Abstract
The present study was focused to isolate the bioactive compounds present in the leaves of Moringa oleifera which contains a high nutritional value. Furthermore, the research was aimed to evaluate the antioxidant, anti-aging, and anti-neurodegenerative properties of M. oleifera using the experimental model Caenorhabditis elegans. The separation of compounds from the crude extract and its identification was carried out through TLC, Column chromatography, UV absorption spectroscopy, and GC–MS. The compounds identified in most abundant fraction of column chromatography were [Phenol-2,4-bis(1,1-dimethylethyl)- phosphite (3:1)] and Tetratetracontane. The result suggests that the leaves extracts and column fraction were able to significantly extend the life span of the N2 wild-type strain of C. elegans. The most potent life span extending effect was displayed by the dichloromethane extract of leaves which was 21.73 ± 0.142 days compared to the control (16.55 ± 0.02 days). It could also extend the health span through improved physiological functions such as pharyngeal pumping, body bending, and reversal frequency with increased age. The treated worms were also exhibited improved resistance to thermal stress, oxidative stress, and reduced intracellular ROS accumulation. Moreover, the leaves extract could elicit neuroprotection as it could delay the paralysis in the transgenic strain of C. elegans ‘CL4176’ integrated with Aβ. Interestingly, The RNAi experiment demonstrated that the extended life span under the treatment of extracts and the compound was daf-16 dependent. In transgenic C. elegans TJ356, the DAF-16 transcription factor was localized in the nucleus under the stress conditions, further supported the involvement of the daf-16 gene in longevity. Overall, the study suggests the potential of M. oleifera as a dietary supplement and alternative medicine to defend against oxidative stress and aging.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02485-x) contains supplementary material, which is available to authorized users.
Keywords: Moringa oleifera, Caenorhabditis elegans, N2 wild-type, CL4176, TJ356, Alzheimer’s disease, ROS, RNAi, DAF-16
Introduction
For several centuries, various types of plants have been utilized globally not only as nutritional supplements but also as conventional treatments for numerous diseases (Njan et al. 2014). Among these plants, the Moringa oleifera is a widely used medicinal plant which is a rapidly growing, deciduous, drought resistant, a small or middle-sized tree about 8–10 m in height. It is native to India and widely cultivated in the tropics & subtropics of Asia and Africa (Paliwal et al. 2011). This plant is commonly known as Moringa, drumstick tree, horseradish tree in English, and Saragvo in local Gujarati language. Moringa is known as ‘Sigru’ in Ayurveda as referenced in The Ayurvedic Pharmacopoeia of India (Ayurvedic Pharmacopoeia Committee 2001). Moringa tree has been in use in conventional Indian medication for over 5000 years. There are, moreover, reports of Moringa being utilized by the antiquated Greeks, Romans, and Egyptians.
Moringa tree is profoundly nutritious with a wide range of pharmacological properties. The leaves of Moringa are a considerable source of protein, calcium and potassium, vitamin C, and β-carotene. It is a rich source of natural antioxidant compounds such as phenolics, flavonoids, carotenoids, and ascorbic acid (Dillard and German 2000; Siddhuraju and Becker 2003). The flowers of Moringa have been accounted for to be a potent source of flavonoids, alkaloids, waxes, fat, vitamins, Ca2+, and K+ (Ramachandran et al. 1980; Kalappurayil and Joseph 2017). It has been demonstrated that various parts of M. oleifera such as leaves, stem, fruit, flowers, seed, and root display diverse biological activities, including analgesic (Sutar et al. 2008), antipyretic (Oliveira et al. 1998), anti-atherosclerotic (Mehta et al. 2003), immune-boosting (Miyoshi et al. 2004), anti-cardiovascular disease (Faizi et al. 1994), antiviral (Murakami et al. 1998), antioxidant (Iqbal and Bhanger 2006; Kumar et al. 2019; Kumar et al. 2012), antimicrobial (Caceres et al. 1991), anti-inflammatory (Martinez-Gonzalez et al. 2017; Gupta et al. 2013) and anti-tumor effects (Murakami et al. 1998; Bharali et al. 2003). It has been reported that this plant was traditionally used for the treatment of neurologic conditions such as muscle spasmodic, epilepsy, headache, hysteria (Fahey 2005), and nervous debility (Mishra et al. 2011). Moringa can be used to provide effective neuroprotection. Inadequate blood flow in the brain prompts a condition called cerebral ischemia. This condition further causes the reperfusion and lipid peroxidation which results in reactive oxygen species (ROS). Moringa can protect the brain by reducing ROS by means of its antioxidant activities (Baker et al. 1998; Kirisattayakul et al. 2013). Previous studies on the effect of M. oleifera in the brain discovered that nootropic activity displayed by leaves can enhance memory (Mohan et al. 2005), possibly by altering brain monoamine levels and electrical activity (Bakre et al. 2013; Ganguly and Guha 2008). The presence of a high amount of potassium in M. oleifera proved advantageous for the functioning of the brain and nerves (Das et al. 2017).
A variety of techniques can be used to determine and estimate the presence of various phytochemical compounds. The most commonly utilized approach for the recovery of bioactive compounds from plants is solvent extraction using various solvents of specific polarity (Chavan and Amarowicz 2013). The isolated compounds can be screened and characterized by various techniques such as thin-layer chromatography (TLC), column chromatography, and gas chromatography-mass spectrometry (GC–MS) (Medic-Saric et al. 2004; Vongsak et al. 2014; Sharma and Paliwal 2013a).
As stated in the free radical theory of aging, the accumulation of cellular ROS leads to the aging with increased risk of diseases and death (Harman 1992). Elevated levels of oxidative stress damage the cellular targets that lead to growth inhibition and an increased mutation rate (Liochev 2013). There are several diseases linked to oxidative stress and aging such as kidney disease, neurodegenerative disease, macular degeneration, cancer, and cardiovascular disease (Lee et al. 2003; Liguori et al. 2018). It has been validated that the life span of an organism can be extended if reactive free radicals are decreased or the concentration of antioxidant substances increased (Gourley et al. 2003; Rahman 2007). The most widely used animal model for the life span study is C. elegans. It is readily accessible, easy to culture in the lab facility, has a short life span, and has a completely sequenced genome, and 40% of genes are associated with human diseases (Corsi et al. 2015). Other than wild-type N2 Bristol, there are distinctive transgenic C. elegans available to study various diseases related to humans. One of these mutants is CL4176 which is used to study Alzheimer’s disease (AD). CL4176 expresses the human Aβ1–42 in muscle tissues under a temperature-inducible system (Drake et al. 2003). One of the causes of AD is an elevated level of ROS and oxidative stress (Huang et al. 2016). Another transgenic C. elegans ‘TJ356’, in which GFP is fused with the daf-16 gene. DAF16 is an ortholog of the FOXO family of transcription factors that are accountable for gene activation involved in longevity, lipogenesis, heat sock survival, and oxidative stress responses. DAF16 is a downstream regulator of insulin/IGF-1 signaling pathway (IIS pathway) which is well recognized for improving the life span in C. elegans, D. melanogaster, and Mus musculus (Zecic and Braeckman 2020). TJ356 is used to determine the localization of the DAF16 transcription factors under applied stress conditions (Shukla et al. 2012).
It has been proven that longevity is regulated through genetic and environmental factors. Such factors can influence the metabolism and free radical production depending upon the organism. One such organism used here to study the genes involved in longevity is C. elegans (Guarente and Kenyon 2000). To identify the genes that control the C. elegans life span, RNA interference is the most widely used process. The RNA interference (RNAi) is primarily based on the principle of antisense, wherein the injected RNA can hybridize with RNA present in the cells, which further manipulate the gene expression (Fire et al. 1998).
The ultimate aim of the present study was to evaluate the bioactive compounds present in leaves extracts of M. oleifera as well as to investigate its impact on life span extension and Alzheimer’s disease using N2 wild-type and CL4176 transgenic C. elegans, respectively. The RNAi experiment was performed to estimate the genes involved in longevity using daf-16 RNAi. Another transgenic C. elegans ‘TJ356’ was employed for the DAF-16 localization assay.
Materials and methods
Preparation of sample for phytochemical characterization
The leaves of M. oleifera were collected from the institutional garden and validated at the Department of Genetics and Plant Breeding, B. A. College of Agriculture, Anand Agricultural University (BACA/GPB/68/18). The leaves were washed and shade dried for 2–3 days. The dried leaves were ground and stored at 4 °C for further use. The crude extracts of M. oleifera leaves were prepared by sequential extraction using the Soxhlet apparatus. The solvents used for extraction were n-hexane, ethyl acetate, dichloromethane, methanol, and water with increasing polarity. The used powdered sample was dried after extraction with each solvent before using a subsequent solvent. All the extracts [Leaves aqueous extract: La, Leaves methanol extract: Lm, Leaves dichloromethane extract: Ld, Leaves ethyl acetate extract: Le, Leaves n-hexane extract: Lh] were dried and stored at 4 °C for further analysis. For experiments performed on C. elegans, each dry extract was dissolved in dimethyl sulfoxide (DMSO) (≤ 2%) (Dengg and van Meel 2004).
Phytochemical separation using TLC method
The principle compounds present in the crude extracts of M. oleifera leaves were analyzed by TLC. Separation of compounds was carried out on pre-coated 20 × 20 cm (0.25 mm thick) TLC plates (F254 silica gel 60). Different solvent systems with a range of polarities were used to achieve better resolution.
Preparation of the mobile phase
The different solvent systems used for TLC were chloroform: glacial acetic acid: methanol: water (5:4:1:2), hexane: ethyl acetate: acetic acid (3:4:1), chloroform: methanol: water (7:3:1), hexane: acetic acid (9:1), toluene: hexane: isopropyl alcohol: water (5:3:1:1). The solvent systems were selected according to the different polarities of various solvents used.
Spotting and development of TLC plate
The plant extracts were applied on a silica plate at a distance of 1 cm from the end using capillary tubes. The chromatographic chamber first saturated with an appropriate solvent and then TLC plates were developed. The plates were removed from the chamber after complete development and the solvent front was marked. The developed TLC plates were air-dried and observed under ultraviolet light (UV). The spots observed were marked and the retardation factor (Rf) was calculated using the following formula:
Column chromatography
The dichloromethane extract of leaves was applied to the column chromatography for further fractionation and eluted using solvents with increasing polarity. The chromatography column was packed with silica gel G (60–120). The required amount of silica was weighted and solvent (hexane) was added to form a slurry. For better separation, the column packing should be consistent and without any air bubble. The adsorbent was uniformly packed by tapping the column gently. The column was arranged in a vertical position and the column packing was supported using glass wool. A piece of filter paper was placed over packed silica followed by cotton. The slurry of the dichloromethane extract of leaves (3 gm) was added at the top of the column. The sample was added carefully over the column without disrupting the column surface. A layer of cotton and filter paper was placed over the sample. The fresh solvent was carefully added to the column and the stop clock was opened so that the solvent flows constantly through the column. Each fraction of 30 ml volume was collected by eluting hexane:ethyl acetate (H:EA), followed by ethyl acetate:dichloromethane (EA:DCM), then followed by dichloromethane: methanol (DCM: M). The order of elution was H:EA (99:1), H:EA (98:2), H:EA (97:3), H:EA (96:4), H:EA (95:5), H:EA (94:6), H:EA (93:7), H:EA (92:8), H:EA (91:9), H:EA (90:10), H:EA (85:15), H:EA (80:20), H:EA (75:25), H:EA (70:30), H:EA (65:35), H:EA (60:40), H:EA (50:50), H:EA (40:60), H:EA (30:70), H:EA (20:80), H:EA (10:90), 100% (EA), EA:DCM (80:20), EA:DCM (60:40), EA:DCM (40–60), EA: DCM (20–80), DCM (100%). The collected fractions were then concentrated by solvent evaporation and used for further analysis.
UV-spectroscopic analysis
To detect the UV spectrum profile, the column fraction eluted by EA: DCM (80:20) was scanned in the wavelength ranging from 200 to 1000 nm using Double Beam spectrophotometer Thermo Scientific Evolution 201. The characteristic peaks were detected and the absorption spectra of the sample were measured and recorded.
Gas chromatography–mass spectrometry
GC–MS analysis was carried out on a Perkin Elmer GC Auto system XL; autosampler and gas chromatograph interfaced with a mass spectrometer Turbomass. The instrument employed the following conditions: Column: PE 5 ms [30 m × 0.25 mm 1D]) operated in electron impact mode at 70 eV; helium was used as carrier gas at a constant flow of 1 ml/min and an injection volume of 3μ1 was employed (split ratio of 10:1); injector temperature 250 °C; and ion source temperature 220 °C. The oven temperature was programmed from 75 °C for 5 min, with an increase of 10 °C/min up to 270 °C. Mass spectra were taken at 70 eV: a scan-interval of 0.5 s and fragments from 20 to 620 amu. The total GC running time was 40 min.
C. elegans strains and cultivation
Various C. elegans strains used in this study were N2 Bristol (wild-type), CL4176 (dvIs27 [pAF29 (myo-3/Ab 1-42/let UTR) + pRF4 (rol-6(su1006)]), and TJ356 (zI356 [daf-16p::daf-16a/b::GFP + rol-6(su1006)]. All strains were grown and maintained at 16 °C on nematode growth medium (NGM) agar plates seeded with the Escherichia coli strain OP50 (Brenner 1974). The E. coli OP50 was grown in Luria–Bertani (LB) medium at 37 °C. Grown cultures were spotted onto NGM plates and incubated at 37 °C for 24 h prior to worm inoculation. All the C. elegans worms and their food source were obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota, USA. The effect of various extracts was studied by providing the extracts with NGM agar at a dose of 100 μg/ml of media (Sonani et al. 2014). The extract containing plates were prepared by addition of extract to NGM after autoclave and prior to pouring.
Worm synchronization and life span assay
The life span assay was performed with the synchronized worms. Synchronization is the process to arrest all the worms at one stage of the life cycle. Synchronization of worms was accomplished by means of bleaching that kills the worms by dissolving the outer cuticle of and releasing the eggs from the guts of gravid hermaphrodites. To get synchronized worms, they were allowed to grow on NGM for 2–3 days. After sufficient eggs/adults were seen, M9 (3 g of KH2PO4, 6 g of Na2HPO4, 5 g of NaCl, 1 ml of 1 M MgSO4, make up the volume to 1 L using H2O) buffer was poured on the plate to dislodge the worms. After washing with M9 buffer, alkaline hypochlorite solution (1 N NaOH + 5% NaOCl) was added and incubated for 5–7 min. The eggs collected after bleaching were washed with M9 buffer for 4–5 times to remove the bleaching solution. Finally, the fresh M9 buffer was added and incubated at 20 °C overnight. The obtained L1 stage worms were then transferred to the NGM plate seeded with E. coli OP50. Incubation was done until the worms reached the L4 larval stage. The synchronized L4 worms were transferred to fresh NGM plates (control and 100 µg/ml extract containing plates) containing 150 μM fluorodeoxyuridine (FUDR) and seeded with E. coli OP50. The FUDR was added to maintain the synchronous population of worms as it does not allow the reproduction. Every experiment was performed in triplicates. The dead worms were counted every alternate day. The worm which did not respond to repeated touching was scored as dead (Kenyon et al. 1993). The experiment was terminated when all worms were scored as dead. The plot of fraction survival against days was plotted and subjected to the log-rank test to find the mean life span.
Health span assays
The health span of C. elegans was determined by observing some physiological movements such as pharyngeal pumping, body bending, and reversal frequency. The health span assay was performed on the same synchronized L4 worms that were used for the life span assay.
Pharyngeal pumping rate
The pharyngeal pumping rate determines the feeding behavior of the worms treated with leaves extracts (100 µg/ml). It was estimated by monitoring an individual worm under a stereo-microscope (Magnus MSZ-TR) on the 5th and 10th days to count the rhythmic contractions of the pharynx over the 1 min. The mean pharyngeal pumping rate was expressed per minute.
Body bending rate
The synchronized L4 worms were used for the experiment. Body bending is related to the locomotion ability of worms. Locomotion assay was performed on 5th and 10th day adult worms by counting the number of body bends monitored during a 1 min time interval (Kumar et al. 2010). A body bend was defined as a change in the reciprocating motion of bending at the mid-body. The assay was performed on control (untreated) and treated worms (100 µg/ml).
Reversal frequency rate
C. elegans moves on an agar plate by making sinusoidal waves along the length of its body. When these waves are propagated from head to tail the animal moves forward, and when propagated from tail to head the animal moves backward. The backward movement is referred to as “reversals frequency.” It can be spontaneous or induced as a direct response to a sensory stimulus. The synchronized L4 worms were used for the experiment. The reversal frequency was monitored on 5th and 10th day adults during the 1 min interval in control and treated (100 µg/ml) worms (Zhao et al. 2003).
Stress resistance assay
The N2 wild-type worms were exposed to temperature and oxidative stress for stress resistance assay. For both assays, age-synchronized young adult worms were transferred to control and treated (100 µg/ml) NGM plates. They were grown till the fourth day (post adulthood) and then subjected to temperature and oxidative stress. For temperature stress, treated and untreated worms were shifted from 20 to 35 °C for 12 h. The dead worms were counted at a regular interval of every 2 h up to 12 h.
For oxidative stress, the worms were washed 3–4 times with M9 buffer and soaked them in 20 mM hydrogen peroxide (H2O2) solution for 2 h. After exposed to oxidative stress, the worms were transferred to fresh NGM plates and incubated for 16 h at 20 °C for recovery. After 16 h, live animals were scored for treated and untreated conditions (Cai et al. 2011).
Measurement of reactive oxygen species (ROS)
Endogenous ROS levels were measured using 2′, 7′-dichlorofluorescein diacetate (DCFH–DA). Age synchronized L4 worms (N2 wild-type) were used for the experiment. Untreated and treated (100 μg/ml extracts) worms were grown under oxidative stress (10 mM paraquat) in liquid media and stained by DCFH-DA after 24 h to detect their intracellular-ROS level. Staining was performed by soaking the worms in 5 μM (final concentration) DCFH-DA solution for 30 min at 20 °C (Chaubey et al. 2020). The DCFHDA stained C. elegans were observed and photographed by the Nikon DS-Ri2 fluorescence microscope (Eclipse Ni-E, Nikon) (Singh et al. 2016). The relative fluorescent intensity was measured and calculated as corrected total cell fluorescence (CTCF) by removing background intensity using ImageJ software (Park et al. 2020).
Paralysis assays
CL4176 transgenic worms were used for paralysis assay which contains heat-inductive transgene of human Aβ1-42 in muscle cells. The synchronized L4 worms were transferred to extract treated (100 µg/ml) and untreated plates and incubated at 16 °C (permissive temperature) for 48 h and then shifted to 25 °C (non-permissive temperature) for 20 h for the expression of amyloid-beta (Aβ), which aggregates in muscle cells and causes paralysis. After 20 h of incubation at 25 °C, numbers of paralyzed worms were scored at every 2 h interval until all the worms were paralyzed. Worms that did not respond to mechanical stimulus were counted as paralyzed (Link et al. 2003).
RNA-mediated interference (RNAi)
In the RNAi experiment, the targeted gene expression can be inhibited by the introduction of sequence-specific double-stranded RNA. Normally, C. elegans feeds on the bacteria and absorb bacterial content in the gut by grinding them into the pharynx. The specific interference effects were detected in the nematode which feeds on E. coli bacteria expressing dsRNA (Fraser et al. 2000).
In this experiment, the E. coli HT115 expressing dsRNA (RNAi) for daf-16 was used for the study of the gene participation in longevity. The synchronized L4 worms (N2 wild-type) were fed with daf-16 (RNAi) expressing bacteria and allowed the proper intestinal ingestion of RNAi. These worms were allowed to lay eggs for a day and the offspring obtained were used for the experiment. The daf-16 RNAi culture was fed to the worms along with extracts and column fraction 33 (100 µg/ml) (Timmons and Fire 1998). The worms were counted until all the worms died and the life span was analyzed using the log-rank test.
DAF-16:: GFP localization assay
The synchronized L4 worms of TJ356 were treated with extracts and column fraction 33 (100 μg/ml). The treated and untreated worms were shifted up to 35 °C for 15 min to induce heat stress. The worms were observed and photographed using a fluorescence microscope (Chiang et al. 2012; Singh et al. 2016) through an excitation filter of 400/30 and an emission filter of 508/20. The percentage relative distribution of DAF-16 in the cytosolic, intermediate, and nuclear state was calculated in all the worms used for the experiment.
Statistical analysis
All the experiments were performed in triplicates and the results obtained were expressed as mean ± standard error of the mean (SEM). The survival curve in life span assay was performed using log-rank tests by the Prism 6.01 software. The data was statistically significant when p < 0.001. Two data sets were compared using a Student’s two-tailed t test and p < 0.05 was considered statistically significant.
Results
Preparation of sample for phytochemical characterization
Sequential extraction of leaves was performed using n-hexane, ethyl acetate, dichloromethane, methanol, and water. The percentage yield obtained in leaves was in the order of dichloromethane (45.4%) > n-hexane (38%) > methanol (28.4%) > water (26.6%) > ethyl acetate (22.2%).
Identification of the presence of various compounds by thin-layer chromatography
TLC is generally used to analyze and isolate the number of active compounds present in the crude extracts. Each extract of leaves was subjected to TLC for the analysis of various compounds present. Five different solvent systems with diverse polarities were used to develop the TLC plates. The compounds present in the extracts were separated on the basis of their polarity. The Rf values of separated compounds in all the extracts subjected to the different solvent systems given in Supplementary Table 1.
In the solvent system of chloroform: glacial acetic acid: methanol: water (5:4:1:2), leaves extracts of M. oleifera revealed the presence of single compound (Rf value of 0.44) in aqueous extract, two compounds (Rf values of 0.68, & 0.55, respectively) in methanol extract, three compounds (Rf values of 0.8, 0.67, & 0.45, respectively) in dichloromethane extract, five compounds (Rf values of 0.8, 0.67, 0.56, 0.53, & 0.44, respectively) in ethyl acetate extract, and two compounds (Rf values of 0.8, & 0.75, respectively) in hexane extract. In the previous study, the same solvent system revealed the presence of four compounds in chloroform extracts of M. oleifera pods (Sharma and Paliwal 2013b).
In the solvent system of hexane: ethyl acetate: acetic acid (3:4:1), leaf extracts of M. oleifera discovered the presence of two compounds (Rf values of 0.86, & 0.14, respectively) in dichloromethane extract, single compound (Rf value of 0.13) in ethyl acetate and two compounds in hexane extract with Rf value of 0.13 and 0.14, respectively. There was no single spot observed in aqueous and methanol extract.
In the solvent system of chloroform: methanol: water (7:3:1), leaves extracts of M. oleifera revealed the presence of a single compound in aqueous, methanol, dichloromethane, and ethyl acetate with Rf values of 0.70, 0.72, 0.69, & 0.68, respectively. There was no single spot observed in aqueous, ethyl acetate, and hexane extract. When the same solvent system was used previously for the separation of ethyl acetate extract of M. oleifera pods, five compounds having Rf values of 0.47, 0.78, 0.87, 0.90, and 0.98 were revealed (Sharma and Paliwal 2013b).
In the solvent system of hexane: acetic acid (9:1), leaf extracts of M. oleifera revealed the presence of nine compounds (Rf values of 0.89, 0.45, 0.34, 0.28, 0.23, 0.20, 0.13, 0.05, & 0.03, respectively) in dichloromethane extract, and nine compounds (Rf values of 0.89, 0.64, 0.43, 0.36, 0.31, 0.27, 0.23, 0.12, & 0.04, respectively) in hexane extract, no spots were obtained in aqueous, methanol, and ethyl acetate extract.
In the solvent system of hexane: acetic acid (9:1), the leaf extract of M. oleifera revealed the presence of five compounds (Rf values of 0.74, 0.65, 0.48, 0.43, & 0.24, respectively) in methanol extract. There was no spot detected in aqueous, dichloromethane, ethyl acetate, and hexane extract.
From the above result, it can be said that the maximum number of compounds were found in the dichloromethane extract of M. oleifera leaves. Furthermore, the most suitable solvent system for dichloromethane extract was proved to be hexane: acetic acid (9:1).
Column chromatography for separation of compounds
As the optimum separation in TLC was achieved in the dichloromethane extract of M. oleifera leaves, it was subjected to the fractionation by column chromatography. The extract was eluted into eighty fractions using 27 different solvent phases with increasing polarity. The collected fractions were mostly colorless, while some fractions ranged from light green to yellow, and black color.
Each fraction was tested by TLC for the presence of compounds. The solvent system used for the TLC was hexane: acetic acid (9:1), and the obtained Rf values are given in Supplementary Table 2. In TLC, the intact single spot was found in fraction number 33 in the solvent system of H: EA (80:20). Furthermore, GC–MS analysis of both the fractions was done for the identification of the compound.
UV-spectrophotometric analysis to identify spectral characteristics
The UV–visible spectrum of the dichloromethane extract of M. oleifera leaves at H: EA (80:20) fraction of column chromatography revealed the two peaks. The spectrum indicated the peaks at 415 nm and 670 nm (Fig. 1).
Fig. 1.
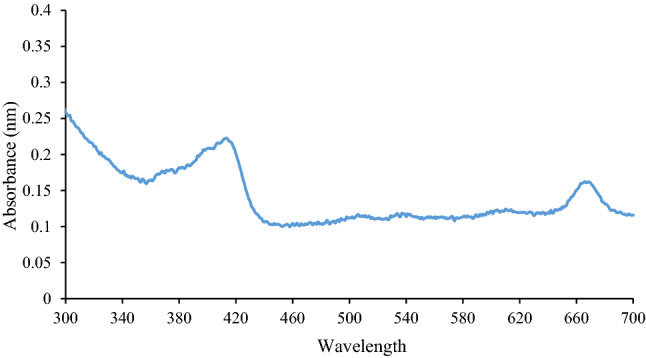
After performing column chromatography of dichloromethane extract of M. oleifera leaves, eluted fraction no. 33 which found most intact was analyzed by UV–visible spectrophotometer through the range from 300 to 700 nm. The spectrum showed the peaks at 415 nm and 670 nm
The absorbance peak at 415 is a characteristic band for flavonoids and specifically for rutin. Another peak at 670 might be due to chlorophyll (Pandian 2018). The absorbance between 405 and 419 nm might be due to hydroxyl and carbonyl groups attached to structures with extended conjugation. The absorbance between 600 and 700 nm indicated the presence of structures with extended conjugation and other functional groups from tannins, flavones, and polyphenols (Spiridon et al. 2011). Further study was needed to confirm the structure of compounds by analysis methods such as GC–MS.
Gas chromatography–mass spectrometry for identification of present compound
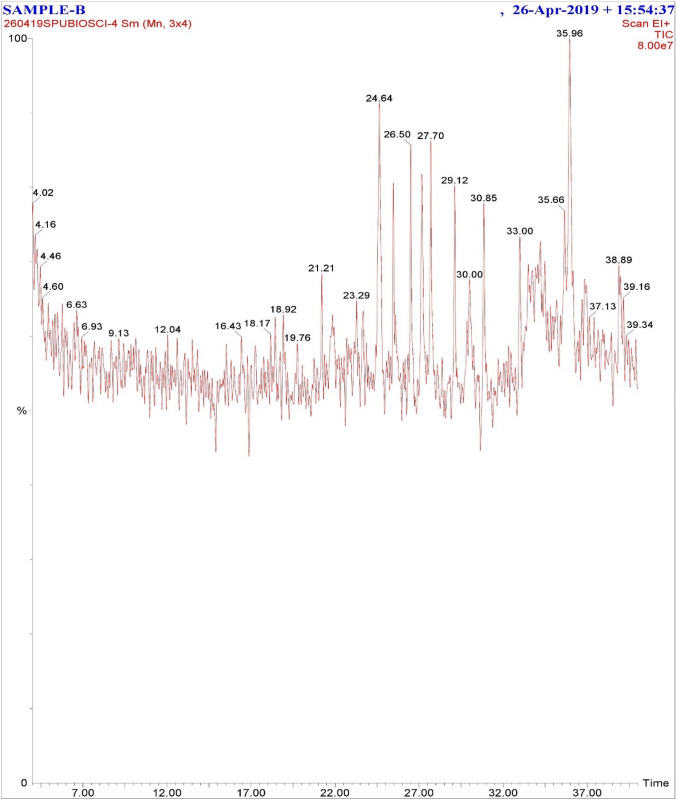
The compounds present in column fraction H:EA (80:20) of dichloromethane extract of M. oleifera leaves were further identified by GC–MS. In GC chromatogram there were twelve major peaks obtained, from which the two most abundant peaks were analyzed through MS and identified using the NIST (National Institute of Standards and Technology) mass spectral library (Fig. 2).
Fig. 2.
Various fractions were collected in column chromatography performed for the dichloromethane extract of M. oleifera leaves. The compounds present in most intact column fraction eluted with H (hexane):EA (ethyl acetate) (80:20) were further analyzed by GC. Twelve major peaks were obtained in GC chromatogram, from which the two most abundant peaks (at retention time 24.64 and 35.97) were further analyzed through MS
The major compounds identified by MS were Tetratetracontane (RT 24.64) (Fig. 3a) and Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) (RT 35.97) (Fig. 3b) with the molecular weight of 618 and 646 g/mol, respectively.
Fig. 3.
Most abundant peaks obtained in GC chromatogram were further analyzed through MS. a Mass spectrum of fraction obtained in GC at retention time 24.64 and the compound identified was Tetratetracontane with the molecular weight of 618 g/mol, and b Mass spectrum fraction obtained in GC at retention time 35.97 and the compound identified as Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) with the molecular weight of 646 g/mol
In the previous study on M. oleifera fruit extract, 28 compounds were found from which the most abundant was 2,6-dihydroxybenzoic acid, 3TMS derivative (38.8%) followed by tetrapentacontane (20.6%) (Shunmugapriya et al. 2017). A similar study was done on dichloromethane extract of roots of M. oleifera which revealed the presence of 63 compounds (58.8%) including oleic acid (46.5%), N-benzyl-N-(7-cyanato heptanamide (38.3%), N-benzyl-N-(1-chlorononyl) amide (30.3%), bis [3-benzyl prop-2-ene (Faizi et al. 2014). The GC–MS analysis revealed the presence of Linalool oxide, Upiol, Adenine, Palmitic acid in ethyl acetate extract of M. oleifera leaves (Karthika et al. 2013).
Phenol, 2,4-bis(1,1-dimethylethyl)-, phosphite (3:1) is also known as Alkanox 240; Hostanox PAR 24; Lowinox 242; Naugard 524; Tris-(2,4-di-t-butylphenyl) phosphite; Tris(2,4-di-tert-butylphenyl) phosphite; Trisdibutylphenyl phosphite; and Irgafos 168. It is an organic compound of trivalent phosphorus. It is used as a processing stabilizer in polyolefin industries. It is frequently used in combination with phenolic antioxidants which can reduce the consumption of these phenolic antioxidants during processing. It is a secondary antioxidant. The secondary antioxidants mainly reduce the unstable hydroperoxides before their homolytic cleavage which further prevents the production of free radicals (Bradley and Coulier 2007).
The beneficial effect of M. oleifera leaves on the life span of C. elegans
The untreated and leaves extracts treated (100 µg/ml) worms of N2 C. elegans were grown at 20 °C. The dead worms were counted at every alternate day until all the worms were dead.
In N2 worms, the mean survival rate was higher in the dichloromethane extract (21.73 ± 0.142 days) compared to a positive control (16.55 ± 0.02 days) and negative control (16.59 ± 0.12 days). The mean survival rate observed in aqueous, methanol, ethyl acetate, and n-hexane extracts were 19.63 ± 0.08, 18.99 ± 0.29, 18.62 ± 0.13, and 17.86 ± 0.05 days, respectively (Fig. 4, Supplementary data 1 & 2). In the previous study, N2 C. elegans treated with methanol extract of M. oleifera leaves revealed an extended life span of up to 17.7 ± 0.4 days which was nearly similar to the present study (Im et al. 2016). Worms under the treatment of fraction 33 exhibited almost similar survival rates as dichloromethane extract, which was 21.68 ± 0.19 days.
Fig. 4.

Treatment of M. oleifera leaves extracts (100 µg/ml) and isolated compounds enhanced the life span of the N2 wild-type strain of C. elegans. The synchronized worms were used for the experiment, which was allowed to grow until the L4 stage and then transferred to extract containing plates. All the treated and untreated worms were maintained at 20 °C and dead worms were counted at every alternate day until all the worms died. The plot of fraction survival against days was plotted and subjected to the log-rank test to find the mean life span. The graph shows the maximum life span in days. The mean life span obtained in La, Lm, Ld, Le, Lh and in fraction 33 (100 µg/ml) was 19.63 ± 0.08, 18.99 ± 0.29, 21.73 ± 0.14, 18.62 ± 0.13, 17.86 ± 0.05, and 21.68 ± 0.19 days, respectively, compared to PC (16.55 ± 0.02) and NC (16.59 ± 0.12) days (p < 0.001, log-rank test). The maximum life span extension was observed in worms treated with dichloromethane extract. The data is represented as mean ± SEM. [PC positive control (untreated), NC negative control (treated with DMSO), La leaves aqueous extract, Lm leaves methanol extract, Ld leaves dichloromethane extract, Le leaves ethyl acetate extract, Lh leaves hexane extract (treated with 100 µg/ml of each extract dried and dissolved in DMSO), and 33 fraction 33 from column chromatography collected, dried and dissolved in DMSO]
Effect of M. oleifera leaves on physiological parameters related to health span of C. elegans
Pharyngeal pumping
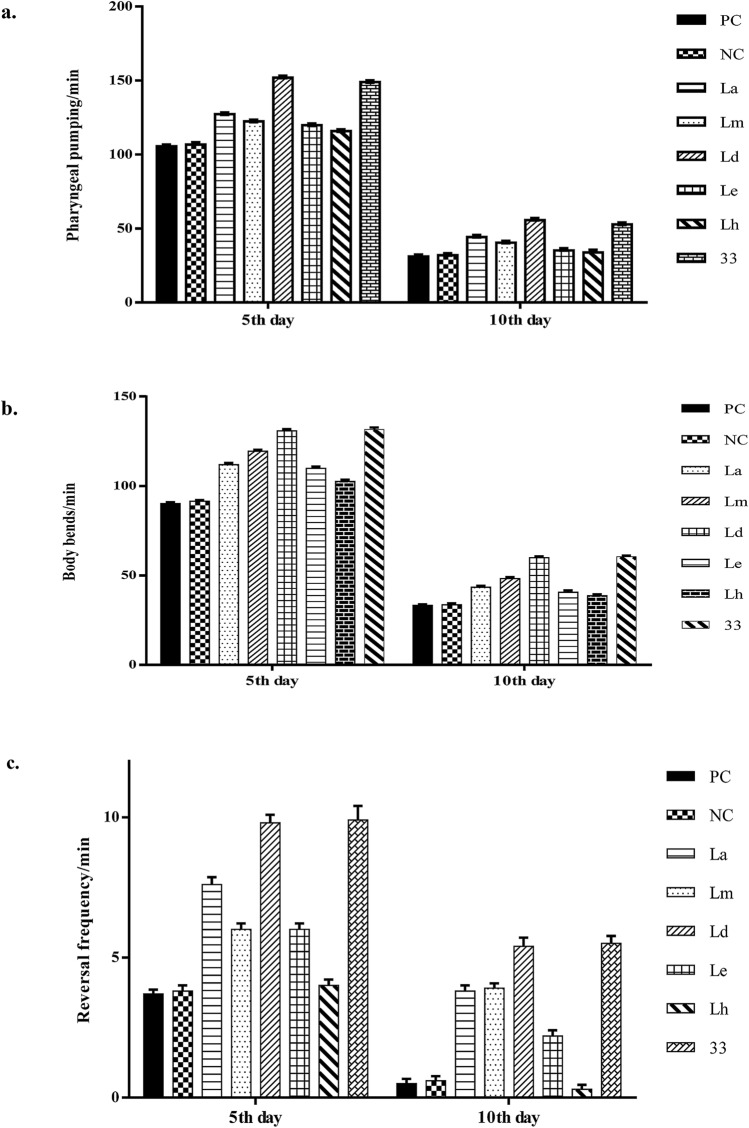
Pharyngeal pumping is one of the markers for aging in C. elegans that declines with increasing age. In N2 worms, the most potent pharyngeal pumping rate was obtained in the dichloromethane extract of M. oleifera leaves (Ld) which was 151.9 ± 0.5 min−1 and 55.7 ± 0.6 min−1 at 5th and 10th days, respectively. The pharyngeal pumping obtained in worms treated with fraction 33 was 149 ± 0.42 min−1 and 52.8 ± 0.46 min−1 at 5th and 10th days, respectively. These results are better compared to control worms in which the pharyngeal pumping obtained was 105.6 ± 0.26 min−1 and 31.1 ± 0.5 min−1 at 5th and 10th day, respectively (Fig. 5a, Supplementary data 1 & 2).
Fig. 5.
Synchronized N2 wild-type C. elegans were used for the experiment, which was allowed to grow until the L4 stage and then transferred to extract containing plates. The progression of a decrease in the rate of some physiological parameters in C. elegans such as pharyngeal pumping, body bonding, and reversal frequency with increasing age was delayed in treated (with leaves extract and column fraction 33–100 µg/ml) worms maintained at 20 °C. All the parameters were counted per minute on the 5th and 10th days after transfer. a Potential pharyngeal pumping rate was obtained in dichloromethane extract of M. oleifera leaves (Ld) which was 151.9 ± 0.5 min−1; p = 3.807 × 10−15 and 55.7 ± 0.6 min−1; p = 2.119 × 10−10 (t test) at 5th and 10th days, respectively. The pharyngeal pumping obtained in worms treated with fraction 33 was 149 ± 0.42 min−1; p = 1.776 × 10−14 and 52.8 ± 0.46 min−1; p = 6.334 × 10−10 (t test) at 5th and 10th days, respectively. The pharyngeal pumping obtained in control worms was 105.6 ± 0.26 min−1 and 31.1 ± 0.5 min−1 at 5th and 10th days, respectively. b Potential body bending rate was obtained in dichloromethane extract of M. oleifera leaves (Ld) which was 130.7 ± 0.98 min−1; p = 2.382 × 10−10 and 59.9 ± 0.64 min−1; p = 1.229 × 10−9 (t test) at 5th and 10th days, respectively. The body bending obtained in worms treated with fraction 33 was 131.6 ± 1.02 min−1; p = 2.177 × 10−10 and 60.5 ± 0.56 min−1; p = 1.925 × 10−9 (t test) at 5th and 10th days, respectively. These body bends obtained in control worms were 90.2 ± 0.68 min−1 and 33.2 ± 0.71 min−1 at 5th, and 10th days, respectively. c Potential reversal frequency rate was obtained in dichloromethane extract of M. oleifera leaves (Ld) which was 9.8 ± 0.29 min−1; p = 8.462 × 10−10 and 5.4 ± 0.3 min−1; p = 8.102 × 10−08 (t test) at 5th and 10th days, respectively. The reversal frequency obtained in worms treated with fraction 33 was 9.9 ± 0.5 min−1; p = 7.136 × 10−07 and 5.5 ± 0.27 min−1; p = 4.266 × 10−08 (t test) at 5th and 10th days, respectively. The reversal frequency obtained in control worms was 3.7 ± 0.15 min−1 and 0.5 ± 0.16 min−1 at the 5th and 10th days, respectively. The data is represented as mean ± SEM
From the above result, it can be stated that the worms treated with extracts showed better pharyngeal pumping compared to untreated. Pharyngeal pumping is a measure of the feeding capacity of worms. The extracts of M. oleifera leaves have the potential to enhance the health of worms by improving the physiological parameter such as pharyngeal pumping. From all the extracts of leaves, dichloromethane extract exhibited the most potent effect. The fraction 33 of column chromatography showed an almost similar rate of pharyngeal pumping as a dichloromethane extract of leaves.
Body bending
Body bending is also counted as a marker for aging which decreases with the increase in age. In N2 worms, the maximum body bending rate was obtained in the dichloromethane extract of M. oleifera leaves (Ld) which was 130.7 ± 0.98 min−1 and 59.9 ± 0.64 min−1 at 5th and 10th days, respectively. The body bending obtained in worms treated with fraction 33 was 131.6 ± 1.02 min−1 and 60.5 ± 0.56 min−1 at 5th and 10th days, respectively. The body bends obtained in control worms were 90.2 ± 0.68 min−1 and 33.2 ± 0.71 min−1 at 5th, and 10th days, respectively (Fig. 5b, Supplementary data 1 & 2).
The above result proves the body bends were improved in all the extracts of M. oleifera leaves. Body bending is the measure of locomotion activity of worms which is related to the health of worm. Additionally, the dichloromethane extract of leaves exhibited the most potent effect than other extracts. The almost similar result was displayed by worms treated with fraction 33 of column chromatography.
Reversal frequency
Reversal frequency is another physiological parameter considered to evaluate the health of the worm and it declines with increasing age. The worms treated with extracts showed a delay in the decrease in reversal frequency.
In N2 worms, the potential reversal frequency rate was obtained in the dichloromethane extract of M. oleifera leaves (Ld) which was 9.8 ± 0.29 min−1 and 5.4 ± 0.3 min−1 at 5th and 10th days, respectively. The reversal frequency obtained in worms treated with fraction 33 was 9.9 ± 0.5 min−1 and 5.5 ± 0.27 min−1 at 5th and 10th days, respectively. The reversal frequency rate of 3.7 ± 0.15 min−1 and 0.5 ± 0.16 min−1 was obtained at 5th and 10th days, respectively, in control worms (Fig. 5c, Supplementary data 1 & 2).
The above result showed the improved reversal frequency in all the extracts of M. oleifera leaves. The worms treated with dichloromethane extract of leaves exhibited the most potent effect compared to other extracts. A similar reversal frequency was obtained in worms treated with fraction 33 of column chromatography.
Stress resistant properties of M. oleifera leaves in C. elegans
Thermal assay
To check how effective the treatment of the extract under stress conditions, the treated (100 µg/ml) and untreated worms were grown at 20 °C up to adulthood and then transferred to 35 °C. The worms were counted every 2 h until the 12th hour and the survival curve was produced against time (hours) (Fig. 6a).
Fig. 6.
Synchronized N2 wild-type worms were used for the experiment. After the worms reached to L4 stage they were transferred to extract containing plates. The percentage survival under thermal stress in N2 worms treated with M. oleifera leaves extracts and column fraction 33 (100 µg/ml). a Thermal stress was given by the transfer of treated and untreated worms from 20 to 35 °C for 12 h. Then, the dead worms were counted at an interval of 2 h until the 12th hour and represented as a survival curve. The increase in survival was observed in treated worms. b Extracts treatment improved stress tolerance. The mean survival is shown in the graph. The potential result was obtained in the dichloromethane extract of M. oleifera leaves (Ld) which was 61.79 ± 0.08%; p < 0.05, t test. The survival rate obtained in worms treated with fraction 33 was 62.16 ± 0.09%; p < 0.05, t test. These results were better compared to the control which was 43.7 ± 0.21; p < 0.05, t test. The data is represented as mean ± SEM
The mean survival percentage was obtained using a survival curve. In N2 worms, the highest mean survival percentage under thermal stress displayed by the worms that were treated with dichloromethane extract of leaves (61.79 ± 0.08%) compared to a positive control (43.7 ± 0.21%) and negative control (44.3 ± 0.04%). The worms treated with fraction 33 of column chromatography showed the thermal stress tolerance up to 62.16 ± 0.09% (Fig. 6b, Supplementary data 1 & 2).
Oxidative stress assay
To explore oxidative stress tolerance the treated (100 µg/ml) and untreated worms were soaked in 20 mM hydrogen peroxide (H2O2) solution for 2 h and then transferred to fresh NGM plates and incubated for 16 h at 20 °C for recovery. After 16 h, live animals were scored.
The highest survival rate of 91.47 ± 1.29% was obtained in N2 worms treated with dichloromethane extract of leaves as compared to a positive control (43.59 ± 1.86%) and negative control (42.13 ± 1.03%). The worms treated with fraction 33 showed the survival rate of 90.18 ± 1.71% which was slightly lower than the dichloromethane extract of leaves but higher than all other extracts (Fig. 7, Supplementary data 1 & 2).
Fig. 7.

Synchronized N2 wild-type worms were used for the experiment, which was then transferred to extract containing plates after the worms reached to the L4 stage. The percentage survival under oxidative stress in N2 worms treated with leaf extracts and column fraction 33 (100 µg/ml). The oxidative stress was generated by the treatment of 20 mM hydrogen peroxide (H2O2) for 2 h. Then the worms were transferred to 20 °C for recovery and were counted post-recovery. The increase in survival was observed in treated worms. The potential result was obtained in the dichloromethane extract of M. oleifera leaves (Ld) which was 91.47 ± 1.29%; p < 0.05, t test. The survival rate obtained in worms treated with fraction 33 was 90.18 ± 1.71%; p < 0.05, t test. These results were improved compared to the control which was 43.59 ± 1.86%. The data is represented as mean ± SEM
Effect of M. oleifera leaves on reactive oxygen species produced in C. elegans
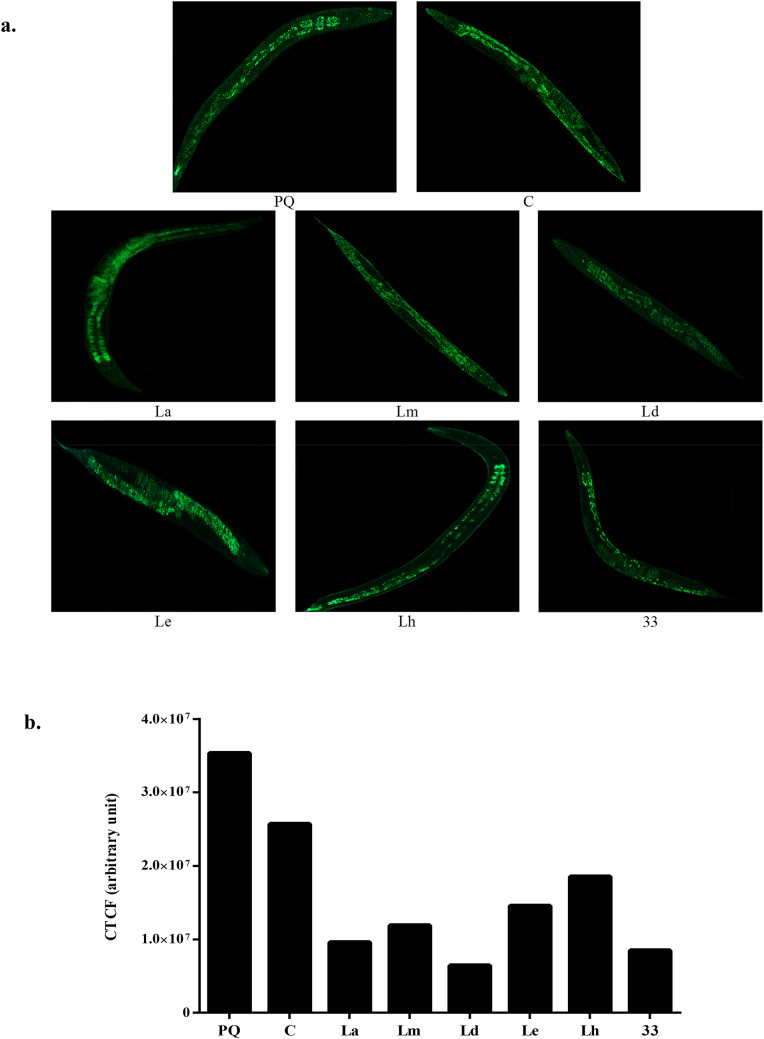
The intracellular ROS in C. elegans was generated by oxidative stress when grown under the treatment of 10 mM paraquat along with extract (100 µg/ml). Paraquat is an herbicide that is used to induce ROS production. The fluorescence generated after DCFH-DA staining was photographed under a fluorescent microscope (Fig. 8a).
Fig. 8.
Synchronized N2 wild-type worms were used for the experiment, which was then transferred to extract containing plates after the worms reached to the L4 stage. The anti-oxidative effect of M. oleifera leaves extracts and column fraction 33 (100 µg/ml) against ROS was analyzed using 2’,7’-dichlorofluorescein diacetate (H2DCF–DA). a Intracellular-ROS level in extracts treated and untreated worms grown in liquid media were detected by H2DCF–DA (5 μM for 30 min) after treatment of 10 mM paraquat for 24 h at 20 °C. Then the worms were observed under a fluorescent microscope. The effect of dichloromethane extract was most efficient. b Relative fluorescent intensity under the paraquat treatment was measured by ImageJ software in the form of corrected total cell fluorescence (CTCF). In N2 worms, the lowest fluorescent intensity of 6.4 × 10−6 was obtained in worms treated dichloromethane extract of leaves compared to paraquat treated (3.5 × 10−7) and untreated (2.5 × 10−7). The intensity obtained in worms treated with fraction 33 and paraquat was 8.4 × 10−6 (PQ paraquat treated, C control without paraquat and extract treatment)
The fluorescent intensity was measured and calculated as corrected total cell fluorescence (CTCF). It is expressed as an arbitrary unit as it is a relative unit of measurement to show the ratio of the amount of intensity in worms to background intensity. In N2 worms, the lowest fluorescent intensity of 6.4 × 10−6 was obtained in worms treated dichloromethane extract of leaves compared to paraquat treated (3.5 × 10−7) and untreated (2.5 × 10−7). The intensity obtained in worms treated with fraction 33 and paraquat was 8.4 × 10−6. The lower intensity compared to paraquat treated worms indicates that the extracts can restrict the ROS production under oxidative stress (Fig. 8b).
Effect of M. oleifera leaves on the decline of paralysis induced by amyloid-β expression
CL4176 C. elegans is a transgenic model for Alzheimer’s disease. This strain (dvIs27[pAF29(myo-3/Ab 1-42/let UTR) + pRF4(rol- 6(su1006)]) is engineered to provide muscle expression of a human β-amyloid peptide (Aβ) transgene, resulting in paralysis due to Aβ toxicity when the temperature was upshifted from 16 to 25 °C. The number of paralyzed worms was counted at every 2 h (Fig. 9, Supplementary data 1).
Fig. 9.

Synchronized CL4176 C. elegans were used for the experiment, which was allowed to grow until the L4 stage and then transferred to extract containing plates. CL4176 are the transgenic worms that contain heat-inductive transgene of human Aβ1-42 in muscle cells and were used as an Alzheimer’s diseased model. The amyloid β started expressing in body wall muscles and initiated paralysis in CL4176 after the worms were upshifted to 25 °C from 16 °C. After 20 h of incubation at 25 °C, numbers of paralyzed worms were scored at every 2 h interval until all the worms were paralyzed. Worms that did not respond to mechanical stimulus were counted as paralyzed. M. oleifera extracts and column fraction 33 were proved effective in the decline of paralysis caused by protein aggregation in CL4176 worms. The most preventing effect was observed in the worms treated with Ld (100 µg/ml) compared to untreated worms (p < 0.05, t test). The isolated compound also showed similar preventive activity
The result showed a significant delay in paralysis in extract-treated worms. The most potent delay was observed in the dichloromethane extract of leaves followed by fraction 33 and dichloromethane extract of leaves compared to positive and negative controls.
Involvement of daf-16 gene in longevity analyzed by RNAi experiment
The set of more than 5600 genes were used for RNAi screening to identify the genes that control the life span in C. elegans. These genes belonged to two of the six C. elegans chromosomes. It has been proved that a large number of genes have critical roles in determining C. elegans life span that is essential for mitochondrial function (Fraser et al. 2000; Kamath et al. 2003).
N2 worms were fed on daf-16 RNAi, which inhibited the daf-16 gene expression. Under the treatment of RNAi along with extracts, the worms were grown and scored until all worms died.
The life span was calculated using the log-rank test and compared to the life span of worms fed on a normal vector. The life span obtained in RNAi experiment were 16.03 ± 0.06, 15.93 ± 0.13, 18.13 ± 0.17, 14.8 ± 0.09, 14.82 ± 0.12, and 18.29 ± 0.05 days under treatment of La, Lm, Ld, Le, Lh, and 33, respectively (Fig. 10a, Supplementary data 1 & 2). The life span obtained in worms fed on control vector (CV) and treated with extracts (100 µg/ml) were 18.2 ± 0.09, 18.3 ± 0.2, 20.6 ± 0.12, 17.5 ± 0.11, 16.9 ± 0.23, and 20.4 ± 0.15 days in La, Lm, Ld, Le, Lh, and fraction 33, respectively.
Fig. 10.
RNAi experiment performed to analyze the involvement of daf-16 gene in longevity. The E. coli HT115 expressing dsRNA (RNAi) for daf-16 were used in the experiment. The synchronized L4 worms (N2 wild-type) were fed with daf-16 (RNAi) expressing bacteria and allowed the proper intestinal ingestion of RNAi. Then the worms were allowed to lay eggs for a day and the offspring obtained were used for the experiment. The daf-16 RNAi culture was fed to the worms along with extracts treatment (100 μg/ml). The worms were counted until all the worms died and the life span was analyzed using the log-rank test. The treatment of M. oleifera leaves extracts and column fraction 33 declined the life span of N2 worms fed on daf-16 RNAi compared to worms without RNAi treatment. a The mean life span obtained in worms treated with La, Lm, Ld, Le, Lh and in fraction 33 (100 µg/ml) and daf-16 RNAi was 16.03 ± 0.06, 15.93 ± 0.13, 18.13 ± 0.17, 14.8 ± 0.09, 14.82 ± 0.12, and 18.29 ± 0.05 days, respectively; (p < 0.001, log-rank test). b Life span obtained in control vector treated with extracts La, Lm, Ld, Le, Lh and in fraction 33 (100 µg/ml) was 18.2 ± 0.09, 18.3 ± 0.2, 20.6 ± 0.12, 17.5 ± 0.11, 16.9 ± 0.23, and 20.4 ± 0.15 days, respectively; (p < 0.001, log-rank test). The treatment of RNAi inhibited the expression of the daf-16 gene and the decline in life span indicated the life span extension effect of extracts and fraction 33 was daf-16 dependent. The data is represented as mean ± SEM
In the present study, the RNAi methodology was used to explore the involvement of daf-16 in longevity under the treatment of leaf extracts and column fraction. Here, the life span under RNAi treatment was decreased compared to the life span of worms fed on the control vector which proves the involvement of the daf-16 gene in the longevity of C. elegans (Fig. 10b, Supplementary data 1 & 2). From the results, it can be said that the extracts of M. oleifera leaf help to improve the life span via the daf-16 gene pathway.
Effect of M. oleifera leaves on DAF-16 localization in TJ356 C. elegans
In TJ356 transgenic worms, GFP is fused with the daf-16 gene which can be used to determine DAF-16 accumulation. The DAF-16 nuclear localization can be induced by stress conditions such as heat stress, oxygen deficiency, oxidative stress, starvation, and exposure to pathogenic bacteria (Senchuk et al. 2018).
The DAF-16 gets activated under stress and it moves to the nucleus from the cytoplasm. In this study, the DAF-16 was localized more in the cytoplasm under thermal stress conditions in control, while in worms treated with extracts it was found localized in the nucleus, and in some cases, it was in the intermediate state (Fig. 11a, Supplementary data 1 & 2).
Fig. 11.
Transgenic TJ356 C. elegans in which the DAF-16 is tagged with GFP were used for the experiment. The synchronized L4 worms were used for the experiment. The treated (with M. oleifera leaves extracts and column fraction 33–100 µg/ml) and untreated worms were shifted up to 35 °C for 15 min to induce heat stress. a As the DAF-16 started expressing under thermal stress, it was seen to be localized in the nucleus in treated worms. The worms were examined and photographed under the fluorescent microscope through an excitation filter of 400/30 and an emission filter of 508/20. b Representative graph displayed the relative distribution of DAF-16 in the cytosol, intermediate, and nuclear state in the worms
The percentage distribution of cytosolic, intermediate, and nuclear localization was calculated (Fig. 11b, Supplementary data 1 & 2). DAF-16 translocated to the nucleus can further trigger the transcriptional activation of genes including sod-3, hsp-16.2, and ctl-1. In the living cells, the enzymes sod-3 and ctl-1 play a significant protective role against ROS, while hsp-16.2 is used for longevity prediction as it can be highly active under stress and serve as stress-sensitive reporters (Wang et al. 2015). However, further study is required to examine the expression of sod-3, hsp-16.2, and ctl-1 genes.
Discussion
It has already been proven that ROS plays a pivotal role in aging and related diseases. To counterbalance ROS generated, the human body produces several antioxidant enzymes. However, the natural antioxidant defense system is not sufficient to balance the elevated level of free radicals (Jayachitra and Krithiga 2012). Nowadays, synthetic antioxidants are used to improve antioxidant capacity. But sometimes synthetic antioxidants proved to be toxic, so the research interest shifted towards the finding of naturally occurring antioxidants. Plants are the greatest source of antioxidants that are beneficial in the cure of many diseases related to aging and oxidative stress. Because of the presence of potent antioxidant compounds in Moringa, we checked the potency of M. oleifera leaves extracts on declining aging and age-related diseases such as Alzheimer’s disease. C. elegans employed during this study, is the most widely used model organism to explore the anti-aging properties of drugs. The similarities in aging patterns between C. elegans and mammals make it a potent model organism to study the aging-related properties.
The leaves of M. oleifera were sequentially extracted through Soxhlet extraction using five different solvents having different polarities such as water, methanol, dichloromethane, ethyl acetate, and n-hexane. This process proved advantageous for the extraction of a different combination of compounds in different solvents according to their polarity (Jeyaseelan et al. 2012). The presence of different compounds was analyzed using TLC, in which dichloromethane extract revealed the presence of the highest number of compounds. The dichloromethane proved to be the best suitable solvent for the extraction of compounds present in leaves of M. oleifera. The dichloromethane extract was further analyzed through column chromatography and the eluted column fractions were collected. The fraction number 33 was found to be most abundant after analyzing all the fractions by TLC. That fraction was further analyzed by UV–Vis spectrophotometer and GC–MS, which revealed the presence of compounds such as [Phenol-2,4-bis(1,1-dimethylethyl)- phosphite (3:1)] and Tetratetracontane. In previous studies, tetratetracontane was found to be present in the ethanolic extract of leaves (Chandasekar and Malathi 2016) and petroleum ether extract of callus associated with rooting cultured from M. oleifera (Mathur et al. 2014). Fraction number 33 was also applied along with leaf extracts in all the experiments performed on C. elegans to test its efficiency.
We have analyzed the life span extending capacity of all the leaf extracts of M. oleifera and the collected fraction in C. elegans. Our results demonstrated that the treatment of leaf extracts and column fraction increased the life span of wild-type N2 C. elegans. The maximum life span extension by 23.83% was obtained in worms treated with dichloromethane extract (Supplementary Table 3). Along with this it also improved the health span, as it delayed the reduction of other physiological markers such as pharyngeal pumping, body bending, and reversal frequency of C. elegans with increasing age. Increased level of ROS production is a major contributing factor in the aging process (Kong et al. 2014). As described previously the higher concentration of antioxidants present in M. oleifera imparts reduced intracellular ROS in C. elegans. Since there is a significant correlation between increased stress tolerance and longevity, the stress resistance capacity of extract fed worms under thermal and oxidative stress was evaluated (Kenyon 2010). In the present study, the extract-treated worms displayed a significant increase in survival rate compared to untreated worms under thermal stress conditions, suggesting that the leaf extract treatment improved the ability to fight ROS generated by thermal stress. The wild-type worms also exhibited the increased survival rate under oxidative stress induced by paraquat. The in vivo antioxidant potential was determined using dichlorofluorescein diacetate (DCFH-DA) dye which reacts with the intracellular ROS and generates the fluorescence which can be measured (Kalyanaraman et al. 2012). The results of the in vivo assay proved the reduced ROS generation under extracts treatment compared to control. The results of the present study are correlated with previous reports indicating that stress resistance and life span are usually connected (Kaletsky and Murphy 2010; Surco-Laos et al. 2012). Hence, the increased life span, heath-span, and stress tolerance might be linked to the potent antioxidant capacity of M. oleifera.
Since the M. oleifera revealed the potent anti-aging activity, we investigated its activity on the Alzheimer’s disease model of C. elegans. AD is one of the neurodegenerative diseases associated with aging. The Amyloid-β (Aβ) is the main cause of AD which is found to accumulate in the brain of AD patients. Amyloid precursor protein (APP) undergoes proteolytic events by α-secretase and γ-secretase which leads to the production of the nonamyloidogenic fragment and Aβ, respectively (Rasmussen et al. 2017; Zhang et al. 2018). The Alzheimer’s disease model of C. elegans CL4176 was used to check the efficacy of leaves and column fraction. These worms are transgenic which expresses human amyloid β upon temperature upshift and induces paralysis in body muscles. In our findings, the leaf extracts and collected column fraction delayed the paralysis proving the beneficial effects of M. oleifera on AD and other age-associated diseases.
In nematode and mammals, the activation of transcription factor DAF-16/FOXO is correlated with the increased life span and stress tolerance (Lamitina and Strange 2005). DAF-16 is a key regulator of the aging process and the downstream transcription factor of the insulin signaling pathway (IIS pathway). To explore the molecular mechanism by which the life span has been extended under the treatment of M. oleifera, we hypothesized that the daf-16 gene might be involved. We performed the RNAi experiments on N2 wild-type C. elegans (Conte et al. 2015). The RNAi gene knockdown was induced by feeding the worms with bacteria producing double-stranded RNA (dsRNA). The expression of the daf-16 gene was inhibited by RNAi treatment and the life span of N2 wild-type worms was analyzed. The results of the RNAi experiment proved the involvement of the daf-16 gene in the longevity of C. elegans under the treatment of M. oleifera extracts and column fraction.
To confirm the involvement of the daf-16 gene, we used TJ356 C. elegans in which the DAF-16 is tagged with GFP. DAF-16 remains inactive in the cytosol under normal conditions; however, it moves from the cytoplasm to the nucleus under the stress condition, which leads to the activation of other genes that participates in stress response (Mukhopadhyay et al. 2006; Murphy et al. 2003). In this study, the DAF-16 in treated TJ356 worms were localized in the nucleus under thermal stress which further validates the involvement of daf-16 gene expression against stress.
Conclusion
From all the above experiments, it tends to be concluded that the dichloromethane extract of M. oleifera leaves exhibits the most potent activities compared to the other extracts. Also, the column fraction number 33 derived from dichloromethane extract through column chromatography showed similar potential effects. GC–MS of fraction revealed some compounds present in the fraction that can be further purified and used for pharmaceutical purposes. It was observed that the leaf extracts and column fraction extended the life span and stress-tolerance in C. elegans. The health of C. elegans was also improved through improved physiological functions. The M. oleifera also proved beneficial for delaying paralysis mediated by Aβ in the Alzheimer’s disease model of C. elegans. The increased life span in C. elegans under the treatment of M. oleifera was dependent on the DAF-16 pathway. As C. elegans has a genome similarity of 65% with humans, the increase in life span, health span, and stress-tolerance in C. elegans indirectly serves for the production of novel medicine to cure the diseases caused by aging and elevated oxidative stress. On the basis of promising activities of extracts of M. oleifera, it could be subjected to isolate the most active components for drug development therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- M. oleifera
Moringa oleifera
- C. elegans
Caenorhabditis elegans
- La
Leaves aqueous extract
- Lm
Leaves methanol extract
- Ld
Leaves dichloromethane extract
- Le
Leaves ethyl acetate extract
- Lh
Leaves n-hexane extract
- TLC
Thin layer chromatography
- M
Methanol
- H
Hexane
- EA
Ethyl acetate
- DCM
Dichloromethane
- GC–MS
Gas chromatography–mass spectrometry
- RT
Retention time
- NGM
Nematode growth medium agar plate
- L1
Larval stage 1
- L4
Larval stage 4
- H2O2
Hydrogen peroxide
- DCFH-DA
2’,7’-dichlorofluorescein diacetate
- ROS
Reactive oxygen species
- Aβ
Amyloid-beta
- Rf
Retardation factor
- h
Hour/hours
- PC
Positive control
- NC
Negative control
- PQ
Paraquat
- GFP
Green fluorescent protein
Authors contributions
Conceptualization: APC, MGC, SNP, DM, NKS; Methodology: APC, MGC, SNP, DM, NKS; Formal analysis and investigation: APC, MGC, SNP, DM, NKS; Writing-original draft preparation: APC; Writing-review and editing: NKS; Resources: Shri. A. N. Patel P. G. Institute of Science and Research, Anand; Post Graduate Department of Bioscience, Sardar Patel University, Anand; Supervision: NKS.
Funding
The research was not supported by any funding.
Compliance with ethical standards
Conflict of interest
Anita Prabhatsinh Chauhan, Mukesh Ghanshyam Chaubey, Stuti Nareshkumar Patel, Datta Madamwar, and Niraj Kumar Singh declare that they have no conflict of interest.
Ethics approval
No human or animal rights are applicable to this study.
References
- Ayurvedic Pharmacopoeia Committee (2001) Ayurvedic Pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare. Department of AYUSH, New Delhi. http://www.ayurveda.hu/api/API-Vol-2.pdf, http://www.ayurveda.hu/api/API-Vol-4.pdf
- Baker K, Marcus CB, Huffman K, et al. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284(1):215–221. [PubMed] [Google Scholar]
- Bakre AG, Aderibigbe AO, Ademowo OG. Studies on neuropharmacological profile of ethanol extract of Moringa oleifera leaves in mice. J Ethnopharmacol. 2013;149(3):783–789. doi: 10.1016/j.jep.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Bharali R, Tabassum J, Azad MR. Chemomodulatory effect of Moringa oleifera Lam on hepatic carcinogen-metabolizing enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac J Cancer Prev. 2003;4:131–139. [PubMed] [Google Scholar]
- Bradley E, Coulier L (2007) An investigation into the reaction and breakdown products from starting substances used to produce food contact plastics. London: Central Science Laboratory FD07/01
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Cabrera O, Morales O, et al. Pharmacological properties of Moringa oleifera. 1: preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991;33:213–216. doi: 10.1016/0378-8741(91)90078-r. [DOI] [PubMed] [Google Scholar]
- Cai WJ, Huang JH, Zhang SQ. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans. PloS One. 2011;6(12):e28835. doi: 10.1371/journal.pone.0028835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandasekar S, Malathi R. Identification of bioactive constituents in Moringa concanensis leaf using gas chromatography and mass spectrometry. Int J Curr Adv Res. 2016;5:1071–1073. [Google Scholar]
- Chaubey MG, Patel SN, Rastogi RP, et al. Cyanobacterial pigment protein allophycocyanin exhibits longevity and reduces Aβ-mediated paralysis in C. elegans: complicity of FOXO and NRF2 ortholog DAF-16 and SKN-1. 3 Biotech. 2020;10(8):1–11. doi: 10.1007/s13205-020-02314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan UD, Amarowicz R. Effect of various solvent systems on the extraction of phenolics, tannins, sugars from beach pea (Lathyrus maritimus L.) Int Food Res J. 2013;20(3):1139–1144. [Google Scholar]
- Chiang WC, Tishkoff DX, Yang B, et al. C. elegans SIRT6/7 homolog SIR-2.4 promotes DAF-16 relocalization and function during stress. PLoS Genet. 2012;8(9):e1002948. doi: 10.1371/journal.pgen.1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte JrD, MacNeil LT, Walhout AJ, Mello CC (2015) RNA Interference in Caenorhabditis elegans. Curr Protoc Mol Biol 109(1):26–3 [DOI] [PMC free article] [PubMed]
- Corsi AK, Wightman B, Chalfie M. A transparent window into biology: a primer on Caenorhabditis elegans. Genetics. 2015;200(2):387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Sahu D, Baruah D, et al. Moringa oleifera (Shigru): a miracle tree for its nutritional, ethnomedicinal and therapeutic importance. Int J Dev Res. 2017;7(11):16823–16827. [Google Scholar]
- Dengg M, van Meel JC. Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J Pharmacol Toxicol Methods. 2004;50(3):209–214. doi: 10.1016/j.vascn.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health: a review. J Sci Food Agric. 2000;80:1744–1756. [Google Scholar]
- Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24(3):415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005;1(5):1–15. [Google Scholar]
- Faizi S, Siddiqui B, Saleem R, et al. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J Nat Prod. 1994;57:1256–1261. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- Faizi S, Sumbul S, Versiani MA, et al. GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac J Trop Biomed. 2014;4(8):650–654. doi: 10.12980/APJTB.4.201414B141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408(6810):325. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Ganguly R, Guha D. Alteration of brain monoamines & EEG wave pattern in rat model of Alzheimer’s disease & protection by Moringa oleifera. Indian J Med Res. 2008;128(6):744–751. [PubMed] [Google Scholar]
- Gourley BL, Parker SB, Jones BJ, et al. Cytosolic aconitase and ferritin are regulated by iron in Caenorhabditis elegans. J Biol Chem. 2003;278:3227–3234. doi: 10.1074/jbc.M210333200. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate aging in model organisms. Nature. 2000;408(6809):255. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Kumar B, Srinivasan BP, et al. Retinoprotective effects of Moringa oleifera via antioxidant, anti-inflammatory, and anti-angiogenic mechanisms in streptozotocin-induced diabetic rats. J Ocul Pharmacol Ther. 2013;29(4):419–426. doi: 10.1089/jop.2012.0089. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging. Mutat Res DNAging. 1992;275(3–6):257–266. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Huang WJ, Zhang XIA, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016;4(5):519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JS, Lee HN, Oh JW. Moringa oleifera Prolongs Lifespan via DAF-16/FOXO Transcriptional Factor in Caenorhabditis elegans. Nat Prod Sci. 2016;22(3):201–208. [Google Scholar]
- Iqbal S, Bhanger MI. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compos Anal. 2006;19:544–551. [Google Scholar]
- Jayachitra A, Krithiga N. Study on antioxidant property in selected medicinal plant extract. Int J Med Arom Plants. 2012;2(3):495–500. [Google Scholar]
- Jeyaseelan EC, Jenothiny S, Pathmanathan MK, Jeyadevan JP. Antibacterial activity of sequentially extracted organic solvent extracts of fruits, flowers and leaves of Lawsonia inermis L. from Jaffna. Asian Pac J Trop Biomed. 2012;2(10):798–802. doi: 10.1016/S2221-1691(12)60232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappurayil TM, Joseph BP. A review of pharmacognostical studies on Moringa oleifera lam. flowers. Pharmacogn J. 2017;9(1):1–7. [Google Scholar]
- Kaletsky R, Murphy CT. The role of insulin/IGF-like signaling in C. elegans longevity and aging. Dis Models Mech. 2010;3(7–8):415–419. doi: 10.1242/dmm.001040. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJ. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421(6920):231. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Karthika S, Ravishankar M, Mariajancyrani J, Chandramohan G. Study on phytoconstituents from Moringa oleifera leaves. Asian J Plant Sci Res. 2013;3(4):63–69. [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, et al. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- Kirisattayakul W, Wattanathorn J, Tong-Un T et al (2013) Cerebroprotective effect of Moringa oleifera against focal ischemic stroke induced by middle cerebral artery occlusion. Oxidative Medicine and Cellular Longevity 2013, Article ID 951415. 10.1155/2013/951415 [DOI] [PMC free article] [PubMed]
- Kong Y, Trabucco SE, Zhang H. Oxidative stress, mitochondrial dysfunction and the mitochondria theory of aging. Aging. 2014;39:86–107. doi: 10.1159/000358901. [DOI] [PubMed] [Google Scholar]
- Kumar J, Choudhary BC, Metpally R, et al. The Caenorhabditis elegans Kinesin-3 motor UNC-104/KIF1A is degraded upon loss of specific binding to cargo. PLoS Genet. 2010;6(11):e1001200. doi: 10.1371/journal.pgen.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Pandey N, Mohan V, Singh RP. Antibacterial and antioxidant activity of the extract of Moringa oleifera leaves—an in vitro study. Int J Pharm Sci Rev Res. 2012;12:89–94. [Google Scholar]
- Kumar G, Gupta R, Sharan S, et al. Anticancer activity of plant leaves extract collected from a tribal region of India. 3 Biotech. 2019;9(11):399. doi: 10.1007/s13205-019-1927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamitina ST, Strange K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am J Physiol Cell Physiol. 2005;288(2):C467–C474. doi: 10.1152/ajpcell.00451.2004. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33(1):40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Taft A, Kapulkin V, et al. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging. 2003;24(3):397–413. doi: 10.1016/s0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez CL, Martinez L, Martinez-Ortiz EJ, et al. Moringa oleifera, a species with potential analgesic and anti-inflammatory activities. Biomed Pharmacother. 2017;87:482–488. doi: 10.1016/j.biopha.2016.12.107. [DOI] [PubMed] [Google Scholar]
- Mathur M, Yadav S, Katariya PK, Kamal R. In vitro propagation and biosynthesis of steroidal sapogenins from various morphogenetic stages of Moringa oleifera Lam., and their antioxidant potential. Acta Physiol Plant. 2014;36(7):1749–1762. [Google Scholar]
- Medic-Saric M, Jasprica I, Smolcic-Bubalo A, Mornar A. Optimization of chromatographic conditions in thin layer chromatography of flavonoids and phenolic acids. Croat Chem Acta. 2004;77(1–2):361–366. [Google Scholar]
- Mehta K, Balaraman R, Amin AH, et al. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolemic rabbits. Ethnopharmacol. 2003;86:191–195. doi: 10.1016/s0378-8741(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Mishra G, Singh P, Verma R, et al. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: an overview. Der Pharm Lett. 2011;3(2):141–164. [Google Scholar]
- Miyoshi N, Takabayashi S, Osawa T, Nakamura Y. Benzyl isothiocyanate inhibits excessive superoxide generation in inflammatory leukocytes: implication for prevention against inflammation-related carcinogenesis. Carcinogenesis. 2004;25(4):567–575. doi: 10.1093/carcin/bgh051. [DOI] [PubMed] [Google Scholar]
- Mohan M, Kaul N, Punekar A, et al. Nootropic activity of Moringa oleifera leaves. J Nat Remedies. 2005;5(1):59–62. [Google Scholar]
- Mukhopadhyay A, Oh SW, Tissenbaum HA. Worming pathways to and from DAF-16/FOXO. Exp Gerontol. 2006;41(10):928–934. doi: 10.1016/j.exger.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Murakami A, Kitazono Y, Jiwajinda S, et al. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation. Planta Med. 1998;64:319–323. doi: 10.1055/s-2006-957442. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Njan AA, Amali MO, Olatunji LO, Olorundare OE. An overview of the ethno-pharmacological potential of Moringa oleifera Lam, “The Miracle Tree”. Arch Basic Appl Med. 2014;2:135–145. [Google Scholar]
- Oliveira JTA, Silveira SB, Vasconcelos IM, et al. Compositional and nutritional attributes of seeds from the multipurpose tree Moringa oleifera Lamarck. J Sci Food Agric. 1998;79:815–820. [Google Scholar]
- Paliwal R, Sharma V, Pracheta J. A review on horseradish tree (Moringa oleifera): a multipurpose tree with high economic and commercial importance. Asian J Biotechnol. 2011;3(4):17–328. [Google Scholar]
- Pandian GS. Ultraviolet spectrum of extract of Centella asiatica (Vallarai) in edible oil as supplement. Asian J Pharm Clin Res. 2018;11(4):425–426. doi: 10.22159/ajpcr.2018.v11i4.23519. [DOI] [Google Scholar]
- Park SY, Jung E, Kim JS, et al. Cancer-specific hNQO1-responsive biocompatible naphthalimides providing a rapid fluorescent turn-on with an enhanced enzyme affinity. Sensors. 2020;20(1):53. doi: 10.3390/s20010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran C, Peter KV, Gopalakrishnan PK. Drumstick (Moringa oleifera): a multipurpose Indian vegetable. Econ Bot. 1980;34(3):276–283. [Google Scholar]
- Rasmussen J, Mahler J, Beschorner N, et al. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc Natl Acad Sci. 2017;114(49):13018–13023. doi: 10.1073/pnas.1713215114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchuk MM, Dues DJ, Schaar CE, et al. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genet. 2018;14(3):e1007268. doi: 10.1371/journal.pgen.1007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Paliwal R. Isolation and characterization of saponins from Moringa oleifera (moringaeceae) pods. Int J Pharm Pharm Sci. 2013;5(1):179–183. [Google Scholar]
- Sharma V, Paliwal R. Preliminary phytochemical investigation and thin layer chromatography profiling of sequential extracts of Moringa oleifera pods. Int J Green Pharm. 2013;7(1):41–45. [Google Scholar]
- Shukla V, Yadav D, Phulara SC, et al. Longevity-promoting effects of 4-hydroxy-E-globularinin in Caenorhabditis elegans. Free Radic Biol Med. 2012;53(10):1848–1856. doi: 10.1016/j.freeradbiomed.2012.08.594. [DOI] [PubMed] [Google Scholar]
- Shunmugapriya K, Vennila P, Thirukkumar S, Ilamaran M. Identification of bioactive components in Moringa oleifera fruit by GC-MS. J Pharmacogn Phytochem. 2017;6(3):748–751. [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera L.) J Agric Food Chem. 2003;15:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Singh NK, Sonani RR, Awasthi A, et al. Phycocyanin moderates aging and proteotoxicity in Caenorhabditis elegans. J Appl Phycol. 2016;28(4):2407–2417. [Google Scholar]
- Sonani RR, Singh NK, Awasthi A, et al. Phycoerythrin extends life span and health span of Caenorhabditis elegans. Age. 2014;36(5):9717. doi: 10.1007/s11357-014-9717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiridon I, Bodirlau R, Teaca CA. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent Eur J Biol. 2011;6(3):388–396. [Google Scholar]
- Surco-Laos F, Duenas M, Gonzalez-Manzano S, et al. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res Int. 2012;46(2):514–521. [Google Scholar]
- Sutar NG, Bonde CG, Patil VV, et al. Analgesic activity of seeds of Moringa oleifera L. Int J Green Pharm. 2008;2:108–110. [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395(6705):854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Vongsak B, Sithisarn P, Gritsanapan W. Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera Lam. J Chromatogr Sci. 2014;52(7):641–645. doi: 10.1093/chromsci/bmt093. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Lu L, Zhou L. The longevity effect of echinacoside in Caenorhabditis elegans mediated through daf-16. Biosci Biotechnol Biochem. 2015;79(10):1676–1683. doi: 10.1080/09168451.2015.1046364. [DOI] [PubMed] [Google Scholar]
- Zecic A, Braeckman BP. DAF-16/FoxO in Caenorhabditis elegans and its role in metabolic remodeling. Cells. 2020;9(1):109. doi: 10.3390/cells9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Fu Z, Meng L, et al. The early events that initiate β-amyloid aggregation in Alzheimer’s disease. Front Aging Neurosci. 2018;10:359. doi: 10.3389/fnagi.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Khare P, Feldman L, Dent JA. Reversal frequency in Caenorhabditis elegans represents an integrated response to the state of the animal and its environment. J Neurosci. 2003;23(12):5319–5328. doi: 10.1523/JNEUROSCI.23-12-05319.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.