Abstract
Background:
Uterine leiomyomas, the most common tumors of the female reproductive system, feature excessive deposition of disordered stiff extracellular matrix (ECM) and fundamentally altered mechanical signaling pathways. Specifically, these alterations impact the normal dynamic state of responsiveness to mechanical cues in the extracellular environment. These mechanical cues are converted via integrins, cell membrane receptors, to biochemical signals including cytoskeletal signaling pathways to maintain mechanical homeostasis. Leiomyoma cells overexpress β1 integrin and other downstream mechanical signaling proteins. We previously reported that simvastatin, an anti-hyperlipidemic drug, has anti-leiomyoma effects through cellular, animal model and epidemiologic studies.
Objective:
The purpose of this study was to examine the hypothesis that simvastatin might influence altered mechanotransduction in leiomyoma cells.
Study Design:
This is a laboratory-based experimental study. Primary leiomyoma cells were isolated from five patients who underwent hysterectomy at the Department of Gynecology and Obstetrics at the Johns Hopkins Hospital. Primary and immortalized human leiomyoma (HuLM) cells were treated with simvastatin at increasing concentrations (0.001, 0.01, 0.1, 1μM or control) for 48 h. Protein and mRNA levels of β1 integrin and ECM components involved in mechanical signaling were quantified by qRT-PCR, western blotting, and immunofluorescence. In addition, we examined the effect of simvastatin on RhoA activity using pull-down assay and gel contraction.
Results:
We found that simvastatin significantly reduced the protein expression of β1 integrin by 44% and type I collagen by 60% compared to untreated leiomyoma cells. Simvastatin-treated cells reduced phosphorylation of FAK down to 26-60% of control whereas it increased total FAK protein expression. Using a RhoA pull-down activation assay, we observed that simvastatin-treated cells had reduced levels of active RhoA by 45-85% compared to control. Consistent with impaired RhoA activation, simvastatin treatment reduced tumor gel contraction where gel area was 122-153% larger compared to control. Furthermore, simvastatin treatment led to reduced levels of mechanical signaling proteins involved in β1 integrin downstream signaling, including AKAP13, ROCK1, MLCK, and cyclin D1.
Conclusion:
The results of this study suggest a possible therapeutic role for simvastatin to restore the altered state of mechanotransduction signaling in leiomyoma. Collectively, the findings are aligned with previous epidemiologic and other reports and support the need for clinical trials.
Keywords: Simvastatin, leiomyoma, mechanotransduction, extracellular matrix, β1 integrin, type I collagen, FAK, activated RhoA, AKAP13, ROCK1, MLCK, cyclin D1
Graphical Abstract

INTRODUCTION
Mechanotransduction is a dynamic process whereby cells respond to mechanical cues from the surrounding environment via cell membrane sensors and transform these mechanical signals into a series of cytoskeletal and intracellular biochemical signals. In turn, these cellular responses modulate several processes including the production of extracellular matrix (ECM) components. Thus, the mechanotransduction process is bidirectional (outside-in and inside-out) where both cells and the surrounding ECM affect each other to maintain homeostasis.1
Uterine leiomyomas, the most common tumors of the female genital tract, are believed to originate from uterine smooth muscle cells.2-4 A dysregulated ECM is a key feature of uterine leiomyomas.5 Studies by our group6 and others7 indicate that altered mechanical signaling and homeostasis play a significant role in uterine leiomyoma development and growth. Uterine leiomyoma demonstrate increased mechanical stress, high ECM density and stiffness6, and altered response to mechanical cues.8
Integrins are transmembrane receptors that play a key role in mechanosensing and mechanotransduction. Aberrations of integrins and integrin-mediated mechanotransduction appear to be major contributors to uterine leiomyoma pathobiology.5 Leiomyoma cells overexpress β1 integrin9, 10 which leads to overphosphorylation of focal adhesion kinase (FAK) compared to matched normal myometrial cells.10 Furthermore, A-kinase anchor protein 13 (AKAP13) is highly expressed in leiomyoma11 and contributes to altered mechanotransduction by activating RhoA (ras homolog family member A)6, 8 (Figure 1). Thus, considerable evidence indicates that fibroids do not respond properly to mechanical cues and suggested that restoration of normal mechanical signaling might be beneficial for leiomyoma treatment.
Figure 1. Altered mechanotransduction pathways in uterine leiomyoma cells.
Human leiomyoma cells surrounded by ECM consisting mainly of collagen I. The figure shows the mechanical interaction between cells and collagen I by heterodimeric (α and β1) integrin receptors. Activated β1 integrin provides mechanical forces for polymerization and organization of actin filaments and biochemical signaling for the formation of the adhesion complex triggers autophosphorylation of FAK. Phosphorylated FAK leads to the activation of AKAP13 and RhoA which further recruits ROCK1 and MLCK activation for altering mechanotransduction pathways in human leiomyoma cells. ECM, extracellular matrix; FAK, focal adhesion kinase; AKAP13, A kinase anchor protein 13; RhoA, Ras homolog gene family, member A; ROCK, RhoA kinase; MLCK, myosin light chain kinase.
Simvastatin is an inhibitor of the 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase enzyme, the rate-limiting step in cholesterol biosynthesis. We previously demonstrated that simvastatin induces apoptosis, inhibits proliferation and suppresses tumor growth in leiomyoma cell culture and animal model.12, 13 In addition, it inhibits the expression of key ECM proteins, including types I and III collagen, fibronectin, versican, and brevican in leiomyoma cells.14 Importantly, simvastatin has been shown to modulate mechanical signaling pathways. For example, simvastatin improves RhoA-mediated cellular response to increased matrix stiffness15 which may prevent atherosclerosis instigated by age-related arterial stiffening.15 Therefore, herein we explored the ability of simvastatin to ameliorate the perturbed mechanotransduction in uterine leiomyoma cells.
MATERIALS AND METHODS
Primary and immortalized leiomyoma cells
Primary leiomyoma cells were isolated from five patients who underwent hysterectomy at the Department of Gynecology and Obstetrics at the Johns Hopkins University Hospital. Institutional review board (IRB) at the Johns Hopkins University reviewed and approved the study and informed consents were obtained from patients. Tissues were brought to the laboratory immediately after surgery and washed several times with a Hanks' Balanced Salt Solution (HBSS, Thermo Fisher Scientific, Waltham, Massachusetts) without calcium or magnesium. Leiomyoma tissue was manually cut into small pieces (1-2 mm3). We then incubated tissues in sterile HBSS (without phenol, calcium, or magnesium) with collagenase (Worthington, Lakewood, New Jersey), deoxyribonuclease (DNase, Sigma-Aldrich), antibiotic-antimycotic mixture (Thermo Fisher Scientific), and HEPES buffer solution (Thermo Fisher Scientific) at 37°C on a shaker for 4-8 h. The digest was filtered through a 100-μm filter and cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM)/F-12 (Thermo Fisher Scientific) medium supplemented with HEPES, L-glutamine, 10 % FBS, and 1% antibiotic-antimycotic. Immortalized human leiomyoma (HuLM) cells were cultured and maintained in smooth muscle cell growth medium (SmGM, Lonza, Walkersville, Maryland) which includes smooth muscle basal medium (SmBM), 5% fetal bovine serum (FBS), 0.1% insulin, 0.2% recombinant human fibroblast growth factor B, 0.1% gentamicin sulfate and amphotericin B mixture, and 0.1% human epidermal growth factor. Cells were incubated at 37 °C in a humidified atmosphere (95% air, 5% CO2) and split at 70-80% confluence.
Simvastatin preparation
We purchased simvastatin from Cayman Chemicals (Ann Arbor, MI), prepared stock solution (10 mM) in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO), and kept it at −20°C until use. The final concentration of DMSO in culture medium was less than 0.1% v/v.
RNA extraction and quantitative Real-Time polymerase chain reaction
Primary and HuLM cells were plated in a 6-well plate at a concentration of 1.2 x 105 cells/well and maintained with complete DMEM/F-12 or SmGM media, respectively, until 60-70% confluence. After overnight serum starvation, cells were treated with complete medium containing 0.001, 0.01, 0.1 and 1μM simvastatin for 48 h. We chose these concentrations based on clinical relevance and previous publications.13, 14 Control cells were exposed to vehicle (DMSO). Cells were harvested and pellets obtained by centrifugation at 1500 rpm for 10 minutes.
The total RNA content of the cells was measured using an RNeasy Mini Kit (Qiagen, Gaithersburg, MD) as per manufacturer’s instructions. RNA concentration and purity were checked by using the NanoVue Plus (Biochrom US, Holliston, MA). Reverse transcription was performed using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) in a Bio-Rad Thermocycler according to the manufacturer’s instructions. The 100 ng/μL of total RNA from each sample was reverse-transcribed in 20 μl reaction volume to complementary DNA (cDNA). The qRT-PCR was performed using the LightCycler® 96 System (Roche Diagnostics, Mannheim, Germany) using FastStart Essential DNA green Master (Roche Diagnostics) according to the manufacturer's protocol. An equal amount of cDNA of each sample was added to the master mix containing appropriate primer sets in a 10 μl reaction volume. All experiments were performed in duplicate and repeated three times. RPLP0 was amplified under the same qRT-PCR conditions for normalizing quantitative data. Forward and reverse primer sequences (IDT, Coralville, Iowa) used for determining the messenger RNA (mRNA) expression of several genes, including β1 integrin, FAK, AKAP13, ROCK1 (Rho associated protein kinase 1), MLCK (myosin light chain kinase), cyclin D1, and type I collagen (Supplementary table 1). The relative mRNA expression was calculated using the ΔΔCT method and is presented as fold increase relative to control levels expressed as 1.00.
Protein extraction and western blot analysis
Primary and HuLM cells were cultured in 100 mm dish (5 x 105 cells/dish) with complete DMEM/F-12 or SmGM media, respectively, until they reach 60-70% confluence. After overnight serum starvation, cells were treated with complete medium containing 0.001, 0.01, 0.1 and 1μM simvastatin or DMSO (control) for 48 h. Cells were lysed in radio immunoprecipitation assay buffer (RIPA, Sigma-Aldrich) containing protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentrations were quantified using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). For western blotting, 30-40 μg of protein lysates were resolved in 4-12% Bis-Tris Protein Gradient Gels (Thermo Fisher Scientific) for electrophoresis and then transferred onto a nitrocellulose membrane (Thermo Fisher Scientific). The membrane was blocked with 5% non-fat-milk with Tris buffered saline with 0.1 % Tween 20 (TBST, Thermo Fisher Scientific) for 1 h at room temperature. Membrane were incubated with specific primary antibodies: anti-collagen I (Thermo Fisher Scientific, #PA5-29569), anti-β1 integrin (Cell Signaling Technology (CST), #4706), anti-FAK (CST, #3285S), anti-phospho-FAK (CST, #8556), anti-ROCK1 (CST, #4035), anti-MLCK (Thermo Fisher Scientific, #PA5-46298), anti-cyclin D1 (CST, #2922) overnight at 4° C. For preparing anti-AKAP13, rabbit primary antisera was focused counter to AKAP13 and a peptide corresponding to the AKAP13 protein (CREKEKDKIKEKEKDS KEKEKDKKTLNGHTF) was generated polyclonal antiserum 6969 using standard techniques. BRX binding was confirmed by enzyme-linked immunosorbent assay (Covance Laboratories, Sterling, VA).16 Membrane were incubated with appropriate horseradish Peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare) for 1 h at room temperature. Immunolabeled proteins were identified by using a SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific) and specific protein bands were visualized using Azure Imager c300 (Azure Biosystems, Dublin, California). The band intensity was quantified using NIH Image J software and normalized against corresponding anti-β-actin antibody (CST, #4970); normalized values were used to create final graphs.
Cytoimmunofluorescence
Primary and HuLM cells were cultured on an 8-well chamber slide (3x103 cells/well). When cells reached 60% confluence, they were serum-starved overnight then treated with 0.001, 0.01, 0.1 and 1μM simvastatin or DMSO (control) for 48 h. Cells were fixed in a 4% formaldehyde solution for 15 minutes at room temperature. After washing three times in 1x PBS for 5 min each, cells were blocked for 1 h using blocking solution with 1x PBS, 5% normal goat serum (CST) and 0.3% Triton X-100 (Sigma-Aldrich) at room temperature. Primary antibodies, anti-pFAK (Thermo Fisher Scientific, #700013) and anti-β1 integrin (Invitrogen, #MA5-13658), and Alexa 647 phalloidin (staining of F-actin, CST) were diluted with antibody dilution buffer containing 1x PBS, 1% BSA (Sigma-Aldrich) and 0.3% Triton X-100 and cells were incubated overnight at 4°C in a moist chamber. After three gentle rinses with 1x PBS, the slide was incubated with anti-mouse Alexa 546 (Invitrogen, #A11030) and anti-rabbit Alexa 488 (Invitrogen, #A11034) conjugated secondary antibody (1 h at room temperature in the dark) for visualizing the pFAK and β1 integrin expression. After the last three rinses, the slide was fixed with Prolong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific) overnight at room temperature. The cells were examined under a confocal microscope (Leica SP8 microsystem, Wetzlar, Germany). The exposure time is 1.9 microseconds per pixel. Each image was 4 megapixels, so the total duration was 7.6 seconds of scanning. The exposure time was similar for each collection. All images were captured with a 20X magnification. Fluorescence integrated density was quantified using NIH Image J software and is presented as fold change relative to control levels expressed as 1.00.
RhoA activation assay
Activated RhoA was measured using a commercially available Rho pull-down activation assay kit (Cytoskeleton Inc., Denver, CO) according to the manufacturer's protocol. The assay principle is that the Rho binding domain (RBD) of the Rhotekin GST-fusion protein binds active (GTP-bound) but not inactive (GPD-bound) Rho. In brief, HuLM cells were cultured and maintained in 100 mm dishes (5x105 cells/dish), serum-starved overnight, then treated with 0.001, 0.01, 0.1, 1 and 10μM simvastatin or DMSO (control) for 48 h. Cells were harvested on ice in lysis buffer with protease inhibitor cocktail. The active RhoA was pulled down using GST-Rhotekin-RBD protein beads after 1 h incubation at 4°C followed by washing the beads with washing buffer. The precipitated active RhoA was detected by western blotting using specific anti-RhoA monoclonal antibody and the band intensity was quantified as a ratio compared to total RhoA protein expressed in the same samples using NIH Image J software.
Gel contraction assay
The gel contraction assay was performed as previously described.17 The principle is that cells are grown in a three-dimensional (3D) collagen gel matrix, which provides a physiological environment for the cells to grow. As cells interact with ECM collagen via integrins, the cell-ECM complex starts to contract, and the gel gets smaller over time. The more the contraction, the smaller the diameter of the gel. In brief, we prepared a total of 5 ml of rat tail collagen I and HuLM cell suspension by adding 2.5 ml of 6 mg/ml rat tail collagen-I (EMD Millipore), 1.5 ml complete SmGM medium, 0.5 ml of 5X PBS, 0.07 ml of 0.1M NaOH, and 0.43 ml medium containing 1x106 cell suspended. We then poured 0.5 ml of the suspension in each well in a 24-well plate (each will have about 1x105 cells). The plate was then incubated at 37°C for 30 minutes for the collagen to polymerize. Then, we added 0.5 ml SmGM and were kept in the incubator until the cells are 50-60% confluent (usually takes around 2 weeks). The medium is changed every 48-72 hours. The 3D HuLM gels were treated with 0.001, 0.01, 0.1, 1μM simvastatin or DMSO (control) for 96 h. Then, gels were moved from 24-well plate to 12-well plate, photographs taken and the gel surface area analyzed by NIH Image J software.
Statistical analysis
All experiments were repeated three separate times and the data were expressed as mean ± standard error of the mean (SEM). Data were compared to control using an unpaired two-tailed Student t test using Prism 5 software (GraphPad Software, Inc., La Jolla, CA). Statistical significance was considered when p value was < 0.05.
RESULTS
Simvastatin treatment reduced β1 integrin expression in leiomyoma cells
Leiomyoma cells overexpress integrins, a phenomenon believed to contribute to altered mechanotransduction and tumor development.9 To explore the effect of simvastatin on β1 integrin expression, we treated primary and HuLM cells for 48 h with different simvastatin concentrations (0.001, 0.01, 0.1, 1μM or control).
We found that simvastatin treatment reduced β1 integrin mRNA transcripts to 22-39% in primary and 17-40% in HuLM cells of controls, though there was some variability (Fig. 2A and 2B). Consistent with the reduced mRNA expression, simvastatin reduced β1 integrin protein expression by 29-38% in primary leiomyoma cells and 30-44% in HuLM cells, compared to untreated cells (Fig. 2C and 2D).
Figure 2. Reduction in steady state levels of β1 integrin in simvastatin-treated leiomyoma cells.
Primary and immortalized (HuLM) human leiomyoma cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. The mRNA levels of β1 integrin genes were measured by quantitative real time PCR (qRT-PCR) (A, B). RPLP0 was amplified under the same qRT-PCR conditions for normalizing quantitative data. C, D: β1 integrin protein expression were determined by western blotting along with quantification and the data were normalized by β-actin. Results are expressed as mean ± standard error of the mean (SEM). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control. The experiments were repeated three times.
Similar to the mRNA and protein expression, we examined the expression of β1 integrin and its co-localization with F-actin by immunofluorescence both in primary and HuLM cells. As shown in Fig.3, β1 integrin (green fluorescence) was present in high levels in primary (Fig. 3A) and HuLM (Fig. 3B) cells, while simvastatin clearly reduced expression in a dose-dependent manner.
Figure 3. Cytoimmunofluorescence images of β1 integrin in control and simvastatin-treated leiomyoma cells.
Primary and immortalized (HuLM) human leiomyoma cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. Cellular distribution of β1 integrin (green fluorescence) (A, B) were examined by immunofluorescence and confocal laser microscopy (20X imaging). Phalloidin (red fluorescence) and DAPI (blue fluorescence) were used as F-actin and nuclear markers. Scale bar = 50 μm. Fluorescence-integrated density was quantified using NIH Image J software and is presented as fold change normalized to control. *, p < 0.05; **, p < 0.01 versus control. The experiments were repeated three times.
Of note, while the effect size in some of the results was large, e.g. about 30% reduction of β1 integrin protein expression at 0.01μM simvastatin in primary cells, in other situations the effect size was small. We should exercise caution when clinically interpreting results with a small effect size, even if statistically significant. This applies here and in other sections.
Simvastatin inhibited phosphorylation of FAK
In leiomyoma cells, FAK phosphorylation was found to be upregulated compared to myometrium and contribute to activation of β1 integrin downstream signaling.9 We found that simvastatin treatment reduced FAK mRNA transcripts in primary and HuLM cells displaying a “U” shaped curve, with maximal reductions observed at 0.01 and 0.1μM (Fig. 4A and 4B). Next, we evaluated the effect of simvastatin on FAK phosphorylation using western blotting. Primary and HuLM cells demonstrated a significant reduction of pFAK to total FAK ratio, to 24-32% and 26-60% normalized to controls (Fig. 4C and 4D). While simvastatin reduced pFAK, it seemed to increase total FAK protein expression at 1 μM, similar to mRNA findings.
Figure 4. Simvastatin treatment reduced phosphorylated FAK expression levels in leiomyoma cells.
Primary leiomyoma and immortalized (HuLM) human leiomyoma cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. The mRNA levels of FAK genes were measured by qRT-PCR (A, B). RPLP0 was amplified under the same qRT-PCR conditions for normalizing quantitative data. The protein expression level of phosphorylated to total FAK ratio (pFAK/FAK) were determined by western blotting and normalized to β-actin (C, D). Results are expressed as the mean ± SEM. *, p < 0.05; **, p < 0.01 versus control. The experiments were repeated triplicate.
Similar to β1 integrin expression, we examined FAK phosphorylation after simvastatin treatment in primary and HuLM cells by using immunofluorescent staining. We found that spotty membranous phosphorylated FAK (pFAK) was greatly reduced in primary (Fig. 5A) and HuLM (Fig. 5B) cells treated with simvastatin compared to control.
Figure 5. Cytoimmunofluorescence images of FAK phosphorylation in simvastatin-treated leiomyoma cells.
Primary and immortalized (HuLM) human leiomyoma cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. Cellular distribution of pFAK (green fluorescence) (A, B) were examined by immunofluorescence and confocal laser microscopy (20X imaging). Phalloidin (red fluorescence) and DAPI (blue fluorescence) were used as F-actin and nuclear counterstain for all the figures. Scale bar = 50 μm. Fluorescence integrated density was quantified using NIH Image J software and is presented as fold change relative to control levels expressed as 1.00. *, p < 0.05; **, p < 0.01 versus control. The experiments were repeated three times.
Simvastatin suppressed activation of RhoA
There is evidence that mechanical stress from disordered ECM is associated with overactivation of RhoA in uterine leiomyoma cells.8 To characterize the effect of simvastatin on RhoA activation, we used RhoA pull down assay after treating HuLM cells for 48 h. As shown in Fig. 6, simvastatin decreased active-to-total RhoA ratio except at lower concentrations.
Figure 6. Simvastatin treated cells showed attenuated levels of active RhoA compared to controls.
Immortalized human leiomyoma (HuLM) cells were cultured and treated with simvastatin (0.001, 0.01, 0.1, 1 and 10μM) and DMSO (control) for 48 h. Cellular lysate was made by ice-cold lysis buffer and incubated with a GST Rhotekin Rho-binding domain (GST-Rhotekin-RBD) and the activated RhoA were pulled down by beads. Western blot analysis was performed to determine the activated RhoA (GTP-bound RhoA) and the data quantified as the ratio of active to total RhoA. The 1st lane shows a 50 ng recombinant His-tagged RhoA standard (His-Rho). The subsequent 2nd to 7th lanes shows the pull-down of activated RhoA after simvastatin treatment. The 8th lane shows active GTP-loaded RhoA (GTP) and the 9th lane shows inactive GDP-loaded RhoA (GDP) from equivalent amounts of cell lysates. Results are expressed as the mean ± SEM. *, p < 0.05; **, p < 0.01 versus control. The experiments were repeated three times.
Simvastatin inhibited collagen gel contraction
Cell-matrix interaction is achieved largely via integrins which physically link cellular cytoskeleton to ECM proteins.18 Since we observed that simvastatin reduced integrin expression, we investigated the effect of simvastatin on cellular response to the ECM in a gel contraction assay.
RhoA/ROCK signal transduction, a vital mediator of contractility19, was found to be overactive in leiomyoma cells.8, 10 Having found that simvastatin reduced levels of active RhoA, we tested the effect of simvastatin in a collagen gel contraction assay. HuLM cells were cultured in 3D using rat-tail collagen for 2 weeks. Thereafter, cells were treated with different concentrations of simvastatin or control for 96 h. Then, gel contraction was examined by capturing the images and comparing the gel surface area of each treated well to control. As shown in Fig.7, different simvastatin doses inhibited collagen gel contraction. Moreover, the higher concentrations of simvastatin induced maximum gel relaxation, with the gel diameter similar to gel without cells. Gel diameter is a convenient technique to quantity cell-ECM contraction force within the collagen matrix.
Figure 7. Reduction of gel contraction in HuLM cells after simvastatin treatment.
Immortalized human leiomyoma (HuLM) cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. HuLM cells were cultured in three-dimensional (3D) in collagen I gel and allowed to grow for 2 weeks before exposure of simvastatin for 96 h. Photograph were taken at the end of the treatment and the gel area was quantified by Image J software. Results are expressed as the mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control. The experiments were repeated three times.
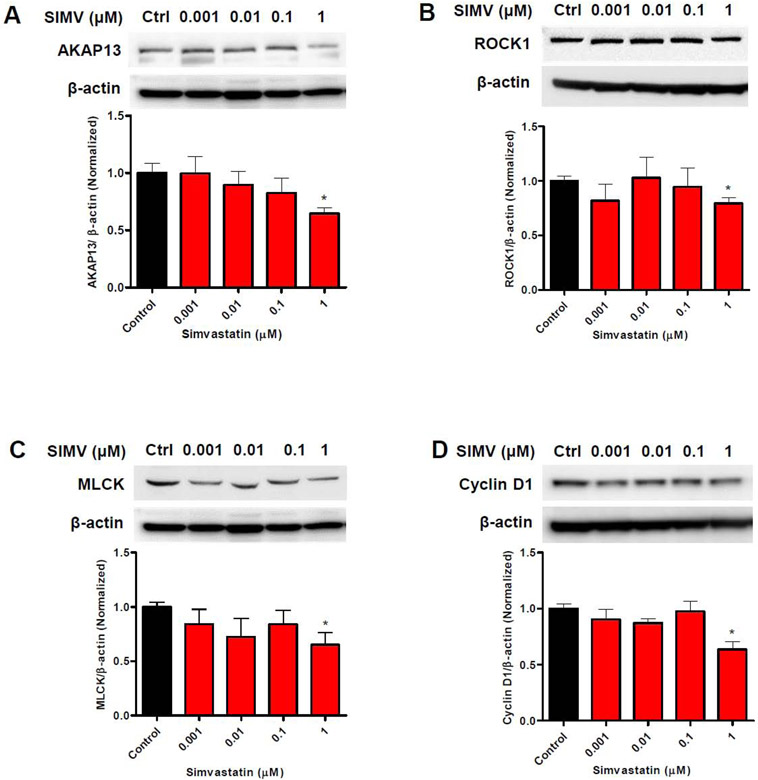
Simvastatin inhibited downstream factors involved in β1 integrin signaling
To test whether the reductions in pFAK and RhoA affected proteins involved in mechanical signaling downstream of β1 integrin, we measured levels of mRNA and proteins of factors downstream of β1 integrin activation in HuLM cells treated with simvastatin for 48 h.
The mRNA level of AKAP13 demonstrated a significant reduction to >50% of controls (Fig. 8A). Similarly, the mRNA levels of ROCK1 and MLCK were significantly decreased to a range of 64% (Fig. 8B), and 49% (Fig. 8C) of control. Additionally, mRNA level of cyclin D1 was significantly downregulated at all simvastatin concentrations by 80% of control (Fig. 8D).
Figure 8. Reduction of steady state mRNA transcripts encoding proteins involved in mechanical signaling following simvastatin treatment.
Immortalized human leiomyoma (HuLM) cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. The mRNA levels of A) AKAP13, B) ROCK1, C) MLCK and D) Cyclin D1 genes were measured by qRT-PCR. RPLP0 was amplified under the same qRT-PCR conditions for normalizing quantitative data. Results are expressed as the mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control. The experiments were repeated three times.
Regarding protein expressions, at 1μM simvastatin concentration, the expression of AKAP13 was suppressed by 38% (Fig. 9A) whereas the expression of ROCK1 and MLCK were decreased by 30% (Fig. 9B) and 35% (Fig. 9C) of control. In addition, the expression of cyclin D1 decreased by 37% at 1μM concentrations (Fig. 9D).
Figure 9. Reduction in levels of protein for key proteins involved in mechanical signaling after simvastatin treatment.
Immortalized human leiomyoma (HuLM) cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. The protein levels of A) AKAP13, B) ROCK1, C) MLCK and D) Cyclin D1 genes were measured by western blotting and quantified using β-actin as the loading control. Results are expressed as the mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control. The experiments were repeated three times.
Simvastatin inhibited type I collagen expression
Type I collagen is known to be overexpressed in leiomyoma and have a significant impact on integrins activation.10, 20 To examine the effect of simvastatin on type I collagen expression, we treated HuLM cells for 48 h with different simvastatin concentrations and measured mRNA and protein expression.
As shown in Fig. 10A, simvastatin treatment was associated with a significant reduction in mRNA transcripts compared to control. In addition, the protein expression of type I collagen was suppressed to 27-60% compared to control (Fig. 10B).
Figure 10. Inhibition of type I collagen expression after simvastatin treatment.
Immortalized human leiomyoma (HuLM) cells were treated with different simvastatin doses (0.001, 0.01, 0.1 and 1μM) and DMSO (control) for 48 h. A) The mRNA level of type 1 collagen were measured by qRT-PCR. RPLP0 was amplified under the same qRT-PCR conditions for normalizing quantitative data. B) Protein expression of type 1 collagen was determined by western blotting. Data was quantified and normalized by β-actin (loading control). Results were expressed as the mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control. The experiments were repeated three times.
COMMENT
Principal findings of the study
This study demonstrated that simvastatin targets altered β1 integrin-mediated mechanotransduction pathway in uterine leiomyoma cells. Treatment of leiomyoma cells with simvastatin reduced β1 integrin expression and FAK phosphorylation. In addition, we observed that simvastatin attenuated RhoA activation and collagen gel contraction. Furthermore, this drug inhibits the downstream targets of β1 integrin signaling, potentially correcting the dysregulated mechanical signaling in leiomyoma. Therefore, this suggests that simvastatin may inhibit uterine leiomyoma growth, at least in part, by ameliorating the altered mechanotransduction process.
Results of the study in the context of what is known
Mechanotransduction process starts when signals from ECM components (such as collagen) activate integrins (transmembrane receptors composed of α and β heterodimers) which transmits signals from outside to inside cells.5, 21 Upon activation, integrins activate two related downstream pathways; a cytoskeleton mechanical pathway and a biochemical signaling one.
In the cytoskeleton mechanical pathway, integrins activation leads to polymerization and organization of G-actin molecules to form F-actin fibers to which integrins are connected via linker proteins. This pathway is involved in cellular processes such as contractility and cytoskeletal rearrangement.22 Integrins are conceivably the target of first choice to modulate mechanosensing. In fact, inhibition of integrin was associated with a significant reduction of uterine leiomyoma cells proliferation and cytoskeletal rearrangement.10 In addition, simvastatin was shown to downregulate β1 integrin, FAK phosphorylation and cellular proliferation in other tumor types.23 In the present study, we found that simvastatin significantly reduces the expression levels of β1 integrin in leiomyoma cells.
In the biochemical signaling pathway, integrin activation and the formation of the focal adhesion complex triggers autophosphorylation of FAK which binds to the Src-homology 2 (SH2) domain of Src which further phosphorylates FAK and creates more binding sites for Src.24 It shows that simvastatin dose-dependently reduces phosphorylation of FAK in leiomyoma cells compared to control. Cell-ECM adhesions are mainly accomplished via integrins. At the adhesion site, integrin bind their ECM ligands and initiates the intracellular recruitment.25 Furthermore, interruption of ECM-β1 integrin signaling have remarkable inhibitory effects on leiomyoma cells progression.9
Phosphorylated FAK leads to activation of AKAP135, 26 and other intermediate cytoplasmic kinases that mediate different downstream signaling pathways, including Erk1/2, p38 MAPK and c-Jun N-terminal kinase (JNK), and PI3K.27 Recently, AKAP13 was reported to augment progesterone-mediated transcriptional response in leiomyoma cells11, suggesting AKAP13 as a leiomyoma therapeutic target. In fact, ulipristal acetate and omega-3-fatty acids downregulate AKAP13 expression in uterine leiomyoma.11, 28 In this study, we also found that simvastatin decreases AKAP13 expression in leiomyoma cells.
AKAP13 contains RhoA GTPase-specific guanine exchange factor (GEF) which activates Rho and its downstream signaling.6 In turn, activated RhoA contributes to the production of ECM, alters the viscoelastic properties of tissue, and contributes to increased ECM stiffness, all are key features of leiomyomas.8 The link between β1 integrin and active RhoA in the mechanotransduction process was further demonstrated by the observation that inhibition of β1 integrin leads to a decrease in active RhoA.10 We found that simvastatin treatment dose-dependently decreases RhoA activation in uterine leiomyoma cells.
ROCK is one of the main effectors of RhoA, which regulates cytoskeleton and cell polarity.29 Fukushima et al. reported that inhibition of the Rho/ ROCK pathway is associated with suppression of collagen production and augmentation of collagenase activity in hepatic stellate cells.30 Fasudil, an inhibitor of ROCK, was reported to decrease expression of ECM proteins fibronectin, procollagen 1A, and versican in leiomyoma cell cultures and relax the contraction of 3D collagen gels.17 In the present study, we found that treatment of leiomyoma cells with simvastatin dose-dependently reduced ROCK1 expression and repressed 3D collagen gel contraction compared to the control.
MLCK is a Ca2+/calmodulin-dependent serine/threonine kinase31, which is involved in cytoskeleton organization, cell contraction, cytokinesis, and aggregation.32 This serine/threonine kinase phosphorylates the regulatory light chain of myosin that allows myosin to bind to actin filaments.33 A significant role of stiff substrate, integrin binding, and MLCK activation in cell spreading and migration have been demonstrated.34 It was shown that MLCK can regulate myosin II-mediated periodic lamellipodial contraction.34 The inhibition of MLCK has been associated with the reduction of ventilator-induced lung injury35, and NADPH oxidase-induced oxidative injury in the brain of rats.36 Treatment of leiomyoma cells with simvastatin also showed reduced levels of MLCK at dose-dependently, suggesting the potential role of simvastatin as a possible MLCK inhibitor.
We have previously shown that simvastatin treatment inhibits the phosphorylation of ERK1/2 in leiomyoma cells.12, 13 Here, we found reduced levels of cyclin D1 after simvastatin that further confirms the inhibitory effect of simvastatin on leiomyoma cell growth. Moreover, collagens serve as a central structural component of ECM and are involved in proliferation, wound healing and fibrosis.37 In our previous study, we found that simvastatin reduces collagen I expression in leiomyoma cells.14 The present study confirms this inhibitory effect and further elucidate on the pathway.
Interestingly, some results showed a U-shaped dose-response curve. In Fig. 4A/4B, the FAK mRNA U-shape is mirrored by a similar pattern in total FAK protein levels (Fig. 4C/4D). We believe this may be due to specific dose-dependent inhibition of regulatory or feedback loops in the signal pathways. For example, at a very low dose, the drug can hit certain target sites. However, at higher concentrations, the drug may hit additional target sites in such a way to change the overall effect. Similar U-shaped responses have been reported in anti-tumor treatments.38 The U-shaped response noted in AKAP13, ROCK1 and MLCK mRNA levels (Fig 8A, B, C) was not paralleled by protein levels (Fig 9A, B, C), possibly due to dose-dependent mRNA post-transcriptional modifications.
Clinical and research implications
This study has several clinical and research implication. It provides a novel mechanism to explain the clinically relevant anti-leiomyoma properties of simvastatin seen in cellular and animal experiments.12, 13 The stiffness and disordered mechanical homeostasis in leiomyoma are major contributors to leiomyoma development and discovering that simvastatin ameliorates them can open the door for further studies. We are currently performing a project to measure the effects of simvastatin on the stiffness of uterine leiomyoma tissue using an explant system.
Simvastatin has been in use for more than 20 years as a treatment of high cholesterol, cardiovascular disease and other conditions. In a nested case-control study using insurance databases, statin users had a lower leiomyoma risk and less symptoms compared to nonusers.39 In addition to providing a mechanism, the findings of the current study validate the case for the currently ongoing phase II clinical of simvastatin in uterine leiomyoma. As an anti-leiomyoma treatment, simvastatin could have several advantages. Its safety profile and pharmacokinetics are well-known and can be used for extended periods of time. It is a non-hormonal option and therefore can be used in many cases where hormonal options such as oral contraceptives are contraindicated. Many patients with uterine fibroids have hyperlipidemia and other conditions where simvastatin can have more than one beneficial effect. In addition, it is inexpensive and is already available in a generic form. Needless to say, the consideration of simvastatin as a therapeutic option for uterine leiomyoma must be only after clinical trials confirm its efficacy and safety.
It is difficult to ignore the striking similarities of the effects of simvastatin on ECM in uterine leiomyomas and atherosclerotic patches. There is also evidence linking the development of uterine leiomyoma with several cardiometabolic risk factors.40 In fact, the atherogenic hypothesis suggests that atherosclerosis and uterine leiomyoma may share several characteristics.40 Similarly, there evidence that statins in general possess several beneficial effects on uterine leiomyoma, endometriosis and other gynecologic conditions.41, 42 The association of benign gynecologic conditions such as leiomyoma and endometriosis with cardiometabolic risk factors seems important and deserve further clinical and mechanistic investigation.
Strengths and Limitations
This is the first report of the beneficial effects of simvastatin on altered mechanotransduction in uterine leiomyoma.
In this study, we used both primary and immortalized human leiomyoma (HuLM) cells. Primary cells were isolated from fibroids after surgery. HuLM cells were previously isolated from fibroid tissue and immortalized by retroviral vector carrying human telomerase reverse transcriptase. Each cell types has its own advantages and disadvantages. The behavior of primary cells is very close to in vivo biology. However, they can be used for only few passages before they start to show morphologic changes. On the other hand, immortalized cells can be maintained for several passages without changes. However, the immortalization process may have introduced some differences. Overall, immortalized cells present an important tool in cellular and molecular biology research. We have decided to do most experiments in both cells types to capitalize on advantages both cell types. We clearly stated which cell type was used in each experiment.
The study may have been impacted by some limitations. Experiments were only performed in vitro. In vivo data (animal models and human tissue) can be valuable to verify in vitro results. We plan xenograft animal experiments. In addition, our ongoing clinical trial (clinicaltrials.gov number NCT03400826) will provide tissues for in vivo studies. Our experiments were limited to simvastatin and other statins were not examined. This decision was based on our previous works using simvastatin. We also have not investigated the exact mechanism by which simvastatin lowers the expression level of β1 integrin and other proteins, which we plan to undertake in future work.
Conclusions and future studies
In summary, our study shows that simvastatin ameliorates several dysregulated steps of the mechanical signaling cascade (Fig. 11). Future studies can examine if these findings will be replicated in leiomyoma tissue from the ongoing phase II clinical trial patients. Furthermore, this clinical trial can show if these molecular and cellular changes are associated with clinical improvement in symptoms, tumors size, or both.
Figure 11. Effect of simvastatin on the mechanotransduction pathways in uterine leiomyoma cells.
A cartoon showing the beneficial effects of simvastatin on the mechanotransduction pathway. Red minus signs denote sites of simvastatin inhibitory effects.
Supplementary Material
Condensation:
Simvastatin treatment may ameliorate the altered mechanical signaling in leiomyoma cells.
AJOG at a Glance (Implications and Contributions).
A. Why was the study conducted?
Altered mechanical signaling and excessive stiff disordered extracellular matrix (ECM) are compelling therapeutic targets in uterine leiomyoma. Simvastatin, an anti-hyperlipidemic drug, inhibits leiomyoma cellular proliferation, tumor growth, and ECM production on leiomyoma cells and xenograft model. This study was undertaken to examine the simvastatin effects on altered mechanical signaling in leiomyoma cells.
B. Key findings
Simvastatin suppresses β1 integrin expression and FAK phosphorylation, both overactive in leiomyoma cells. This effect can be beneficial and bring mechanical signaling back toward normal.
Simvastatin attenuates RhoA activation which is elevated in leiomyoma and contributes to its pathogenesis, stiffness, and altered mechanical homeostasis.
Simvastatin downregulates β1 integrin-mediated downstream mechanotransduction signaling which is perturbed in leiomyoma cells and influences cell contractility, ECM stiffness, and mechanical stress.
C. What does this add to what is known?
Our study results support the therapeutic potential of simvastatin for uterine leiomyoma by targeting dysregulated mechanotransduction. In addition, this support a clinical trial of simvastatin in uterine leiomyoma that is currently in progress.
Acknowledgments
Financial support
This work was supported, in part, by National Institutes of Health grant 1R01HD094380-01.
ABBREVIATIONS
- AKAP13
A-kinase anchor protein 13
- cDNA
complementary DNA
- DMSO
dimethyl sulfoxide
- ECM
extracellular matrix
- ERK
extracellular-signal-regulated kinase
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- HBSS
hanks' balanced salt solution
- MAPK
mitogen activate protein kinase
- MLCK
myosin light chain kinase
- qRT-PCR
quantitative real-Time polymerase chain reaction
- RhoA
ras homolog family member A
- ROCK
Rho associated protein kinase
Footnotes
Conflict of interest
William H. Catherino declares the following conflict of interest: Abbvie (Consultant); Allergan, now owned by Abbvie (Consultant); American Board of Obstetrics and Gynecology (Boards Examiner, Subject Matter Expert); American Society for Reproductive Medicine (Subject Matter Expert); Bayer (Consultant); EMD Serono (Consultant, wife is Senior Medical Director); Myovant (Consultant). Mostafa A. Borahay serves as Advisory Board member for Myovant Sciences.
Other authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 2014;15:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borahay MA Al-Hendy A, Kilic GS, Boehning D. Signaling pathways in leiomyoma: understanding pathobiology and implications for therapy. Mol Med 2015;21:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam MS, Protic O, Stortoni P, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril 2013;100:178–93. [DOI] [PubMed] [Google Scholar]
- 4.Bulun SE. Uterine fibroids. The New England journal of medicine 2013;369:1344–55. [DOI] [PubMed] [Google Scholar]
- 5.Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update 2018;24:59–85. [DOI] [PubMed] [Google Scholar]
- 6.Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol 2008;198:474. e1–74. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norian JM, Owen CM Taboas J, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol 2012;31:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H-M, Lin Y-H, Cheng Y-M, Wing L-YC, Tsai S-J. Overexpression of integrin-beta1 in leiomyoma promotes cell spreading and proliferation. J Clin Endocrinol Metab 2013;98:E837–E46. [DOI] [PubMed] [Google Scholar]
- 10.Malik M, Segars J, Catherino WH. Integrin β1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol 2012;31:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng SSM, Jorge S, Malik M, et al. A-kinase anchoring protein 13 (AKAP13) augments progesterone signaling in uterine fibroid cells. J Clin Endocrinol Metab 2018;104:970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borahay MA, Vincent K, Motamedi M, et al. Novel Effects of Simvastatin on Uterine Fibroids: In vitro and Patient-Derived Xenograft Mouse Model Study. Am J Obstet Gynecol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borahay MA, Kilic GS, Yallampalli C, et al. Simvastatin Potently Induces Calcium-Dependent Apoptosis of Human Leiomyoma Cells. J Biol Chem 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik M, Britten J, Borahay M, Segars J, Catherino WH. Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production. Fertil Steril 2018;110:1398–407. e1. [DOI] [PubMed] [Google Scholar]
- 15.Lampi MC, Faber CJ, Huynh J, Bordeleau F, Zanotelli MR, Reinhart-King CA. Simvastatin ameliorates matrix stiffness-mediated endothelial monolayer disruption. PLoS ONE 2016;11:e0147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayers CM, Wadell J, McLean K, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem 2010;285:12344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik M, Britten J, Segars J, Catherino WH. Leiomyoma cells in 3-dimensional cultures demonstrate an attenuated response to fasudil, a rho-kinase inhibitor, when compared to 2-dimensional cultures. Reprod Sci 2014;21:1126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 2002;4:E65. [DOI] [PubMed] [Google Scholar]
- 19.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 2009;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril 2004;82 Suppl 3:1182–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol 2005;17:509–16. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci 2000;25:388–91. [DOI] [PubMed] [Google Scholar]
- 23.Takeda I, Maruya Si, Shirasaki T, et al. Simvastatin inactivates β1-integrin and extracellular signal-related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer science 2007;98:890–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huveneers S, Danen EH. Adhesion signaling–crosstalk between integrins, Src and Rho. J Cell Sci 2009;122:1059–69. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011;475:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, Leppert PC. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol Reprod 2015;92:25, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islam MS, Castellucci C, Fiorini R, et al. Omega-3 fatty acids modulate the lipid profile, membrane architecture, and gene expression of leiomyoma cells. J Cell Physiol 2018;233:7143–56. [DOI] [PubMed] [Google Scholar]
- 29.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010;67:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukushima M, Nakamuta M, Kohjima M, et al. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int 2005;25:829–38. [DOI] [PubMed] [Google Scholar]
- 31.Sharanek A, Burban A, Burbank M, et al. Rho-kinase/myosin light chain kinase pathway plays a key role in the impairment of bile canaliculi dynamics induced by cholestatic drugs. Sci Rep 2016;6:24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka H, Ishimaru S, Nagatsuka Y, Ohashi K. Smooth muscle-like Ca(2+)-regulation of actin-myosin interaction in adult jellyfish striated muscle. Sci Rep 2018;8:7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassianidou E, Hughes JH, Kumar S. Activation of ROCK and MLCK tunes regional stress fiber formation and mechanics via preferential myosin light chain phosphorylation. Molecular biology of the cell 2017;28:3832–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannone G Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell 2004;116:431–43. [DOI] [PubMed] [Google Scholar]
- 35.Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol (1985) 2000;89:2241–8. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HF, Li TB, Liu B, et al. Inhibition of myosin light chain kinase reduces NADPH oxidase-mediated oxidative injury in rat brain following cerebral ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol 2015;388:953–63. [DOI] [PubMed] [Google Scholar]
- 37.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds AR. Potential relevance of bell-shaped and u-shaped dose-responses for the therapeutic targeting of angiogenesis in cancer. Dose Response 2010;8:253–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borahay MA, Fang X, Baillargeon JG, Kilic GS, Boehning DF, Kuo YF. Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study. Am J Obstet Gynecol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AlAshqar A, Patzkowsky K, Afrin S, Wild R, Taylor HS, Borahay MA. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obstet Gynecol Surv 2019;74:661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeybek B, Costantine M, Kilic GS, Borahay MA. Therapeutic Roles of Statins in Gynecology and Obstetrics: The Current Evidence. Reproductive sciences 2018:1933719117750751. [DOI] [PubMed] [Google Scholar]
- 42.Fritton K,Borahay MA. New and Emerging Therapies for Uterine Fibroids. Semin Reprod Med 2017;35:549–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.