Abstract
Chronic pain is a major health problem and the effective treatment for chronic pain is still lacking. The recent crisis created by the overuse of opioids for pain treatment has clearly shown the need for non-addictive novel pain medicine. Conventional pain medicines usually inhibit peripheral nociceptive transmission and reduce central transmission, especially pain-related excitatory transmission. For example, both opioids and gabapentin produce analgesic effects by inhibiting the release of excitatory transmitters and reducing neuronal excitability. Here, we will review recent studies of central synaptic plasticity contributing to central sensitization in chronic pain. Neuronal selective adenylyl cyclase subtype 1 (AC1) is proposed to be a key intracellular protein that causes both presynaptic and postsynaptic forms of long-term potentiation (LTP). Inhibiting the activity of AC1 by selective inhibitor NB001 blocks behavioral sensitization and injury-related anxiety in animal models of chronic pain. We propose that inhibiting injury-related LTPs will provide new mechanisms for designing novel medicines for the treatment of chronic pain and its related emotional disorders.
Electronic supplementary material
The online version of this article (10.1007/s13311-020-00927-1) contains supplementary material, which is available to authorized users.
Keywords: Long-term potentiation, Chronic pain, ACC, Adenylyl cyclase subtype 1, NB001
Introduction
Basic research has provided a basic mechanism for our understanding of pain. From peripheral nociceptors to cortical regions involved in pain perception, we have obtained cumulative evidence for how pain is transmitted, modulated and possibly stored as one special experience in the brain. Furthermore, we also recognize the importance of distinguishing acute pain from chronic pain, and the differences between molecular mechanisms underlying these two processes. Unfortunately, the colloquial terms “pain” and “analgesia” fail to distinguish acute and chronic pain, and even cause confusion among the public. For example, a novel acute pain receptor (or more appropriate, nociceptor) may not serve well as a drug target for treating chronic pain; and drugs that do not act on acute pain may be proved to be beneficial for alleviating chronic pain. Although the discovery of selective proteins and pathways for physiological pain or acute pain has greatly improved our knowledge of pain, there are still limitations for the treatment of chronic pain. Recent discoveries from research have demonstrated that molecular and synaptic mechanisms of chronic pain are different from that of acute pain and simply reducing neuronal excitability or transmission may not be enough to block chronic pain.
In this review, we will summarize recent progress made on basic mechanisms of chronic pain, especially at the synaptic level of cortical regions that play key roles in pain perception. Synaptic potentiation plays a critical role in cortical excitation in animal models of chronic pain. Neuronal adenylyl cyclase subtype 1 (AC1), a key protein for producing activity-dependent long-term potentiation (LTP), is a potential novel target for treating chronic pain.
LTPs May Contribute to Exaggerated Chronic Pain Responses
LTP was originally proposed as a key cellular mechanism for learning and memory [1, 2]. Recent studies have consistently indicated that LTP may also take place in memory-independent excitatory synapses, and contribute to physiological and pathological functions [1, 3]. For nociceptive transmission and chronic pain, it has been reported that peripheral injury can trigger LTP along somatosensory pathways contributing to pain transmission, relay, and perception, including the spinal cord dorsal horn, thalamus, and cortical regions [1, 4, 5]. Depending on the type of injury, onset time and duration of pain may be different.
In spinal dorsal horn neurons, electrophysiological experiments have generated some important findings related to spinal LTP. Strong tetanic stimulation of the dorsal root induces LTP of synaptic responses to presynaptic stimulation [6]. Postsynaptic depolarization is critical for the induction of dorsal horn neuron LTP. Pairing postsynaptic depolarization also induces long-lasting enhancement of synaptic responses in the spinal cord. The level of postsynaptic depolarization may be important in determining whether synaptic transmission will be potentiated or depressed [7]. The induction of spinal LTP requires activation of N-methyl-D-aspartate receptors (NMDARs) and/or the substance P receptors (NK1). The contribution of neuropeptide substance P to spinal LTP may act by enhancing NMDAR-mediated currents in spinal dorsal projecting neurons [8]. The intracellular signal pathways of spinal LTP remain to be fully mapped. Evidence from other studies indirectly indicates that several protein kinases may be important for spinal LTP, such as phospholipid-dependent protein kinase C (PKC). Phorbol ester induces long-lasting facilitation of evoked excitatory postsynaptic potentials (EPSPs) or excitatory postsynaptic currents (EPSCs) amplitude to stimulation of presynaptic fibers [9, 10]. One possible mechanism for PKC-dependent spinal LTP is through the recruitment of spinal silent synapses or the insertion of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptors (AMPARs). Brain-derived neurotrophic factor (BDNF) can induce NMDAR-dependent LTP in the spinal dorsal horn via different signaling pathways [11]. Interestingly, the activity of AC1 has been found to be important for serotonin (5-HT) induced potentiation [12].
Both in vitro and in vivo studies have consistently demonstrated that cortical synapses that receive sensory inputs can also undergo LTP. These cortical regions include the anterior cingulate cortex (ACC), insular cortex (IC), somatosensory cortex, and prefrontal cortex (PFC). The intracellular mechanism for the induction and expression of LTP has been extensively investigated in the ACC, but less so in other cortical regions. Recent studies have begun to report evidence for other pain-related cortical areas. In pain-related ACC and IC of adult animals (mostly mice, but also rats and tree shews), different stimulation protocols can induce long-lasting LTP [5, 13, 14]. For example, it has been reported that the pairing training protocol (synaptic activity paired with postsynaptic depolarization), spike-EPSPs pairing protocol, and theta burst stimulation (TBS) protocol all induce LTP in the ACC pyramidal neurons [15, 16]. Similar results have been recently reported in the IC [14]. These findings indicate that excitatory synapses in the ACC and IC are plastic and can undergo LTP.
Lack of Selective Drugs for Inhibiting Pain-Related LTP
Most of translational pain research is focusing on identifying selective pain receptors or pain-related transmission. Drugs that have been identified often target the release of transmitters, postsynaptic receptors, and related neuromodulators (that modulate excitatory/inhibitory transmission). In addition, reducing neuronal excitability can also be used as a drug target mechanism. For example, the popular drug gabapentin, is used for treating neuropathic pain and its related compounds act by inhibiting excitatory transmission (by inhibiting voltage-gated calcium channels, for example). In the ACC, we have recently reported that gabapentin reduced excitatory synaptic transmission in layer II/III pyramidal cells [17]. Opioids are known to also reduce the release of transmitters by inhibiting presynaptic calcium channels, as well as postsynaptic plasticity [18, 19]. However, the addiction and tolerance effects accompanying the use of opioids have caused significant medical and social problems, and have nearly limited the usage of opioids for long term care of chronic pain. One possible avenue for reducing chronic pain that has not been extensively explored so far is the central plasticity. Although the potential side effects of targeting such key mechanisms have been a major concern, its side effect profiles are not more than those that are commonly used currently in pain care such as gabapentin and opioids. Furthermore, it may be possible to identify some selective molecular targets that are preferentially involved in injury-related central plasticity to avoid central side effects. Recent studies using genetic and neuroscientific approaches have demonstrated that it is possible to target such novel proteins without causing side effects. In this review, we will focus on one major protein AC1, which has met most of these safety requirements.
Basic Mechanisms Contributing to Cortical LTPs
Two major advances have been made regarding cortical potentiation after injury. First, adult cortical synapses in the pain-related cortical areas are highly plastic. Second, two key forms of LTP have been identified: presynaptic and postsynaptic forms of LTP (or called pre-LTP and post-LTP).
For post-LTP, NMDARs, including GluN1 and GluN2 (GluN2A-D) isoforms, have been reported to contribute to the induction of LTP. Since it is completely blocked by the NMDAR antagonist, we also refer to post-LTP as NMDAR-dependent LTP [1, 20]. LTP induction protocols such as TBS, pairing training, and spike-EPSPs can induce NMDAR-dependent LTP. The expression of NMDAR-dependent LTP requires postsynaptic modification or insertion of GluA1-containing AMPARs. AC1-dependent, protein kinase A (PKA) phosphorylation of AMPARs GluA1 contributes to LTP [21–23]. A recent study using selective knock-in mice demonstrates that phosphorylation of AMPAR GluA1 plays an important role in synaptic potentiation. However, the same LTP did not require a CaMKII/PKC phosphorylation site serine 831 (Ser831). These results demonstrate that ACC LTP employs a different mechanism than hippocampal LTP [24].
In addition to post-LTP, an NMDAR-independent form of LTP can also be readily induced in the ACC by paired-pulse low-frequency stimulation. This form of LTP is resistant to NMDAR blockade and was inhibited in mice lacking the kainate receptor (KAR) GluK1 subunit [25]. Pharmacological experiments using a potent GluK1-selective KAR antagonist, UBP310, further confirmed that this form of LTP is KAR dependent. Both genetic and pharmacological evidence consistently indicates that this form of LTP is pre-LTP. Pre-LTP in the ACC is sensitive to a hyperpolarization-activated cyclic nucleotide-gated (HCN) channel inhibitor, ZD7288 [25]. In addition, p42/p44 mitogen-activated protein kinase inhibitors PD98059 and U0126 suppressed the induction of pre-LTP and did not affect the maintenance of pre-LTP. The activation of presynaptic extracellular signal-regulated kinase (ERK) was required for the induction of pre-LTP [26].
Top-down Cortical-Spinal Facilitation: Positive Feedback for Chronic Pain
It is well known that spinal sensory transmission can be affected by receiving top-down biphasic modulation. Integrative studies using different experimental approaches reveal that descending modulation of spinal sensory transmission is biphasic, including inhibitory and facilitatory influences. Recent studies have indicated that descending influences from the ACC, IC, PFC, amygdala, periaqueductal gray (PAG), and rostroventral medial medulla (RVM) may exert excitatory, inhibitory, or mixed biphasic modulation of spinal nociceptive transmission [27]. The RVM is a subcortical structure that is thought to be one of key relays for descending modulation from the supraspinal region to the spinal cord. The PAG-RVM is known to play a key analgesic effect in descending inhibition of pain. Few studies report that PAG may exert descending facilitatory effects on spinal transmission [28, 29]. A key feature of descending facilitation is its intensity dependence. At biphasic sites of stimulation, it is typical that electrical stimulation facilitates spinal nociceptive transmission at lesser intensities and inhibits responses of the same neurons at greater intensities [30]. 5-HT is the major neurotransmitter for triggering biphasic effects on spinal nociceptive transmission [31]. In addition, it has been known that cortical neurons could project to brainstem neurons and lead to the excitation of descending facilitation. For the ACC, neurons in the deeper layers of the ACC send their axonal projections to neurons in the RVM of the brainstem, which then sends its modulatory projection to the spinal cord dorsal horn [32]. Our recent studies imply that ACC neurons can project to the spinal cord dorsal horn directly through corticospinal pathways and that glutamate is likely to be the transmitter. In vivo electrophysiological experiments found that activation of ACC enhances spinal sensory transmission, and this facilitation is independent of RVM activity [32, 33].
In other pain-related cortical areas, such as IC, amygdala and PFC were also reported to modulate pain in a top-down manner. In the rostral agranular IC, locally increasing GABA by using an enzyme inhibitor or gene transfer mediated by a viral vector produced lasting analgesia by enhancing the descending inhibition of spinal nociceptive neurons [34]. Recently, Huang et al. reported a BLA–PFC–PAG–spinal cord pathway that alters pain behaviors by reducing descending noradrenergic and serotoninergic modulation of spinal pain signals [35]. Our group recently found an ascending projection from locus coeruleus (LC) to the ACC modulating pain behaviors. We found that the ascending noradrenergic projection from the LC to the ACC may contribute to the enhancement of pain/itching by potentiating glutamatergic synaptic transmissions in the ACC, which is opposite to the well-known descending LC-spinal inhibitory modulation (in press). Consequently, cortical-spinal top-down facilitation, including those relayed through brainstem neurons, provides powerful positive feedback control for pain transmission at the level of the spinal cord.
AC1 Is a Neuronal Selective and Novel Protein for Chronic Pain
Previous studies show that Ca2+-calmodulin (CaM) dependent signaling pathways play crucial roles in biological systems, such as learning and memory, chronic pain, and emotional fear. Adenylyl cyclases (ACs) is the enzyme that catalyzes ATP to cAMP and is activated by Ca2+-CaM. There are two major families of ACs expressed in the central nervous system: nine membrane-bound ACs (AC1–9) and one soluble AC. Among these ten ACs subunits, AC1 and AC8 are two major ACs subtypes that respond positively to Ca2+-CaM. Compared with AC8, AC1 is more sensitive to Ca2+ increases [36, 37]. In the hippocampus, early studies using gene knockout (KO) mice have shown that AC1 is not required for hippocampal LTP, Morris water maze performance, anxiety-like behaviors, and motor functions. It is likely that other signaling proteins such as CaMKII, CaMKIV, cAMP response element (CRE)-binding protein (CREB) contribute to hippocampal LTP and learning memory [38]. Compared with the role of AC1 in the hippocampus, AC1 KO mice showed reduced inflammatory, deep muscle pain and neuropathic pain in the ACC (Fig. 1) [39]. AC1 is highly expressed in pyramidal neurons located in most of the layers of the ACC [39]. Gene deletion of AC1 does not affect basal glutamate transmission in the ACC. However, AC1 activity is required for TBS or pairing stimulation induced LTP in ACC pyramidal neurons. Pharmacological inhibition of AC1 in the ACC neurons also consistently abolished LTP induced by pairing training [1, 17].
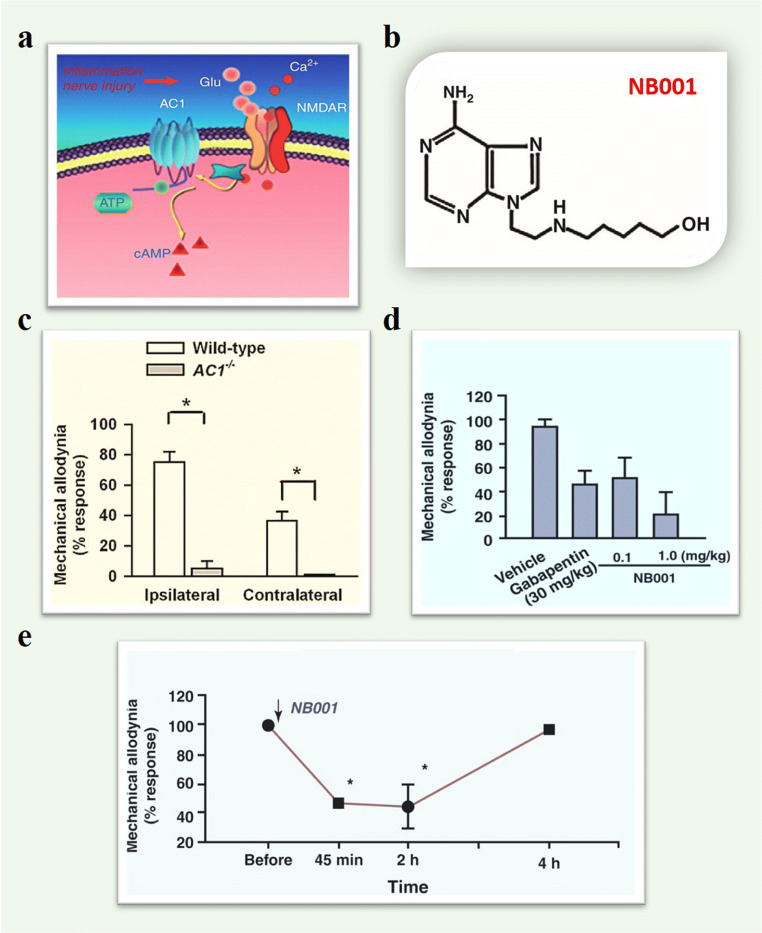
Fig. 1.
Inhibition of AC1 activitycan reduce behavioral sensitization on neuropathic pain. (a) A model shows that AC1 acts downstream of the glutamate NMDARsand is activated in a calcium-dependent manner. (b) Chemical structure of NB001. (c) Reduced both ipsilateral and contralateral sides mechanical allodynia in AC1 KOmice with nerval ligation. (d) Effectof NB001 on allodynia after nerve ligation. Intraperitoneal administration ofNB001 (0.1mg/kg body weight) produced a significant analgesic effect.NB001 at a high dose of 1mg/kg produced a greater inhibition ofbehavioral allodynia. Intraperitoneal injection of gabapentin (30mg/kg)produced significant analgesic effects in the animals with neuropathic pain.The inhibitory effects were comparable to those produced by NB001 at 0.1mg/kg. (e) Oral application of NB001 (1mg/kg,3ml per injection) produced significant analgesic effects in adult ratswith neuropathic pain. Allodynia was measured at 45min, 2h, and 4hafter oral application (Adapted from Hansen Wang et al., [46]; Hui Xu et al., [64])
In addition to its contribution to the ACC, AC1 activity is likely to contribute to other pain-related cortical areas, such as PFC, IC, and somatosensory cortex. In AC1 KO mice, we found that the amount of synaptic GluA1 and its phosphorylation at the Ser845 site remained unchanged in the IC after the injury. Furthermore, no upregulation of AKAP79/150 and PKA was detected from the AC1 KO mice with nerve ligation [40]. It has been reported that AC1 activity is required for injury-activated immediate early gene activity in these areas. Similar LTP induction protocols also induce LTP in PFC, somatosensory cortex, and IC areas. Considering that AC1 is mainly expressed in the central nervous system, we propose that AC1 may be a suitable neuron-specific drug target for treating chronic pain.
AC1 Versus NMDA GluN2B Receptor
NMDAR is one type of ionotropic glutamate receptors and is known to be important for triggering long-lasting changes in synapses. NMDAR-dependent synaptic plasticity plays a role not only in physiological functions such as learning and memory but also in unwanted pathological conditions such as chronic pain. A previous study using genetic overexpression mice provides the first direct evidence that forebrain GluN2B-containing NMDARs may contribute to chronic pain [41]. Subsequent investigation has found that the forebrain, including ACC and IC GluN2B can undergo upregulation after peripheral injury [42–44]. For example, after peripheral nerve injury, GluN2B-mediated responses in the ACC neurons were significantly enhanced and inhibiting NMDA GluN2B receptors within the ACC produced analgesic effects in animal models of neuropathic pain [43]. Due to its fewer side effects, NMDA GluN2B receptors have been selected as a potential target for treating chronic pain, especially neuropathic pain. In addition to being upregulated after the injury, GluN2B has been found to be critical for producing LTP in different brain areas related to the pain process, including ACC, IC, spinal cord, and amygdala [16, 42, 45].
Basic investigations using animal models have revealed that AC1 is likely acting downstream of NMDARs, including GluN2B receptors. In central neurons, activation of NMDARs can activate several key intracellular pathways, including CaMKII, tyrosine kinase, PKC, etc. For cognitive functions, genetic deletion of AC1 failed to impair key memory functions, indicating that such functions can be taken over by other signaling pathways [38, 46]. Thus, targeting AC1 is likely to cause fewer cognitive side effects compared with GluN2B receptors. Finally, recent studies show that AC1 activity is required for LTP of GluN2B-containing NMDARs [42–44]. Therefore, AC1 plays an essential role in postsynaptic upregulation of both AMPARs and NMDA GluN2B receptors.
AC1 Is Essential for Cortical LTPs
Recent studies consistently demonstrate that excitatory synapses within the ACC and IC are highly plastic. Both pre-LTP and post-LTP have been reported in excitatory synapses in the ACC and IC [21, 25, 26, 47]. Using genetic and selective pharmacological inhibitors, it has been demonstrated that AC1 is essential for both forms of LTPs in these cortical areas. Liauw et al. (2005) reported that genetic deletion of AC1 completely abolished LTP induced by TBS and forskolin perfusion [23]. Wang et al. further showed that LTP was also blocked by a selective AC1 inhibitor NB001 [46]. This data consistently suggests that AC1 activity is required for LTP (or post-LTP) in the ACC. Similar results have been reported in the IC recently [21, 22]. Furthermore, Chen et al. reported that strong TBS-induced late-phase LTP (L-LTP) also requires AC1 activity (Fig. 2 and Table I) [17]. Since NMDAR-mediated responses were not affected by AC1 deletion or NB001, it is thus likely that AC1 acts downstream of NMDARs or KARs to contribute to LTPs. Recent studies using gene knock-in mice showed that AC1 likely acts upstream of PKA. The phosphorylation of AMPARs at Ser845 is critical for the potentiation in the ACC [24].
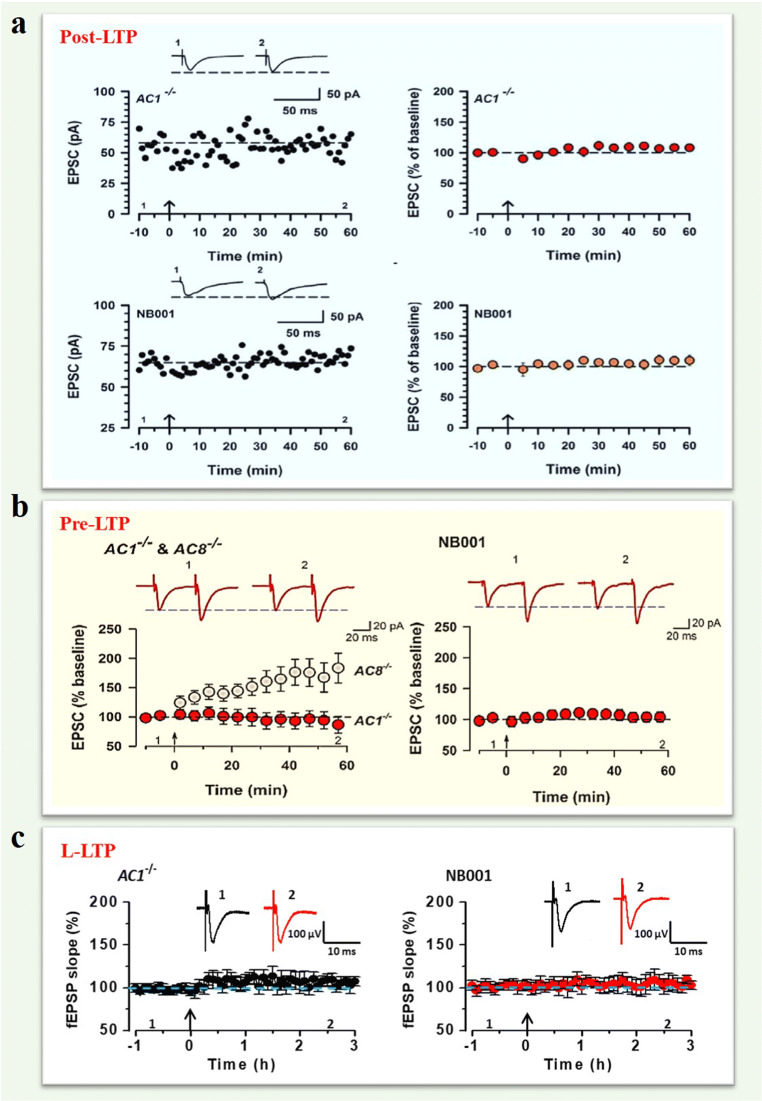
Fig. 2.
AC1 is required fordifferent forms of cortical LTPs. (a) AC1 KO and NB001 blocked the induction of pairing stimulation-induced post-LTPin the IC of mice. Top: Data of one neuron (left) and pooled data (right)showed that post-LTP failed to induce LTP in the IC of AC1 KO mice. Bottom:NB001 blocked the induction of LTP in one neuron (left) and pooled data ofNB001 (right). (b) AC1 KO (left) andNB001 (right) blocked the induction of pre-LTP in the ACC of mice. Left: Sampletraces of EPSCs with paired-pulse stimulation at 50 ms and pooled data showedthat pre-LTP was absent in AC1 KO mice (red). AC8 KO mice showed normal pre-LTP(gray). Right: A specific AC1 inhibitor, NB001, blocked pre-LTP (red circle). (c) The summarized L-LTP of the fEPSPslopes showed that AC1 KO (left) and NB001 application (right) blocked theL-LTP induction. (Adapted from Manabu Yamanaka et al., [22]; Kohei Koga et al., [25]; Tao Chen et al., [17])
Table I.
The role of AC1 in different forms of central synaptic plasticity in the ACC and IC
| AC1 KO | NB001 | References | |
|---|---|---|---|
| Post-LTP | Attenuated LTP in the ACC and IC | Blocked LTP in the ACC and IC | [22, 23, 46, 78] |
| Pre-LTP | Attenuated pre-LTP in the ACC and IC | Blocked pre-LTP in the ACC and IC | [21, 25] |
| L-LTP | Attenuated L-LTP in the ACC | Blocked L-LTP in the ACC | [17, 76] |
| LTD | No data | No data | No data |
| Depotentiation | No data | No data | No data |
AC1 Is Required for the Upregulation of PKMzeta (PKMζ) in Chronic Pain
Among several protein kinases that may contribute to the expression of L-LTP, protein kinase Mζ (PKMζ) is mostly reported. Inhibiting PKMζ activity reduced L-LTP and long-term memory. In the ACC, the expression of PKMζ can be detected [48–50], PKMζ peptide inhibitor can erase L-LTP [51]. The possible contribution of PKMζ to neuropathic pain has also been investigated. Interestingly, we found that PKMζ and its phosphorylation were upregulated within the ACC after peripheral nerve injury. The AC1 activity is critical for such upregulation. By contrast, the baseline level of PKMζ expression was not affected by AC1 deletion, indicating that AC1 is not topically regulating the expression of PKMζ. In AC1 KO mice, such injury-induced increases in the protein expression of PKMζ and/or phosphorylation of PKMζ was abolished. PKMζ inhibitory peptide (ZIP) can reduce mechanical allodynia responses in mice with 1-month-old nerve injuries. Inhibition of PKMζ in the ACC reduced the GluA1 trafficking in the synapse, but not postsynaptic GluA2 levels [52]. However, some studies report that the consequences of PKMζ inhibition within the rostral ACC are not permanent in neuropathic pain, possibly because the re-establishment of amplification mechanisms by ongoing activity of injured nerves [53]. The activity-dependence of PKMζ in the ACC is also needed in other chronic pain model, including inflammatory pain [54] and diabetic neuropathic pain [55], and AC1 activity is critical for these kinds of chronic pain [46, 56].
In other brain regions, the mouse was also reported in the modulation of chronic pain. In the IC, the mechanical allodynia was significantly decreased by ZIP microinjection into the IC after nerve injury. The levels of GluA1, GluA2, and p-PKMζ were decreased after ZIP microinjection [57]. Our recent results suggested that AC1, but not AC8, can be a trigger of the induction and maintenance of LTP in the IC. Inhibition of PKMζ reduced the expression of LTP [22]. In the spinal cord, Asiedu et al. reported that spinal inhibition of PKMζ completely abolished prolonged allodynia induced by hindpaw injection of prostaglandin E(2) [58]. Inhibition of PKCζ/PKMζ activity decreased the expression of c-Fos in response to formalin and complete Freund’s adjuvant (CFA) in both superficial and deep laminae of the dorsal horn. Recent studies have shown that PKMζ was also implicated in the formation of visceral hypersensitivity with irritable bowel syndrome (IBS). The expression of hippocampal p-PKMζ significantly increased in rats of visceral hypersensitivity generated by neonatal maternal separation (NMS). ZIP could inhibit the maintenance of CA1 LTP and attenuate the visceral hypersensitivity in rats of NMS [59, 60]. In addition, the expression of p-PKMζ also increased in the thoracolumbar and lumbosacral spinal cord in the IBS-like rats with notable concomitant chronic visceral pain [61]. And more notably, recent studies provide novel behavioral evidence that there is a sex difference in PKMζ involved in the maintenance of nociceptive sensitization. Pharmacological inhibition or genetic ablation of PKMζ effectively reduced referred visceral and muscle pain in males, but not in female mice and rats [62, 63]. These findings suggest that females may rely on a factor independent of PKMζ for the maintenance of nociceptive sensitization. Future studies are needed to be carried out to determine whether AC1 is important or not for the upregulation of PKMζ in female mice in chronic pain.
AC1 Is Required for Injury-Induced Changes in the Cortical Regions
Injury-induced changes in excitatory transmission, including postsynaptic and presynaptic changes, have been investigated in the ACC. AMPAR-mediated responses recorded from ACC pyramidal cells were significantly enhanced after peripheral nerve injury or inflammation [64, 65]. This enhancement is mainly mediated by GluA1-containing AMPARs, including phosphorylation at Ser845 site [64] and increased membrane expression of GluA1. AC1 activity is absolutely required for this postsynaptic modulation [1]. In AC1 KO mice, injury triggered the upregulation of GluA1, and AMPAR-mediated responses were blocked [40, 64]. In addition to the enhancement of AMPAR-EPSCs, peripheral nerve injury, and inflammation also led to increased GluN2B-containing NMDAR-mediated responses in the ACC [16, 43, 66]. The upregulation of NMDARs after inflammation is relatively selective for the GluN2B subtype; GluN2A-mediated responses were not significantly affected [43]. The expression enhancement of GluN2B, p-CREB, and CREB after visceral pain was reduced in AC1 KO mice in a visceral pain model of IBS [44]. Recent studies of the IC have shown that upregulation of GluN2B-containing NMDARs after nerve injury also occurred. Injury-induced upregulation of the synaptic NMDARs subunit was completely blocked in AC1 KO mice in the IC. The phosphorylation of GluN2B at the Tyr1472 site was also blocked [42]. These results indicate that AC1 contributes to the upregulation of synaptic NMDARs after nerve injury in the IC. In AC1 KO mice, the amount of synaptic GluA1 and its phosphorylation at the Ser845 site remained unchanged in the IC after the injury. Furthermore, no upregulation of AKAP79/150, PKA Cα, or PKA RIIβ was detected from the AC1 KO mice with nerve ligation [40].
In addition to postsynaptic changes, the presynaptic enhancement of excitatory synaptic transmission in the ACC after neuropathic pain and inflammation pain also depends on AC1. In AC1 KO mice, the decrease of the paired-pulse ratio after nerve injury or inflammation was blocked in the ACC. Furthermore, no increase of miniature EPSCs (mEPSCs) frequency or amplitude of AMPAR-mediated mEPSCs occurred in the ACC neurons in AC1 KO mice after peripheral nerve injury or inflammation [64, 67]. In suffering chronic pain, long-term changes in intrinsic electrical properties have also been noted in ACC neurons. The temporal precision of action potential firing in the ACC is reduced after peripheral inflammation or nerve injury, as reflected in increased jitter (the standard deviation of spikes latency). Activation of ACs by bath application of forskolin increased jitter, whereas genetic deletion of AC1 eliminated the change of jitter caused by CFA inflammation [68].
In addition to its important roles in cortical LTP related to injury, there are several reports that AC1 may also contribute to injury-related facilitation or potentiation. For example, it has been known that 5-HT is involved in spinal facilitatory modulation of sensory transmission [9, 27, 69]. The application of 5-HT produces facilitation of synaptic transmission between primary afferent fibers and dorsal horn neurons. Such facilitation requires AC1 activity in the spinal cord [12]. Furthermore, Wei et al. reported that AC1 activity is required for the spinal activation of ERK induced by inflammation. AC1 and AC8 act upstream of ERK activation in spinal dorsal horn neurons and the calcium-AC1/AC8-dependent ERK signaling pathways may contribute to spinal sensitization, an underlying mechanism for the development of persistent pain after injury [70]. In a recent study, Corder et al. reported that AC1 may contribute to hypersensitivity induced by long term activation of μ-opioid receptors [71].
New Cellular Mechanism: AC1 Positive Feedback Mechanisms
AC1 is thought to be an activity-dependent signaling protein that is essential to produce the second messenger cAMP. However, there is less knowledge about whether it is also regulated in the disease condition. A recent study demonstrates that AC1 itself is also activity-dependent for regulation in cortical neurons. Liu et al. (2019) reported that AC1 significantly increased in the ACC in an animal model of IBS and persisted for at least a few weeks. By contrast, acute pain did not affect the protein level of AC1. Inhibiting AC1 activity by NB001 significantly reduced the upregulation of AC1 protein in the ACC, suggesting that AC1 activity itself is critical for AC1 protein upregulation [44]. This finding indicates that AC1 may form a positive regulation in the cortex during chronic visceral pain. Fig. 3 is a proposed model for AC1 positive feedback control in disease conditions.
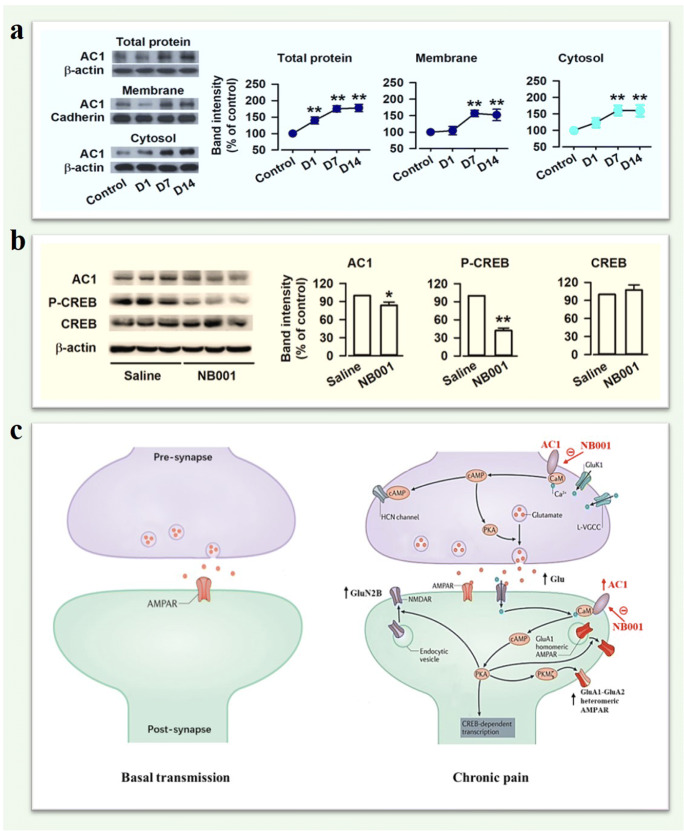
Fig. 3.
Upregulation of AC1 in chronic visceral pain. (a) Representative Western blots for total, membrane, and cytosol AC1 in the ACC of control and zymosan-induced chronic visceral pain mice. Total, membrane, and cytosol AC1 levels in the ACC were markedly increased on Day 7 and 14 after zymosan injection. (b) Representative Western blots for AC1, p-CREB, and CREB in the ACC of chronic visceral pain mice treated with saline or NB001. NB001 injection reduced the levels of AC1 and p-CREB in the ACC, but not total CREB of model mice. (c) Signaling pathways of the AC1-dependent LTP in the ACC of chronic pain. Synapses in the ACC undergo long-term presynaptic and postsynaptic changes after chronic pain. The presynaptic glutamate release, expression of GluA1-GluA2 containing AMPARs and GluN2B-containing NMDARs are upregulated in models of chronic pain. AC1 is essential for both long-term presynaptic and postsynaptic potentiation. NB001 inhibited the AC1-dependent LTP as a drug target for the treatment of chronic pain. (Adapted from Shuibing Liu et al., 2019)
Preclinical Studies of AC1 and AC1 Inhibitor NB001
The original discovery of the requirement of AC1 in chronic pain was made using AC1 KO mice. The lack of phenotype in learning-related LTP and behavioral memory made it more attractive for candidates of chronic pain [12]. Like other cognitive, emotional, and motor functions, acute sensory functions are intact in AC1 KO mice. However, Wei et al. reported that behavioral responses to peripheral injection of two inflammatory stimuli, formalin and CFA, were reduced or abolished in AC1 KO mice [39]. In a neuropathic pain model, several studies show that behavioral allodynia and synaptic changes were also reduced in AC1 KO mice [39, 42, 46, 64]. By using an acute persistent inflammatory muscle pain model, Vadakkan et al. showed that behavioral nociceptive responses of both the late phase of acute muscle pain and the chronic muscle inflammatory pain were significantly reduced in AC1 KO mice [72]. In a zymosan-induced chronic visceral pain model of IBS, the pain behaviors and vertical counts are markedly attenuated in AC1 KO mice [44]. In the diabetic neuropathy model, AC1 KO mice abolished methylglyoxal-induced hyperalgesia (Table II) [56, 73].
Table II.
Indication of AC1/NB001 in clinical chronic pain treatments
| Pain model | Inhibition AC1 activity | Biochemical marker | Electrophysiological marker | Behavioral marker | References |
|---|---|---|---|---|---|
| Neuropathic pain | AC1 KO |
•Did not change the expression of GluA1, GluA2/3, p-GluA1, PKMζ, and p-PKMζ in the ACC after nerve injury •Blocked the upregulation of GluN1, GluN2B, or GluN2A and the p-GluN2B at Tyr1472 in the IC •Blocked the forskolin- and rolipram-induced enhancement of GluN2A and GluN2B in the IC |
Did not change the frequency and amplitude of mEPSCs, paired-pulse ratio, and rectification index of AMPAR-mediated current in the ACC after nerve injury |
•Reduced nerve injury-induced mechanical allodynia •Reduced nerve injury-induced mechanical sensitivity of both ipsi- and contralateral hindpaw |
[51] [39, 42, 64] |
| NB001 | Blocked the forskolin- and rolipram-induced enhancement of GluN2A and GluN2B |
•No effect on AMPAR-mediated EPSCs, paired-pulse ratio, and NMDAR-mediated EPSCs and I-V curves in wild-type mice •Blocked the LTP in the ACC, spinal cord, but not in the hippocampus. |
•Reduced nerve injury-induced mechanical allodynia with intraperitoneal and oral application •No effect on anxiety-like behavior, locomotor activity, and motor function test by open field and EPM in wild-type mice •No effect on acute nociception tested by hot-plate, tail-flick and von Frey filaments in wild-type mice |
[42, 46] | |
| Inflammatory pain | AC1 KO |
•Blocked the enhancement of p-CREB after formalin injection in the ACC, IC and spinal dorsal horn •Blocked the activation of ERK by glutamate and capsaicin |
No data |
•Reduced behavioral responses induced by CFA and formalin injection in the hot-plate and tail-flick reflex test. •Reduced mechanical allodynia induced by with CFA and formalin injection |
[39, 70] |
| NB001 | No data | No data | Reduced CFA-induced mechanical allodynia | [46] | |
| Cancer pain | AC1 KO | No data | No data | No data | no data |
| NB001 | Blocked the upregulation of cAMP, GluN2A, GluN2B, p-GluA1 (Ser831), p-GluA1 (Ser845) and GluA1 after pain model | Inhibited the increase of mEPSC frequency in pain model | Decreased the number of spontaneous lifting and increased the mechanical paw withdrawal threshold | [74] | |
| Visceral pain | AC1 KO | Abolished the enhancements of AC1, GluN2B, p-CREB, and CREB after visceral pain | Inhibited the increase of GluN2B EPSCs after visceral pain | Attenuated pain behaviors and vertical counts | [44] |
| NB001 | Abolished the enhancements of AC1, p-CREB, and CREB after visceral pain | No data |
•Reduced pain behaviors and vertical counts •Reduced zymosan-induced anxiety-like behaviors tested by EPM and light/dark box |
[44, 75] | |
| Chronic muscle pain | AC1 KO | No data | No data | Reduced behavioral nociceptive responses of muscle pain | [72] |
| NB001 | No data | No data | Blocked behavioral responses in both acute persistent and chronic muscle pain. | [72] | |
| Arthralgia | AC1 KO | No data | No data | No data | |
| NB001 | Did not attenuate CFA-induced ankle joint and knee joint structural destruction | No data |
•Reduced the joints edema, spontaneous flinches in both ankle and knee joints induced by CFA •Inhibited mechanical allodynia and hyperalgesia in both ankle and knee joints induced by CFA •No effect on CFA-induced joint stiffness |
[79] | |
| Migraine | AC1 KO | No data | Blocked CGRP-induced LTP in the ACC | No data | [76] |
| NB001 | No data | Blocked CGRP-induced LTP in the ACC and IC | No data | [76, 77] | |
| Painful diabetic neuropathy | AC1 KO | No data | No data | Abolished methylglyoxal-induced heat and mechanical hyperalgesia but not immediate pain-like behaviors | [56] |
| NB001 | No data | No data | Attenuated methylglyoxal-induced hyperalgesia | [73] |
While genetic deletion provides direct evidence for the involvement of a selective gene in behavioral functions before selective inhibitors can be identified, selective pharmacological inhibitors are the most effective way to translate animal discovery to potential clinical applications. Through rational drug design and chemical screening, Wang et al. (2011) identified a lead candidate AC1 inhibitor, NB001, which is relatively selective for AC1 over other AC isoforms. Using a variety of behavioral tests and toxicity studies, Wang et al. showed that NB001, when administered intraperitoneally or orally, had an analgesic effect in animal models of neuropathic pain, without any apparent side effects [46]. Except in neuropathic pain, NB001 was reported to have analgesic effects on other pain models. NB001 markedly decreased the amount of spontaneous lifting and increased the mechanical paw withdrawal threshold in mice suffering bone cancer pain by injecting osteolytic murine sarcoma cell NCTC 2472. The upregulation of cAMP, GluN2A, GluN2B, p-GluA1 (Ser831), p-GluA1 (Ser845), and GluA1 were decreased by NB001 in bone cancer pain mice [74]. NB001 also produced a significant analgesic effect in both acute persistent and chronic inflammatory muscle pain [72]. Zhang et al. reported that NB001 produced inhibition of injury-induced behavioral anxiety and spontaneous pain in a visceral pain model of IBS [75]. In addition, Taylor’s group reported that spinal inhibition of AC1 by NB001 reduced hypersensitivity using db/db mice, an animal model of type 2 diabetes [56]. Recent human brain imaging studies showed that ACC activities increased during or after chronic headache. Our latest studies on calcitonin gene-related peptide (CGRP), a critical molecule for inducing headache, suggest that AC1 may be a potential target for the treatment of headache. We found that CGRP-induced chemical LTP and recruited inactive circuit are AC1 activities dependent in the ACC. Both genetic deletion of AC1 using AC1 KO mice or AC1 inhibitor NB001 can block CGRP-induced LTP [76]. In addition, these effects were also found in the IC, another cortical area related with headache (Table II) [77]. Finally, these results indicate that AC1 inhibitor NB001 may be a potential drug for the treatment of chronic pain.
Conclusion and Future Directions
Recent progress made in basic research of chronic pain have clearly demonstrated that chronic pain is different from acute pain. Unpleasantness can be caused by non-noxious stimuli that normally do not cause pain, or can be triggered by emotional discomfort, anxiety or depression. Cortical excitation or LTP in cortical synapses are likely to play important roles in the amplification or generation of central pain. Inhibiting signaling pathways that contribute to cortical excitation is a key to treat chronic pain. Among several potential targets, AC1 is a good candidate with few or no side effects. We hope that future studies of AC1 inhibitors in patients with different types of chronic pain will provide firsthand information about the function of sensory LTP in chronic pain, and the use of novel AC1 inhibitors may help to treat patients with different illnesses such as chronic pain and drug addiction.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Abbreviations
- 5-HT
Serotonin
- AC1
Adenylyl cyclase subtype 1
- ACC
Anterior cingulate cortex
- AMPARs
2-Amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptors
- BDNF
Brain-derived neurotrophic factor
- CaM
Calmodulin
- CFA
Complete Freund’s adjuvant
- CGRP
Calcitonin gene-related peptide
- CREB
cAMP response element-binding protein
- EPSCs
Excitatory postsynaptic currents
- EPSPs
Excitatory postsynaptic potentials
- ERK
Extracellular signal-regulated kinase
- GluN1
Glutamate NMDA receptor subunit 1
- GluN2
Glutamate NMDA receptor subunit 2
- HCN
Hyperpolarization-activated cyclic nucleotide-gated
- IBS
Irritable bowel syndrome
- IC
Insular cortex
- KAR
Kainate receptor
- LC
Locus coeruleus
- LTP
Long-term potentiation
- NMDARs
N-Methyl-d-aspartate receptors
- NMS
Neonatal maternal separation
- PAG
Periaqueductal gray
- PFC
Prefrontal cortex
- PKA
Protein kinase A
- PKC
Protein kinase C
- PKMζ
Protein kinase Mζ
- RVM
Rostroventral medial medulla
- TBS
Theta burst stimulation
Footnotes
The original online version of this article was revised to replace Figure 3 with the correct figure send by the author.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/4/2021
A Correction to this paper has been published: 10.1007/s13311-021-01065-y
Contributor Information
Xu-Hui Li, Email: lixuhui0207@163.com.
Qi-Yu Chen, Email: chenqiyu@stu.xjtu.edu.cn.
Min Zhuo, Email: min.zhuo@utoronto.ca.
References
- 1.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature reviews Neuroscience. 2016;17(8):485–96. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Molecular brain. 2013;6:5. doi: 10.1186/1756-6606-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature reviews Neuroscience. 2007;8(11):844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 4.Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Molecular pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369(1633):20130146. doi: 10.1098/rstb.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299(5610):1237–40. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 7.Liu XG, Zhou LJ. Long-term potentiation at spinal C-fiber synapses: a target for pathological pain. Current pharmaceutical design. 2015;21(7):895–905. doi: 10.2174/1381612820666141027115949. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312(5780):1659–62. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Kerchner GA, Sala C, Wei F, Huettner JE, Sheng M, et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nature neuroscience. 1999;2(11):972–7. doi: 10.1038/14771. [DOI] [PubMed] [Google Scholar]
- 10.Hori Y, Endo K, Takahashi T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. The Journal of physiology. 1996;492(Pt 3):867–76. doi: 10.1113/jphysiol.1996.sp021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou LJ, Zhong Y, Ren WJ, Li YY, Zhang T, Liu XG. BDNF induces late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. Experimental neurology. 2008;212(2):507–14. doi: 10.1016/j.expneurol.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Wang GD, Zhuo M. Synergistic enhancement of glutamate-mediated responses by serotonin and forskolin in adult mouse spinal dorsal horn neurons. Journal of neurophysiology. 2002;87(2):732–9. doi: 10.1152/jn.00423.2001. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo M. Cortical excitation and chronic pain. Trends in neurosciences. 2008;31(4):199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhuo M. Contribution of synaptic plasticity in the insular cortex to chronic pain. Neuroscience. 2016;338:220–9. doi: 10.1016/j.neuroscience.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Li XH, Song Q, Chen T, Zhuo M. Characterization of postsynaptic calcium signals in the pyramidal neurons of anterior cingulate cortex. Molecular pain. 2017;13:1744806917719847. doi: 10.1177/1744806917719847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47(6):859–72. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Chen T, O'Den G, Song Q, Koga K, Zhang MM, Zhuo M. Adenylyl cyclase subtype 1 is essential for late-phase long term potentiation and spatial propagation of synaptic responses in the anterior cingulate cortex of adult mice. Molecular pain. 2014;10:65. doi: 10.1186/1744-8069-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446(7139):1086–90. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- 19.Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325(5937):207–10. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- 20.Li XH, Miao HH, Zhuo M. NMDA Receptor Dependent Long-term Potentiation in Chronic Pain. Neurochemical research. 2019;44(3):531–8. doi: 10.1007/s11064-018-2614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao HH, Li XH, Chen QY, Zhuo M. Calcium-stimulated adenylyl cyclase subtype 1 is required for presynaptic long-term potentiation in the insular cortex of adult mice. Molecular pain. 2019;15. [DOI] [PMC free article] [PubMed]
- 22.Yamanaka M, Matsuura T, Pan H, Zhuo M. Calcium-stimulated adenylyl cyclase subtype 1 (AC1) contributes to LTP in the insular cortex of adult mice. Heliyon. 2017;3(7):e00338. doi: 10.1016/j.heliyon.2017.e00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liauw J, Wu LJ, Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. Journal of neurophysiology. 2005;94(1):878–82. doi: 10.1152/jn.01205.2004. [DOI] [PubMed] [Google Scholar]
- 24.Song Q, Zheng HW, Li XH, Huganir RL, Kuner T, Zhuo M, et al. Selective Phosphorylation of AMPA Receptor Contributes to the Network of Long-Term Potentiation in the Anterior Cingulate Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017;37(35):8534–48. doi: 10.1523/JNEUROSCI.0925-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, et al. Coexistence of Two Forms of LTP in ACC Provides a Synaptic Mechanism for the Interactions between Anxiety and Chronic Pain. Neuron. 2015;86(4):1109. doi: 10.1016/j.neuron.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Yamanaka M, Tian Z, Darvish-Ghane S, Zhuo M. Pre-LTP requires extracellular signal-regulated kinase in the ACC. Molecular pain. 2016;12. [DOI] [PMC free article] [PubMed]
- 27.Zhuo M. Descending facilitation. Molecular pain. 2017;13:1744806917699212. doi: 10.1177/1744806917699212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson DA, Calejesan AA, Wei F, Gebhart GF, Zhuo M. Endogenous facilitation: from molecular mechanisms to persistent pain. Curr Neurovasc Res. 2004;1(1):11–20. doi: 10.2174/1567202043480189. [DOI] [PubMed] [Google Scholar]
- 29.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in neurosciences. 2002;25(6):319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo M, Gebhart GF. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Journal of neurophysiology. 1992;67(6):1599–614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393(6686):695–8. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- 32.Chen T, Koga K, Descalzi G, Qiu S, Wang J, Zhang LS, et al. Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Molecular pain. 2014;10:33. doi: 10.1186/1744-8069-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nature communications. 2018;9(1):1886. doi: 10.1038/s41467-018-04309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424(6946):316–20. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Gadotti VM, Chen L, Souza IA, Huang S, Wang D, et al. A neuronal circuit for activating descending modulation of neuropathic pain. Nature neuroscience. 2019;22(10):1659–68. doi: 10.1038/s41593-019-0481-5. [DOI] [PubMed] [Google Scholar]
- 36.Xia M, Sreedharan SP, Bolin DR, Gaufo GO, Goetzl EJ. Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. The Journal of pharmacology and experimental therapeutics. 1997;281(2):629–33. [PubMed] [Google Scholar]
- 37.Zhuo M. Targeting neuronal adenylyl cyclase for the treatment of chronic pain. Drug discovery today. 2012;17(11-12):573–82. doi: 10.1016/j.drudis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23(4):787–98. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 39.Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36(4):713–26. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 40.Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, et al. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(40):13505–15. doi: 10.1523/JNEUROSCI.1431-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, et al. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nature neuroscience. 2001;4(2):164–9. doi: 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- 42.Qiu S, Chen T, Koga K, Guo YY, Xu H, Song Q, et al. An increase in synaptic NMDA receptors in the insular cortex contributes to neuropathic pain. Science Signaling. 2013;6(275):ra34. doi: 10.1126/scisignal.2003778. [DOI] [PubMed] [Google Scholar]
- 43.Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(48):11107–16. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu SB, Wang XS, Yue J, Yang L, Li XH, Hu LN, et al. Cyclic AMP-dependent positive feedback signaling pathways in the cortex contributes to visceral pain. Journal of neurochemistry. 2019. [DOI] [PubMed]
- 45.Liu MG, Kang SJ, Shi TY, Koga K, Zhang MM, Collingridge GL, et al. Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings. Journal of neurophysiology. 2013;110(2):505–21. doi: 10.1152/jn.01104.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, et al. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Science Translational Medicine. 2011;3(65):65ra3. doi: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
- 47.Koga K, Liu MG, Qiu S, Song Q, O'Den G, Chen T, et al. Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(5):2033–43. doi: 10.1523/JNEUROSCI.2644-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li XY, Ko HG, Chen T, Collingridge GL, Kaang BK, Zhuo M. Erasing injury-related cortical synaptic potentiation as a new treatment for chronic pain. Journal of molecular medicine. 2011;89(9):847–55. doi: 10.1007/s00109-011-0768-9. [DOI] [PubMed] [Google Scholar]
- 49.Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493(7432):420–3. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, et al. Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. The Journal of comparative neurology. 2000;426(2):243–58. doi: 10.1002/1096-9861(20001016)426:2<243::aid-cne6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330(6009):1400–4. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- 52.Ko HG, Ye S, Han DH, Park P, Lim CS, Lee K, et al. Transcription-independent expression of PKMzeta in the anterior cingulate cortex contributes to chronically maintained neuropathic pain. Molecular pain. 2018;14:1744806918783943. doi: 10.1177/1744806918783943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King T, Qu C, Okun A, Melemedjian OK, Mandell EK, Maskaykina IY, et al. Contribution of PKMzeta-dependent and independent amplification to components of experimental neuropathic pain. Pain. 2012;153(6):1263–73. doi: 10.1016/j.pain.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du J, Fang J, Wen C, Shao X, Liang Y, Fang J. The Effect of Electroacupuncture on PKMzeta in the ACC in Regulating Anxiety-Like Behaviors in Rats Experiencing Chronic Inflammatory Pain. Neural plasticity. 2017;2017:3728752. doi: 10.1155/2017/3728752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Wang P, Li H. Upregulation of glutamatergic transmission in anterior cingulate cortex in the diabetic rats with neuropathic pain. Neuroscience letters. 2014;568:29–34. doi: 10.1016/j.neulet.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 56.Griggs RB, Santos DF, Laird DE, Doolen S, Donahue RR, Wessel CR, et al. Methylglyoxal and a spinal TRPA1-AC1-Epac cascade facilitate pain in the db/db mouse model of type 2 diabetes. Neurobiol Dis. 2019;127:76–86. doi: 10.1016/j.nbd.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han J, Kwon M, Cha M, Tanioka M, Hong SK, Bai SJ, et al. Plasticity-Related PKMzeta Signaling in the Insular Cortex Is Involved in the Modulation of Neuropathic Pain after Nerve Injury. Neural plasticity. 2015;2015:601767. doi: 10.1155/2015/601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, et al. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(18):6646–53. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen A, Bao C, Tang Y, Luo X, Guo L, Liu B, et al. Involvement of protein kinase zeta in the maintenance of hippocampal long-term potentiation in rats with chronic visceral hypersensitivity. Journal of neurophysiology. 2015;113(9):3047–55. doi: 10.1152/jn.00929.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan F, Tang Y, Dai H, Cao Y, Sun P, Chen Y, et al. Blockade of BDNF signalling attenuates chronic visceral hypersensitivity in an IBS-like rat model. European journal of pain. 2020. [DOI] [PMC free article] [PubMed]
- 61.Tang Y, Chen A, Chen Y, Guo L, Dai H, Huang Y, et al. Zeta Inhibitory Peptide as a Novel Therapy to Control Chronic Visceral Hypersensitivity in a Rat Model. PloS one. 2016;11(10):e0163324. doi: 10.1371/journal.pone.0163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nasir H, Mahboubi H, Gyawali S, Ding S, Mickeviciute A, Ragavendran JV, et al. Consistent sex-dependent effects of PKMzeta gene ablation and pharmacological inhibition on the maintenance of referred pain. Molecular pain. 2016;12. [DOI] [PMC free article] [PubMed]
- 63.George NC, Laferriere A, Coderre TJ. Sex differences in the contributions of spinal atypical PKCs and downstream targets to the maintenance of nociceptive sensitization. Molecular pain. 2019;15:1744806919840582. doi: 10.1177/1744806919840582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(29):7445–53. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu LJ, Steenland HW, Kim SS, Isiegas C, Abel T, Kaang BK, et al. Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Molecular Pain. 2008;4:40. doi: 10.1186/1744-8069-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang GD, Zhuo M. Forebrain NMDA receptors contribute to neuronal spike responses in adult mice. Sheng li xue bao : [Acta physiologica Sinica]. 2006;58(6):511–20. [PubMed] [Google Scholar]
- 67.Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(35):8923–30. doi: 10.1523/JNEUROSCI.2103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li XY, Wang N, Wang YJ, Zuo ZX, Koga K, Luo F, et al. Long-term temporal imprecision of information coding in the anterior cingulate cortex of mice with peripheral inflammation or nerve injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(32):10675–87. doi: 10.1523/JNEUROSCI.5166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397(6715):161–4. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 70.Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(3):851–61. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, et al. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341(6152):1394–9. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vadakkan KI, Wang H, Ko SW, Zastepa E, Petrovic MJ, Sluka KA, et al. Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Molecular pain. 2006;2:7. doi: 10.1186/1744-8069-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griggs RB, Laird DE, Donahue RR, Fu W, Taylor BK. Methylglyoxal Requires AC1 and TRPA1 to Produce Pain and Spinal Neuron Activation. Front Neurosci. 2017;11:679. doi: 10.3389/fnins.2017.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang WB, Yang Q, Guo YY, Wang L, Wang DS, Cheng Q, et al. Analgesic effects of adenylyl cyclase inhibitor NB001 on bone cancer pain in a mouse model. Molecular pain. 2016;12. [DOI] [PMC free article] [PubMed]
- 75.Zhang MM, Liu SB, Chen T, Koga K, Zhang T, Li YQ, et al. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Molecular brain. 2014;7:47. doi: 10.1186/1756-6606-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li XH, Matsuura T, Liu RH, Xue M, Zhuo M. Calcitonin gene-related peptide potentiated the excitatory transmission and network propagation in the anterior cingulate cortex of adult mice. Molecular pain. 2019;15. [DOI] [PMC free article] [PubMed]
- 77.Liu Y, Chen QY, Lee JH, Li XH, Yu S, Zhuo M. Cortical potentiation induced by calcitonin gene-related peptide (CGRP) in the insular cortex of adult mice. Molecular brain. 2020;13(1):36. doi: 10.1186/s13041-020-00580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng F, Zhang M, Ding Q, Sethna F, Yan L, Moon C, et al. Voluntary running depreciates the requirement of Ca2+-stimulated cAMP signaling in synaptic potentiation and memory formation. Learning & memory. 2016;23(8):442–9. doi: 10.1101/lm.040642.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian Z, Wang DS, Wang XS, Tian J, Han J, Guo YY, et al. Analgesic effects of NB001 on mouse models of arthralgia. Molecular brain. 2015;8(1):60. doi: 10.1186/s13041-015-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)