Abstract
Sex steroids regulate insulin sensitivity and glucose metabolism. We had characterized a lean type 2 diabetes (T2D) rat model using gestational low-protein (LP) diet programming. Our objective was to identify if endocrine dysfunction leading to decreased sex hormone levels will precede the development of T2D and if steroid replacement will prevent the onset of the disease. Pregnant rats were fed control or isocaloric LP diet from gestational day 4 until delivery. Normal diet was given to all mothers after delivery and to pups after weaning. LP offspring developed glucose intolerance and insulin resistance at 4 months. We measured sex steroid hormone profiles and expression of key genes involved in steroidogenesis in testis and ovary. Furthermore, one-month old rats were implanted with 90-day slow release T and E2 pellets for males and females, respectively. Glucose tolerance test (GTT) and euglycemic hyperinsulinemic clamp was performed at 4 months. LP-programmed T2D males had low T levels and females had low E2 levels due to dysregulated gene expression during steroidogenesis in gonads. GTT and euglycemic hyperinsulinemic clamp showed that LP males and females were glucose intolerant and insulin resistant; however, steroid supplementation prevented the onset of glucose intolerance and insulin resistance. Rats that developed T2D by LP programming have compromised gonadal steroidogenesis leading to low T and E2 in males and females, respectively. Sex steroid supplementation prevented the onset of glucose intolerance and insulin resistance indicating low sex steroid levels could cause compromised glucose metabolism ultimately leading to T2D.
Keywords: developmental programing, glucose intolerance, insulin resistance, testosterone, estrogen, sex steroids
Low-protein-programmed type 2 diabetes is mediated by testosterone and estradiol in males and females, respectively, in rats.
Introduction
Gestational low-protein (LP) programming causes glucose intolerance and insulin resistance in an adult offspring. We have earlier characterized a lean type 2 diabetes (T2D) rat model using gestational LP programming and showed that both male and female offspring develop progressively worsening T2D [1–3]. Interestingly, we observed sex differences in the severity, progression, and mechanism of glucose intolerance [1, 2, 4]. Our studies showed that females develop glucose intolerance and insulin resistance faster with a severe phenotype than males [1]. Furthermore, the mechanism of insulin resistance in the skeletal muscles of males and females was different. In males, glucose-transport mechanism was dysregulated [2] whereas in females, glucose-disposal-associated mechanisms were dysregulated [3]. Also, we found sex differences in the suppression of glucose production in liver with greater dysregulation in females when compared to males [5]. Although the exact mechanisms for the development of T2D and sex differences are not known, various studies have shown that sex steroids affect insulin sensitivity and glucose metabolism and possibly could be involved in this process.
Human studies have shown that both androgens and estrogens play key roles in modulating glucose metabolism. Studies show that men with decreased testosterone (T) and women with decreased estradiol (E2) and increased T exhibit varying degree of insulin resistance [6–9]. In men, low T levels have been implicated in the development of T2D and T treatment has restored insulin sensitivity [7, 8, 10]. A recent study showed a strong association between low T levels and insulin resistance, both cross-sectionally and longitudinally [11]. Furthermore, various clinical and epidemiological studies have shown that low T is associated with insulin resistance, poor glucose disposal, and T2D in males [6, 12, 13]. Interestingly, a 26-week T treatment leading to physiologically relevant serum concentrations improved insulin resistance and glycemic control in these men along with improvements in diabetic complications [7]. Lower E2 in females has been shown to cause impaired glucose tolerance and insulin resistance in humans and animal models [14]. Estrogen replacement in post-menopausal women with low E2 showed improved insulin sensitivity [15, 16], and estrogen treatment has a favorable effect on glucose metabolism [16]. Studies using mice show that estrogen improved insulin sensitivity and suppressed gluconeogenesis in females [17].
Based on our observation of sex differences in the disease presentation, we hypothesized that gestational low-protein programming will dysregulate gonadal steroidogenesis leading to low T in males and low E2 in females, with associated insulin resistance and that supplementation with their respective hormones, will prevent the development of insulin resistance. We also assessed changes in the expression levels of steroidogenic enzymes in gonads from control and LP-programmed offspring.
Materials and methods
Animals
Female Wistar rats (Envigo, Indianapolis, IN) weighing 175–225 g were mated with males of proven fertility. Sperm-positive rats were identified and separated. They were fed with isocaloric control (C, 20% casein; Harlan Teklad) or a low-protein (LP, 6% casein) diet from day 4 throughout pregnancy. Rats were given unlimited access to food and water and were housed in a temperature-controlled room (23 °C) with a 12:12-h light/dark cycle [1, 2]. Standard diet (20% protein) was given to all dams (including LP) after delivery. Litter sizes were culled to eight pups/dam (pups with weights at each extreme were euthanized, four males and four females were maintained when possible) to maintain uniformity. Pups received standard chow after weaning. All procedures including euthanasia of female rats were done during the diestrus phase of estrous cycle. Estrous cycle was determined by vaginal cytology as described by Cora et al. [18]. Two cohorts of identically programmed animals were used for this study. Animals from cohort 1 (males: control n = 9 and LP, n = 9; females: control n = 6 and LP, n = 6) were used for sex steroid hormone analysis, glucose tolerance test (GTT), qPCR, and western blot. Animals from cohort 2 (males: control n = 6, LP, n = 6 and LPT, n = 5; females: control n = 7, LP, n = 7 and LPE, n = 7) were utilized for sex steroid treatment studies along with GTT and euglycemic hyperinsulinemic clamp. Animals were euthanized at the end of the experiments. After euthanasia, testis and ovary were collected, snap frozen in liquid nitrogen, and stored at −80 °C until analysis. All experimental procedures involving rats were approved by the Institutional Animal Care and Use Committee of the Baylor College of Medicine.
Hormone measurements
Plasma T was measured by using an ELISA kit (ADI-900-065, Enzo Life Sciences, Inc. NY. USA) following manufacturer’s instruction as reported earlier [19]. Briefly, 100 μL of plasma (diluted 1:40) and standards were added in each wells pre-coated with antibody. Antibody solution (50 μL) was pipetted into each well, except the blank, total activity (TA), and non-specific wells and incubated for 1 h at room temperature with constant shaking (~500 rpm). Conjugate solution was added into each well, except the TA and blank wells followed by an incubation for 1 h at room temperature with constant shaking. The contents were emptied and washed thrice. Conjugate solution (5 μL) was added to the TA well followed with the addition of 200 μL of the substrate solution to every well. The plate was then incubated at 37 °C for 1 h without shaking. Stop solution (50 μL) was added to arrest the color development. The plate was then read at 405 nm using a plate reader (CLARIOstar, BMG Labtech Inc. NC, USA). The assay had a sensitivity of 5.67 pg/ml and had a co-efficient of variation of <10% (intra-assay variation of 7.8% and inter-assay variation of 9.3%).
Plasma T measurements were also performed using LC/MS at the metabolomics core facility, Baylor College of Medicine as reported earlier [20]. Briefly, T was extracted from rat serum by preparing 50 μL of each dilution of standard calibrator, rat serum, and quality-control serum 5 μL of T-d3. To this 1 mL of the methyl-tertiary butyl, ether was added and vortexed for 10 min and centrifuged. The organic layer was collected and dried at a low boiling point. The dried samples were reconstituted with 100 μL of 0.1% formic acid in methanol: water (30:70) and subjected to LC–MS. ESI-positive mode was used in the method. The HPLC column was from Agilent Infinity Lab Poroshell 120 HILIC-OH5 2.1 × 50, 2.7 μM. Mobile phases A and B were 0.1% formic acid in water and acetonitrile, respectively. Gradient used was as follows: at 0 min-30% B, 3.5 min-60% of B, 9 min-80% B, 12 min-98% of B, 15 min-30% of B, followed by re-equilibration at end of the gradient 17 min to the initial starting condition 30% of B. Flow rate: 0.2 mL/min. Testosterone was quantified using 6495B triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA) coupled to an HPLC system (Agilent Technologies, Santa Clara, CA) in multiple reaction monitoring mode (MRM). Source parameters were as follows: gas temperature: 250 °C; gas flow: 12 l/min; nebulizer: 20 psi; sheath gas temperature: 350 °C; sheath gas flow: 12 l/min; capillary 3000 V positive and 3000 V negative; nozzle voltage: 1500 V positive and 1500 V. Approximately, 8–11 data points were acquired per detected T and calculated using the Agilent software.
Plasma estradiol was assessed using Mouse/Rat Estradiol ELISA kit (ES180S-100, Calbiotech, CA, USA), following manufacturers instruction. The assay had a sensitivity of 2 pg/ml and had an intra-assay co-efficient of variation of <5% and inter-assay variation of <10%. Briefly, 25 μL of standards, plasma samples, and controls were pipetted into appropriate wells followed by 100 μL of estradiol enzyme conjugate into each well. The plate was then incubated at room temperature for 120 min. The wells were then washed thrice with wash buffer. TMB reagent (100 μL) was pipetted into each well followed by incubation for 30 min at room temperature for color development. Stop solution was added (50 μL) and mixed well. Absorbance was read at 450 nm with a microplate reader (CLARIOstar, BMG Labtech Inc. NC, USA).
Steroid pellet implantation
LP-programmed males and females from cohort 2 received T and E2, respectively, in the form of pellets capable of controlled release of hormones for 90 days, as reported by our group earlier [21–23]. Steroid pellets were implanted on the fifth week after birth just prior to the time of puberty as evident by the opening of vagina (~35–38 days of age) in females and preputial separation in males (~38–40 days of age), to offset the expected decreases in steroid hormones in LP rats. A 90-day slow release 17β-estradiol pellet (providing 1 μg/day, NE-121, Innovative Research America, USA) for LP female and a 90-day slow release T pellet (providing 0.5 mg/day, NA-151, Innovative Research America) for LP male offspring were inserted under the skin at the nape. These doses were chosen based upon previous publications [24–26] and based on our pilot data with a goal of achieving plasma E2 levels targeted to 40 pg/ml in females and T levels to 6 ng/ml in males. A placebo pellet (NC-111) was be inserted for controls.
Real-time quantitative PCR
Total RNA was isolated from testes and ovary using TRIzol reagent (Life Technologies, Carlsbad, CA) and further purified with RNeasy clean-up kit (Qiagen, Valencia, CA). All RNA samples were treated with DNase. RNA concentration and purity were determined using an ND-1000 model Nanodrop spectrophotometer (Thermo Fisher Scientific, Newark, DE). Two micrograms of total RNA were reverse transcribed (RT) using a modified Maloney murine leukemia virus-derived RT (New England Biolabs Inc., MA, USA) and random hexamer primers (Life Technologies, CA, USA) as reported earlier [2]. cDNA was amplified by real-time PCR using SYBR Green (Bio-Rad, Hercules, CA) in a CFX96 model real-time thermal cycler (Bio-Rad). Specific pairs of primers were designed and purchased (IDT, IA, USA) (Table 1). PCR conditions used were 10 min at 95 °C for 1 cycle, 15 s at 95 °C, 30 s at 60 °C, and 15 s at 72 °C for 40 cycles, followed by a melt curve analysis (0.5 °C/5 s from 65 to 95 °C). Results were calculated using 2–ΔΔCT method and expressed as fold changes of expression of genes of interest. Briefly, CT values were obtained by drawing an arbitrary threshold line across all the amplification curves for each gene and the point at which the curve intersects the threshold is the CT for each sample. Replicate CT values from each animal were averaged for both genes of interest and the reference gene. ΔCT values for each animal were obtained by calculating the difference between CT values of the gene of interest and the reference gene. ΔΔCT was then obtained by calculating the difference between the average CT values of each animal of both the groups with the average of the control group. Fold differences were then obtained by converting ΔΔCT values to 2–ΔΔCT. All reactions were performed in duplicate, and cyclophilin A was used as an internal control.
Table 1.
Oligonucleotide primers used for real-time PCR
| Gene | Accession number | Primers F = forward; R = reverse | Amplicon size (bp) | Primer efficiency (%) |
|---|---|---|---|---|
| Sf-1 | NM_001191099.1 | F: 5’-TGTCTGTCTCAAGTTCCTCATCCTC-3′ | 272 | 109.9 |
| R: 5’-TGGCCTGCAGCATCTCAAT-3’ | ||||
| Star | NM_031558.3 | F: 5’-AGGAAAGCCAGCAGGAGAATG-3’ | 101 | 105.5 |
| R: 5’-GTCCATGGGCTGGTCTAGCA-3’ | ||||
| Hsd17b1 | NM_012851.2 | F: 5’-ACTCCGGGCGTGTGCTGGTGA-3’ | 517 | 106.0 |
| R: 5’-GGCGTGTCTGGATCCCCTGAAACTT-3’ | ||||
| Hsd17b7 | NM_017235.3 | F: 5’-GAACGCCGGAATCATGCCTAACC-3’ | 551 | 99.9 |
| R: 5’-GGAAAAGCCACACCAATGCCTCTG-3’ | ||||
| Cyp11a1 | NM_017286.3 | F: 5’-TCAAGCAGCAAAACTCTGGA-3’ | 97 | 96.6 |
| R: 5’-CGCTCCCCAAATACAACACT-3’ | ||||
| Cyp19a1 | NM_017085.2 | F: 5’-CCTGGAGATGACGTGATTG-3’ | 197 | 105.5 |
| R: 5’-CGATGTACTTCCCAGCACAG-3’ | ||||
| Cyclophilin A | NM_017101.1 | F: 5’-TATCTGCACTGCCAAGACTGAGTG-3’ | 127 | 99.3 |
| R: 5’-CTTCTTGCTGGTCTTGCCATTCC-3’ |
Protein extraction
Total protein extraction from ovary and testis were performed as reported earlier [2]. Briefly, tissues were weighed and homogenized in1X RIPA buffer (Cell Signaling technologies, MA, USA) containing 1 mM PMSF, protease inhibitor cocktail (Roche, IN, USA) and phosphatase inhibitor cocktails (Sigma, MO, USA). The lysates were then sonicated, centrifuged (14,000 × g for 10 min), and the supernatants were stored in −80°C until further analysis. Proteins concentrations were quantified using Pierce BCA kit (Pierce Biotechnology, IL, USA).
Western blot
Western blots for steroidogenic enzymes were performed as reported earlier [3]. Briefly, 10–30 μg of protein extract was resolved on 4–15% precast gradient polyacrylamide gels (Mini-PROTEAN TGX Precast Gels; Bio-Rad, Hercules, CA). Resolved proteins were transferred to a polyvinylidine fluoride membrane (Millipore, Billerica, MA). When multiple antibodies were probed from the same blot, the blots were stripped using Restore PLUS western blot stripping buffer (Thermo Scientific, Waltham, MA) for 5–15 min followed by three washes with Tris-buffered saline containing 0.1% Tween 20. Primary antibodies were incubated overnight at 4 °C after blocking the membranes in 5% bovine serum albumin or nonfat dried milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h at room temperature. Details of primary antibodies and their dilutions are as follows: Aromatase (Cat # Ab 124776, 1:1000), StAR (Cat # ab 58013, 1:1000), Hsd17b1 (Cat # ab217851, 1:500), SF1 (Cat # ab168380, 1:500) and GAPDH (Cat # ab 8245, 1:1000) were obtained from Abcam, Cambridge, MA USA. Antibodies for CYP11A1 (Cat # 14217, 1:1000) and Hsd17b7 (Cat # 393936, 1:500) were obtained from cell signaling, MA, USA and Santa Cruz Inc. CA. USA, respectively. After primary antibody incubations, membranes were washed and incubated for 60 min at room temperature with horseradish peroxidase conjugated secondary antibodies (Abcam, Cambridge, MA USA). Membranes were washed and incubated in ECL western blotting detection reagents (Pierce Biotechnology, Waltham, MA USA) for a minute and imaged using the Odyssey Fc imaging system (LI-COR). Densitometric analyses were performed using Image Studio software from LI-COR or ImageJ.
GTT
GTT was performed on LP-programmed offspring of both sexes belonging to both cohorts at 4 months of age to identify the presence of glucose intolerance as reported earlier [1]. Briefly, rats were fasted for 6 h and were administered glucose (1 g/kg body weight i.p.). Blood glucose levels were measured using ACCU-CHEK Nano (Roche USA) at 0, 15, 30, 60, 120, and 180 min via saphenous puncture.
Euglycemic-hyperinsulinemic clamp
Tail vein euglycemic-hyperinsulinemic clamp was done at 4 months of age for as reported earlier [5]. Briefly, rats were fasted for 6 h and were restrained in an appropriately sized restrainer (Kent Scientific Corporation, CT, USA). Tail vein catheter was inserted using BD Insyte Autoguard Shielded IV Catheter (BD Biosciences, IL, USA) proximal end of the tail. The catheter was connected to a “Y” connector, which was connected to syringes filled with 50% glucose solution and insulin (HumulinR, Eli Lilly and Company, IN, USA). The syringes were mounted onto a syringe pump (Harvard Apparatus, MA, USA). Insulin was constantly infused at a rate of 4 mU/(kg·min) (flow rate of 200 and 150 μL/h for males and females, respectively). Blood samples for measuring glucose were obtained every 10–15 min from the tail tip. Glucose infusion rates were adjusted by trial and error until a steady state of blood glucose concentration was reached. Three consecutive readings within a range of ~1 mM blood glucose concentrations were considered to have reached a steady state. Glucose levels were clamped between 5–6 mM.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software. Data are presented as mean ± SEM. Comparison between two groups was performed using unpaired students t-test. Comparisons between multiple groups were done using one-way or two-way ANOVA with repeated measures followed by Bonferroni test. Differences were considered significant when P < 0.05.
Results
LP-programmed males have low T and females have low E2
Our results show that in utero LP programming affects circulating sex steroid concentrations in both males and females at 4 months of age. In males, plasma T levels were significantly reduced in LP with 1.5 ± 0.33 ng/ml when compared to 5.7 ± 1.6 ng/ml in controls, (P < 0.05, n = 5) using ELISA (Figure 1A). Furthermore, LC/MS measurements also showed similar results with 2.4 ± 0.3 ng/ml in LP and 5.2 ± 0.8 ng/ml in controls (P < 0.01, n = 9; Figure 1B). Similarly, females also displayed low E2 levels (2.3 ± 0.3 pg/ml in LP vs. 4.4 ± 0.6 pg/ml in controls P < 0.01, n = 6; Figure 1C) in comparison with their respective controls when measured during diestrus phase using ELISA.
Figure 1.
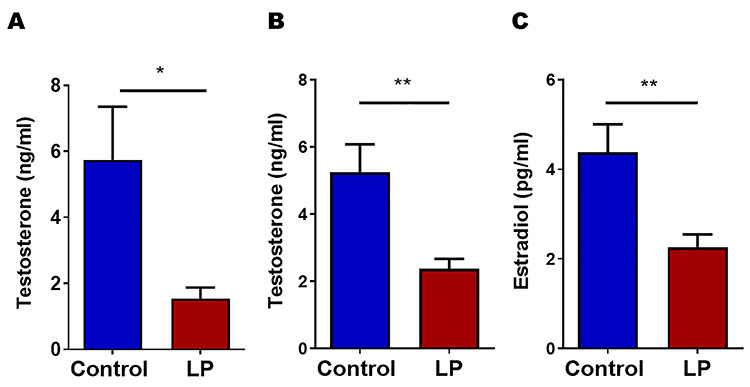
Plasma testosterone and estradiol concentrations in male and female in LP-programmed 4-month-old offspring. (A and B) Testosterone (n = 5 for ELISA and = 9 for LC/MS) levels in male as measured by ELISA and LC/MS, respectively. (C) 17β-Estradiol (n = 5–6) levels in female control and LP rat offspring (*P < 0.05 and **P < 0.01).
Impaired expression of steroidogenic genes in gonads
Expression of key genes involved in steroidogenesis in testis and ovary were investigated using qPCR and western blot. qPCR analysis was performed for Cyp11a1, StAR, Hsd17b1, Hsd17b7, Cyp19 (Aromatase), and steroidogenic factor 1 (Sf-1) in both ovary and testis. Results show that Cyp11a1, Hsd17b1, and Sf-1 were downregulated and, StAR and Cyp19 were upregulated in testis (Figure 2A). Furthermore, western blot analysis also showed the similar results (Figure 2B). In females, mRNA levels of Hsd17b7 and Cyp19 were upregulated and, StAR was downregulated in ovaries (Figure 3A). Western blot analysis also showed results like qPCR (Figure 3B).
Figure 2.
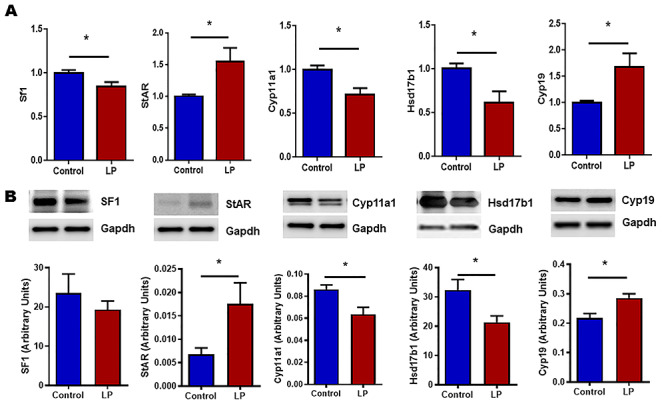
mRNA and protein expression levels of molecules involved in steroidogenesis measured by qPCR and western blot, respectively, in testis. (A) Figure showing the mRNA expression of steroidogenic factor 1 (Sf-1), Star, Cyp11a1, Hsd17b1, and Cyp19 (Aromatase). qPCR data were normalized with cyclophilin A as an internal control. (B) Representative blots and their densitometric analyses showing the protein levels of Sf-1, StAR, Cyp11a1, Hsd17b1 and Cyp19. GAPDH was used as a loading control for western blots, and its values were used for normalization. Results are presented as mean ± SEM, n = 4–6 per group (*P < 0.05).
Figure 3.
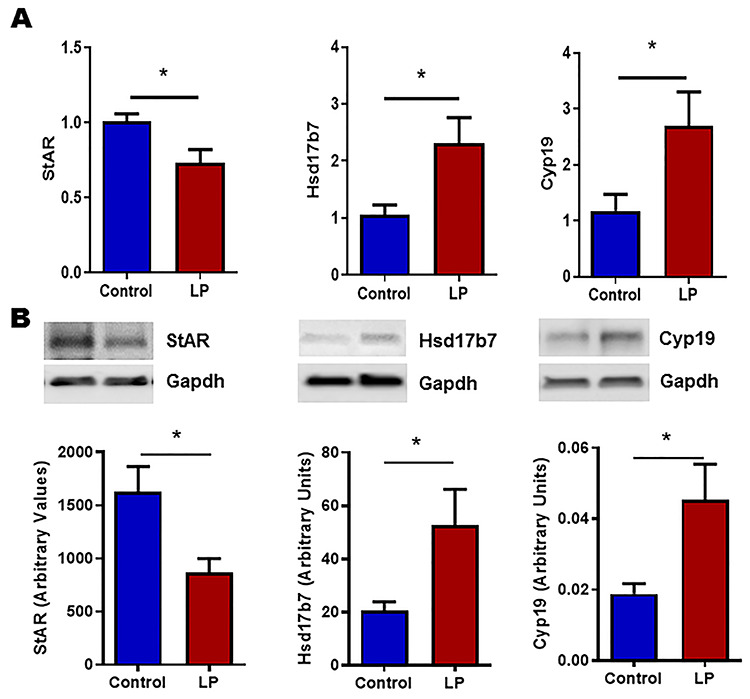
mRNA and protein expression levels of molecules involved in steroidogenesis measured by qPCR and western blot, respectively, in ovary. (A) Figure showing the mRNA expression of Star, Hsd17b7 and Cyp19 (Aromatase), and qPCR data were normalized with cyclophilin A as an internal control. (B) Representative blots and their densitometric analyses showing the protein levels of StAR, Hsd17b7, and Cyp19. GAPDH was used as a loading control for western blots, and its values were used for normalization. Results are presented as mean ± SEM, n = 4–6 per group (*P < 0.05).
Steroid replacement prevents glucose tolerance in both sexes
Results from GTT show that steroid supplementation prior to the onset of the disease clearly prevented the animals from developing glucose intolerance in both males and females (Figure 4A–D). In males (Figure 4A), fasting glucose levels did not show any differences between the groups (controls: 6.1 ± 0.2 mmol/L vs. LP: 6.0 ± 0.1 mmol/L vs. LPT: 5.9 ± 0.2 mmol/L). At 15 min, blood glucose levels rose in all three groups, but there were no differences between the groups (controls: 13.5 ± 0.5 mmol/L vs. LP: 14.6 ± 0.5 mmol/L vs. LPT: 12.9 ± 0.9 mmol/L). However, at 30 min (controls: 12.7 ± 0.5 mmol/L vs. LP: 15.4 ± 0.9 mmol/L vs. LPT: 13.4 ± 1.0 mmol/L) and 60 min (controls: 11.2 ± 0.6 mmol/L vs. LP: 14 ± 0.9 mmol/L vs. LPT: 10.1 ± 0.9 mmol/L), there was a significant increase (P < 0.05) in the blood glucose levels in the LP males when compared to the controls and at 60-min LPT males had lower (P < 0.01) blood glucose levels when compared to LP males. There were no changes in the blood glucose levels between the groups at 120 min (controls: 8.9 ± 0.4 mmol/L vs. LP: 10.0 ± 0.5 mmol/L vs. LPT: 8.4 ± 0.4 mmol/L) or 180 min (controls: 7.0 ± 0.2 mmol/L vs. LP: 7.6 ± 0.2 mmol/L vs. LPT: 7.3 ± 0.4 mmol/L). The overall differences in the blood glucose concentrations after administering GTT were analyzed by calculating the area under the curve (AUC) (mmol/L·180 min), which showed that overall glucose levels were significantly (P < 0.05) higher (glycemia: 2066 ± 63) in LP males compared with controls (glycemia: 1784 ± 40) and LPT males (glycemia: 1714 ± 106) (Figure 4B).
Figure 4.
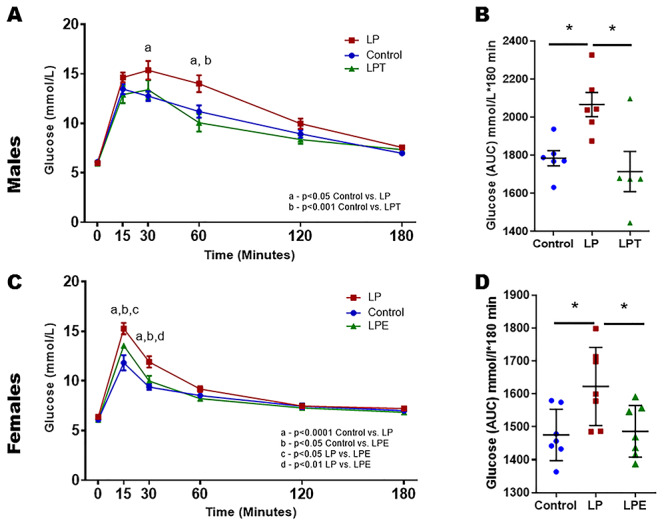
GTT in 4-month-old males (A and B) and females (C and D) offspring from control and LP-programmed rats along with offspring from LP group supplemented with T for males (LPT) and 17β estradiol for females (LPE). Figure showing glucose and insulin levels along with their respective AUC during GTT in control, LP and LPT for male and LPE for female offspring. (n = 5–6 per group).
In females (Figure 4C), fasting glucose levels were similar between the groups (controls: 6.1 ± 0.2 mmol/L vs. LP: 6.4 ± 0.1 mmol/L vs. LPT: 6.1 ± 0.1 mmol/L). At 15 min, blood glucose levels rose significant higher in the LP females when compared to the controls (P < 0.0001). Interestingly, estradiol supplementation suppressed the glucose increase and the values were lower than the LP females (P < 0.05) but were higher than the controls (P < 0.05) (controls: 11.8 ± 0.8 mmol/L vs. LP: 15.3 ± 0.6 mmol/L vs. LPE: 13.6 ± 0.1 mmol/L). Similar findings were observed at 30 min with a significant increase in the blood glucose levels in LP group when compared to the controls (P < 0.0001), but estradiol supplementation prevented the glucose increase and the values were lower than the LP females (P < 0.01) but were higher than the controls (P < 0.05) (controls: 9.4 ± 0.3 mmol/L vs. LP: 11.9 ± 0.6 mmol/L vs. LPE: 10.0 ± 0.5 mmol/L). There were no differences in the blood glucose levels between the groups at 60 min (controls: 8.5 ± 0.2 mmol/L vs. LP: 9.2 ± 0.3 mmol/L vs. LPE: 8.2 ± 0.3 mmol/L), 120 min (controls: 7.5 ± 0.3 mmol/L vs. LP: 7.5 ± 0.2 mmol/L vs. LPE: 7.3 ± 0.2 mmol/L) or 180 min (controls: 7.0 ± 0.2 mmol/L vs. LP: 7.2 ± 0.2 mmol/L vs. LPE: 6.8 ± 0.2 mmol/L). The overall differences in the blood glucose concentrations after administering GTT were analyzed by calculating the AUC (mmol/L·180 min) showed that overall glucose levels were significantly (P < 0.01) higher (glycemia: 1623 ± 45) in LP females compared with controls (glycemia: 1475 ± 29) and LPE females (glycemia: 1486 ± 30) (Figure 4D).
Steroid supplementation prevents LP-programmed insulin resistance
Euglycemic hyperinsulinemic clamp was performed to assess the peripheral insulin resistance in both males (Figure 5) and females (Figure 6). Comparisons between the groups were performed by comparing the infusion rates of glucose to maintain a steady blood glucose under constant insulin infusion. At steady state, controls and LPT/LPE animals were able to withstand the infusion of more glucose when compared to LP, indicating the ability of steroid treatment to prevent insulin resistance. Analysis of the steady state averages (control: 15.6 ± 2.7 μmol/kg·min, LP: 6.6 ± 1.2 μmol/kg·min and LPT: 17.9 ± 2.4 μmol/kg·min) in males reveals that the infusion rate of LP was lower (P < 0.05) than that of the controls and LPT (Figure 5A–D). Similarly in females, steady state averages (control: 18.7 ± 2.5 μmol/kg·min, LP: 4.2 ± 1.6 μmol/kg·min and LPE: 29.6 ± 5.8 μmol/kg·min) show that the infusion rate of LP was significantly lower when compared to the controls (P < 0.05) and LPE (P < 0.001) (Figure 6A–D). Thus, our results show that steroid supplementation prior to the onset of insulin resistance protects the LP-programmed rats from getting the disease and restores insulin sensitivity in both males and females.
Figure 5.
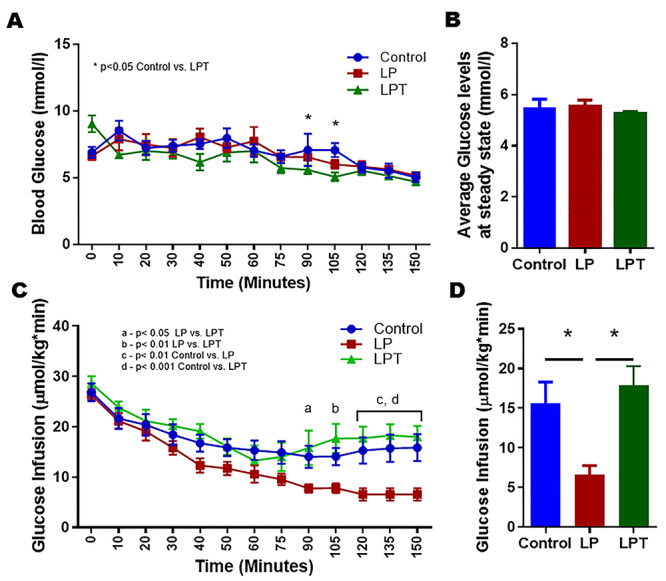
Euglycemic hyperinsulinemic clamp in 4-month-old male offspring from control and LP-programmed rats along with offspring from LP group supplemented with T (LPT). Figures showing the blood glucose (A), average glucose levels at steady state (B), and their corresponding glucose infusion rates (C). Infusion rates were compared between the groups at the steady state (D). (n = 5–6 per group) *P < 0.05.
Figure 6.
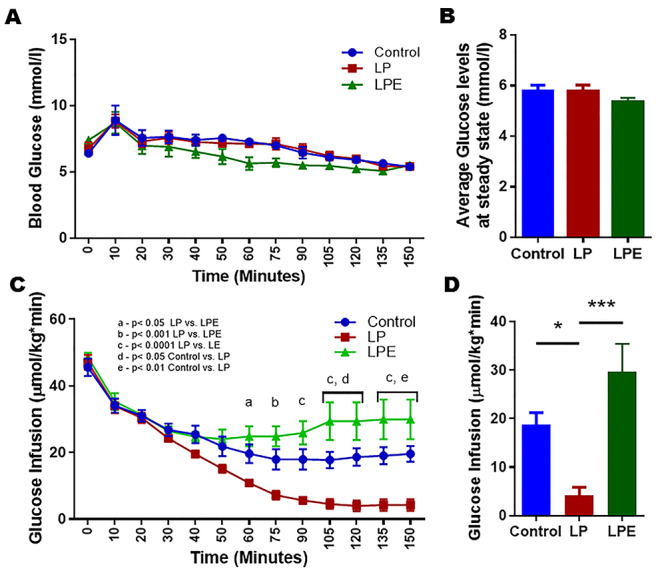
Euglycemic hyperinsulinemic clamp in 4-month-old female offspring from control and LP-programmed rats along with offspring from LP group supplemented with 17β estradiol (LPT). Figures showing the blood glucose (A), average glucose levels at steady state (B), and their corresponding glucose infusion rates (C). Infusion rates were compared between the groups at the steady state (D). (n = 7 per group) *P < 0.05, and ***P < 0.001.
Discussion
We and others have shown sex differences in the onset, progression, and severity of metabolic disorders induced by developmental programming [1, 5, 27]. One of the possible explanation for the sex differences is likely to be a mechanism involving sex steroids. There are various experimental and anecdotal evidences in animal models and human studies linking sex steroids T and E2 with glucose homeostasis. T and E2 are involved in modulating insulin sensitivity [6–9, 28]. In men, low T causes glucose intolerance and insulin resistance, and T supplementation restored glucose tolerance and insulin sensitivity [7, 8]. Lower E2 in women is implicated in T2D [29] and E2 supplementation improved their insulin sensitivity [15]. In a rat model using dietary manipulations involving both gestational and lactation periods, male offspring were insulin resistant at an earlier age (20 weeks) and with faster disease progression compared to females [30–34]. Thus, maintaining physiologically relevant sex steroid levels is expected to prevent the onset of glucose intolerance and insulin resistance. In the present study, we explored if sex steroids T and E2 play a direct role in the development of glucose intolerance and insulin resistance.
We show for the first time that gestational LP-diet-induced developmental programming of T2D is sex steroid dependent. Our present study shows that rats that develop glucose intolerance and insulin resistance by LP programming have compromised gonadal steroidogenesis leading to reduced circulating levels of T and E2 in males and females, respectively. Similar observations have been noticed in human studies [7, 8]. We further wanted to identify the mechanism involved in compromised gonadal steroidogenesis. Our data show that in males, T production was affected in the testes by the downregulation of key enzymes and regulatory genes involved in T synthesis like Cyp11a1, Hsd17b1, and Sf1, despite upregulation by StAR. Furthermore, it showed increased expression of Cyp19 (Aromatase) suggests possible increased conversion of androgens to estrogens. In females, ovarian estrogen levels were downregulated due to the possible downregulation of StAR (rate limiting step) in spite of the compensatory upregulation of Cyp19 and Hsd17b7. Thus, T and E2 production is compromised by a series of suboptimal expression of genes in the gonads. Interestingly, treatment with physiologically relevant doses of T and E2 to males and females, respectively, prevented the onset of glucose intolerance and insulin resistance as evident by our GTT and euglycemic clamp data. This clearly indicates that T and E2 play a direct role in the etiology of the disease.
It is not clear how prenatal LP programing affect the expression of these genes involved in sex steroid synthesis. It may be possible that these genes are epigenetically regulated with an expression capacity based on the nutritional availability during in utero developmental stages. Over the years, various investigations have shown epigenetic regulations of genes involved in steroidogenesis. SF1 is essential for the steroidogenic gene syntheses in both testis and ovary [35]. SF-1 increases the expression of the steroidogenic machinery, and it has binding sites in the promoter regions of Star and Cyp family genes [36, 37]. It is regulated in a tissue specific and spatio-temporal manner by the methylation of its promoter region [38, 39]. SF-1 regulates steroidogenic acute regulatory protein (STAR), which facilitates transport of cholesterol to the outer mitochondrial membrane. This is an important rate-limiting step. Although Star gene does not have CpG islands, it has proximal promoters that can be modified by histone acetylation leading to gene activation and repression in both Leydig cells and granulosa cells [40, 41]. Interestingly, Star is also regulated by mRNA stability. Star gene is transcribed as 1.6 and 3.5 kb mRNAs differing in alternative polyadenylation sites in exon 7 causing the 3.5 kb mRNA to degrade rapidly when compared to the 1.6 kb mRNA variant [42]. Thus, preferential expression of one variant over other can facilitate rapid changes in Star expression in response to external changes [42]. Cyp11a1 otherwise known as cytochrome P450 cholesterol side-chain cleavage enzyme, which is present in mitochondria and facilitates the conversion of cholesterol into pregnenolone. Recent evidence shows that Cyp11a1 gene is regulated by histone modifications and chromatin remodeling of the promoter region but not by DNA methylation [43]. Hsd17b1 catalyzes and preferentially converts low-potent 17keto-steroids to highly potent 17beta-hydroxysteroids, E2, and T. Hsd17b1 is expressed by Sertoli cells contributes to steroid synthesis and is essential for male fertility [44]. Epigenetic modification by methylation of this gene has been reported in various cancers but is not well studied in reproductive tissues [45–48]. Hsd17b7 is essential for cholesterol synthesis and in the steroid synthesis as a 17-beta-hydroxysteroid dehydrogenase [49]. Its promoter region contains nuclear factor 1 binding site where estrogen receptors can bind to mediate estradiol action in breast cancer cells [50]. It is not known if this gene is epigenetically regulated in ovary. Cyp19 also known as aromatase converts androgens to estrogen. It has been shown to be widely regulated by promoter methylation in various tissues [37]. It is clear based on the existing evidences that most the key genes involved in the steroidogenesis can be epigenetically regulated. However, further investigations are required to bridge the gap between LP diet and epigenetic regulation of these genes. Three major possibilities are considered as potential mechanism of developmental programming although DNA methylation is considered to be the primary process. They are epigenetic modification on DNA by methylation, chromatin structure alteration by histone modifications, and uterine environment. Each regulated gene has to be individually assessed further for these mechanisms in order to understand how gestational diet affects gene expression.
We had earlier demonstrated that LP pups were small and exhibited intrauterine growth restriction in our lean T2D rat model [1]. The brain sparing effect in intra uterine growth restriction is well studied, where the developing fetus adapts its circulation of nutrients and oxygen preferentially to the brain compared to other systems [51]. It is likely that the reproductive system is given a lower priority during early development when there is a nutritional deficiency. The evidence for such a thrifty phenotype is mixed and warrants further investigations. Although some authors conclude there is no definite relationship [52], other recent studies show evidences in human and animal studies [53–55]. These studies show that the reproductive potential of the offspring is affected due to nutritional deficiency during early development. The fact that sex steroids are involved in both reproduction and metabolism makes this a critical system to investigate further in order to understand the programming mechanism.
Our studies do have some weaknesses, which we will address in subsequent studies some of which are currently in process. We do not have enzyme activities of the steroidogenic enzymes. We also do not have data on the fertility of these animals as they have low sex steroid levels. LP-programmed females do have normal estrus cycles (data not shown), but we have not bred them to see their fertility. We speculate that they may be sub-fertile. Furthermore, we do not know if steroid supplementation after the onset of the disease will reverse previously established glucose intolerance and insulin resistance.
Taken together, the current study clearly demonstrates that prenatal LP programming compromised the offspring’s gonadal synthesis of T and E2 in males and females, respectively, which further affects the glucose homeostasis leading to the onset of glucose intolerance and insulin resistance. Thus, our study shows that maternal protein restriction causes decreased sex hormone concentrations in the offspring and leads to glucose intolerance and peripheral insulin resistance.
Conflict of interest
The authors have declared that no conflict of interest exists.
Disclosure statement: Authors have nothing to declare.
Author Contributions
CSB and CY were involved in planning, designing of the study, and drafting of the manuscript. CSB, AKS, DTT, PRM, and AB were involved in animal work. CSB, VAV, DTT, PRM, and MB were involved in bench work performing hormone analysis, qPCR, and WB.
References
- 1. Blesson CS, Schutt AK, Balakrishnan MP, Pautler RG, Pedersen SE, Sarkar P, Gonzales D, Zhu G, Marini JC, Chacko SK, Yallampalli U, Yallampalli C. Novel lean type 2 diabetic rat model using gestational low protein programming. Am J Obstet Gynecol 2016; 214:540.e1–540.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blesson CS, Sathishkumar K, Chinnathambi V, Yallampalli C. Gestational protein restriction impairs insulin-regulated glucose transport mechanisms in gastrocnemius muscles of adult male offspring. Endocrinology 2014; 155:3036–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blesson CS, Chinnathambi V, Kumar S, Yallampalli C. Gestational protein restriction impairs glucose disposal in the gastrocnemius muscles of female rats. Endocrinology 2017; 158:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blesson CS, Chinnathambi V, Sathishkumar K, Yallampalli C. Gestational protein restriction causes hyperglycemia by affecting IRS-1 tyrosine phosphorylation and the dysregulation of Akt-GSK3 signaling in adult female offspring In: Reproductive Sciences, vol. 22 USA: Sage Publications Inc; 2015: 98A–98A. [Google Scholar]
- 5. Blesson CS, Schutt A, Chacko S, Marini JC, Mathew PR, Tanchico D, Balakrishnan M, Yallampalli C. Sex dependent dysregulation of hepatic glucose production in lean type 2 diabetic rats. Front Endocrinol (Lausanne) 2019; 10:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haffner SM, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 1994; 43:212–219. [DOI] [PubMed] [Google Scholar]
- 7. Janjgava S, Zerekidze T, Uchava L, Giorgadze E, Asatiani K. Influence of testosterone replacement therapy on metabolic disorders in male patients with type 2 diabetes mellitus and androgen deficiency. Eur J Med Res 2014; 19:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kovac J, Pastuszak AW, Lamb DJ, Lipshultz LI. Testosterone supplementation therapy in the treatment of patients with metabolic syndrome. Postgrad Med 2014; 126:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) 2002; 102:151–166. [DOI] [PubMed] [Google Scholar]
- 10. Zarotsky V, Huang MY, Carman W, Morgentaler A, Singhal PK, Coffin D, Jones TH. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology 2014; 2:819–834. [DOI] [PubMed] [Google Scholar]
- 11. Ottarsdottir K, Nilsson AG, Hellgren M, Lindblad U, Daka B. The association between serum testosterone and insulin resistance: A longitudinal study. Endocr Connect 2018; 7:1491–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapoor D, Malkin CJ, Channer KS, Jones TH. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf) 2005; 63:239–250. [DOI] [PubMed] [Google Scholar]
- 13. Simon D, Charles MA, Nahoul K, Orssaud G, Kremski J, Hully V, Joubert E, Papoz L, Eschwege E. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The telecom study. J Clin Endocrinol Metab 1997; 82:682–685. [DOI] [PubMed] [Google Scholar]
- 14. Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Curr Atheroscler Rep 2004; 6:180–185. [DOI] [PubMed] [Google Scholar]
- 15. Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab 2003; 285:E311–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slopien R, Wender-Ozegowska E, Rogowicz-Frontczak A, Meczekalski B, Zozulinska-Ziolkiewicz D, Jaremek JD, Cano A, Chedraui P, Goulis DG, Lopes P, Mishra G, Mueck A et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018; 117:6–10. [DOI] [PubMed] [Google Scholar]
- 17. Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, Han G, Newell-Fugate A, Tian Y, Majeti R, Liu W, Xu Y et al. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes 2019; 68:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 2015; 43:776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones SL, Ismail N, King L, Pfaus JG. The effects of chronic administration of testosterone propionate with or without estradiol on the sexual behavior and plasma steroid levels of aged female rats. Endocrinology 2012; 153:5928–5939. [DOI] [PubMed] [Google Scholar]
- 20. Matysik S, Liebisch G. Quantification of steroid hormones in human serum by liquid chromatography-high resolution tandem mass spectrometry. J Chromatogr A 2017; 1526:112–118. [DOI] [PubMed] [Google Scholar]
- 21. Gao H, Yallampalli U, Yallampalli C. Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries. Biol Reprod 2012; 86:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross GR, Chauhan M, Gangula PR, Reed L, Thota C, Yallampalli C. Female sex steroids increase adrenomedullin-induced vasodilation by increasing the expression of adrenomedullin2 receptor components in rat mesenteric artery. Endocrinology 2006; 147:389–396. [DOI] [PubMed] [Google Scholar]
- 23. Ross GR, Yallampalli U, Gangula PR, Reed L, Sathishkumar K, Gao H, Chauhan M, Yallampalli C. Adrenomedullin relaxes rat uterine artery: Mechanisms and influence of pregnancy and estradiol. Endocrinol 2010; 151:4485–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowman AR, Sass DA, Dissanayake IR, Ma YF, Liang H, Yuan Z, Jee WS, Epstein S. The role of testosterone in cyclosporine-induced osteopenia. J Bone Miner Res 1997; 12:607–615. [DOI] [PubMed] [Google Scholar]
- 25. Poulsen RC, Moughan PJ, Kruger MC. Docosahexaenoic acid and 17 beta-estradiol co-treatment is more effective than 17 beta-estradiol alone in maintaining bone post-ovariectomy. Exp Biol Med (Maywood) 2008; 233:592–602. [DOI] [PubMed] [Google Scholar]
- 26. Kitson AP, Marks KA, Shaw B, Mutch DM, Stark KD. Treatment of ovariectomized rats with 17beta-estradiol increases hepatic delta-6 desaturase enzyme expression and docosahexaenoic acid levels in hepatic and plasma phospholipids. Prostaglandins Leukot Essent Fatty Acids 2013; 89:81–88. [DOI] [PubMed] [Google Scholar]
- 27. Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction 2013; 145:R1–R13. [DOI] [PubMed] [Google Scholar]
- 28. Andersson B, Mattsson LA, Hahn L, Marin P, Lapidus L, Holm G, Bengtsson BA, Bjorntorp P. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997; 82:638–643. [DOI] [PubMed] [Google Scholar]
- 29. Suba Z. Low estrogen exposure and/or defective estrogen signaling induces disturbances in glucose uptake and energy expenditure. J Diabetes Metab 2013; 4:2. [Google Scholar]
- 30. Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol 2002; 175:757–767. [DOI] [PubMed] [Google Scholar]
- 31. Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, Hartil K, Petry CJ, Buckley AJ, Mosthaf-Seedorf L. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol 2003; 177:235–241. [DOI] [PubMed] [Google Scholar]
- 32. Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: From measuring insulin to the control of fetal organogenesis. Biochem Soc Trans 1996; 24:341–350. [DOI] [PubMed] [Google Scholar]
- 33. Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res 2001; 2:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol 2005; 288:R368–R373. [DOI] [PubMed] [Google Scholar]
- 35. Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development 2012; 139:4561–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bamberger AM, Ezzat S, Cao B, Wong M, Parker KL, Schulte HM, Asa SL. Expression of steroidogenic factor-1 (SF-1) mRNA and protein in the human placenta. Mol Hum Reprod 1996; 2:457–461. [DOI] [PubMed] [Google Scholar]
- 37. Martinez-Arguelles DB, Papadopoulos V. Epigenetic regulation of the expression of genes involved in steroid hormone biosynthesis and action. Steroids 2010; 75:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoivik EA, Aumo L, Aesoy R, Lillefosse H, Lewis AE, Perrett RM, Stallings NR, Hanley NA, Bakke M. Deoxyribonucleic acid methylation controls cell type-specific expression of steroidogenic factor 1. Endocrinol 2008; 149:5599–5609. [DOI] [PubMed] [Google Scholar]
- 39. Hoivik EA, Bjanesoy TE, Bakke M. Epigenetic regulation of the gene encoding steroidogenic factor-1. Mol Cell Endocrinol 2013; 371:133–139. [DOI] [PubMed] [Google Scholar]
- 40. Hiroi H, Christenson LK, Chang L, Sammel MD, Berger SL, Strauss JF, 3rd. Temporal and spatial changes in transcription factor binding and histone modifications at the steroidogenic acute regulatory protein (stAR) locus associated with stAR transcription. Mol Endocrinol 2004; 18:791–806. [DOI] [PubMed] [Google Scholar]
- 41. Hiroi H, Christenson LK, Strauss JF, 3rd. Regulation of transcription of the steroidogenic acute regulatory protein (StAR) gene: Temporal and spatial changes in transcription factor binding and histone modification. Mol Cell Endocrinol 2004; 215:119–126. [DOI] [PubMed] [Google Scholar]
- 42. Zhao D, Duan H, Kim YC, Jefcoate CR. Rodent StAR mRNA is substantially regulated by control of mRNA stability through sites in the 3′-untranslated region and through coupling to ongoing transcription. J Steroid Biochem Mol Biol 2005; 96:155–173. [DOI] [PubMed] [Google Scholar]
- 43. Okada M, Lee L, Maekawa R, Sato S, Kajimura T, Shinagawa M, Tamura I, Taketani T, Asada H, Tamura H, Sugino N. Epigenetic changes of the Cyp11a1 promoter region in granulosa cells undergoing Luteinization during ovulation in female rats. Endocrinol 2016; 157:3344–3354. [DOI] [PubMed] [Google Scholar]
- 44. Hakkarainen J, Zhang FP, Jokela H, Mayerhofer A, Behr R, Cisneros-Montalvo S, Nurmio M, Toppari J, Ohlsson C, Kotaja N, Sipila P, Poutanen M. Hydroxysteroid (17beta) dehydrogenase 1 expressed by Sertoli cells contributes to steroid synthesis and is required for male fertility. FASEB J 2018; 32:3229–3241. [DOI] [PubMed] [Google Scholar]
- 45. Drzewiecka H, Galecki B, Jarmolowska-Jurczyszyn D, Kluk A, Dyszkiewicz W, Jagodzinski PP. Increased expression of 17-beta-hydroxysteroid dehydrogenase type 1 in non-small cell lung cancer. Lung Cancer 2015; 87:107–116. [DOI] [PubMed] [Google Scholar]
- 46. Frycz BA, Murawa D, Wysocki-Borejsza M, Marciniak R, Murawa P, Drews M, Jagodzinski PP. Expression of 17beta-hydroxysteroid dehydrogenase type 1 in gastric cancer. Biomed Pharmacother 2013; 67:651–657. [DOI] [PubMed] [Google Scholar]
- 47. Bhavani V, Srinivasulu M, Ahuja YR, Hasan Q. Role of BRCA1, HSD17B1 and HSD17B2 methylation in breast cancer tissue. Cancer Biomark 2009; 5:207–213. [DOI] [PubMed] [Google Scholar]
- 48. Rawluszko AA, Horbacka K, Krokowicz P, Jagodzinski PP. Decreased expression of 17beta-hydroxysteroid dehydrogenase type 1 is associated with DNA hypermethylation in colorectal cancer located in the proximal colon. BMC Cancer 2011; 11:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marijanovic Z, Laubner D, Moller G, Gege C, Husen B, Adamski J, Breitling R. Closing the gap: Identification of human 3-ketosteroid reductase, the last unknown enzyme of mammalian cholesterol biosynthesis. Mol Endocrinol 2003; 17:1715–1725. [DOI] [PubMed] [Google Scholar]
- 50. Shehu A, Albarracin C, Devi YS, Luther K, Halperin J, Le J, Mao J, Duan RW, Frasor J, Gibori G. The stimulation of HSD17B7 expression by estradiol provides a powerful feed-forward mechanism for estradiol biosynthesis in breast cancer cells. Mol Endocrinol 2011; 25:754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cohen E, Baerts W, Bel F. Brain-sparing in intrauterine growth restriction: Considerations for the neonatologist. Neonatology 2015; 108:269–276. [DOI] [PubMed] [Google Scholar]
- 52. Gardner DS, Lea RG, Sinclair KD. Developmental programming of reproduction and fertility: What is the evidence? Animal 2008; 2:1128–1134. [DOI] [PubMed] [Google Scholar]
- 53. Chadio S, Kotsampasi B. Maternal undernutrition and developmental programming: implications for offspring reproductive potential In: Preedy V, Patel V (eds.), Handbook of Famine, Starvation, and Nutrient Deprivation. Springer International Publishing AG; 2017: 1–17. [Google Scholar]
- 54. Thorsted A, Lauridsen J, Hoyer B, Arendt LH, Bech B, Toft G, Hougaard K, Olsen J, Bonde JP, Ramlau-Hansen C. Birth weight for gestational age and the risk of infertility: A Danish cohort study. Hum Reprod 2020; 35:195–202. [DOI] [PubMed] [Google Scholar]
- 55. Wang N, Huang Y, Wen J, Su Q, Huang Y, Cai L, Lin W, Zong L, Huang H, Qian X, Zhu F, Sun H et al. Early life exposure to famine and reproductive aging among Chinese women. Menopause 2019; 26:463–468. [DOI] [PubMed] [Google Scholar]


