfolded proteins. Multiple cellular disturbances can cause ER stress including disturbances in redox regulation, calcium regulation, glucose deprivation, and viral infection. The ER stress response is initiated when the capacity of ER-resident chaperone proteins is exceeded by the load of misfolded proteins. Prolonged ER stress typically results in cell death by apoptosis
- mTOR
The mammalian target of rapamycin, is a kinase that is encoded by the Mtor gene. mTOR belongs to the phosphatidylinositol 3-kinase-related kinase family of protein kinases. mTOR regulates an array of cellular function including cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription. mTOR also promotes the activation of insulin receptors and insulin-like growth factor 1 receptors. The mTOR pathway, a central pathway that regulates metabolism and physiology, play important roles in the function of tissues including liver, muscle, white and brown adipose tissue, and the brain, and its dysregulation is manifested in human diseases, such as diabetes, obesity, depression, and certain cancers. p70S6K1 is a downstream effector of mTOR because mTOR activation leads to p70S6K1 activation (phosphorylation)
- Autophagosome
is a spherical structure with double layer membranes. It is the key structure in macroautophagy, the intracellular degradation system for cytoplasmic contents (e.g., abnormal intracellular proteins, excess or damaged organelles, invading microorganisms). After formation, autophagosomes deliver cytoplasmic components to the lysosomes. The outer membrane of an autophagosome fuses with a lysosome to form an autolysosome. The lysosome’s hydrolases degrade the autophagosome-delivered contents and its inner membrane
- siRNA
Small interfering RNA, sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA non-coding RNA molecules, 20–25 base pairs in length, similar to miRNA, and operating within the RNA interference (RNAi) pathway. It interferes with the expression of specific genes with complementary nucleotide sequences by degrading mRNA after transcription, preventing translation
- UPR
The unfolded protein response is a cellular stress response related to the endoplasmic reticulum (ER) stress. The UPR is activated in response to an accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum. In this scenario, the UPR has three aims: initially to restore normal function of the cell by halting protein translation, degrading misfolded proteins, and activating the signaling pathways that lead to increasing the production of molecular chaperones involved in protein folding. If these objectives are not achieved within a certain time span or the disruption is prolonged, the UPR aims towards apoptosis
Footnotes
Disclosure: None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Developmental cell 2004;6:463–77. [DOI] [PubMed] [Google Scholar]
- 2.Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryotic cell 2002;1:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nature reviews Molecular cell biology 2005;6:439–48. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell death and differentiation 2005;12 Suppl 2:1528–34. [DOI] [PubMed] [Google Scholar]
- 5.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science 2004;306:990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nature reviews Drug discovery 2007;6:304–12. [DOI] [PubMed] [Google Scholar]
- 7.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011;147:728–41. [DOI] [PubMed] [Google Scholar]
- 8.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell death and differentiation 2005;12 Suppl 2:1509–18. [DOI] [PubMed] [Google Scholar]
- 9.Meijer AJ, Codogno P. Autophagy: regulation and role in disease. Critical reviews in clinical laboratory sciences 2009;46:210–40. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Livingston MJ, Liu Z, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cellular and molecular life sciences : CMLS 2018;75:669–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging 2009;1:515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Chen Y, Ji Y, et al. Gemcitabine induces apoptosis and autophagy via the AMPK/mTOR signaling pathway in pancreatic cancer cells. Biotechnology and applied biochemistry 2018. [DOI] [PubMed] [Google Scholar]
- 13.Bhuiyan MS, Pattison JS, Osinska H, et al. Enhanced autophagy ameliorates cardiac proteinopathy. The Journal of clinical investigation 2013;123:5284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei Y, Thompson MD, Cohen RA, Tong X. Autophagy and oxidative stress in cardiovascular diseases. Biochimica et biophysica acta 2015;1852:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Molecular cell 2008;30:678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochimica et biophysica acta 2009;1792:3–13. [DOI] [PubMed] [Google Scholar]
- 17.Bujak AL, Crane JD, Lally JS, et al. AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell metabolism 2015;21:883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Molecular and cellular biology 2005;25:1025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C, Li X, Wang F, Weng H, Yang P. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. American journal of physiology Endocrinology and metabolism 2013;305:E667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Xu C, Reece EA, et al. Protein kinase C-alpha suppresses autophagy and induces neural tube defects via miR-129–2 in diabetic pregnancy. Nature communications 2017;8:15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nager M, Sallan MC, Visa A, et al. Inhibition of WNT-CTNNB1 signaling upregulates SQSTM1 and sensitizes glioblastoma cells to autophagy blockers. Autophagy 2018;14:619–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanninen TT, de Andrade Ramos BR, Witkin SS. The role of autophagy in reproduction from gametogenesis to parturition. European journal of obstetrics, gynecology, and reproductive biology 2013;171:3–8. [DOI] [PubMed] [Google Scholar]
- 23.Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science 2008;321:117–20. [DOI] [PubMed] [Google Scholar]
- 24.Fimia GM, Stoykova A, Romagnoli A, et al. Ambra1 regulates autophagy and development of the nervous system. Nature 2007;447:1121–5. [DOI] [PubMed] [Google Scholar]
- 25.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett 2010;584:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C, Chen X, Reece EA, Lu W, Yang P. The increased activity of a transcription factor inhibits autophagy in diabetic embryopathy. American journal of obstetrics and gynecology 2019;220:108 e1–08 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu C, Chen X, Sheng WB, Yang P. Trehalose restores functional autophagy suppressed by high glucose. Reproductive toxicology 2019;85:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. American journal of obstetrics and gynecology 2013;209:345 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. American journal of obstetrics and gynecology 2015;213:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Reece EA, Yang P Oxidative stress is responsible for maternal diabetes-impaired transforming growth factor beta signaling in the developing mouse heart. American journal of obstetrics and gynecology 2015;212:650 el–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Weng H, Quon MJ, et al. Dominant negative FADD dissipates the proapoptotic signalosome of the unfolded protein response in diabetic embryopathy. American journal of physiology Endocrinology and metabolism 2015;309:E861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weng H, Li X, Reece EA, Yang P SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. American journal of obstetrics and gynecology 2012;206:448 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wentzel P, Jansson L, Eriksson UJ. Diabetes in pregnancy: uterine blood flow and embryonic development in the rat. Pediatric research 1995;38:598–606. [DOI] [PubMed] [Google Scholar]
- 34.Wentzel P, Eriksson UJ. Ethanol-induced fetal dysmorphogenesis in the mouse is diminished by high antioxidative capacity of the mother. Toxicological sciences : an official journal of the Society of Toxicology 2006;92:416–22. [DOI] [PubMed] [Google Scholar]
- 35.Wentzel P, Rydberg U, Eriksson UJ. Antioxidative treatment diminishes ethanol-induced congenital malformations in the rat. Alcoholism, clinical and experimental research 2006;30:1752–60. [DOI] [PubMed] [Google Scholar]
- 36.Wentzel P, Eriksson UJ. Genetic influence on dysmorphogenesis in embryos from different rat strains exposed to ethanol in vivo and in vitro. Alcoholism, clinical and experimental research 2008;32:874–87. [DOI] [PubMed] [Google Scholar]
- 37.Zabihi S, Wentzel P, Eriksson UJ. Altered uterine perfusion is involved in fetal outcome of diabetic rats. Placenta 2008;29:413–21. [DOI] [PubMed] [Google Scholar]
- 38.Eriksson UJ. Congenital anomalies in diabetic pregnancy. Seminars in fetal & neonatal medicine 2009;14:85–93. [DOI] [PubMed] [Google Scholar]
- 39.Nash P, Olovsson M, Eriksson UJ. Placental dysfunction in Suramin-treated rats: impact of maternal diabetes and effects of antioxidative treatment. Journal of the Society for Gynecologic Investigation 2005;12:174–84. [DOI] [PubMed] [Google Scholar]
- 40.Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. American journal of obstetrics and gynecology 2010;203:75 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, Li X, Xu C, et al. Maternal hyperglycemia activates an ASK1-Fox03a-caspase 8 pathway that leads to embryonic neural tube defects. Science signaling 2013;6:ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. American journal of obstetrics and gynecology 2008;198:130 e1–7. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Wu Y, Yang P. High glucose-induced oxidative stress represses sirtuin deacetylase expression and increases histone acetylation leading to neural tube defects. J Neurochem 2016;137:371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Dong D, Reece EA, Wang AR, Yang P. Oxidative stress-induced miR-27a targets the redox gene nuclear factor erythroid 2-related factor 2 in diabetic embryopathy. American journal of obstetrics and gynecology 2018;218:136 el–36 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong J, Xu C, Reece EA, Yang P The green tea polyphenol EGCG alleviates maternal diabetes-induced neural tube defects by inhibiting DNA hypermethylation. American journal of obstetrics and gynecology 2016;215:368 e1–68 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Weng H, Reece EA, Yang P SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. American journal of obstetrics and gynecology 2011;205:84 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Weng H, Xu C, Reece EA, Yang P Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes 2012;61:2084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Xu C, Yang P c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes 2013;62:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin X, Yang P, Reece EA, Yang P Pregestational type 2 diabetes mellitus induces cardiac hypertrophy in the murine embryo through cardiac remodeling and fibrosis. American journal of obstetrics and gynecology 2017;217:216 e1–16 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang P, Xu C, Reece EA, et al. Tip60- and sirtuin 2-regulated MARCKS acetylation and phosphorylation are required for diabetic embryopathy. Nature communications 2019;10:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chappell JH Jr., Wang XD, Loeken MR. Diabetes and apoptosis: neural crest cells and neural tube. Apoptosis : an international journal on programmed cell death 2009;14:1472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Reece EA, Zhong J, et al. Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. American journal of obstetrics and gynecology 2016;215:366 e1–66 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong D, Reece EA, Lin X, Wu Y, AriasVillela N, Yang P. New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects. American journal of obstetrics and gynecology 2016;214:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang TI, Horal M, Jain SK, Wang F, Patel R, Loeken MR. Oxidant regulation of gene expression and neural tube development: insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia 2003;46:538–45. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Borg LA, Eriksson UJ. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. The Anatomical record 1995;241:255–67. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y, Wang F, Reece EA, Yang P. Curcumin ameliorates high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. American journal of obstetrics and gynecology 2015;212:802 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F, Wu Y, Gu H, et al. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes 2015;64:973–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology 2009;50:216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apoptosis Thorburn A. and autophagy: regulatory connections between two supposedly different processes. Apoptosis : an international journal on programmed cell death 2008;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nature reviews Molecular cell biology 2014;15:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J 1998;17:6649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong D, Fu N, Yang P. MiR-17 Downregulation by High Glucose Stabilizes Thioredoxin-Interacting Protein and Removes Thioredoxin Inhibition on ASK1 Leading to Apoptosis. Toxicological sciences : an official journal of the Society of Toxicology 2016;150:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Yang P. The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicological sciences : an official journal of the Society of Toxicology 2015;144:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Ali H, Ding Y, Slepak T, et al. The mTOR Substrate S6 Kinase 1 (S6K1) Is a Negative Regulator of Axon Regeneration and a Potential Drug Target for Central Nervous System Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 2017;37:7079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. The Journal of biological chemistry 1995;270:2320–6. [DOI] [PubMed] [Google Scholar]
- 67.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. The international journal of biochemistry & cell biology 2011;43:47–59. [DOI] [PubMed] [Google Scholar]
- 68.Klionsky DJ, Meijer AJ, Codogno P. Autophagy and p70S6 kinase. Autophagy 2005;1:59–60; discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 69.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes care 2007;30 Suppl 2:S112–9. [DOI] [PubMed] [Google Scholar]
- 70.Qin L, Wang Z, Tao L, Wang Y ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 2010;6:239–47. [DOI] [PubMed] [Google Scholar]
- 71.Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes 1997;46:1189–97. [DOI] [PubMed] [Google Scholar]
- 72.Mishra R, Karande AA. Endoplasmic reticulum stress-mediated activation of p38 MAPK, Caspase-2 and Caspase-8 leads to abrin-induced apoptosis. PloS one 2014;9:e92586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 1992;68:33–51. [DOI] [PubMed] [Google Scholar]
- 74.Lei J, Zhong W, Almalki A, et al. Maternal Glucose Supplementation in a Murine Model of Chorioamnionitis Alleviates Dysregulation of Autophagy in Fetal Brain. Reproductive sciences 2017:1933719117734321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanninen TT, Sisti G, Witkin SS. Induction of the 70 kDa heat shock protein stress response inhibits autophagy: possible consequences for pregnancy outcome. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2016;29:159–62. [DOI] [PubMed] [Google Scholar]
- 76.Scholl J, Nasioudis D, Boester A, Speleotes M, Grunebaum A, Witkin SS. Group B streptococcus alters properties of vaginal epithelial cells in pregnant women. American journal of obstetrics and gynecology 2016;214:383 e1–5. [DOI] [PubMed] [Google Scholar]
- 77.Ilekis JV, Tsilou E, Fisher S, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. American journal of obstetrics and gynecology 2016;215:S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maiti K, Sultana Z, Aitken RJ, et al. Evidence that fetal death is associated with placental aging. American journal of obstetrics and gynecology 2017;217:441 e1–41 e14. [DOI] [PubMed] [Google Scholar]
- 79.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. American journal of obstetrics and gynecology 2017;217:592 e1–92 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? American journal of obstetrics and gynecology 2018;218:S762–S73. [DOI] [PubMed] [Google Scholar]
- 81.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. American journal of obstetrics and gynecology 2018;218:S745–S61. [DOI] [PubMed] [Google Scholar]
- 82.Kanninen TT, de Andrade Ramos BR, Jaffe S, et al. Inhibition of autophagy by sera from pregnant women. Reproductive sciences 2013;20:1327–31. [DOI] [PubMed] [Google Scholar]
- 83.Clerici G, Antonelli C, Rizzo G, Kanninen TT, Di Renzo GC. Atypical hemodynamic pattern in fetuses with hypercoiled umbilical cord and growth restriction. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2013;26:558–62. [DOI] [PubMed] [Google Scholar]
- 84.Donepudi R, Akkermans J, Mann L, et al. Recurrent Twin-Twin Transfusion Syndrome (rTTTS) and Twin Anemia Polycythemia Sequence (TAPS) after fetoscopic laser surgery (FLS): size (of the cannula) does matter. Ultrasound in obstetrics & gynecology : the official journal of the international Society of Ultrasound in Obstetrics and Gynecology 2017. [DOI] [PubMed] [Google Scholar]
- 85.Ginsberg Y, Khatib N, Saadi N, Ross MG, Weiner Z, Beloosesky R. Maternal pomegranate juice attenuates maternal inflammation-induced fetal brain injury by inhibition of apoptosis, neuronal nitric oxide synthase, and NF-kappaB in a rat model. American journal of obstetrics and gynecology 2018;219:113 e1–13 e9. [DOI] [PubMed] [Google Scholar]
- 86.McMaster-Fay RA. Oxidative stress and inflammatory biomarkers in normal and preeclamptic pregnancies. American journal of obstetrics and gynecology 2017;217:492–93. [DOI] [PubMed] [Google Scholar]
- 87.Reece EA, Ma XD, Zhao Z, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. American journal of obstetrics and gynecology 2005;192:967–72. [DOI] [PubMed] [Google Scholar]
- 88.Reece EA, Wu YK, Zhao Z, Dhanasekaran D. Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. American journal of obstetrics and gynecology 2006;194:580–5. [DOI] [PubMed] [Google Scholar]